Abstract
Objective
Epidemiologic studies comparing the incidence and prevalence of systemic lupus erythematosus (SLE) and isolated cutaneous lupus erythematosus (CLE) are few. Olmsted County, Minnesota provides a unique setting for such a study owing to resources of the Rochester Epidemiology Project. We sought to describe and compare the incidence and prevalence of SLE and CLE from 1993 to 2005.
Methods
SLE cases were identified from review of medical records and fulfilled the 1982 ACR classification criteria. CLE cases included patients with classic discoid LE (CDLE), subacute cutaneous LE (SCLE), lupus panniculitis and bullous LE. Age-and sex-adjusted incidence and prevalence were standardized to 2000 US white population.
Results
The age- and sex-adjusted incidence of SLE (2.9 per 100,000; 95% CI 2.0, 3.7) was similar to that of CLE (4.2 per 100,000; 95% CI 3.1, 5.2, p= 0.10). However, incidence of CLE was three times higher than SLE in males (2.4 versus 0.8 per 100,000, p=0.009). The age- and sex-adjusted prevalence of CLE on January 1, 2006 was higher than that of SLE (70.4 versus 30.5 per 100,000; p<0.001). The prevalence of CLE and SLE in women were similar but the CLE prevalence was higher in men than in women (56.9 versus 1.6 per 100,000, p<0.001). The incidence of CLE rose steadily with age and peaked at 60-69 years.
Conclusion
The incidences of CLE and SLE are similar but CLE is more common than SLE in males and in older adults. These findings may reflect differences in genetic or environmental etiology of CLE.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous, multisystem disease with many clinical phenotypes, including drug induced lupus, neonatal lupus, secondary antiphospholipid antibody syndrome and isolated cutaneous lupus (CLE). The Gilliam classification divides CLE lesions into lupus specific, acute cutaneous LE, subacute cutaneous lupus (SCLE) and chronic variants like discoid lupus (DLE) and non-specific skin lesions e.g. urticarial vasculitis[1]. In general, the prognosis of CLE is considered more favorable than SLE but it may evolve into SLE in about 20% of individuals. Presence of arthritis, leucopenia and anti-dsDNA are considered risk factors for development of SLE in patients with CLE [2]. Skin involvement in SLE patients is also very common and is observed in up to 70% of patients [2].
There is controversy as to whether SLE and CLE represent different spectrum of the same disease or are distinct disease phenotypes. Epidemiologic studies on natural history of CLE and SLE may provide important clues on etiology of these conditions. The incidence and prevalence of SLE have been examined in numerous population-based studies. Annual incidence of SLE is about 1-10 per 100,000 and the prevalence is about 5.8 -130 per 100,000 from 1970s to 2000s [3, 4]. In contrast, epidemiologic studies of CLE are rare [5, 6]. There are no studies that directly compare the incidence of SLE and CLE in the same population. Olmsted County, Minnesota provides a unique setting to study the epidemiology of SLE and CLE owing to resources of the Rochester Epidemiology Project (REP). As described previously [7], the REP provides access to the linked medical records of Olmsted County residents for over 5 decades and consequently, provides an ideal setting to examine the epidemiology of SLE and CLE concurrently in the same underlying population. Within the Olmsted County population, we have previously reported that the incidence of SLE tripled from 1.51 to 5.56 per 100,000 over 4 decades from 1950 to 1992 [8, 9]. The incidence of CLE and subtypes in this population between 1965 and 2005 was 4.30 per 100,000 [6]. Although the reported incidence of CLE between 1965 and 1992 was similar to SLE, a direct comparison of the SLE and CLE cohorts was not performed. Therefore, we undertook this study to compare the epidemiology and characteristics of SLE and CLE between 1993 and 2005.
Patients and Methods
This is a population-based retrospective cohort study in Olmsted County, Minnesota, which had a population of 124,277 according to the 2000 census. The racial composition of Olmsted County in 2000 was 90.3% white, 2.7% blacks or African- Americans, 0.3 % American Indians, 4.3% Asian/Native Hawaiian/Pacific Islander and 2.4% Hispanic (US Census Data).
SLE case definition
Potential SLE cases (n=438) were identified using the ICD-9 codes, Hospital International Classification of Disease Adaptation (HICDA) and Berkson codes. Medical records were reviewed manually for determination of organ involvement and presence or absence of ACR criteria. In case of doubtful cases adjudication was made based on discussion between authors (SJ, DH and VC). All study forms were then reviewed by VC to determine if patients were classified as “definite” SLE (4 or more ACR criteria), “probable” (3 or less criteria) or “possible (2 or less criteria) [10]. Data was double-checked (by CC) to determine residency status and fulfillment of ACR criteria.
An incident SLE case was defined as an individual who had been a resident of Olmsted County, Minnesota for at least 1 year prior to diagnosis and who fulfilled four out of eleven 1982 ACR criteria for classification of SLE between January 1, 1993 and December 31, 2005. The incidence date was the date of fulfillment of the fourth ACR criterion. An individual, who was a resident of Olmsted County, Minnesota as of January 1, 2006 and who prior to that date, fulfilled the 1982 ACR criteria for SLE was defined as a prevalent SLE case. Patients with drug-induced lupus, CLE only and overlap connective tissue diseases were excluded from the SLE cohort.
The medical records of all 438 individuals were reviewed and 366 of these individuals were excluded for the following reasons: 17 denied authorization of their medical records for research per Minnesota statute, 104 CLE only, 2 drug induced lupus, 4 duplicate records, 130 did not have SLE, 33 less than 4 ACR criteria, 11 lupus nephritis only, 40 non-residents, and 25 other rheumatic illnesses (5 undifferentiated connective tissue disease, 6 Sjögren's syndrome, 2 scleroderma, 1 overlap connective tissue disease, 6 mixed connective tissue disease, 1 polymyositis, 2 rheumatoid arthritis, 1 antiphospholipid antibody syndrome, 1 psoriatic arthritis). Of the 959 individuals with nonspecific codes, we reviewed the medical records of a random subset of 120 individuals and no SLE cases were identified. Therefore, no further review was done. The final SLE cohort comprised 45 incident SLE cases between January 1, 1993 and December 31, 2005 and 72 prevalent SLE cases on January 1, 2006.
