Abstract
Intrathecal (IT) delivery of nicotinic agonists evokes dose dependent nocifensive behavior and cardiovascular responses. Previous studies suggested that these effects may be attenuated by the loss of substance P positive (sP(+)) primary afferents. To further characterize these cell systems, we examined the effect of selectively destroying neurokinin 1 receptor bearing (NK1-r (+) ) dorsal horn neurons on IT nicotinic agonist evoked responses. In the dorsal spinal cord, confocal immunohistochemical microscopy revealed that nAChR subunits (α3, α4, α5, β2 and β4), NeuN B (neuronal marker) and NK1-r were all co-expressed in the superficial dorsal horn; however α3, α5, β2 and β4 exhibited the highest degree of colocalization with NK1-r expressing neurons. After intrathecal substance P-saporin (sP-SAP), NK1-r(+) cell bodies and dendrites in the superficial dorsal horn were largely abolished. The greatest loss in co-expression of nAChR subunits with NK1-r was observed with α3, α5, β2 and β4 subunits. Following intrathecal sPSAP, the nocifensive responses to all nicotinic agonists were reduced; however, in contrast, while cardiovascular responses evoked by IT nicotine were unaltered, IT cytisine and epibatidine exhibited enhanced tachycardia and pressor responses. These results indicate subunit-specific relationships between the NK1-r and nicotinic receptor systems. The loss of nocifensive activity after destruction of the NK1-r bearing cells in spite of the persistence of nicotinic subunits on other cells, emphasizes the importance of the superficial marginal neuron in mediating these nicotinic effects. Further, the exaggerated cardiovascular responses to cytisine following loss of NK1-r bearing cells suggest the presence of a nicotinic receptor-mediated stimulation of inhibitory circuits at the spinal level.
Keywords: spinal cord, cardiovascular responses, nicotinic receptor, substance P, spinal inhibitory circuits
Introduction
Neuronal nicotinic receptors are associated with spinal systems which underlie nociceptive signal processing and autonomic efferent responses (Dussor et al., 2004; Dussor et al., 2005; Khan et al., 2001; Khan et al., 2004). Thus, spinal administration of various nicotinic agonists elicits distinct cardiovascular, nocifensive and antinociceptive responses through different nicotinic receptor subtypes (Khan et al., 2003; Khan et al., 2004; Bradaia et al., 2005). Binding studies show that expression of these receptor proteins is higher in the dorsal than in the ventral horn (Khan et al., 2003; Khan et al., 2004; Perry et al., 2002), with binding largely localizing within the superficial layer of the dorsal horn where peptidergic primary afferent terminals are present. Neuronal nicotinic receptors are pentameric structures composed of various combinations of protein subunits. Current evidence indicates the selective expression of these subunits in several spinal cord regions (Genzen et al., 2001; Ishii et al., 2005; Khan et al., 2003). At the cellular level, nicotinic receptor subunits (notably α3, α4, α5, β2 and β4) have been localized on primary afferent terminals IB4(+) and IB4(−)-peptidergic(+) terminals. These IB4(+) and IB4(−) afferents are considered to encode high threshold stimuli and play a major role in mediating nociceptive responses to spinally administered nicotinic agonists through the release of excitatory amino acid neurotransmitters (EAA) and EAA plus neuropeptides, respectively (Fundytus et al., 2002; Khan et al., 1996; Khan et al., 2004; Puttfarcken et al., 1997). These results suggest a possible presynaptic regulation of excitatory neurotransmitter release by nicotinic receptors. After neonatal capsaicin treatment, which destroys IB4(−) peptidergic afferent neurons, there is a loss of substance P and a reduction in α3, α4, α5, β2 and β4 nicotinic receptor subunits. In such lesioned models, the agitation behavior and hypertension evoked by IT nicotinic agonists are attenuated. An important question relates to the role of the second order NK1-r positive neurons in the superficial layer which are driven by peptidergic high threshold C fibers. These neurons are believed to be important for pain processing. Not only do they provide ascending input into the spinothalamic system (Li et al., 1997), but it now appears that these lamina I neurons are the initial element in spinobulbospinal facilitatory loops that regulate dorsal horn excitability (Suzuki et al., 2002; Suzuki et al., 2006) and autonomic outflow (Benarroch, 2001; Cortelli and Pierangeli, 2003). Therefore, in the present study we examined the role of second order NK1-r positive neurons on the effects of spinally administered nicotinic agonists. This was accomplished through selective destruction of these neurons in the spinal cord using intrathecal -substance P-saporin conjugate (sP-SAP). This material binds to NK1 receptor positive neurons. The internalization of the NK1 receptor initiated by ligand binding serves to internalize the conjugate leading to the death of that particular NK-1-r bearing neuron (Mantyh et al., 1997).
Materials and Methods
Experimental Animals
Male Sprague-Dawley rats (300-350 g) rats were purchased from Harlan Co. (Indianapolis, IN) and housed in the campus vivarium maintained on 12-hr light/dark cycles with standard rat chow and water ad libitum. All studies were carried out according to protocols reviewed and approved by the University of California San Diego Institutional Animal Care and Use Committee.
Selective Ablation of Substance P Receptive Neurons
Male eleven-week-old rats were implanted with IT catheters under isoflurane-oxygen anesthesia (Khan et al., 1996). Four to five days following catheter implantation, the animals were re-anesthetized and 10 μl of 10 μM substance P-Saporin (sP-SAP) conjugate (Advanced Targeting Systems, San Diego, CA) was administered intrathecally, as previously described, while control animals received 10 μl of 10 μM unconjugated saporin. Eleven to fifteen days following the treatment, cardiovascular and nociceptive responses to IT nicotinic agonists were examined. Separate groups of IT sP-SAP and control rats were used for the immunohistochemical assays.
Immunofluorescence and Confocal Microscopy
Antibodies to nAChR subunits
Rabbit polyclonal antibodies to neuronal nAChR subunits (α3, α4, α5, β2 and β4) were generated using either synthetic peptide fragments, specifically 22 to 26 amino acids in length from within the cytoplasmic domain between transmembrane segments 3 and 4, or the GST-nicotinic receptor subunit fusion protein which constituted the cytoplasmic domain between transmembrane segments 3 and 4 (Khan et al., 2003; Khan et al., 2004). The antibodies were affinity purified by coupling the antigen(s) to a solid phase support using the UltraLink Immobilization kit (Pierce). As determined by Western blot analysis and immunohistochemical assays, these antibodies exhibit specificity for the specific subunit and/or subunit sequence to which they were generated (Khan et al., 2003).
