Abstract
Background
Anesthetic isoflurane has been reported to induce caspase-3 activation. The underlying mechanism(s) and targeted intervention(s), however, remain largely to be determined. Vitamin C (VitC) inhibits oxidative stress and apoptosis. We therefore employed VitC to further determine the up-stream mechanisms and the down-stream consequences of the isoflurane-induced caspase-3 activation.
Methods
H4 human neuroglioma cells over expressed human amyloid precursor protein (H4-APP cells) and rat neuroblastoma cells were treated either with: 1) 2% isoflurane or 2) the control condition, plus saline or 400 mM VitC for three or six hours. Western blot analysis and fluorescence assay was utilized at the end of the experiments to determine caspase-3 activation, levels of reactive oxygen species and ATP, and mitochondrial function. The interaction of isoflurane (1.4% for two hours) and VitC (80 mg/kg) on cognitive function in mice was also assessed in the Fear Conditioning System.
Results
Here we show for the first time that the VitC treatment attenuated the isoflurane-induced caspase-3 activation. Moreover, VitC mitigated the isoflurane-induced increase in the levels of reactive oxygen species, opening of mitochondrial permeability transition pore, reduction in mitochondrial membrane potential, and the reduction in ATP levels in the cells. Finally, VitC ameliorated the isoflurane-induced cognitive impairment in the mice.
Conclusion
Pending confirmation from future studies, these results suggested that VitC attenuated the isoflurane-induced caspase-3 activation and cognitive impairment by inhibiting the isoflurane-induced oxidative stress, mitochondrial dysfunction, and reduction in ATP levels. These findings would promote further research into the underlying mechanisms and targeted interventions of anesthesia neurotoxicity.
Introduction
Alzheimer’s disease (AD) is one of the greatest public health problems in the United States and in the world, and its impact will only increase with demographic changes anticipated in the coming decades. Anesthesia may potentially facilitate the development of AD dementia [1–12]. Specifically, in a retrospective study, Chen et al. investigated one million patients and found that previous exposure to surgery and anesthesia could contribute to the development of AD [11]. In a different study, Liu et al. reported that surgery under sevoflurane anesthesia could facilitate the progression of cognitive function decline in patients who already had mild cognitive impairment [13]. In another retrospective study, Chen et al. included 24,901 patients in the anesthesia/surgery group and 110,972 participants in the control group, and found that the hazard ratio of anesthesia and surgery as risk factors for dementia was 1.99 [12]. However, other findings have also suggested the opposite that there is no association between anesthesia and dementia [14–17]. Further clinical investigation is required in order to determine whether anesthesia and surgery can contribute to AD neuropathogenesis and progression.
Despite this need for greater clinical investigations, it is a fact that conducting more clinical studies, and analyzing the results of these studies, would necessitate a longer time frame. Therefore, it is equally important to investigate the potential neurotoxicity of anesthesia in animals. Understanding anesthesia’s potential role in promoting AD neuropathogenesis in animals, as well as learning the underlying mechanisms and targeted interventions, of anesthesia in vitro and in animals, may lead to findings with translational potential for future human studies.
The commonly used inhalation anesthetic, isoflurane, has been shown to induce caspase-3 activation, oligomerization and accumulation of β-amyloid protein (Aβ), and learning and memory impairment [18–27]. The up-stream mechanisms, down-stream consequences and targeted interventions of this isoflurane-induced caspase activation, however, remain largely to be determined.
We have previously shown that isoflurane can increase the levels of reactive oxygen species (ROS), induce mitochondrial dysfunction [e.g., opening of mitochondrial permeability transition pores (mPTP) and reduction in mitochondrial membrane potential (MMP)], and decrease adenosine triphosphate (ATP) levels, which may subsequently cause caspase-3 activation, leading to learning and memory impairment [27,28]. Vitamin C (VitC) has been shown to inhibit oxidative stress and apoptosis [29–36]. We therefore assessed in our studies whether or not VitC could attenuate the isoflurane-induced capase-3 activation through a ROS-, mitochondria- and ATP-associated mechanism in cultured cells, and whether VitC could also ameliorate the associated isoflurane-induced cognitive impairment seen in rodents. The primary hypothesis in the current studies was that VitC would attenuate the isoflurane-induced caspase-3 activation. The secondary hypothesis was that VitC would attenuate the isoflurane-induced ROS accumulation, mitochondrial dysfunction and ATP reduction (potential up-stream mechanisms of the isoflurane-induced caspase-3 activation), as well as would ameliorate the isoflurane-induced cognitive impairment (potential down-stream consequence of the isoflurane-induced caspase-3 activation). The objectives of the current studies were to seek targeted intervention into the neurotoxicity of isoflurane and its related neurobehavioral deficits, as well as to further demonstrate the up-stream mechanisms and the down-stream consequences of isoflurane-induced caspase-3 activation. We performed the studies both in cultured cells and in mice.
Methods
Cell Line
We employed H4 human neuroglioma cells, stably transfected to express full-length human amyloid precursor protein (H4-APP cells), in the studies to determine whether VitC could attenuate isoflurane-induced cellular neurotoxicity. We chose this particular cell line (H4-APP cells), because we have already shown the isoflurane-induced neurotoxicity in the H4-APP cells [27,28,22,20]. The cells were cultured in DMEM (high glucose) containing 9% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 ug/ml streptomycin and 2 mM L-glutamine, and were supplemented with 220 μg/ml G418. H4-APP cells may not be suitable for flowcytometry studies owing to their potential auto-fluorescence [27]. Therefore, we also used rat neuroblastoma cells, ((B104 cells), generous gifts from Dr. Dora Kovacs and Dr. Doo Kim of the Massachusetts General Hospital and Harvard Medical School), in the mitochondrial permeability transition pore (mPTP) experiments, as demonstrated in our previous studies [27]. The B104 cells were cultured in DMEM containing 9% heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine.
