Significance
Appropriate homeostatic regulation of catecholamines (dopamine, norepinephrine) is important for the maintenance of normal brain function and mental state. The dysregulation of dopamine systems has been correlated with a hyperactive phenotype and social abnormalities, which are frequently observed in patients with psychiatric disorders. In this report, we found that IRBIT regulates catecholamine homeostasis by binding to calcium calmodulin-dependent kinase II alpha and subsequently controlling tyrosine hydroxylase phosphorylation. In addition, mice lacking IRBIT present with increased locomotor activity and social abnormalities. Our finding provides new insight into the homeostatic regulation of catecholamines.
Keywords: IRBIT, CaMKIIα, catecholamine, hyperactivity
Abstract
Inositol 1,4,5-trisphosphate receptor (IP3R) binding protein released with IP3 (IRBIT) contributes to various physiological events (electrolyte transport and fluid secretion, mRNA polyadenylation, and the maintenance of genomic integrity) through its interaction with multiple targets. However, little is known about the physiological role of IRBIT in the brain. Here we identified calcium calmodulin-dependent kinase II alpha (CaMKIIα) as an IRBIT-interacting molecule in the central nervous system. IRBIT binds to and suppresses CaMKIIα kinase activity by inhibiting the binding of calmodulin to CaMKIIα. In addition, we show that mice lacking IRBIT present with elevated catecholamine levels, increased locomotor activity, and social abnormalities. The level of tyrosine hydroxylase (TH) phosphorylation by CaMKIIα, which affects TH activity, was significantly increased in the ventral tegmental area of IRBIT-deficient mice. We concluded that IRBIT suppresses CaMKIIα activity and contributes to catecholamine homeostasis through TH phosphorylation.
Inositol 1,4,5-trisphosphate receptor (IP3R) binding protein released with IP3 (IRBIT) was originally identified as a molecule that interacts with the intracellular calcium channel, IP3R. IRBIT binds to and suppresses IP3R activity in the resting state by blocking IP3 access to IP3R (1, 2). Our group and others have reported that IRBIT contributes to electrolyte transport, mRNA processing, and the maintenance of genomic integrity (3–9) through its interaction with multiple targets. However, little is known about the physiological role of IRBIT in the brain, where it is most highly expressed (1).
Calcium calmodulin (CaM) dependent kinase II alpha (CaMKIIα) is a Ser/Thr kinase that is abundant in the central nervous system and is activated by the binding of Ca2+–CaM. CaMKIIα phosphorylates various target proteins and is involved in the regulation of synaptic transmission and plasticity (10, 11). CaMKIIα is expressed in the hippocampus, neocortex, thalamus, hypothalamus, olfactory bulb, cerebellum, and basal ganglia (12, 13). Many studies involving mutant mice and also pharmacological studies have indicated that CaMKIIα activity is essential for the acquisition of memory and learning (14, 15). In addition, the appropriate regulation of CaMKIIα is required for cognitive function and mood control (16–18). Thus, aberrant CaMKIIα activity is associated with several neuronal disorders such as schizophrenia, autism spectrum disorder, attention-deficit hyperactivity disorder (ADHD), and drug addiction, in which hyperactivity and social abnormalities are frequently observed (19–23). However, the precise mechanism linking CaMKIIα dysregulation and mental disorders is poorly understood.
Recent behavioral studies using knockout (KO) mouse models or pharmacological approaches have revealed that the dysregulation of dopamine (DA) systems is correlated with a hyperactive phenotype and social abnormalities (24–26). The catecholamines, DA and norepinephrine (NE) are biosynthesized from the amino acids phenylalanine and tyrosine. The sequence of steps starts with the enzyme, tyrosine hydroxylase (TH). Thus, TH is the rate-limiting enzyme for both DA and NE synthesis. The appropriate regulation of TH activity is important for the maintenance of normal brain function and mental state (27).
In this study, we identified CaMKIIα as an IRBIT-interacting molecule in the central nervous system. IRBIT binds to and suppresses the kinase activity of CaMKIIα by inhibiting the binding of CaM to CaMKIIα. In addition, we found that mice deficient in IRBIT present with hyperactivity and social abnormalities. In IRBIT KO mice, we observed increased catecholamine levels and hyperphosphorylation of Ser19 on TH, which is known to enhance TH activity and increase the biosynthesis of DA and NE (27, 28). Thus, we have concluded that IRBIT regulates CaMKIIα activity and contributes to catecholamine homeostasis through TH phosphorylation.
Results
IRBIT Interacts with CaMKIIα and Is Dissociated by an Excess of Ca2+–CaM.