CLE case definition
Individuals with incident CLE (without systemic features) were identified from a previous cohort from January 1, 1965 and December 31, 2005 [6]. Potential CLE cases were searched using ICD-9 code 695.4 (lupus erythematosus) and diagnosis of all forms of CLE was determined by review of clinical, serologic, histopathologic, and immunopathologic findings (MD, OS). The subtypes of CLE included classic discoid LE (CDLE), lupus panniculitis, bullous LE, and subacute cutaneous LE (SCLE). An SCLE lesion was described, using the definition by Sontheimer et al, as a photosensitive, nonscarring, non– atrophy producing, annular, erythematous, papulosquamous, or psoriasiform rash [11]. Patients were sub-classified as having annular or psoriasiform SCLE. Classic discoid LE was defined, by the classification criteria of Gilliam and Sontheimer, as the presence of discoid lesions without SCLE or SLE [1]. Localized discoid lupus was defined as lesions confined to the head and neck and as the generalized form if it occurred both above and below the neck. Lupus panniculitis was defined as circumscribed subcutaneous nodules. Date of diagnosis was determined as the date of fulfillment of criteria for subtype definition.
An incident CLE case was defined as an Olmsted County resident first diagnosed with a subtype of CLE by a dermatologist and who did not fulfill the 1982 ACR criteria for SLE at the time of diagnosis. A prevalent CLE case was defined as an Olmsted County resident on January 1, 2006 and who, prior to that date, had a diagnosis of CLE made by a dermatologist, without co-existent SLE. CLE cases who were previously incident in 1965-2005 were included in the prevalent cohort; whereas patients who moved into Olmsted County with a prior diagnosis of CLE were not included per previous study criteria [6]. The rationale for these stringent criteria was to reduce the diagnostic uncertainty as for many patients the clinical morphology of skin lesions had changed and histology was unavailable. Data on demographic characteristics, extent of skin involvement, treatment and serological findings were collected.
Statistical analysis
Age and sex specific incidence were estimated assuming the entire population of Olmsted County from 1993-2005 to be at risk. The age- and sex-specific denominators for each year were estimated from decennial census data. Incidence per 100,000 population were age and sex adjusted to the US White 2000 population. Comparisons of incidence and prevalence between cohorts were performed using Poisson regression models. Prevalence was calculated by dividing the number of prevalent cases by the number of persons living in Olmsted County in 2006. As the prevalent CLE cohort includes only incident cases from 1965 to 2005, our CLE prevalence estimate is an underestimate of the true prevalence of CLE. To compare the prevalence of SLE and CLE, we removed prevalent SLE cases which were not incident SLE to match the prevalence definition used in the CLE cohort.
The distribution of survival times after the SLE/CLE incidence date was estimated using the Kaplan–Meier method. The expected number of deaths was determined from the National Center for Health Statistics life tables for the US population, according to the age, sex and calendar year of the SLE/CLE cohorts. The one-sample log rank test was used to test for differences between observed and expected survival rates. The standardized mortality ratio was estimated by dividing the observed number of deaths by the expected number of deaths. Ninety-five percent confidence intervals for the standardized mortality ratios were calculated assuming that the expected rates were fixed and the observed rates followed a Poisson distribution. Cox proportional hazards models were used to compare survival between cohorts adjusting for age and sex. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Results
The study population comprised of 45 incident and 72 prevalent cases of SLE, and 62 incident and 92 prevalent cases of CLE.
Incidence and prevalence of SLE and CLE
The age- and sex-adjusted incidence of SLE (2.9 per 100.000, 95% CI 2.0-3.7) was similar to that of CLE (4.2 per 100,000, 95% CI 3.1 -5.2, p=0.10) (table 1). Yet, CLE was three times more common than SLE in men (2.4 versus 0.8 per 100,000, p=0.009). Incidence of SLE and CLE was similar in women (5.8 versus 5.1 per 100,000). The age-and sex-adjusted prevalence of SLE was 53.5 per 100,000. The prevalence of SLE was nine times higher in women than in men (94.2 versus 10.7 per 100,000). We recalculated the prevalence of SLE by including only the 41 SLE prevalent cases that were also incident to ensure comparability. With the adjusted calculations, the prevalence of CLE per 100,000 on January 1, 2006 was higher than SLE at 70.4 versus 30.5 respectively (p<0.001). The prevalence of CLE in women was higher than the prevalence of SLE (85.1 versus 58.4 per 100,000) but this did not reach statistical significance (p=0.06). Men had a significantly higher prevalence of CLE than SLE (56.9 versus 1.6 per 100,000, p<0.001).
Table 1. Incidence and prevalence of systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE) in Olmsted County between in 1993-2005 and prevalence of SLE and CLE in Olmstead County on January 1, 2006.
| Incidence per 100,000* (95% CI) N=no. of cases | Prevalence per 100,000 (95% CI) N=no. of cases | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SLE | CLE | P value | SLE | Adjusted SLE Prevalence ** | CLE | P value+ | |
| Female | 5.1 (3.5, 6.6) N = 41 |
5.8 (4.1, 7.5) N= 45 |
0.67 | 94.2 (71.2,117.1) N = 65 |
58.4 (40.2, 76.5) N=40 |
85.1 (63.3, 106.8) N=59 |
0.06 |
| Male | 0.8 (0.0, 1.6) N= 4 |
2.4 (1.2, 3.6) N= 17 |
0.009 | 10.7 (2.8, 18.7) N=7 |
1.6 (0.0, 4.7) N=1 |
56.9 (37.1, 76.7) N=34 |
<0.001 |
| Total | 2.9 (2.0, 3.7) N= 45 |
4.2 (3.1, 5.2) N=62 |
0.10 | 53.5 (41.1, 65.9) N=72 |
30.5 (21.1, 39.9) N=41 |
70.4 (55.9, 84.8) N=92 |
0.001 |
incidence rates were derived for the time period 1993 and 2005 and age and sex adjusted to the United States White 2000 population
Prevalence of SLE was recalculated based on the 41 prevalent SLE cases who were also incident SLE, to match the definition used in the previous CLE study to ensure comparability {Durosaro, 2009 #106}. CI = confidence interval.
Comparison between adjusted SLE prevalence and CLE prevalence.