Treatments for Cells
Isoflurane was delivered from an anesthesia machine to a sealed plastic box in a 37°C incubator containing 6-well plates or 96-well plates; the 6-well plates were seeded with one million cells in 1.5 ml cell culture media per well, and the 96-well plates were seeded with fifty thousand cells in 200 ul cell culture media per well, as described in our previous studies [27]. A Datex infrared gas analyzer (Puritan-Bennett, Tewksbury, MA) was used to continuously monitor the delivered concentrations of carbon dioxide, oxygen and isoflurane. The cells were treated with 2% isoflurane, plus 21% O2 and 5% CO2, for a duration of six hours for the studies of caspase-3 activation and reactive oxygen species (ROS), and for a duration of three hours for the studies of mPTP opening, mitochondrial membrane potential (MMP) and adenosine triphosphate (ATP,) as described by Xie et al. [22] and Zhang et al. [37]. These treatments of isoflurane were chosen according to findings from our previous studies, which revealed that treatment with 2% isoflurane for six hours had induced caspase-3 activation and ROS accumulation, and treatment with 2% isoflurane for three hours had induced mPTP opening, MMP reduction and decrease in ATP levels [22,37]. VitC (400 uM) [38] was given to the cells 30 minutes before the isoflurane treatments.
Cell Lysis and Protein Quantification
The pellets of the harvested cells were detergent-extracted on ice using an immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40), plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A), as described in our previous studies [39]. The lysates were collected, centrifuged at 13,000 rpm for fifteen minutes, and quantified for total protein amount by a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Western Blot Analyses
The harvested cells were subjected to Western blot analyses, as described in our previous studies [28,27,39]. Specifically, a caspase-3 antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA) was used to recognize full-length caspase-3 (35 – 40 kDa) and caspase-3 fragments (17 – 20 kDa) resulting from cleavage at aspartate position 175. Antibody anti-β-Actin (1:10,000, Sigma, St. Louis, MO) was used to detect β-Actin (42 kDa). Each band in the Western blot represented an independent experiment. The results were averaged from six independent experiments. The intensity of signals was analyzed using the National Institute of Health image program. We quantified the Western blots in two steps. First, we used β-Actin levels to normalize protein levels (e.g., determining the ratio of caspase-3 fragment to β-Actin amount) and to control for loading differences in the total protein amount. Secondly, we presented protein level changes in cells exposed to anesthesia as a percentage of those in the control group. 100% of the protein level changes refer to the control levels, for the purpose of comparison to the experimental conditions.
Reactive Oxygen Species (ROS) Measurement
An OxiSelect Intracellular ROS Assay Kit and an OxiSelect In Vitro ROS/RNS Assay Kit (Cell Biolabs, San Diego, CA) were used to measure the amount of ROS in cells, according to protocol provided by the company and our previous studies [28,27,39]. In short, cultured H4-APP cells were placed in a clear 96-well cell culture plate in the incubator overnight. We then added the 2′,7′-dichlorfluorescein-diacetate (DCFH-DA) media solution to the cells. The DCFH-DA loaded H4-APP cells were subsequently exposed to 2% isoflurane for six hours. These treated cells were first lysed by adding 100 μL of cell lysis buffer, and then mixed thoroughly and incubated for five minutes at room temperature. 150 μL of the mixture was transferred to each well of a 96-well plate to be used for fluorescence measurement. Finally, the fluorescence was read with a fluorometric plate reader at 480 nm/530 nm.
Flow Cytometric Analysis of mPTP Opening
B104 cells were treated with 2% isoflurane for three hours. The opening of mPTP was determined by flowcytometry, using the MitoProbe™ Transition Pore Assay Kit (Invitrogen, Carlsbad, CA), as described in our previous studies [27]. Specifically, in normal conditions, the non-fluorescent acetoxymethyl ester (AM) of calcein dye (calcein AM) and cobalt can enter the cells. The acetoxymethyl ester (AM) groups are cleaved from calcein via non-specific esterase, and calcein can then show fluorescent signals in both the cytosol and mitochondria. Cobalt can quench the cytosolic calcein signal. However, cobalt cannot enter healthy mitochondria freely, and therefore cannot quench the mitochondrial calcein signal. When the opening of mPTP occurs, cobalt enters through the pore and subsequently quenches the mitochondrial calcein signal. Flowcytometry was used to detect the amount of cells that exhibit quenched calcein signals inside the mitochondria. The location of the curves indicates the amount of cells with quenched calcein signals, which suggests the opening of mPTP. Dead cells and debris were excluded from analysis by gates set on forward and side angle light scatter. We used inomycin as a positive control in the mPTP assay, as described our previous studies [27].
Determination of Mitochondrial Membrane Potential (MMP)
MMP level was calculated by JC-1 fluorescence ratio detection. Specifically, H4-APP cells were placed into 96-well plates with densities of 50,000 per well into the incubator overnight. The cells were washed with 100 μl DPBS twice before isoflurane treatment. At the end of the treatments, cells were incubated with JC-1 reagents at 37°C for fifteen minutes and washed twice with HBSS. Finally, fluorescence was read with a fluorometric plate reader for red fluorescence (excitation 590 nm, emission 610 nm) and with a fluorescence plate reader for green fluorescence (excitation 490 nm, emission 520 nm). The level of MMP was calculated by the ratio of red fluorescence to green fluorescence. We also used tetramethylrhodamine ethyl ester and perchlorate (TMRE) to measure levels of MMP. TMRE is a cationic dye that is rapidly and reversibly accumulated by healthy mitochondria. A decrease in levels of TMRE immunostaining indicates reduction in MMP levels. The TMRE studies were performed as described by Zhang et al. [27]. Briefly, at the end of the treatment, the cells were treated with 100 nM TMRE (Sigma, St. Louis, MO) for 30 minutes at 37°C. The cells were washed with Hanks Balanced Salt Solution (HBSS) twice and then analyzed under a 40X objective lens fluorescence microscope.
ATP Measurement
We employed the ATP Determination Kit (Invitrogen, Carlsbad, CA) in the experiments to detect ATP levels, as described in our previous studies [27,28]. In short, H4-APP cells were placed in 6-well plates in the incubator overnight. The cells were then exposed to isoflurane treatment for three hours. At the end of the treatment, the amount of fluorescence was measured and the levels of ATP in the experimental samples were calculated from the standard curve made from samples containing known amounts of ATP.