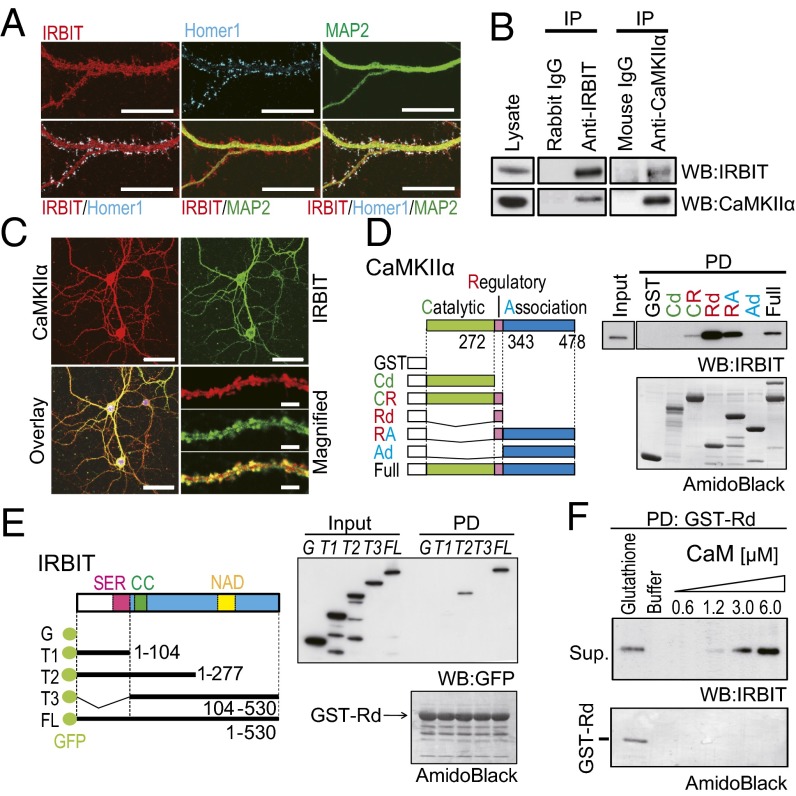
Because IRBIT is highly expressed in dendrites and spine-like structures and colocalizes with Homer 1, which is a postsynaptic marker in hippocampal neurons (29) (Fig. 1A), we speculate that IRBIT interacts with synaptic molecules and contributes to neuronal function. To identify the proteins that interact with IRBIT, we immunoprecipitated (IP) IRBIT from a crude membrane fraction of the hippocampus, and IRBIT-associated proteins were identified using mass-spectrometry analysis. We found several peptides that matched the sequence of CaMKIIα (SI Materials and Methods and Fig. S1A). Physical interactions between IRBIT and CaMKIIα have also been reported in proximal tubule cells (7). To confirm the interaction between IRBIT and CaMKIIα in the brain, we performed a co-IP assay of samples from the hippocampus using anti-IRBIT and anti-CaMKIIα antibodies. IRBIT was co-IP with CaMKIIα using anti-CaMKIIα antibody, and vice versa (Fig. 1B). Moreover, the immunostaining of primary-cultured hippocampal neurons showed that IRBIT and CaMKIIα were extensively colocalized in spines and dendrites (Fig. 1C).
Fig. 1.
IRBIT interacted with CaMKIIα. (A) Cultured hippocampal neurons were stained with antibodies against IRBIT (red), Homer 1 (blue), and MAP2 (green). (Scale bar, 20 μm.) (B) Co-IP of IRBIT and CaMKIIα from the hippocampus. (C) The expression of CaMKIIα (red) and IRBIT (green) antibodies. (Scale bar, 50 μm.) The magnified images of the dendrite are shown. (Scale bar, 5 μm.) (D) Identification of CaMKIIα binding region to IRBIT in vitro. (Left) Schematic diagram of GST–CaMKIIα truncated mutants. CR, catalytic domain plus regulatory domain; Rd, regulatory domain; RA, regulatory domain plus associated domain; Cd, catalytic domain; Ad, associated domain. (Right) Pull-down assay using various GST–CaMKIIα mutants and purified IRBIT (from Sf9 cells). (E) Identification of the binding region of IRBIT to CaMKIIα. (Left) Schematic diagram of GFP–IRBIT truncated mutants. (Right) Pull-down assay using GST–Rd and GFP–IRBIT truncation mutants. (F) Ca2+–CaM and IRBIT competitively bind to CaMKIIα. Purified IRBIT was pulled down with GST–Rd and eluted with various concentration of Ca2+–CaM. Elution with glutathione was used as a positive control.
To further verify the direct interaction between IRBIT and CaMKIIα, we performed a pull-down (PD) assay with the purified CaMKIIα deletion mutants and purified IRBIT (from Spodoptera frugiperda 9 (Sf9) cells) and determined their binding domains. IRBIT bound to full-length CaMKIIα protein in vitro (Fig. 1D). We also found that IRBIT binds to CaMKIIα fragments that contain the regulatory domain (CR, Rd, and RA), but not to fragments containing the catalytic domain (Cd) or associated domain (Ad). As a control, no binding of IRBIT was observed to GST alone. To determine the region of IRBIT that is responsible for the interaction with CaMKIIα, we performed a PD assay using various GFP–IRBIT deletion mutants and the regulatory domain of CaMKIIα. CaMKIIα bound to the T2 fragment and full-length (FL) IRBIT, but not to the T1 and T3 fragments, or GFP alone (Fig. 1E). These results indicate that IRBIT directly binds to CaMKIIα in vitro. The amino acid residues 272–343 of CaMKIIα (Rd) are sufficient for binding to IRBIT, and the two segments including the amino acid residues 1–104 and 105–277 of IRBIT are both necessary for binding to CaMKIIα. Because the Ca2+–CaM complex also binds to the regulatory domain of CaMKIIα, we next tested whether IRBIT and the Ca2+–CaM complex bind competitively to the regulatory domain. An excess of Ca2+–CaM dissociated IRBIT from the regulatory domain of CaMKIIα (Fig. 1F).
IRBIT Inhibits Kinase Activity of CaMKIIα in Vitro.