Immunohistochemical Assays
The procedure for immunohistochemical assays by confocal microscopy has been described previously (Khan et al., 2003). Briefly, spinal cord tissue fixed in 4% paraformaldehyde was sectioned (100 μm thickness) with a Vibratome and the tissue sections incubated overnight with anti-nAChR subunit antibodies (∼ 1μg/ml). Other antibodies used in the assays were as follows: anti-Substance P receptor (NK-1 r) (1:1000 of source stock, Chemicon, CA); anti-NeuN B (1:200 of source stock, Chemicon, CA).
After incubation with primary antibodies, sections were incubated with appropriate secondary antibodies (1:75 in buffer) conjugated to Cy™5 (Cyanine), FITC or Lissamine-Rhodamine fluorescence probes (Jackson Immuno Research, PA). Immunofluorescence labeling of the spinal tissue was observed using a Bio-Rad MRC-1024 confocal system attached to a Zeiss Axiovert 35 M microscope. The collected images were processed using Adobe PhotoShop. For each comparison of paired samples conducted in the same experiment, spinal sections from sP-SAP-treated and control rats were matched for treatment, post-tissue collection manipulations, and immunoassay protocols. To compare fluorescence intensities between two images, images were collected with the same laser intensity and gain setting on the same day. Moreover, the images were taken as single optical sections with identical confocal aperture setting. Post-capture manipulation of the images for gray levels and contrast employed identical settings. As samples did not exhibit significant changes in label intensities (for a particular antibody) when scanned at different times or with different microscopes, there was no evidence of significant photobleaching in this time frame. Differences in mean pixel values from treated and control rats were analyzed from matched areas for statistical significance by paired t-test. A one-tailed ‘p’ value of < 0.05 was considered as significant.
Quantification
To quantify co-expression of nAChR subunits, NK1-r proteins in neurons, triple channel confocal images containing pixel intensities corresponding to neuronal marker (NeuN B); NK1-r protein and a specific nAChR subunit were analyzed. Initially, a grid was placed on the merged image using Adobe Photoshop and neuronal bodies were identified in that grid in the channel for anti-NeuN B staining. Similarly, all the grid boxes in the superficial layer (encompassing laminae I to III) were observed and the neuronal bodies were counted in that region. Secondly, using the same co-ordinates to identify a specific neuron, it was determined if pixel densities were evident in the channel for nAChR subunit. Thus, the number of neurons expressing a specific nAChR subunit was identified. This was followed by identifying these nAChR subunit protein expressing neurons that also stained for NK1-r protein in it's respective channel. Accordingly, the co-expression of NK1-r and the specific nAChR subunit in a specific neuron was determined. The co-localization of NK1-r with nAChR subunit was expressed both with respect to total number of neurons and neurons also expressing the specific nAChR subunit (labeled as % of nAChR + neurons).
Surgical Procedures, Arterial Blood Pressure and Heart Rate Recordings
For both the treated and control rats, at the time of testing, tail arteries were catheterized to monitor blood pressure and heart rate as previously described (Khan et al., 1996). Nicotinic agonists were administered intrathecally according to a previously detailed procedure (Khan et al., 1996). Cardiovascular parameters were recorded by, and data analyzed with the Ponemah Physiology Platform-3 (Gould Instruments Systems, Valley View, OH).
Quantification of nocifensive behaviors
As the long-term objective of this research is to examine the relationships between nocifensive behaviors and autonomic control of associated cardiovascular responses, a behavioral measure which can provide quantification of both responses, over time, was developed (Khan et al., 2004; Khan et al., 1996). This method involves observational scoring of selected behavioral responses accepted as potentially reflecting a nociceptive response in rodents. Briefly, a score of 1 was given for each of the following behavioral responses: 1) brisk movement of the head and grooming activity of the front limbs; 2) brisk ambulatory movement of the hind limbs; 3) whole body twisting and turning; and 4) tail erection and/or high pitched squeaking. The responses typically occurred in the sequence described, and the maximum assignable score per minute was 4. This provided both a measure of the time-dependent behavioral responses observed, and the duration of the nocifensive response pattern. Areas under the intensity-time curve were analyzed yielding an estimate of the maximum nocifensive response (defined as nocifensive index) following nicotinic agonist administration (Khan et al., 2004). Similarly, the cardiovascular response profile over the ten minute period was analyzed, by the minute average, permitting analysis of the temporal nature of the two response patterns.
RESULTS
Dorsal horn Neurokinin 1 and nicotinic receptor subunit protein distribution
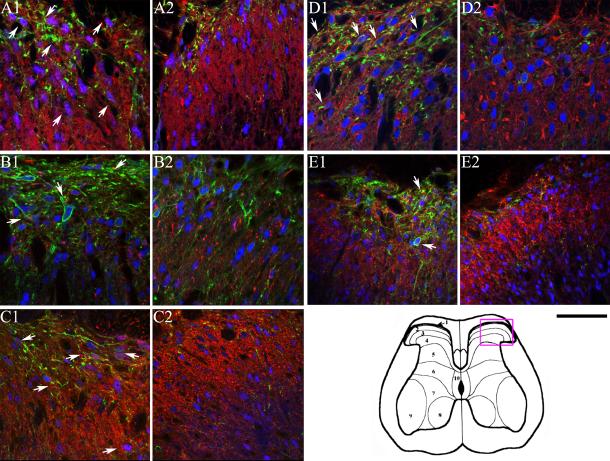
In control animals, NK1-receptor staining was identified on both fibers and cell bodies throughout the superficial dorsal horn localizing primarily to lamina I and to a lesser degree in laminas II and III (Figure 1A1). The green stain represents NK1 receptors, whereas the blue stain represents neuronal structures (Neun B positive). With respect to the nAChR subunit proteins, anti-sera to α3, α4, α5, β2 and β4 exhibited staining patterns in control rats that were consistent with previously reported observations (composites in Figures 2 and 3, also cf. Khan et al. 2003). In brief, α3, α4, α5, β2 and β4 subunit protein immunoreactivities (red stains) were observed throughout the dorsal horn with dense staining in the superficial layer (Figures 2 and 3).
Figure 1.
