Abstract
Rapid fluctuations in contrast are common in our modern visual environment. They arise, for example, in a room lit by a fluorescent light, when viewing a CRT computer monitor and when watching a movie in a cinema. As we are unconscious of the rapid changes, it has been assumed that they do not affect the operation of our visual systems. By periodically reversing the contrast of a fixed pattern at a rapid rate we render the pattern itself, as well as the modulations, invisible to observers. We show that exposure to these rapidly contrast-modulated patterns alters the way subsequent stationary patterns are processed; patterns similar to the contrast-modulated pattern require more contrast to be detected than dissimilar patterns. We present evidence that the changes are cortically mediated. Taken together, our findings suggest that cortical stages of the visual system respond to the individual frames of a contrast-reversed sequence, even at rates as high as 160 frames per second.
Keywords: adaptation, consciousness, primary visual cortex, temporal frequency response
Introduction
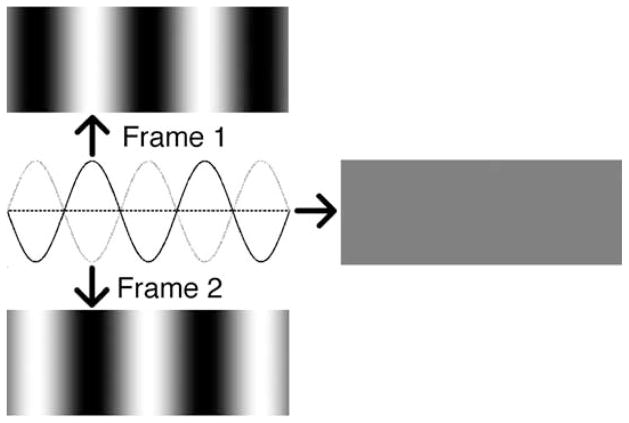
We tend not to see the flicker in a room lit by a fluorescent light because, prior to conscious registration, our visual systems integrate the visual scene (which is lit in brief pulses separated by dark intervals) across time. Due to temporal integration by our visual systems it is possible to compose a sequence of patterned images that, when presented in rapid succession, appears uniform. Periodically reversing the contrast of a black and white stripy (grating) pattern (the sequence is referred to as a counterphasing grating) will produce this effect, as long as the reversal rate is high enough (de Lange, 1958; Robson, 1966). This is because “blending together” or, more accurately, averaging the pair of images that make up the sequence leads to a uniform intermediate gray across the entire image (see Figure 1). Although it is the blended scene that arrives at the level of consciousness, it is possible that a more detailed representation—one containing the individual frames—is registered elsewhere in the cortex. We designed experiments to test whether the oriented patterns, unseen by observers, nevertheless activated cortical neurons.
Figure 1.
Counterphasing sine-wave grating. Consecutive frames are 180° out of phase. Averaging consecutive frames leads to a uniform intermediate gray (represented by the horizontal dashed line).
Viewing a pattern for a few seconds or more lowers sensitivity to similar patterns. We require more contrast to see a pattern that resembles its predecessor than if the predecessor is dissimilar. For instance, viewing a high contrast vertical grating pattern produces an orientation-specific loss in sensitivity: a subsequent faint vertical grating requires more contrast to be detected than a faint grating of a different orientation. This is thought to arise from desensitization of neurons that are specifically activated by the oriented pattern. Orientation specificity first arises in primary visual cortex so this site is implicated in these adaptation effects. Perhaps surprisingly, we demonstrate orientation-specific adaptation to rapidly contrast-reversed grating patterns, even when the rate of reversal is so high that both the contrast modulations and the patterns themselves are invisible. This strongly suggests activation of orientation-tuned cortical neurons by rapidly modulated patterns in the absence of conscious awareness.
Methods
Apparatus
All experiments were performed using a desktop PC with VSG/5 graphics board and an Iiyama Vision Master Pro 514 CRT monitor (model HM204DT, 20 inch visible, 22 inch nominal). For all experiments the monitor was run with 600 × 800 pixel resolution and 160-Hz refresh rate. Stimuli were created and responses were collected using Matlab R2007a software.
Observers
All observers had normal or corrected-to-normal vision. Consent was obtained prior to participation.
Adaptation to sine-wave gratings
Data were collected over a series of short sessions. There were breaks of minutes or days between sessions. Each session consisted of 4 sequential blocks. In the first session, the adapting orientation was left diagonal, in the second, it was vertical, in the third, right diagonal, and in the fourth, horizontal. Each block consisted of 100 s of adaptation material interrupted every 4 s by a test stimulus. During each 4-s adaptation interval, observers fixated a black bar that appeared at the center of the display after removal of the preceding test stimulus and that made a slow out and back excursion covering half a period of the counterphasing grating during the adapting interval. The excursion was designed to prevent significant retinal adaptation.
The test grating stimulus onset was marked by a beep. During the test interval, a pair of stable sine-wave grating test patterns that flanked the fixation point was simultaneously presented for 300 ms (following 120-ms blank interval). Both test patterns had the same spatial frequency as the adapting grating (0.63 cycles per degree of visual angle) but one was parallel to the adapting grating and the other orthogonal. Both test patterns started at the same low contrast—just above threshold. Observers responded after each test presentation by pressing one or more arrow keys during the following adaptation interval. Button presses indicated the observer’s perception as per the diagram in Figure 2. An up arrow decreased the contrast of both patterns on the next presentation, the down arrow increased both contrasts and a left/right arrow produced a decrease in the contrast of the left/right test pattern and an increase in the right/left pattern. Multiple presses of the same key increased the amount of change. A given test pattern (either parallel or orthogonal to the adaptor) was randomly assigned to the left or right of the screen at each presentation.