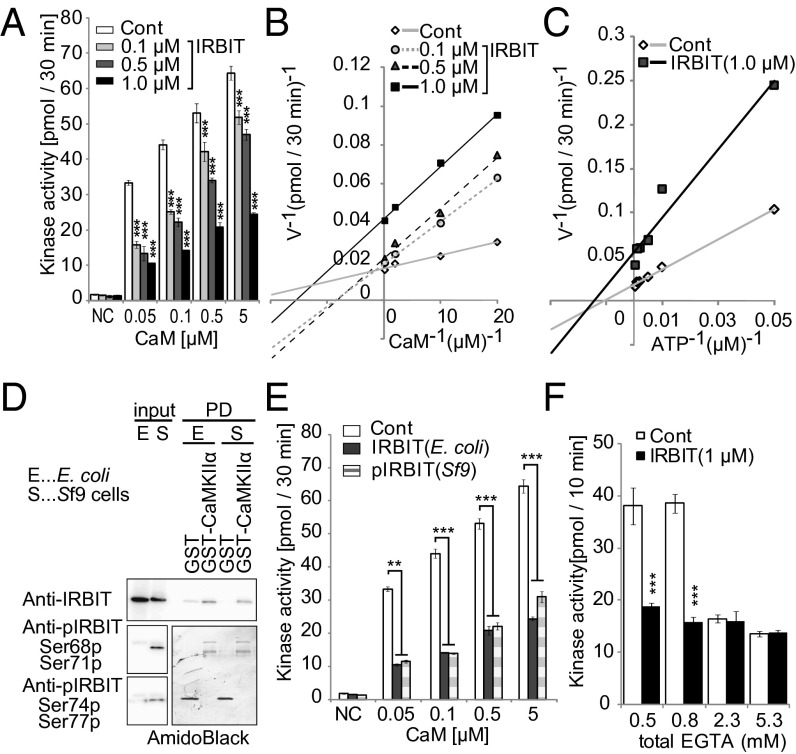
Although a previous report showed that IRBIT is a substrate of CaMKIIα (7), the effect of IRBIT on CaMKIIα activity is unknown. To explore the functional effect of IRBIT binding on CaMKIIα kinase activity, we performed an in vitro kinase assay using purified IRBIT (from Sf9 cells). IRBIT significantly inhibited CaMKIIα kinase activity in a concentration-dependent manner (Fig. 2A). When we added a relatively lower concentration of IRBIT (0.1 or 0.5 μM), the double-reciprocal analysis of these data yielded straight lines intersecting on the y axis, indicating simple competitive inhibition of CaMKIIα by IRBIT with respect to the Ca2+–CaM complex (Fig. 2B). Interestingly, the double-reciprocal plot for 1.0 μM IRBIT showed complex inhibition. Boiled IRBIT did not affect the CaMKIIα kinase activity (Fig. S1B). In addition, a large excess of CaM (50 μM) completely blocked the inhibition of CaMKIIα by 1.0 μM IRBIT (Fig. S1C). In contrast, the inhibition by IRBIT was noncompetitive with respect to ATP (Fig. S1D and Fig. 2C) and was not affected by an excess of substrate (Fig. S1 E and F). Therefore, we concluded that IRBIT inhibits CaMKIIα kinase activity by competing with the Ca2+–CaM complex.
Fig. 2.
IRBIT inhibits the CaMKIIα kinase activity in vitro. (A) Effect of IRBIT on the Ca2+–CaM dependency of CaMKIIα activity. Synthetic peptide (syntide-2) was phosphorylated by purified CaMKIIα (4 nM) with or without IRBIT. CaM and IRBIT concentrations are indicated. Negative control (NC): kinase assay without CaM. ATP: 200 μM. Syntide-2: 10 μM. (B) The double-reciprocal plot analysis of Fig. 2A. (C) Effect of IRBIT on the ATP dependency of CaMKIIα activity. CaM: 0.1 μM. Syntide-2: 10 μM. (D) Effect of IRBIT phosphorylation on the IRBIT–CaMKIIα interaction. Purified IRBIT from E. coli. or Sf9 cells were pulled down with GST–CaMKIIα. (E) Effect of nonphospho-IRBIT (E. coli) and phospho-IRBIT (Sf9 cells) on the CaMKIIα activity. ATP: 200 μM. Syntide-2: 10 μM. (F) Effect of IRBIT on the Ca2+–CaM-independent CaMKIIα activity. In vitro kinase assay was performed with various concentrations of EGTA. n = 3. ATP: 200 μM. Syntide-2: 10 μM.
Because IRBIT interacts with various binding partners in a phosphorylation-dependent manner (2–4), we next examined the phosphorylation dependency of the IRBIT–CaMKIIα interaction using nonphospho-IRBIT and phosphorylated IRBIT, purified from Escherichia coli and Sf9 insect cells, respectively. Both nonphospho-IRBIT and phospho-IRBIT interacted similarly with GST–CaMKIIα (Fig. 2D). Phosphorylation of IRBIT was confirmed using antibodies specific for phospho-IRBIT (S68p/S71p and S74p/S77p). In addition, nonphospho-IRBIT inhibited CaMKIIα kinase activity as effectively as phospho-IRBIT (Fig. 2E). These results indicated that the phosphorylation of IRBIT (at S68/S71 and S74/S77), which is essential for other known interactions, is not necessary for the regulation of CaMKIIα.
CaMKIIα has Ca2+–CaM-independent activity in its T286 autophosphorylated form (30). We therefore investigated the effect of IRBIT on the Ca2+–CaM-independent activity of CaMKIIα. Purified CaMKIIα was preincubated with Ca2+–CaM and ATP to achieve autophosphorylation. Phosphorylated CaMKIIα was mixed with substrate and IRBIT in the presence of various concentrations of EGTA (details in Fig. S1G). As shown in Fig. 2F, IRBIT inhibited the CaMKIIα kinase activity at low concentrations of EGTA (0.3, 0.8 μM). However, IRBIT did not affect kinase activity at higher concentrations of EGTA (2.3, 5.3 μM). Thus, IRBIT did not regulate Ca2+–CaM-independent activity of CaMKIIα. In addition, we confirmed the effect of IRBIT on CaMKIIα kinase activity using recombinant Homer 3 protein, which is a substrate for CaMKIIα (31). IRBIT significantly inhibited the phosphorylation of Homer 3 in a concentration-dependent manner (Fig. S1 H and I). Therefore, we concluded that IRBIT negatively regulates CaMKIIα kinase activity in vitro.
IRBIT Regulates Kinase Activity of CaMKIIα in Living Cells.