Figure 1 shows the age- and sex-specific incidence of SLE and CLE. The incidence of SLE was highest at in persons 20-29 years and 50-59 years of age (4.1 per 100,000 in both age groups). The incidence of CLE rose steadily across each decade of life and peaked at 60-69 years (9.3 per 100,000). The female to male ratio was 10:1 for SLE and 3:1 for CLE. Among females, we observed a nearly constant SLE incidence rate for ages 20-59 years. At 20-29 years, SLE was 3 times more common than CLE (p=0.10). Conversely, CLE was 3 times more common than SLE at 60-69 years but this was not statistically significant (p=0.12). The disease onset was after 59 years in all male SLE cases whereas the incidence of CLE in males (and females) increased with increasing age.
Figure 1. Age- and sex-specific incidence of SLE and CLE.
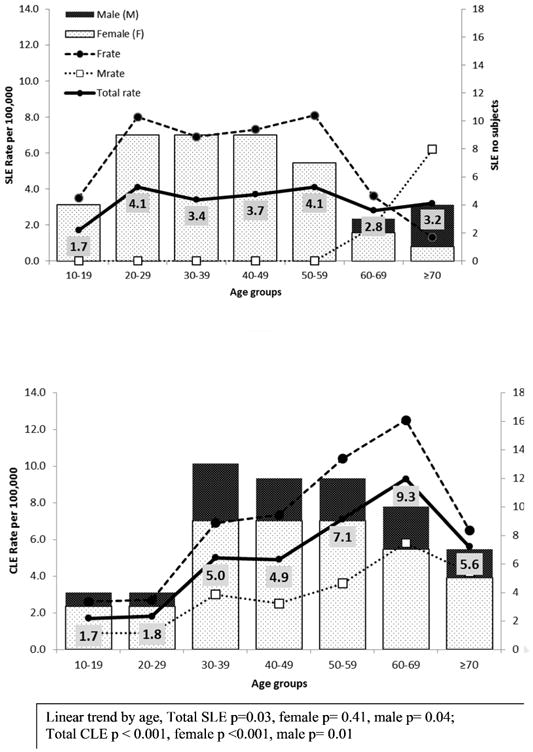
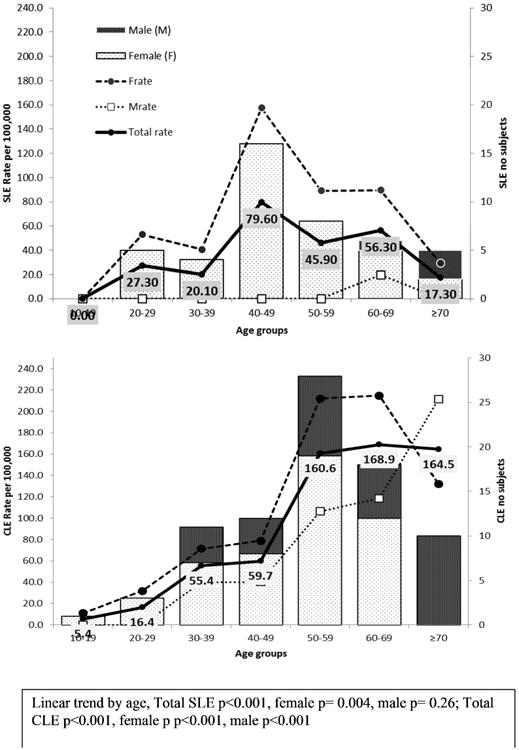
Figure 2 shows age- and sex-specific prevalence of SLE and CLE. The prevalence of CLE increased with increasing age (p<0.001). The prevalence of SLE was highest at 40-49 years (79.6 per 100,000) and lowest at ≥70 years (17.3 per 100,000). The prevalence of CLE was significantly higher than SLE at 50-59 years (160.6 versus 45.9 per 100,000, p=0.002), 60-69 years (168.9 versus 56.3 per 100,000, p=0.02) and ≥ 70 years of age (164.5 versus 17.3 per 100,000, p<0.001). The highest SLE prevalence among females was observed at 40-49 years (157.5 per 100,000) whereas the highest for CLE was at 60-69 years (214.7 per 100,000). In males, prevalence of CLE increased by age (p<0.001) with the highest prevalence at ≥70 years (211.3 per 100,000).
Figure 2. Age- and sex-specific prevalence of SLE and CLE.
Clinical Features
The demographics and clinical features of incident SLE and CLE patients are summarized in table 2. Mean age of patients with incident SLE and CLE was similar (42 and 47.6 years, respectively, p=0.10). Majority of patients with SLE were female (91% versus 73%, p=0.017). The female to male ratio was 10:1 for SLE and 3:1 for CLE. The majority of SLE cases were white (80 %) and a small number were of Asian and African American descent. Of the CLE patients, 71% were white, 6% African-American and 3% were Asian.
Table 2. Clinical characteristics of incident patients with systemic lupus erythematosus (SLE n=45) and Cutaneous Lupus Erythematosus (CLE, n=62).
