Significance
Bacteria self-organize into a dense multicellular community known as a biofilm, in which cells are embedded in self-secreted extracellular polymeric substances (EPSs). A number of processes can contribute to spatial heterogeneity in a growing biofilm; among them, the effect of macromolecular crowding enhanced by the EPSs has largely remained unexplored. To understand the effect of macromolecular crowding in spontaneous spatial organization, we develop a computational model to investigate the explicit role of mechanical interactions in driving the collective behavior of bacterial cells in the presence of EPS particles in a colony growing on a solid substrate. Our findings demonstrate that an entropy-driven depletion interaction between bacteria and EPSs can induce significant phase separation and spatial heterogeneity in a biofilm.
Keywords: biofilms, extracellular polymeric substance, depletion interaction, mechanical interaction, phase separation
Abstract
Secretion of extracellular polymeric substances (EPSs) by growing bacteria is an integral part of forming biofilm-like structures. In such dense systems, mechanical interactions among the structural components can be expected to significantly contribute to morphological properties. Here, we use a particle-based modeling approach to study the self-organization of nonmotile rod-shaped bacterial cells growing on a solid substrate in the presence of self-produced EPSs. In our simulation, all of the components interact mechanically via repulsive forces, occurring as the bacterial cells grow and divide (via consuming diffusing nutrient) and produce EPSs. Based on our simulation, we show that mechanical interactions control the collective behavior of the system. In particular, we find that the presence of nonadsorbing EPSs can lead to spontaneous aggregation of bacterial cells by a depletion attraction and thereby generates phase separated patterns in the nonequilibrium growing colony. Both repulsive interactions between cell and EPSs and the overall concentration of EPSs are important factors in the self-organization in a nonequilibrium growing colony. Furthermore, we investigate the interplay of mechanics with the nutrient diffusion and consumption by bacterial cells and observe that suppression of branch formation occurs due to EPSs compared with the case where no EPS is produced.
A common underlying theme of biophysics of living matter is the quest to understand how local interactions of individual components lead to collective behavior and formation of highly self-organized systems (1–6). In this regard, bacterial systems are an especially interesting example. Bacteria are known to self-organize into multicellular communities, commonly known as biofilms, in which microbial cells live in close association with a solid surface or liquid–air interface and are embedded in a self-produced extracellular matrix. Extracellular polymeric substances (EPSs) play an important role in determining the structural and mechanical architecture of a biofilm (7–12). Generally, the collective dynamics of bacterial colony involves a complex interplay of various physical, chemical, and biological mechanisms, such as growth and differentiation of cells, production of EPSs, the collective movement of cells determined by interacting physical forces and chemical cues, e.g., chemotaxis, motility, cell–cell signaling, adhesion, and gene regulation (13–19). At low density, communication among cells occurs mainly through chemical signals (20). However, at a higher density, as bacteria aggregate and form dense communities, direct mechanical interaction becomes increasingly relevant in the self-organization (21–23). In this regard, microfluidics-based experiments coupled with continuum modeling of cellular dynamics by Volfson et al. (24) was a pioneering study emphasizing the role of cell–cell mechanical interaction in the growth of a highly organized bacterial colony. As a significant extension, Farrell and coworkers (25, 26) have recently proposed a theoretical model for bacterial cells, the colony of which grows purely based on cell–cell mechanical interaction; they have studied both circular and branched colonies. However, none of the previous studies has explicitly considered the presence of extracellular matrix; the potentially important role of direct mechanical interaction of EPS with bacterial cells has remained unexplored.
In this article, we investigate the role of direct mechanical interaction of EPSs with bacterial cells in a dense bacterial colony on a solid substrate. These EPSs could contribute to patterning the system either by bridging (27) or by a depletion attraction (28, 29), as observed in colloid–polymer mixtures. Bridging is a common phenomenon where polymers adsorb or adhere to multiple colloidal particles and pull them closer. On the other hand, in the latter case of depletion, which is the central focus of the present study, the polymer coils are nonadsorbing/nonadhering and the center of the coil cannot approach the surface of colloidal particles by a distance less than the size of its own radius (e.g., the radius of gyration). Due to this depletion effect, large objects experience mutual effective attraction via an entropy-driven mechanism (for an explanation, see Fig. 2), possibly leading to aggregation and phase separation in the system. This depletion effect is well appreciated as one of the driving forces of structure formation in biological systems (30–34). For instance, it has been suggested to play an important role in the compression of chromatin fibers and formation of nuclear bodies such a nucleoli and adhesion of red blood cells, etc. (30–34). Recently, a number of studies revealed that crowding effects by extracellular substances can operate in bacterial cultures as well (35–40). However, these earlier works study the role of depletion attraction operating in an equilibrium mixture of fixed bacterial cells and polymer concentration. Our focus instead is on how a nonequilibrium growing bacterial colony undergoes spontaneous aggregation of bacterial cells in the presence of self-produced EPSs.