Figure 2.
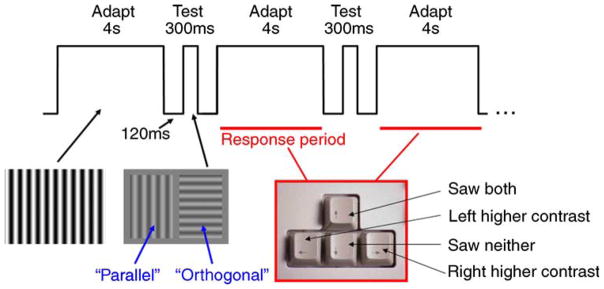
Adaptation experiment procedure.
The mean of the test contrast settings made between 80 and 100 s of exposure was used to calculate the adaptation effect size; we found that contrast settings had generally stabilized by the beginning of this interval (Blakemore & Campbell, 1969; Daugman, 1983). We used the log of the ratio between the contrast of the parallel test pattern and the contrast of the orthogonal pattern as our measure of the orientation-specific adaptation effect. This is equivalent to subtracting the log of the contrast of the orthogonal from the log of the contrast of the parallel (see, e.g., vertical axis of Figure 3). Results were averaged across all 4 adaptation orientations (blocks); no systematic differences between orientations were evident. At least three sessions were completed for each adaptation contrast/ temporal frequency combination. Error bars in Figure 4 in the main text represent between-session Standard Errors of the Means (±1 SEM).
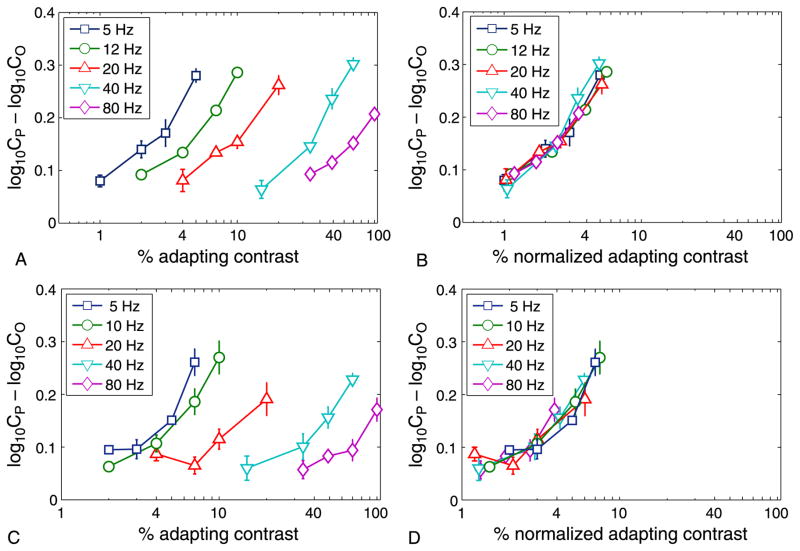
Figure 3.
Contrast adaptation functions at different temporal frequencies. Left column (A, C): Orientation-specific adaptation effect as a function of adapting contrast, for a range of temporal frequencies for observers (A) MF and (C) HD. Horizontal axis: Contrast of the adapting pattern, on a logarithmic scale. Vertical axis: Orientation-specific adaptation effect, expressed as the log contrast required to detect a test grating parallel to the adapting pattern minus the log contrast required for an orthogonal test grating; this is equivalent to the log of the ratio of the two contrasts. High temporal frequencies require greater adapting contrasts for the same orientation-specific adaptation effect. Right column (B, D): Same curves displaced leftward to lie on top of the 5-Hz curve. Amount of displacement is a measure of the sensitivity of the adaptation effect at the frequency in question—it is the factor by which the adapting contrast has to be multiplied to produce the same sized adaptation effects as in the most sensitive (5 Hz) condition.
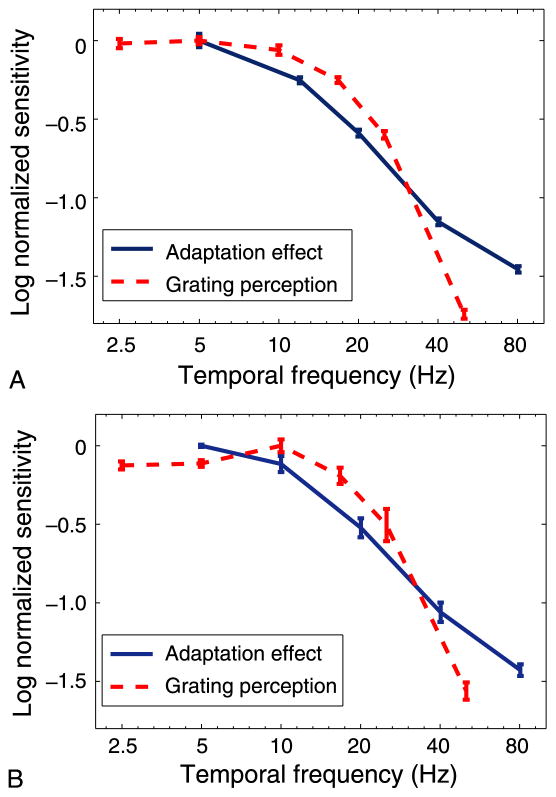
Figure 4.