Mice
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, Massachusetts. Wild-type C57BL/6J mice (8-month-old, The Jackson Laboratory, Bar Harbor, ME) were randomly assigned to the anesthesia group or the control group, and then were further divided into the saline group or the VitC treatment group. The mice were housed in a controlled environment (20 – 22°C; 12 hour light: dark on a reversed light cycle) for one week prior to the studies. The maintenance and handling of the mice was consistent with the guidelines set forth by the National Institute of Health, and all efforts were made to minimize the number of animals utilized in the studies. Power analyses used to establish experimental group sizes are described below in the Statistics section.
Mice Anesthesia
The mice (ten per experiment) were randomized by weight and gender into experimental groups that received either 1.4% isoflurane plus 100% oxygen for two hours, or into control groups that received 100% oxygen for two hours, at identical flow rates and in identical anesthetizing chambers. The size of the induction chamber in the current study was 20 × 20 × 7 centimeters. The induction flow rate was two liters per minute for the first three minutes (for the induction) and then 0.2 litters per minute afterwards (for maintenance). The anesthetic and oxygen concentrations were measured continuously by a gas analyzer (Ohmeda, GE Healthcare, Tewksbury, MA). The temperature of the anesthetizing chamber was controlled by the DC Temperature Control System (FHC, Bowdoinham, Maine), which is a feedback-based system for monitoring and controlling temperature, to maintain the rectal temperature of the mice at 37 ± 0.5 °C. In the interaction studies, dehydroascorbic acid, the oxidized form of VitC, which can enter into the brain through the blood-brain barrier (100 mg/kg), or saline, was administered to the mice via tail vein injection 30 minutes before the isoflurane anesthesia. The dosage of VitC was chosen, with modification, according to previous studies [40,41].
Fear Conditioning System (FCS)
The FCS was performed just as described in previous studies [42,27,43,44], with modification. Briefly, the pairing of the FCS was performed two hours after the isoflurane anesthesia. The first context and tone tests were performed 24 hours after the end of the pairing. The second and third context and tone tests were performed 48 hours and seven days after the anesthesia, respectively. The pairing in the FCS (Stoelting Co., Wood Dale, IL) was performed two hours after the isoflurane anesthesia. Each mouse was allowed to explore the FCS chamber for 180 seconds before presentation of a 2-Hz pulsating tone (80 dB, 3,600 Hz) that persisted for 60 seconds. The tone was followed immediately by a mild foot shock (0.8 mA for 0.5 seconds). The first context test was performed 24 hours after the end of the pairing. Each mouse was allowed to stay in the chamber for a total of 390 seconds. Learning and memory function in the context test was assessed by measuring the amount of time the mouse demonstrated “freezing behavior,” which is defined as a completely immobile posture, except for respiratory efforts, during the second period of 180 seconds. The first tone test was also performed 24 hours after the end of the pairing. Each mouse was allowed to stay in the chamber for a total of 390 seconds. The same tone was presented for the second 180 seconds without the foot shock. Learning and memory function in the tone test was also assessed by measuring the amount of time the mouse demonstrated “freezing behavior,” which is defined as a completely immobile posture, albeit for respiratory efforts, during the second period of 180 seconds. The second and third context and tone tests were performed 48 hours and seven days after the anesthesia, respectively. The “freezing behavior” was then analyzed by Any-Maze (freezing on threshold: 10; freezing off threshold: 20; minimum freezing duration: one second) (Stoelting).
Statistical Analyses
Data were expressed as means ± standard deviation (SD). The number of samples was ten per group for the mice experiments, and six per group for the in vitro studies. The power calculation was performed using information collected from a preliminary study that was conducted under the same conditions. Based on the preliminary data, assuming a two-sided Student-t test, samples of six and ten for each control and treatment group for the in vitro studies and the mice studies, respectively, would lead to 90% power and 95% significance. A two-way ANOVA was used to assess the interaction of VitC with isoflurane, and to test the hypothesis that VitC would mitigate the effects of isoflurane on caspase-3 activation, ROS, mPTP, MMP, ATP and freezing time. Post hoc analyses were conducted if the main effects were found to be statistically significant. The cut-off p-value was Bonferroni adjusted to correct for sub-set analysis, e.g., comparing the level of isoflurane caspase-3 activation between VitC and saline treatments. The nature of the hypothesis testing was two-tailed. P values less than 0.05 were considered statistically significant. SAS software (Cary, NC) and Prism 6 software (La Jolla, CA) were used to analyze the data.
Results
VitC attenuates isoflurane-induced caspase-3 activation
Anesthetic isoflurane has been suggested to induce both caspase-3 activation and cognitive impairment by causing mitochondrial dysfunction and oxidative stress [28,27]. VitC, on the other hand, has been reported to inhibit oxidative stress. Thus, we set out to determine whether VitC could attenuate isoflurane-induced neurotoxicity and neurobehavioral deficits in cultured cells (e.g., H4-APP cells) and in mice. Immunoblotting of caspase-3 showed that treatment with 2% isoflurane plus saline for six hours (lane 2) induced a visible increase in the level of the Western blot band representing the caspase-3 fragment compared to the control condition plus saline for six hours (lane 1) (Figure 1A). The treatment with VitC alone (lane 3) did not significantly alter the level of caspase-3 fragment compared to the control condition (lane 1). However, there was a lesser visible band representing the caspase-3 fragment following the treatment with isoflurane plus VitC (lane 4) compared to the treatment with isoflurane plus saline (lane 2) (Figure 1A). There were no significant differences in the levels of full-length caspase-3 or β-Actin among the above treatments. Quantification of the Western blots, based on the ratio of caspase-3 fragments to full length caspase-3, showed that the treatment with 2% isoflurane plus saline for six hours (black bar) induced caspase-3 activation compared to the control condition treatment plus saline (white bar) (Figure 1B). A two-way ANOVA illustrated a significant interaction between the group (control condition versus isoflurane) and the treatment (saline versus VitC) on caspase-3 activation (F = 4.712, P = 0.042). A post-hoc Bonferroni test showed that there was greater caspase-3 activation in the H4-APP cells following the treatment with isoflurane (black bar) as compared to the control condition (white bar) (P = 0.020, Figure 1B), and there was less caspase-3 activation in the H4-APP cells following treatment with isoflurane plus VitC (net bar) compared to treatment with isoflurane plus saline (black bar) (P = 0.023, Figure 1B).
