Abstract
Objectives:
The role of radiation therapy in the management of unresectable pancreatic cancer is controversial. One concern about concurrent chemoradiation relates to the timing of chemotherapy. In contrast to conventional radiation therapy, stereotactic body radiation therapy (SBRT) delivers high doses in a shorter duration resulting in minimal disruption in chemotherapy. Here, we report our results of patients treated with SBRT and chemotherapy for inoperable pancreatic cancer.
Materials and Methods:
Thirty-eight patients treated with SBRT and chemotherapy for locally advanced, borderline resectable, and medically inoperable pancreatic cancer at our institution from January 2008 to December 2012 were included in this retrospective analysis. Treatment was delivered in 5 fractions of 5 or 6 Gy per fraction over 5 days. Toxicities were scored using the Common Terminology Criteria for Adverse Events version 3. Survival was calculated using the Kaplan-Meier method.
Results:
The median age was 70 years (range, 45 to 90 y). Eastern Cooperative Oncology Group performance status ranged from 0 to 3. Thirty-four patients received concurrent chemotherapy. Four patients received sequential chemotherapy. Median overall survival was 14.3 months and median progression-free survival was 9.2 months from diagnosis. From radiation, overall survival and progression-free survival were 12.3 and 6.8 months, respectively. The overall local control rate was 79%. Acute toxicity was minimal. Severe late SBRT-related toxicities included 1 grade 3 gastric outlet obstruction, 1 grade 4 biliary stricture, and 1 grade 5 gastric hemorrhage.
Conclusions:
SBRT combined with chemotherapy for unresectable pancreatic cancer is convenient, feasible, and generally well tolerated. Outcomes of SBRT combined with chemotherapy compare favorably to results obtained with chemotherapy and conventional radiation therapy.
Key Words: pancreatic cancer, stereotactic body radiation therapy, radiation, gemcitabine, mFOLFOX
Pancreatic cancer carries a poor prognosis with a 5-year overall survival (OS) of <5%. Up to 70% of patients die with widespread metastatic disease and 30% die with locally destructive pancreatic cancer.1 Resection provides the only chance of cure, offering 5-year OS rates of 18% to 24%, but unfortunately only one fifth of patients present with resectable disease.2,3
Concurrent chemoradiation (CRT) is often employed in patients with localized pancreatic cancer, who are not considered candidates for upfront surgical resection. Randomized clinical trials evaluating the role of chemoradiation have shown conflicting results, with some trials showing a survival benefit with chemoradiation4–7 and others demonstrating no advantage.8,9 These trials have all used conventionally fractionated external-beam radiation therapy.
Stereotactic body radiation therapy (SBRT) uses high doses of radiation delivered over a few number of sessions to a limited target volume. The accuracy, precision, and a rapid dose fall off of SBRT minimizes doses to the adjacent normal tissues. High rates of local control have previously been reported using SBRT for the liver and lung tumors.10,11 Recent studies have demonstrated the feasibility of SBRT for the treatment of pancreatic cancer. SBRT allows for dose escalation, and single-institution studies have demonstrated excellent local control rates without excessive toxicity.12–14
The advantages of SBRT include shorter treatment duration and better integration with chemotherapy. Conventionally fractionated radiation therapy requires approximately 5 to 6 weeks of treatment and often necessitates alternations in chemotherapy. Given the high rates of distant failure in localized pancreatic cancer, fewer interruptions in chemotherapy may improve treatment outcomes. Herein, we report our experience with 5 fractions of SBRT combined with chemotherapy in patients with localized adenocarcinoma of the pancreas.
MATERIALS AND METHODS
This retrospective review includes patients with biopsy-proven, nonmetastatic pancreatic adenocarcinoma, who were unresectable, borderline resectable, medically inoperable, or refused surgery, treated with SBRT and chemotherapy at Medstar Georgetown University Hospital from January 2008 to December 2012. Unresectable and borderline resectable were defined according to the AHPBA/SSO consensus statement.15 Comorbidity scores were calculated using the Charlson Comorbidity Index (CCI).16 The chemotherapy regimen was chosen at the discretion of the treating medical oncologist. Patients were considered to have received concurrent chemotherapy if it was given within the week before SBRT. In patients receiving “concurrent” gemcitabine, SBRT was administered during the off week of chemotherapy, which was typically week 4 of the first cycle, and the second cycle was begun without delay. Similarly, in patients receiving “concurrent” mFOLFOX, 5-FU, or capecitabine, SBRT was generally given during an off week. Patients who did not receive chemotherapy within the week before SBRT were considered to have received sequential chemotherapy.