Fig. 2.
Schematic representation of how depletion attraction operates in a colony of bacteria producing exopolysaccharide. Bacterial cells are large spherocylinders (magenta) and coiled polymers (EPSs) are assumed as small spheres (yellow). The dashed lines around each bacteria are zones of excluded volume as shown in A and B, which is not accessible to the center of mass of a given EPS after it has been secreted into the medium. In A, as bacterial cells are separated from each other, the area of excluded volume is large, an area that could be partially accessible for the EPS particles if the cells come closer and form aggregates, as is shown in B.
To investigate this question, we construct a particle-based model to study the growth of a colony of nonmotile bacterial cells in two dimensions, which consume nutrient from the solid substrate to grow and divide and produce EPSs that are secreted into the medium. We use a coarse-grained approach to treat surrounding polymeric EPS coils as small spherical particles. Via computer simulation, we show that it is the cell–EPS mechanical interaction that drives the nature of the morphology of the growing assembly: whereas at a low cell–EPS mechanical interaction, the bacterial colony remains homogeneous and isotropic, a high cell–EPS mechanical interaction drives the system into a patterned colony. In addition, our results show that the secretion rate of EPS also plays an important role; the resultant pattern depends on the ratio of EPS to cell density and on the growth velocity as determined by the level of nutrient.
The Model and Method
To explore the mechanical effects of bacterial cells and the surrounding self-secreted extracellular polymers in a growing colony, we construct a particle-based simulation model in two dimensions. Following the work of Farrell and coworkers (25), an individual bacterial cell is modeled as a nonmotile growing spherocylinder having a constant diameter () and variable length l. The EPS is composed of polysaccharides, proteins, and nucleic acids (extracellular DNA), although the exact composition depends on the strain of the bacterium and the type of nutrients present in the culture medium. On the scale of the bacterial colony, it is clearly impossible to resolve the conformations of individual polymer molecules. Instead, we approximate the EPSs as small spherical particles having a radius equal to their radius of gyration (38, 39). For the sake of computational ease, we will typically take this radius to be around half that of a bacterial cell (38), as shown in Fig. 1. This size is clearly larger than the actual physical size, and hence, one should perhaps assume that each large sphere represents an aggregate of EPS particles. We verified that this size is not a critical determinant of the pattern as long as it is significantly smaller than the long dimension of the bacterial cells. In what follows, “cell” will refer to a model bacterium and “particle” to a model EPS polymer.
Fig. 1.
Representation of (A) bacterial cell as spherocylinder, (B) coiled polymers (EPSs) are assumed as spheres (red), (C) repulsive interaction between two cells, and (D) repulsive interaction of a cell and an EPS particle.
In our model, each individual cell is restricted to a 2D surface and therefore is represented by two coordinates () and unit vectors representing the orientation of the symmetry axis of a bacterial cell. The colony expands due to consumption of nutrient with concentration [], governed by a diffusion equation linked to a sink term
| [1] |
where is the area of cell i, is the radius of end-cap, li is the length of the cell, and is its spatial coordinates. Initially, everywhere, and c is kept constant at the edges of the simulation box, which is taken as large enough to ensure that there is no direct effect of the boundary. The nutrient is consumed by the bacterial cells at a rate per unit biomass density, where is a monotonically increasing dimensionless function. In our simulations, we assume , a Monod function with half-saturation constant equal to 1 (in arbitrary units).
A cell grows by elongation in accordance with the relation , where ϕ is the growth parameter and is the average area (25). Once a cell reaches a critical length, , it splits at a rate into two independent cells with orientations roughly the same as the mother cell but with small random kicks. This randomness in the orientation incorporates the effect of various irregularities in the system, e.g., roughness of the agar surface and slight bending of the cells, and ensures that cells will not grow as long filaments but instead will generate a quasi-circular colony. To simulate EPS production in the growing colony, we assume that each cell has a probability of producing EPS particles at the nearby area. In simulations, EPS particles are inserted at certain rate and placed at a small distance away randomly from the center of the cell. To prevent the uncontrolled production of EPS, we restrict the EPS production by considering that it starts at a location once the local cell area density reaches a certain threshold and stops once the local EPS area density reaches a maximum concentration of EPS. In the simulation protocol, we discretize the 2D space of the colony (Lx = Ly = 200 μm) into certain number of coarse square grids. Then, we compute the cumulative area of all cells and EPSs that spatially belong to a particular grid point . From simulations of growing colony of only cells (i.e., without EPSs; not shown here), we a priori have determined that the average cumulative area of cells at a particular grid point is about . In our simulations, we use this average cell area density () as a lower bound above which EPSs will be produced at a particular location. Similarly, by giving a threshold value of (Table 1), we can limit the amount of EPSs produced at a certain time at a particular location in the colony.