Figure 1. VitC attenuates the isoflurane-induced caspase-3 activation in H4-APP human neuroglioma cells.
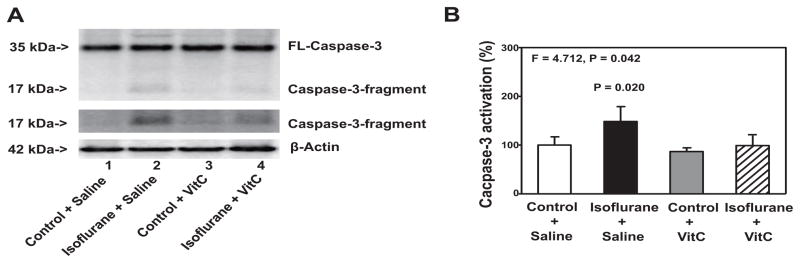
A. The treatment with 2% isoflurane plus saline for six hours (lane 2) induces caspase-3 activation as compared to the control condition plus saline for six hours (lane 1) in the H4-APP cells. The treatment with 400 uM VitC alone (lanes 3) does not induce caspase-3 activation as compared to the control condition plus saline (lanes 1). There is lesser caspase-3 activation following the treatment with 2% isoflurane plus 400 uM VitC for six hours (lane 4) than that following the treatment with 2% isoflurane plus saline for six hours (lane 2). There is no significant difference in the levels of full length caspase-3 and β-Actin among the above treatments. B. The quantification of the Western blots shows that the treatment with 2% isoflurane plus saline for six hours (black bar), but not the control condition plus 400 uM VitC for six hours (gray bar), induces caspase-3 activation as compared to the control condition plus saline for six hours (white bar) in H4-APP cells. The treatment with 400 uM VitC (net bar) attenuates the isoflurane-induced caspase-3 activation (black bar). N = 6 in each group. FL, full length; APP, amyloid precursor protein; VitC, Vitamin C.
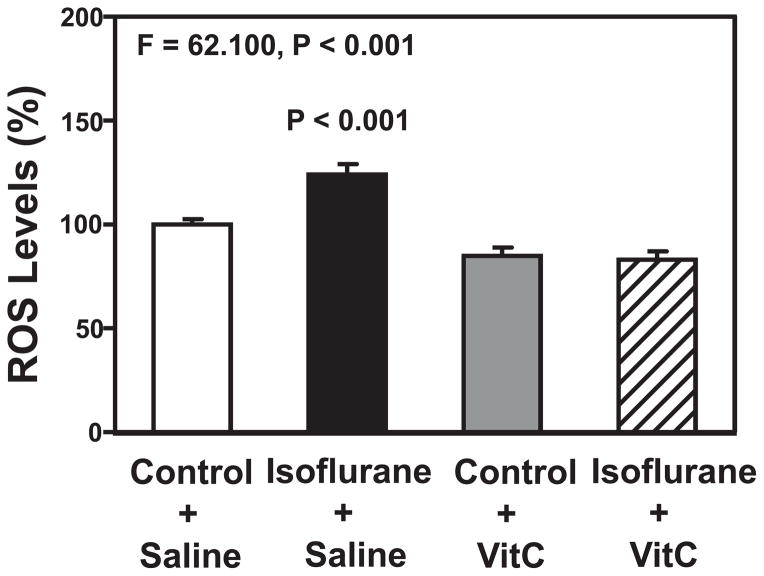
VitC attenuates isoflurane-induced increase in ROS levels
Isoflurane has been shown to increase ROS levels, which leads to caspase-3 activation [27,28]. We, therefore, determined the effects of VitC on isoflurane-induced increases in ROS levels in the H4-APP cells. The quantitative ROS assay showed that there was a significant interaction between group (control condition versus isoflurane) and treatment (saline versus VitC) on ROS levels (Figure 2): F = 62.100, P < 0.001. A post-hoc Bonferroni test showed that there were greater ROS levels in the H4-APP cells following the treatment with isoflurane (black bar) as compared to the control condition (white bar) (P < 0.001, Figure 2), and that there were lower ROS levels in the H4-APP cells following the treatment with isoflurane plus VitC (net bar) as compared to the treatment with isoflurane plus saline (black bar) (P < 0.001, Figure 2). VitC decreased the baseline amounts of ROS levels in the control condition. These data suggest that VitC could mitigate isoflurane-induced ROS accumulation.
Figure 2. VitC attenuates the isoflurane-induced increase in ROS levels in H4-APP cells.
A fluorescence staining of ROS shows that the treatment with 2% isoflurane plus saline for six hours (black bar) increases ROS levels as compared to the control condition plus saline for six hours (white bar). The treatment with 400 uM VitC for six hours alone (grey bar) does not significantly alter the ROS levels. There are lesser ROS levels following the treatment with isoflurane plus VitC (net bar) as compared to those following the treatment with isoflurane plus saline (black bar). N = 6 in each group. ROS, reactive oxygen species; APP, amyloid precursor protein; VitC, Vitamin C.
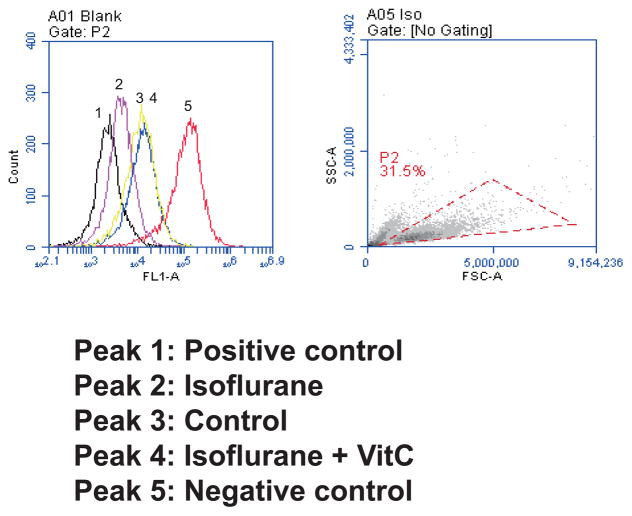
VitC attenuates isoflurane-induced opening of mPTP
Given that VitC was able to mitigate isoflurane-induced ROS accumulation and caspase-3 activation, we then set out to determine whether VitC could also attenuate isoflurane-induced mitochondrial dysfunction. We specifically assessed the interaction of isoflurane and VitC on the opening of mPTP, and levels of MMP and ATP in the B104 and H4-APP cells, respectively.