Sensitivity as a function of temporal frequency. Log sensitivity for the adaptation effect (solid line) and for the perception of the grating (dashed line), as a function of temporal frequency. Each curve is normalized by its peak value. The fall-off for the adaptation effect at mid-high frequencies is shallower than the fall-off for perception. (A) Observer HD. (B) Observer MSF. Error bars represent ±1 SEM.
Temporal response function
Observers adjusted the contrast of the counterphase sine-wave grating to just above the maximum contrast at which the screen appeared uniform. At this point observers typically saw a flickering or moving grating-like pattern. Ten settings were made for each temporal frequency (2.5, 5, 10, 16.7, 25, 50 Hz) condition.
Adaptation to phase-contrast gratings
The procedure for measuring adaptation to our phase-contrast (dot) gratings (Figure 5A) was identical to that for the sine-wave gratings except that we used only two orientations for the adapting gratings—horizontal and vertical. Note that the traveling fixation point was used just as for the sine-wave gratings to reduce retinal adaptation. Observers participated in between 6 and 16 adaptation blocks per condition (represented by a single bar in Figure 5B).
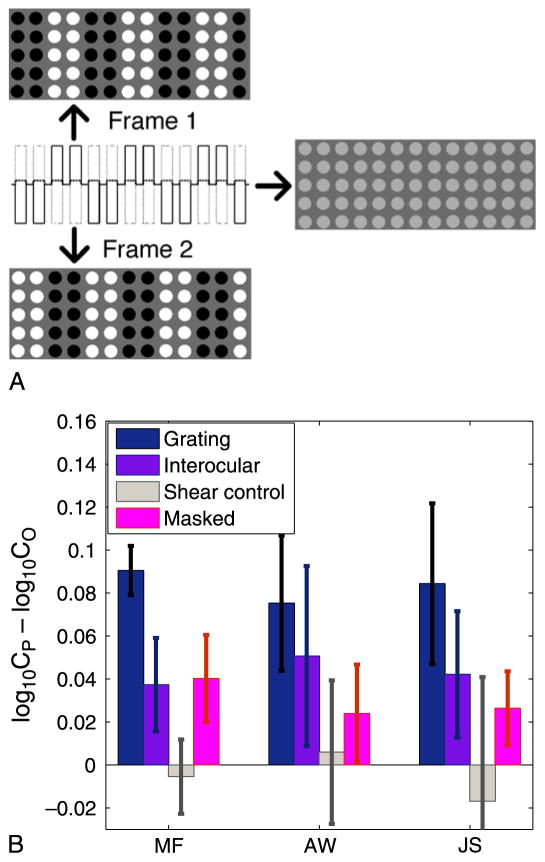
Figure 5.
Adaptation effects for 80-Hz counterphasing “dot” grating. (A) “Phase-contrast grating” stimulus used to produce an unseen pattern. Toggling rapidly between the two frames led to the appearance depicted on the right. The white and black dot luminances were set so that they averaged out to a slightly higher luminance than the background. (B) An orientation-specific adaptation effect was calculated by subtracting log detection thresholds for test gratings orthogonal to the adapting grating from log thresholds for test gratings that were parallel as in Figure 3. Blue bars: normal viewing conditions; purple bars: interocular condition (adapt pattern presented to one eye and test patterns presented to the other). In both cases, the adaptation effects were statistically significant (p < 0.025) for each observer. Taken together, the results support cortically mediated adaptation to the invisible counterphasing dot gratings involving neural mechanisms that are orientation-selective and binocularly driven. Gray bars: Control experiment; the counterphasing dots were replaced with steady light gray dots that moved occasionally in replication of a shearing pattern seen infrequently when observers performed a rapid saccadic eye movement during adaptation. Pink bars: Adaptation to a sinusoidal grating pattern whose orientation was masked (see text). All error bars depict 95% confidence intervals based on between-trial variation.
Interocular transfer of phase-contrast adaptation effect
The procedure and stimuli were exactly the same as that for measuring adaptation to our phase-contrast gratings except in the following ways: The screen was divided down the midline. An adapting grating was assigned randomly to either the left or the right side of the screen. The associated test patterns were presented on the opposite side. Thus, the physical size of the adaptor and test patterns was half that used in the other experiments although spatial frequency and other parameters stayed the same. Observers viewed the screen through a stereo-viewer that, using a simple arrangement of mirrors, effectively widened the separation between eyes so that each eye could look directly ahead at the center of the respective half of the screen. A black divider extended from the midline of the stereo-viewer to the midline of the screen so that each eye only saw one side of the screen. Observers practiced with the apparatus to the point that they were comfortable with the task before testing began. Observers participated in 12 adaptation blocks (6 per orientation; results averaged across orientations).
