Supplemental Digital Content is available in the text.
Keywords: pertussis, whooping cough, seroprevalence, sub-Saharan Africa, vaccine
Abstract
Background:
Bordetella pertussis can cause severe respiratory disease and death in children. In recent years, large outbreaks have occurred in high-income countries; however, little is known about pertussis incidence in sub-Saharan Africa.
Methods:
We evaluated antibody responses to pertussis toxin (Ptx) from individuals aged between 2 and 90 years in rural Gambia. IgG-Ptx was measured using luminex xMAP technology. IgG-Ptx geometric mean concentrations (GMC) and their 95% confidence intervals were calculated. The proportion seropositive (>20 EU/mL or ≥62.5 EU/mL) and GMCs were compared by age, sex, ethnic group, vaccination status, birth order and number of siblings per household using logistic and linear regression.
Results:
76.3% had anti-Ptx levels <20 EU/mL, 17.5% had concentrations between 20 and 62.5 EU/mL, 4.4% had concentrations between 62.5 and 125 EU/mL and 1.8% had concentrations ≥125 EU/mL. The overall Ptx antibody GMC was 6.4 EU/mL (95% confidence interval: 5.8–6.9). Higher antibody concentrations were observed in older populations with evidence for an increase in infection risk with increasing age (1.9% yearly increase, 95% confidence interval: 1.3–2.5). No child under 6 years of age had GMC above 62.5 EU/mL but 29.5% had concentrations between 20 and 62.5 EU/mL.
Conclusions:
These data provide evidence that B. pertussis is being transmitted within this population despite high vaccination coverage. Re-infection may occur implying that immunity from childhood vaccination may not be lifelong. In the absence of data on actual clinical cases of pertussis, seroprevalence studies remain valuable tools to assess the transmission dynamics of B. pertussis.
The bacterium Bordetella pertussis which causes whooping cough can lead to severe respiratory disease and death mainly in infants. Recent mathematical models suggest that annually 16 million cases of pertussis occur worldwide, with 95% in low-income countries,1 and an estimated 81,400 die from this disease.2 The introduction of whole cell pertussis vaccines (WCVs) in the 1950s in industrialized countries resulted in a dramatic decrease in pertussis cases. Although efficacious, reactogenicity of WCVs led to suboptimal vaccine uptake, therefore, less reactogenic acellular vaccines (ACVs) were introduced.3 However, recent large outbreaks among infants and adults have been observed in several high-income countries.3–5 This resurgence of pertussis has most likely arisen through a combination of factors: improved diagnostics; pathogen adaptation which may have reduced the efficacy of pertussis vaccines6; waning immunity occurring after vaccination; vaccination which induces short duration of protection compared with natural infection with B. pertussis and finally, there is now evolving evidence to suggest that immunity induced by ACVs is less long lasting compared with WCVs vaccines.7–9
The substantial increase especially among adolescents and young adults, despite high vaccination coverage,5,10,11 is of public health concern as these individuals are important sources of infection for infants too young to be (fully) vaccinated.12,13 In high-income countries, young infants account for the majority of hospital admissions for pertussis and incidence in this age group is increasing.3,4,14These changes in the epidemiology of pertussis have raised concern that current vaccination strategies against pertussis might not be optimal and alternative strategies such as adolescent booster vaccinations, cocooning or maternal vaccination are now being considered and already implemented in some settings.1
To understand to what extent the findings from industrialized countries also reflect the situation in sub-Saharan Africa and whether pertussis has re-emerged as a threat in a population vaccinated with a WCV only, insight into the levels of infection in populations is required. However, there have been only a few studies in Africa: they were conducted either in the context of a vaccine trial,15,16 or hospital-based and provide no information on less severe or subclinical cases17 or focused only on children under 10 years of age.18 To assess immunity and potential susceptibility to pertussis at population levels, we evaluated antibody concentrations to pertussis toxin (Ptx) in a cross-sectional study in sera from individuals aged between 2 and 90 years of age living in rural Gambia.