Treatment with 2% isoflurane for three hours induced the opening of mPTP in B104 cells as evidenced by the peak following the isoflurane treatment (peak 2) located to the left, as compared to the peak following the control condition (peak 3) (Figure 3). The peak following treatment with isoflurane plus VitC (peak 4) is located to the right of the peak following treatment with isoflurane plus saline (peak 2). Peak 1 was the positive control peak of mPTP opening; peak 5 was the peak following treatment with calcein, the negative control of mPTP opening. These data suggest that the isoflurane opened the mPTP and that VitC was able to mitigate this isoflurane-induced opening of mPTP.
Figure 3. VitC attenuates the isoflurane-induced opening of mitochondrial permeability transition pore (mPTP) in B104 cell line.
Left panel: Flow cytometric analysis shows changes in mitochondria of B104 cells stained with calcein AM or calcein AM plus cobalt, which indicates mPTP opening. Peak 1, positive controls in B104 cells; peak 2, B104 cells treated with calcein AM plus cobalt and 2% isoflurane; peak 3, negative control (treatment of calcein AM plus cobalt); peak 4, the treatment of 2% isoflurane plus 400 uM VitC with calcein AM and cobalt. Peak 5, calcein AM treated B104 cells. The treatment of 400 uM VitC attenuates the isoflurane-induced opening of mPTP, as demonstrated by the position of the peak of 2% isoflurane plus saline treatment is shifted to the right following the 400 uM VitC treatment. Right panel: Dead cells and debris were excluded from analysis by gates set on forward and side angle light scatter. Stained cells were chosen by the red dotted triangle. mPTP, mitochondrial permeability transition pore; VitC, Vitamin C.
VitC attenuates isoflurane-induced reduction in MMP
Next, we assessed whether or not VitC could inhibit the isoflurane-induced decrease in the levels of MMP in the H4-APP cells. Immunocytochemistry staining of TMRE, the indicator of MMP, showed that the isoflurane treatment (b) decreased levels of MMP, detected by confocal microscopy, as compared to the control condition (a) in the H4-APP cells (Figure 4A). Moreover, VitC inhibited the isoflurane-induced reduction in MMP, as evidenced by greater TMRE staining in the cells treated with isoflurane plus VitC (d) compared to the cells treated with isoflurane plus saline (b) (Figure 4A).
Figure 4. VitC attenuates the isoflurane-induced reduction in MMP in H4-APP cells.
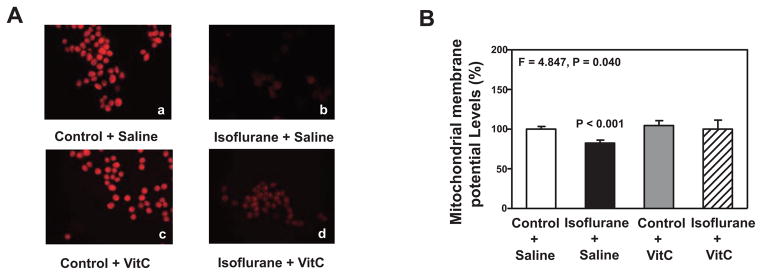
A. Staining of tetramethylrhodamine ethyl ester and perchlorate, the MMP-dependent fluorescent indicator, shows that the treatment with 2% isoflurane plus saline for three hours (b) decreases MMP levels as compared to the control condition plus saline for three hours (a). Treatment with 2% isoflurane plus 400 uM VitC for three hours (d) attenuates the isoflurane-induced MMP reduction (b). B. Tetraethylben-zimidazolylcarbocyanine iodide (JC-1) fluorescence analysis shows that the treatment with 2% isoflurane plus saline for three hours (black bar) decreases the levels of mitochondrial membrane potential (MMP) as compared to the control condition plus saline for three hours (white bar) in the H4-APP cells. The treatment with 400 uM VitC for three hours (gray bar) does not significantly alter the MMP levels as compared to the control condition plus saline for three hours (white bar). The treatment with 2% isoflurane plus 400 uM VitC for three hours (net bar) leads to a lesser reduction in MMP levels as compared to the treatment with 2% isoflurane plus saline for three hours (black bar). N = 6 in each group. MMP, Mitochondrial membrane potential; VitC, Vitamin C.
JC-1 fluorescence ratio detection showed that the treatment with 2% isoflurane plus saline (black bar) for three hours decreased the levels of MMP as compared to the treatment with the control condition plus saline (white bar) (Figure 4B). A two-way ANOVA showed that there was a significant interaction between the group (control condition and isoflurane) and the treatment (saline and VitC) (F = 4.847, P = 0.040, Figure 4B). A post-hoc Bonferroni test showed that there was a smaller MMP level in the H4-APP cells following treatment with isoflurane (black bar) compared to the control condition (white bar) (P < 0.001, Figure 4B), and there was a higher MMP level in the H4-APP cells following treatment with isoflurane plus VitC (net bar) compared to treatment with isoflurane plus saline (black bar) (P = 0.017, Figure 4B).
