Abstract
Despite aggressive efforts to contain it, methamphetamine use disorder continues to be major public health problem; and with generic behavioral therapies still the mainstay of treatment for methamphetamine abuse, rates of attrition and relapse remain high. This review summarizes the findings of structural, molecular, and functional neuroimaging studies of methamphetamine abusers, focusing on cortical and striatal abnormalities and their potential contributions to cognitive and behavioral phenotypes that can serve to promote compulsive drug use. These studies indicate that individuals with a history of chronic methamphetamine abuse often display several signs of corticostriatal dysfunction, including abnormal gray- and white-matter integrity, monoamine neurotransmitter system deficiencies, neuroinflammation, poor neuronal integrity, and aberrant patterns of brain connectivity and function, both when engaged in cognitive tasks and at rest. More importantly, many of these neural abnormalities were found to be linked with certain addiction-related phenotypes that may influence treatment response (e.g., poor self-control, cognitive inflexibility, maladaptive decision-making), raising the possibility that they may represent novel therapeutic targets.
Keywords: methamphetamine, addiction, corticostriatal circuitry, positron emission tomography, magnetic resonance imaging, diffusion tensor imaging
Methamphetamine use disorder constitutes a major public health problem, associated with high rates of attrition, crime, relapse, and mortality. Although the prevalence of illicit methamphetamine use in the U.S. declined sharply in the late 2000s following legislation limiting access to precursors, the estimated number of current users has increased since 2010, totaling 595,000 in 2013 (SAMHSA, 2014a)). Furthermore, with 144,000 Americans estimated to have tried methamphetamine for the first time in 2013 (SAMHSA, 2014a) and established supply connections to Mexican cartels (Shukla et al., 2012), the problem may continue to grow. Methamphetamine has also become more prevalent throughout Asia and the Pacific region in recent years, leading to it being ranked as the primary or secondary drug of use in 13 of the 15 countries from that region surveyed in 2012 (UNODC, 2013). While many users seek treatment for methamphetamine abuse, which accounts for well over 100,000 admissions to drug treatment facilities annually in the U.S. alone (SAMHSA, 2014b), the vast majority relapse (Brecht and Herbeck, 2014; McKetin et al., 2012). Still, despite their poor therapeutic efficacy, generic behavioral interventions (e.g., cognitive behavioral therapy, contingency management, motivational interviewing) remain the mainstay of treatment for methamphetamine use disorder.
Chronic abuse of methamphetamine is often associated with a constellation of behavioral problems (e.g., Cartier et al., 2006; Cohen et al., 2003; McKetin et al., 2008; Zweben et al., 2004), including mood disturbances (London et al., 2004; Newton et al., 2004; Shen et al., 2012), persistent craving (Zorick et al., 2010) and psychosis (Grant et al., 2012). Cognitive deficits are also common among individuals with a history of methamphetamine abuse, particularly involving executive functions (e.g., mental flexibility, self-control), which are important for suppressing habitual behaviors (Dean et al., 2012; Monterosso et al., 2005; Scott et al., 2007; Simon et al., 2010). As outlined below, neuroimaging studies have demonstrated that methamphetamine abusers also typically display several signs of corticostriatal dysfunction. Moreover, these studies provide suggestive evidence that many of these corticostriatal abnormalities may underlie certain cognitive and behavioral phenotypes that can serve to promote compulsive drug use, raising the possibility that they may represent novel therapeutic targets.
Structural Brain Imaging of Methamphetamine Users
Structural magnetic resonance imaging has provided evidence for cortical and striatal gray- and white-matter abnormalities associated with methamphetamine abuse. A substantial number of studies have linked structural brain abnormalities with cognitive dysfunction in methamphetamine users.
Methamphetamine users generally exhibit smaller cortical but larger striatal gray-matter volumes than non-users (Berman et al., 2008a). Following brief abstinence (< 3 weeks), gray-matter volumes in anterior cingulate cortex (ACC), dorsolateral prefrontal (DLPFC), orbitofrontal (OFC), and superior temporal cortices as well as the hippocampus are smaller than in never-users (Nakama et al., 2011; Thompson et al., 2004); and a bilateral deficit in gray-matter density of the insula has been observed with abstinence up to 6 months (Schwartz et al., 2010). In contrast, subjects who are abstinent from methamphetamine for an average of 3-4 months show greater gray-matter volume in the parietal cortex, caudate nucleus, lenticular nucleus, nucleus accumbens (Jernigan et al., 2005), putamen and globus pallidus (Chang et al., 2005) than never-users. Putamen volume also is larger among non-abstinent users than non-users (Jan et al., 2012b).
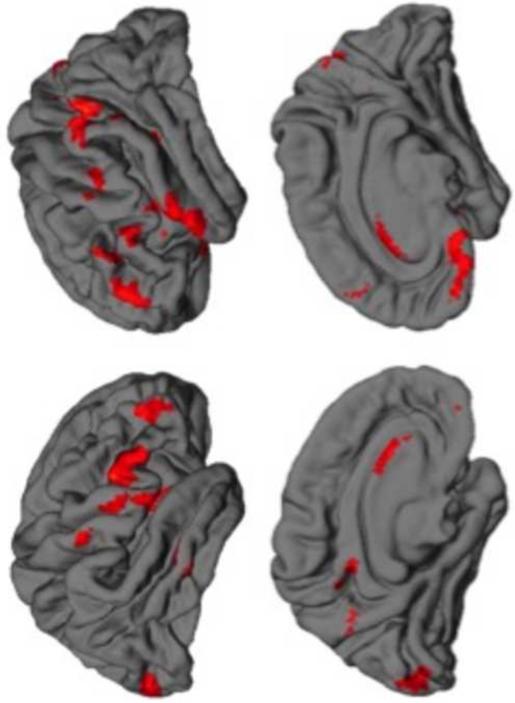
Cortical gray-matter deficits appear to reverse with protracted abstinence from methamphetamine. Supporting this view is the observation that gray-matter volume increased in inferior frontal, angular, and superior temporal gyri, precuneus, insula, and occipital pole in methamphetamine users following one month of abstinence (Figure 1) (Morales et al., 2012). In addition, participants abstinent for 6 months or more have higher gray-matter density in the bilateral middle frontal gyrus than those abstinent for shorter periods (Kim et al., 2006).
Figure 1. Changes in gray matter during early abstinence from methamphetamine.