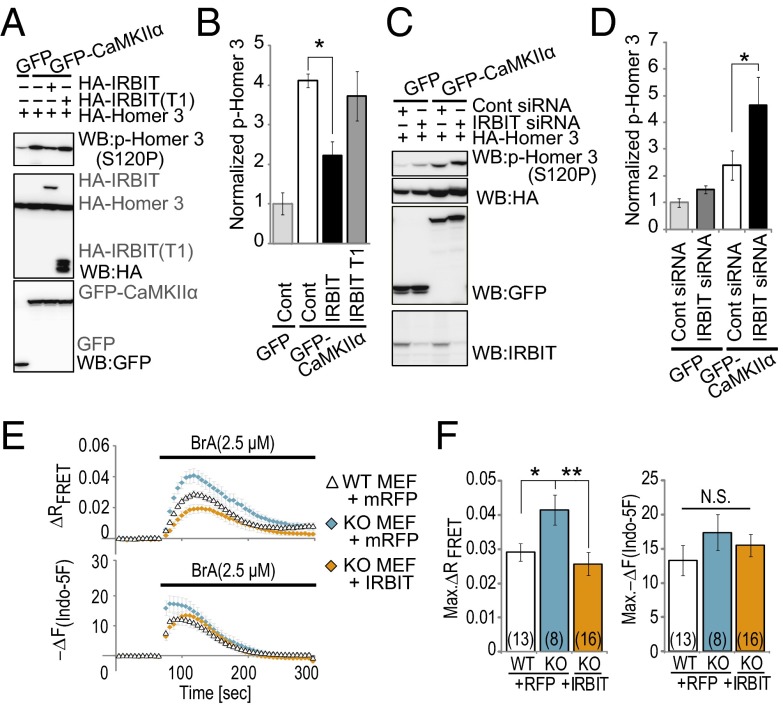
To further explore the functional effect of IRBIT on CaMKIIα activity in living cells, we examined the effect of IRBIT overexpression on the Ca2+-ionophore (4-Bromo-A23187, BrA) induced phosphorylation of Homer 3 in HEK-293 cells that stably express GFP–CaMKIIα (GFP–CaMKIIα cells). Because IRBIT has been reported as a CaMKIIα substrate (7), we used an IRBIT T1 fragment (indicated in Fig. 1E), with disrupted binding to CaMKIIα, but containing the consensus sequence for the CaMKIIα substrate motif as a negative control. The magnitude of the BrA-induced increase in intracellular calcium was not affected by the overexpression of IRBIT or the IRBIT T1 fragment compared with control cells (Fig. S2 A and B). However, overexpression of IRBIT significantly reduced the BrA-induced phosphorylation of Homer 3 compared with the control (Fig. 3 A and B), whereas overexpression of the IRBIT T1 fragment did not. We then investigated the effect of IRBIT knockdown on CaMKIIα kinase activity within cells. Transfection with siRNAs targeting IRBIT clearly abolished the IRBIT signal in GFP–CaMKIIα cells (Fig. S2C), but did not affect the calcium increase induced by BrA (Fig. S2 D and E). Then we expressed HA–Homer 3 with control siRNAs or IRBIT siRNAs in GFP–CaMKIIα cells and stimulated the cells using 2.5 μM BrA. The knockdown of IRBIT significantly increased the BrA-induced phosphorylation of Homer 3 compared with the control siRNA (Fig. 3 C and D).
Fig. 3.
IRBIT suppresses the CaMKIIα kinase activity in living cells. (A) IRBIT overexpression inhibited phosphorylation of Homer 3. GFP or GFP–CaMKIIα cells were transfected with HA–Homer 3 and HA–IRBIT or HA–IRBIT (T1, 1–104). After 24 h, cells were stimulated with 2.5 μM BrA for 2 min. (B) Quantitative analysis of phospho-Homer 3 in Fig. 3A. n = 3. (C) Phosphorylation of Homer 3 was enhanced in IRBIT knock-down cells. GFP–CaMKIIα cells were transfected with HA–Homer 3 and control siRNA or IRBIT siRNA. After 48 h, cells were stimulated with 2.5 μM BrA for 2 min. (D) Quantitative analysis of phospho-Homer 3 in Fig. 3C. n = 4. (E) Simultaneously imaging of FRET and Ca2+ change in IRBIT WT or KO MEF cells transfected with mRFP or IRBIT/mRFP. (Upper) Representative FRET changes are shown. (Lower) Representative Ca2+ responses (indo-5F) are shown. (F) Quantitation of FRET and Ca2+ peak amplitude. Peak amplitude FRET (Max. ΔR FRET) and Ca2+ responses [Max. −ΔF(Indo-5F)] that were expressed as the averaged amplitude of 0–50 s are equal to zero. Results show three independent experiments. The total cell numbers are indicated in each graph.
To further confirm the effect of IRBIT on CaMKIIα activity, we expressed a fluorescence resonance energy transfer (FRET)-based CaMKIIα activity probe (Camuiα) (32) in mouse embryonic fibroblast (MEF) cells from IRBIT KO and wild-type (WT) mice. The morphology of IRBIT KO MEF cells was similar to WT MEF cells (Fig. S3A). Both WT and KO MEF cells showed roughly normal growth rates and expressed other Ca2+-associated proteins at a similar level (Fig. S3B). Interactions between IRBIT and Camuiα were confirmed by co-IP (Fig. S3C). We performed simultaneous imaging of the Ca2+ change and Camuiα FRET. We found that the overexpression of IRBIT in WT MEF cells significantly inhibited the BrA-induced Camuiα FRET change, but did not affect the Ca2+ change (Fig. S3 D and E). In addition, the BrA-induced Camuiα FRET change was significantly increased in IRBIT KO MEF cells compared with WT MEF cells (Fig. 3 E and F). This enhancement of the FRET change was diminished by exogenous IRBIT expression in IRBIT KO MEF cells. On the other hand, we found that point mutation of CaMKIIα regulatory domain (T286A and T286D) attenuated interaction between IRBIT and CaMKIIα (Fig. S3F) and the overexpression of IRBIT did not affect BrA-induced FRET change of these Camuiα mutants in WT MEF cells (Fig. S3 G and H). Thus, the effect of IRBIT on Camuiα FRET change correlates with interaction between IRBIT and CaMKIIα.
NMDA Receptor Activation Causes Prolonged CaMKIIα Activity in IRBIT KO Hippocampal Neurons.
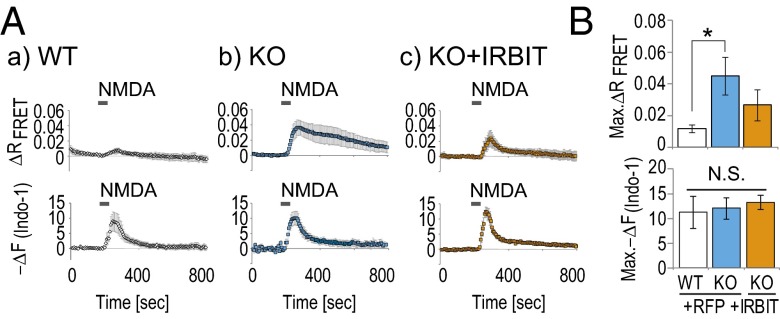
To verify that IRBIT regulates CaMKIIα activity in neurons, we transfected Camuiα into cultured hippocampal neurons derived from WT or IRBIT KO mice and performed simultaneous imaging of FRET and Ca2+ change. Transfected neurons were transiently stimulated with 25 μM NMDA with 1 μM glycine. The transient NMDA-induced FRET change was significantly enhanced and more persistently observed in IRBIT KO neurons, whereas the Ca2+ change was not affected (Fig. 4 A and B). This enhancement of the FRET change was inhibited by exogenous IRBIT expression in IRBIT KO neurons. From these results, we concluded that IRBIT inhibits CaMKIIα activity in neurons.
