Summary
Background
Ipilimumab is a fully human monoclonal antibody that binds cytotoxic T-lymphocyte antigen 4 to enhance antitumour immunity. Our aim was to assess the use of ipilimumab after radiotherapy in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel chemotherapy.
Methods
We did a multicentre, randomised, double-blind, phase 3 trial in which men with at least one bone metastasis from castration-resistant prostate cancer that had progressed after docetaxel treatment were randomly assigned in a 1:1 ratio to receive bone-directed radiotherapy (8 Gy in one fraction) followed by either ipilimumab 10 mg/kg or placebo every 3 weeks for up to four doses. Non-progressing patients could continue to receive ipilimumab at 10 mg/kg or placebo as maintenance therapy every 3 months until disease progression, unacceptable toxic effect, or death. Patients were randomly assigned to either treatment group via a minimisation algorithm, and stratified by Eastern Cooperative Oncology Group performance status, alkaline phosphatase concentration, haemoglobin concentration, and investigator site. Patients and investigators were masked to treatment allocation. The primary endpoint was overall survival, assessed in the intention-to-treat population. This trial is registered with ClinicalTrials.gov, number NCT00861614.
Findings
From May 26, 2009, to Feb 15, 2012, 799 patients were randomly assigned (399 to ipilimumab and 400 to placebo), all of whom were included in the intention-to-treat analysis. Median overall survival was 11·2 months (95% CI 9·5–12·7) with ipilimumab and 10·0 months (8·3–11·0) with placebo (hazard ratio [HR] 0·85, 0·72–1·00; p=0·053). However, the assessment of the proportional hazards assumption showed that it was violated (p=0·0031). A piecewise hazard model showed that the HR changed over time: the HR for 0–5 months was 1·46 (95% CI 1·10–1·95), for 5–12 months was 0·65 (0·50–0·85), and beyond 12 months was 0·60 (0·43–0·86). The most common grade 3–4 adverse events were immune-related, occurring in 101 (26%) patients in the ipilimumab group and 11 (3%) of patients in the placebo group. The most frequent grade 3–4 adverse events included diarrhoea (64 [16%] of 393 patients in the ipilimumab group vs seven [2%] of 396 in the placebo group), fatigue (40 [11%] vs 35 [9%]), anaemia (40 [10%] vs 43 [11%]), and colitis (18 [5%] vs 0). Four (1%) deaths occurred because of toxic effects of the study drug, all in the ipilimumab group.
Interpretation
Although there was no significant difference between the ipilimumab group and the placebo group in terms of overall survival in the primary analysis, there were signs of activity with the drug that warrant further investigation.
Funding
Bristol-Myers Squibb.
Introduction
Prostate cancer is the second most frequently diagnosed cancer worldwide and is the second leading cause of cancer deaths in men.1 Prostate cancer that progresses despite castrate concentrations of testosterone is termed castration-resistant prostate cancer.2,3 Recently, several treatments have been approved for metastatic castration-resistant prostate cancer after progression with docetaxel chemotherapy, each of which extended overall survival compared with controls.4–7 However, new treatments that provide durable disease control are still needed.
Prostate cancer tissues are often infiltrated by inflammatory cells,8,9 suggesting that this cancer is the target of a host immune response. This response can be constrained by various mechanisms that undermine antitumour immunity.9–13 As such, a goal of immunotherapy is to overcome these constraints to enhance tumour regression.14,15 Currently, the only approved immunotherapeutic approach for prostate cancer is a vaccine that targets prostatic acid phosphatase, sipuleucel-T, which has been shown to extend overall survival for patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer, but does not affect prostate-specific antigen (PSA) concentration, induce tumour regression, or increase progression-free survival.16 Prostvac VF, a vaccinia virus-based PSA vaccine, is another antigen-specific immunotherapy17 that is currently in phase 3 testing for minimally symptomatic metastatic castration-resistant prostate cancer (ClinicalTrials.gov number NCT01322490), but also has not shown an effect on PSA concentration, tumour regression, or progression-free survival.
Ipilimumab is a fully human monoclonal antibody that binds to the inhibitory cytotoxic T-lymphocyte antigen 4 (CTLA-4) and thereby enhances antitumour immunity.18 Responses triggered by ipilimumab are not believed to be antigen specific, and instead result from prevention of the CTLA-4-mediated downregulation of T-lymphocyte activity. On the basis of results showing increased overall survival in patients with advanced melanoma in phase 3 trials, ipilimumab has been approved for the treatment of advanced melanoma in more than 40 countries at a dose of 3 mg/kg.19,20 Notably, a proportion of patients with melanoma who received ipilimumab within the clinical development programme remained alive more than 2 years after treatment, suggesting the potential for long-term survival.21–23 Ipilimumab has side-effects that are related to its immune-based mechanism of action, but these are generally manageable using standard treatment algorithms, including proactive monitoring and early intervention with corticosteroids.
Prostate cancer was one of the first malignant diseases in which CTLA-4 blockade was tested in preclinical models.15,24 Clinical potential for this treatment was subsequently confirmed in several studies of patients with metastatic castration-resistant prostate cancer,25–27 including a phase 1/2 dose-escalation trial of ipilimumab in chemotherapy-naive and chemotherapy-pretreated patients.27 Evidence from both preclinical and clinical studies suggests that radiotherapy might activate the immune system.28–32 Thus, radiotherapy is a rational modality to stimulate immune priming amenable to subsequent amplification by ipilimumab. Consistent with this hypothesis, the combination of immunotherapy and radiotherapy in a transgenic mouse model that spontaneously develops prostate cancer was shown to result in antitumour T-cell activation, but only when radiotherapy was given before immunotherapy.28 Although the precise mechanisms by which radiotherapy promotes immunotherapeutic responses remain unknown, radiotherapy might sensitise tumour cells to killing by antigen-specific cytotoxic T lymphocytes.29 Radiotherapy also promotes the local infiltration of immune cells into the tumour,30 and might trigger antitumour immune responses at locations distant from the original site of irradiation (abscopal effect).31,32 In the clinical setting, the combination of radiotherapy and ipilimumab at 10 mg/kg is well tolerated in patients with metastatic castration-resistant prostate cancer.27
On the basis of existing evidence, phase 3 trials of ipilimumab for patients with metastatic castration-resistant prostate cancer were initiated in chemotherapy-naive (ClinicalTrials.gov number NCT01057810) and post-docetaxel populations. Here, we report results from a phase 3 trial of ipilimumab after radiotherapy in patients with metastatic castration-resistant prostate cancer who progressed after docetaxel chemotherapy.
Methods
Study design and patients
In this randomised, double-blind, controlled phase 3 trial (CA184-043), done in 191 centres across 26 countries, we enrolled male patients aged 18 years or older with histologically or cytologically confirmed adenocarcinoma of the prostate, at least one bone metastasis that could be irradiated or warranted irradiation in the clinical judgment of the investigator, testosterone concentration less than 1·74 nmol/L, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients must have received at least one previous docetaxel-containing regimen for metastatic castration-resistant prostate cancer, consisting of at least two cycles of docetaxel, and progressed while receiving, or within 6 months of receiving, the docetaxel regimen. Disease progression was assessed on the basis of the Prostate Cancer Clinical Trials Working Group’s recommendations.2 Patients were excluded if they had received more than two cytotoxic chemotherapy regimens for castration-resistant prostate cancer or if they had brain metastases, an autoimmune disease, or a known HIV, hepatitis B, or hepatitis C infection.
The institutional review boards at all participating centres approved the study, which was done in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent to participate in the study.
Randomisation and masking
Random assignment was done with an interactive voice response system via the Pocock and Simon minimisation algorithm, stratified by ECOG performance status (0 vs 1), alkaline phosphatase concentration (<1·5 times the upper limit of normal [ULN] vs ≥1·5 times ULN), haemoglobin concentration (<110 g/L vs ≥110 g/L), and investigator site.