MATERIALS AND METHODS
Study Area and Population
Antibody concentrations against pertussis were measured in banked serum samples of an existing longitudinal cohort. This prospective cohort was created in 1984, when HBV vaccination was initiated in two Gambian villages in the Kiang West region (Keneba and Manduar). Since then, cross-sectional serological surveys at approximately 4- to 5-year intervals have been conducted to assess long-term HBV vaccine effectiveness.19 At time of the original 2008 survey informed consent was given for use of stored blood samples for additional research. Stored samples from the 2008 serosurvey from adults and children aged >2 years of age were available to us. For children below the age of 2, a finger prick sample was collected and no serum remained for this study, hence this age group could not be included. For the remainder of the population, an age stratified sample was randomly selected, powered to achieve a 3% confidence interval (CI) for an estimate 5% seroprevalence. The current Expanded Programme on Immunisation schedule for the Gambia uses threedoses of the Pentavalent vaccine [Diphtheria–Tetanus–whole cell Pertussis (DTwP)–hepatitis B(HepB)-Hib] at 2, 3 and 4 months. A further fourth dose of DTwP is also given routinely at 18 months of age.20
Laboratory Methods
The banked sera were stored at -80°C until the time of testing. IgG antibodies directed against pertussis toxin (IgG-Ptx) were measured using luminex xMAP technology with a validated multiplex immunoassay for diphtheria, tetanus and pertussis as described previously.21 In brief, reconstituted freeze-dried Ptx (National Institute for Biological Standards and Control, South Mimms, United Kingdom) was conjugated to activated carboxylated microsphere (Bio-plex COOH beads, Bio-rad, Hemel Hempstead, United Kingdom) following published methods.22 The in-house pertussis standard (calibrated against the US reference pertussis anti-serum human lot 3 FDA) was diluted fourfold in 6 dilution steps (1:200–1:204,800), whereas unknown sera were diluted 1:200 and 1:4000. The detection antibody, goat anti-human IgG (γ chain specific) R-phycoerythrin conjugated (Jackson ImmunoResearch Laboratories Inc., Westgrove, PA) was diluted 1:200. Results were generated with a Bio-plex 200 system in combination with Bio-plex Manager software (version 4.1.1, Bio-rad, United Kingdom). Median fluorescent intensity was converted to EU/mL by interpolation from a 5-parameter logistic standard curve.
IgG-Ptx concentrations have been widely used to assess the prevalence of B. pertussis infections. Previous studies show that concentrations of anti-Ptx antibodies ≥62.5 and ≥125 EU/mL were evidence of a previous infection in the past 12 and 6 months, respectively.11,23–25 We categorized anti-Ptx IgG levels into 4 groups according to the estimated average time since infection; 0 to <20 EU/mL (no recent infection), 20 to <62.5 EU/mL (infection possibly more than a year ago and/or vaccination response), 62.5 to <125 (infection in the last year) and ≥125 EU/mL (infection in the past 6 months).
Statistical Analyses
Logarithms of anti-Ptx IgG levels and antibody geometric mean concentrations (GMC) and their 95% CIs were calculated. The proportion of seropositivity and GMCs were compared by age group. Logistic regression and χ2 tests were used to compare and test the statistical significant of the difference of Ptx seropositivity (as defined by ≥20 or ≥62.5 EU/mL) between covariates of interest (age, sex, ethnic group, vaccination status, birth order and number of siblings per household). Student’s t test and linear regression were used to estimate differences in log concentrations between covariate groups. Covariates were retained in the model if associations were observed at the P < 0·05 level and/or if they altered substantially the associations of other effect variables in multivariable analysis. Data on DwPT vaccination status were only systematically available for those born after January 1, 1996; thus, the above analysis was repeated to include vaccination status and restricted to this age group. Statistical analyses were performed using STATA 12·0 statistical software (http://www.stata.com).
Ethical Approval
Approval was obtained from The Government of The Gambia/Medical Research Council Joint Ethics Committee (SCC1261).
RESULTS
A total of 1893 individuals from Keneba and Manduar were included in the initial 2008 HBV serosurvey. One thousand and sixty-seven (56·4%) of these samples were randomly selected throughout all age categories and included in this analysis. The general characteristics of the study population and levels of Ptx concentrations are shown in Table 1. 95.5% were of Mandinka ethnic origin. Available data with vaccine records in the cohort (n = 298) show that 86% had received at least one dose and 77% at least 3 doses of DwPT vaccine (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C30).
TABLE 1.