Fig. 4.
CaMKIIα activity is enhanced in hippocampal neurons from IRBIT KO mice. (A) Simultaneously imaging of FRET and Ca2+ change (Indo-1) after NMDA stimulation in WT or IRBIT KO cultured hippocampal neurons transfected with mRFP or IRBIT/mRFP. (B) Quantitation of FRET and Ca2+ peak amplitude [Max. −ΔF(Indo-1)] that were expressed as the averaged amplitude of 0–50 s are equal to zero.
IRBIT KO Mice Show Hyperactivity and Social Abnormalities.
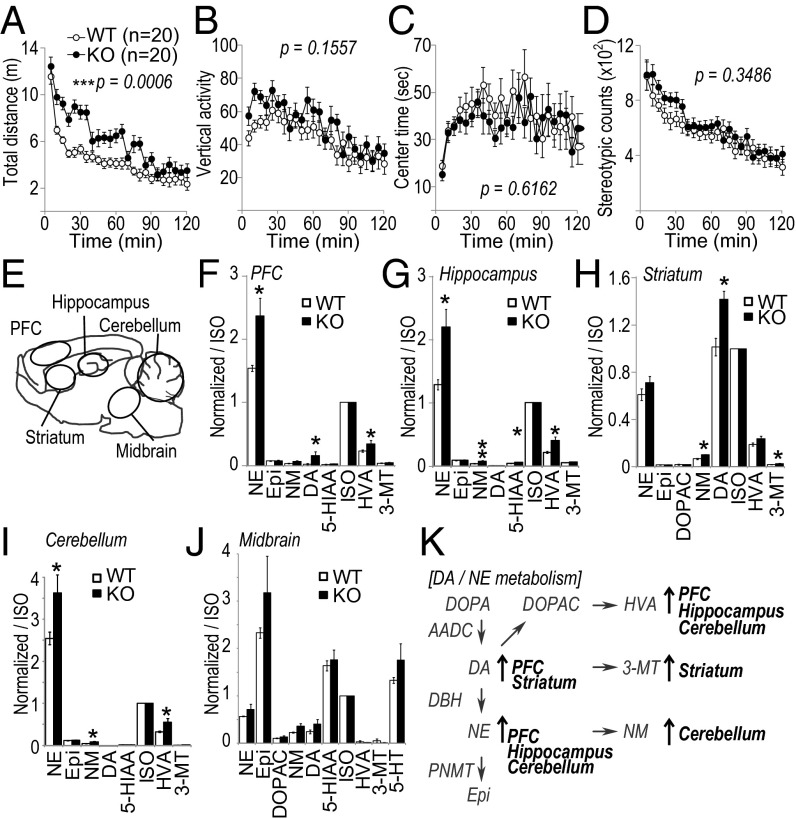
To examine the role of IRBIT on brain function, we performed behavioral testing on the IRBIT KO mouse (6). IRBIT KO mice showed slight dwarfism and low body weight, whereas their brain size and histological structures appeared normal (Fig. S4). We did not observe any significant differences between the WT and KO mice in tests of muscle strength, motor function, pain response, and fear responses (Fig. S5 A–I). However, interestingly, IRBIT KO mice showed increased locomotor activity compared with WT mice (Fig. 5 A–D). To evaluate the effect of environmental factors on the hyperactivity of IRBIT KO mice, we further tested their social interactions and monitored their activity in the home cage (Fig. S5 J and K). Consistent with the results of open field tests, IRBIT KO mice showed enhanced activity in the home cage (Fig. S5 J and K, Lower). In addition, IRBIT KO mouse showed an increment in contact between mice (Fig. S5 J and K, Upper). We also measured the social interaction in a novel environment. IRBIT KO mice also displayed enhanced social interaction in an open field (Fig. S5 L–P). From these results, we concluded that the mice lacking IRBIT present with hyperactivity and social abnormalities.
Fig. 5.
IRBIT KO mice exhibit hyperactivity and have increased dopamine and norepinephrine levels in the brain. (A–D) Locomotor activity in open field test. Total distance (A), vertical activity (B), center time (C), and stereotypic counts (D) were recorded. The P values indicate genotype effect in two-way ANOVA (A–D). (E) Schematic illustration of brain regions for monoamine analysis by HPLC. (F–J) Monoamine level of prefrontal cortex (PFC) (F), hippocampus (G), striatum (H), cerebellum (I), midbrain, including raphe nucleus (J). ISO, isoproterenol, internal control; NE, norepinephrine; Epi, epinephrine; DOPAC, dihydroxyphenylacetic acid; NM, normethanephrine; DA, dopamine; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; 3-MT, 3-methoxytyramine; 5-HT, serotonin. (K) Schematic illustration of altered DA/NE metabolism in IRBIT KO mice.
Dopamine and Norepinephrine Levels in IRBIT KO Mice.
Recent behavioral studies using either KO mice or pharmacological approaches have revealed a correlation between dysregulated monoamine homeostasis and the hyperactive phenotype with social abnormalities (24–26, 33). Therefore, we examined monoamine levels in the brains of IRBIT KO mice. The sampling locations are illustrated in Fig. 5E. DA, NE, and their metabolites were significantly increased in the prefrontal cortex (PFC), hippocampus, striatum, and/or cerebellum of IRBIT KO mice (Fig. 5 F–K). By contrast, the level of serotonin and its metabolites showed no difference in the midbrain, including the raphe nucleus, which is the main source of serotonergic innervation. These results suggested that dysregulation of catecholamine homeostasis could be an explanation for the hyperactivity and the social abnormality of IRBIT KO mice.