In methamphetamine-dependent individuals, gray matter increased between the first and fourth weeks of abstinence from methamphetamine (results displayed at a statistical threshold of p < 0.005 uncorrected with a cluster extent > 200 voxels). No changes in gray matter were detected in healthy control participants who underwent two scanning sessions approximately 1 month apart. The left hemisphere is displayed on the left side of the image.
Factors other than methamphetamine use per se may contribute to cerebral structural abnormalities. For example, while only 22% of the general population comprises smokers, smoking is more prevalent among methamphetamine users (87-92%) (Weinberger and Sofuoglu, 2009). One study found that smokers, both those who did and those who did not use methamphetamine, had smaller gray-matter volume in the OFC and caudate nucleus than nonsmokers who did not use methamphetamine (Morales et al., 2012). Participants who engaged in both behaviors, however, had smaller gray-matter volumes in superior and inferior temporal, supramarginal, and precentral gyrus than smokers who did not use methamphetamine. Thus, while some cortical deficits appear to result from either methamphetamine use or its combination with smoking, gray-matter volume deficits in the OFC and caudate may be in part attributable to cigarette smoking or pre-morbid conditions in methamphetamine users.
White matter abnormalities have also been linked to methamphetamine use. Fractional anisotropy, a measure of the extent to which water diffusion is restricted to a single direction, depends on axon caliber, fiber density and organization, and myelination (Beaulieu, 2009). Methamphetamine users have lower fractional anisotropy in prefrontal white matter (Chung et al., 2007; Tobias et al., 2010), the genu of the corpus callosum (Kim et al., 2009; Salo et al., 2009a; Tobias et al., 2010), midcaudal superior corona radiata, and the perforant path (Tobias et al., 2010). These findings are consistent with drug-induced white-matter damage. Prominent generalized white-matter hypertrophy, found in early abstinent methamphetamine users (Thompson et al., 2004), may result from altered myelination and adaptive changes, including gliosis secondary to neuronal damage. Significant effects are observed in temporal and occipital cortices, adjacent to some of the regions where hippocampal and cortical gray-matter changes are detected; and right cingulate gray-matter loss accompanies frontal horn expansion in the right lateral ventricles (Thompson et al., 2004).
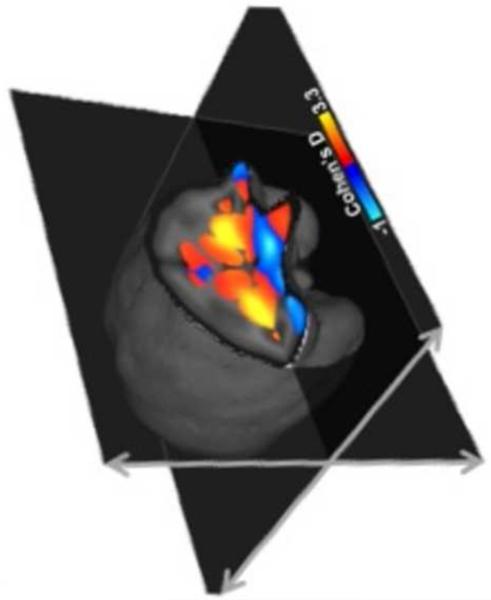
Although human studies generally do not allow assignment of structural abnormalities to methamphetamine use per se, this question can be addressed in animal studies. In vervet monkeys, exposure to a methamphetamine-dosing regimen that resembles a common pattern of human drug-taking results in increased gray-matter volume in the putamen, and this increase is associated with reductions in D2-type dopamine receptor and dopamine transporter (DAT) availability, and decrements in cognitive flexibility (Figure 2) (Groman et al., 2013). These observations suggest that at least the gray-matter abnormalities detected in the putamen in human methamphetamine users may partly be a consequence of drug exposure.
Figure 2. Exposure to escalating methamphetamine causes structural changes in the vervet monkey brain.
In the effect size (Cohen’s d) map, positive values (warmer colors) indicate brain regions where gray matter increased in the methamphetamine-exposed group relative to the saline controls; negative values (cooler colors) depict the converse relationship. Methamphetamine exposure produced a significant increase in gray matter in the striatum compared to saline (p < 0.05 corrected for multiple comparisons). The left hemisphere is depicted right side of the image.
As structural features of the brain (Peper et al., 2007; Thompson et al., 2002; Thompson et al., 2001) and addiction (Agrawal and Lynskey, 2008; Li and Burmeister, 2009; Uhl et al., 2008) are both heritable, methamphetamine users may differ in brain structure from drug non-users before they initiate drug use, and the differences may be linked to genetic vulnerability for drug use. In a test of this hypothesis, 50 cocaine- or amphetamine-dependent individuals and their siblings without histories of chronic drug use were compared to healthy controls (Ersche et al., 2012). Stimulant-dependent individuals and their unaffected siblings had greater gray-matter volume in putamen, amygdala and hippocampus than controls, but only the stimulant-dependent individuals showed smaller volumes of OFC and anterior insula than controls. Stimulant-dependent individuals and their unaffected siblings also had lower fractional anisotropy than healthy controls, suggesting that white-matter abnormalities shared by individuals with familial history of substance use disorders predispose them to drug abuse. Collectively, these findings suggest that some but not all of the structural brain abnormalities in methamphetamine users may predate drug use.
Deficits in Brain Structure and Impaired Executive Functions
Considerable evidence indicates that structural integrity of the prefrontal cortex contributes to cognitive control. The pars opercularis of the right inferior frontal gyrus (IFG) has been linked to inhibitory control (Aron et al., 2004), and gray-matter structural deficits in this region occur in methamphetamine users (Tabibnia et al., 2011; Thompson et al., 2004). Moreover, white-matter integrity, inferred from fractional anisotropy in fiber tracts proximal to the right IFG, is positively related to motor inhibitory control capacity on the Stop-signal task in stimulant users, their unaffected siblings, and healthy controls with no familial history of stimulant dependence (Ersche et al., 2012). Gray-matter integrity of the pars opercularis has also been linked with capacity for motor response inhibition on the Stop-signal task and with success in an emotion-regulation task in healthy adults (Tabibnia et al., 2011). Methamphetamine users show impairment on this task, their performance being negatively correlated with methamphetamine craving, which is negatively associated with gray-matter volume in the pars opercularis (Monterosso et al., 2005). A positive association between putamen volume and performance on a test of psychomotor inhibitory control also has been observed in methamphetamine users (Jan et al., 2012b).
