Abstract
Isolated cases in which HIV infection was claimed to have been eradicated generated renewed interest in HIV reservoirs in the brain particularly since attempts to reproduce the findings using genetically engineered stem cells and immune or myeloablation have failed. A clear understanding of the cell types in which the virus resides in the brain, the mechanism of viral persistence, restricted replication and latency and the turnover rate of the infected cells is critical for us to develop ways to control or get rid of the virus in the brain. The brain has several unique features compared to other reservoirs. There are no resident T cells in the brain; the virus resides in macrophages and astrocytes where the viral infection is non-cytopathic. The virus evolves in the brain and since the turnover rate of these cells is low, the virus has the potential to reside in these cells for several decades and possibly for the life of the individual. This review discusses the HIV reservoirs in brain, issues related to eradication of the virus from sanctuaries in brain and current challenges faced by neuroscientists in finding a cure.
Keywords: compartmentalization, astrocyte, microglia, macrophage, antiretroviral, latency
Introduction
Even though the advent of combined antiretroviral therapy (cART) has been hailed as a major success in modern medicine for its ability to prolong the lives of individuals infected with the human immunodeficiency virus (HIV), it is becoming abundantly clear that neurocognitive manifestations cannot be fully controlled with the treatment1. It was originally thought that the virus had been eliminatied from at least one patient which led to the enthusiasm that a cure for HIV infection may be possible 2-4. Subsequently it was found that HIV sequences could be found in this patient however HIV replication has not been detected, hence he is now referred to as “functional cure”. This enthusiasm has been tempered by the inability to reproduce this in other patient populations. While there may be several explanations for this failure, one likely possibility is that these strategies are ineffective in eradicating or controlling HIV reservoirs in the brain. Strategies developed to eliminate the reservoirs include the enhancement of immune responses against cells that harbor the virus and creating genetically engineered cells that would be resistant to viral infection with the hope that these cells will eventually replace the viral reservoirs 5. However, for these strategies to be successful they need to take into account the fact that the brain is an important reservoir for HIV where the virus can reside indefinitely in glial cells. While the presence of the virus in the brain was shown soon after the discovery of the virus, important questions regarding the timing of viral entry into the brain and the mechanisms involved in viral entry, persistence and turn over still remain unanswered. It is hence important to address these issues as a prerequisite to finding a cure for HIV-1.
The mechanisms by which HIV causes damage to the brain seems to be multifactorial. In general, the number of productively infected cells in the brain is small compared to the amount of neuronal damage and the neurons are not infected with the virus. Extensive studies show that viral proteins released from HIV-infected cells can cause glial cell activation which may in turn cause neuronal injury. Activated macrophages and astrocytes produce factors with neurotoxic potential like free radicals, peroxynitrite, tumor necrosis factor-alpha and arachidonic acid metabolites 6 that appear to ultimately lead to glutamate-mediated toxicity or direct neuronal injury via oxidative stress 7. Alternatively, HIV proteins may also directly interact with neurons to cause excitotoxicity or travel along neuronal pathways to cause injury at distant sites 8. Several viral proteins have been implicated as having neurotoxic effects and in particular the effects of gp120 and Tat have been characterized well in vitro and in limited in vivo studies 9-13. Co-morbidities are often associated with HIV infection such as hyperlipidemia, vasculopathies, drug, alcohol and nicotine abuse and even some of the antiretroviral drugs themselves may be neurotoxic. Host genetic factors have also been implicated neuronal vulnerability to the virus and other neurotoxic agents.