Phosphorylation of TH Was Elevated in IRBIT KO Mice.
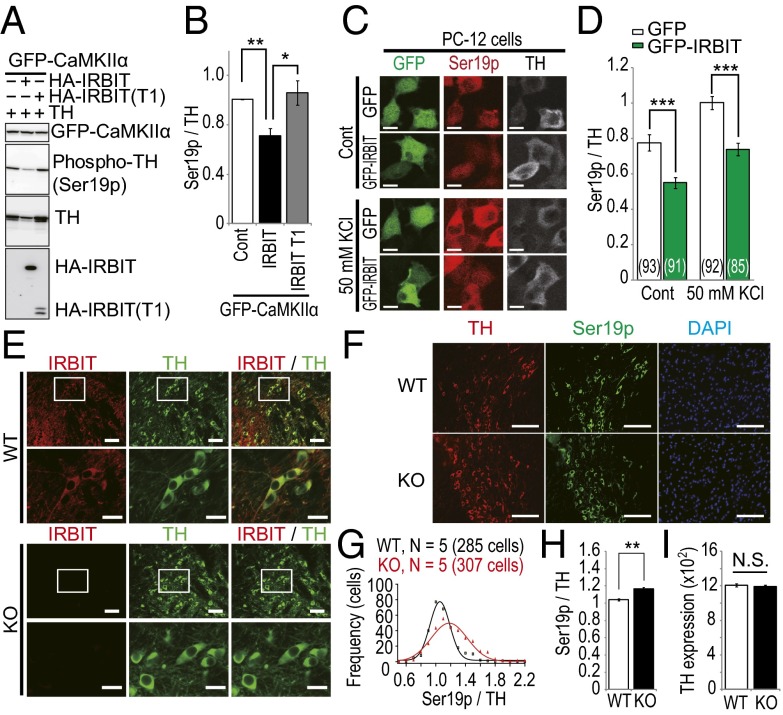
To reveal the molecular mechanism by which IRBIT deletion leads to dysregulation of catecholamine homeostasis, we focused on TH, the rate-limiting enzyme for both DA and NE synthesis. Because CaMKIIα regulates TH activity by phosphorylation (34), we first examined whether the expression of IRBIT affects TH phosphorylation via CaMKIIα in GFP–CaMKIIα cells. The overexpression of IRBIT significantly decreased the BrA-induced phosphorylation of TH compared with the control, whereas the IRBIT T1 fragment that lacked the ability to bind CaMKIIα had no effect (Fig. 6 A and B). Next, we transfected GFP–IRBIT into rat pheochromocytoma-12 (PC-12) cells and evaluated the effect of IRBIT on endogenous TH phosphorylation. In both the basal and stimulated states (50 mM KCl, 10 min), the phosphorylation level of endogenous TH was significantly decreased in PC-12 cells transfected with GFP–IRBIT compared with GFP alone (Fig. 6 C and D). We also examined TH phosphorylation levels in dopaminergic neurons in the ventral tegmental area (VTA) of IRBIT KO mice, where significant IRBIT expression was detected (Fig. 6E). We used anti-TH and antiphospho-TH (S19) specific antibodies to evaluate TH phosphorylation levels by immunohistochemistry. The phosphorylation level of TH was slightly but significantly increased in IRBIT KO dopaminergic neurons (Fig. 6 F–H), whereas the expression level of TH was not changed (Fig. 6I). These data suggested that the expression level of IRBIT determines the phosphorylation level of TH via CaMKIIα, which might lead to increased TH activity and subsequently, to aberrant catecholamine homeostasis in IRBIT KO mice.
Fig. 6.
IRBIT regulates the phosphorylation level of tyrosine hydroxylase (TH) in the dopaminergic neurons of the VTA. (A) GFP–CaMKIIα cells were transfected with TH and HA–IRBIT or HA–IRBIT T1 fragment (1–104). After 24 h, cells were stimulated with 2.5 μM BrA for 2 min. (B) Quantitative analysis of phospho-TH in A. (C) PC-12 cells were transfected with GFP or GFP–IRBIT. After 24 h, cells were stimulated with 50 mM KCl for 10 min. Nonstimulated and KCl-stimulated cells were fixed and stained with indicated antibodies. (D) Quantitative analysis of phospho-TH in C. Total number of cells is indicated in each bar. (E) Adult WT and IRBIT KO mice brain sections were stained with anti-IRBIT (red) and anti-TH (green) antibodies. (Scale bar, 50 μm.) The boxed regions are shown at increased magnification. (Scale bar, 20 μm.) (F) Adult WT and IRBIT KO mice brain sections were stained with anti-TH (red), anti–phospho-TH (Ser19p, green) antibodies, and DAPI (blue). (Scale bar, 100 μm.) (G–I) Quantitative analysis of phospho-TH levels of dopaminergic neurons in the VTA of IRBIT KO mice. (G) Histogram of normalized TH phosphorylation. (H) Average of normalized TH phosphorylation. (I) Average of TH expression. n = 5. Total cell numbers of quantified TH positive neurons were 285 (WT) and 307 (KO).
Discussion
We have investigated the physiological function of IRBIT in the brain. We showed that (i) IRBIT bound to CaMKIIα and inhibited CaMKIIα kinase activity in vitro and in living cells, (ii) IRBIT KO mice exhibited hyperactivity and social abnormalities, (iii) catecholamine levels were increased in IRBIT KO brain, (iv) TH phosphorylation in VTA neurons was increased in IRBIT KO mice, and (v) the expression level of IRBIT determined the phosphorylation of TH by CaMKIIα. These data support a model in which IRBIT regulates CaMKIIα activity and contributes to catecholamine homeostasis through TH phosphorylation, and dysfunction of IRBIT causes hyperactivity and abnormal social phenotypes in mice.