General Characteristics of the Keneba and Manduar Population, 2008 Serosurvey
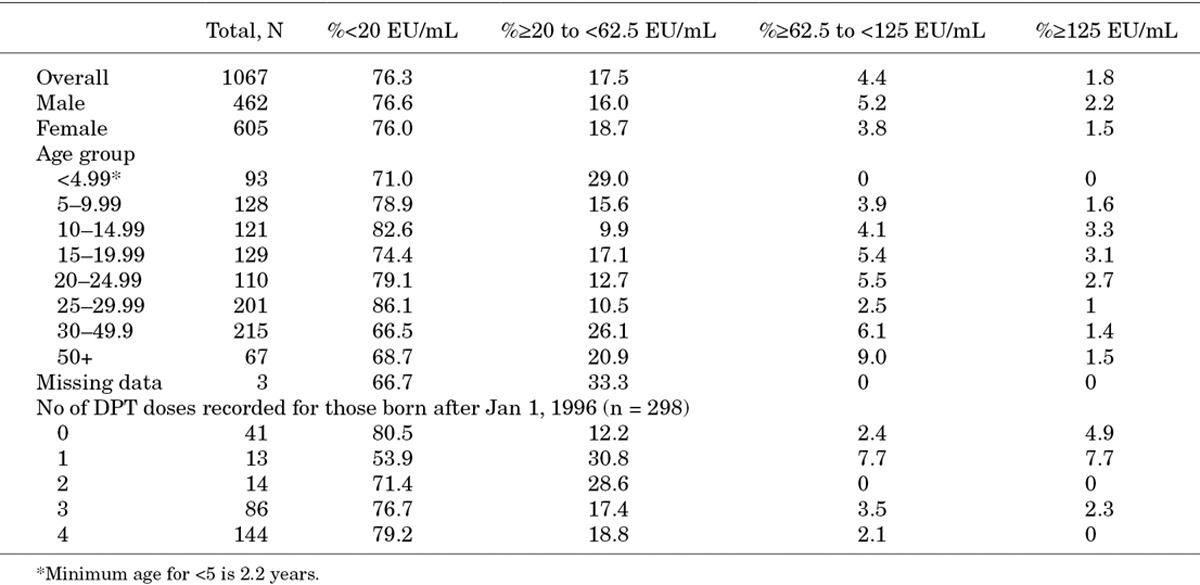
Ptx IgG Antibody Levels
Overall, 76·3% had anti-Ptx concentrations <20 EU/mL, 17.5% had concentrations between 20 and 62.5 EU/mL, 4.4% had concentrations between 62.5 and 125 EU/mL. Fewer than 2% of the population had concentrations ≥125 EU/mL. Ptx IgG concentrations by age groups are shown in Figures 1 and 2.
FIGURE 1.
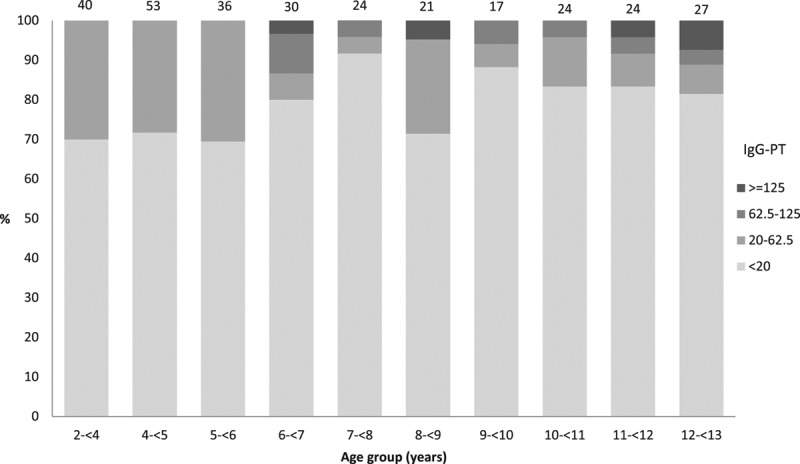
Concentrations of Ptx IgG by age group for those with vaccination records (born after January 1, 1996), 2008 serosurvey in Keneba and Manduar, Kiang West Region, The Gambia. Numbers on top of bars Total number sampled by age group.
FIGURE 2.