| SLE Number (Percentage, %) |
CLE Number (Percentage, %) |
||
|---|---|---|---|
| Age, years (mean ± SD) | 42.0 ± 17.9 | 47.6± 16.9 | |
| Sex, female | 41 (91) | 45 (73) | |
| Follow up, years (mean ± SD) | 7.8 ± 4.9 | 10.0 ± 6.0 | |
| Race | |||
| White | 36 (80) | 44 (71) | |
| Asian | 3 (7) | 2 (3) | |
| African American | 2 (4) | 4 (6) | |
| Other | 1 (2) | 4 (6) | |
| Unknown | 3 (7) | 8 (13) | |
| ACR Criteria | Subtypes of CLE | ||
| Malar rash | 14 (31) | Discoid Lupus | |
| Discoid rash | 3 (7) | Localized | 34 (55) |
| Photosensitivity | 23 (51) | Generalized | 12 (19) |
| Oral ulcers | 12 (27) | SCLE | |
| Arthritis | 32 (71) | - Annular/Polycyclic | 5 (8) |
| Serositis | 12 (27) | - Psoriasiform | 8 (13) |
| Renal (Cellular casts and or | 12 (27) | ||
| proteinuria) | 24 (53) | Lupus Panniculitis | 2 (3) |
| Hematologic disorder* | 5 (11) | Bullous LE | 1 (2) |
| Hemolytic anemia | 15 (33) | ||
| Leukopenia | 9 (20) | ||
| Lymphopenia | 11(24) | ||
| Thrombocytopenia | 3 (7) | ||
| Psychosis/seizure | 33(73) | ||
| Immunologic Disorder | |||
| Positive ANA | 45 (100) | 32 (52) | |
| Positive dsDNA | 29 (64) | 1 (2) | |
| anti- Smith (Sm) | 4 (9) | Not available | |
| Anticardiolipin (aCL) | 10 (22) | Not available |
Total number exceeds patient number as many patients had simultaneous prevalence of various cytopenias
The average length of follow up of SLE patients was 7.8 ± 4.9 years. Arthritis (71%) and hematologic manifestations (53%) were the most common clinical manifestations followed by photosensitivity (51%). Renal involvement (cellular casts and/or proteinuria) was seen in 27%. All patients had positive ANA antibodies, and anti-dsDNA antibodies were seen in 64%. Of the 62 CLE patients, 46 had discoid lupus, 13 had sub-acute cutaneous lupus, 2 had lupus panniculitis and one patient had bullous LE. Localized discoid lesions were seen in 55% and generalized in 19%, 8% had annular SCLE and 13% had psoriasiform. ANA was positive in 52% patients. The average length of follow up from date of diagnosis was 10.0 ± 6.0 years.
Patient survival
Six out of 45 SLE patients died during follow-up (table 3). Cause of death was renal failure in 2 patients and infections in 4 patients (septic arthritis, pseudomonas bacteremia, Pneumocystis jirovecii pneumonia and endocarditis). All of the deceased SLE patients were ≥ 50 years of age. Average disease duration at the time of death was 6.2 years (range 0.5- 14.8 years). The 5, 10 and 15-year survival rates (± standard error; %) were 93.0 ±3.9, 89.2 ±5.2 and 63.5 ± 16.5 respectively. Overall standardized mortality ratio (SMR) was 2.6 (95% CI 1.0-5.6, table 3). The 5, 10 and 15 year survival rates (± standard error; %) of CLE patients were 96.6 ± 2.4, 92.3 ± 3.7 and 92.3 ± 3.7 respectively with a corresponding SMR of 0.6 (95% CI 0.2, 1.4).
Table 3. Standardized mortality ratios (SMR) among incident patients with systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE).
| SLE | CLE | |||
|---|---|---|---|---|
|
| ||||
| Observed no. deaths/Total number | SMR (95% CI) | Observed no. deaths/Total number | SMR (95% CI) | |
| Overall | 6/45 | 2.6 (1.0, 5.6) | 5/62 | 0.6 (0.2-1.4) |
| Age ≥ 50 years | 6/14 | 3.3 (1.2, 7.1) | 4/29 | 0.6 (0.2, 1.4) |
| Age < 50 years | 0/31 | -- | 1/33 | 0.9 (0.02, 4.8) |
Discussion
We compared the incidence and prevalence of CLE and SLE in a predominantly white US population. Our findings indicate that, although overall incidence of CLE and SLE were similar, CLE was three times more common than SLE in males. CLE was also more prevalent than SLE, especially in males. Age-and sex-specific incidence of SLE in females showed a nearly constant incidence rate for ages 20-59 years whereas the onset of SLE in males began after 59 years. The highest prevalence of SLE was in the 40-49 age groups. The incidence and prevalence of CLE were distinct from SLE with a steady rise in incidence and prevalence across each decade of life in both females and males.
Although it is difficult to compare the epidemiology of SLE across studies due to methodological differences (i.e., case definition, case ascertainment, geographic differences, population versus hospital based, rural versus urban), our incidence and prevalence estimates are identical to several studies from Norway and Iceland and quite similar to many US and Scandinavian studies (table 4). Incidence in these previous studies ranged from 2.4 to 3.3 per 100,000 [12-16]. Studies from Spain, Sweden, UK and US using administrative claims databases report a higher incidence at 3.6 - 7.2 per 100000 whereas incidence is much lower in Denmark (1.04 per 100,000)[17-27]. Studies from Marshfield clinic, a predominantly rural area from Midwest, very similar to ours, have reported a higher incidence of lupus of 5.1 per 100 000 from 1991-2001 and 5.03 cases per 100,000 from 2002-2008 [25, 28]. The exact reason for this discrepancy is unknown but could possibly be due to differences in case definition. We used a stricter definition of SLE including them only if they were residents of Olmsted County for at least one year prior to diagnosis. The first study included patients with overlap disease and it is unclear if other cutaneous LE cases except discoid were excluded or not. However, similar to our study, the disease was more common in older adults.
Table 4. Incidence and prevalence of systemic lupus erythematosus (SLE) in various populations.