Radiation planning and delivery techniques have been detailed previously.17 All patients underwent an esophagogastroduodenoscopy with endoscopic ultrasound with placement of 3 to 4 gold fiducial markers. Seven days after fiducial placement, a treatment planning computed tomography (CT) with oral and intravenous contrast was obtained during a breath hold. The planning target volume (PTV) included the gross tumor volume (GTV) plus a 3- to 5-mm margin (excluding the bowel). The PTV was modified at the discretion of the treating physician to include the adjacent vasculature. The adjacent vasculature consisted of the superior mesenteric vessels, celiac axis, and/or para-aortic nodes at the level of the pancreatic mass when involved or immediately adjacent to the GTV. Enlarged lymph nodes were not routinely included in the GTV. Before 2011, the prescribed dose was 25 Gy in 5 fractions. This was subsequently increased to 30 Gy in 5 fractions because of our previous report demonstrating poor local control but also a low toxicity profile with 25 Gy in 5 fractions.17 The prescription isodose line encompassed at least 95% of the PTV. The stomach, duodenum, and bowel constraints were as follows: volume of organ receiving the prescribed dose, 90% prescribed dose, 80% prescribed dose, and 50% prescribed dose were <1 mL, 20%, 40%, and 90%, respectively. SBRT was delivered using the CyberKnife system (Accuray, Sunnyvale, CA) with respiratory tracking as previously described.18 Treatment was typically during 5 consecutive days.
Toxicity was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Baseline characteristics and adverse events were tabulated. Each symptom was counted once per patient at the highest grade it occurred in the acute and late setting. Biliary strictures were attributed to radiation if the patient did not have biliary obstruction before treatment or local progression at the time of stricture.
Patients had follow-up imaging by CT scan every 3 months when possible. Local control was determined by Response Evaluation Criteria In Solid Tumors (RECIST) criteria.19 Patients who did not have follow-up imaging at our institution were excluded from the RECIST analysis. Overall survival (OS) and progression-free survival (PFS) were calculated from diagnosis and from the start date of radiation to date of death or progression by the Kaplan-Meier method. PFS was determined radiographically and/or by clinical decline defined as decreasing performance status or development of ascites prohibiting therapeutic treatment in the absence of objective progression. Spearman rank correlation was used to determine if age, Eastern Cooperative Oncology Group (ECOG) performance status, CCI, baseline CA 19-9, dose, chemotherapy regimen, number of chemotherapy cycles before radiation, or time to radiation from diagnosis influenced outcomes. Log-rank was used to examine survival differences among subgroups. Fisher exact test was used to determine if local control and toxicity were affected by radiation dose or chemotherapy regimen. This study was approved by the Georgetown University Institutional Review Board.