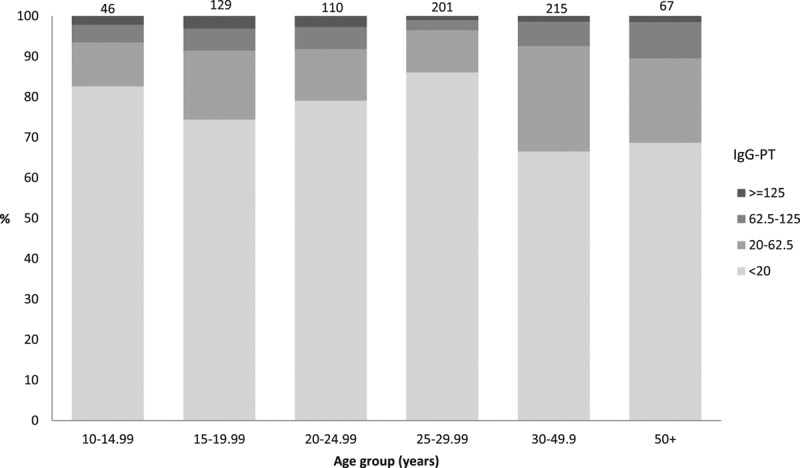
Concentrations of Ptx IgG by age group for those without vaccination records (born before January 1, 1996), 2008 serosurvey in Keneba and Manduar, Kiang West Region, The Gambia. Numbers on top of bars Total number sampled by age group.
Overall Ptx antibody GMC was 6·4 EU/mL (95% CI: 5.8–6.9), but 6.2% had anti-Ptx antibody concentrations ≥62.5 EU/mL (Fig. 3). Increasing age was associated with increasing GMC (1.9 % yearly increase, 95% CI: 1.3–2.5). The odds of having antibody concentrations ≥62.5 EU/mL were greater among those over 50 years of age compared with those younger than 10 years [odds ratio: 10·4 (95% CI: 4.3–20.3)]. There was no evidence for other risk factors (gender, ethnic group, birth order or sibling order) associated with Ptx antibody concentrations and therefore no multivariable analysis was performed (Table, Supplemental Digital Content 2, http://links.lww.com/INF/C29).
FIGURE 3.
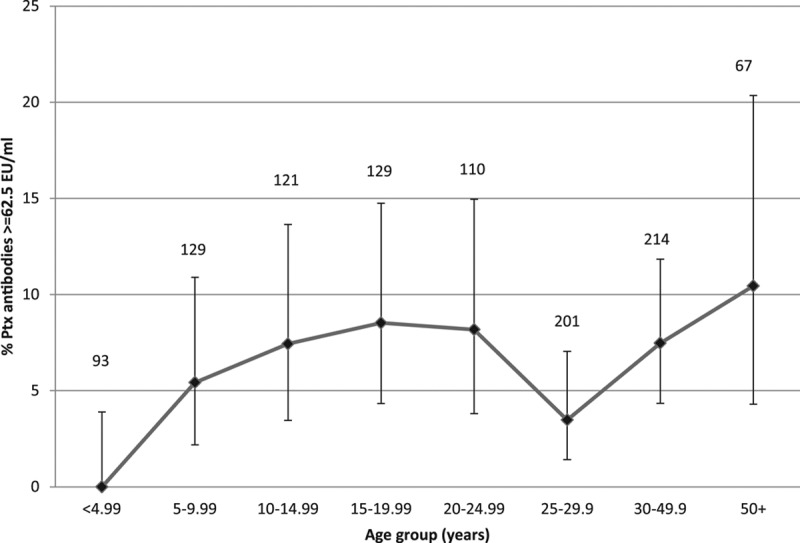
Proportion with Ptx IgG concentration ≥62.5 EU/mL by age group in years: reflecting infection within the last year, 2008 serosurvey in Keneba and Manduar, Kiang West Region, The Gambia. Vertical bars 95% CIs, numbers on top of bars total number sampled by age group.
For those born after January 1, 1996, 22.8% (95% CI: 18–28) of children less than 13 years of age had anti-Ptx antibody concentrations ≥20 EU/mL. The overall anti-Ptx antibody GMC was 4.9 EU/mL (95% CI: 4.1–5.8) (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C30). No child under 6 years of age (0/93) had GMC above 62.5 EU/mL but 29.5% (95% CI: 21.8–38.1) had concentrations between 20 and 62·5 EU/mL. Children aged between 6 and 10 years had lower odds of having concentrations above 20 EU/mL (OR: 0.5, 95% CI: 0.3–1.0) and a 35.5% (95% CI: 3.9–56.7) decrease in GMC compared with children aged less than 6 years of age. This association was not observed among those aged between 10 and 13 years (P = 0·069). Being Mandinka was associated with lower antibody concentrations [47·2% decrease (95% CI: 3.7–71.0)]. There was no evidence for other risk factors (gender, birth order, number of siblings per household or the number of DPT doses recorded) associated with Ptx antibody concentrations and therefore no multivariable analysis was performed (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C30).
