Much of our understanding of disease processes and treatments begins with preclinical studies that use nonhuman animals and cell cultures. Such studies are integral to biomedical research and the development pipeline for drugs, devices, and biologics. Most preclinical biomedical research, however, has been conducted with inadequate consideration of sex (1–3).
Why is sex so important? Sex represents one of the most evolutionarily well-conserved differences in biology; yet, it is one of the most underappreciated differences in biomedical research. During this era of “personalized medicine,” sex is a fundamental variable that can be used to disaggregate data and explain heterogeneous disease outcomes. Although many factors can influence an outcome, sex is evolutionarily fundamental and affects the whole of the population.
Across diverse disciplines, researchers risk drawing erroneous conclusions when they extrapolate outcome data from one sex to another. Male sex bias is most extreme in pharmacology and neuroscience, whereas female sex bias is most pervasive in immunology (4).
To remedy this situation, the National Institutes of Health (NIH) announced policies in May 2014 that “require applicants to report their plans for the balance of male and female cells and animals in preclinical studies in all future applications, unless sex-specific inclusion is unwarranted, based on rigorously defined exceptions” (5, 6). We suggest that other biomedical funders and institutions follow suit. Here we show why such changes are crucial, and why researchers should not only include male and female animals in biomedical research, but also analyze sex and gender in preclinical research.
Including both sexes at the less expensive preclinical end of the science pipeline will save money and time compared with assessing sex differences during the more costly clinical trial phase. It also avoids an even more expensive and dangerous scenario: companies being forced to remove drugs from the market due to adverse events in one sex.
Numerous objections have been made about the inclusion of females in preclinical research. Some maintain that requiring study of both sexes would result in unnecessary duplication and slow progress due to added workloads (7). Others argue that the reproductive cycle renders females (rodents and other mammals) intrinsically more variable than males, which prejudices investigators against female models (8).
However, a meta-analysis comparison of male and female mice, with no regard to estrous cycle stage, established that variability on most traits was equivalent in females and males (9). In fact, for most end points, it is unnecessary to stage the estrous cycle. If and when evidence shows that reproductive hormones affect specific traits in humans or animal models, researchers should incorporate female reproductive phases in study design. Regardless, hormonal variability does not justify exclusion of females.
Inclusion of sex as a research variable will promote discovery of disease mechanisms and has the potential to save lives. Inattention to sex as a variable in preclinical research could present real risks to women, for example. About 80% of rodent drug studies are conducted only on males (10), and 8 of 10 drugs withdrawn from the US market from 1997 to 2000 posed greater health risks for women than for men (11). Just as the US Food and Drug Administration (FDA) recognized the need to lower the recommended dosage for zolpidem-containing medications in women compared with men (12), many other drugs may require reconsideration of unisex dosing.
Even if analyzing sex as a variable in preclinical research costs more money in a subset of studies in individual laboratories, it won’t cost more in the majority of studies. And it will likely save money in the long run by minimizing the failure of drug candidates and other therapeutic approaches. In addition to animal studies that can obviously be conducted in one sex (e.g., prostate cancer in males and models of most breast cancers in females), if an effect of sex has been ruled out through rigorous experimentation, data from females and males can be combined without increasing overall sample size or cost. If, however, a substantial sex effect is detected, sample sizes should be increased to generate sufficient power for statistical analysis to ensure that findings apply equally to, or differ substantially between, the sexes. To address the cost concerns, the NIH has launched a program of administrative supplements for research on sex/gender differences (PA-15-034).
It is impossible, in absolute terms, to prove no sex difference. This is why we rely on statistics to quantify the strength of a difference between the sexes. Confidence about the strength of a sex difference will come from the effect size (often called Cohen’s d). For instance, if males and females differ significantly with respect to a given end point, but the effect size is small (<0.2), the difference may not be worth pursuing. Conversely, if males and females differ modestly for a specific outcome, but the effect size is large (>0.5), indicating a high reliability (i.e., low variance), sex should be considered as a contingent variable. By reporting effect sizes and P values for outcomes, the significance of a sex difference may be critically evaluated and neither over- nor underinterpreted.
Finding no sex difference is as important as confirming one; such null hypotheses should be reported in peer-reviewed journals, noting that clear evidence of sex parity requires avoidance of accepting a false negative (type I error) and not concluding a false positive (type II error). However, detecting no differences in an outcome measure does not give an investigator license to use only one sex in follow-up research. Sex is a complex variable, and the underlying mechanisms of an outcome measure may differ between the sexes (i.e., different means to the same end point) (13). Excluding one sex may obscure discoveries pertinent to the etiology and treatment of disease. In the presence and even in the absence of robust or measurable differences between males and females, we propose inclusion of both sexes in all experiments.
The sex of primary cells may also explain variability in responses to experimental perturbation and be of clinical importance. For example, skeletal muscle stem cells derived from females regenerated new tissue faster than did those from males (14), and athero-protective capabilities of bone marrow mononuclear cells in atherosclerotic apolipoprotein-E KO males have only been observed when female cells were used (15). Thus, the sex of primary cells should be considered when designing experiments. [Note, however, that this may not be a factor in experiments using immortalized cell lines, which may have become chromosomally unstable, rendering it impossible to determine their original sex (16).]