Table 1.
Parameters, constants, and expression of repulsive forces used in the simulations
| Parameter | Symbol | Simulations |
| Maximum length | 5.0 μm | |
| Diameter of cell | 1.0 μm | |
| Diameter of EPS particle | 0.5 μm | |
| Linear growth rate | ϕ | 3.5 μm/h |
| Cell division rate | 0.1/h | |
| EPS production rate | 0.35/h | |
| Elastic modulus (cell and EPS) | E | to |
| Friction coefficient (cell) | ζ | 200 Pa⋅h |
| Friction coefficient (EPS) | 200 Pa⋅h | |
| Nutrient concentration | 1.0 fg⋅μm3 | |
| Nutrient consumption rate | k | 4.0/h |
| Diffusion rate of nutrient | D | 300 μm2/h |
| Threshold area-density of cell | 4.0 μm2 | |
| Threshold area-density of EPS | 0.3 μm2 | |
| Component pair | Repulsive forces | |
| Cell–cell | ||
| EPS–EPS | ||
| Cell–EPS |
Our goal is to understand the role of mechanical interactions in biofilm structure formation. We assume cells interact mechanically in accordance with the Hertzian theory of elastic contact (22, 24) and follow overdamped dynamics. The force between two spherocylinders is approximated by the force between two spheres placed along the major axis of the rods at such positions that their distance is minimal, as depicted in Fig. 1C. If the shortest distance between the two spherocylinders is r and is the overlap, then the force is assumed to be , where E parametrizes the strength of the repulsive interaction proportional to the elastic modulus of cell. implies perfectly hard cells, but in fact, our simulation uses a finite value of E (Table 1), allowing for some deformation. In a similar manner, we also assume bacterial cells and EPS particles interact mechanically.
Assuming overdamped dynamics of the system, the velocity and angular velocity are proportional to the force and torque, respectively. The equation of motion for the individual bacterial cells are given by
| [2] |
| [3] |
where ζ is the friction per unit length of cell, and are the cell’s velocity and force on it, and ω and τ are the corresponding angular velocity and torque on it. The EPS particles also follow the similar equation of motions, but the friction is taken as a separate constant . The force is assumed to be independent of the orientation of the rod relative to its velocity, and Eq. 2 is obtained by assuming that the rod is made up of independent elements each experiencing a Stoke’s drag proportional to its velocity. Particle positions are evaluated by solving the equation of motions using a simple Euler time stepping method along with solving the diffusion equation for the nutrient with constant boundary condition taken far from colony front.
Results and Discussion
Depletion-Induced Phase Separation and Pattern Formation.
The main thrust of the current study is the treatment of EPSs as depleting particles. In this regard, we explore the interplay of mechanical interactions between bacterial cells and nonadsorbing EPSs, which serve as depletants in the growing medium. This interaction is implemented by allowing the repulsion between bacterial cells and EPS spheres such that it prevents the center of the particles from coming into an excluded volume zone surrounding the bacteria (Fig. 2 and Table 1). The width of the exclusion zone is taken as the radius of gyration of the EPS particles. Similar to what occurs in a colloid–polymer mixture, we expect the depletion effect to be a key ingredient for giving rise to a heterogeneous distribution in the growing colony. The origin of this depletion effect is an entropy gain due to the increased accessible volume of the small depleting EPS particles when large objects (bacterial cells) approach one another and reduce the excluded volume for the depletants. Inspired by recent experimental finding of Dorken and coworkers (38) of enhanced aggregation and phase separation of a mutant bacterium Sinorhizobium meliloti that overproduces EPSs, we study the effect of EPS particles in the growing bacterial colony.
We begin our study by simulating the growth of a colony starting from a single cell on a 2D flat surface in a fixed domain (). As depicted in Movie S1 and Fig. 3A, starting from a single cell, the colony spreads due to mechanical pushing of the bacterial cells and EPS particles, and cell division and EPS secretion occur in tandem, finally developing an almost circular isotropic colony of a total number of interacting agents (), with bacterial cells () smeared out homogeneously in a matrix of EPS particles (). A circular colony spreads linearly with time as evident from Fig. S1. It is worth noting that the main result of forming a patterned colony over a long time does not differ significantly if we begin our simulation from a number of cells instead of a single cell.
Fig. 3.