Other dimensions of inhibitory control include the ability to suppress or resist irrelevant information, commonly measured using the Stroop task, and the ability to respond flexibly to changes in the environment, which can be tested using the Wisconsin Card Sorting Task (WCST). On the Stroop task, methamphetamine users exhibit greater interference, measured as the Stroop reaction time, than controls (Salo et al., 2009b). And on the WCST, methamphetamine users make more errors and achieve fewer categories (Hosak et al., 2012; Kim et al., 2006). These deficits have been found to be correlated with increased fractional anisotropy in the genu of the corpus callosum, a white-matter bundle carrying fibers originating in the prefrontal cortex (Kim et al., 2006; Salo et al., 2009a). Gray-matter density in the middle frontal gyrus of methamphetamine users is also associated with errors on the WCST (Kim et al., 2006).
The pattern of smaller gray-matter volume in prefrontal cortex and larger striatal volumes has been linked both to impulsive choice and impulsive action. Temporal discounting paradigms measure the rate at which the subjective value of a reward diminishes with time, assessing preference for smaller rewards available sooner over larger, more delayed alternatives. Methamphetamine users exhibit steeper temporal discounting than healthy controls (Monterosso et al., 2007; Schwartz et al., 2010); and this characteristic has been associated with lower gray-matter density in the superior frontal gyrus but greater gray-matter density in putamen, ventral striatum and posterior cingulate in methamphetamine-dependent individuals as well as non-users (Schwartz et al., 2010).
Molecular Imaging of Methamphetamine Users
Studies employing positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance spectroscopy (MRS) have identified neurochemical abnormalities in the cortices and striata of individuals with a history of methamphetamine abuse. These include markers of deficient monoaminergic signaling capacity, poor neuronal integrity, neuroinflammation, and abnormal glucose metabolism.
Monoamine systems
Methamphetamine is a psychostimulant that acts to increase release and sustain extracellular concentrations of dopamine, serotonin and norepinephrine (rev. Sulzer et al., 2005). These actions primarily underlie effects of the drug on mood, cognition and physiology, including its addictive properties and, in part, its capacity to induce neurotoxicity (rev. Cadet et al., 2010; Panenka et al., 2013). Accordingly, much of the relevant molecular imaging research has focused on neurochemical markers for monoaminergic, particularly dopamine, systems.
Extending findings from animal studies and postmortem human brain analyses indicating that methamphetamine alters monoaminergic nerve terminals (rev. Seiden and Ricaurte, 1987), PET has been used to assess presynaptic neurochemical markers in vivo. Striatal levels of DAT were generally lower in abstinent methamphetamine users than in matched non-users: deficits of 11-28% were measured using [11C]WIN-35,428 and [11C]d-threo-methylphenidate (Johanson et al., 2006; McCann et al., 1998; McCann et al., 2008; Sekine et al., 2001; Volkow et al., 2001b; Volkow et al., 2001d). Although some participants in these studies were abstinent for up to 25 years (rev. McCann et al., 2008), a Tc-99nm TRODAT SPECT study found evidence of up to a 38% DAT recovery in the first two weeks of abstinence among five methamphetamine abusers (Chou et al., 2007), and another with PET and [11C]d-threo-methylphenidate found that DAT availability increased 19% and 16% in the caudate and putamen, respectively, in five individuals between early (< 6 months) and later abstinence (12-17 months) (Volkow et al., 2001b). Notably, in some (McCann et al., 2008; Volkow et al., 2001d) but not all (Johanson et al., 2006) of these studies, measures of DAT availability were correlated with cognitive and psychomotor performance, and there is preliminary evidence that DAT recovery with abstinence may correspond to recovery of executive function, as indicated by improved performance on the WCST (Chou et al., 2007). Lower DAT availability has also been found in the anterior prefrontal cortex (PFC), OFC, DLPFC and amygdala of methamphetamine users (Sekine et al., 2001; Sekine et al., 2003). DAT availability in the striatum and PFC were negatively associated with psychiatric symptoms and duration of methamphetamine use (Sekine et al., 2001; Sekine et al., 2003).
The serotonin and type-2 vesicular monoamine transporters (SERT and VMAT2, respectively) are other presynaptic markers that have been measured using PET in methamphetamine users. Consistent with laboratory rodent (Kovachich et al., 1989; Reichel et al., 2012) and postmortem human findings (Kish et al., 2009), a study using [11C]trans-1,2,3,5,6,10-beta-hexahydro-6-[4-(methylthio)phenyl]pyrrolo-[2,1-a]isoquinoline ([11C](+)McN-5652) to measure SERT levels showed lower availability in cortical and subcortical structures, including the striatum, among methamphetamine users abstinent for less than one year; reductions in the OFC, temporal cortices, and ACC were related to a measure of aggression (Sekine et al., 2006). Findings pertaining to VMAT2 are less clear, with one study using [11C]dihydrotetrabenazine finding that striatal availability was higher (+22%, caudate; +12%, putamen; +11%, ventral striatum) in methamphetamine users during early abstinence (< 3 weeks) compared to controls, the group differences diminishing with longer abstinence (Boileau et al., 2008), and another finding 10% lower striatal availability among users who were abstinent for over 3 months than non-users (Johanson et al., 2006). The former finding fits with work in rats showing reductions in striatal VMAT2 protein following an excitotoxic methamphetamine dosing regimen; although, no change was found following a behaviorally sensitizing regimen (Frey et al., 1997), and a postmortem brain analysis found no difference in striatal VMAT2 levels between methamphetamine users and non-users (Wilson et al., 1996). Molecular imaging studies of norepinephrine transporters or markers of monoamine synthesis in methamphetamine users have not been conducted in vivo.