Several reports have stated that CaMKIIα activity is related to locomotor activity in mice. The transient overexpression of CaMKIIα in the nucleus accumbens enhanced the hyperlocomotion response to amphetamine and AMPA (17, 35). The expression of CaMKIIα was increased in the VTA after repeated cocaine injections, which induced locomotor hyperactivity (36). Moreover, the administration of the CaMKIIα inhibitor into the VTA prevented the augmentation of cocaine-induced hyperactivity (37). In addition, CaMKIIα activity was shown to be excessively elevated in the medial prefrontal cortex of ADHD model rats (38). On the other hand, CaMKIIα heterozygous mice and autophosphorylation-deficient mice (T286A) also present with locomotor hyperactivity (18, 36). It has also been reported that overexpression of CaMKIIα using the CaMKIIα promoter leads to decreased locomotor activity in mice (39). Thus, both hyper- and hypoactivation of CaMKIIα seems to cause hyperactive phenotypes in mice. Although the precise reasons for these contradictory observations are unknown, the contribution of the serotonergic pathway may reconcile the two observations. The serotonergic pathway plays a crucial role in the regulation of locomotor activity. For example, serotonin transporter KO mice show increased 5-HT release and hypolocomotion (40). It has been reported that CaMKIIα heterozygous and homozygous mice show a reduction in 5-HT release (41).
In IRBIT KO mice, DA, NE, and their metabolites were significantly increased in the PFC, hippocampus, striatum, and/or cerebellum. However, the levels of serotonin and its metabolites showed no difference in the midbrain, including the raphe nucleus (Fig. 5). In addition, we observed appreciable expression of IRBIT in the dopaminergic neurons of the VTA (Fig. 6A), but the immunosignal for IRBIT in 5HT-positive neurons of the raphe nucleus was very weak and below the limit of detection (Fig. S4G). Thus, it is possible that enhanced CaMKIIα activity in the dopaminergic pathway, but not in the serotonergic pathway, contributes to the altered catecholamine homeostasis and increased locomotor activity of IRBIT KO mice.
The regulation of CaMKIIα by IRBIT was competitive with respect to the Ca2+–CaM complex, because IRBIT bound to the regulatory domain of CaMKIIα and was released by an excess of Ca2+–CaM (Fig. 1). Double-reciprocal analysis of the in vitro kinase assays indicated simple competitive inhibition of CaMKIIα by IRBIT with respect to Ca2+–CaM at relatively lower concentrations of IRBIT (Fig. 2B, 0.1 or 0.5 μM). However, the double-reciprocal plot at a higher concentration of IRBIT (1.0 μM) apparently showed complex inhibition. The autophosphorylation of regulatory domain regulates CaMKIIα activity (42–44). CaMKIIα forms a dodecameric holoenzyme, and autophosphorylation of CaMKIIα seems to occur in trans, between two subunits of the same holoenzyme (45). Because IRBIT is bound to the regulatory domain of CaMKIIα (Fig. 1), it is possible that IRBIT affects the efficiency of autophosphorylation of CaMKIIα and apparently leads to complex inhibition at a high concentration. We performed a binding assay using IRBIT and CaMKIIα T286A or T286D mutants to separate the effects of Ca2+–CaM competition and the effects on autophosphorylation, but these mutations themselves affected the interaction between IRBIT and CaMKIIα (Fig. S3F). This means that the interaction between IRBIT and CaMKIIα is in close proximity to the T286 autophosphorylation site of CaMKIIα. A detailed analysis of the kinetics using simulations is our next priority, to reveal the precise mechanism by which IRBIT regulates CaMKIIα, because CaMKIIα forms a dodecameric holoenzyme with multiple activity states brought about by phosphorylation, and IRBIT also seems to form a multimer (2).
In summary, we found that IRBIT regulates CaMKIIα activity and contributes to catecholamine homeostasis through TH phosphorylation. IRBIT was originally identified as an IP3R-interacting molecule and inhibited IP3R–Ca2+ signaling. Deletion of IRBIT results in both enhancement of Ca2+ release from IP3R and a lower threshold for CaMKIIα activation. Thus, dysfunction of IRBIT would synergistically activate IP3R–Ca2+–CaMKIIα signaling. IRBIT has multiple targets in cells. Further studies using an IRBIT knock-in mutant mouse that specifically disrupts the interaction with each target molecule would help us to reveal the precise pathogenic mechanism underlying hyperactivity and social abnormalities. In addition, a multitude of mutant mouse studies and pharmacological studies has clearly demonstrated that CaMKIIα activity is essential for memory and learning acquisition. It will be interesting to see how IRBIT contributes to the regulation of synaptic plasticity, learning, and memory in the brain.
Materials and Methods
A full description of the materials and methods can be found in SI Materials and Methods.
Animals.
All animal experiments were performed in accordance with the RIKEN guidelines for animal experiments. Every effort was made to minimize the number of animals used.
In Vitro Kinase Assay.
The kinase reaction was performed in kinase assay buffer [25 mM Hepes, pH 7.4, 1 mM MgCl2, 0.3 mM EGTA, 0.33 mM CaCl2, 1 mM 2-mercaptoethanol, 0.005% (wt/vol) Triton X-100, 0.87 μM BSA]. Reactions were performed on ice to avoid the autophosphorylation-dependent decrease in kinase activity. It is described in a previous report that performing experiments at 0 °C prevents autophosphorylation at the inhibitory sites on CaMKIIα (T305 and T306) (46). Recombinant Homer 3 or synthetic peptide, syntide-2 in kinase assay buffer, was incubated with 0.2 ng/μL recombinant CaMKIIα, various concentration of CaM, and with 200 μM ATP and [γ- 32P] ATP in the presence or absence of IRBIT. The reaction was stopped by addition of 83 mM EDTA. For phosphorylation of Homer 3, reaction mixture was separated by SDS/PAGE and phosphorylation level of Homer 3 was detected by Western blotting analysis with the antibody against phospho-Homer 3 (p-Ser120). For phosphorylation of syntide-2, reaction mixture was spotted onto the P81 phosphocellulose paper (GE Healthcare) and radioactivity was counted with a liquid scintillation counter.
Statistical Analysis.