The study funder, patients, and site staff were masked with respect to each patient’s assignment until the database cutoff date for this report (ie, when the required number of events [deaths] for the primary analysis had occurred). Local pharmacists and the pharmacy monitors (who prepared and dispensed study drugs to the blinded research staff ) were not masked. An independent data monitoring committee had access to unblinded data to enable review of emerging safety data. In the event of a medical emergency in an individual patient, the treating physician could be informed of the assigned treatment if knowledge of the investigational product was crucial to the patient’s management.
Procedures
We randomly assigned patients in a 1:1 ratio to receive bone-directed radiotherapy followed by either ipilimumab (Bristol-Myers Squibb, Princeton, NJ, USA) at 10 mg/kg or placebo every 3 weeks for up to four doses. Both ipilimumab and placebo (0·9% sodium chloride or 5% dextrose) were given by intravenous infusion over a 90-min period. Non-progressing patients could continue to receive ipilimumab at 10 mg/kg or placebo as maintenance therapy every 3 months. Treatment with either ipilimumab or placebo continued until confirmed disease progression, intolerable toxic effects, clinical deterioration, death, withdrawal of consent, or loss to follow-up. No dose reductions were permitted. A dose of study drug could be skipped for any grade 2 or higher non-skin-related adverse event, any grade 3 or higher laboratory abnormality, or any grade 3 or higher skin-related adverse event, irrespective of cause. Treatment could be discontinued at any time because of an unacceptable toxic effect (ie, an adverse event attributable to the study drug that required permanent discontinuation of the drug or an adverse event that, in the investigator’s opinion, presented a substantial risk to the patient with continued administration), clinical deterioration, or confirmed disease progression (by PSA or radiographic assessment). Patients were treated as per local standard of care after discontinuing study treatments; crossover was not permitted.
All patients received a single dose of radiotherapy of 8 Gy for at least one, and up to five, bone fields, at the investigator’s discretion. This single-administered radiation dose (8 Gy in one treatment fraction) was previously shown to be therapeutically equivalent to a fractionated regimen (30 Gy in ten treatment fractions over 2 weeks) with respect to pain palliation.33 Radiotherapy was done some time within the 2 days before initiation of the study drug regimen, and palliative radiotherapy was allowed for any bone lesion while on study. Sites of radiotherapy included the arm, leg, pelvis, spine, rib, and skull. We did not assess the efficacy of the radiotherapy with respect to pain palliation or lesional regression as part of the study, because it was given to stimulate immune response. Until database lock, investigators assessing disease progression (including by radiographic assessment) remained masked to treatment allocation.
Tumour assessments by radiographic imaging (eg, MRI or CT of chest, abdomen, pelvis, and other soft tissue, and bone scans, as applicable) were done every 12 weeks. Progression per bone scan was defined as the appearance of two or more new lesions in two consecutive assess ments; nodal or visceral disease progression in target lesions was assessed by modified Response Evaluation Criteria In Solid Tumors version 1.0. PSA concentrations were measured in serum panels by use of an automated immunochromatographic membrane assay at a central laboratory every 6 weeks until the patient received subsequent therapies, after which they were measured every 12 weeks. Pain response was assessed every 12 weeks with the Brief Pain Inventory (Short Form), and was defined as a decrease in average daily worst pain intensity (question 3) by at least 30% from baseline, maintained over two consecutive assessments, without any rescue drug treatment, or increase in analgesic use in the same time period. Safety characterisation was based on events that occurred throughout the on-study period (from the first dose of study drug to 70 days after the last dose of study drug, including maintenance therapy). Adverse events were graded in accordance with the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0).
Outcomes
The primary endpoint of the trial was overall survival, defined as time from random assignment to death from any cause. Secondary endpoints included progression-free survival, pain response, and safety profile. Progression-free survival was a composite endpoint based on confirmed PSA progression, which was defined as a PSA increase of at least 25% and at least 2 µg/L above PSA concentration nadir on or after week 12 (confirmed over two subsequent assessments), confirmed radiological progression, clinical deterioration, or death. Clinical deterioration was defined as a persistent decrease in performance status (ie, lasting more than 14 days) of at least 2 points from baseline performance that was attributable to: disease progression; any symptomatic clinical event attributable to disease progression that, in the investigator’s opinion, suggested that the patient was not benefiting from study treatment and could be managed by supportive care (eg, bisphosphonates or bone-directed radiotherapy); or any change of antineoplastic therapy because of prostate cancer. Exploratory endpoints included PSA response rate and quality of life.
Statistical analysis
The primary analysis was a between-group comparison of overall survival by use of a stratified log-rank test in the intention-to-treat population (all randomly assigned patients). The study was designed to ensure 90% power (two-sided α=0·05) to reject the null hypothesis of no effect of ipilimumab, assuming a true hazard ratio (HR) of 0·76. Under an exponential distribution, this HR would correspond to a median overall survival of 15·8 months for the ipilimumab group and 12·0 months in the placebo group. We planned to enrol 800 patients to observe 560 events (deaths). The study database was locked for the primary analysis on Feb 6, 2013, when a total of 574 deaths had occurred. All efficacy endpoints were based on the intention-to-treat population, apart from the PSA analyses (done in PSA-assessable patients), and safety was assessed in all treated patients. For PSA response, assessable patients were defined as randomly assigned patients with both a non-missing PSA baseline value and at least one on-study value.
We used the Kaplan-Meier product-limit method34 to estimate the overall and progression-free survival probabilities for each treatment group.34,35 We examined proportional-hazards assumptions by testing the period-by-treatment interaction term in a time-dependent Cox model,36 with period as a binary variable (before or after the timepoint when obvious separation of the curves began). In an exploratory analysis, we used a piecewise hazard model to further assess the assumptions of the Cox model, and to provide estimates of the HR over time (ie, before, during, and after crossover of the survival curves). We also did post-hoc analyses of overall survival in protocol-specified subgroups, based on clinically known prognostic risk factors for metastatic castration-resistant prostate cancer, using a multivariate Cox proportional hazards model. Additionally, we did post-hoc treatment interaction tests using baseline prognostic factors as covariates; separate Cox models were used (one for each baseline factor), with treatment and each baseline factor as main effects and the treatment-by-baseline factor interaction included to test whether the treatment effect differed for patients with different levels of the specific factor.
The protocol specified that a statistical comparison of progression-free survival between groups was to be done only if the groups differed significantly for the primary endpoint of overall survival. Although there was no significant difference in overall survival between patients groups, we decided to compare progression-free survival anyway to allow us to further assess the potential activity of ipilimumab in metastatic castration-resistant prostate cancer. Accordingly, statistical findings for secondary and exploratory analyses are reported for descriptive purposes only. Demographic and baseline characteristics, safety, and outcome research instruments (eg, pain intensity) are summarised descriptively.
Statistical analyses were done with SAS version 8.2.
This trial is registered with ClinicalTrials.gov, number NCT00861614.