In addition to assessments of monoaminergic nerve terminal integrity, PET studies have shown deficits in dopamine D2-type receptor availability, a putative biomarker of postsynaptic dopamine function. One study using the D2/D3-preferring ligand [11C]raclopride demonstrated lower striatal availability among methamphetamine users who were abstinent for between 2 weeks and 35 months (−16%, caudate; −13%, putamen; −8%, nucleus accumbens) (Volkow et al., 2001b), and similar results have been obtained with [18F]fallypride among individuals abstinent for 4-10 days (Lee et al., 2009). Furthermore, striatal D2-type receptor availability was positively correlated with regional glucose metabolism in the OFC, measured with [18F]fluorodeoxyglucose (Volkow et al., 2001b), and with trait impulsivity (Lee et al., 2009). D2-type receptor availability also has predicted treatment outcome, in that high baseline D2-type receptor availability in the caudate and putamen predicted the maintenance of abstinence for participants in an outpatient rehabilitation program, and low baseline D2-type receptor availability predicted relapse to methamphetamine abuse during a nine-month follow-up period (Wang et al., 2011). Preliminary evidence also suggests that low striatal D2-type receptor availability is associated with steeper temporal discounting among methamphetamine users (Ballard et al., 2014). There is some evidence that striatal D2-type receptor availability recovers with protracted abstinence in rodents (Segal et al., 2005) and nonhuman primates (Groman et al., 2012), although longitudinal studies investigating D2-type receptor availability with methamphetamine abstinence over time not been conducted in humans.
A PET study with [11C](+)4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol ([11C]-(+)-PHNO), which shows higher differential binding at D3 than at D2 dopamine receptors (Wilson et al., 2005) showed that individuals with a history of methamphetamine use exhibited only a slight reduction in binding in striatal regions compared to controls, a finding interpreted as evidence that increased D3 receptor levels overshadowed the reduction in D2 receptor levels (Boileau et al., 2012). Most of the individuals sampled in that study, however, had abused drugs in addition to methamphetamine, limiting the conclusions that can be drawn regarding the effects of methamphetamine. Aside from a study that found no difference in binding of a ligand that selectively binds both D2 and type-2 serotonin receptors ([11C]-N-methylspiperone) between six men with a history of methamphetamine psychosis and healthy non-user controls (Iyo et al., 1993), no other molecular imaging studies have yet investigated levels of D1-like, serotonin, or norepinephrine receptors in methamphetamine users.
Neuronal integrity and neuroinflammation
Mounting evidence that repeated exposure to methamphetamine can produce neurotoxicity (rev. Cadet et al., 2010; Jan et al., 2012a) has prompted evaluation of chemical markers of neuronal integrity and neuroinflammation with MRS in methamphetamine users. These studies have shown lower levels of n-acetyl-aspartate (NAA) metabolites and a lower ratio of NAA to creatine and phosphocreatine (Cr) metabolites – indicating poorer neuronal integrity and viability – among methamphetamine abusers relative to healthy controls in frontal brain regions, particularly the ACC (Ernst et al., 2000; Nordahl et al., 2002; Nordahl et al., 2005; Sailasuta et al., 2010; Sung et al., 2007). Moreover, the NAA/Cr ratio in the ACC is positively correlated with attentional control (Salo et al., 2007) and negatively correlated with years of methamphetamine use (Nordahl et al., 2005) among methamphetamine users, and appears to recover, albeit slowly, with extended abstinence (Nordahl et al., 2005; Salo et al., 2011). Higher levels of choline (Cho) metabolites have been found in frontal regions and the basal ganglia of methamphetamine users than non-users (Ernst et al., 2000; Nordahl et al., 2002; Nordahl et al., 2005; Sekine et al., 2002), and evidence that the level of frontal glutamate is increased (Ernst and Chang, 2008; Sailasuta et al., 2010) implicates glutamate excitotoxicity as a potential contributing factor. Inverse correlations between months of abstinence and Cho/Cr and Cho/NAA ratios in the ACC suggest that some recovery is possible after prolonged abstinence (Nordahl et al., 2005). In addition, higher myo-inositol in frontal gray matter – indicative of glial cell proliferation – has been found using MRS in methamphetamine users compared to non-users (Ernst et al., 2000; Sung et al., 2007). Corroborating MRS findings, a PET study that used [11C]N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide ([11C]PK11195), a ligand for the translocator protein, found more binding, suggestive of increased neuroinflammation, in the midbrain, striatum, thalamus, OFC, and insular cortex among abstinent methamphetamine abusers than non-users (Sekine et al., 2008). As well, a longer duration of abstinence from methamphetamine was inversely associated with binding in the midbrain, striatum, and thalamus (Sekine et al., 2008). Methamphetamine-induced inflammation and neurodegeneration are likely behaviorally relevant, as peripheral markers of immune activation are associated with impaired cognitive functioning (Loftis et al., 2011), and Cr/Cho ratios in the basal ganglia are positively correlated with duration of methamphetamine use and the severity of residual psychiatric symptoms (Sekine et al., 2002).
Regional Brain Function
The radiotracer [18F]fluorodeoxyglucose is used with PET to map regional cerebral metabolism of glucose, an index for local brain function (Phelps et al., 1979; Reivich et al., 1977; Sokoloff et al., 1977). This procedure has been used to compare methamphetamine users with non-users and to evaluate how functional abnormalities are related to mood, and how they change over time. In the first of these studies, global metabolic rate was higher among methamphetamine users, who had a range in duration of abstinence from 2 weeks to 35 months; with an elevation in normalized metabolism in the parietal cortex reaching statistical significance (Volkow et al., 2001c). In the same study, deficits in normalized metabolism were observed in subcortical regions that have dopaminergic innervation (thalamus, caudate, putamen), consistent with the notion of disrupted dopaminergic function.
When the abstinence period was limited to the first week, a different pattern emerged, with methamphetamine users exhibiting less relative glucose metabolism in the ACC and insula but greater relative regional glucose metabolism in the amygdala, ventral striatum, and OFC (London et al., 2004). In another study, glucose metabolism in the OFC was positively correlated with personality measures of inhibitory control, as indexed by the harm avoidance scale and constraint superfactor of Tellegen’s Multidimensional Personality Questionnaire, among recently abstinent methamphetamine users (Goldstein et al., 2002), suggesting that OFC function contributes to stable personality predispositions in methamphetamine users. Recently abstinent methamphetamine users also perform more poorly than non-users on tests of sustained attention; and their performance on these tasks correlates negatively with metabolic activity in the anterior and middle cingulate gyri and the insula, suggesting that PFC and insular dysregulation in the early abstinence period could underlie some of the cognitive deficits (London et al., 2004).