| Study Location(ref) | Study period | Incidence per 100000 Overall female (F) male (M) | Prevalence per 100000 Overall female (F) male (M) | MeanAge(years) | Comments | |
|---|---|---|---|---|---|---|
| Present | 1993-2005 | 2.9 F 5.1 M 0.8 |
52.9 F 94.7 M 9.2 |
42 | ||
| Oslo, Norway (12) | 1999-2008 | 3.0 | 51.8 F 91 M 10.7 |
Bimodal pattern 16-29 and 50-59 years of age. Incidence 4 per 100 000 in Asian and Turkish immigrants compared to 2.6 in Europeans. | ||
| Arctic region of Norway (13) | 1978-1995 | 2.9 F 5.1 M 0.7 |
49.7 F 89.3 M 9.7 |
Peak incidence in women 30-49. Increased Mortality in those > 50 years of age. | ||
| Northern Norway (14) | 1978-1995 & | 2.6 F 5.1 M 0.9 & |
64.1 F 108.6 M 20.0 |
39.4 | 1982 ACR criteria used for 1978-1995 cohort and 1997 ACR criteria for 1996-2006. Use of 1997 criteria did not change incidence. | |
| 1996-2006 | 3.0 F 4.6 M 0.8 |
41.7 | ||||
| Iceland (15) | 1975-1984 | 3.3 F 5.9 M 0.8 |
36 F 62 M 7 |
46.6 | Low incidence of kidney with nephritis in 20%. 5 year survival 84% and 10 year 78%, higher rates seen in older population. |
|
| Allegheny county, Pennsylvania (16) | 1985-1990 | Whites 2.4 F 3.5 M 0.4 |
- | 39.8 whites 35.2 AA | Incidence was 2.5 times higher in AA females compared to white. | |
| African-American (AA) 5.3 F 9.2 M 0.7 |
F: M ratio 9 times higher in whites and 13 times higher in AA. | |||||
| Northwestern Spain (17) | 1987-2006 | 3.6 F 5.9 M 1.1 |
17.5 F 29.2 M 5.8 |
46.1 | Arthritis most commonmanifestation, renal in 27.3%. In age groups 60 years and older the incidence was similar in men and women. |
|
| Southern Sweden (18) | 1981-86 | 4 F 5.4 M 1 |
- | Prospective study; highest incidence in 55-64 and 65-74 age groups. | ||
| Southern Sweden, (19) | 1981-1986 & 1987-1991 |
4.5 4.8 |
42 68 |
49 47 |
Highest incidence seen in ages 65-74 and no difference in sex ratio in older individuals. Arthritis was seen in 71 %, kidney had decreased from previous cohort of 41 to 19%. | |
| UK General practice research database (GPRD) (20) | 1990-1999 | 4.71 Female 7.8 Male 1.53 |
47.3 F 46.3 M 52.2 |
Peak incidence at 50-54 for females and 70-74 in males. Patients with SCLE were retained. Data on race, ethnicity and birth location is not available. ACR criteria were not applied. | ||
| GPRD (1992-1998) (21) | 1992-1998 | - | F 70.8 M 10.1 |
- | ||
| Nottingham, (22) | 1989-1990 | 4.0 F 6.5 M 1.5 |
24 F 45.4 M 3.7 |
47 F 55.5M |
More in Afro-Caribbeans. Peak age of diagnosis was 50-59 years. |
|
| Birmingham, UK (23) | 1991 | 3.8 F 6.8 M 0.5 |
27.7 | F 37 | Prevalence was 197.2 in Afro-Caribbean females and 96.5 in Asians (Caucasians 36.3). Afro-Caribbean 25.8 incidence and Asians 20.7. |
|
| US managed care plan, Administrative database (24) | 2003-2008 | 7.22 F 11.89 M 2.21 |
81.07 -102.94 | Based on insurance claim codes, chart review not done. Incidence higher in those over 65. compared to 45-64 or 18-44. Consists of more geographically diverse population, data on age, ethnic group not available. | ||
| Rural Wisconsin (25) | 1991-2001 | 5.1 F 8.2 M 1.9 |
78.5 F 131.5 M 24.8 |
51.7 | Hematologic, arthritis and renal common, older age at presentation, including 3 overlap cases. All deaths occurred in older individuals. |
|
| Funen county, Denmark Voss et al (26) | 1980-1994 | 3.6 | 21.7 F 37.9 M 4.7 |
39.2 | Most new cases diagnosed in persons 40-49 years of age; 98% white Europeans | |
| Funen county Denmark (27) | 1995-2003 | 1.04 | 28.3 | Prevalence increased slightly compared to previous cohort. | ||
| Georgia lupus registry (29) | 2000-2004 | ACR definition 5.6 F 9.2 M 1.8 Whites 2.7 F 4.7 M 0.7 Combined case definition* 6.9 F 11.7 M 1.9 whites 3.3 F 5.8 M 0.8 |
ACR definition 73.0 F 127.6 M 14.7 Whites 32.7 F 59.0 M 7.5 Combined case definition*92.1 F 159.8 M 19.6 Whites 43.1 F 77.7 M 9.6 |
40.5 | Blacks younger at diagnosis, rates 3 times higher in black women compared to white females. Prevalence rates nine times higher than men and in blacks compared to whites. Arthritis (62.5%), hematologic (80%), and serologic disorders were most common. Renal disorder and serositis commoner in blacks. | |
| MILES Michigan (30) | 2000-2004 | ACR definition 5.5 F 9.3 M 1.5 Whites 3.7 F 6.3 M 1.2 rheumatologist definition 4.7 F 7.9 M 1.4 Whites 2.7 F 4.5 M 0.9 |
ACR definition 72.8 F 128 M 12.8 Whites 47.5 F 86.7 M 8.7 rheumatologist definition 64.6 F 113.4 M 12.5 Whites 39.8 F 72.1 M 7.8 |
39.3 Whites 43.9 | Age specific incidence rates in black females rose from early childhood and peaked in twenties while rates for white females increased gradually and plateaued from thirties to fifties. Blacks had higher proportion of renal involvement and ESRD, discoid rash, and neurologic disorder more common in blacks whereas malar rash, photosensitivity, oral ulcers are more common in whites. | |
| Curaco island (31) | 1980-1989 | 4.6 F 7.86 M 1.13 |
47 | 34.6 | Milder cases may have been missed. In women aged 15-44 years the annual incidence (12/100 000) was highest, whereas in women aged 44-65 years the 1990 point prevalence rate (1 in 526; CI 469 to 625) was highest. | |
| Barbados lupus registry (32) | 2000-2009 | 6.59 F 12.21 M 0.84 |
84.1 F 152.6 M 10.1 |
Most cases in younger years, between 20-39 years of age. | ||
| African-Caribbean population Vilar, Natal City, Brazil (33) | January 1, 2000 – December 31 2000 | 8.7 F 14.1 M 2.2 |
- | 31.8 | Peak incidence was observed in females between 35 -39 years (32.7/100 000). The incidence rate was high for females in all age groups, except for the 55 – 59 years-old. | |
| Taiwan (34) | 2000-2007 | 8.1 | 67.4, F 122.7 M 67.4 |
National Health Insurance research database. Highest incidence in 20-54 years age group in women | ||
| Hong Kong (35) | 2000-2006 | 3.1 F 5.4 |
- | 32.3 | ||
| Cutaneous lupus | ||||||
| Study Location (ref) | Study period | Overall Incidence per 100000 female (F) male (M) | Overall Prevalence per 100000 female (F) male (M) | Age (years) | Comments | |
| Present study | 1993-2005 | 4.2 F 5.8 M 2.4 |
71.7 F 85.1 M 58.4 |
48 | ||
| Swedish National register (6) | 2005-2007 | 4.0 | 54 F 54 M 53 |
3:1 female to male ratio. 24% already had a diagnosis of SLE at the time of CLE diagnosis. For SCLE patients, increase from 45 years for women and 65 years for men. | ||
| Stockholm county Ro/SSA positive SCLE only (41) | 1996-2002 | 0.7 | 6.2-14 | Majority were women 51-60 years of age. | ||
| French Guiana (42) | 1995-1999 | 2.6 F 4.72 M 0.5 |
- | 32 | Only chronic cutaneous lupus cases were included. Underestimate of cases likely. | |