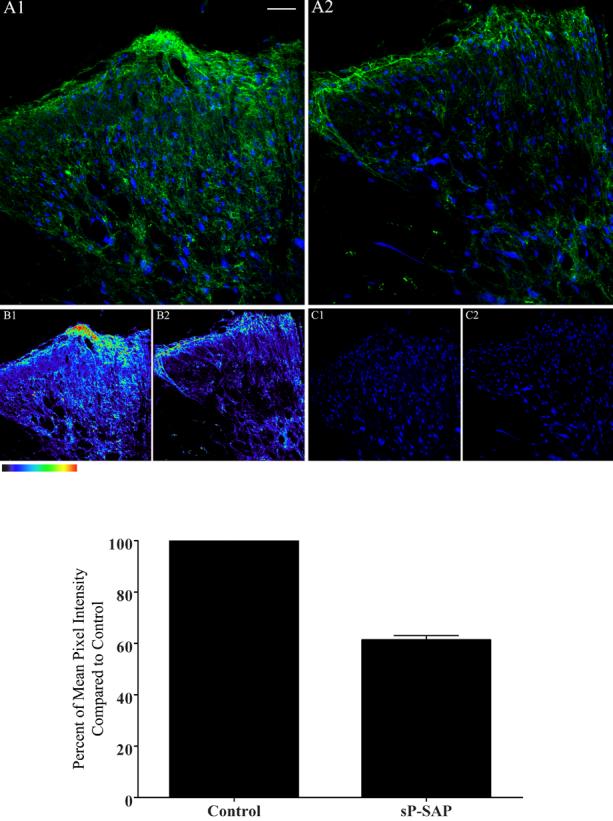
Confocal images illustrating immunofluorescence of lumbar spinal sections from controls (saporin only, panel A1) and sP-SAP (saporin-substance P conjugate) treated rats (panels A2) using anti-neuron specific nuclear protein antisera (Neun B) (in blue), and anti-NK1 receptor antisera (in green). Panels B1 and B2 depict the immunofluorescence in color-coded intensities of the actual fluorescence intensities of the anti-NK1 receptor binding (panels A1 and A2); whereas panels C1 and C2 represent NeuN B staining alone as depicted in panels A1 and A2 respectively. The color table gradient bar (rectangular bar below panel B1) corresponds to the green fluorescence intensity in figures 1A1 and 1A2, which represents zero to 255 pixels. White bars represent 50 μm for panels A1 and A2 and 100μm for panels B1, B2, C1 and C2. The area of the spinal cord photographed is represented by the rectangular box on the cartoon of the cross-section of lumbar spinal cord as shown in bottom right corner of figure 3.
Figure 2.
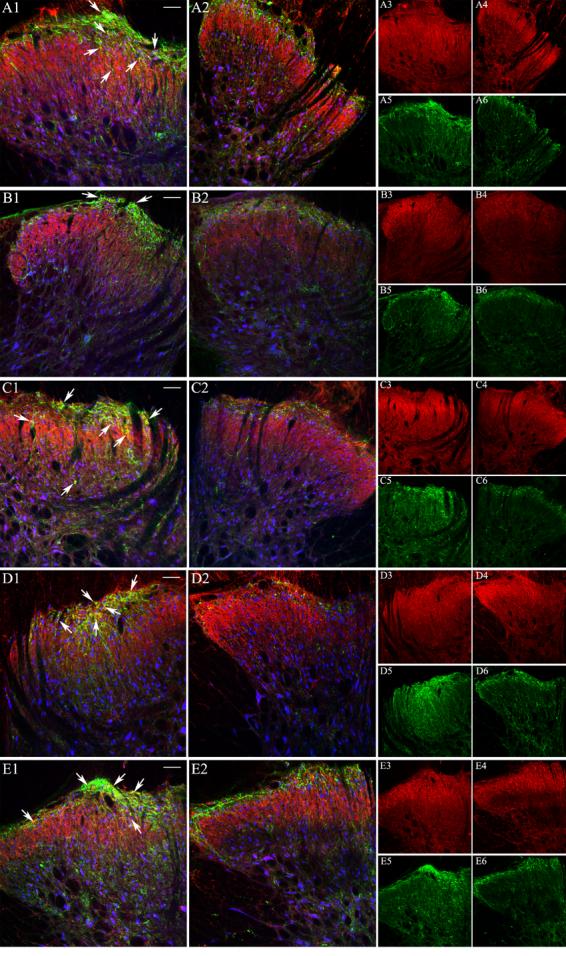
Confocal microscopy images depicting immunofluorescence labeling in control (panels A1, B1, C1, D1, and E1) and IT sP-SAP (saporin-substance P conjugate) treated (panels A2, B2, C2, D2, and E2) rats following binding with anti-nAChR subunit antibodies (in red), anti-NK1 receptor (in green) and anti-NeuNB (in blue). In panel pairs A1 and A2, B1 and B2, C1 and C2, D1 and D2 and E1 and E2, red color represents staining with anti-α3, anti-α4, anti-α5, anti-β2 and anti-β4 subunit antibodies, respectively. Panel pairs A3 and A4, B3 and B4, C3 and C4, D3 and D4, and E3 and E4 are identical to panels A1 and A2, B1 and B2, C1 and C2, D1 and D2, and E1 and E2, respectively except they represent fluorescence labeling by the anti-nAChR subunit only. Similarly, Panel pairs A5 and A6, B5 and B6, C5 and C6, D5 and D6, and E5 and E6 represent fluorescence labeling by anti-NK1 receptor antibody. White bars represent 50 μm and 100 μm for left two columns and right two columns of panels, respectively. The area of the spinal cord photographed is represented by the rectangular box on the cartoon of the cross-section of lumbar spinal cord as shown in Figure 3.
Figure 3.
Confocal microscopy images depicting immunofluorescence labeling in control (panels A1, B1, C1, D1, and E1) and IT sP-SAP (saporin-substance P conjugate) treated (panels A2, B2, C2, D2, and E2) rats following binding with anti-nAChR subunit antibodies (in red), anti-NK1 receptor (in green), and anti-NeuNB (in blue). In panel pairs A1 and A2, B1 and B2, C1 and C2, D1 and D2 and E1 and E2, red color represents staining with anti-α3, anti-α4, anti-α5, anti-β2 and anti-β4 subunit antibodies, respectively. The black bar under panel E2 represents 50 μm. The approximate area of the spinal cord photographed is represented by the rectangular box on the cartoon of the cross-section of lumbar spinal cord as shown in the bottom right corner.