Snapshot of growing bacterial colony in presence of EPS particles. Bacterial cells are represented by spherocylinders (color: magenta) and EPS particles as spheres (color: yellow). Snapshot of cell-eps assembly after time t = 190: (A) in the presence of low repulsive interaction among the components of biofilm: ; (B) in the presence of moderate repulsive interaction: ; and (C) in the presence of high cell-EPS repulsive interaction: . (D) Enlarged view of a small rectangular segment taken from the center of A. (E) Enlarged view of a small rectangular segment taken from the center of B. (F) Enlarged view of a small rectangular segment taken from the center of C. The rest of the parameters of the simulations are given in Table 1.
To understand to what extent the generation of this self-organized colony depends on the strength of repulsive mechanical interaction between cells and EPSs, we carry out our growing simulation by increasing the elastic repulsive forces of the interacting components, keeping the EPS production rate and other parameters the same as used in Fig. 3A. As shown in Fig. 3B, for moderate repulsive force constants in the bacterial colony, there is an occurrence of somewhat weak cell–EPS phase segregation, with relatively homogeneously distributed cells in the EPS medium. In this figure, the final state contains a total number of interacting agents (), made up of bacterial cells () and EPS particles ().
To further investigate the role of mechanical interaction between cells and EPS particles, we now increase the repulsive force constant (), keeping the rest of the parameters the same as used in Fig. 3B and carry out a simulation of a growing bacterial colony. This idea was motivated by wanting to explore whether enhanced depletion, as might arise by a more complete description of the polymer coils (whose spatial extent is not bounded by the radius of gyration and hence might be repelled more effectively than previously assumed), would increase the degree of phase separation. As shown in Fig. 3C and demonstrated in Movie S2, we indeed find that an increase in the cell–EPS repulsive force leads to considerable aggregation of cells and thereby results in more pronounced phase separation and self-organization in the colony. The formation of a self-organized patterned biofilm (now with is quite evident. This high force is required to push out the EPS particles from the inner region of two approaching cells. One caveat of our simulations is that if the force becomes extremely strong, the repulsion may not be enough to prevent cells from significantly overlapping and on rare occasions may even cross through each other. Actually, this would occur anyway in a colony as cells could climb over each other if the repulsion becomes strong.
To make sure of the primary role of repulsive elastic force between cells and EPS particles, we increase the EPS production rate, keeping the repulsive forces the same as used in simulating the growing colony (Fig. 3B). The result of the corresponding simulation is depicted in Fig. S2, which again shows almost homogeneously distributed bacterial cells in a medium of EPS particles and very little segregation. It is thus evident that a strong repulsive mechanical interaction is needed to drive the system, leading to aggregation of bacterial cells by depletion attraction, even if there are excess depletants in the medium. In brief, the idea of depletion-induced equilibrium phase separation is well studied in the context of colloid–polymer mixtures. However, although those prior studies of colloid–polymer mixtures are performed in an equilibrium setup at a fixed concentration, our current system of a growing colony is inherently nonequilibrium in nature. Thus, the phase separation occurs even as the colony grows and gives rise to a patterned biofilm due to mechanically driven depletion-induced aggregation of bacterial cells.
Effect of Exclusion Zone in Phase Separation of the Biofilm.
In our simulation protocol, we only considered the repulsive mechanical interaction among the components of the bacterial colony. Moreover, we assume that the interaction between bacterial cells (cell–cell) and EPS particles (EPS–EPS) is rather soft compared with the cell–EPS interaction. There is another approach to get enhanced depletion by directly increasing the size of the repulsive zone for the EPS particles, again to take into account more complex conformational constraints. We therefore generalized our model by varying the exclusion zone between the EPS particle and cell, defining a variable by , where varied from 0 to . In Fig. S3, we show the effect of varying the exclusion zone in the formation of the patterned biofilm. Whether by directly changing the repulsion strength or the repulsion geometry, the inhomogeneity in the biofilm structure is significantly increased if we enhance the effectiveness of the depletion.
Characterization of Organization of the Biofilm.
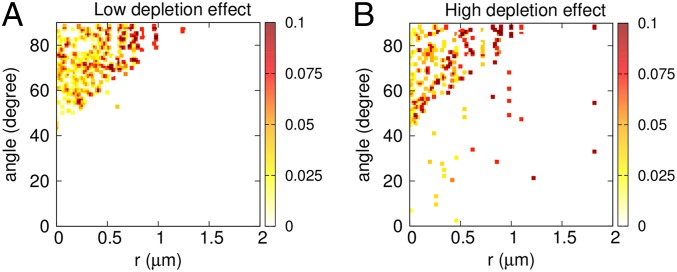
To shed light on how the depletion force influences the local organization of cells, we compute the 2D distribution of the relative angle of cells as a function of their center of mass separation (r). Fig. 4 compares the angle distribution in the presence of low and high repulsive forces between cells and EPSs. The results are obtained for the corresponding simulations shown in Fig. 3 A and C. We find that there is a major population of cells that makes a very wide range of angles between 50° and 90°. In the presence of high depletion forces, however, there is a significantly larger density of cells within a shorter distance of separation, with angle varying between 0° and 40°. This angle distribution implies that the aggregates of bacterial cells formed in the presence of high depleting forces exhibit significant orientational ordering, increasing the likelihood of neighboring cells remaining parallel.