RESULTS
Patient Characteristics
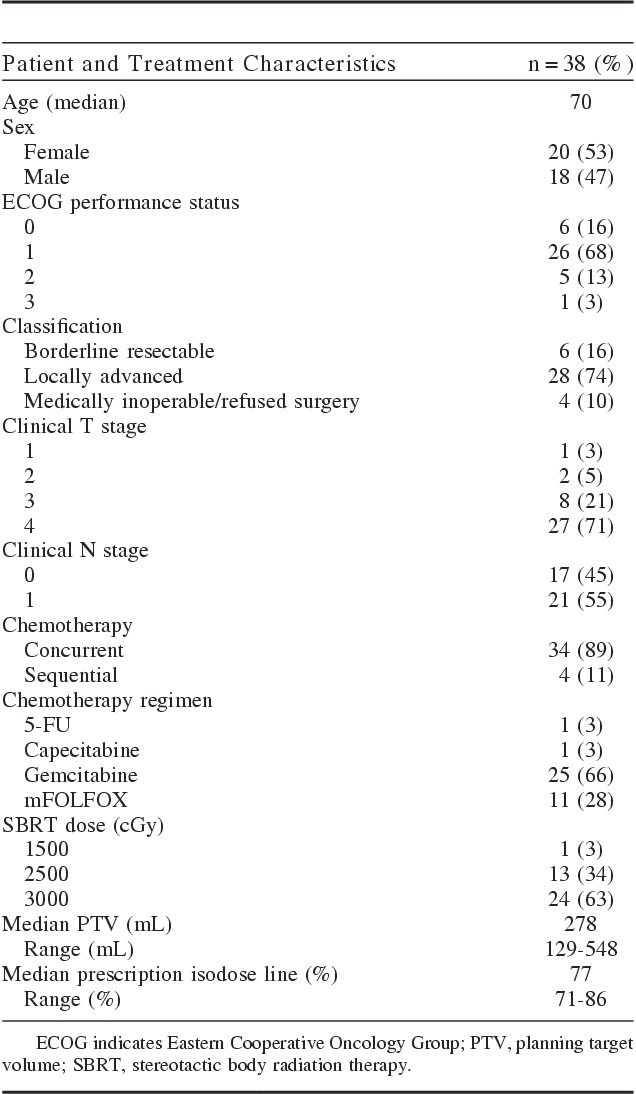
From January 1, 2008 and December 31, 2012, 38 patients were treated with SBRT and chemotherapy for unresected, nonmetastatic pancreatic adenocarcinoma. The first 10 patients were treated on a phase I study and the remainder were treated off protocol. Patient characteristics are given in Table 1. Median age was 70 years (range, 45 to 90 y). ECOG performance status ranged from 0 to 3 and the median CCI was 4 (range, 0 to 8). The majority of patients (n=28) were unresectable, 6 patients were borderline resectable, and the rest were medically inoperable or refused surgery, but had resectable disease. Twenty-seven patients had T4 primary tumors and 21 patients had nodal disease. Median baseline CA 19-9 of the cohort was 463 U/mL.
TABLE 1.
Patient and Treatment Characteristics
Treatment
Most patients received concurrent chemotherapy with gemcitabine (21 patients). Other regimens included mFOLFOX, 5-FU, and capecitabine. Four patients included in this study received sequential chemotherapy. Median time from diagnosis to radiation treatment was 1.9 months. Early on patients were treated with 25 Gy (n=13) in 5 consecutive fractions. Later, patients received 30 Gy (n=24). One patient did not complete the prescribed course of SBRT and is not included in the outcomes analysis. This patient was on study and withdrew for unknown reasons. Median PTV volume was 278 mL (range, 129 to 548 mL) and median prescription isodose line was 77% (range, 71% to 86%) (Table 1). Only 2 patients, who were both borderline resectable, in this cohort went onto surgery. One had a R0 and the other had a R1 resection. The remaining 4 borderline resectable patients did not go on to surgery for various reasons. One patient refused surgery after neoadjuvant treatment was completed, 2 patients had distant progression during neoadjuvant treatment, and finally, 1 patient had a decline in performance status due to age and medical comorbidities, which precluded surgical resection.
Local Control
Local RECIST response was evaluable for 33 patients. Only 1 patient had a partial response, but then had local progression at a later time. All other patients had stable local disease as their best response, except 1 who had progressive local disease at first radiographic follow-up. At a median radiographic follow-up time of 7.2 months from radiation, 7 patients failed locally for an overall local control rate of 79%. The 6-month local control was 82%. Local failure occurred as the first site of failure in 3 patients. Three patients simultaneously failed locally and distantly and 1 patient experienced local failure after distant failure. Higher dose, 30 Gy as opposed to 25 Gy, was almost significant for local control (P=0.07) by Fisher exact test.
Survival
Median OS was 14.3 months and median PFS was 9.2 months from diagnosis (Fig. 1A). From the start date of radiation, OS and PFS were 12.3 and 6.8 months, respectively (Fig. 1B). There was not a significant difference in survival when comparing borderline resectable patients to unresectable (P=0.08). Patients with a baseline CA 19-9 below the median before SBRT had significantly better PFS (hazard ratio=0.2800, P=0.0002) (Fig. 2). CA 19-9 remained significant when tested in rank correlation (P=0.0005). Higher dose (30 vs. 25 Gy) was almost a significant factor for PFS from radiation with a P value of 0.0637 (Fig. 3). No other factors tested were found to significantly influence survival outcomes.
FIGURE 1.