Role of the funding source
The trial was designed jointly by the funder and academic authors (EDK, TMB, and WRG). Data were collected by the funder and were analysed and interpreted by the funder in collaboration with the authors. The funder was involved in the writing of the report. A professional medical writer employed by the funder helped to produce the initial draft of this report. PG, DL, and MBM had access to all the raw data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
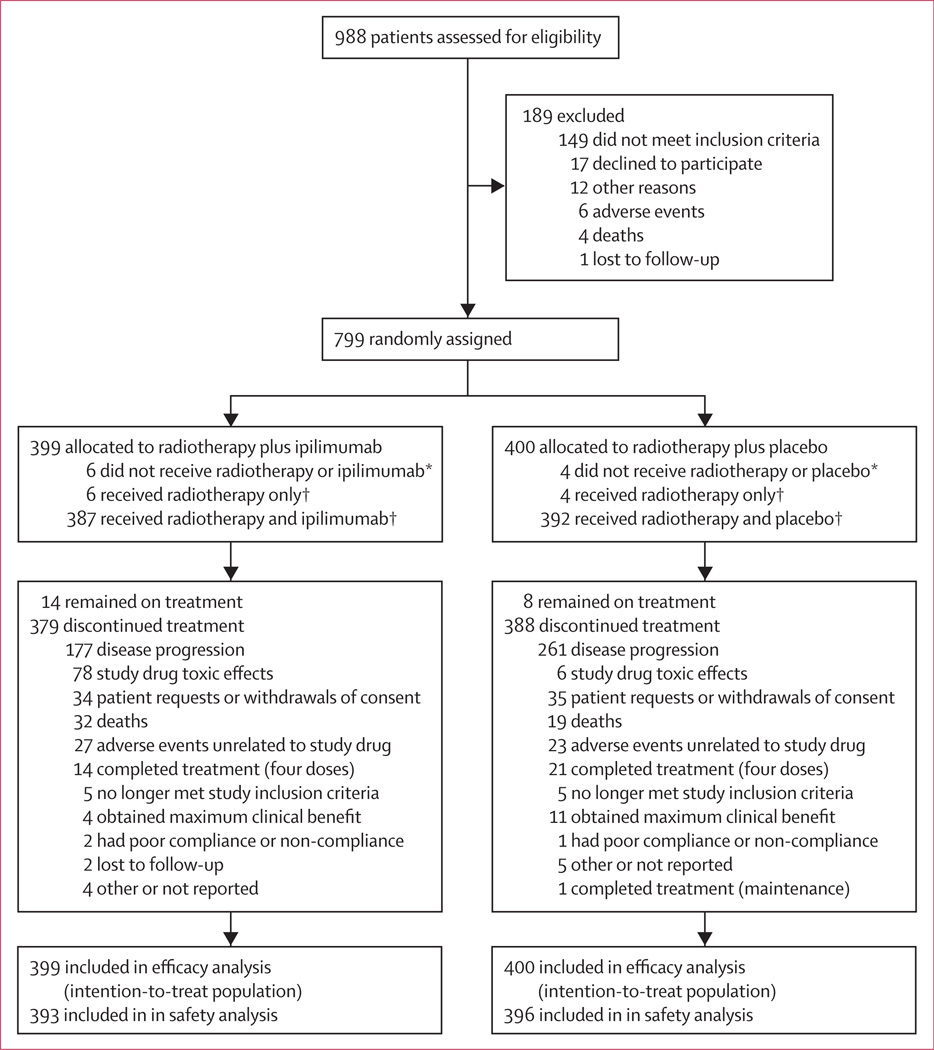
From May 26, 2009, to Feb 15, 2012, 799 patients were randomly assigned: 399 to the ipilimumab group and 400 to the placebo group (figure 1). Baseline patient characteristics were balanced across groups, and were indicative of advanced disease (table 1). 12 (3%) of 399 patients in the ipilimumab group and eight (2%) of 400 patients in the placebo group discontinued before receiving the assigned treatment; of these patients, six (2%) and four (1%), respectively, received radiotherapy only. Of the 387 patients who received radiotherapy and ipilimumab, and the 392 who received radiotherapy and placebo, 198 (51%) patients in the ipilimumab group and 262 (67%) in the placebo group received at least four doses of study drug. Patients did not receive all four initial doses of study drug for various reasons, most often disease progression (118 [30%] of 387 patients in the ipilimumab group, and 221 [56%] of 392 patients in the placebo group), or toxic effects of the study drug (71 [18%] and six [2%], respectively). Of 393 patients in the ipilimumab group, 95 (24%) received maintenance therapy, as did 62 (16%) of 396 patients in the placebo group. Use of subsequent anticancer therapy was similar between the ipilimumab and placebo groups (161 [41%] of 393 vs 185 [47%] of 396, respectively; appendix p 1), with median times to first subsequent therapy of 171 (IQR 100–284) and 119 days (87–183), respectively (data not shown).
Figure 1. Trial profile.
*Excluded from safety analyses. †Included in safety analyses.
Table 1.
Demographic and baseline characteristics
| Ipilimumab group (n=399) |
Placebo group (n=400) |
|
|---|---|---|
| Median age (range), years | 69·0 (47·0–86·0) | 67·5 (45·0–86·0) |
| Age group | ||
| <70 years | 215 (54%) | 234 (59%) |
| ≥70 years | 184 (46%) | 166 (42%) |
| Alkaline phosphatase concentration | ||
| <1·5 times ULN | 225 (56%) | 232 (58%) |
| ≥1·5 times ULN | 163 (41%) | 151 (38%) |
| Missing data | 11 (3%) | 17 (4%) |
| Gleason score | ||
| ≤7 | 174 (44%) | 190 (48%) |
| >7 | 192 (48%) | 187 (47%) |
| Missing data | 33 (8%) | 23 (6%) |
| Haemoglobin concentration | ||
| <110 g/L | 116 (29%) | 111 (28%) |
| ≥110 g/L | 267 (67%) | 269 (67%) |
| Missing data | 16 (4%) | 20 (5%) |
| ECOG performance status | ||
| 0 | 168 (42%) | 170 (43%) |
| 1 | 216 (54%) | 220 (55%) |
| 2* | 3 (1%) | 0 |
| Missing data | 12 (3%) | 10 (3%) |
| Number of bone metastases | ||
| ≤5 | 276 (69%) | 253 (63%) |
| >5 | 103 (26%) | 111 (28%) |
| Missing data | 20 (5%) | 36 (9%) |
| Visceral metastases | ||
| No | 280 (70%) | 275 (69%) |
| Yes | 113 (28%) | 114 (29%) |
| Missing data | 6 (2%) | 11 (3%) |
| Lactate dehydrogenase concentration | ||
| ≤2 times ULN | 326 (82%) | 325 (81%) |
| >2 times ULN | 58 (15%) | 53 (13%) |
| Missing data | 15 (4%) | 22 (6%) |
| PSA concentration | ||
| Number of patients with PSA data available (%) | 338 (85%) | 334 (84%) |
| Median (range), µg/L | 138·5 (0–4576) | 176·5 (0–13768) |
| Average daily worst bone pain intensity score | ||
| <4 | 152 (38%) | 150 (38%) |
| ≥4 | 197 (49%) | 186 (47%) |
| Missing data | 50 (13%) | 64 (16%) |
| Pretreatment steroid (prednisone) use | ||
| No | 331 (83%) | 338 (85%) |
| Yes | 68 (17%) | 62 (16%) |
Data are n (%), unless otherwise indicated. ECOG=Eastern Cooperative Oncology Group. ULN=upper limit of normal. PSA=prostate-specific antigen.
Assigned in error (protocol deviation).