cART fails to completely control progression of HIV-1 associated neurocognitive disorders or viral pathogenesis in brain
Nearly one third of HIV-infected individuals develop neurocognitive deficits despite adequate cART and excellent virological control in blood 1. These range of neurocognitive deficits are collectively referred to as HIV-1 associated neurocognitive disorders (HAND). Although, cART is successful in most cases in rapidly reducing HIV RNA to <50 copies/ml, the virus typically rebounds back quickly, sometimes within two weeks of cessation of therapy 14. Highly sensitive assays capable of detecting 1 copy of HIV RNA /ml have revealed that around 80% of patients continue to have low level viremia of around 3-5 copies/ml despite several years of cART 15,16. Such studies have strengthened the belief that latent infections persist in certain cells within the host and that latent viral genomes can be reactivated to produce infectious viral particles 17-19 that perhaps are responsible for the rebound of the virus. Multiple mechanisms have been proposed as to how low level viral replication may lead neurocognitive disorders. These include neurotoxicity and glal cell activation by viral proteins such as gp120 and Tat. In particular antiretroviral drugs do not impact the production of Tat protein once the proviral DNA has been formed and the viral reservoir has been established20, 21. Tat can also travel along neuronal pathways and thus have far reaching effects from the site of production (reviewed in8).
The failure to eradicate HIV from its reservoirs in host tissues is one of the major hurdles towards curing HIV infection and cells in the brain constitute one such reservoir. cART therapy is successful in controlling HIV-1 replication in active CD4+ immune cells but fails to target infected quiescent cells. Microglia, perivascular and meningeal macrophages, astrocytes, and neural stem cells are sites of viral infection in human brain 22-27. It is now clear that a cure for HIV infection is not possible unless safe heavens of the virus are purged and total eradication of HIV from the host is achieved. Recognition of this stumbling block has prompted several investigators to focus their research efforts on the paramount issue of eradication of HIV from its reservoirs.
HIV infection of the brain
HIV may traffic into brain via blood monocytes termed the Trojan horse phenomenon early in the course of infection long before symptoms of AIDS appear 28. In fact, the virus can be detected in the CSF soon after a primary infection 29. Phylogenetic studies suggest that the virus enters the brain early in the course of infection and subsequent viral entry may be inhibited by establishment of an immune barrier 30. Virus may enter the brain again in the later stages of infection when there is a general immune failure 31. The ability of the virus to replicate depends on the cells type and its state of activation. If complete viral particles are formed by the cell, it is termed, productive infection. In the context of brain infection, if p24 immunostaining is present, it has been interpreted to mean “productive infection”. In contrast, “latent infection” means the presence of proviral DNA but the absence of any HIV proteins being formed. It is uncertain if this form of true latency exists in HIV-infected brain tissues since detection of such cells is technically challenging. The term “restricted infection” has been used to describe the production of some viral proteins in the absence of production of infectious viral particles. HIV infected astrocytes may immunostain for nef protein but not p24 and hence this term is most often used to describe these cells.
Once inside the brain parenchyma, it resides in perivascular macrophages and microglial cells that provide the site of productive replication and evolution for HIV. Importantly however, the virus appears to not enter the brain in all individuals. In a small study of 13 patients it was found that nearly 50% of patients do not have HIV in the brain as detected by PCR at the time of death 32. While larger and more rigorous studies are needed to validate these findings, it would be critically important to try and identify these patients as they may have the best chance for curative therapies.
Eradication of HIV from human brain is challenging due to the selectively permeable blood brain barrier (BBB) that interferes with bioavailability of cART in brain. Poor penetration of most of cART drugs into brain is attributed to an highly efficient drug efflux systems in the brain 33, 34. However, a recent study showed that further intensification of antiviral therapy with raltegravir, an integrase inhibitor which achieves high CSF concentrations 35, did not reduce HIV RNA levels in CSF or intrathecal immunoactivation 36.