Adaptation and discrimination for masked sine-wave grating
Thirteen observers participated in 2 sessions consisting of between 250 and 430 (median 310) trials depending on their response rates. The experimenter was present with the observer at all times. On each trial, a masked counter-phasing sine-wave adaptation stimulus (Figure 6A) was presented for 4 s followed by two test gratings as in the standard sine-wave grating adaptation experiments described above. Sessions were divided into pairs of blocks. In one block the adapting patterns were randomly either vertical or horizontal on each trial and in the other they were either left or right diagonal. A traveling fixation point was used during adaptation as in the previous experiments. Following presentation of each adaptor and pair of test patterns observers responded verbally to three questions: (1) Which of the two test stimuli was higher contrast, (2) what was the orientation of the grating in the adaptation stimulus, and (3) how confident are you in your response to question 2? The confidence ratings did not correlate with likelihood of correct response to question 2. Although this is interesting, it is not considered further in this report.
Figure 6.
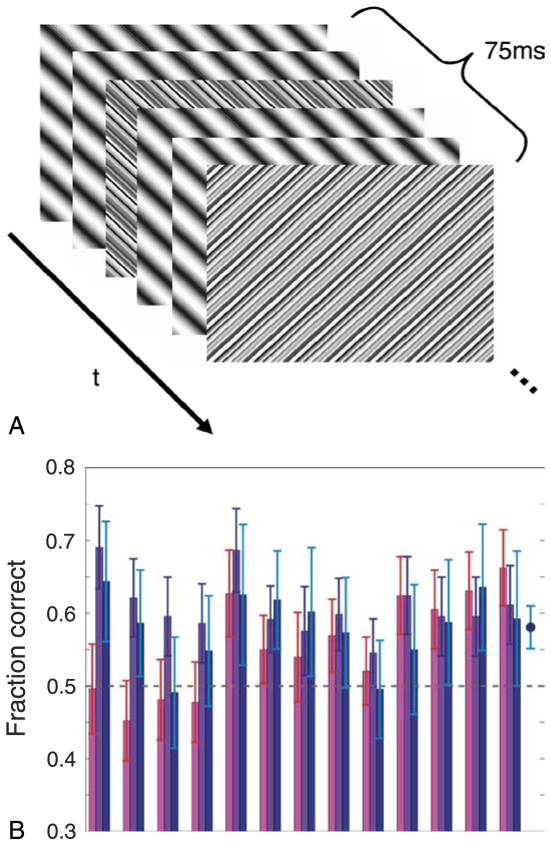
Ambiguous sine-wave grating—stimulus and results. (A) Masked counterphasing sine-wave grating stimulus. Two out of every six frames were “noise” frames as described in the text. (B) Fraction of correct responses for various tasks related to the adaptation stimulus depicted in (A). For each task, a choice was made between two orthogonal alternatives so chance level performance is 0.5, depicted by the horizontal dashed line. Error bars represent 95% confidence intervals. Pink bars: Discerning the orientation of the counterphasing grating in the adaptation stimulus. Purple bars: Choosing which of two orthogonal test patterns was higher contrast following the adaptation stimulus. Response was flagged “correct” if the chosen pattern was orthogonal to the adaptation stimulus. Blue bars: Same as purple bars but only considers trials where the orientation of the adaptation stimulus was chosen incorrectly. The blue point on the far right depicts the average for these data. It suggests significant adaptation on trials where the orientation of the adaptation stimulus was clearly indiscernible.
Results
Sine-wave gratings
Orientation-specific adaptation in the absence of conscious awareness can only occur if there is dissociation between the mechanisms driving the two effects. This possibility was explored by investigating, in separate experiments, how both the visibility of a contrast-reversed pattern and the resulting pattern-specific sensitivity loss vary with the temporal frequency of the reversals. Counter-phasing sine-wave gratings like that depicted in Figure 1 were used.
In the first experiment, pattern-specific sensitivity loss was examined. We wanted to compare the strength of adaptation across various reversal rates. The measure used was the relative amounts of adapting contrast required to get similar sized adaptation effects for a range of temporal frequencies (c.f. Temporal Response Function). The more contrast required to get a certain adaptation effect at a particular temporal frequency, the less sensitive the adaptation mechanisms at that frequency. For a range of temporal frequencies, we have plotted the adaptation effect as a function of adapting contrast in Figures 3A and 3C. The first thing to note is that significant levels of adaptation occur for rapidly contrast-reversed gratings, even 80-Hz gratings, which suggests cortical activation by the grating patterns at these rates. Whether this modulation is too rapid for perception is considered in detail below. The second thing to note is that the curves for the higher frequencies are of a similar form and are parallel to those of lower frequencies but displaced rightward. This means that the effectiveness of any adapting stimulus, whatever its contrast, is multiplied by the same frequency-dependent contrast sensitivity factor, indicated by the horizontal displacement of the curve on the logarithmic axis of Figure 3. This suggests a common underlying adaptation process, but with a frequency-dependent sensitivity.
It may come as a surprise that the adaptation effect, as a function of contrast, is similar for all temporal frequencies—the only difference being the need to multiply the set of contrasts by a higher factor as temporal frequency is increased. Consider an undulating signal and a neuron or system of neurons that is responding to that signal. One might expect that as the amplitude of the signal increased, the ability of the neuron/s to follow the signal to its extremities might decrease. Thus, neural activity in response to high contrast undulating patterns might be lower than expected. How would this effect manifest itself in our results? Essentially, there would be a second mechanism besides the contrast/adaptation effect mechanism modeled by a single curve in Figure 3 that decreased the effectiveness of high contrast adaptors. This would manifest itself as a drop in the curves as you move from the leftmost to the rightmost curve. There does not appear to be such a drop. The points of inflection for the various curves appear to be at a similar point on the y-axis.