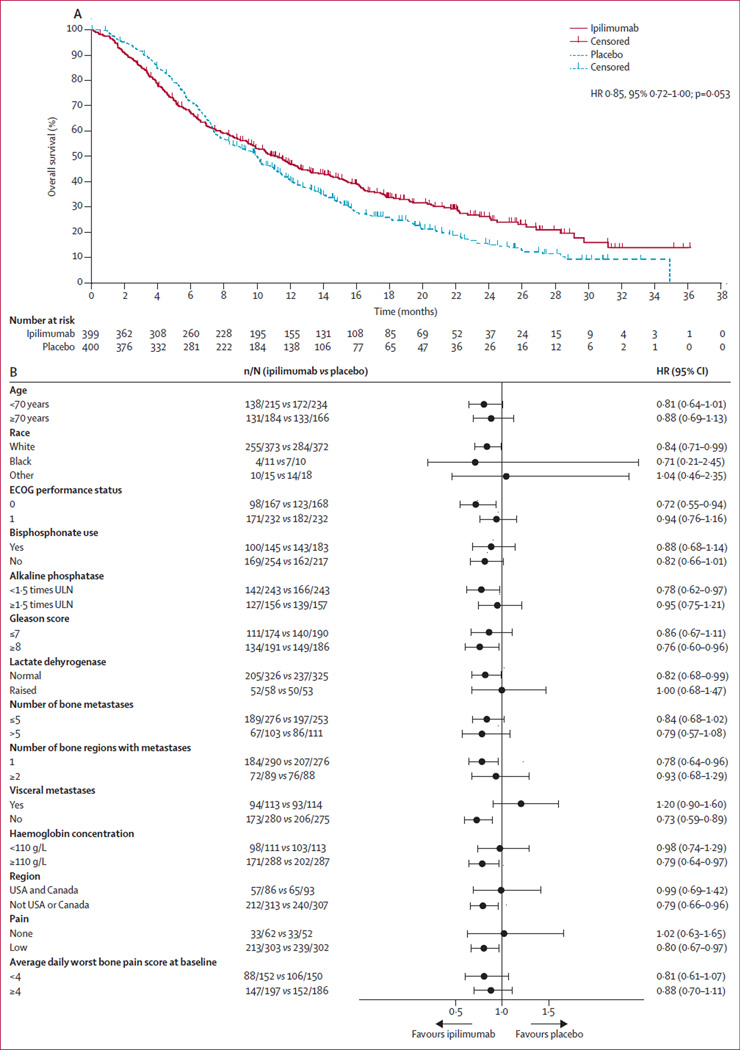
After median follow-up of 9·9 months (IQR 4·3–16·7) in the ipilimumab group and 9·3 months (IQR 5·4–14·6) in the placebo group, 269 deaths had occurred in the ipilimumab group and 305 in the placebo group. Median overall survival was 11·2 months (95% CI 9·5–12·7) for ipilimumab and 10·0 months (95% CI 8·3–11·0) for placebo (HR 0·85, 95% CI 0·72–1·00; p=0·053; figure 2A). Crossover of the Kaplan-Meier survival curves occurred at 7–8 months. An assessment of the proportional hazards assumption showed that it was violated (p=0·0031). We therefore implemented an exploratory piecewise hazard model, which showed that the HR decreased over time: the estimated HR before 5 months was 1·46 (95% CI 1·10–1·95), from 5 to 12 months inclusive was 0·65 (95% CI 0·50–0·85), and beyond 12 months was 0·60 (95% CI 0·43–0·86).
Figure 2. Overall survival in the intention-to-treat population (A) and by patient subgroup (B).
ECOG=Eastern Cooperative Oncology Group. ULN=upper limit of normal.
1-year overall survival was 46·8% (95% CI 41·8–51·8) for ipilimumab and 40·4% (95% CI 35·4–45·3) for placebo. 2-year overall survival was 26·2% (95% CI 21·0–31·4) for ipilimumab and 15·0% (95% CI 10·6–19·4) for placebo; however, this finding should be interpreted with caution because of the high proportion of censored patients at the time of the database lock (180 [80%] of 225 censored patients were censored before 2 years).
The results of prespecified subgroup analyses suggest that ipilimumab might provide a benefit for some subgroups of patients, particularly those with favourable prognostic features (figure 2B). The largest difference was between patients with and without visceral metastases— the presence of visceral metastases was the only prognostic feature that showed a significant interaction with treatment (table 2). Furthermore, a prespecified multivariate Cox analysis was done to identify covariates associated with overall survival among the features measured at baseline. We used log adjustments to normalise the continuous variables (alkaline phosphatase concentration and PSA). Treatment with ipilimumab, age, log of alkaline phosphatase concentration, log of PSA concentration, haemoglobin concentration, number of bone regions with metastases, presence of visceral metastases, and average daily worst bone pain intensity were all associated with overall survival after adjustment for other factors in the model (appendix p 2).
Table 2.
Tests for interaction between baseline prognostic features and treatment
| HR (95% CI)* | p value* | |
|---|---|---|
| Age (<70 years vs ≥70 years) | 1·073 (0·772–1·491) | 0·6764 |
| ECOG performance status (0 vs 1) | 1·271 (0·906–1·782) | 0·1655 |
| Alkaline phosphatase concentration (<1·5 times ULN vs ≥ 1·5 times ULN) | 1·178 (0·847–1·637) | 0·3304 |
| Gleason score (≤7 vs ≥8) | 0·888 (0·631–1·250) | 0·4971 |
| Lactate dehydrogenase concentration (≤2 times ULN vs >2 times ULN) | 1·214 (0·789–1·870) | 0·3778 |
| Visceral metastases (no vs yes) | 1·644 (1·157–2·336) | 0·0056 |
| Haemoglobin concentration (<110 g/L vs ≥110 g/L) | 0·842 (0·597–1·187) | 0·3257 |
| Average daily worst bone pain intensity score (<4 vs ≥4) | 1·057 (0·735–1·519) | 0·7645 |
| Log of PSA concentration | 0·951 (0·845–1·071) | 0·4105 |
| Number of bone metastases (≤5 vs >5) | 0·954 (0·655–1·391) | 0·8077 |
| Number of bone regions with metastases (1 vs ≥2) | 1·156 (0·792–1·689) | 0·4526 |
No adjustments were made for multiplicity. Log of prostate-specific antigen (PSA) concentration was treated as a continuous variable, whereas all other prognostic features were treated as categorical variables. ECOG=Eastern Cooperative Oncology Group. ULN=upper limit of normal. PSA=prostate-specific antigen.
Hazard ratios and p values are for exploratory purposes only.
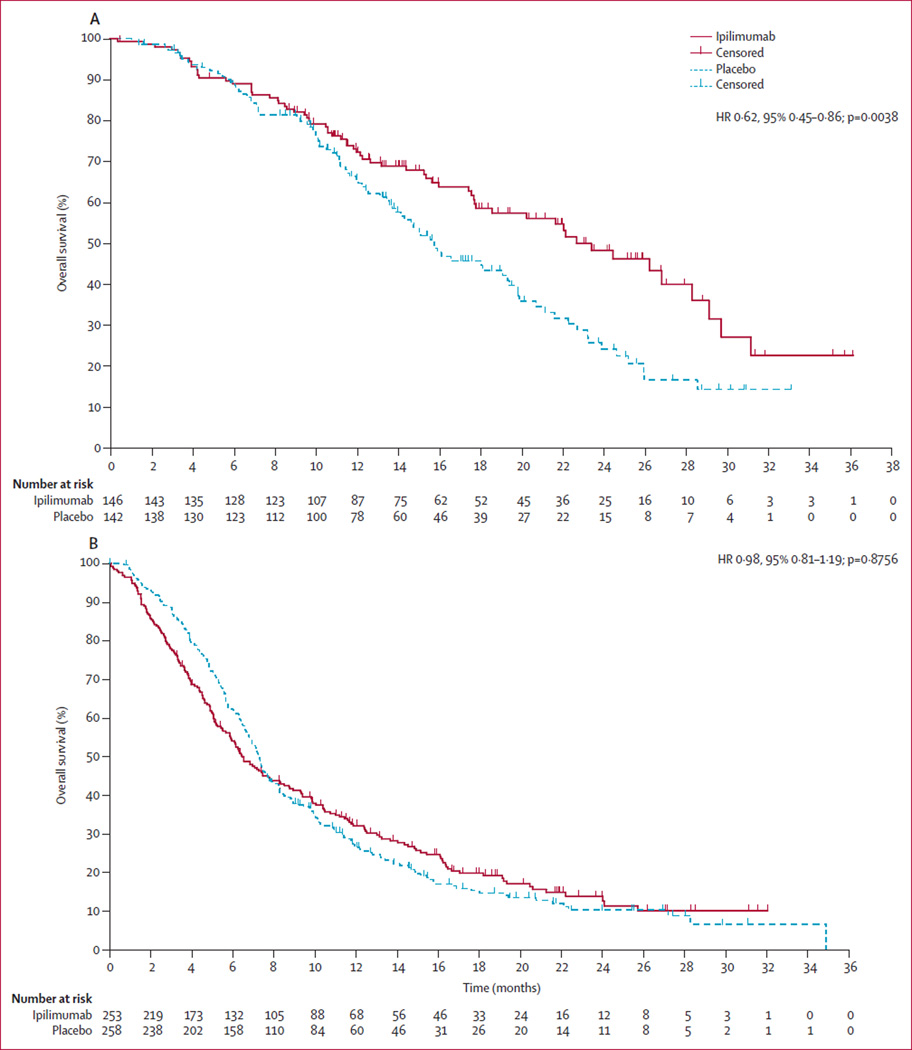
On the basis of these findings, we did a post-hoc assessment of predefined subgroups of patients with multivariate analyses on all baseline prognostic features. These results also suggest that ipilimumab could be most active in patients with favourable prognostic features, particularly those with an alkaline phosphatase concentration of less than 1·5 times ULN, a haemoglobin concentration of 110 g/L or higher, and no visceral metastases. For this subset of patients, median overall survival was 22·7 months (95% CI 17·8–28·3) with ipilimumab (n=146) compared with 15·8 months (13·7–19·4) with placebo (n=142; HR 0·62, 95% CI 0·45–0·86; p=0·0038; figure 3A). In all other patients—ie, a mixed population in which all patients had at least one adverse prognostic feature—median overall survival was 6·5 months (5·7–7·9) with ipilimumab (n=253) compared with 7·3 months (6·7–7·8) with placebo (n=258; HR 0·98, 0·81–1·19; p=0·8756; figure 3B). The results of additional multivariate analyses done on baseline prognostic features are reported in the appendix (pp 19–22).