Control colocalization
Colocalization of NK1-r subunits (green stain) with a specific nAChR subunit (red stain) was determined by identifying neurons (anti-NeuNB positive, blue stain in figures 2 and 3) that also expressed green and red stains. According to this protocol, we observed that α3 is expressed in almost all neurons (structures with blue stains) in this region. However, only 30% of these neurons in the superficial layer also expressed NK1 receptor along with α3 (Fig 3A1; Table 1). Similar to anti-α3, antibodies to α5, and β2 subunit also showed significant colocalization with NK1-r (Figures 2 and 3; also Table 1). Interestingly, as previously observed, anti-β4 showed very limited co-expression with NK1-r in neurons (approximately 10% of the total number of neurons; Table 1, also cf. 15); however, when colocalization of anti-β4 with NK1-r in β4 positive neurons were only considered, it represented greater than 50% of the β4 positive neurons (Figures 2E1 and 2E2; and 3E1 and E2, Table 1). Compared to α3, α5 and β2 subunits, α4 subunit exhibited limited co-expression with NK1-r proteins in the second order neurons of the superficial layer of the dorsal horn. Although α3, α5 and β2 showed co-localization with NK1-r, no significant differences in staining of these subunits was observed between the sP-SAP-treated and control rats in the dorsal horn region, indicating that the preponderancy in expression of these subunits were on non NK1 (+) cells (Figures 2A through 2E, and 3A through 3E; Table 2). We note that under the conditions used in the above quantification of co-localization, NK1-r staining could be observed in 24% of total neurons in the control rats as compared to 9% in the sp-SAP-treated rats in the superficial layers of the dorsal horn.
Table 1.
Co-expression of NK1-r proteins and various nAChR subunit proteins in the neuronal cell bodies of the dorsal thoraco-lumbar spinal cord regions.
| Percent of total Neurons (Neun B positive) that express |
Percent of nAChR positive cell bodies that also express |
||||||
|---|---|---|---|---|---|---|---|
| nAChR | nAChR + NK1-r |
nAChR | nAChR + NK1-r |
Neun B + NK1-r |
Neun B + NK1-r |
||
| nAChR subunits |
Laminae | Control | Sp-SAP | Control | Sp-SAP | ||
| α3 | I-III | 99.4+1.0 | 34.8+2.4 | 96.0+1.0 | 12.6+2.0** | 34.9+2.4 | 13.2+2.0** |
| IV-V | 97.1+1.2 | 30.7+5.9 | 97.8+1.0 | 15.6+2.2* | 31.7+5.9 | 15.9+2.1** | |
| α4 | I-III | 97.2+1.0 | 10.6+1.6 | 96.8+1.2 | 6.6+0.9* | 11.8+2.1 | 6.9+1.3* |
| IV-V | 90.5+2.6 | 9.5+2.4 | 91.4+6.4 | 8.8+1.7 | 10.7+3.0 | 9.7+1.8 | |
| α5 | I-III | 97.9+0.7 | 34.1+5.7 | 93.8+2.5 | 6.7+1.0*** | 34.9+5.9 | 7.3+1.3*** |
| IV-V | 99.8+0.2 | 42.8+7.4 | 99.5+0.5 | 18.2+5.9** | 42.7+7.4 | 18.2+5.9** | |
| β2 | I-III | 90.6+8.3 | 28.9+2.6 | 84.9+13.3 | 9.4+2.7*** | 33.6+5.2 | 12.4+3.0** |
| IV-V | 99.2+0.5 | 33.9+5.3 | 95.9+3.3 | 18.6+4.3** | 34.9+5.3 | 19.7+5.3* | |
| β4 | I-III | 25.1+3.8 | 10.8+1.8 | 24.7+3.1 | 4.5+0.4* | 44.1+2.1 | 19.3+2.4*** |
| IV-V | 19.8+3.0 | 10.2+2.1 | 23.9+3.9 | 5.9+1.2 | 52.6+9.9 | 26.2+4.4** | |
Each value, shown as a mean±sem, represents the percent of neurons that show expression of the specific nAChR subunit, NeuNB (neuronal marker) and NK1 receptor combinations in sP-SAP-treated and vehicle-treated control rats. The treated and control groups were compared by unpaired t-test. Each value represents at least 4 pairs of comparisons. ***, ** and * denote ‘p’ values of <0.001, <0.01 and <0.05 as compared to controls for the same matching column category as in sP-SAP treated rats.
Table 2.
Mean Pixel intensity per area of various nicotinic receptor subunits in control and sP-SAP treated rats.
| Control | sP-SAP | |
|---|---|---|
| α3 | 1.08±0.23 | 1.28±0.37 n.s. |
| α4 | 0.53±0.39 | 0.39±0.09 n.s. |
| α5 | 0.73±0.12 | 0.50±0.04 n.s. |
| β2 | 0.41±0.05 | 0.48±0.10 n.s. |
| β4 | 0.43±0.04 | 0.32±0.08 n.s. |
Each value, shown as a mean±sem, represents the mean pixel intensity (0 to 255 pixels scale) per unit area in the fluorescence microscopy images from superficial dorsal horn region of sP-SAP-treated and only saporin-treated control rats. The treated and control groups were compared by paired ‘t-test’ (two-tailed). Each value represents at least 5 pairs of comparisons. n.s.= not significant.
sP-SAP Treated animals
Following sP-SAP lesioning there was a significant reduction of NK1-r protein in all three lamina of the dorsal horn (Figures 1A1 and 1A2 and pseudo color coded intensities, Figure 1B1 and 1B2) at the level of the lumbar spinal cord Pixel density analysis also indicated that the lesioning effect was significant across the entire superficial dorsal horn section (Figure 1, bottom bar graph). Though not shown here, comparable analyses carried out at more rostral thoracic spinal segments failed to show any changes in the presence of superficial or deep dorsal horn NK1(+) neurons, including in those neurons present in the intermediolateral cell column or in lamina X (data not shown). As shown, sP-SAP treatment resulted in approximately 40% reduction of the pixel density from NK1-r staining in the treated rats (p<0.001 as compared to controls) in the dorsal horn region (lamina I to IV). Similar to previously reported studies (Mantyh et al. 1997), we did not observe any significant differences in the number of neurons (as indicated by blue stains, Figure 1, C1 and C2) in the superficial dorsal horn region between sp-SAP treated and control rats (data not shown) emphasizing the limited number of NK1-r (+) neurons in this region. However, in rats treated with IT sP-SAP, colocalization of anti-α3 with NK-1 receptor staining was lost as expression of NeuNB (blue in neurons), NK1-r (green) and α3 (red) in the same neuron was reduced to 5% of the total neurons in a specific region of the dorsal lumbar spinal cord (Figures 3A2 and Table 1) in the treated rats. Similar to α3, the various degree of colocalization of other subunit proteins was also significantly reduced in treated compared to control rats (Figures 2 and 3, Table 1).