This is emphasized in Figures 3B and 3D where the curves for higher frequencies have been displaced to lie on top of the 5-Hz curve. Note that the x-axis here represents normalized contrast where the contrasts for each original curve have been multiplied by some factor specific to each curve. Each multiplication factor is represented by a leftward displacement on the logarithmic x-axis. Displacements are larger for higher temporal frequencies. The appropriate displacement for each curve was found by taking the log of the y-axis data (to straighten out the curve), fitting a line to the curve thus created then finding the displacement that minimizes the RMS difference between the fitted line and the fitted 5-Hz line. The required leftward displacement defines the frequency-dependent, relative sensitivity of the adaptation effect. This relative sensitivity, as a function of temporal frequency, is depicted by the blue solid curves of Figure 4.
Next, for a range of modulation frequencies, we measured the visibility of the counterphasing gratings by determining how much contrast was required by observers to tell that they were seeing a counterphasing grating pattern rather than a blank gray field. Observers adjusted the contrast of each grating to the point where it was barely visible. When visible, it appeared to either flicker or move as the bright and dark bars were exchanged (Kelly, 1966). Consistent with previous studies (Watson, 1986), we found that beyond about 10 Hz, as temporal frequency increased more contrast was required to see the undulating grating (see Figure 4, red curves). For example, for the grating to be seen at 50 Hz, it needed to be about forty times the contrast of the 10-Hz grating.
Figure 4 compares the frequency-dependent sensitivity of the adaptation effect to the same observers’ direct perceptual sensitivity to counterphasing gratings. The decline in sensitivity with increasing temporal frequency is significantly more rapid for perception of the counter-phasing gratings than it is for the adaptation effect produced by these gratings. This suggests two things. The first is that the mechanisms driving the adaptation effect precede the mechanisms mediating conscious perception of the scene. The reasoning is given as follows: Neural activation results from an integration process whereby afferent signals from a range of axons are combined over some time window. This temporal integration process generally leads to decreased sensitivity to high temporal frequencies as you move downstream along a neural pathway (supported, e.g., in McKeeff, Remus, & Tong, 2007). As the “conscious system” is less sensitive to high temporal frequencies than the adaptation mechanism we might conclude that it is further downstream. The second thing the data suggests is that, with a high enough temporal frequency, observers might exhibit an adaptation effect even though they cannot see the counterphasing grating producing the effect.
A modulation rate of 80 Hz is well above the Critical Fusion Frequency (CFF)—the rate at which a flickering uniform field is indistinguishable from a stationary uniform field (Watson, 1986). It is also above the rate at which counterphasing grating patterns are indistinguishable from a stationary uniform field (Kelly, 1966; Robson, 1966). Consistent with this, in the above experiments we found that an 80-Hz counterphasing grating pattern (despite its ability to produce adaptation) was not visible, even if it was presented at full contrast. However, at this contrast observers did perceive a shimmering and extremely faint grating pattern that had twice the spatial frequency of the actual adapting grating. Double-frequency gratings have been reported previously for the case of low spatial frequencies and temporal frequencies just below the point at which a counterphasing grating is indistinguishable from a uniform field (approximately 50 Hz; Kelly, 1966). The gratings were ascribed to a particular combination of linear and non-linear operations in the visual system. In our case, the double frequency gratings were real—they were measurable with a photometer. Note that the monitors were gamma-corrected for monitor non-linearity using standard procedures; in addition, the gamma correction was further refined by preliminary experiments in which the visibility of the grating was minimized by having observers independently adjust the second and fourth harmonics of the underlying grating to make the counter-phasing pattern appear as uniform as possible. We were unable, however, to completely eliminate the impression of a faint and unstable frequency-doubled grating during the presentation of the 80-Hz adaptation stimulus. To determine whether adaptation can result under conditions where there are no visible cues at all to the presence of the counterphasing grating, we designed a new stimulus that was free of this faint pattern.
Phase-contrast gratings
In the new stimulus, a square-wave counterphasing grating was sampled using stationary circular windows arranged on a grid so that there were spaces between the visible parts of the grating (see Figure 5A). To make a vertical grating, the dots were grouped by columns. Alternate pairs of columns were toggled between light and dark in opposite temporal phase, creating a “phase contrast” grating in which all dots undergo the same modulations and are only distinguished by their temporal phase. At 80 Hz, this phase-contrast dot grating looked like a steady field of identical light gray dots on a mid-gray background (the dots were slightly lighter gray than the background; approximately 2% contrast). To confirm that the dots were perceptually identical, we presented 3 naive observers with 3 horizontally arranged counter-modulated dots where all dots were identical except that either the left or the right dot was modulated 180° out of phase with the other two. When asked to identify the odd one out, observers performed at chance levels; the number of correct responses compared to the number of trials was 172/306, 98/204, and 100/204, which corresponds to p-values of 0.15, 0.77, and 0.92, respectively, using Yates correction. This result does not exclude the possibility that, when many counterphasing dots are arranged as in our phase-contrast pattern, some clues about the pattern are available (more on this below). It does indicate that individual dots will be indistinguishable from their neighbors in such a pattern. We can therefore ask: Is this perceptually uniform field of dots capable of producing orientation-specific desensitization—an effect usually associated with visible grating patterns?