Fig. 4.
Comparison of 2D distribution of angles of orientation between two cells and corresponding intercell distance in presence of (A) low and (B) high depletion effect provided by varying repulsive force constants. The parameters are kept same as in Fig. 3 A and C.
The higher probability of parallel orientation among neighbors is also reflected in the collective orientational and radial order prevalent in the biofilms. As far as orientational order is concerned, we determine the so-called Steinhardt–Nelson order parameter q6 (SI Text) for both cases: with low and high repulsive forces (41, 42). This orientational order parameter q6 is known to distinguish between particles based on their liquid-like or solid-like order. The value of q6 parameter ranges between 0 (pure liquid) and 1 (pure solid). In Fig. 5, we compare the probability distribution of q6 parameters of cells in the presence of low and high depletion effect induced by EPS particles. We clearly see that in the presence of high repulsive force between cells and EPS particles, i.e., the high depletion effect, the relative probability of higher q6 values is significantly larger than that in the case of a low repulsive interaction, implying that the mechanically driven depletion effect induces ordering among the cells.
Fig. 5.
Comparison of local orientational order parameter q6 in presence of low and high depletion effect. The parameters are kept the same as used in Fig. 3 A and C. The probability distribution is normalized such that .
To gain further insight into the spatial ordering of bacterial cells, EPS particles, and the whole biofilms, we compute the radial distribution function, , and the static structure factor , among different biofilm components for both cases: with and without the depletion effect. Fig. 6 A–C compares the radial distribution function between cell–cell, EPS–EPS, and all components of the bacterial colony in the presence of low and high depletion effects due to the nonadsorbing EPS, once the colony attains a steady state. As observed in all three figures, there is a clear difference observed in the case of a low and high depletion effect depending on the repulsive elastic force of the interaction between cells and EPS particles, which is responsible for the depletion-induced phase segregation. When the cell–EPS interaction is highly repulsive, among cell–cell or EPS–EPS or any particles of biofilms is accompanied by a sharper peak at a short distance, followed by a well-defined shoulder at a slightly far-off distance, implying formation of ordered aggregate with well-defined layering of the coordination shell. On the other hand, when the cell–EPS repulsive interaction is low and hence the depletion effect could not generate aggregation of bacterial cells, we find that radial distribution functions among different components are relatively broad and lack the presence of any well-defined shoulder, signifying an almost homogeneous colony spread of the cells, EPSs, and overall biomass. Static structure factors [] among different components of the biofilms reveal complementary insights from those obtained from radial distribution functions. As depicted in Fig. 7 A–C, when there is a high repulsive interaction between cell and EPS particles causing a depletion-mediated patterned colony, the static structure factors between different components of the colony are found to be accompanied by sharper, well-defined, and slowly decaying peaks, indicative of long-range orders.
Fig. 6.

Comparison of radial distribution function [g(r)] for different components of the colony: (A) cell, (B) EPS, and (C) entire biofilm in the presence of low (cyan) and high (red) depletion effect obtained from varying the repulsive force constant of the components of biofilm. Parameters are same as used in simulations of Fig. 3 A and C.
Fig. 7.

Comparison of static structure factor [S(q)] for different components of the colony: (A) cell, (B) EPS, and (C) entire biofilm in the presence of low (cyan) and high (red) depletion effect. x and y axes are in logarithmic scale. Parameters are same as used in simulations of Fig. 3 A and C.
Effect of EPS over Production.