Figure 3. Post-hoc subgroup analyses of overall survival in patients with good (A) and poor prognostic features (B).
(A) Overall survival in patients with alkaline phosphatase concentration less than 1·5 times the upper limit of normal (ULN), haemoglobin concentration of 110 g/L or more, and no visceral metastases (ipilimumab, n=146; placebo, n=142). (B) Overall survival in patients with at least one adverse prognostic feature— ie, alkaline phosphatase concentration of 1·5 times ULN or higher, haemoglobin concentration less than 110 g/L, or presence of visceral metastases (ipilimumab, n=253; placebo, n=258).
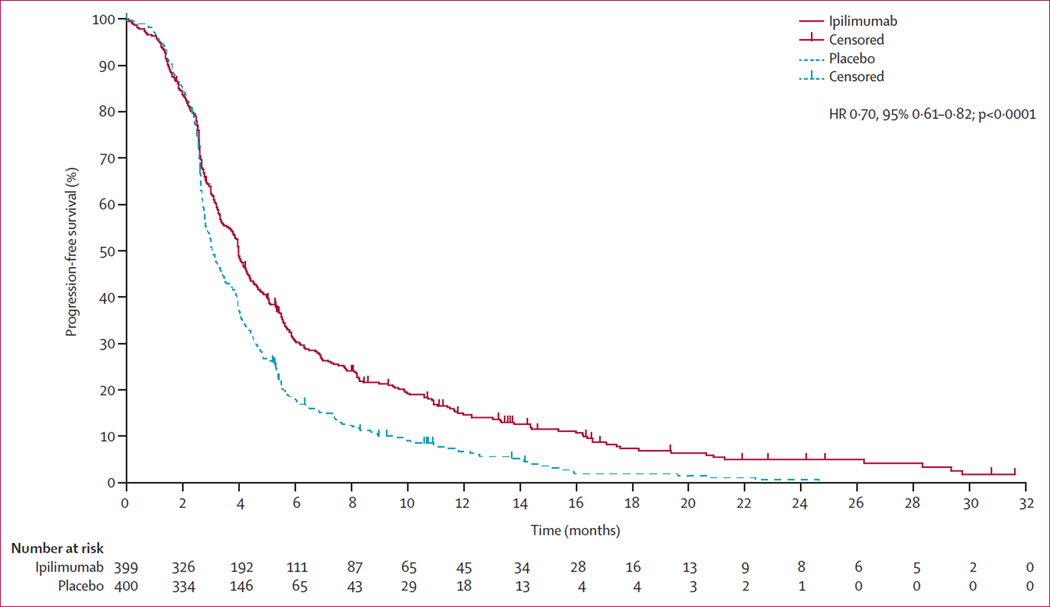
At data cutoff , 347 patients in the ipilimumab group had progressed or died, as had 369 in the placebo group. Treatment with ipilimumab improved progression-free survival compared with placebo (median 4·0 [95% CI 3·6–4·3] vs 3·1 [2·9–3·4] months; HR 0·70, 95% CI 0·61–0·82; p<0·0001; figure 4). 323 progressions or deaths had occurred before 3 months (34 censored): 139 in the ipilimumab group (102 progressions and 37 deaths) and 184 in the placebo group (171 progressions and 13 deaths). 42 additional deaths occurred within the first 3 months after progression: 22 in the ipilimumab group and 20 in the placebo group. Progression-free survival at 6 months was 30·7% (95% CI 26·0–35·3) for the ipilimumab group and 18·1% (14·2–22·0) for the placebo group. Post-treatment PSA reductions were more frequent in the ipilimumab group (appendix p 23), as assessed by the proportion of patients with a confirmed reduction of 50% or more at any time (39/297 [13·1%, 95% CI 9·5–17·5] for ipilimumab and 16/305 [5·2%, 3·0–8·4] for placebo; appendix p 3). The numbers of patients with a pain response were too small to draw meaningful conclusions about differences between the groups (appendix p 3).
Figure 4. Progression-free survival in the intention-to-treat population.
The safety analyses included 393 patients in the ipilimumab group and 396 patients in the placebo group. 385 (98%) patients in the ipilimumab group and 364 (92%) in the placebo group had an on-study adverse event; grade 3–4 on-study adverse events occurred in 232 (59%) patients in the ipilimumab group and 162 (41%) in the placebo group, and 66 (17%) patients in the ipilimumab group and 45 (11%) in the placebo group died on study because of adverse events. On-study adverse events of any grade that led to discontinuation of treatment occurred in 137 (35%) patients in the ipilimumab group and 62 (16%) in the placebo group.
Table 3 summarises the most common adverse events and immune-related adverse events; a full list of events is reported in the appendix (pp 4–14). Drug-related adverse events of any grade were more frequent in the ipilimumab group (295 [75%] of 393 patients) than in the placebo group (180 [45%] of 396 patients). The most common drug-related adverse events were immune-related: 249 (63%) patients in the ipilimumab group and 86 (22%) in the placebo group had an immune-related adverse event of any grade, with grade 3–4 immune-related adverse events occurring in 101 (26%) and 11 (3%) of patients, respectively (appendix pp 4–14). The most frequently reported on-study immune-related adverse events of any grade in the ipilimumab group were diarrhoea, pruritus, rash, colitis, increased aspartate aminotransferase, and increased alanine aminotransferase (table 3). Diarrhoea was the only on-study immune-related adverse event reported by more than 5% of patients in the placebo group.
Table 3.