A subset of the methamphetamine users who were tested in early abstinence were re-tested after another month of abstinence, when greater variance in task performance accompanied a global increase in cerebral glucose metabolism in the parietal cortex, as well as other cortical regions and the thalamus (Berman et al., 2008b). Considering the earlier study of participants who were abstinent for up to about 2.5 years (Volkow et al., 2001c), these findings suggest either that cortical abnormalities in glucose metabolism evolve after the first week of abstinence, or that a chronic elevation in glucose metabolism is masked by residual effects of the drug during early abstinence. The change may reflect adaptive processes, such as reactive proliferation of glial cells (which have a higher metabolic demand than neurons) in response to cortical (especially parietal) damage. In both studies, parietal metabolism in the methamphetamine users correlated with task performance (Grooved Pegboard Task (Volkow et al., 2001c) or Continuous Performance Task (Berman et al., 2008b)), suggesting that this dysregulation of both dopaminergic and non-dopaminergic systems contributes to the cognitive, motor, and mood deficits observed with long-time methamphetamine abuse.
There is also evidence that cerebral glucose metabolism remains abnormal throughout protracted abstinence. Significantly greater thalamic, although not striatal, metabolism was seen following 12 – 17 months of abstinence compared to values after less than 6 months, and this increase was associated with improved performance in motor and verbal memory tests (Wang et al., 2004). In addition, lower glucose metabolism in PFC white matter has been observed among male methamphetamine users abstinent 19 months (SD = 27), compared to non-users, and the severity of this deficit correlated with impairment on the WCST, which relies on prefrontal integrity (Kim et al., 2006). Still, a study employing technetium-99m-hexamethyl-propyleneamineoxime ([99mTc]HMPAO) SPECT that showed lower relative regional cerebral blood flow, another functional index, in the right ACC among abstinent methamphetamine users than in healthy controls found signs of recovery with abstinence. In that study, binding was lower among the subset of 13 users who had been abstinent an average of 3 months (SD = 2) than 27 users who were abstinent an average of 36 months (SD = 40) (Hwang et al., 2006).
Functional Imaging of Brain Activation Using fMRI in Methamphetamine Users
The use of functional magnetic resonance imaging (fMRI) has greatly advanced the understanding of brain function as it provides a non-invasive approach to indirectly measure neuronal activity. Consistent with the reports that chronic methamphetamine use results in changes in biological markers of neuronal function in cortical and subcortical areas (London et al., 2004), fMRI studies have shown that methamphetamine users have impairments in prefrontal and striatal activation during tests of executive functioning.
On the Stroop task for example, methamphetamine users exhibit less activation than controls in the right IFG, supplementary motor cortex/ACC and the anterior insular cortex during the incongruent condition relative to rest but greater activation in the ACC, medial PFC and frontal pole than control subjects in the incongruent than congruent conditions (Nestor et al., 2011). These results, in combination with findings that impaired Stroop performance was related to a deficit in integrity of white-matter fibers originating in the prefrontal cortex (see above), suggest that loss of integrity and connectivity of PFC regions may underlie deficits in executive function in methamphetamine users.
Methamphetamine users show functional deficits while performing tasks that assess decision-making in the presence of varying likelihoods of risk and reward magnitude. An fMRI study found a link between greater temporal discounting and abnormality in neural recruitment within the left DLPFC and intraparietal sulcus of methamphetamine users (Monterosso et al., 2007). While non-users showed increasing BOLD signal with more difficult choices, methamphetamine users showed similar recruitment for easy and difficult choices (Monterosso et al., 2007). In another study, healthy controls exhibited greater activation in DLPFC, ACC and caudate nucleus than methamphetamine users while choosing between immediate and delayed alternatives (Hoffman et al., 2008). The results suggest that discounting of delayed rewards in methamphetamine users may represent reward-driven behavior due to impairments in frontostriatal activation.
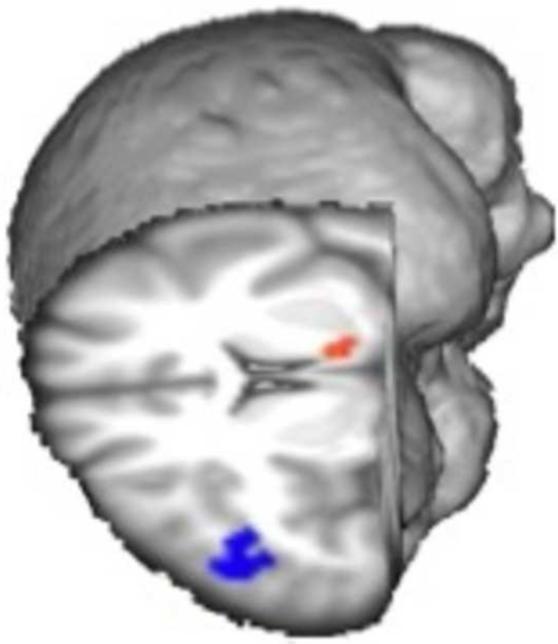
Stimulant users also show impairments in brain activation during risky decision-making, drug users consistently showing impairments in the striatum and in the OFC, DLPFC, medial frontal cortex and ACC (Gowin et al., 2013). In a recent study, a linear relationship between level of risk and activation in the DLPFC during decision-making was greater among non-user controls than methamphetamine users, but the relationship with the ventral striatal activation was greater among the methamphetamine users (Figure 3) (Kohno et al., 2014). Inasmuch as frontostriatal circuitry plays a critical role in integrating motivational and cognitive processes to produce optimal behavior (Jentsch and Taylor, 1999), frontostriatal impairments may contribute to deficits in executive functioning that have been observed in methamphetamine users (Dean et al., 2012; Jentsch and Taylor, 1999; Nordahl et al., 2003).
Figure 3. Differences in brain function during risky decision-making between methamphetamine-dependent and healthy control participants.
The linear relationship between risk levels and activation in the dorsolateral prefrontal cortex (blue) was greater in control group than in the methamphetamine-dependent group; however, the opposite relationship was found in the greater in the ventral striatum (red) (p < 0.05, cluster corrected). Results were controlled for age, sex, smoking status, and marijuana use.
Methamphetamine-related impairments in frontal function have also been linked with dysregulated mood and emotional processing deficits. Threatening or fearful visual scenes have shown to evoke less activation in bilateral DLPFC and insula of methamphetamine users than in non-user controls (Kim et al., 2011). Methamphetamine users also exhibit less activation in the IFG during the presentation of fearful and angry faces (Payer et al., 2011) and while performing an empathy task (Kim et al., 2010). As methamphetamine users exhibit less empathy (Kim et al., 2010) but greater aggressive behavior (Payer et al., 2011), the results suggest that impairments in PFC control may contribute to deficits in the evaluation of internal states, and that they precipitate dysregulated emotional processing in methamphetamine-abusing individuals.