Effect of IT sP-SAP on cardiovascular and nociceptive responses to spinally administered nicotinic agonist
To determine whether substance P and/or NK1-r bearing neurons/ terminals in the spinal cord plays a role in mediating the nocifensive or the cardiovascular responses to spinal nicotinic agonists, the responses to IT nicotinic agonists were compared between sP-SAP treated and vehicle-treated control rats. Prior studies established the concurrent response profiles of three nicotinic receptor agonists (nicotine, cytisine and epibatidine) following IT administration in various rat strains (Khan et al., 1996; Khan et al., 1994a; Khan et al., 2004). Those dose-ranging studies demonstrated that 0.05 μg of IT nicotine or cytisine elicit minimal cardiovascular and behavioral responses while a 50.0 μg dose of the agonists produced unacceptable nocifensive responses. Accordingly, only the middle two doses for nicotine and cytisine were used in this study. The basal blood pressure or heart rate in the SAP-treated rats as compared to control rats were not different from each other (data not shown).
IT Nicotine
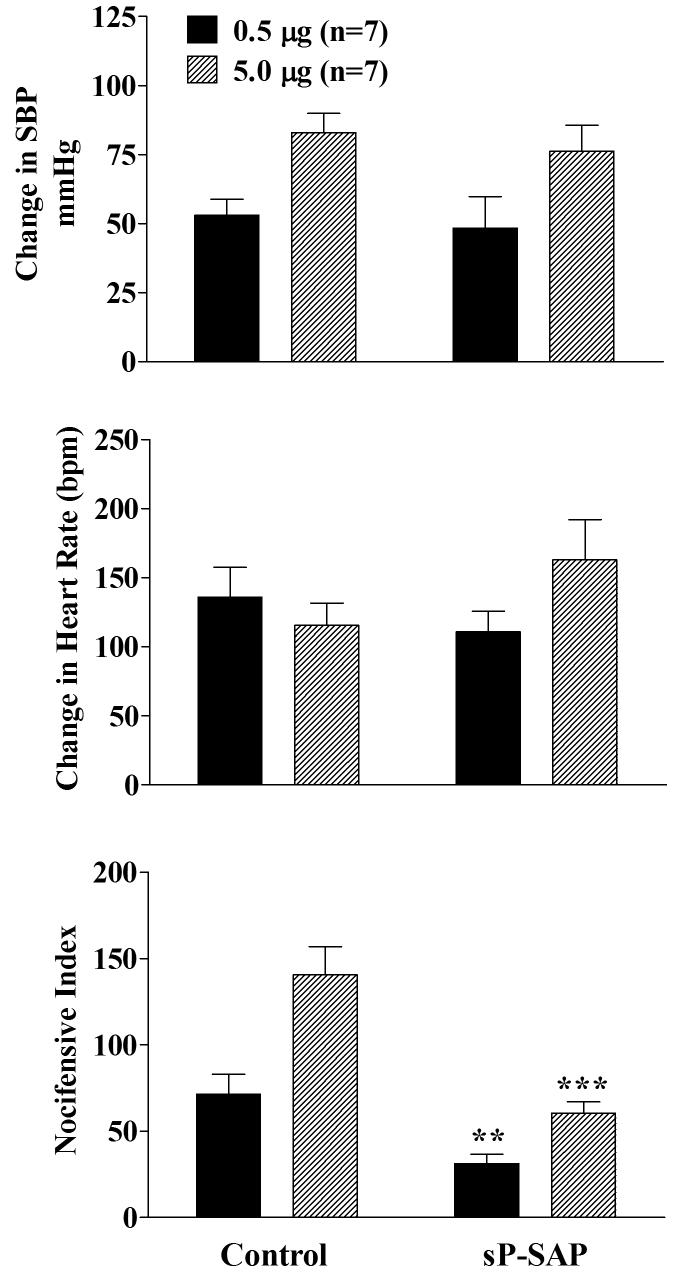
As shown in figure 4, no differences in the change in blood pressure or heart rate response were observed between control and sP-SAP groups following sequentially increasing doses of nicotine given 30 minutes apart. However, the behavioral nocifensive response (agitation) was significantly attenuated for both doses in sP-SAP treated rats.
Figure 4.
Effects of 0.5 μg (solid) and 5.0 μg (hatched) of intrathecal nicotine administrations on systolic blood pressure, heart rate and on the nocicefensive responses in control (saporin only) and sP-SAP (saporin-substance P conjugate) pretreated rats. All responses denote the maximum response measured during the 10 minute period following administration of each dose of the nicotinic agonist. The second dose of nicotine was administered 30 min following the initial dosing. Each value represents the mean ± SEM. **p<0.01 and ***p<0.001 as compared to control rats for the specific dose as determined by two-tailed unpaired Student's t-test.