Statistical comparison between two independent groups of data were performed with the Student’s t test. Other statistical analysis was conducted using IgorPro 4.0 or StatView (SAS Institute). Data were analyzed by two-way ANOVA, or two-way repeated measures ANOVA, or one-way ANOVA followed by Bonferroni–Dunn test unless noted otherwise. Values in graphs were expressed as mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Dr. Nobuhiro Hayashi (Tokyo Institute of Technology) for rat CaM vector; Dr. Tomoko Tashiro (Aoyamagakuin University), Dr. Charles Yokoyama, Dr. Takeo Yoshikawa (RIKEN Brain Science Institute, BSI), Dr. Shizuka Kobayashi, and Dr. Toshiya Manabe (The University of Tokyo) for valuable suggestions; all members of our laboratories, especially Dr. Takeyuki Sugawara, Dr. Hideki Nakamura, and Dr. Hideaki Ando for fruitful discussions; Miwa Takamura for technical assistance; and RIKEN BSI Research Resources Center for help with FANTOM3 clone distribution, HPLC analysis, cell sorting, and DNA sequencing analysis. This study was supported by Grant-in-Aid for Scientific Research (S) 25221002 (to K.M.), for Young Scientists (B) 24700389 (to K.K.), and for Scientific Research (C) 24590332 (to A.M.) from Japan Society for the Promotion of Science; the Moritani Scholarship Foundation (C.H.); Japan Foundation for Applied Enzymology (K.K.); and a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan (to T.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503310112/-/DCSupplemental.
References
- 1.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278(12):10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 2.Ando H, et al. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22(6):795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Kiefer H, et al. Inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) associates with components of the mRNA 3′ processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation. J Biol Chem. 2009;284(16):10694–10705. doi: 10.1074/jbc.M807136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirakabe K, et al. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3- cotransporter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103(25):9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando H, Kawaai K, Mikoshiba K. IRBIT: A regulator of ion channels and ion transporters. Biochim Biophys Acta. 2014;1843(10):2195–2204. doi: 10.1016/j.bbamcr.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Park S, et al. Irbit mediates synergy between ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology. 2013;145(1):232–241. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2010;285(36):27869–27878. doi: 10.1074/jbc.M110.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, et al. IRBIT coordinates epithelial fluid and HCO3- secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119(1):193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaoutov A, Dasso M. Enzyme regulation. IRBIT is a novel regulator of ribonucleotide reductase in higher eukaryotes. Science. 2014;345(6203):1512–1515. doi: 10.1126/science.1251550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulman H. Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization. J Neurosci. 2004;24(39):8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhang C, Szábo G, Sun QQ. Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 2013;1518:9–25. doi: 10.1016/j.brainres.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5(12):3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 15.Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35(10):607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak G, Seeman P. Hyperactive mice show elevated D2(High) receptors, a model for schizophrenia: Calcium/calmodulin-dependent kinase II alpha knockouts. Synapse. 2010;64(10):794–800. doi: 10.1002/syn.20786. [DOI] [PubMed] [Google Scholar]
- 17.Loweth JA, et al. Transient overexpression of alpha-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J Neurosci. 2010;30(3):939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki N, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1(1):6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leo D, Gainetdinov RR. Transgenic mouse models for ADHD. Cell Tissue Res. 2013;354(1):259–271. doi: 10.1007/s00441-013-1639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veenstra-VanderWeele J, Blakely RD. Networking in autism: Leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology. 2012;37(1):196–212. doi: 10.1038/npp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelc K, Cheron G, Dan B. Behavior and neuropsychiatric manifestations in Angelman syndrome. Neuropsychiatr Dis Treat. 2008;4(3):577–584. doi: 10.2147/ndt.s2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalsgaard S, et al. Association between Attention-Deficit Hyperactivity Disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry. 2014;29(4):259–263. doi: 10.1016/j.eurpsy.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Gadow KD. Schizophrenia spectrum and attention-deficit/hyperactivity disorder symptoms in autism spectrum disorder and controls. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1076–1084. doi: 10.1016/j.jaac.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148(1-2):185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 25.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 26.van der Kooij MA, Glennon JC. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2007;31(4):597–618. doi: 10.1016/j.neubiorev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J Neurochem. 2004;91(5):1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- 28.Bevilaqua LR, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW. Phosphorylation of Ser(19) alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of Ser(40) J Biol Chem. 2001;276(44):40411–40416. doi: 10.1074/jbc.M105280200. [DOI] [PubMed] [Google Scholar]
- 29.Xiao B, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21(4):707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 30.Fong YL, Taylor WL, Means AR, Soderling TR. Studies of the regulatory mechanism of Ca2+/calmodulin-dependent protein kinase II. Mutation of threonine 286 to alanine and aspartate. J Biol Chem. 1989;264(28):16759–16763. [PubMed] [Google Scholar]
- 31.Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. J Neurosci. 2008;28(20):5369–5382. doi: 10.1523/JNEUROSCI.4738-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takao K, et al. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25(12):3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gainetdinov RR, et al. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283(5400):397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T, Nakata H, Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981;256(11):5404–5409. [PubMed] [Google Scholar]
- 35.Singer BF, Loweth JA, Neve RL, Vezina P. Transient viral-mediated overexpression of alpha-calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell leads to long-lasting functional upregulation of alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors: dopamine type-1 receptor and protein kinase A dependence. Eur J Neurosci. 2010;31(7):1243–1251. doi: 10.1111/j.1460-9568.2010.07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Easton AC, et al. αCaMKII autophosphorylation controls exploratory activity to threatening novel stimuli. Neuropharmacology. 2011;61(8):1424–1431. doi: 10.1016/j.neuropharm.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci. 2004;19(2):405–414. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 38.Yabuki Y, et al. Aberrant CaMKII activity in the medial prefrontal cortex is associated with cognitive dysfunction in ADHD model rats. Brain Res. 2014;1557:90–100. doi: 10.1016/j.brainres.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa S, et al. Transgenic up-regulation of alpha-CaMKII in forebrain leads to increased anxiety-like behaviors and aggression. Mol Brain. 2009;2(1):6. doi: 10.1186/1756-6606-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6(4):389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science. 1994;266(5183):291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- 42.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 43.Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3(1):59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 44.Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268(10):7163–7170. [PubMed] [Google Scholar]
- 45.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279(5348):227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 46.Singla SI, Hudmon A, Goldberg JM, Smith JL, Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276(31):29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.