DISCUSSION
Our data from a 2008 rural population cohort of over 1000 individuals aged between 2 and 90 years in The Gambia showed that 6% of individuals had antibody concentrations indicative of a pertussis infection within the last year (≥62.5 EU/mL). These data provide evidence that B. pertussis continues to be transmitted within this population despite high vaccination coverage. High IgG-Ptx concentrations (≥62.5 EU/mL) are a highly specific criterion for a pertussis infection within the last year.24 Following infection with B. pertussis, an increase of IgG-Ptx is observed which reaches a maximum within a few weeks and then declines steadily for 6–12 months after infection.26 Interpretation of IgG-Ptx concentrations is complicated by the fact that all pertussis vaccines contain Ptx. However, most WCVs hardly induce IgG-Ptx antibodies and high concentrations of IgG-Ptx induced by ACVs rapidly wane within 6 months.23 The definitive level of antibodies required to protect against pertussis infection is not established, and evidence indicates an additional role of T-cell mediated immunity.27,28 Unfortunately, no samples were available to conduct T cell-based stimulation assays in this Gambian cohort.
Despite the population differences, our results are comparable with data from a resource-rich country: a seroprevalence study conducted in the Netherlands in 1995/1996 by our co-authors using samples of WCV vaccinated individuals analyzed with identical laboratory methods showed that 4.0% of individuals (95% CI: 3.3–4.7) above >9 years of age had IgG-Ptx concentrations ≥62.5 EU/mL.11 For both studies, MIA Luminex was used, the correlation between MIA and Elisa was excellent in previous studies, and cut-off values are similar for both assays and based on the same international standard.11,21,29
Pertussis vaccine update in our cohort was generally high. The Expanded Programme on Immunisation was initiated in The Gambia in May 1979, initially administering BCG, Diphtheria–Tetanus–whole cell Pertussis (DTwP), measles, oral polio and yellow fever. HepB vaccine was phased in between 1986 and 1990 with Haemophilus influenzae type b (Hib) vaccine added to the schedules in 1997. In 2009, the pentavalent vaccine (DwPT-HepB-Hib) was introduced, and lately PCV13 and rotavirus vaccination were added. Data from our demographic surveillance system in Kiang West between 2005 and 2012 show that in general, over 95% of infants received 3 doses of DwPT and 83% of infants received a fourth dose.20 These data are comparable with our study participants albeit with slightly lower coverage in our study population; in children <6 years of age, 93% were vaccinated with at least one dose of DwPT and 81.4% had received 3 or 4 doses. No child in this age group showed serological evidence of recent pertussis infection, possibly as they remain protected by the primary immunizations. In children aged between 6 and 10 years, overall anti-Ptx IgG concentrations were lower compared with the younger age group and 5.5% in this age group had concentrations ≥62.5 EU/mL. Both of these observations suggest waning of vaccine-induced immunity and evidence for increasing susceptibility to wild-type infection. The lack of very high concentrations observed in younger age groups could potentially be due to a blunted response, due to recent vaccination and hence resulting in less severe infection.