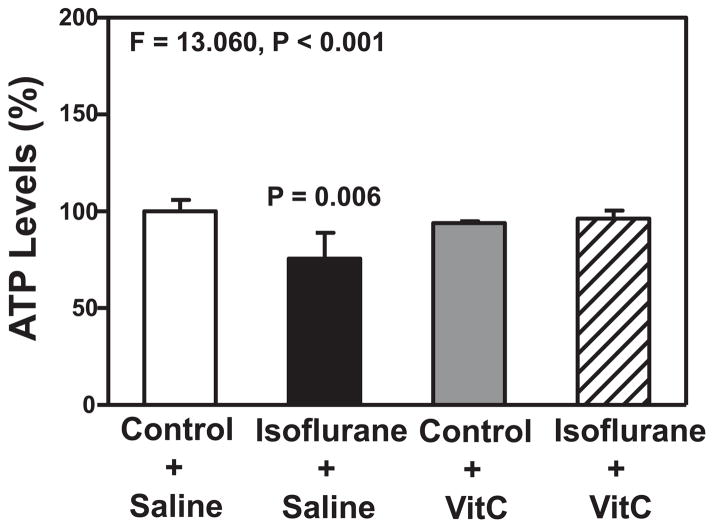
VitC attenuates isoflurane-induced reduction in ATP levels
Finally, we assessed the possible interaction of isoflurane and VitC on ATP levels in the H4-APP cells. A two-way ANOVA showed a significant interaction between group (control condition versus isoflurane) and treatment (saline versus VitC) on ATP levels (F = 13.060, P < 0.001, Figure 5). A post-hoc Bonferroni test showed that there was a lower level of ATP in the H4-APP cells following treatment with isoflurane (black bar) as compared to the control condition (white bar) (P = 0.006, Figure 5), and there was a higher level of ATP in the H4-APP cells following treatment with isoflurane plus VitC (net bar) as compared to treatment with isoflurane plus saline (black bar) (P = 0.014, Figure 5). Collectively, these results suggest that VitC was able to mitigate isoflurane-induced mitochondrial dysfunction, including both mPTP opening and the reduction in the levels of MMP and ATP, in the H4-APP cells.
Figure 5. VitC attenuates the isoflurane-induced reduction in ATP levels in H4-APP cells.
The treatment with 2% isoflurane plus saline for three hours (black bar) decreases ATP levels as compared to the control condition plus saline for three hours (white bar). The treatment with 400 uM VitC for three hours (gray bar) does not significantly alter the ATP levels as compared to the control condition plus saline for three hours (white bar). Finally, the treatment with 2% isoflurane plus 400 uM VitC for three hours (net bar) leads to lesser reduction in ATP levels as compared to the treatment of 2% isoflurane plus saline for three hours (black bar). N = 6 in each group. ATP, adenosine triphosphate; VitC, Vitamin C.
VitC ameliorates isoflurane-induced cognitive impairment
After discovering that VitC could mitigate isoflurane-induced cellular neurotoxicity, which can lead to cognitive impairment [27], we consequently asked whether VitC would also be able to ameliorate the isoflurane-induced cognitive impairment seen in mice. Anesthesia, with 1.4% isoflurane for two hours, induced cognitive impairment in mice, as evidenced by the isoflurane anesthesia-induced decreased freezing time of mice in the context test of FCS at seven days (F = 8.032, P = 0.008, two-way ANOVA; P = 0.0034, post-hoc Bonferroni test, Figure 6A), and in the tone test of FCS at two days (F = 10.050, P = 0.003, two-way ANOVA; P = 0.041, post-hoc Bonferroni test, Figure 6B). A post-hoc Bonferroni test showed that there were greater freezing times for the mice treated with isoflurane plus VitC than for the mice treated with isoflurane plus saline in the context test of FCS at 7 days (P = 0.015, Figure 6A) and in the tone test of FCS at two days (P = 0.004, Figure 6B). These findings suggest that VitC has the ability to ameliorate isoflurane-induced cognitive impairment in mice.
Figure 6. VitC attenuates the isoflurane-induced learning and memory impairment in mice.
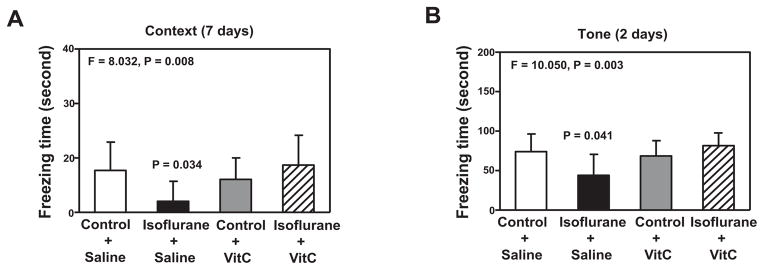
A. The treatment with isoflurane plus saline (black bar) decreases the freezing time in the context test of the fear conditioning system (FCS) as compared to the control condition plus saline (white bar) at 7 days after isoflurane anesthesia in mice. VitC treatment alone (gray bar) does not significantly affect freezing time as compared to the control condition plus saline (white bar) in the context test of the at 7 days after the isoflurane anesthesia. The treatment with isoflurane plus VitC (net bar) attenuates the isoflurane-induced reduction in freezing time in the context test of the FCS at 7 days after the treatment. B. The treatment with isoflurane plus saline (black bar) decreases freezing time in the tone test of the FCS as compared to the control condition plus saline (white bar) at 2 days after the isoflurane anesthesia in mice. The VitC treatment alone group (gray bar) does not significantly affect freezing time as compared to the control condition plus saline (white bar) in the tone test of the FCS at 48 hours after the isoflurane anesthesia. However, VitC (net bar) attenuates the isoflurane-induced reduction in freezing time in the tone test of the FCS at 2 days after the treatment. N = 10 in each group. FCS, fear conditioning system; VitC, Vitamin C.
Discussion
ROS accumulation, mitochondrial dysfunction and reduction in ATP levels have all been proposed as up-stream mechanisms of isoflurane-induced caspase-3 activation, while cognitive impairment has been suggested as the down-stream consequence of this isoflurane-induced caspase-3 activation [27,28]. Vitamin C (VitC) may protect against the negative effects of oxidative stress and apoptosis [29–36]. Therefore, in the present study, we assessed the interaction between isoflurane and VitC on caspase-3 activation, ROS accumulation, mitochondrial function and ATP levels, in order to further illustrate the up-stream mechanisms and the down-stream consequences of isoflurane-induced caspase-3 activation. Moreover, we wished to explore the potential for targeted interventions into this isoflurane-induced neurotoxicity phenomenon and its associated neurobehavioral deficits.
We found that the VitC treatment attenuated the isoflurane-induced caspase-3 activation in the H4-APP cells (Figure 1). These data suggest that VitC could attenuate the cellular neurotoxicity of the anesthetic, isoflurane, and therefore may serve as a targeted intervention of this anesthesia’s neurotoxicity. Future studies are needed to further investigate the application of VitC in treating and preventing anesthesia neurotoxicity. The outcomes of these studies would lead to safer anesthesia care for patients and better postoperative outcomes, particularly in AD patients and senior adults. Moreover, the results suggested that VitC could be used as a tool to investigate the up-stream mechanisms and the down-stream consequences of isoflurane-induced caspase-3 activation. For example, we may use VitC to further assess whether oxidative stress and mitochondrial dysfunction are parts of the up-stream mechanisms and whether inflammation, DNA damages and synaptic dysfunction, are parts of the down-stream consequences.