The combined case definition consists of the following 3 criteria: ≥4 American College of Rheumatology (ACR) criteria; 3 ACR criteria plus a final diagnosis of SLE made by a rheumatologist; or <4 ACR criteria and either biopsy findings consistent with World Health Organization class II–VI lupus nephritis or end-stage renal disease secondary to SLE that required dialysis/renal transplantation
Recently, two large epidemiologic studies have been performed in Georgia (GLR - Georgia Lupus Registry) and Michigan (MILES -The Michigan Lupus Epidemiology and Surveillance Program) using novel methodologies for case ascertainment from multiple sites[29, 30]. The overall incidence and prevalence estimates per 100,000 in both studies were similar at 5.6 (GLR), 5.5 (MILES) and 73 (GLR) and 72.8 (MILES) respectively and notably higher than ours. The racial composition of these populations is different from ours with 38% blacks in Michigan and 49% blacks in Georgia. Not surprisingly, the rates in white individuals were similar to ours (table 4). The overall age-adjusted incidence of SLE in whites was 2.7 -3.7 per 100,000 in MILES and 2.7-3.3 per 100,000 in GLR cohorts. The prevalence per 100,000 of SLE in whites was also quite similar to ours in both studies with 32.7- 43.1 in GLR and 39.8- 47.5 in MILES. These comparisons corroborate the validity of our estimates from Olmsted County.
The expression of SLE is influenced by race with much higher incidence (4.6-8.7 per 100,000) among Afro-Caribbean's, Asians, African Americans and Native Americans [16, 31-39]. In these racial groups, disease onset is typically earlier, and patients have a greater degree of renal involvement and higher mortality compared to whites.
Epidemiology of CLE
Although CLE is considered to be 2-3 times more common than SLE, our data show that the overall incidence of SLE and CLE is similar [2]. There are few studies on the epidemiology of CLE. Estimates from a population based Swedish cohort [5] were similar to ours with an incidence of 4.0 per 100,000 and female to male ratio of 3:1. The mean age at diagnosis was 54 years but patients with SCLE were older at 59 years. The age and gender specific incidence of SCLE showed an increase in age from 45 years for women and from 65 years for men. One postulated explanation for the higher rates was drug-induced SCLE, as many middle aged patients are prescribed anti-hypertensive medications, several of which are associated with SCLE. Like ours, DLE was the most common group. In another Swedish study, the incidence of CLE and SLE was reported to be similar but a direct comparison was not performed [5, 40]. Using a registry of Ro/SSA positive individuals, the incidence of SCLE was 0.7 per 100,000 in Stockholm from 1996-2002 and prevalence of 6.2-14 per 100000 [41]. Interestingly in this study 29% of patients were smokers compared to 18% of adult population supporting current belief that smoking plays a role in CLE pathogenesis. The incidence of chronic CLE in French Guiana was estimated at 2.59 per 100,000 and was similar to the incidence of chronic CLE and lupus panniculitis in our published cohort from 1965- 2005 [6, 42]. Unlike our study, the population was predominantly young (75% age < 40 years) and African. CLE may be underestimated in this group either due to lack of diagnosis or reluctance to biopsy due to increased risk of keloid formation. Otherwise, due to insufficient data in literature, it is hard to compare incidence of CLE across different races.
Older Age at Presentation
We found that in white individuals, the incidence of SLE in males and CLE overall, increases with age. This may be secondary to aging of population, better access to health care and increased serologic monitoring or reflect unique pathophysiology of disease in this racial group. Immunosenescence, cumulative effects of photodamage, reactive oxygen intermediates in aging skin may increase the incidence of autoimmune diseases [43, 44]. Increased exposure of aging population to numerous drugs, many of which can flare lupus, as postulated in a Swedish study may be another explanation [5]. The increasing incidence of SLE in males with increasing age suggests a possible protective role of androgens in disease pathogenesis. Hypoandrogenicity has been described in many chronic diseases including SLE and androgens are postulated to be anti-inflammatory [45].
Mortality
Overall standardized mortality ratio of SLE was 2.6 (95% CI 1.0, 5.6). Majority of deaths were seen in patients older than 50 years and were infectious in etiology. Late onset lupus in spite of lower rates of kidney involvement may be associated with higher mortality due to higher prevalence of co-morbidities, greater disease activity and damage [46]. Presence of cutaneous lupus had no effect on mortality and the overall SMR of CLE was 0.6 (95% CI, 0.2-1.4).
Strengths and Limitations
Limitations of our study include small number of patients; the prevalence rate for SLE was “adjusted” to compare with CLE cases. However studies are needed with larger cohorts to confirm our findings. The study population comprised of predominantly white individuals, with increasing racial diversity in Olmsted County from immigration, racial composition in 2010 was 86 % white, 5 % AA, 0.2% American Indian, Asian 6%, and Hispanic 4 % future studies will be crucial to examine the trends (US Census Data). This was a retrospective study relying on diagnosis and clinical signs and symptoms in medical records. The case ascertainment depends on the diagnosis being made by the physician and the exact burden of undiagnosed disease remains unknown. Milder cutaneous lupus rashes may not be recognized and diagnosed correctly and thus the number presented here may be an underestimate. There are many clinical mimics of cutaneous lupus erythematosus and in absence of histology the diagnosis of CLE may not be certain. The prevalence of CLE in our study is likely an underestimate due to study criteria as only cases that were incident were included in the prevalent cohort. Due to increased recognition and usage of serologic surveillance, we do not believe there is an under ascertainment of SLE cases. The ACR criteria are biased towards picking up milder and cutaneous cases (4 cutaneous criteria whereas other organ systems have 1). Patients with SCLE may fulfill ACR criteria with limited mucocutaneous disease. There is also a need for an improved case definition as proposed in the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria that includes cases with lupus nephritis alone (or other major organ manifestations) but fulfilling less than 3 ACR criteria [47]. We excluded patients with isolated lupus nephritis in this cohort but the overall age- and sex- adjusted incidence and prevalence of SLE+ isolated lupus nephritis were not too different from SLE alone at 3.1 (95% CI: 2.2 – 4.0) and 57.5 (95% CI: 44.6 – 70.3) respectively.