Not only is activation in frontal and striatal systems central to decision-making and emotional processing, it has been associated with success of treatment for methamphetamine abuse. An fMRI study of decision-making using a 2-choice prediction task, found that activation of the insula, DLPFC, as well as parietal, and temporal cortices predicted maintained abstinence from methamphetamine, with lack of activation heralding relapse (Paulus et al., 2005). In another study, differences in activation of the striatum, insula and IFG between abstainers and relapsers were obtained while participants performed the paper-scissors-rock task (Stewart et al., 2013). These findings provide evidence that corticostriatal and limbic dysfunction relate to impaired higher-order cognitive and motivational functions that promote the maintenance of addiction (Goldstein and Volkow, 2002)
Resting-state Functional Abnormalities in Brain Demonstrated Using fMRI
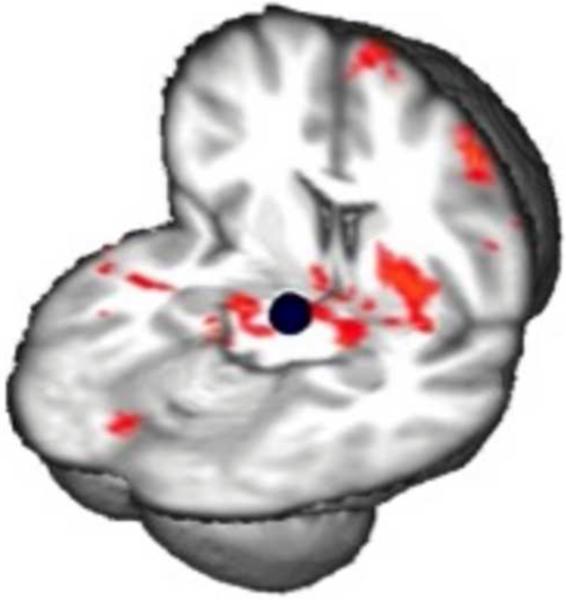
Advances in assessing network dynamics through resting-state functional connectivity (RSFC) have contributed to new insights in the functional organization of brain systems (Greicius, 2008), and recent studies have interrogated RSFC within the mesocorticolimbic system in individuals with various substance use disorders (Gu et al., 2010; Tomasi et al., 2010; Upadhyay et al., 2010; Wilcox et al., 2011). These studies have indicated various differences in mesocorticolimbic RSFC between drug users and nonusers, presumably in part as a function of drug use history and recency of drug taking (Konova et al., 2013) . In another study, abstinent methamphetamine users exhibited stronger RSFC than controls of the midbrain with amygdala, hippocampus, striatum, insula and PFC (Kohno et al., 2014). It is plausible that this effect contributes to psychostimulant sensitization (Robinson and Berridge, 1993). As RSFC was related to brain function during decision-making (Kohno et al., 2014), the study has important implications for mesocorticolimbic sensitization on brain function and behavior (Figure 4).
Figure 4. Relationship between prefrontal brain function during risky decision-and resting-state connectivity of the midbrain in methamphetamine-dependence.
Connectivity maps show a negative correlation between modulation of activation in right dorsolateral prefrontal cortex during risky decision-making and the connectivity between midbrain seed (shown in blue) and nucleus accumbens, putamen, amygdala, hippocampus, orbitofrontal cortex, anterior cingulate, and superior frontal gyrus in methamphetamine-dependent individuals (p < 0.05, whole-brain cluster corrected). Results controlled for age, sex, smoking status and marijuana use.
In addition to the aforementioned evidence for dopamine system dysfunction, increases of local tonic dopamine concentrations, presynaptic glutamate release and reduction of long-term depression in dopamine neurons (Jones et al., 2000) disrupt striatal and cortical function. Dopaminergic efferents from the ventral tegmental area (VTA) and glutamatergic efferents from the PFC often terminate on the same postsynaptic cell in the ventral striatum (Beaulieu and Gainetdinov, 2011) and efferents from the PFC contact dopaminergic neurons of the VTA that project to the nucleus accumbens directly (Bunney et al., 1991). As adaptive decision-making would require a balance between behavioral control and reward-seeking behavior, impairments in the corticostriatal circuit of methamphetamine users may attenuate PFC regulation of ventral-limbic response to reward. Thus, the functional deficits during risky decision-making (Gowin et al., 2013; Lane and Cherek, 2000) and temporal discounting (Hoffman et al., 2008; Monterosso et al., 2007) seen in methamphetamine abusers could be attributed to a disruption in the corticostriatal circuit leading to abnormal evaluation of a stimuli and the assignment of value.
Implications for Therapy
Dopamine system deficiencies and associated behavioral phenotypes may be a critical barrier to success in treating methamphetamine use disorders. As reviewed above, the effects of methamphetamine on dopaminergic markers and associated signaling pathways may promote dysfunctions of cognitive control and decision-making. In line with this view, low striatal D2-type receptor availability has been linked with poor inhibitory control (Monterosso et al., 2005) and impulsivity (Lee et al., 2009) in methamphetamine users, as well as with decreased activity in prefrontal regions important for executive functioning (Volkow et al., 2001a).
Direct and indirect dopamine agonists, including modafanil, methylphenidate and d-amphetamine have not consistently proven to be efficacious (Brensilver et al., 2013; Miles et al., 2013). This may in part be due to methamphetamine-induced neurotoxicity to dopamine nerve terminals. Consistent with studies in animals, neuroimaging studies have detected evidence for neuroinflammation in humans with chronic methamphetamine exposure (Ernst et al., 2000; Sekine et al., 2008). Given the evidence that minocycline, a powerful anti-inflammatory drug, ameliorates methamphetamine-induced neurotoxicity to dopamine nerve terminals and behavioral changes (Zhang et al., 2006), anti-inflammatory agents may be considered in the treatment of methamphetamine dependence.