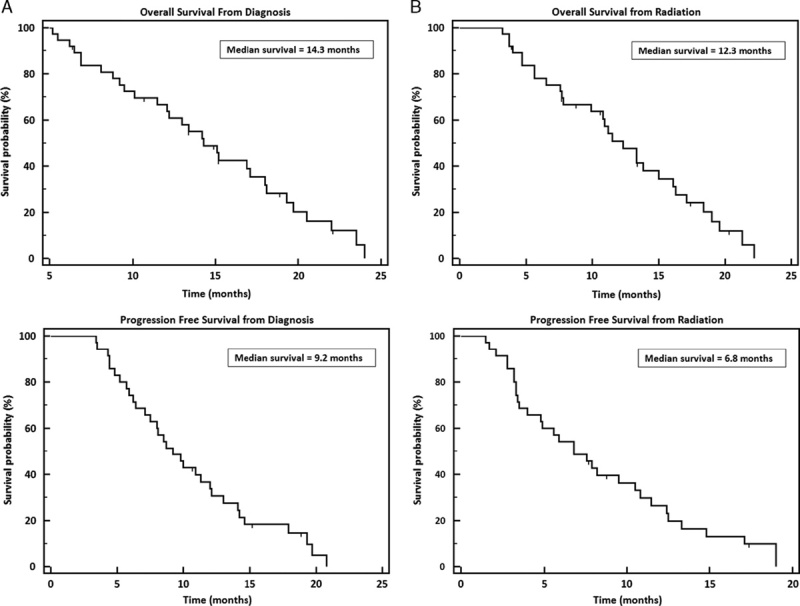
Kaplan Meier overall survival and progression-free survival curves. A, Results calculated from diagnosis. B, Results calculated from radiation.
FIGURE 2.
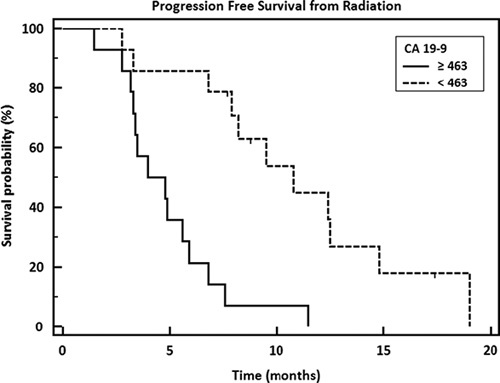
Progression-free survival from radiation by CA 19-9 level before radiation treatment. Median CA 19-9=463; hazard ratio=0.2800; 95% CI, 0.1149-0.6822; P=0.0002.
FIGURE 3.
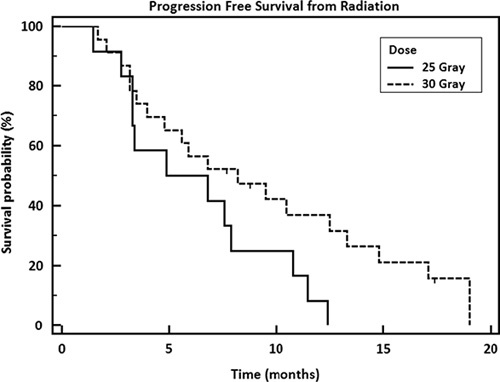
Progression-free survival from radiation by dose (P=0.0637).
Toxicity
In the acute setting, patients generally experienced grade 1 or 2 fatigue, nausea, abdominal pain, and appetite loss. Two patients experienced grade 3 toxicity due to abdominal pain. In the late setting there was grade 5 hemorrhage in a patient who had no radiographic progression on previous scans 2 months prior. This patient had a pancreatic tail lesion and no evidence of duodenal invasion before treatment. Other late events included 3 grade 2 biliary strictures and 1 grade 4 biliary stricture, where the patient presented with ascending cholangitits and was admitted to the ICU. Finally, 1 patient experienced gastric outlet obstruction and required stent placement. All late toxicities occurred in patients who received 30 Gy except one of the grade 2 biliary strictures. This was not significantly different (P=0.39).
DISCUSSION
Despite the high rates of distant metastases in pancreas cancer, local control is an important factor in the management of the disease. Local progression adversely affects quality of life and may lead to chronic pain, bleeding, and gastric obstruction. Due to the controversy regarding the role of conventionally fractionated radiation therapy for locally advanced pancreatic cancer, SBRT is an appealing alternative that offers significantly reduced treatment duration and better integration with chemotherapy.