The higher antibody concentrations observed in the older age groups are consistent with increased risk of infection with increasing age, implying that immunity from childhood vaccination or disease may not be lifelong. However, because it is not possible to distinguish between antibodies induced by vaccination or infection using our current assays, it is also plausible that observed Ptx antibody concentrations may be the result of natural boosting. Our findings are consistent with previous studies in Senegal,18 and further add to the evidence that the current pertussis vaccination strategies in sub-Saharan Africa may not be sufficient to provide long-term immunity. Alternatively increasing antibody levels with age could be due to age-related cumulative exposure to B. pertussis over time, possibly resulting in boosting of specific memory immune responses by continuous encounters with B. pertussis antigens.30
Because there are no current data on pertussis incidence or case-fatality rates from low-income countries, expert opinion and modeling remain as the only methods available to provide such estimates.2 Only one study in the 1980s to mid-1990s from Senegal carried out active surveillance within communities to measure changes in pertussis incidence pre- and post-vaccination introduction.15,16 Children less than 15 years of age were followed over a 13-year period for the occurrence of pertussis.16 The crude pertussis incidence before vaccination was 183 per 1000 child-years, with a 2.8% case-fatality rate. After the introduction of the vaccination program, overall incidence dropped by 27% after 3 years and 46% after 6 years. A recent hospital study from Niger has reported 11.2% out of 305 children aged <5 years admitted with respiratory symptoms at the National Hospital of Niamey were positive for a Bordetella species detected by PCR, but only 1.3% were positive for B. pertussis.17 The more recent Senegalese population-based data reported no cases of pertussis disease in children less than 10 years of age.18 Anecdotally and according to hospital admission data, very little pertussis disease is being observed in any age group in The Gambia (Dr. S. Anderson, Head of Clinical Services, MRC Unit-The Gambia, personal communication). Misdiagnosis could be an important reason for the lack of clinical diagnosis of pertussis in The Gambia and other similar settings, as well as possibly underreporting of milder cases which may not be considered severe enough to report to health facilities, particularly in an African rural context.18 Pertussis diagnosis in adolescents and adults is often delayed or missed because of mild symptoms.3 Pertussis in infants can also present atypically; there may be no cough,17 and children may die from extreme leukocytosis and pulmonary hypertension or even other causes (later) due to weight loss and secondary infections. Thus, without laboratory confirmation the cause of severe morbidity or death can often not be attributed directly to a pertussis infection. This remains a challenge in particular in resource-poor settings.
An important question is whether WCV may be better at preventing severe disease compared with ACV when given during childhood, even if not preventing infection.7,9 Evolving evidence suggests that immunity induced by ACVs is less long lasting compared with WCVs vaccines.7–9 Infant priming with WCV has been shown to be associated with a lower risk of subsequent pertussis than ACV only primed infants,7,9 with the effect of protection through WCV persisting for more than a decade.9 Long-term protection against disease was also observed when immunity was induced by natural infection, and evidence from human and animal studies suggest that natural infection is more effective than vaccination in conferring protection against subsequent (secondary) infection. The baboon model of pertussis suggests that both WCV- and ACV-immunized baboons can be re-infected, but B. pertussis is cleared faster in the former group.8 Further studies of immunity in countries where WCVs are still used, such as the Gambia, will provide an opportunity to further clarify the interdependence of cellular and humoral mechanisms of protection.
Very little is known about currently circulating strain types in the Gambia and their virulence factors in comparison to those in higher income countries. Further studies of strain diversity and its impact on host responses may be warranted to compare populations that use either WCV or ACV to better understand the epidemiological differences between high- and low-income countries, and to guide public health efforts to protect the population against severe pertussis infections.
In conclusion, our serological study shows that B. pertussis is still circulating within populations of The Gambia. In the absence of data on actual clinical cases of pertussis, seroprevalence studies remain valuable tools to assess the transmission dynamics of B. pertussis. However, without data from case findings studies the long-term impact of vaccination on pertussis incidence and severity of illness in low-income countries remains unclear.
ACKNOWLEDGMENTS
The authors extend their thanks to the residents of Keneba and Manduar villages in the Kiang West Region of the Gambia who agreed to take part in the 2008 serosurvey. The authors also acknowledge Sabine de Greeff for her valuable input and discussions in the design of this study. Sir Andy Hall, Hilton Whittle, Sophie Moore, the data and field team at MRC Keneba and all those involved in the Hepatitis B cohort.
Supplementary Material
Footnotes
The study was funded by UK Medical Research Council/Dutch Centre for Infectious Disease Control.