Summary of on-study adverse events
| Ipilimumab (n=393) |
Placebo (n=396) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Grade 5 | Any grade | Grade 3 | Grade 4 | Grade 5 | |
| Any adverse events* | ||||||||
| Diarrhoea | 199 (51%) | 63 (16%) | 1 (<1%) | 0 | 97 (24%) | 7 (2%) | 0 | 0 |
| Fatigue | 150 (38%) | 34 (9%) | 6 (2%) | 0 | 123 (31%) | 31 (8%) | 4 (1%) | 0 |
| Nausea | 127 (32%) | 11 (3%) | 0 | 0 | 106 (27%) | 7 (2%) | 0 | 0 |
| Decreased appetite | 120 (31%) | 15 (4%) | 0 | 0 | 98 (25%) | 12 (3%) | 0 | 0 |
| Vomiting | 111 (28%) | 7 (2%) | 0 | 0 | 85 (21%) | 9 (2%) | 0 | 0 |
| Pruritus | 99 (25%) | 1 (<1%) | 0 | 0 | 22 (6%) | 0 | 0 | 0 |
| Anaemia | 90 (23%) | 37 (9%) | 3 (1%) | 0 | 88 (22%) | 35 (9%) | 8 (2%) | 0 |
| Decreased weight | 90 (23%) | 5 (1%) | 0 | 0 | 56 (14%) | 1 (<1%) | 0 | 0 |
| Pyrexia | 89 (23%) | 5 (1%) | 0 | 0 | 51 (13%) | 1 (<1%) | 0 | 0 |
| Rash | 83 (21%) | 2 (1%) | 0 | 0 | 27 (7%) | 0 | 0 | 0 |
| Asthenia | 82 (21%) | 25 (6%) | 1 (<1%) | 0 | 68 (17%) | 12 (3%) | 1 (<1%) | 0 |
| Constipation | 69 (18%) | 2 (1%) | 0 | 0 | 83 (21%) | 3 (1%) | 0 | 0 |
| Back pain | 59 (15%) | 14 (4%) | 0 | 0 | 77 (19%) | 17 (4%) | 3 (1%) | 0 |
| Peripheral oedema | 51 (13%) | 0 | 0 | 0 | 37 (9%) | 2 (1%) | 0 | 0 |
| Dyspnoea | 50 (13%) | 15 (4%) | 3 (1%) | 0 | 36 (9%) | 5 (1%) | 2 (1%) | 0 |
| Arthralgia | 44 (11%) | 6 (2%) | 0 | 0 | 58 (15%) | 7 (2%) | 0 | 0 |
| Headache | 39 (10%) | 2 (1%) | 0 | 0 | 32 (8%) | 2 (1%) | 0 | 0 |
| Dehydration | 38 (10%) | 21 (5%) | 0 | 0 | 24 (6%) | 8 (2%) | 1 (<1%) | 1 (<1%) |
| Pain | 37 (9%) | 8 (2%) | 2 (1%) | 0 | 49 (12%) | 15 (4%) | 2 (1%) | 0 |
| Cough | 36 (9%) | 2 (1%) | 0 | 0 | 27 (7%) | 0 | 0 | 0 |
| Abdominal pain | 36 (9%) | 6 (2%) | 0 | 0 | 26 (7%) | 7 (2%) | 0 | 0 |
| Increased aspartate aminotransferase | 36 (9%) | 12 (3%) | 2 (1%) | 0 | 21 (5%) | 6 (2%) | 1 (<1%) | 0 |
| Bone pain | 34 (9%) | 9 (2%) | 0 | 0 | 55 (14%) | 13 (3%) | 0 | 0 |
| Decreased haemoglobin | 34 (9%) | 10 (3%) | 3 (1%) | 0 | 23 (6%) | 8 (2%) | 1 (<1%) | 0 |
| Musculoskeletal pain | 32 (8%) | 1 (<1%) | 0 | 0 | 47 (12%) | 9 (2%) | 0 | 0 |
| Pain in legs or arms | 32 (8%) | 4 (1%) | 0 | 0 | 43 (11%) | 9 (2%) | 0 | 0 |
| Insomnia | 31 (8%) | 0 | 0 | 0 | 34 (9%) | 3 (1%) | 0 | 0 |
| Urinary tract infection | 31 (8%) | 12 (3%) | 1 (<1%) | 0 | 27 (7%) | 4 (1%) | 0 | 0 |
| Increased alanine aminotransferase | 29 (7%) | 9 (2%) | 0 | 0 | 9 (2%) | 3 (1%) | 0 | 0 |
| Colitis | 27 (7%) | 15 (4%) | 3 (1%) | 0 | 3 (1%) | 0 | 0 | 0 |
| Pneumonia | 24 (6%) | 11 (3%) | 1 (<1%) | 4 (1%) | 9 (2%) | 2 (1%) | 1 (<1%) | 0 |
| Dizziness | 23 (6%) | 1 (<1%) | 0 | 0 | 18 (5%) | 1 (<1%) | 0 | 0 |
| Hypokalaemia | 22 (6%) | 10 (3%) | 1 (<1%) | 0 | 10 (3%) | 4 (1%) | 0 | 0 |
| Hypertension | 20 (5%) | 1 (<1%) | 0 | 0 | 13 (3%) | 1 (<1%) | 0 | 0 |
| General deterioration of physical health | 19 (5%) | 12 (3%) | 2 (1%) | 3 (1%) | 15 (4%) | 7 (2%) | 1 (<1%) | 2 (1%) |
| Chest pain | 15 (4%) | 1 (<1%) | 0 | 0 | 18 (5%) | 2 (1%) | 0 | 0 |
| Immune-related adverse events | ||||||||
| Diarrhoea | 155 (39%) | 58 (15%) | 1 (<1%) | 0 | 55 (14%) | 3 (1%) | 0 | 0 |
| Pruritus | 80 (20%) | 1 (<1%) | 0 | 0 | 15 (4%) | 0 | 0 | 0 |
| Rash | 68 (17%) | 2 (1%) | 0 | 0 | 16 (4%) | 0 | 0 | 0 |
| Colitis | 27 (7%) | 15 (4%) | 3 (1%) | 0 | 3 (1%) | 0 | 0 | 0 |
| Increased aspartate aminotransferase | 22 (6%) | 8 (2%) | 1 (<1%) | 0 | 13 (3%) | 3 (1%) | 1 (<1%) | 0 |
| Increased alanine aminotransferase | 20 (5%) | 6 (2%) | 0 | 0 | 8 (2%) | 3 (1%) | 0 | 0 |
| Hypothyroidism | 9 (2%) | 2 (1%) | 0 | 0 | 1 (<1%) | 0 | 0 | 0 |
| Adrenal insufficiency | 6 (2%) | 2 (1%) | 0 | 0 | 2 (1%) | 1 (<1%) | 0 | 0 |
| Hepatitis | 3 (1%) | 1 (<1%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased lipase | 2 (1%) | 0 | 1 (<1%) | 0 | 2 (1%) | 2 (1%) | 0 | 0 |
| Hepatotoxicity | 2 (1%) | 2 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Motor dysfunction | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 | 0 | 0 | 0 |
Data are number of patients (%); patients could have had more than one event.
Only events that occurred at a frequency of 5% or higher (as rounded to the nearest 1%) in either group are shown.
Most immune-related adverse events, including severe immune-related adverse events, occurred during the period in which patients were given the initial four doses of study drug. After standard ipilimumab management algorithms were followed, 91 (89%) of 102 immune-related adverse events in the ipilimumab group and seven (70%) of ten in the placebo group resolved. In the ipilimumab group, the median time to onset of grade 3–4 immune-related adverse events ranged from 3·7 weeks (95% CI 2·6–6·4, for skin disorders) to 11·1 weeks (not estimable [NE], for neurological disorders); the median time to resolution ranged from 2·9 weeks (1·6–4·7, for gastrointestinal disorders) to 11·1 weeks (2·4–NE, for endocrine disorders; appendix p 16).