We have previously reported the early results of a phase I study of 10 patients with locally advanced pancreatic cancer treated with SBRT (25 Gy in 5 fractions) and concurrent gemcitabine (1000 mg/m2) given the week before and after radiation for a total of 6 cycles.17 In this series, patients underwent serial endoscopies every 2 months postradiation to rigorously assess mucosal toxicities and no severe acute toxicity or late toxicities occurred; however, local control was low at 40%. In the current pooled analysis, the OS and PFS rates were not significantly different for patients treated on protocol versus those treated off protocol (P>0.05). In contrast, there was a trend toward improved PFS and local control with 30 Gy as compared with 25 Gy, which was the dose used during our earlier experience. Higher doses did not result in improved OS, which may indicate that metastatic disease progression has a greater impact on survival than local control. There was no significant difference in late toxicities with higher doses, but a detailed dose-volume histogram analysis is necessary to determine the impact of dose on complications rates.
There was a grade 5 hemorrhage that occurred 3.7 months after radiation in a patient with a large (PTV=369.2 mL) pancreatic tail lesion, who received 30 Gy in 5 fractions. On previous CT imaging 2 months prior there was no evidence of local progression of disease. The exact location of the bleeding was not determined because autopsy was declined. Review of the dose-volume histogram revealed that dose constraints were met. Although tumor progression could not be excluded as the cause the bleeding, this toxicity was considered as possibly related to treatment.
Others have reported on the incidence of gastrointestinal (GI) bleeding after radiation with concurrent chemotherapy for pancreatic cancer. In series with conventional or intensity modulated radiation therapy radiation, the rates vary from 7.5% to 20% for grade 3 or higher GI hemorrhage.20–22 In comparison, published rates of GI bleeding after SBRT range from 4% to 5%.14,23–25 To reduce rates of late GI complications we assessed the duodenal mucosal by endoscopy before SBRT and excluded patients with biopsy-proven duodenal invasion. Further study is necessary to characterize risk factors that are predictive for late radiation-related GI toxicity, including a dose-volume histogram analysis.
Other limitations of this study are because of its retrospective nature and small size. As with any retrospective analysis there is selection bias. Furthermore, most patients were not on a protocol resulting in variable record keeping. Also, there is heterogeneity among the chemotherapy timing and regimens. Although a comparison was made and no difference in outcomes was discovered between number of cycles, time to SBRT, or regimen, our numbers are small and underpowered to detect one if it exists.
The clinical outcomes in this study are similar to the previously published reports of SBRT for pancreatic cancer. A summary of selected trials is shown in Table 2. One of the earliest reports by Schellenberg et al12 administered gemcitabine for 1 cycle followed by SBRT, 25 Gy in 1 fraction with a week break before and after radiation. Patients then received adjuvant gemcitabine until progression. A median OS of 11.4 months and median PFS of 9 months were reported. The local control rate was 81% with all failures occurring after 1 year. There were 2 grade 3 or greater late toxicities which included a duodenal perforation requiring surgery and a duodenal stricture that required stent placement. In an updated report, 73 patients were treated with 25 Gy in 1 fraction, and the 6- and 12-month grade 2 or higher rate of gastrointestinal toxicity was 11% and 29%, respectively.13
TABLE 2.