As in the adaptation experiment described above, two sinusoidal grating test patterns—one parallel to the unseen adapting grating and the other orthogonal—appeared after every 4 s of exposure to the adapting field of dots. An orientation-specific adaptation effect was measured just as in the adaptation experiment above. All three observers exhibited a moderate but significant adaptation effect (see Figure 5B, blue bars). These results indicate that the orientation-selective neurons in the observers’ visual systems were responding to patterns that the observers themselves were unable to see. Viewing the 80-Hz phase-contrast grating produced an orientation-selective adaptation effect even though the grating itself was invisible.
Where in the visual system is the structure that adapts? This system of neurons apparently has access to properties of the adaptation stimulus that an observer cannot see. Thus, knowing where the adaptation effect is mediated tells us where consciousness is not—it helps delineate the “neural correlates of consciousness” (Crick & Koch, 1990, 1998). As the adaptation effect is orientation specific, we can say with some confidence that the effect is mediated in the visual cortex, where pronounced orientation selectivity first arises (Hubel & Wiesel, 1962; Reid & Alonso, 1995; Shou & Leventhal, 1989). Can we further constrain the possibilities?
To do this, we tested whether the adaptation effects reported here were interocularly transferable. If the adaptation stimulus is presented to one eye, will an adaptation effect be observed when you test with the other eye? If so, the adaptation effect must occur at least as late as the binocular cell stage (layers 2, 3, and 4B in area V1 and beyond) of visual processing, since these are the first visual neurons to get input from both eyes. Figure 5B (purple bars) demonstrates that adaptation does result under these testing conditions, so the adaptation effect is at least partially mediated by neurons at or above the binocular cell stage. This excludes these cells from direct participation in conscious awareness—at least under the conditions of this particular experiment.
Although the phase-contrast gratings were invisible, occasional abrupt eye movements (saccades) as the observer followed the slow moving fixation point (see Methods section) produced a slight impression of shearing motion at the boundary between same-phase columns (or rows) of dots in the direction of the underlying grating, thereby revealing the stimulus orientation. This could result if at the time of the saccade a difference in processing times for the black versus the white dots on the screen (e.g., Cattell, 1886; Kammer, Lehr, & Kirschfeld, 1999) differentially displaces out-of-phase dots. Such saccades were infrequent (approximately one per few seconds of exposure) and the gratings as such remained invisible to observers during the saccades—each dot remained an indistinguishable gray. To ensure that the adaptation effects were not due to the shearing artifact, we recreated the shearing effect using a field of uniform light gray dots that shifted a small amount relative to one another in a manner that matched the perceived shift. As expected, there was no measurable adaptation effect following this stimulus (Figure 5B, gray bars).
Even so, we wanted to be sure that adaptation could result under conditions where the adapting stimulus was completely invisible to observers—where they were unable to discern the orientation of the grating. To this end, we designed a new adaptation stimulus.
The stimulus was, in all respects, the same as that used in the experiments just described except that in every 5th frame of the stimulus sequence the black and white dots defined a lattice of squares rather than a grating. The squares were two dots across and were spaced 2 dots apart. The inclusion of this frame was sufficient to eliminate awareness of the orientation of the adaptation grating. All three observers performed at chance levels in a 2-alternative forced-choice discrimination task; the number of correct responses compared to the number of trials was 55/101, 98/202, and 92/202. Corresponding p-values using Yates correction were 0.623, 0.842, and 0.426, respectively. Nevertheless, adaptation still resulted. The size of the adaptation effects is depicted by the pink bars in Figure 5B. All mean effects are significant: observers thus exhibited orientation-selective adaptation to a stimulus whose orientation was completely indiscernible.
Masked sine-wave gratings
A masking procedure proved similarly helpful in eliminating orientation cues with the continuous sine-wave gratings of Figure 1. The masking effect was created by inserting two “noise” frames every 6 frames of the counterphasing grating sequence (see Figure 6A). A noise frame followed each pair of consecutive frames in the counterphasing grating sequence. In one of the noise frames, each cycle of the grating was replaced by a set of 14 narrow strips of random luminance—the same set of luminance values repeated each cycle; the next noise frame, presented 3 frames later, was similar but was rotated by 90 degrees from the adapting orientation. The sequence was repeated (with a fixed grating orientation) for an adapting period of 4 s, which preliminary experiments had shown was sufficient to produce measurable adaptation effects. On each trial the adapting orientation was randomly either horizontal or vertical (or in other blocks of trials, randomly left or right diagonal).
Since the mask frames were not cancelled by a counterphase presentation (whereas the gratings were), the orthogonally crossed masking line patterns dominated perception. Indeed, although five out of every six frames of any given adaptation sequence shared a common orientation (the adapting grating orientation) and only one out of six was orthogonal, the observers performed at or near chance when asked to report the orientation of the adapting grating after each 4-s exposure in a 2-alternative forced-choice task (see Figure 6B, pink bars). As a measure of adaptation, observers also reported which of two subsequent faint test patterns, one parallel to the adapting grating and one orthogonal, appeared to have higher contrast. For all observers the orthogonal test grating was perceived as having higher contrast more often than the parallel grating (purple bars, Figure 6B). Performance was significantly above chance (p < 0.05) for all but one observer (p = 0.174), indicating highly significant adaptation effects, despite the fact that the orientation was indiscernible for almost half of the observers and barely discernible for the others.