Another therapeutic approach might be to augment dopaminergic signaling using nonpharmacological methods. Given relationships between striatal D2-type receptor availability and decision-making (Kohno et al., 2013), inhibitory control (Ghahremani et al., 2012) and trait impulsivity (Buckholtz et al., 2010) in healthy controls, an upregulation of D2 receptors may provide clinical improvements in executive function for individuals affected with stimulant-use disorders. In patients with Parkinson’s disease intensive exercise leads to increased D2-type receptor BPND (Fisher et al., 2013), and preliminary findings suggest a positive finding in methamphetamine users as well (Robertson et al., 2013). Future studies may also benefit from therapeutic approaches targeted at novel targets in the dopamine system, such as the D3 receptor (Baladi et al., 2014; Paterson et al., 2014), as chronic methamphetamine exposure is associated with reduced levels of D2 receptors but elevated levels of D3 receptors (Boileau et al., 2012; Payer et al., 2014).
Despite high rates of attrition and relapse (Rawson et al., 2004; Smout et al., 2010), cognitive behavioral therapy, contingency management, and motivational interviewing (Carroll and Onken, 2005; Rawson et al., 2006) are the mainstay of treatment for methamphetamine users. As cognitive impairment is related to poorer treatment outcomes for drug dependence, cognitive remediation may augment the efficacy of behavioral therapy for drug dependence (Vocci, 2008). Cognitive remediation therapies requires the practice of skills that are essential for daily functioning such as attention, memory, executive skills, visuoperceputal skills and problem-solving (Kurtz et al., 2007). Improvements with cognitive remediation therapies have been shown in patients with schizophrenia (Bell et al., 2001), attention-deficit hyperactivity disorder (O'Connell et al., 2006), and anorexia nervosa (Wood et al., 2011). The potential of cognitive remediation for treatment of methamphetamine dependence lies in the probability of transfer of improvement in one cognitive task to another task that uses similar underlying neural structures. As an emerging literature suggests experience-dependent neural plasticity can be detected in vivo using magnetic resonance imaging (Ilg et al., 2008; May and Gaser, 2006; May et al., 2007; Scholz et al., 2009), future studies might address whether cognitive remediation can facilitate recovery of brain structure and function in methamphetamine users.
Highlights.
Chronic methamphetamine abuse is linked to abnormalities in frontostriatal circuitry
Neural abnormalities linked with methamphetamine exposure, abstinence, and executive control
Brain structure, chemistry and function are potential therapeutic targets
Acknowledgements
This work was supported by an endowment from the Thomas P and Katherine K Pike Chair in Addiction Studies and a gift from the Marjorie M Greene Trust (EDL), T32 DA 024635 (MEB), F31 DA0331-17 (AMM), F31 DA033120-02 (MK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–81. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, et al. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–73. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Baladi MG, et al. Dopamine D(3) receptors contribute to methamphetamine-induced alterations in dopaminergic neuronal function: role of hyperthermia. Eur J Pharmacol. 2014;732:105–10. doi: 10.1016/j.ejphar.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, et al. Low striatal dopamine D2/D3 receptor availability predicts steeper delay discounting in methamphetamine abusers; Poster session presented at the meeting of the College on Problems of Drug Dependence; San Juan, PR. Jun, 2014. [Google Scholar]
- Beaulieu C. In: Diffusion MRI: From Quantitiative Measurement to In Vivo Neuroanatomy. Johansen-Berg H, Behrens TE, editors. Elsevier; London: 2009. pp. 105–126. [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bell M, et al. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Arch Gen Psychiatry. 2001;58:763–8. doi: 10.1001/archpsyc.58.8.763. [DOI] [PubMed] [Google Scholar]
- Berman S, et al. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008a;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, et al. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008b;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–6. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–9. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25. doi: 10.1016/j.drugalcdep.2014.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev. 2013;32:449–60. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Cadet JL, et al. Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets. 2010;9:526–38. doi: 10.2174/187152710793361496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–60. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier J, Farabee D, Prendergast ML. Methamphetamine use, self-reported violent crime, and recidivism among offenders in California who abuse substances. J Interpers Violence. 2006;21:435–45. doi: 10.1177/0886260505285724. [DOI] [PubMed] [Google Scholar]
- Chang L, et al. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–74. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, et al. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: A SPECT study. Eur Neuropsychopharmacol. 2007;17:46–52. doi: 10.1016/j.euroneuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chung A, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–75. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Cohen JB, et al. Abuse and violence history of men and women in treatment for methamphetamine dependence. Am J Addict. 2003;12:377–85. [PubMed] [Google Scholar]
- Dean AC, et al. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2012;38:259–74. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, et al. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–72. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–4. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fisher BE, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. NeuroReport. 2013. Publish Ahead of Print, 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed]
- Frey K, Kilbourn M, Robinson T. Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol. 1997;334:273–9. doi: 10.1016/s0014-2999(97)01152-7. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32:7316–24. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, et al. The orbitofrontal cortex in methamphetamine addiction: involvement in fear. Neuroreport. 2002;13:2253–7. doi: 10.1097/01.wnr0000044215.09266.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132:13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KM, et al. Methamphetamine-associated psychosis. J Neuroimmune Pharmacol. 2012;7:113–39. doi: 10.1007/s11481-011-9288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Groman SM, et al. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–52. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, et al. Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 2013;229:527–38. doi: 10.1007/s00213-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosak L, et al. Comparison of Wisconsin Card Sorting Test results between Czech subjects dependent on methamphetamine versus healthy volunteers. Psychiatr Danub. 2012;24:188–93. [PubMed] [Google Scholar]
- Hwang J, et al. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006;82:177–81. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Ilg R, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–5. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, et al. Dopamine D2 and serotonin S2 receptors in susceptibility to methamphetamine psychosis detected by positron emission tomography. Psychiatry Res. 1993;50:217–31. doi: 10.1016/0925-4927(93)90002-y. [DOI] [PubMed] [Google Scholar]
- Jan RK, Kydd RR, Russell BR. Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2012a;2:434–82. doi: 10.3390/brainsci2040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan RK, et al. Striatal volume increases in active methamphetamine-dependent individuals and correlation with cognitive performance. Brain Sci. 2012b;2:553–72. doi: 10.3390/brainsci2040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–72. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Johanson CE, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–38. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones LB, et al. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–14. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, et al. Reduced corpus callosum white matter microstructural integrity revealed by diffusion tensor eigenvalues in abstinent methamphetamine addicts. Neurotoxicology. 2009;30:209–13. doi: 10.1016/j.neuro.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kim SJ, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–8. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kim YT, et al. Alterations in cortical activity of male methamphetamine abusers performing an empathy task: fMRI study. Hum Psychopharmacol. 2010;25:63–70. doi: 10.1002/hup.1083. [DOI] [PubMed] [Google Scholar]