The authors have no other conflicts of interest or funding to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:385–400. [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008;9:201–211. doi: 10.1016/j.prrv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Galanis E, King AS, Varughese P, et al. investigators I. Changing epidemiology and emerging risk groups for pertussis. CMAJ. 2006;174:451–452. doi: 10.1503/cmaj.050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celentano LP, Massari M, Paramatti D, et al. Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24:761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 6.Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect. 2014;142:685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liko J, Robison SG, Cieslak PR. Priming with whole-cell versus acellular pertussis vaccine. N Engl J Med. 2013;368:581–582. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- 8.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan SL, Ware RS, Grimwood K, et al. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308:454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 10.Skowronski DM, De Serres G, MacDonald D, et al. The changing age and seasonal profile of pertussis in Canada. J Infect Dis. 2002;185:1448–1453. doi: 10.1086/340280. [DOI] [PubMed] [Google Scholar]
- 11.de Greeff SC, de Melker HE, van Gageldonk PG, et al. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One. 2010;5:e14183. doi: 10.1371/journal.pone.0014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Greeff SC, Mooi FR, Westerhof A, et al. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis. 2010;50:1339–1345. doi: 10.1086/652281. [DOI] [PubMed] [Google Scholar]
- 13.Kowalzik F, Barbosa AP, Fernandes VR, et al. Prospective multinational study of pertussis infection in hospitalized infants and their household contacts. Pediatr Infect Dis J. 2007;26:238–242. doi: 10.1097/01.inf.0000256750.07118.ee. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Vitek CR, Pascual FB, et al. Trends in pertussis among infants in the United States, 1980–1999. JAMA. 2003;290:2968–2975. doi: 10.1001/jama.290.22.2968. [DOI] [PubMed] [Google Scholar]
- 15.Simondon F, Preziosi MP, Yam A, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997;15:1606–1612. doi: 10.1016/s0264-410x(97)00100-x. [DOI] [PubMed] [Google Scholar]
- 16.Preziosi MP, Yam A, Wassilak SG, et al. Epidemiology of pertussis in a West African community before and after introduction of a widespread vaccination program. Am J Epidemiol. 2002;155:891–896. doi: 10.1093/aje/155.10.891. [DOI] [PubMed] [Google Scholar]
- 17.Jusot V, Aberrane S, Ale F, et al. Prevalence of Bordetella infection in a hospital setting in Niamey, Niger. J Trop Pediatr. 2014;60:223–230. doi: 10.1093/tropej/fmu001. [DOI] [PubMed] [Google Scholar]
- 18.Gaayeb L, Sarr JB, Ndiath MO, et al. Seroprevalence of pertussis in Senegal: a prospective study. PLoS One. 2012;7:e48684. doi: 10.1371/journal.pone.0048684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendy M, Peterson I, Hossin S, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. doi: 10.1371/journal.pone.0058029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott S, Odutola A, Mackenzie G, et al. Evaluation of the EPI programme in the Gambia: implications of the current immunisation schedule and for new vaccine introduction. PLoS One. 2014;9:e107280. doi: 10.1371/journal.pone.0107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gageldonk PG, van Schaijk FG, van der Klis FR, et al. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008;335:79–89. doi: 10.1016/j.jim.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 23.Hallander HO, Ljungman M, Storsaeter J, et al. Kinetics and sensitivity of ELISA IgG pertussis antitoxin after infection and vaccination with Bordetella pertussis in young children. APMIS. 2009;117:797–807. doi: 10.1111/j.1600-0463.2009.02530.x. [DOI] [PubMed] [Google Scholar]
- 24.de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, et al. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol. 2000;38:800–806. doi: 10.1128/jcm.38.2.800-806.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Greeff SC, Teunis P, de Melker HE, et al. Two-component cluster analysis of a large serodiagnostic database for specificity of increases of IgG antibodies against pertussis toxin in paired serum samples and of absolute values in single serum samples. Clin Vaccine Immunol. 2012;19:1452–1456. doi: 10.1128/CVI.00229-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallander HO, Gustafsson L, Ljungman M, et al. Pertussis antitoxin decay after vaccination with DTPa. Response to a first booster dose 3 1/2-6 1/2 years after the third vaccine dose. Vaccine. 2005;23:5359–5364. doi: 10.1016/j.vaccine.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Mahon BP, Brady MT, Mills KH. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J Infect Dis. 2000;181:2087–2091. doi: 10.1086/315527. [DOI] [PubMed] [Google Scholar]
- 28.Higgins SC, Jarnicki AG, Lavelle EC, et al. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control. External quality assurance scheme for Bordetella pertussis serology 2013. Stockholm: ECDC; 2014. [Google Scholar]
- 30.Versteegh FG, Mertens PL, de Melker HE, et al. Age-specific long-term course of IgG antibodies to pertussis toxin after symptomatic infection with Bordetella pertussis. Epidemiol Infect. 2005;133:737–748. doi: 10.1017/s0950268805003833. [DOI] [PMC free article] [PubMed] [Google Scholar]