In addition to the deaths noted above, one ipilimumab-related death (from pneumonia) occurred more than 70 days after the last ipilimumab dose (during survival follow-up) and thus was not regarded as an on-study event. Two patients in the ipilimumab group (one within 70 days and the other more than 70 days after the last dose) and one in the placebo group (within 70 days of last dose) had serious adverse events related to the study drug with fatal outcomes (cholangitis and gastrointestinal perforation or peritonitis in the ipilimumab group, and multiorgan failure in the placebo group). The causes of death in the remaining patients were unknown (appendix p 17).
Five (1%) cases of gastrointestinal perforation were reported in the ipilimumab group, whereas none were reported in the placebo group. Of these five cases, two occurred on study and were classified as drug-related events, one of which had led to patient death, and the other resolved. One on-study gastrointestinal perforation was not drug-related and resolved. The two other gastrointestinal perforations were drug-related but occurred after the study treatment was discontinued (more than 70 days from the last dose), of which one resolved, and the other led to patient death. Among ipilimumab-treated patients, frequency and severity of gastrointestinal adverse events were numerically similar between those who received baseline pelvic radiotherapy and those who did not (appendix p 15).
Among treated patients, 266 (68%) deaths occurred in the ipilimumab group and 304 (77%) in the placebo group, with most caused by disease progression (204 [77%] and 243 [80%], respectively; appendix p 17). 81 (30%) of 266 deaths in the ipilimumab group and 62 (20%) of 304 deaths in the placebo group occurred within 70 days of the last dose of study drug (ie, on study). Of these deaths, 73 (90%) in the ipilimumab group and 52 (84%) in the placebo group occurred within the first 5 months of the study. Among patients who died or were censored on study within the first 5 months, fatal (grade 5) adverse events were reported in 56 (14%) of 393 patients in the ipilimumab group and 39 (10%) of 396 patients in the placebo group. Of the on-study deaths, three in the ipilimumab group were related to toxic effects of the study drug. These three deaths were caused by pulmonary embolism, perforated diverticulitis, and performance status decrease with heart failure and respiratory distress, respectively. Most of the other deaths were unrelated to study drug, and were attributed mainly to disease progression; other causes of death included cardiac disorders, respiratory disorders, and infections (appendix p 17). We noted an imbalance in baseline prognostic features in favour of the placebo group in the subset of patients who died or were censored within 5 months of random assignment (appendix p 18). Ipilimumab-treated patients who died or were censored within 5 months were not more likely to have severe immune-related adverse events than were those who lived longer than 5 months.
Discussion
The primary objective of this phase 3 study was to assess the ability of ipilimumab to extend overall survival compared with placebo in patients with metastatic castration-resistant prostate cancer who had progressed after docetaxel chemotherapy, due to the strong evidence of an endogenous immune response against prostate cancer cells (panel). The proportional hazards assumption was violated in the primary analysis, in which no difference in overall survival was noted between patients who received ipilimumab and those who received placebo after bone-directed radiotherapy. However, an exploratory piecewise hazard model suggested that the hazard ratio for overall survival decreased over time, with ipilimumab seeming to be associated with better survival than placebo at later timepoints.
Length of follow-up for the primary analysis (median of less than 1 year) precluded an assessment of whether a durable survival benefit exists, as has been shown for ipilimumab-treated patients with advanced melanoma.21–23 Such an effect for patients with metastatic castration-resistant prostate cancer will be assessed in continuing survival follow-up. Notably, median overall survival in both treatment groups was less than was predicted during the statistical design of the study. Although the reasons for this finding are unknown, we might have enrolled patients with worse than expected survival outcomes because of the presence of one or more adverse prognostic features for metastatic castration-resistant prostate cancer (eg, a baseline bone pain score of 4 or higher was reported in 48% of patients). Since administration of bone-directed radiotherapy was included in both study groups and was not controlled for in the study, its effect on overall survival or other efficacy outcomes is unclear (although because it was balanced between both groups it is unlikely to have had any differential effect).
Ipilimumab was associated with reductions in PSA concentration and an improvement in progression-free survival. The proportion of patients showing a PSA response in the ipilimumab group (13·1%) is consistent with previous findings. In a phase 1/2 study27 in which 50 patients received ipilimumab at 10 mg/kg with or without radiotherapy, the proportion of patients with a PSA response was higher among the chemotherapy-naive patients (six [26%] of 23 patients) than among the chemotherapy-pretreated patients (two [7%] of 27 patients). These results suggest that the PSA response induced by ipilimumab could be negatively affected by more advanced disease and previous chemotherapy. Whether the reductions in PSA concentrations in the present study were caused, at least partly, by the use of corticosteroids for the management of immune-related adverse events is unclear. Moreover, the results of the present study are unable to address potential associations between PSA reductions and incidence of immune-related adverse events, number of bone lesions irradiated, or other efficacy outcomes (progression-free and overall survival).
More patients in the placebo group had early disease progression (ie, before 3 months) than in the ipilimumab group. However, the clinical significance of these findings is unclear and will need further investigation. Although prolongation of progression-free survival in this population with advanced metastatic castration-resistant prostate cancer could be a reflection of immune-mediated responses against prostate cancer cells, additional studies will be necessary to assess whether the antitumour clinical activity seen in the present study is indeed immune related, tumour specific, and consistent with the putative mechanism of action of ipilimumab (ie, CTLA-4 blockade).
In the exploratory and post-hoc subgroup analyses, we noted an apparent overall survival benefit for ipilimumab in a subset of patients without visceral metastases, with non-raised or mildly raised alkaline phosphatase, and without anaemia. Thus our results suggest that ipilimumab treatment might be effective in patients with favourable prognostic features. We did not investigate the safety profile in patient subgroups, and further analyses will be necessary to determine whether safety profiles differ between patient subgroups. A phase 3 study currently in progress (CA184-095; ClinicalTrials.gov NCT01057810) is assessing the efficacy and safety of ipilimumab in chemotherapy-naive, asymptomatic or minimally symptomatic patients with metastatic castration-resistant prostate cancer (patients with visceral metastases are excluded). As such, although the CA184-095 trial was designed before the results of the present study were known, it will allow for testing of important hypotheses generated from our study.
In the treated study population, less than 2% of fatal adverse events in either treatment group were regarded as drug-related, but more on-study deaths occurred overall and in the first 5 months in the ipilimumab group than in the placebo group. Treatment-related adverse events were common and mainly consisted of immune-related adverse events, which were generally manageable with standard ipilimumab treatment algorithms. Although comparisons across studies are not statistically valid, the incidence and severity of immune-related adverse events was similar to those reported in previous studies of ipilimumab monotherapy at 10 mg/kg for melanoma,37,38 apart from a higher incidence of gastrointestinal immune-related adverse events attrib utable to diarrhoea and colitis, and a lower incidence of skin immune-related adverse events. In the present study, one death in each treatment group was attributed to an immune-related adverse event. The incidence of drug-related on-study gastrointestinal perforation was low, and most gastrointestinal immune-related adverse events responded to standard management, including cortico steroid treatment. The frequency of gastrointestinal perforations in this study was consistent with the use of ipilimumab for the treatment of advanced melanoma, for which the frequency of gastrointestinal perforation is roughly 1%.39 In view of the possibility that pretreatment radiotherapy could increase the incidence of colitis associated with ipilimumab treatment, we compared the frequency and severity of gastrointestinal adverse events in ipilimumab-treated patients who received pretreatment bone-directed radiotherapy to the pelvis and those who did not, but detected no meaningful differences between these groups. This finding suggests that pretreatment radiotherapy to the pelvis did not have a major effect on gastrointestinal adverse events associated with ipilimumab in this study.
In summary, this study did not meet its primary endpoint of improved overall survival in a population with advanced metastatic castration-resistant prostate cancer. However, we did identify some evidence of antitumour activity with ipilimumab treatment as assessed by prespecified secondary and exploratory endpoints, including reductions in PSA concentration and improved progression-free survival. Other exploratory endpoints, such as quality of life, will be reported elsewhere.