IT Cytisine
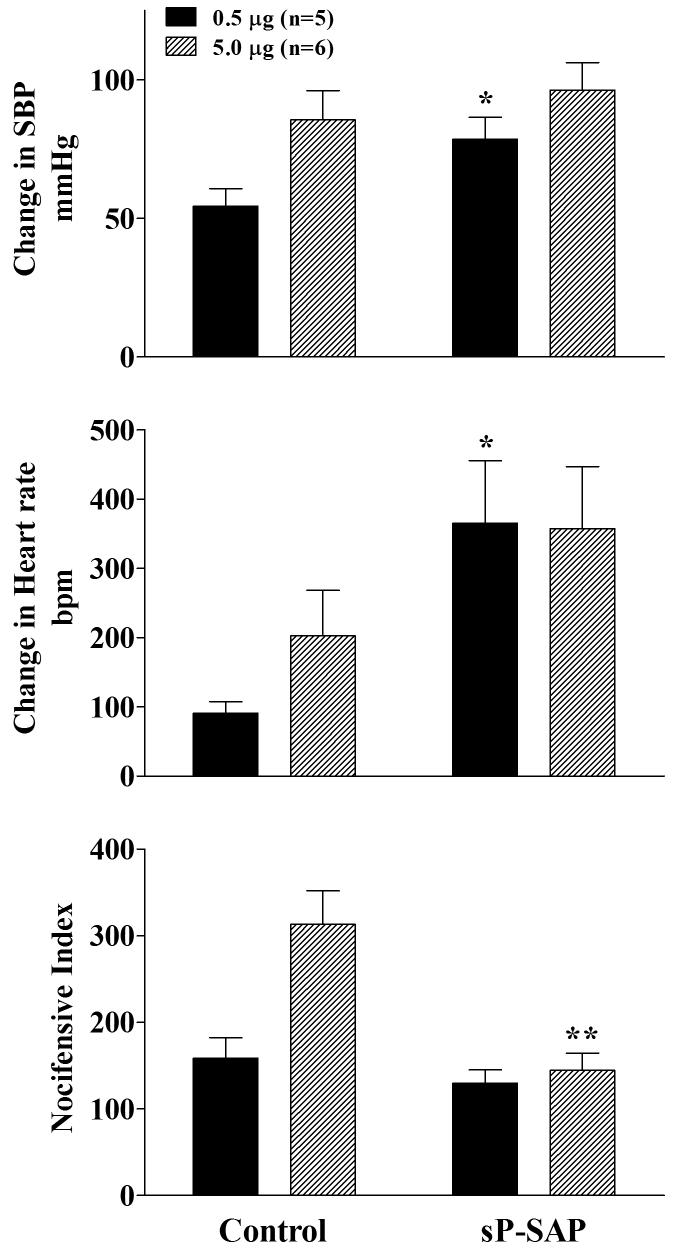
In marked contrast to nicotine, both the pressor and tachycardia responses to cytisine, an α3β4 specific agonist, were potentiated in the sP-SAP treated versus control rats. Thus, IT cytisine resulted in greater increases in blood pressure and heart rate in treated rats, but the potentiation was significant only for the lower dose of cytisine (Figure 5). With the nocifensive response, sP-SAP reduced the magnitude of the IT cytisine as it did for nicotine.
Figure 5.
Effects of 0.5 μg (solid) and 5.0 μg (hatched) of intrathecal cytisine administrations on systolic blood pressure, heart rate and nocifensive responses in control (saporin only) and sP-SAP (saporin-substance P conjugate) pretreated rats. All responses denote the maximum response measured during the 10 minute period following administration of each dose of the nicotinic agonist. The second dose of cytisine was administered 30 min following the initial dosing. Each value represents the mean ± SEM. *p<0.05 and **p<0.01 as compared to vehicle-treated rats for the specific dose as determined by two-tailed unpaired Student's t-test.
IT Epibatidine
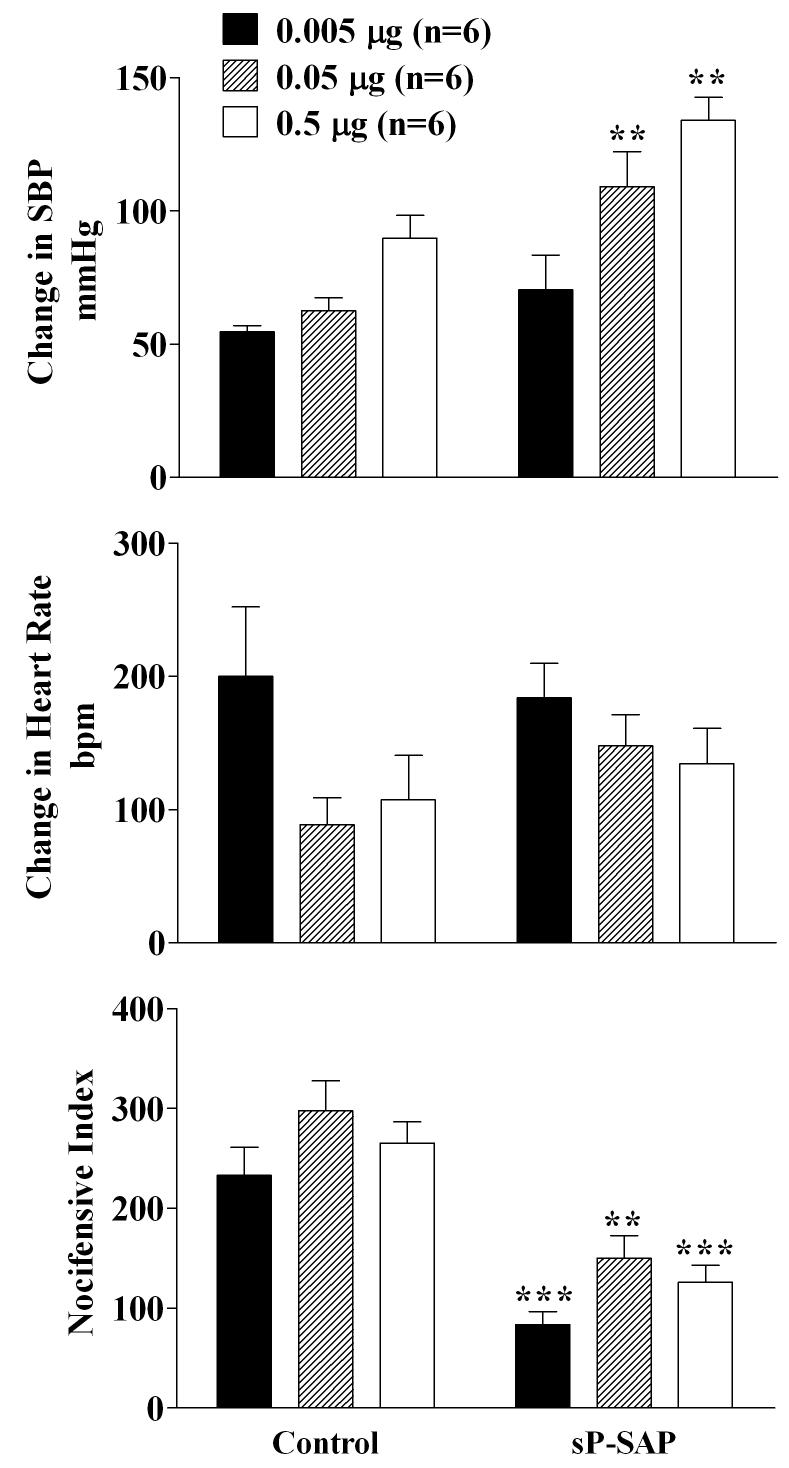
Based on our previous studies that epibatidine in contrast to nicotine or cytisine binds to at least two different receptor subtypes that differ in affinity, we employed three doses of epibatidine with the intent of determining the effect of sP-SAP treatment on the various subtypes of receptors stimulated by epibatidine. A dose-dependent increase in SBP was observed in control and this effect was potenitated in the sP-SAP-treated rats (Figure 6). The HR response to IT epibatidine was unaltered by sP-SAP treatment (Figure 6). As with the other two agonists, the nocifensive responses were significantly reduced by sP-SAP treatment as compared to controls (Figure 6).