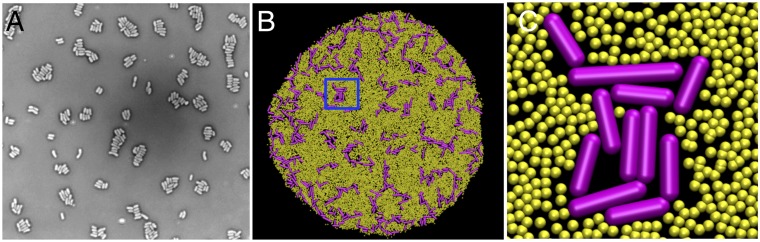
In a recent experiment, a bacterial strain with EPS overproduction (specifically, exopolysaccharide succinoglycan in the S. meliloti bacteria) (38) clearly shows evidence that bacterial cells can form small clusters; an image of their experiment is shown in Fig. 8A. We are thus motivated to ask about what would be expected to occur in our nonequilibrium growth scenario with such an overproducer. To investigate the role of relative numbers of bacterial cells and EPS particles, we carry out a simulation by increasing the rate of EPS production to a very high value , keeping the other parameters and conditions the same as before. In Fig. 8B, the corresponding result of EPS overproduction is demonstrated; small clusters of bacterial cell aggregates are observed in the growing colony. To get a better view of the bacterial cluster and surrounding EPS environment, an enlarged view of the cropped area (shown in a solid blue line) of Fig. 8B is also shown in Fig. 8C. The predicted morphology, including the higher cluster density at the colony edge, could be tested in growth experiments with the aforementioned strain.
Fig. 8.
Effect of high production rate of EPSs. (A) Modified with permission of the Royal Society, where white cylindrical objects are S. meliloti bacteria and the gray region is the extracellular medium. Note that this picture is not produced by a growth experiment and hence cannot be directly compared with our simulations. (B) Snapshot of growing bacterial colony with small cellular aggregates. (C) Enlarged view of a cluster and surrounding EPS particles indicated by the blue-colored rectangular segment of the colony shown on the left. All other parameters are the same as Fig. 3C except .
Interplay of Nutrient Diffusion and EPS in Colony Morphology.
In the process of simulating a growing biofilm colony, our current study uses a nutrient coupled to cell growth (Eq. 1). To illustrate the role of nutrient diffusion in colony dynamics and phase separation, we explore two other possible scenarios for the cell–EPS assembly in the form of two control simulations: (i) using a fixed concentration of non diffusing nutrient that does not affect growth of the bacterial cells and is instead kept as a constant value and (ii) using a diffusing nutrient but with a high consumption rate of nutrient by the bacterial cells, (i.e., a high ratio), thereby creating a situation of diffusion-limited growth. In the first scenario, each cell grows at a constant rate ϕ to the critical length and then divides at a rate . This situation is unlike the original case, where due to nutrient diffusion and consumption, the cells at the center of the colony might not grow to their maximum length and hence cell division might be arrested. The corresponding spatial organization of cell–EPS assemblies are shown in the presence of low and high depletion effects in Fig. 9 A and B, respectively. The fact that cell growth dominates the EPS production rate gives rise to patterns in which depletion creates small clusters of EPS particles. On increasing EPS production, we observe relatively higher phase separation as depicted in Fig. 9C.
Fig. 9.
Snapshot of cell–EPS assembly in the absence of a nutrient effect assuming the constant growth of cells in presence of depletion effect varying repulsive elastic force constants. (A) , . (B) . (C) , . Other parameters are kept the same as given in Table 1, except that the effect of nutrient is absent.
Conversely, the second scenario of a high value for nutrient consumption is particularly interesting in the light of the recent work of Farrell and coworkers (25). Their work suggested the formation of branched colonies at high value and quasi-circular colonies at low value, both in the absence of EPSs. To explore the effect of EPSs at a high value on morphology, we increase the consumption rate k of the nutrient by bacterial cells and repeat the simulation. Fig. 10 compares the morphology of the colony at a high value in the absence and presence of EPSs. In the absence of EPSs (Fig. 10A), we also observe branching, in agreement with the observations by Farrell and coworkers (25). However, irrespective of the presence of low and high depletion effects, the presence of EPSs enhances the spreading and fills the gaps created by the cell branches, thereby significantly suppressing the emergence of a branched colony shape (as observed in Fig. 10 B and C). However, the colony is not perfectly circular at high and does have a little roughness (Fig. 10 B and C).
Fig. 10.
Suppression of the branching effect in the presence of EPS. Simulating bacterial colonies for the same amount of time (A) if there is no production of EPS; production of EPS along with growth of bacterial colony in the presence of (B) low depletion effect and (C) high depletion effect, for a high value of consumption rate . Other parameters are similar as in Fig. 3 A and C.
Finally, we tested the robustness of the self-organized colony formation by studying to what extent the assumed size of the EPS particles affects the morphology of the growing colony. Toward this end, we simulated the formation of a colony by reducing the diameter of EPS particles and simultaneously increasing the rate of EPS secretion during growth and obtained similar phase-aggregated patterns as represented in Fig. S4. However, for the sake of computational efficiency, we carry out most of our simulations using a relatively large size of EPS particles (diameter half of a bacterial cell). As argued above, the relative parameters governing EPS exclusion vs. bacterial cell exclusion are the key controlling factors, and these are not altered by modifying the particle size.