Isoflurane-induced ROS elevation, mitochondrial dysfunction and ATP reduction have been shown, at least partially, to be the up-stream mechanisms of the isoflurane-induced caspase-3 activation [27,28]. We found that VitC attenuated the isoflurane-induced accumulation in ROS levels in the H4-APP cells (Figure 2), inhibited the isoflurane-induced opening of mPTP in B104 cells (Figure 3), mitigated the isoflurane-induced reduction in MMP in H4-APP cells (Figure 4), and attenuated the isoflurane-induced reduction in ATP levels in H4-APP cells (Figure 5). These data suggest that VitC could mitigate the isoflurane-induced caspase-3 activation by inhibiting the isoflurane-induced oxidative stress, mitochondrial dysfunction and ATP reduction. In addition, these data further suggested that ROS accumulation, mitochondrial dysfunction (e.g., opening of mPTP and reduction in MMP) and ATP reduction could be the up-stream mechanisms of the isoflurane-induced caspase-3 activation.
Finally, we were able to demonstrate that VitC ameliorated the isoflurane-induced cognitive impairment observed in mice. These findings suggested that VitC could be used to ameliorate the neurobehavioral deficits induced by anesthesia, pending further studies. Moreover, these findings further demonstrate that isoflurane-induced cognitive impairment could be one of the consequences of the isoflurane-induced caspase-3 activation.
VitC has been shown to attenuate UV-induced apoptosis in human epidermoid carcinoma A431 cells [33]. Consistent with that previous finding, we were able to show that VitC attenuated the isoflurane-induced caspase-3 activation in H4-APP cells. The studies by Lin et al. showed that VitC inhibited the UV-induced apoptosis via regulating Tet activity, DNA demethylation and tumor suppressor gene activation. The data from our current studies suggested that VitC might attenuate the isoflurane-induced caspase-3 activation by inhibiting the isoflurane-induced ROS accumulation, mitochondrial dysfunction and reduction in ATP levels. Moreover, we found that VitC was able to ameliorate the isoflurane-induced cognitive impairment in mice. Future studies should investigate other cellular and molecular mechanisms by which VitC might attenuate the isoflurane-induced neurotoxicity and neurobehavioral deficits. Results from these studies would lead to further investigations into the underlying mechanisms behind anesthesia’s neurotoxicity, and potential targeted interventions.
There are several limitations in the current studies. First, we did not assess the effects of different concentrations of VitC on the isoflurane-induced caspase-3 activation. It is possible that different concentrations of VitC may have different effects on the isoflurane-induced caspase-3 activation, increases in ROS levels, mitochondrial dysfunction and reductions in ATP levels. Second, we did not determine whether VitC could attenuate the isoflurane-induced cognitive impairment through other behavioral methods, e.g., Morris Water Maze. Finally, we did not determine whether other antioxidant agents might also be able to attenuate isoflurane-induced neurotoxicity and neurobehavioral deficits. Nevertheless, the primary objective of the current study was to establish a system and to generate a concept. We will use this established system to systematically investigate the interaction of VitC (as well as other antioxidant agents, e.g., Vitamin E) and isoflurane (as well as other anesthetics, e.g., sevoflurane) on neurotoxicity and neurobehavioral deficits in the future.
In conclusion, we found that VitC attenuated the isoflurane-induced caspase-3 activation, increases in ROS levels, opening of mPTP and decreases in MMP, and reduction in ATP levels in the cells. Moreover, VitC was able to ameliorate the isoflurane-induced cognitive impairment in mice. Pending further studies, these results suggested that VitC might mitigate the isoflurane-induced caspase-3 activation via ROS-, mitochondria- and ATP-associated mechanisms, which may lead to the inhibition of isoflurane-induced cognitive impairment. Ultimately, our hope is that the findings from these studies will promote more research into the underlying mechanisms behind anesthesia’s neurotoxicity, as well as targeted interventions of anesthesia neurotoxicity.
Acknowledgments
This study was supported by R21AG038994, R01GM088801, and R01AG041274 from the National Institutes of Health, Bethesda, Maryland; Investigator-initiated Research grant from Alzheimer’s Association, Chicago, Illinois; and Cure Alzheimer’s Fund, Wellesley, Massachusetts to Zhongcong Xie. The Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital and Harvard Medical School, generously provided the cost of inhalation anesthetic isoflurane.
Footnotes
Conflict of Interests: The authors deny any conflict of interests.
Author Contribution: Z.X. and Y.Z. conceived and designed the project. B.C., A.W., Y.Z and Y.D. performed all the experiments and prepared the figures. Z.X. and Y.Z wrote the manuscript. All authors reviewed the manuscript.