Supplementary Material
Panel: Research in context.
Systematic review
We did a systematic review of the scientific literature as part of the planning for this trial. We searched Medline and PubMed for reports published in English using the search terms “castration-resistant prostate cancer”; “metastatic prostate cancer”; “advanced prostate cancer”; “prostate cancer” AND “bone metastasis”; “prostate cancer” AND “docetaxel”; “prostate cancer” AND “testosterone”; “prostate cancer” AND “immunotherapy”; “prostate cancer” AND “hormonal therapy”; AND “prostate cancer” AND “immune response”. We focused on reports published in the 10-year period before the start of the trial. We reviewed all evidence for treatments currently being assessed in patients with post-docetaxel metastatic castration-resistant prostate cancer, with a particular emphasis on immunotherapy. We identified strong evidence for an endogenous immune response against prostate cancer cells, and identified an unmet clinical need for approaches that enhance antitumour immunity to prostate cancer.
Interpretation
Ipilimumab did not significantly improve overall survival in our study (the primary endpoint). However, we did identify some evidence of antitumour activity as assessed by prespecified secondary and exploratory endpoints, including reductions in prostate-specific antigen concentration and improved progression-free survival. Additionally, prespecified and post-hoc subgroup analyses suggested that ipilimumab might be more effective in patients with favourable prognostic features.
Acknowledgments
We thank the patients who volunteered to participate in this study and the staff who cared for them. We also thank Olaf Christensen and Jingli Song of Bristol-Myers Squibb (Princeton, NJ, USA) for their contributions to the scientific content of the reports and their critical review of statistical analyses, and Ward A Pedersen and Ami Modi of StemScientific (Lyndhurst, NJ, USA) for writing support and editorial assistance (funded by Bristol-Myers Squibb) in preparation of this report.
Footnotes
Contributors
EDK, TMB, and WRG contributed to the study design. EDK, SS, AMB, SH, TMB, PG, DL, and WRG contributed to data analysis. PG and DL oversaw the design, conduct, and analysis of the study on behalf of the funder. MBM did the statistical analyses. EDK, MM, SS, AMB, TEC, EK, LS, SH, TMB, PG, DL, and WRG contributed to data interpretation. All authors contributed to data collection and assembly. EDK and SS searched the scientific literature. EDK, SN, SS, NA, AMB, LS, TMB, MBM, PG, and WRG participated in the writing of the report, an initial draft of which was produced by a publication steering committee (academic authors) and a professional medical writer employed by the funder. EDK edited the report, with all authors reviewing and providing comments on draft versions. All authors reviewed and approved the final submitted draft of the report.
Declaration of interests
EDK has received technology royalties and licence payments from Bristol-Myers Squibb, and has donated payments related to participation in a Bristol-Myers Squibb publication steering committee to his institution. CGD has received consultant fees from Bristol-Myers Squibb, Celgene, Pfizer, Compugen, and Janssen. HIS has served as an uncompensated consultant for Bristol-Myers Squibb and his institution received non-financial support from Bristol-Myers Squibb during the study. He has also received consultant fees from Dendreon and Takeda Millennium; donated consultant fees from Ortho Biotech Oncology Research & Development to his institution; been an uncompensated consultant for Aragon, Janssen, Johnson & Johnson Pharmaceuticals & Development, Medivation, Pfizer, and Sanofi; and received research support (to his institution) from Aragon, Medivation, Janssen Research & Development, Janssen Services, and Veridex. KF has received consultant fees and lecture fees from Bristol-Myers Squibb. AB received grant support from Bristol-Myers Squibb during the study and has received consultant fees from Astellas and Janssen, as well as non-financial support from ISSECAM (unrelated to the present report). AJME has received consultant fees from Bristol-Myers Squibb, Astellas, Sanofi, and Janssen-Cilag. NH has received consultant fees from Sanofi , Janssen, Astellas, Takeda, Ipsen, and Pierre Fabre, and grant support from Astellas. HM has received payments for educational presentations from Bristol-Myers Squibb, Bayer, Novartis, Sanofi -Aventis, and Amgen. He has also served on boards for Amgen and Sanofi -Aventis and has received travel support from Amgen, Novartis, Bayer, Pfizer, and Roche. MM received patients’ fees for Azienda Ospedaliera Universitaria Senese (Siena, Italy) during the study. He has also received research grants for investigator-sponsored trials from Bristol-Myers Squibb, and consultant fees from Bristol-Myers Squibb, GlaxoSmithKline, and Roche for serving on boards. SS has received honoraria from Amgen, Sanofi -Aventis, Bayer, and Astellas; non-financial support from Sanofi -Aventis; and conference sponsorship (non-financial support) from Janssen. NA has received honoraria from Dendreon and research support to his institution from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, ImClone Systems, Medivation, Millennium, Novartis, and Pfizer. TEC has received fees from Bristol-Myers Squibb for consultancy, lectures, and serving on a board. LS received grants and non-financial support from Bristol-Myers Squibb during the study. She has also received grants and non-financial support from Sanofi -Aventis and Ipsen (unrelated to the present report). SH received consultant fees from Bristol-Myers Squibb during the study. TMB received grant support from Bristol-Myers Squibb during the study. MBM, PG, and DL are employees of Bristol-Myers Squibb. WRG received reimbursement for travel expenses from Bristol-Myers Squibb during the study, has received speaker fees from Bristol-Myers Squibb and Janssen-Cilag, and has received consultant fees for serving on boards for Bristol-Myers Squibb, Astellas, and Janssen-Cilag. The other authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwich A, Hugosson J, de Reijke T, et al. Prostate cancer: ESMO Consensus Conference guidelines 2012. Ann Oncol. 2013;24:1141–1162. doi: 10.1093/annonc/mds624. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, et al. , for the TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.Fizazi K, Scher HI, Molina A, et al. , for the COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, et al. , for the AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Parker C, Nilsson S, Heinrich D, et al. , for the ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 8.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 12.Diener KR, Woods AE, Manavis J, Brown MP, Hayball JD. Transforming growth factor-β-mediated signaling in T lymphocytes impacts on prostate-specific immunity and early prostate tumor progression. Lab Invest. 2009;89:142–151. doi: 10.1038/labinvest.2008.123. [DOI] [PubMed] [Google Scholar]
- 13.Degl’Innocenti E, Grioni M, Boni A, et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35:66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 14.Drake CG. Immunotherapy for prostate cancer: emerging treatment modality. Urol Clin North Am. 2010;37:121–129. doi: 10.1016/j.ucl.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantoff PW, Higano CS, Shore ND, et al. , for the IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 17.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 21.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebbé C, Weber JS, Maio M, et al. Long-term survival in patients with metastatic melanoma who received ipilimumab in four phase II trials. Proc Am Soc Clin Oncol. 2013;31(suppl 15) doi: 10.1093/annonc/mdt161. abstract 9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maio M, Bondarenko I, Robert C, et al. European Cancer Congress. Netherlands: Amsterdam; 2013. [Sept 27 to Oct 1, 2013]. Survival analysis with 5 years of follow-up in a phase III study of ipilimumab and dacarbazine in metastatic melanoma. (abstr) 3704. [Google Scholar]
- 24.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 27.Slovin S, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris TJ, Hipkiss EL, Borzillary S, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 30.Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191. doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 36.Fleming TR, Harrington DP. Counting processes and survival analysis. New York: John Wiley and Sons; 1991. [Google Scholar]
- 37.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 38.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 39.Yervoy (ipilimumab) package insert. [accessed Jan 14, 2014];Princeton: Bristol-Myers Squibb. 2013 http://packageinserts.bms.com/pi/pi_yervoy.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.