Concluding Remarks
Aggregation and alignment phenomena are fairly common in the realm of self-organization of both nonliving and living objects. The most pervasive and well-known examples involve self-organization of active particles (3). Here we show that a nonmotile microorganism growing on a solid surface, using only the growth and division forces along with a self-production of extracellular substances, can show a similar type of aggregation in a growing colony. In detail, we studied a particle-based simulation model of bacterial cells and EPSs to show how a nonequilibrium growing colony can generate structural heterogeneity spontaneously in the form of aggregation of bacterial cells and consequently a phase-separated heterogeneous biofilm. This spatial heterogeneity stems purely from mechanical interplay between the two species. The driving force behind this phase separation comes from entropy-favored aggregation of bacterial cells to make extra volume accessible for the EPS particles. Our model differs from previous biofilm simulations that do not explicitly model mechanical forces and the presence of extracellular polymeric substances. To the best of our knowledge, this is the first case where depletion-induced spontaneous aggregation of bacterial cells and phase separation are shown in a growing colony. We also find that the overlap envelope of the bacterial colony can be significantly altered by the presence of EPSs.
There are several logical extensions of our work. It would certainly be interesting to extend our model to 3D, including the buckling transition that drives cells upward. The role of cell death (9) also needs to be investigated. Our model could easily be extended to include self-propelled motile bacteria (18) in cases where they remain motile in the biofilm. Finally, our approach could eventually be extended to study the dynamics of cells vs. the extracellular matrix (ECM) in animal tissues under conditions where some cells are actively secreting ECM material.
Supplementary Material
Acknowledgments
We thank F. D. C. Farrell for useful discussions. This work was supported by Center for Theoretical Biological Physics Grant PHY-1427654 and National Science Foundation Molecular and Cellular Biology (MCB) Division Grant MCB-1241332.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504948112/-/DCSupplemental.
References
- 1.Cross MC, Hohenberg PC. Pattern formation outside of equilibrium. Rev Mod Phys. 1993;65(3):851–1123. [Google Scholar]
- 2.Vicsek T, Zafeiris A. Collective motion. Phys Rep. 2012;517(3-4):71–140. [Google Scholar]
- 3.Marchetti MC, et al. Hydrodynamics of soft active matter. Rev Mod Phys. 2013;85(3):1143–1189. [Google Scholar]
- 4.Zhang HP, Be’er A, Florin EL, Swinney HL. Collective motion and density fluctuations in bacterial colonies. Proc Natl Acad Sci USA. 2010;107(31):13626–13630. doi: 10.1073/pnas.1001651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basan M, Elgeti J, Hannezo E, Rappel WJ, Levine H. Alignment of cellular motility forces with tissue flow as a mechanism for efficient wound healing. Proc Natl Acad Sci USA. 2013;110(7):2452–2459. doi: 10.1073/pnas.1219937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, Sokolov A, Lavrentovich OD, Aranson IS. Living liquid crystals. Proc Natl Acad Sci USA. 2014;111(4):1265–1270. doi: 10.1073/pnas.1321926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 8.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Asally M, et al. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc Natl Acad Sci USA. 2012;109(46):18891–18896. doi: 10.1073/pnas.1212429109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminara A, et al. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc Natl Acad Sci USA. 2012;109(4):1116–1121. doi: 10.1073/pnas.1109261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloag ES, et al. Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci USA. 2013;110(28):11541–11546. doi: 10.1073/pnas.1218898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh P, Ben-Jacob E, Levine H. Modeling cell-death patterning during biofilm formation. Phys Biol. 2013;10(6):066006. doi: 10.1088/1478-3975/10/6/066006. [DOI] [PubMed] [Google Scholar]
- 13.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30(2):285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 14.Cates ME, Marenduzzo D, Pagonabarraga I, Tailleur J. Arrested phase separation in reproducing bacteria creates a generic route to pattern formation. Proc Natl Acad Sci USA. 2010;107(26):11715–11720. doi: 10.1073/pnas.1001994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, et al. Sequential establishment of stripe patterns in an expanding cell population. Science. 2011;334(6053):238–241. doi: 10.1126/science.1209042. [DOI] [PubMed] [Google Scholar]