Replication and non-replicating viral DNA in brain
Following infection, HIV RNA gets reverse transcribed into a strand of DNA that can either get integrated in to the chromosomal DNA or it may reside episomally either as a circular DNA or a linear strand of DNA. This differentiation is important since integrated viral DNA is capable of producing viral products and can be silenced by epigenetic changes whereas episomal DNA may either be non-functional or the cell may eventually release the DNA extracellularly. In a study from the pre-cART era, the amount of unintegrated DNA in the brain was found to be 6-81 fold compared to integrated DNA 37. The unintegrated DNA was largely in a linear form and only 01-1% of the viral DNA was circular. Importantly, the levels of unintegrated DNA did not correlate with the amount of viral antigen in the brain suggesting that the unintegrated DNA was either latent or dysfunctional. In contrast, in T cells, HIV is predominantly in an integrated state however central memory T cells may harbor the virus in a non-induced proviral state. Since this cannot be easily induced replication competent, it poses a significant barrier to eradication of HIV38.
DNA has been shown to modulate resting T cell activity 39. The high levels of unintegrated DNA may be suggestive of reinfection or superinfection of cells potentially making the brain an important reservoir for the virus. Another study shows that the presence of unintegrated HIV DNA and detection of HIV proteins in the brain is associated with dementia 40. Importantly, the viral load in the brain on patients on prolonged cART may be very low 41.
Evolution of HIV sequences in brain
Since the brain is a relatively immune privileged site and is devoid of resident lymphoid cells, the selective pressure on the virus is different compared to other lymphoid organs. The virus evolves in the brain over time and thus acquires unique genetic and functional features. For example, the envelope protein evolves to become more macrophage tropic 42 with unique brain specific mutations43. The macrophage tropic strains of HIV in the brain are functionally different compared to macrophage tropic strains derived from the immune system 44. A database (http://www.HIVBrainSeqDB.org) of envelope sequences has been created which contains 2517 envelope sequences from 90 patients. 1272 sequences are from brain; the remaining are from non-brain tissues 45. Similarly, the Tat protein of HIV also evolves in the brain and while it maintains its HIV activation properties 46 it may vary in its neurotoxic potential 47. The nef gene acquires unique sequences in patients with dementia compared to those without 48. Normalized nonsynonymous substitutions in the nef gene are more frequent in brain compared to lymphoid tissue49. The brain-specific nonsynonymous substitutions are in regions of functional importance resulting in efficient replication in macrophages49. Viral sequences derived from brain macrophages and astrocytes show compartmentalization suggesting that cell specific evolution occurs in the brain 50. Viral sequencing shows that the meninges harbor virus from both the brain and peripheral tissues suggesting that HIV is capable of migrating out of the brain, and the meninges are the most likely primary transport tissue 51. The effect of antiretroviral therapy on evolution of HIV in the brain is not well understood. Poor penetration of antiretrovirals across the blood brain barrier might result in low frequency of antiretroviral resistant sequences in the brain and hence cART might drive the compartmentalization of HIV in the brain. cART has been shown to induce a switch HIV co-receptor usage from CRR5 to CXCR4 which appears later in the CNS compartment compared to the periphery 52. Importantly, even in patients on antiretroviral drugs analysis maximal viral evolution occurs within brain tissues of individuals with dementia compared to without dementia 27.
Regional compartmentalization of HIV in brain
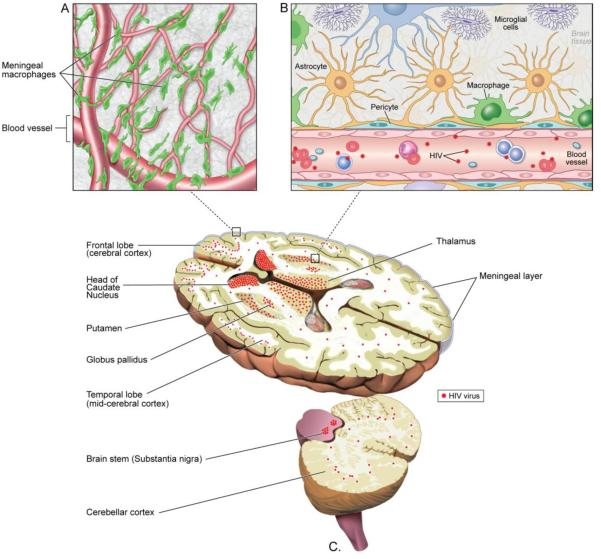
The virus can be found in any part of the brain. However, maximal viral loads have been found in the basal ganglia, in particular in the caudate and globus pallidus, as well as in the medial temporal lobes, the hippocampus, and the frontal lobes 53, 54 The reason for this predilection is not clear, though several viral encephalitides that are spread hematogenously also target these areas preferentially 55Recent studies suggest that there is a substantial viral load in the meninges as well where there is a rich collection of macrophages 51. These meningeal macrophages also get infected with HIV and this may be a yet important reservoir for the virus. It is also possible that some of the infected macrophages might traverse the perivascular spaces from the meningeal blood vessels into the brain parenchyma where a network of macrophages have been shown to communicate between the two spaces56. The infected cells may be present in foci called microglial nodules and in some patients the infected macrophages may fuse to form multinucleated giant cells.