Despite considerable variation among observers, the group data suggest that observers judged the adapted orientation “fainter” more often than they correctly identified the orientation of the adaptation pattern (paired t-test, t = 2.52, df = 12, p = 0.027; the t-test is applicable because the observed proportions, being near 0.5 and based on hundreds of trials, are nearly normally distributed with equal variance). Thus the most reliable indicator of the orientation of the adapting pattern was not the observer’s direct report of the orientation, but rather which one of the subsequent test patterns they considered to be lower contrast.
Of particular interest are the trials in which observers guessed the orientation of the adaptation grating incorrectly. On these trials, we can be fairly certain that the grating was not seen. Did observers still get adaptation on these trials? The blue bars in Figure 6B indicate the proportion of choices suggesting adaptation (i.e., fraction of times the orthogonal test grating was seen as having higher contrast) for this subset of trials for each observer. The fraction of adaptation-consistent judgments for these trials exceeded 0.5 reliably (at p < 0.05) for 8 of the 13 individual observers. The across-observer mean and 95% confidence interval is depicted by the rightmost point on the graph in Figure 6B. In a total of 1843 trials in which observers reported the adapting orientation incorrectly, the adaptation effect was in the direction expected for the true adapting orientation in 1063 trials. This degree of overall consistency could not occur by guessing (p < 10−6).
These results indicate that although the orientation of the masked adaptation stimulus was ambiguous, a weak but substantial orientation-specific loss in sensitivity followed. Even on trials where observers clearly did not see the grating (as they discerned the orientation incorrectly), significant levels of adaptation were measurable. This, in turn, indicates activation of orientation-tuned neurons in the cortex in the absence of conscious awareness.
Discussion
It has been shown previously that rapid flicker of a uniform field alters flicker sensitivity even when the flicker is undetected (Shady, MacLeod, & Fisher, 2004), but there was no evidence that the rapid modulations activated cortex, nor that the individual fields were processed by cortex as the result can be ascribed to retinal adaptation mechanisms. EEG (Lyskov, Ponomarev, Sandstrom, Mild, & Medvedev, 1998) and electrophysiological (Krolak-Salmon et al., 2003; Williams, Mechler, Gordon, Shapley, & Hawken, 2004) studies have revealed that cortical neurons can undergo entrainment—phase locking of neural firing patterns—to the flicker in 60–100 Hz flickering video displays. Whether specific sets of neurons, for example, those tuned to the patterns in each frame, are activated and whether the entrainment leads to perceptual effects is unclear from these findings. Our results thus constitute the first clear demonstration of cortically mediated perceptual effects by contrast modulations too rapid to be seen.
Both the orientation-specific and the interocular-transferable nature of the adaptation effect strongly suggest that the modulated patterns are processed in at least the input layer (layer 4) of area V1. This precludes these neurons from direct participation in perception—at least under the conditions used in these experiments. This is conducive with earlier claims that V1 activity is not tied directly to consciousness (Crick & Koch, 1995; Pollen, 1995) although our results do not preclude a subset of V1 neurons beyond the first binocular cell stage that are linked to consciousness. Our results are consistent with schemes in which the mechanisms mediating adaptation precede those that mediate consciousness (discussed, e.g., in a review by Rees, Kreiman, & Koch, 2002).
The simplest mechanism for the reported effects is one where the cortical representation, at the level of the adapting mechanism, is veridical. The two frames of a given counterphasing sequence are likely to be represented by two distinct sets of neurons up to the simple cell stage. Expressly, an “on-center” retinal ganglion cell might represent one portion of image 1 leaving an “off-center” cell to represent the same portion in image 2. The simple cell representation will be similarly apportioned. A phase zero simple cell might represent an area of image 1 and an opposite phase cell the same area in image 2. Thus both images would be represented in V1, each by a different subset of neurons. Is peak activity likely to oscillate between the two sets of neurons just as the scene alternates between the two images? This is difficult to establish from our results, but the EEG and electrophysiological results cited above have shown that, when exposed to flickering video displays, neurons in the cortex phase-lock their activity to the flicker, including simple and complex cells—even up to 100 Hz. Such a display consists of a single image that is turned on and off. In our case, we alternate between two images and expect that the sets of neurons representing each would oscillate at 80 Hz with a phase difference of 180 degrees between the two sets.
This work is closely related to other studies showing orientation-specific aftereffects for unseen patterns (Blake & Fox, 1974; He, Cavanagh, & Intriligator, 1996; He & MacLeod, 2001; Vul & MacLeod, 2006). Note though that here our focus is on showing pattern-specific adaptation for contrast-modulated patterns in the case that the modulations are too rapid to be seen, rather than the case where the pattern itself is imperceptible. Note though that we did go to some lengths to remove the pattern from awareness because of the potential for a visible pattern to influence our aftereffects. We achieved complete pattern imperceptibility in the case of our phase-contrast pattern with a mask and showed that our aftereffect still persisted. In all other cases at least some hint about the nature of the underlying pattern was available—at least occasionally. In the last experiment (masked sine-wave grating), where the pattern was sometimes seen, we demonstrated significant adaptation on the trials where it was not seen.