Figure 6.
Effects of 0.005 μg (solid), 0.05 μg (hatched) and 0.5 μg (open) of intrathecal epibatidine administrations on systolic blood pressure, heart rate and nocifensive responses in control (saporin only) and sP-SAP (saporin-substance P conjugate) pretreated rats. All responses denote the maximum response measured during the 10 minute period following administration of each dose of the nicotinic agonist. The three consecutive doses of epibatidine were administered 30 minutes apart. Each value represents the mean ± SEM. ***p<0.001 and **p<0.01 as compared to control rats for the specific dose as determined by two-tailed unpaired Student's t-test.
DISCUSSION
The challenges in identifying how and where neuronal nicotinic receptors mediate and/or modulate afferent and efferent signaling in the spinal cord is exacerbated by the synaptic connectivity within the spinal cord which is itself, exceedingly complex. In addition, neuronal nicotinic receptors are comprised of combinations of alpha and beta subunits, some of which bind agonists while others serve to modulate receptor functions. Genetically engineered murine strains have provided insight into the importance of specific nicotinic subunits; however, compensatory adjustment of expression of other subunits to fill the void of the targeted subunit may blur identifying the functional actions of specific receptor subunit compositions in the animal. The experimental approach utilized here was to target neurons expressing the NK1 substance P receptor using saporin-substance P since prior studies by this laboratory and others had established that nicotinic receptors on primary afferent terminals mediate spinally administered nicotinic agonist elicited nociceptive responses (Fundytus et al., 2002; Khan et al., 1996; Khan et al., 2004; Puttfarcken et al., 1997). The present experimental approach was useful in identifying areas of co-localization of NK1-r and nicotinic receptor subunits and equally important, for the first time demonstrating the involvement of these interactions in dictating both the magnitude of the nocifensive and cardiovascular responses.
In the present studies, a significant immunochemical colocalization of α3, α4, α5 and β4 nAChR subunits were found with cells expressing the NK1-r in the superficial dorsal horn. Treatment with IT sP-SAP resulted in a significant reduction in NK1-r (+) cells, but this resulted in only a modest reduction in the total expression of dorsal horn nAChR subunits. This modest effect is consistent with the fact that majority of the dorsal horn neurons do not express the NK1-r and would thus not be responsive to IT sP-SAP. The importance of these few NK1(+) superficial dorsal horn neurons and their associated nicotinic receptors is emphasized, however, by the prominent changes observed in the effects produced by IT nicotinic agonists (epibatidine, cytisine and nicotine ) in the sP-SAP treated animals as compared to controls. The nocifensive response to all nicotinic agonists tested was reduced by sP-SAP treatment. In contrast, the cardiovascular responses were agonist-specific. In the intact animal, all three nicotinic agonists largely resulted in both an increased HR and BP after IT delivery, reflecting heightened sympathetic activity. However, following loss of NK1 (+) neurons, the effect of IT cytisine and epibatidine on BP was potentiated while the effect to IT nicotine was not altered. The difference in the effect of sP-SAP treatment on these agents will be discussed further below, but the differences emphasize two points. First, that the agitated state initiated by the IT agonists reflect the role of nicotinic receptors on these superficial NK1 (+) lamina I and II neurons which contribute to spinofugal projection to brainstem and higher centers. Secondly, the change in blood pressure initiated by the IT agonists is not a simple function of the nociceptive state concurrently initiated by the intrathecal delivery of these agents. Thus, in the sP-SAP treated animals the BP pressure effect was actually enhanced inspite of the reduction in concurrent nocifensive behavior.
Colocalization of NK1-r and nicotinic receptor subunits in spinal neurons
All of the five nAChR subunit antibodies were to some degree co-expressed with NK1-r expression; however, α3, α5 and β2 showed the highest percentage of co-localization with the tachykinin receptor in the total neurons counted in a specific region of the dorsal horn. In our previous studies, we have observed that α3, α5, and β2 show expression in both presynaptic terminals and on the soma of post-synaptic neurons (Khan et al., 2003). Thus, colocalization of these three subunits with NK1-r receptors in the marginal neurons in the superficial layer as well as neurons in lamina II and III is consistent with our previous observations. Similarly, a small degree of co-localization of β4 subunit antibody with NK1-r positive second order neurons in the superficial layers of the dorsal horn is consistent with our previous observations that β4 staining is mostly on the presynaptic sites. Interestingly, while α4 subunit protein is present mostly in post-synaptic locations in the upper layers of the dorsal horn (Khan et al., 2003; Khan et al., 2004), very little colocalization of α4 with NK1-r proteins was observed in the superficial layers. Thus, in relation to functional mapping of the nicotinic receptors in the spinal cord, the present study demonstrate that nicotinic receptor subtypes, most probably a composite of α3, α5 and β2 and to a limited extent β4 are expressed in both NK1-r positive marginal neurons and other NK1-r positive neurons in the superficial layers of the dorsal horn post-synaptic to primary afferent terminal. Secondly, α4 and β4 alone or in combination with α3, α5 and β2 are expressed in post-synaptic neurons that do not express NK1-r receptors. The potential involvement of these receptors became evident as the cardiovascular and behavioral responses to spinal nicotinic agonists were altered (enhanced) in the sP-SAP treated rats.