Perivascular and meningeal macrophages have been shown to be sites of active viral replication in human brain. Most studies do not distinguish between macrophages and microglia in the brain and many of the commonly used cellular makers stain both cell types and infection of parenchymal cells has been interpreted as microglial cell infection57. In an SIV model using a panel of makers to differentiate subtypes of macrophages and microglia, it was claimed that the parenchymal microglia do not get infected 58. However, using similar markers it was found that about two thirds of productively infected cells in patients with HIV encephalitis were parenchymal microglia. 59. Unfortunately, cART is not as effective in controlling HIV replication in microglial cells 59.
In addition to microglia, astrocytes, the most abundant cells in brain, are sites for HIV latent or persistent infection. The evidence for harboring of HIV in astrocytes comes from detection of viral DNA and RNA in post mortem brain tissues from AIDS patients 60-62 . Astrocytes in vivo have been demonstrated to contain integrated HIV 26. Astrocytes may be efficiently infected by cell to cell contact with HIV-infected lymphocytes 63. Studies using laser capture dissection microscopy with amplification of viral genes have demonstrated the frequency of astrocyte infection to be up to 20%. The frequency of infection correlates with both the severity of HIV encephalitis and proximity to perivascular macrophages and multinucleated giant cells64. HIV proteins can be detected in astrocytes with over-expression of the Nef protein 25, 65. Astrocytes produce new viral particles when challenged with inflammatory cytokines 66. It remains unknown when in the course of HIV infection do these cells get infected. The timing of astrocyte infection may be critical, since these cells are considered to be long lived cells. Studies looking at astrocyte turnover are limited and a single murine study suggests a rate of 0.4% per day in the corpus callosum suggesting that it would take nearly half the life span of a mouse to turn over all the astrocytes 67. However it is not known if the turnover of perivascular astrocytes maybe different compared to parenchymal astrocytes and if the rate may be altered in pathological states.
Turnover of HIV reservoirs in brain
Once the virus has entered the brain and has infected resident brain cells, the turnover rate of these cells would be critical to determine if they can be replaced by uninfected cells if genetic approaches to making HIV resistant cells are to be employed. Animal studies suggest that the turnover rate of perivascular macrophages may be quite rapid and within 14 weeks all perivascular cells get replaced 68 How this timeframe translates from mice to humans is not known. Further, in sites of neuronal injury, these macrophages migrate from the blood to the site and assume microglial markers and morphology within three days suggesting that the turnover rate of microglia may also be high in case of neuronal injury 69. This is important because HIV-infected patients may have ongoing neuronal injury and in the case of patients who are additionally treated with radiation and chemotherapy 3 there may be an acceleration of microglial turnover.