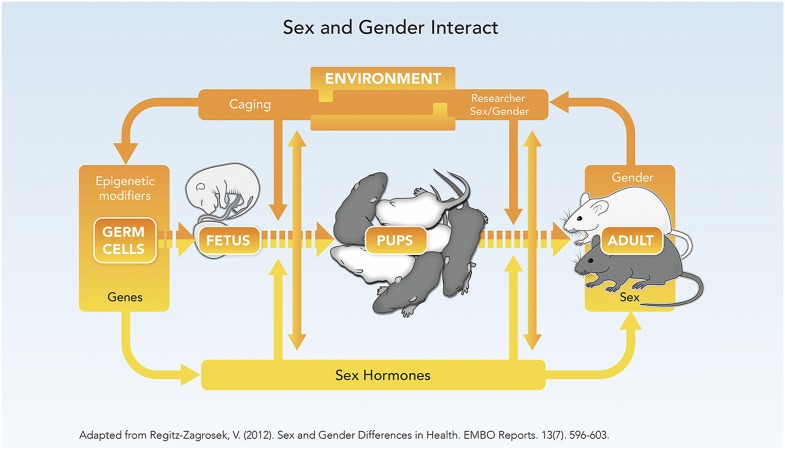
Gender, by contrast to sex, is a constellation of socio-cultural processes that interact with and influence biology (see Fig. 1). In humans, gender refers to cultural attitudes that shape “feminine” and “masculine” behaviors that are learned and vary by culture, historical era, and ethnicity (17, 18). In animal research, “gender” refers to investigators’ differing attitudes toward and handling of male or female animals. Housing male and female mice in groups rather than singly, for example, increases variability in many traits (9). Gender may also refer to differing interactions between male and female investigators and animals.
Fig. 1.
The rodent life cycle exhibits a complex interdependency of sex and gender. Modified with permission from ref. 21.
Exposure of mice and rats to male compared with female investigators, for instance, has been associated with stress and pain inhibition (19). Further, domestication may have impacted females more than males and erased many behavioral traits of ancestral wild female mice (20). Cultural attitudes toward males and females with ensuing differences in treatment of male and female subjects in addition to biological factors may play a primary role in these cases.
The new NIH policy to integrate the biological variable of sex into preclinical research has the potential to transform science and medicine by increasing the likelihood and pace of new discoveries, diminishing errors of extrapolation between sexes, and mitigating adverse events in the drug, devices, and biologics development pipeline. Such policies will help create a more robust research pipeline. The NIH Center for Scientific Review study section members should monitor these issues, as should journal editors during the review process. Along these lines, we support development of training modules to raise awareness of the importance of sex as a biological variable by funding agencies and publishers in an effort to educate clinicians and the scientific community. Ultimately, sex inclusion will increase the pace and quality of the science needed to diagnose, treat, and cure disease.
Acknowledgments
We thank Laurence C. Baker, Stanford University, for assistance conceptualizing cost implications. These perspectives emerged from an NSF-funded workshop held at Stanford University in September 2014. The statements are those of the authors and do not necessarily reflect the views of the NSF.
Footnotes
Any opinions, findings, conclusions, or recommendations expressed in this work are those of the authors and do not necessarily reflect the views of the National Academy of Sciences.
References
- 1.Becker JB, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 2.Yoon DY, et al. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156(3):508–516. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 3.McCullough LD, et al. NIH initiative to balance sex of animals in preclinical studies: Generative questions to guide policy, implementation, and metrics. Biology of Sex Differences. 2014;5(15):1–7. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Office of Research on Women’s Health, National Institutes of Health (2014) Methods and techniques for integrating the biological variable “sex” in preclinical research. https://videocast.nih.gov/summary.asp?live=14501&bhcp=1. Accessed February 24, 2015.
- 7.Sandberg K, Verbalis JG, Yosten GL, Samson WK. Sex and basic science. A Title IX position. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R361–R365. doi: 10.1152/ajpregu.00251.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald C, Wu C. Biomedical research. Of mice and women: the bias in animal models. Science. 2010;327(5973):1571–1572. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- 9.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Hughes RN. Sex does matter: Comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav Pharmacol. 2007;18(7):583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- 11.US General Accounting Office . Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women. Government Publishing Office; Washington, DC: 2001. [Google Scholar]
- 12. US Federal Drug Administration (2013) A drug safety communication: Risk of next-morning impairment after use of insomnia drugs. www.fda.gov/Drugs/DrugSafety/ucm334033.htm. Accessed February 18, 2015.
- 13.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 14.Deasy BM, et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: Female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177(1):73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson WD, et al. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ Res. 2007;101(12):1319–1327. doi: 10.1161/CIRCRESAHA.107.155564. [DOI] [PubMed] [Google Scholar]
- 16.Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306(1):C3–C18. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz SA, et al. First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. 2014;28(1):4–13. doi: 10.1096/fj.13-233395. [DOI] [PubMed] [Google Scholar]
- 18. Schiebinger L, Klinge I, Sánchez de Madariaga I, Schraudner M, Stefanick ML, eds. (2011–2013) Gendered innovations in science, health & medicine, engineering, and environment. genderedinnovations.stanford.edu. Accessed February 18, 2015.
- 19.Sorge RE, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 20.Chalfin L, et al. Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nat Commun. 2014;5:4569. doi: 10.1038/ncomms5569. [DOI] [PubMed] [Google Scholar]
- 21.Regitz-Zagrosek V. Sex and gender differences in health. European Molecular Biology Organization Reports. 2012;13(7):596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]