Study Comparison of Stereotactic Body Radiation Therapy (SBRT) for Pancreatic Cancer
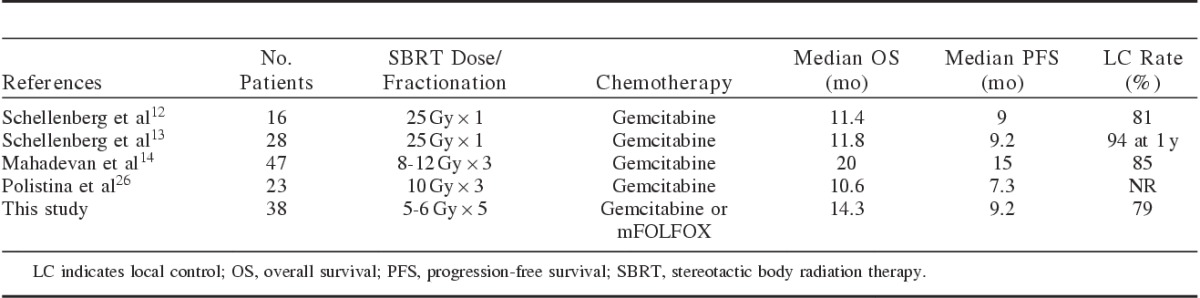
Chuong et al25 have also reported the results of chemotherapy combined with a 5-fraction SBRT course. Using an integrated boost technique, the tumor received 25 to 30 Gy and the involved vasculature received 35 to 50 Gy. There was no standard adjuvant treatment. Median OS for locally advanced patients was 15 months and median PFS was 9.7 months. Thirty-two of 57 patients with borderline resectable disease underwent resection, demonstrating the feasibility of surgery following SBRT. The median overall OS and PFS for borderline resectable patients were 16.4 and 9.8 months, respectively. For patients who did not undergo resection, local control was 81% at 1 year. Late toxicity included 3 grade 3 GI bleeds and 1 patient who required feeding tube placement for anorexia.
Conventionally fractionated radiation therapy is delivered over 5 to 6 weeks and often requires alternations in chemotherapy. In our study, 34 patients received chemotherapy within 1 week of initiating SBRT, which significantly minimized interruptions in systemic therapy. Mahadevan et al14 similarly describes excellent outcomes with SBRT applied within a week of receiving chemotherapy. Patients were treated with 24 to 36 Gy in 3 fractions given during the off week between the third and fourth cycle of gemcitabine. The median OS was 20 months and the median PFS was 15 months. Local control was 85% at 21 months. Late toxicity included 2 grade 3 GI bleeds and 1 grade 3 gastric outlet obstruction. Polistina et al26 reports somewhat inferior results with a median OS of 10.6 months and median PFS of 7.3 months using a very similar regimen; however, there was a larger break between chemotherapy and SBRT. The SBRT fractionation was slightly different with all patients receiving 30 Gy in 3 fractions. Interestingly, this study found that quality of life and pain improved after treatment in patients who responded to therapy.
These studies of SBRT, predominantly involving patients with locally advanced pancreatic cancer, demonstrate outcomes that compare favorably with contemporary conventional chemoradiation trials. A meta-analysis, which included 274 patients treated with SBRT from single-institution series demonstrated a median survival of 12.6 months.27 Recent trials of conventionally fractionated radiation therapy have been designed with induction chemotherapy followed by chemoradiation. The SCALOP trial reported a median OS of 15.2 months for patients receiving concurrent CRT with capecitabine after induction gemcitabine and capecitabine.28 Patients in this trial, who received concurrent gemcitabine, had a slightly lower median OS of 13.4 months. Median PFS was 12.0 months in the capecitabine group and 10.4 months in the gemcitabine group. The 12-month local control rate was 70% for the entire group. The GERCOR pooled analysis of patients receiving induction chemotherapy followed by CRT reports median OS of 15 months and median PFS of 10.8 months.29 Other CRT trials without induction chemotherapy report median OS rates of 10.2 to 15.5 months.30–32
The role of conventionally fractionated radiation in locally advanced pancreatic cancer has been questioned with the early results of the LAP07 trail.33 In this study, patients with locally advanced pancreatic cancer were initially randomized to gemcitabine or gemcitabine plus erlotinib. Participants with controlled disease were subsequently randomized to further chemotherapy or conventional chemoradiation with capecitabine. Median OS in the chemotherapy alone arm was 16.4 months compared with 15.2 months for the chemoradiotherapy group. No significant differences in PFS were observed: 11.8 months for chemotherapy compared with 12.5 months for chemoradiotherapy. However, only OS and PFS survival outcomes are presented thus far. The quality of life and local control results have not been reported at this time.