Strategies for eradication of viral reservoirs
Several strategies are currently being pursued (Table). The variety of approaches are rapidly expanding and hence it is prudent to take a closer look at them to see what impact they might have on the brain and HIV reservoirs in brain cells. A popular approach is to activate viral replication in the reservoirs in the presence of cART to prevent the virus from spreading to other cells. Viral proteins produced by these latent reservoirs will be recognized by the immune cells and reservoir would then be eliminated. Broadly, these include drugs that could modulate epigenetic changes such as histone deacetylase inhibitors or immune activation therapies 70. A concern with this strategy might be that if the reservoir in the brain has been established then activation and production of viral products could lead to an infiltration of cytotoxic T cells. Infiltration of activated lymphocytes in the brain could be injurious to neurons 71 leading to a devastating encephalitis termed, CNS-immune reconstitution syndrome72 . Further, similar strategies have failed to eliminate other persistent CNS viral infections such as JC virus and herpes viruses 72. However, there may be a window of opportunity for viral eradication via this strategy before it enters the brain. Immune ablation is also being considered for elimination the reservoirs however, due to the associated toxicity and immune suppression it has been used only in HIV-infected individuals who have developed leukemia or lymphoma. This approach targets dividing cells hence brain cells that are terminally differentiated may not be eliminated. Yet another approach being used is to engineer HIV resistant stem cells by creating mutations in the chemokine receptor CCR5 73. However since HIV can enter cells using the CXCR4 chemokine receptor this mutation alone may not be sufficient and creating additional blocks for viral replication post-viral entry should be considered. Whatever strategy is used for either achieving a sterilizing or functional cure, it is critical that close attention be given to similarly controlling the CNS viral reservoir or else there would be a theoretical risk for re-seeding periphery with the virus from the CNS. Experimental studies to evaluate this risk are critically needed.
Imaging HIV reservoirs
If viral reservoirs are to be eliminated, then they need to be monitored in real time. Since tissue reservoirs including those in the brain cannot be sampled, imaging techniques will be critical. Although currently, there are no such available techniques, several methods are being considered. This includes developing ligands for positron emission tomography using molecular probes, antibodies to the envelope protein, gp120 or antiviral drugs. However, the ability of the probes and antibodies to cross the blood brain barrier is limited and if viral loads are low such techniques may not be sensitive enough, hence further research along these lines is necessary.
Summary and Future Directions
In summary, while substantial research is still necessary to understand how viral reservoirs in the brain can be manipulated, eradication of HIV may be possible under certain circumstances. In particular we need to identify when the virus enters the brain and if there are certain individuals in whom the virus does not enter the brain at all. Further, if patients are going to be treated with agents that will lead to viral activation, some measure of CNS reservoirs are necessary. This may require the development of techniques for imaging the reservoirs and patients should be closely monitored for any signs of CNS inflammation so that appropriate treatment can be initiated. Genetically modified cells that are resistant to HIV infection might be useful, if the turnover of the HIV-infected cells is brain is faster that the resident parenchymal cells. Alternatively, in patients where CNS reservoirs have been established the possibility of developing a functional cure should be considered. The immune system may be capable of maintaining the virus in a latent state. Certainly, the immune system keeps in check multiple viral and other microbial organisms that manifest themselves as opportunistic infections only when the immune system fails. This requires sustained immune responses against the organism. However, in HIV-infected individuals, in whom viral replication is controlled with cART, the immune responses wean 74, hence periodic immunization may be necessary to maintain a sustained immune response against the virus 75.
Sites of HIV Reservoir in the Brain: HIV in compartmentalized in the subcortical white matter and the midline structures of the brain. This includes the frontal and temporal lobe, the basal ganglia and the brain stem. The posterior parts of the brain are relatively spared. Within these regions HIV infects the macrophages/microglia and astrocytes most commonly located in the perivascular regions where they constitute the blood brain barrier. A previously under appreciated site of viral infection in the brain is the subarachnoid space where the meningeal macrophages form a rich plexus of cells surrounding the meningeal blood vessels. Viral sequencing suggests that the meninges harbor both brain and peripheral blood derived viruses, suggesting that this may be a route for viral transmission from brain to blood. Alternatively, the virus can spread along the meningeal macrophages to the brain parenchyma
Table.