The strength of our study includes a long ascertainment and follow-up period. Studies with observation periods of less than 5 years may overestimate incidence by as much as 238% and underestimate prevalence by 66% [48, 49]. Similarly, misclassification is a concern in administrative database studies; in one study the specificity of administrative database for identifying cases of SLE was only 72.5% [50].
Ours is the first study that has directly compared the incidence and prevalence of CLE with SLE in the same population and geographic area thereby assessing the role of similar genetic factors and possibly similar environmental agents in the pathophysiology of the conditions.
In conclusion, the incidence of SLE and CLE is similar in a predominantly white population but CLE is three times more common in males. Not surprisingly, SLE and CLE are seen in older age groups and more common in females. The prevalence of CLE is higher than SLE, especially in males.
Significance and Innovation.
There are no studies that directly compare the incidence and prevalence of systemic and cutaneous lupus
The overall incidence of systemic and cutaneous lupus are similar but cutaneous lupus is three times more common in men
The prevalence of cutaneous lupus, especially in men, is higher than systemic lupus
The incidence of cutaneous lupus increases steadily with age
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. Funding for this study was also provided by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. VRC is supported by National Institute of Arthritis and Musculoskeletal Diseases - K23 AR057815-01A1 and John M Nasseff Sr. Award in Rheumatology honoring Dr. Harvinder Luthra Research Award, Mayo Foundation.
Footnotes
Disclosures: There are no relevant financial disclosures or conflict of interests.
References
- 1.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4(4):471–475. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 2.Tebbe B, Orfanos CE. Epidemiology and socioeconomic impact of skin disease in lupus erythematosus. Lupus. 1997;6(2):96–104. doi: 10.1177/096120339700600204. [DOI] [PubMed] [Google Scholar]
- 3.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2002;16(5):847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 4.Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–1341. doi: 10.1111/j.1365-2133.2011.10272.x. [DOI] [PubMed] [Google Scholar]
- 6.Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145(3):249–253. doi: 10.1001/archdermatol.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michet CJ, Jr, McKenna CH, Elveback LR, Kaslow RA, Kurland LT. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota, 1950 through 1979. Mayo Clinic proceedings. 1985;60(2):105–113. doi: 10.1016/s0025-6196(12)60294-8. [DOI] [PubMed] [Google Scholar]
- 9.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950-1992. Arthritis Rheum. 1999;42(1):46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 11.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115(12):1409–1415. [PubMed] [Google Scholar]
- 12.Lerang K, Gilboe I, Garen T, Thelle DS, Gran JT. High incidence and prevalence of systemic lupus erythematosus in Norway. Lupus. 2012;21(12):1362–1369. doi: 10.1177/0961203312458168. [DOI] [PubMed] [Google Scholar]
- 13.Nossent HC. Systemic lupus erythematosus in the Arctic region of Norway. J Rheumatol. 2001;28(3):539–546. [PubMed] [Google Scholar]
- 14.Eilertsen GO, Becker-Merok A, Nossent JC. The influence of the 1997 updated classification criteria for systemic lupus erythematosus: epidemiology, disease presentation, and patient management. J Rheumatol. 2009;36(3):552–559. doi: 10.3899/jrheum.080574. [DOI] [PubMed] [Google Scholar]
- 15.Gudmundsson S, Steinsson K. Systemic lupus erythematosus in Iceland 1975 through 1984. A nationwide epidemiological study in an unselected population. J Rheumatol. 1990;17(9):1162–1167. [PubMed] [Google Scholar]
- 16.McCarty DJ, Manzi S, Medsger TA, Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38(9):1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 17.Alonso MD, Llorca J, Martinez-Vazquez F, Miranda-Filloy JA, Diaz de Teran T, Dierssen T, Vazquez-Rodriguez TR, Gomez-Acebo I, Blanco R, Gonzalez-Gay MA. Systemic lupus erythematosus in northwestern Spain: a 20-year epidemiologic study. Medicine (Baltimore) 2011;90(5):350–358. doi: 10.1097/MD.0b013e31822edf7f. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson H, Nived O, Sturfelt G, Silman A. Estimating the incidence of systemic lupus erythematosus in a defined population using multiple sources of retrieval. Br J Rheumatol. 1990;29(3):185–188. doi: 10.1093/rheumatology/29.3.185. [DOI] [PubMed] [Google Scholar]
- 19.Stahl-Hallengren C, Jonsen A, Nived O, Sturfelt G. Incidence studies of systemic lupus erythematosus in Southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J Rheumatol. 2000;27(3):685–691. [PubMed] [Google Scholar]
- 20.Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ. Incidence of systemic lupus erythematosus in the United Kingdom, 1990-1999. Arthritis Rheum. 2007;57(4):612–618. doi: 10.1002/art.22683. [DOI] [PubMed] [Google Scholar]
- 21.Nightingale AL, Farmer RD, de Vries CS. Systemic lupus erythematosus prevalence in the UK: methodological issues when using the General Practice Research Database to estimate frequency of chronic relapsing-remitting disease. Pharmacoepidemiol Drug Saf. 2007;16(2):144–151. doi: 10.1002/pds.1253. [DOI] [PubMed] [Google Scholar]
- 22.Hopkinson ND, Doherty M, Powell RJ. The prevalence and incidence of systemic lupus erythematosus in Nottingham, UK, 1989-1990. Br J Rheumatol. 1993;32(2):110–115. doi: 10.1093/rheumatology/32.2.110. [DOI] [PubMed] [Google Scholar]