Although there is controversy regarding a survival benefit for conventionally fractionated radiation therapy, quality of life may be another relevant endpoint for future studies in unresectable pancreatic cancer. We have previously reported improvements in cancer-related gastrointestinal symptoms and no significant decrement in global quality with chemotherapy and SBRT.17 Several other studies have demonstrated improved quality of life after SBRT.26,34 SBRT can also be delivered with minimal acute treatment-related toxicity to elderly patients and those with a poor performance status. Kim et al35 demonstrated that SBRT is even safe in elderly patients older than 80 years and offered symptom relief in 80% of patients presenting with abdominal pain. In our study, the median age was 70 years old; 6 patients had an ECOG performance status of 2 or 3; and several patients had multiple medical comorbidities. Survival was not negatively affected by age, comorbidity, or performance status, indicating that SBRT is well tolerated in patients who may not be candidates for conventional radiation therapy.
Local control may become more relevant in LAPC as improvements in chemotherapy emerge. Eleven patients in our study received mFOLFOX with SBRT, which did not result in improved outcomes as compared with gemcitabine with SBRT. However, the small number patients in this study limits the power to detect a difference in outcomes. One new regimen, FOLFIRINOX has an objective response rate of 31.6% and improved survival rates in the metastatic setting.36 With the increased use of FOLFIRINOX in localized disease, patients with extended survival may benefit from durable local control. Future studies of SBRT should integrate FOLFIRINOX into the treatment paradigm.
In summary, SBRT combined with chemotherapy for unresectable pancreatic cancer is convenient, feasible, and generally well tolerated. Our findings support the use of 30 Gy as opposed to 25 Gy due to the improvement in local control. The outcomes of SBRT combined with chemotherapy compare favorably to the results of treatment with chemotherapy and conventional radiation therapy. SBRT for pancreatic cancer should be considered for use in randomized trials.
Footnotes
S.P.C. is an Accuray clinical consultant. The other authors declare no conflicts of interest.
REFERENCES
- 1.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–124discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731discussion 31-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Childs DS, Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. [DOI] [PubMed] [Google Scholar]
- 5.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 6.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads+5-fluorouracil), and high dose radiation+5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. [DOI] [PubMed] [Google Scholar]
- 8.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. [DOI] [PubMed] [Google Scholar]
- 10.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. [DOI] [PubMed] [Google Scholar]
- 11.Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. [DOI] [PubMed] [Google Scholar]
- 13.Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81:e615–e622. [DOI] [PubMed] [Google Scholar]
- 15.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17.Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Robertson JM, Ye H, et al. Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83:1120–1125. [DOI] [PubMed] [Google Scholar]
- 21.Kelly P, Das P, Pinnix CC, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:e143–e149. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura A, Shibuya K, Matsuo Y, et al. Analysis of dosimetric parameters associated with acute gastrointestinal toxicity and upper gastrointestinal bleeding in locally advanced pancreatic cancer patients treated with gemcitabine-based concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:369–375. [DOI] [PubMed] [Google Scholar]
- 23.Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469–e474. [DOI] [PubMed] [Google Scholar]
- 24.Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. [DOI] [PubMed] [Google Scholar]
- 25.Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–522. [DOI] [PubMed] [Google Scholar]
- 26.Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17:2092–2101. [DOI] [PubMed] [Google Scholar]
- 27.Jessica Varley TBD, Kresl JJ, Lee CL, et al. SBRT is Non-Inferior to Standard Chemoradiation for Locally Advanced, Non-Metastatic Pancreas Cancer: A Meta-Analysis of Published Data. 2013Carlsbad, CA: SRS/SBRT Scientific Meeting 2013. [Google Scholar]
- 28.Mukherjee S, Hurt C, Griffiths G, et al. SCALOP: results of a randomized phase II study of induction chemotherapy followed by gemcitabine (G) or capecitabine (Cap) based chemoradiation (CRT) in locally advanced pancreatic cancer (LANPC). J Clin Oncol. 2012;30.(suppl 34; abstr LBA146). [Google Scholar]
- 29.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. [DOI] [PubMed] [Google Scholar]
- 30.Li CP, Chao Y, Chi KH, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. [DOI] [PubMed] [Google Scholar]
- 31.Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haddock MG, Swaminathan R, Foster NR, et al. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: results of the North Central Cancer Treatment Group Phase II Study N9942. J Clin Oncol. 2007;25:2567–2572. [DOI] [PubMed] [Google Scholar]
- 33.Hammel PHF, Van Laethem J. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31 [Google Scholar]
- 34.Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. [DOI] [PubMed] [Google Scholar]
- 35.Kim CH, Ling DC, Wegner RE, et al. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. 2013;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]