Approaches to curing HIV infection
| Strategy | Relevance for Brain Reservoirs |
|
|---|---|---|
| Sterilizing Cure | ||
| Create HIV resistant cells |
Need to create multiple blocks to HIV replication |
|
| HIV excision using gene editing tools |
Delivery to all infected cells in brain would be needed |
|
| Blocking HIV entry into brain |
Window of opportunity following primary infection needs to be defined |
|
| Functional Cure | ||
| Immune ablation | HIV resides in terminally differentiated cells in brain, hence unlikely to be effective |
|
| Genetic silencing | Would need to take into account viral evolution in brain |
|
| Enhancement of cytotoxic immune responses in presence of cART |
Poses risk of immune reconstitution inflammatory syndrome in brain |
Acknowledgement
Dr. Dorian McGavern provided a photomicrograph of the meningeal macrophages. Supported by intramural funds from NINDS, NIH. The author declares no conflict of interest.
Bibliography
- 1.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutter G, Ganepola S. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. TheScientificWorldJournal. 2011;11:1068–76. doi: 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 4.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine. 2009;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5(6):788–97. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 6.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26(3):981–90. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. The Journal of infectious diseases. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 9.Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, et al. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10(3):141–51. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177(3):1397–410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74(2):255–65. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- 12.Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879(1-2):42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 13.Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47(2):186–94. [PubMed] [Google Scholar]
- 14.Davey RT, Jr., Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96(26):15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS pathogens. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 18.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 19.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 20.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(33):13588–93. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infectious disorders drug targets. 2012;12(1):81–6. doi: 10.2174/187152612798994939. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, et al. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13(3):274–83. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Current HIV research. 2006;4(3):319–27. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78(14):7319–28. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44(3):481–7. doi: 10.1212/wnl.44.3_part_1.481. Pt 1. [DOI] [PubMed] [Google Scholar]
- 26.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. Journal of neurovirology. 2006;12(2):146–52. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 27.Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, et al. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. Journal of neurovirology. 2010;16(3):230–41. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews Immunology. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 29.Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. Journal of virology. 2010;84(5):2395–407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–62. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 31.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14(4):318–26. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Galligan DC, Lamers SL, Yu S, Shagrun L, Salemi M, et al. High level HIV-1 DNA concentrations in brain tissues differentiate patients with post-HAART AIDS dementia complex or cardiovascular disease from those with AIDS. Science in China Series C, Life sciences / Chinese Academy of Sciences. 2009;52(7):651–6. doi: 10.1007/s11427-009-0085-5. [DOI] [PubMed] [Google Scholar]
- 33.Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4(4):432–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Sawchuk RJ, Yang Z. Investigation of distribution, transport and uptake of anti-HIV drugs to the central nervous system. Advanced drug delivery reviews. 1999;39(1-3):5–31. doi: 10.1016/s0169-409x(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 35.Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrobial agents and chemotherapy. 2010;54(12):5156–60. doi: 10.1128/AAC.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. The Journal of infectious diseases. 2011;204(12):1936–45. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang S, Koyanagi Y, Miles S, Wiley C, Vinters HV, Chen IS. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990;343(6253):85–9. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- 38.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–51. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293(5534):1503–6. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- 40.Teo I, Veryard C, Barnes H, An SF, Jones M, Lantos PL, et al. Circular forms of unintegrated human immunodeficiency virus type 1 DNA and high levels of viral protein expression: association with dementia and multinucleated giant cells in the brains of patients with AIDS. J Virol. 