Our study thus relates to a broader class of studies that use psychophysical techniques to remove stimuli from awareness (Kim & Blake, 2005). In a sense, rapidly reversing the contrast of a pattern might be considered a “trick” for rendering the pattern invisible. We prefer to consider a spatiotemporal state space of all possible stimuli where sufficiently far along the temporal frequency axis modulations are imperceptible and sequences of stimuli are averaged or “blurred” together. We have provided strong evidence that patterned stimuli in this region of state space can be discerned by cortex even when they are not perceived. Our masking techniques certainly fit neatly among the set of “tricks” available for rendering the visible invisible.
Conclusions
An orientation-specific decrease in sensitivity can occur following exposure to a grating pattern whose contrast is reversed so rapidly that the contrast modulations as well as the grating itself cannot be seen. As such adaptation effects are thought to be cortically mediated, this strongly suggests that rapid, even unseen, modulations of contrast activate the cortex. Further, it is not just the existence of modulations that is signaled in the cortex, it appears that the individual images between contrast changes are processed as it is only these images—not the average of them—that contain orientation information in our experiments. The view that rapid contrast modulations—too fast to be seen—do not affect the cortex is erroneous. As such modulations are common in our environment, the potential for adverse effects should be explored.
Acknowledgments
We would like to thank the anonymous reviewers for insightful comments on a previous version of the text.
Footnotes
Commercial relationships: none.
Contributor Information
Michael Falconbridge, Psychology Department, UC San Diego, La Jolla, CA, USA.
Adam Ware, Psychology Department, UC San Diego, La Jolla, CA, USA.
Donald I. A. MacLeod, Psychology Department, UC San Diego, La Jolla, CA, USA
References
- Blake R, Fox R. Adaptation to invisible gratings and the site of binocular rivalry suppression. Nature. 1974;249:488–490. doi: 10.1038/249488a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. The Journal of Physiology. 1969;203:237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell JM. The influence of the intensity of the stimulus on the length of the reaction time. Brain. 1886;9:512–515. [Google Scholar]
- Crick F, Koch C. Towards a neurobiological theory of consciousness. Seminars in Neuroscience. 1990;2:263–275. [Google Scholar]
- Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Consciousness and neuroscience. Cerebral Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- Daugman JG. Dynamics of spatial channel adaptation. Boston: Harvard University; 1983. [Google Scholar]
- de Lange H. Research into the dynamic nature of the human fovea–cortex systems with intermittent and modulated light: I. Attenuation characteristics with white and colored light. Journal of the Optical Society of America. 1958;48:777–784. doi: 10.1364/josa.48.000777. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- He S, MacLeod DI. Orientation-selective adaptation and tilt after-effect from invisible patterns. Nature. 2001;411:473–476. doi: 10.1038/35078072. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. The Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T, Lehr L, Kirschfeld K. Cortical visual processing is temporally dispersed by luminance in human subjects. Neuroscience Letters. 1999;263:133–136. doi: 10.1016/s0304-3940(99)00137-8. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Frequency doubling in visual responses. Journal of the Optical Society of America. 1966;56:1628–1632. [Google Scholar]
- Kim CY, Blake R. Psychophysical magic: Rendering the visible ‘invisible’. Trends in Cognitive Sciences. 2005;9:381–388. doi: 10.1016/j.tics.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Tallon-Baudry C, Yvert B, Guenot M, Vighetto A, et al. Human lateral geniculate nucleus and visual cortex respond to screen flicker. Annals of Neurology. 2003;53:73–80. doi: 10.1002/ana.10403. [DOI] [PubMed] [Google Scholar]
- Lyskov E, Ponomarev V, Sandstrom M, Mild KH, Medvedev S. Steady-state visual evoked potentials to computer monitor flicker. International Journal of Psychophysiology. 1998;28:285–290. doi: 10.1016/s0167-8760(97)00074-3. [DOI] [PubMed] [Google Scholar]
- McKeeff TJ, Remus DA, Tong F. Temporal limitations in object processing across the human ventral visual pathway. Journal of Neurophysiology. 2007;98:382–393. doi: 10.1152/jn.00568.2006. [DOI] [PubMed] [Google Scholar]
- Pollen DA. Cortical areas in visual awareness. Nature. 1995;377:293–295. doi: 10.1038/377293b0. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nature Review Neuroscience. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Robson JG. Spatial and temporal contrast-sensitivity functions of the visual system. Journal of the Optical Society of America. 1966;56:1141–1142. [Google Scholar]
- Shady S, MacLeod DI, Fisher HS. Adaptation from invisible flicker. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5170–5173. doi: 10.1073/pnas.0303452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou TD, Leventhal AG. Organized arrangement of orientation-sensitive relay cells in the cat’s dorsal lateral geniculate nucleus. Journal of Neuroscience. 1989;9:4287–4302. doi: 10.1523/JNEUROSCI.09-12-04287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, MacLeod DI. Contingent aftereffects distinguish conscious and preconscious color processing. Nature Neuroscience. 2006;9:873–874. doi: 10.1038/nn1723. [DOI] [PubMed] [Google Scholar]
- Watson AB. Temporal Sensitivity. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of perception and human performance. New York: Wiley; 1986. pp. 6-1–6-43. [Google Scholar]
- Williams PE, Mechler F, Gordon J, Shapley R, Hawken MJ. Entrainment to video displays in primary visual cortex of macaque and humans. Journal of Neuroscience. 2004;24:8278–8288. doi: 10.1523/JNEUROSCI.2716-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]