References
- 1.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. Journal of Alzheimer’s disease : JAD. 2005;7 (4):319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 2.Bufill E, Bartes A, Moral A, Casadevall T, Codinachs M, Zapater E, Carles Rovira J, Roura P, Oliva R, Blesa R. Genetic and environmental factors that may influence in the senile form of Alzheimer’s disease: nested case control studies. Neurologia. 2009;24(2):108–112. 20091090216 [pii] [PubMed] [Google Scholar]
- 3.Chen CC, Chiu MJ, Chen SP, Cheng CM, Huang GH. Patterns of cognitive change in elderly patients during and 6 months after hospitalisation: A prospective cohort study. Int J Nurs Stud. 2010 doi: 10.1016/j.ijnurstu.2010.03.011. S0020-7489(10)00113-6 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Gruber-Baldini AL, Zimmerman S, Morrison RS, Grattan LM, Hebel JR, Dolan MM, Hawkes W, Magaziner J. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. Journal of the American Geriatrics Society. 2003;51(9):1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. 51406 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Mecocci P, von Strauss E, Cherubini A, Ercolani S, Mariani E, Senin U, Winblad B, Fratiglioni L. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20 (4):262–269. doi: 10.1159/000087440. 87440 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA : the journal of the American Medical Association. 2010;303 (8):763–770. doi: 10.1001/jama.2010.167. 303/8/763 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer’s disease. Int J Neurosci. 1994;77 (3–4):181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- 8.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115 (4):727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline RP, Pirraglia E, Cheng H, De Santi S, Li Y, Haile M, de Leon MJ, Bekker A. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116 (3):603–612. doi: 10.1097/ALN.0b013e318246ec0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M. Inhaled Sevoflurane May Promote Progression of Amnestic Mild Cognitive Impairment: A Prospective, Randomized Parallel-Group Study. Am J Med Sci. 2013 doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 11.Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case-control study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013 doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed] [Google Scholar]
- 12.Chen PL, Yang CW, Tseng YK, Sun WZ, Wang JL, Wang SJ, Oyang YJ, Fuh JL. Risk of dementia after anaesthesia and surgery. The British journal of psychiatry : the journal of mental science. 2013 doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. The American journal of the medical sciences. 2013;345 (5):355–360. doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 14.Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer’s disease and cumulative exposure to anesthesia: a case-control study. Journal of the American Geriatrics Society. 1994;42 (2):198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65 (7):986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. 01.wnl.0000171954.92119.c7 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC, Evers AS. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111 (5):964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprung J, Jankowski CJ, Roberts RO, Weingarten TN, Aguilar AL, Runkle KJ, Tucker AK, McLaren KC, Schroeder DR, Hanson AC, Knopman DS, Gurrieri C, Warner DO. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clinic proceedings. 2013;88 (6):552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101 (3):703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112 (4):834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27 (6):1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61 (12):1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104(5):988–994. doi: 10.1097/00000542-200605000-00015. 00000542-200605000-00015 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Annals of neurology. 2008;64 (6):618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu Y, Zhou Z, Wan Y, Sanders RD, Li M, Pac-Soo CK, Maze M, Ma D. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45 (2):743–750. doi: 10.1016/j.nbd.2011.10.021. S0969-9961(11)00353-6 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neuroscience letters. 2007;425 (1):59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037 (1–2):139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Annals of neurology. 2012;71 (5):687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. The Journal of biological chemistry. 2010;285 (6):4025–4037. doi: 10.1074/jbc.M109.065664. M109.065664 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyacioglu M, Sekkin S, Kum C, Korkmaz D, Kiral F, Yalinkilinc HS, Ak MO, Akar F. The protective effects of vitamin C on the DNA damage, antioxidant defenses and aorta histopathology in chronic hyperhomocysteinemia induced rats. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2014 doi: 10.1016/j.etp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Guaiquil VH, Vera JC, Golde DW. Mechanism of vitamin C inhibition of cell death induced by oxidative stress in glutathione-depleted HL-60 cells. The Journal of biological chemistry. 2001;276 (44):40955–40961. doi: 10.1074/jbc.M106878200. [DOI] [PubMed] [Google Scholar]
- 31.Hanada Y, Iomori A, Ishii R, Gohda E, Tai A. Protection of free radical-induced cytotoxicity by 2-O-alpha-d-glucopyranosyl-l-ascorbic acid in human dermal fibroblasts. Bioscience, biotechnology, and biochemistry. 2014;78 (2):301–306. doi: 10.1080/09168451.2014.882756. [DOI] [PubMed] [Google Scholar]
- 32.Kc S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19 (12):1657–1667. doi: 10.1096/fj.05-4107com. [DOI] [PubMed] [Google Scholar]
- 33.Lin JR, Qin HH, Wu WY, He SJ, Xu JH. Vitamin C Protects Against UV Irradiation-Induced Apoptosis Through Reactivating Silenced Tumor Suppressor Genes p21 and p16 in a Tet-Dependent DNA Demethylation Manner in Human Skin Cancer Cells. Cancer biotherapy & radiopharmaceuticals. 2014;29 (6):257–264. doi: 10.1089/cbr.2014.1647. [DOI] [PubMed] [Google Scholar]
- 34.Siow RC, Richards JP, Pedley KC, Leake DS, Mann GE. Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arteriosclerosis, thrombosis, and vascular biology. 1999;19 (10):2387–2394. doi: 10.1161/01.atv.19.10.2387. [DOI] [PubMed] [Google Scholar]
- 35.Vissers MC, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. The Journal of biological chemistry. 2001;276 (50):46835–46840. doi: 10.1074/jbc.M107664200. [DOI] [PubMed] [Google Scholar]
- 36.Sasazuki S, Hayashi T, Nakachi K, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Tsugane S. Protective effect of vitamin C on oxidative stress: a randomized controlled trial. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2008;78 (3):121–128. doi: 10.1024/0300-9831.78.3.121. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. The Journal of biological chemistry. 2008;283 (18):11866–11875. doi: 10.1074/jbc.M800199200. M800199200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardaway CM, Badisa RB, Soliman KF. Effect of ascorbic acid and hydrogen peroxide on mouse neuroblastoma cells. Molecular medicine reports. 2012;5 (6):1449–1452. doi: 10.3892/mmr.2012.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Shao H, Dong Y, Swain CA, Yu B, Xia W, Xie Z. Chronic treatment with anesthetic propofol attenuates beta-amyloid protein levels in brain tissues of aged mice. Translational neurodegeneration. 2014;3 (1):8. doi: 10.1186/2047-9158-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES., Jr Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proceedings of the National Academy of Sciences of the United States of America. 2001;98 (20):11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekici F, Ozyurt B, Erdogan H. The combination of vitamin D3 and dehydroascorbic acid administration attenuates brain damage in focal ischemia. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2009;30 (3):207–212. doi: 10.1007/s10072-009-0038-6. [DOI] [PubMed] [Google Scholar]
- 42.Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113(5):1061–1071. doi: 10.1097/ALN.0b013e3181f5622800000542-201011000-00018. [pii] [DOI] [PubMed] [Google Scholar]
- 43.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Peripheral surgical wounding and age-dependent neuroinflammation in mice. PloS one. 2014;9 (5):e96752. doi: 10.1371/journal.pone.0096752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Scientific reports. 2014;4:3766. doi: 10.1038/srep03766. [DOI] [PMC free article] [PubMed] [Google Scholar]