1997;71(4):2928–33. doi: 10.1128/jvi.71.4.2928-2933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. Aids. 2009;23(11):1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray LR, Gabuzda D, Cowley D, Ellett A, Chiavaroli L, Wesselingh SL, et al. CD4 and MHC class 1 down-modulation activities of nef alleles from brain- and lymphoid tissue-derived primary HIV-1 isolates. Journal of neurovirology. 2011;17(1):82–91. doi: 10.1007/s13365-010-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunfee RL, Thomas ER, Gorry PR, Wang J, Taylor J, Kunstman K, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15160–5. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Perez MP, O'Connell O, Lin R, Sullivan WM, Bell J, Simmonds P, et al. Independent evolution of macrophage-tropism and increased charge between HIV-1 R5 envelopes present in brain and immune tissue. Retrovirology. 2012;9:20. doi: 10.1186/1742-4690-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holman AG, Mefford ME, O'Connor N, Gabuzda D. HIVBrainSeqDB: a database of annotated HIV envelope sequences from brain and other anatomical sites. AIDS research and therapy. 2010;7:43. doi: 10.1186/1742-6405-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowley D, Gray LR, Wesselingh SL, Gorry PR, Churchill MJ. Genetic and functional heterogeneity of CNS-derived tat alleles from patients with HIV-associated dementia. Journal of neurovirology. 2011;17(1):70–81. doi: 10.1007/s13365-010-0002-5. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, et al. NMDA receptor activation by HIV-Tat protein is clade dependent. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(47):12190–8. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PloS one. 2011;6(2):e16659. doi: 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivieri KC, Agopian KA, Mukerji J, Gabuzda D. Evidence for adaptive evolution at the divergence between lymphoid and brain HIV-1 nef genes. AIDS research and human retroviruses. 2010;26(4):495–500. doi: 10.1089/aid.2009.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, et al. Astrocyte specific viral strains in HIV dementia. Annals of neurology. 2004;56(6):873–7. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- 51.Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11(1):31–7. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vissers M, Stelma FF, Koopmans PP. Could differential virological characteristics account for ongoing viral replication and insidious damage of the brain during HIV 1 infection of the central nervous system? Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010;49(4):231–8. doi: 10.1016/j.jcv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, et al. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8(2):277–84. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, et al. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(3):146–52. doi: 10.1097/00042560-199711010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RK, Soni N, Kumar S, Khandelwal N. Imaging of central nervous system viral diseases. J Magn Reson Imaging. 2012;35(3):477–91. doi: 10.1002/jmri.22830. [DOI] [PubMed] [Google Scholar]
- 56.Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. The Journal of comparative neurology. 2002;451(2):170–88. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 57.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus research. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. The Journal of experimental medicine. 2001;193(8):905–15. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12(4):442–55. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, et al. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13(2):144–54. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Annals of neurology. 1996;39(6):705–11. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. Journal of neurovirology. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- 63.Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. Journal of neuropathology and experimental neurology. 1995;54(3):320–30. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Annals of neurology. 2009;66(2):253–8. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 65.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. Aids. 1995;9(9):1001–8. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. Journal of virology. 1991;65(11):6094–100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271(4):589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- 68.Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14(10):1651–8. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 69.Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simburger E, Naftolin F, et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. Faseb J. 2005;19(6):647–9. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- 70.Cohen J. Understanding HIV latency to undo it. Science. 2011;332(6031):786. doi: 10.1126/science.332.6031.786. [DOI] [PubMed] [Google Scholar]
- 71.Wang T, Lee MH, Choi E, Pardo-Villamizar CA, Lee SB, Yang IH, et al. Granzyme B-induced neurotoxicity is mediated via activation of PAR-1 receptor and Kv1.3 channel. PloS one. 2012;7(8):e43950. doi: 10.1371/journal.pone.0043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Current opinion in neurology. 2011;24(3):284–90. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- 73.Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nature biotechnology. 2010;28(8):839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stranford SA, Ong JC, Martinez-Marino B, Busch M, Hecht FM, Kahn J, et al. Reduction in CD8+ cell noncytotoxic anti-HIV activity in individuals receiving highly active antiretroviral therapy during primary infection. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):597–602. doi: 10.1073/pnas.021550598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]