- Kim YT, et al. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: functional MRI study. NMR Biomed. 2011;24:1392–400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- Kish SJ, et al. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202:649–61. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Kohno M, et al. Risk-Taking Behavior: Dopamine D2/D3 Receptors, Feedback, and Frontolimbic Activity. Cereb Cortex. 2013 doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, et al. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–20. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, et al. Effects of Methylphenidate on Resting-State Functional Connectivity of the Mesocorticolimbic Dopamine Pathways in Cocaine Addiction. JAMA Psychiatry. 2013:1–11. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effects of high-dose methamphetamine administration on serotonin uptake sites in rat brain measured using [3H]cyanoimipramine autoradiography. Brain Res. 1989;505:123–9. doi: 10.1016/0006-8993(89)90122-4. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, et al. Computer-assisted cognitive remediation in schizophrenia: what is the active ingredient? Schizophr Res. 2007;89:251–60. doi: 10.1016/j.schres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR. Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend. 2000;60:179–87. doi: 10.1016/s0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- Lee B, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–31. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, et al. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, et al. Mood Disturbances and Regional Cerebral Metabolic Abnormalities in Recently Abstinent Methamphetamine Abusers. Archives of General Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. PhD. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr Opin Neurol. 2006;19:407–11. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- May A, et al. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–10. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- McCann UD, et al. Reduced Striatal Dopamine Transporter Density in Abstinent Methamphetamine and Methcathinone Users: Evidence from Positron Emission Tomgraphy Studies with [11C]WIN-35,428. The Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McKetin R, et al. Hostility among methamphetamine users experiencing psychotic symptoms. Am J Addict. 2008;17:235–40. doi: 10.1080/10550490802019816. [DOI] [PubMed] [Google Scholar]
- McKetin R, et al. Evaluating the impact of community-based treatment options on methamphetamine use: findings from the Methamphetamine Treatment Evaluation Study (MATES) Addiction. 2012;107:1998–2008. doi: 10.1111/j.1360-0443.2012.03933.x. [DOI] [PubMed] [Google Scholar]
- Miles SW, et al. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:1279–86. doi: 10.1111/add.12109. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, et al. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–93. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, et al. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–8. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H, et al. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011 doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–95. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, et al. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–55. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–25. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–52. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, et al. Cognitive remediation in ADHD: effects of periodic non-contingent alerts on sustained attention to response. Neuropsychol Rehabil. 2006;16:653–65. doi: 10.1080/09602010500200250. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–79. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Paterson NE, et al. Dopamine D3 receptors as a therapeutic target for methamphetamine dependence. Am J Drug Alcohol Abuse. 2014;40:1–9. doi: 10.3109/00952990.2013.858723. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Payer D, Balasubramaniam G, Boileau I. What is the role of the D3 receptor in addiction? A mini review of PET studies with [(11)C]-(+)-PHNO. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:4–8. doi: 10.1016/j.pnpbp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry. 2011;68:271–82. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, et al. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–73. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME, et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–88. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Rawson RA, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–17. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, et al. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–26. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich M, et al. Measurement of local cerebral glucose metabolism in man with 18F-2-fluoro-2-deoxy-d-glucose. Acta Neurol Scand Suppl. 1977;64:190–1. [PubMed] [Google Scholar]
- Robertson CL, et al. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2013. 2013. Dopamine D2/D3 receptor recovery with methamphetamine abstinence and exercise. Program No. 817.05. Online. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, et al. Metabolic Abnormalities in Abstinent Methamphetamine Dependent Subjects. Subst Abuse. 2010;2010:9–20. doi: 10.4137/sart.s4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61:1272–80. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry. 2009a;65:122–8. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, et al. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat. 2009b;37:292–7. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, et al. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend. 2011;113:133–8. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA, Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2014. [PubMed] [Google Scholar]
- SAMHSA, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality . Treatment Episode Data Set (TEDS): 2002-2012. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2014. [Google Scholar]
- Scholz J, et al. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Segal DS, et al. Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology (Berl) 2005;180:501–12. doi: 10.1007/s00213-005-2188-4. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Ricaurte GA. Neurotoxicity of methamphetamine and related drugs. In: Meltzer HY, editor. Psychopharmacology: A Second Generation of Progress. Raven Press; New York, NY: 1987. pp. 359–366. [Google Scholar]
- Sekine Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–14. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sekine Y, et al. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;27:453–61. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- Sekine Y, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Sekine Y, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Sekine Y, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–61. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, et al. Negative moods correlate with craving in female methamphetamine users enrolled in compulsory detoxification. Subst Abuse Treat Prev Policy. 2012;7:44. doi: 10.1186/1747-597X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla RK, Crump JL, Chrisco ES. An evolving problem: methamphetamine production and trafficking in the United States. Int J Drug Policy. 2012;23:426–35. doi: 10.1016/j.drugpo.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Simon SL, et al. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–44. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MF, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31:98–107. doi: 10.1080/08897071003641578. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stewart JL, et al. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals. Addiction. 2013;109:460–71. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sung YH, et al. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–10. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Cannon TD, Toga AW. Mapping genetic influences on human brain structure. Ann Med. 2002;34:523–36. doi: 10.1080/078538902321117733. [DOI] [PubMed] [Google Scholar]
- Thompson PM, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–8. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias MC, et al. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, et al. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify "connectivity constellation" and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–81. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC . Patterns and Trends of Amphetamine-Type Stimulants and Other Drugs: Challenges for Asia and the Pacific 2013: A Report from the Global SMART Programme. United Nations Office on Drugs and Crime; 2013. [Google Scholar]
- Upadhyay J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Exp Clin Psychopharmacol. 2008;16:484–97. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–8. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001c;158:383–9. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001d;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang GJ, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–8. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wang GJ, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011;17:918–25. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–7. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, et al. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–44. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–60. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Wilson JM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wood L, Al-Khairulla H, Lask B. Group cognitive remediation therapy for adolescents with anorexia nervosa. Clin Child Psychol Psychiatry. 2011;16:225–31. doi: 10.1177/1359104511404750. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–93. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Zorick T, et al. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105:1809–18. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, et al. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–90. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]