- 17.McBride MJ. Bacterial gliding motility: Multiple mechanisms for cell movement over surfaces. Annu Rev Microbiol. 2001;55:49–75. doi: 10.1146/annurev.micro.55.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Jacob E, Cohen I, Levine H. Cooperative self-organization of microorganisms. Adv Phys. 2000;49(4):395–554. [Google Scholar]
- 19.Lushi E, Wioland H, Goldstein RE. Fluid flows created by swimming bacteria drive self-organization in confined suspensions. Proc Natl Acad Sci USA. 2014;111(27):9733–9738. doi: 10.1073/pnas.1405698111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, et al. Self-organization in high-density bacterial colonies: Efficient crowd control. PLoS Biol. 2007;5(11):e302. doi: 10.1371/journal.pbio.0050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer D, et al. Buckling instability in ordered bacterial colonies. Phys Biol. 2011;8(2):026008. doi: 10.1088/1478-3975/8/2/026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wensink HH, et al. Meso-scale turbulence in living fluids. Proc Natl Acad Sci USA. 2012;109(36):14308–14313. doi: 10.1073/pnas.1202032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volfson D, Cookson S, Hasty J, Tsimring LS. Biomechanical ordering of dense cell populations. Proc Natl Acad Sci USA. 2008;105(40):15346–15351. doi: 10.1073/pnas.0706805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell FDC, Hallatschek O, Marenduzzo D, Waclaw B. Mechanically driven growth of quasi-two-dimensional microbial colonies. Phys Rev Lett. 2013;111(16):168101. doi: 10.1103/PhysRevLett.111.168101. [DOI] [PubMed] [Google Scholar]
- 26.Grant MAA, Wacław B, Allen RJ, Cicuta P. The role of mechanical forces in the planar-to-bulk transition in growing Escherichia coli microcolonies. J R Soc Interface. 2014;11(97):20140400. doi: 10.1098/rsif.2014.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RH, Mitchell R. The role of polymers in microbial aggregation. Annu Rev Microbiol. 1973;27:27–50. doi: 10.1146/annurev.mi.27.100173.000331. [DOI] [PubMed] [Google Scholar]
- 28.Asakura S, Oosawa F. Interaction between particles suspended in solutions of macromolecules. J Polym Sci Polym Phys Ed. 1958;33(126):183–192. [Google Scholar]
- 29.Yodh AG, et al. Entropically driven self-assembly and interaction in suspension. Phil Trans R Soc A. 2001;359(1782):921–937. [Google Scholar]
- 30.Neu B, Meiselman HJ. Depletion-mediated red blood cell aggregation in polymer solutions. Biophys J. 2002;83(5):2482–2490. doi: 10.1016/S0006-3495(02)75259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffen P, Verdier C, Wagner C. Quantification of depletion-induced adhesion of red blood cells. Phys Rev Lett. 2013;110(1):018102. doi: 10.1103/PhysRevLett.110.018102. [DOI] [PubMed] [Google Scholar]
- 32.Richter K, Nessling M, Lichter P. Experimental evidence for the influence of molecular crowding on nuclear architecture. J Cell Sci. 2007;120(Pt 9):1673–1680. doi: 10.1242/jcs.03440. [DOI] [PubMed] [Google Scholar]
- 33.Hancock R. A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J Struct Biol. 2004;146(3):281–290. doi: 10.1016/j.jsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuinier R, Dhont JKG, De Kruif CG. Depletion-induced phase separation of aggregated whey protein colloids by an exocellular polysaccharide. Langmuir. 2000;16(4):1497–1507. [Google Scholar]
- 36.Schwarz-Linek J, et al. Polymer-induced phase separation in suspensions of bacteria. Europhys Lett. 2010;89(6):68003. [Google Scholar]
- 37.Schwarz-Linek J, et al. Polymer-induced phase separation in escherichia coli suspensions. Soft Matter. 2010;6:4540–4549. [Google Scholar]
- 38.Dorken G, Ferguson GP, French CE, Poon WCK. Aggregation by depletion attraction in cultures of bacteria producing exopolysaccharide. J R Soc Interface. 2012;9(77):3490–3502. doi: 10.1098/rsif.2012.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz-Linek J, et al. Phase separation and rotor self-assembly in active particle suspensions. Proc Natl Acad Sci USA. 2012;109(11):4052–4057. doi: 10.1073/pnas.1116334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dilanji GE, Teplitski M, Hagen SJ. 2014. Entropy-driven motility of sinorhizobium meliloti on a semi-solid surface. Proc R Soc B Biol Sci 281(1784):20132575. [DOI] [PMC free article] [PubMed]
- 41.Rein ten Wolde P, RuizMontero MJ, Frenkel D. Numerical calculation of the rate of crystal nucleation in a lennardjones system at moderate undercooling. J Chem Phys. 1996;104(24):9932–9947. [Google Scholar]
- 42.Steinhardt PJ, Nelson DR, Ronchetti M. Bond-orientational order in liquids and glasses. Phys Rev B. 1983;28(2):784–805. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.