- 23.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth Arthritis Rheum. 1995;38(4):551–558. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 24.Furst DE, Clarke AE, Fernandes AW, Bancroft T, Greth W, Iorga SR. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2013;22(1):99–105. doi: 10.1177/0961203312463110. [DOI] [PubMed] [Google Scholar]
- 25.Naleway AL, Davis ME, Greenlee RT, Wilson DA, McCarty DJ. Epidemiology of systemic lupus erythematosus in rural Wisconsin. Lupus. 2005;14(10):862–866. doi: 10.1191/0961203305lu2182xx. [DOI] [PubMed] [Google Scholar]
- 26.Voss A, Green A, Junker P. Systemic lupus erythematosus in Denmark: clinical and epidemiological characterization of a county-based cohort. Scand J Rheumatol. 1998;27(2):98–105. doi: 10.1080/030097498440958. [DOI] [PubMed] [Google Scholar]
- 27.Laustrup H, Voss A, Green A, Junker P. Occurrence of systemic lupus erythematosus in a Danish community: an 8-year prospective study. Scand J Rheumatol. 2009;38(2):128–132. doi: 10.1080/03009740802419073. [DOI] [PubMed] [Google Scholar]
- 28.Bartels CM, Buhr KA, Goldberg JW, Bell CL, Visekruna M, Nekkanti S, Greenlee RT. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol. 2014;41(4):680–687. doi: 10.3899/jrheum.130874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357–368. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66(2):369–378. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nossent JC. Systemic lupus erythematosus on the Caribbean island of Curacao: an epidemiological investigation. Ann Rheum Dis. 1992;51(11):1197–1201. doi: 10.1136/ard.51.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flower C, Hennis AJ, Hambleton IR, Nicholson GD, Liang MH. Systemic lupus erythematosus in an African Caribbean population: incidence, clinical manifestations, and survival in the Barbados National Lupus Registry. Arthritis Care Res (Hoboken) 2012;64(8):1151–1158. doi: 10.1002/acr.21656. [DOI] [PubMed] [Google Scholar]
- 33.Vilar MJ, Sato EI. Estimating the incidence of systemic lupus erythematosus in a tropical region (Natal, Brazil) Lupus. 2002;11(8):528–532. doi: 10.1191/0961203302lu244xx. [DOI] [PubMed] [Google Scholar]
- 34.Chiu YM, Lai CH. Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus. 2010;19(10):1250–1255. doi: 10.1177/0961203310373780. [DOI] [PubMed] [Google Scholar]
- 35.Mok CC, To CH, Ho LY, Yu KL. Incidence and mortality of systemic lupus erythematosus in a southern Chinese population, 2000-2006. J Rheumatol. 2008;35(10):1978–1982. [PubMed] [Google Scholar]
- 36.Molokhia M, McKeigue PM, Cuadrado M, Hughes G. Systemic lupus erythematosus in migrants from west Africa compared with Afro-Caribbean people in the UK. Lancet. 2001;357(9266):1414–1415. doi: 10.1016/S0140-6736(00)04580-3. [DOI] [PubMed] [Google Scholar]
- 37.Samanta A, Feehally J, Roy S, Nichol FE, Sheldon PJ, Walls J. High prevalence of systemic disease and mortality in Asian subjects with systemic lupus erythematosus. Ann Rheum Dis. 1991;50(7):490–492. doi: 10.1136/ard.50.7.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mok CC. Epidemiology and survival of systemic lupus erythematosus in Hong Kong Chinese. Lupus. 2011;20(7):767–771. doi: 10.1177/0961203310388447. [DOI] [PubMed] [Google Scholar]
- 39.Barnabe C, Joseph L, Belisle P, Labrecque J, Edworthy S, Barr SG, Fritzler M, Svenson LW, Hemmelgarn B, Bernatsky S. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res (Hoboken) 2012;64(1):138–143. doi: 10.1002/acr.20656. [DOI] [PubMed] [Google Scholar]
- 40.Nived O, Sturfelt G, Wollheim F. Systemic lupus erythematosus in an adult population in southern Sweden: incidence, prevalence and validity of ARA revised classification criteria. Br J Rheumatol. 1985;24(2):147–154. doi: 10.1093/rheumatology/24.2.147. [DOI] [PubMed] [Google Scholar]
- 41.Popovic K, Nyberg F, Wahren-Herlenius M. A serology-based approach combined with clinical examination of 125 Ro/SSA-positive patients to define incidence and prevalence of subacute cutaneous lupus erythematosus. Arthritis Rheum. 2007;56(1):255–264. doi: 10.1002/art.22286. [DOI] [PubMed] [Google Scholar]
- 42.Deligny C, Clyti E, Sainte-Marie D, Couppie P, Huong du LT, Piette JC, Arfi S, Pradinaud R. Incidence of chronic cutaneous lupus erythematosus in French Guiana: a retrospective population-based study. Arthritis Care Res (Hoboken) 2010;62(2):279–282. doi: 10.1002/acr.20079. [DOI] [PubMed] [Google Scholar]
- 43.Peters T, Weiss JM, Sindrilaru A, Wang H, Oreshkova T, Wlaschek M, Maity P, Reimann J, Scharffetter-Kochanek K. Reactive oxygen intermediate-induced pathomechanisms contribute to immunosenescence, chronic inflammation and autoimmunity. Mech Ageing Dev. 2009;130(9):564–587. doi: 10.1016/j.mad.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5(2):136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Harle P, Pongratz G, Weidler C, Buttner R, Scholmerich J, Straub RH. Possible role of leptin in hypoandrogenicity in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 2004;63(7):809–816. doi: 10.1136/ard.2003.011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalani S, Pope J, de Leon F, Peschken C. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37(1):38–44. doi: 10.3899/jrheum.080957. [DOI] [PubMed] [Google Scholar]
- 47.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng R, Bernatsky S, Rahme E. Observation period effects on estimation of systemic lupus erythematosus incidence and prevalence in Quebec. J Rheumatol. 2013;40(8):1334–1336. doi: 10.3899/jrheum.121215. [DOI] [PubMed] [Google Scholar]
- 49.Ward MM. Estimating disease prevalence and incidence using administrative data: some assembly required. J Rheumatol. 2013;40(8):1241–1243. doi: 10.3899/jrheum.130675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38(8):1612–1616. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]