Substance P and the role of NK1-r bearing neurons in physiological responses to spinally administered nicotinic agonists
Nocifensive Responses
Given that IT nicotinic agonists elicited nocifensive effects are diminished in capsaicin treated animals (Khan et al. 2004), the loss of the nocifensive response with IT sP-SAP confirms the importance of the linkage of peptidergic afferent with the superficial second order NK1(+) neurons. These results suggest the possibility that IT nicotinic agonists may evoke the nocifensive response by a presynaptic effect upon nicotinic receptors on capsaicin-sensitive-peptidergic C fiber terminals (e.g.,) or by a direct effect upon the sP-SAP sensitive second order neuron (Fig. 7). Based on our present and previous studies combining pharmacological agents and capsaicin treatment (Khan et al., 2003; Khan et al., 2004), we hypothesize that α3, α5, or β4 containing pre-synaptic receptors on the primary afferent terminals (that show selectivity for cytisine) release Substance P and glutamate from the primary afferent terminals. In addition, those post-synaptic neurons expressing α4 subunits in combination with other nicotinic receptor subunits mediate the substance P independent nociceptive response (that is not nicotinic agonist selective). This latter pathway might include second order neurons that are present in the superficial layer and the dorsal horn and release EAA upon stimulation (Alvarez et al., 2004; Benoliel et al., 2000). Our earlier findings indeed demonstrated that IT nicotinic agonists evoked significant spinal glutamate release (Khan et al., 1996; Khan et al., 2001). The fact that the α4 subunit exhibited a smaller degree of co-localization with NK-1 receptor than α3 subunit protein (Figures 2 and 3) provides credence to such an interpretation.
Figure 7.
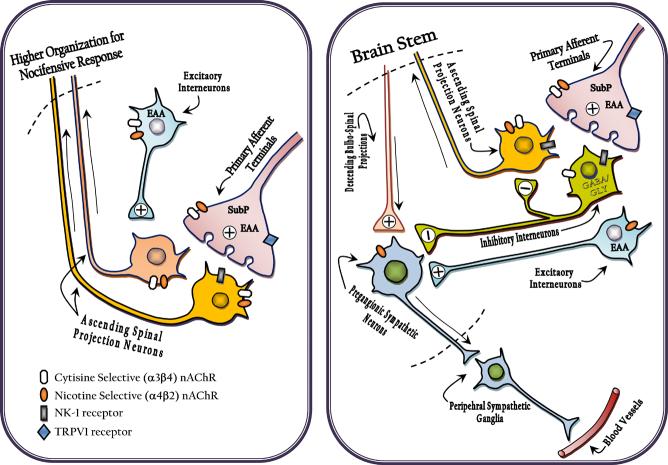
Proposed organization of dorsal horn circuitry underlying nocifensive behavior (left and cardiovascular response (right) evoked by local intrathecal cytisine (acting though primarily α3β4) and nicotine (acting though primarily α4β2) receptors. The location of these subunits are hypothesized based on changes observed in the behavior after pretreatment with IT capsaicin (Khan et al., 2004), IT sP-SAP (present study) and spinal transection (Khan et al., 1994b).
Nocifensive behavior (left) : In brief, presynaptic receptor effects on TRPV1 (+) afferent terminals and post synaptic NK1(+) neurons leads to activation of spinofugal outflow to higher centers initiating organized nocifensive behaviors.
Cardiovascular (right) The block by spinal transfection of the effect of cytisine, but not nicotine, suggests respectively, a bulbospinal linakge and a local (direct or by excitatory interneurons ) activation of preganglionic interneurons. The enhanced effect of IT cytisine in sP-Sap treated rats may reflect the normal activation of local NK-1(+) bearing inhibitory(GABA-ergic) interneurons, which serve to regulate preganglionic outflow.
Cardiovascular Responses
The most intriguing data in the present study is the effect of sP-SAP treatment on the cardiovascular responses to spinal nicotinic agonists. Prior studies demonstrated that the cardiovascular responses to spinally administered nicotinic agonists involved direct stimulation of IML neurons at the spinal level (Khan et al., 1994b; Chen and Su, 2006). Previously, we argued that the increase in pressor and heart rate responses following IT cytisine resulted, in part, from a reflex increase in sympathetic activity elicited by nociceptive receptors (Khan et al., 1994b; Khan et al., 2004) as spinal transection eliminated the cardiovascular responses to spinal cytisine along with the nociceptive response. Moreover, depletion of primary afferent terminals by capsaicin pretreatment significantly attenuated the pain response to cytisine and also depressed the cardiovascular responses, particularly the heart rate response (Khan et al., 2004). However, in the present study, sP-SAP treatment resulted in an enhancement of both the tachycardia and pressor responses to spinal cystine thereby dissociating the cardiovascular and nociceptive responses to spinal cytisine (see Figure 5). We note that the effects were not the result of a general effect upon preganglionic sympathetic neurons. A loss of such NK1(+) cells in the IML region or lamina X was not noted in these studies (a fact consistent with a limited rostral redistirbution of the 10μL injection volume (see Yaksh and Rudy, 1977). Moreover, such an effect would have in fact resulted in a reduction in the cardiovascular response.
We hypothesize that the present results are best interpreted in terms of a “disinhibition” of specific spinal neuronal circuits following sP-SAP treatment. The results of this study suggest that in control rats intrathecal cytisine activated a neuronal circuit which normally inhibits autonomic efferent activity at the spinal level (Fig. 7). Following lesioning of NK1-r bearing neurons, the inhibition was removed, resulting in an exaggerated cardiovascular response despite the absence of effects on the nociceptive response. Such a spinal pathway would be expected to exert a negative feed-back influence preventing excessive reflex sympathetic activity resulting from both direct and indirect (reflexive) activation. Such a local spinal pathway may also involve other inhibitory circuits so as to fine-tune the nociceptive signal processing to modulate reflex activities in the central nervous system (spinal) control of autonomic activity. Others have postulated the existence of such inhibitory interneurons in the spinal cord that regulate both the descending bulbospinal projections as well as the IML neurons directly (Tang et al., 2003, 2004). In relation to the present study, it appears that these neurons may possess NK1-r receptors as well as selected subsets of nAChR subunits (i.e. α3, α5, β2 and β4 subunits). In this regard, it may be noted that although spinal administration of Substance P leads to a pressor response in rats (Burman et al., 2001; Solomon et al., 1999), a depressor response has also been observed by others (Yang and Coote, 2003). The depressor response has been shown to be mediated by GABA/glycine positive inhibitory interneurons, since GABA antagonists significantly inhibit the response (Yang and Coote, 2003).
As nicotine, did not potentiate the autonomic activity following sP-SAP treatment when compared to cytisine or epibatidine, these spinal nicotinic receptors must show subtype selectivity in activating the inhibitory neurons. It is accepted that cytisine exhibits selectivity for composite nAChR that contain α3 subunits. In the present study a significant colocalization of α3 subunits with NK1-r receptors in the interneurons would be consistent with an interpretation that specific inhibitory neurons are selective for cytisine.
In conclusion, our data indicate that nociceptive responses to nicotinic agonists are mediated by excitatory neuronal circuits and involve both NK1-r and selective nicotinic receptor subtypes. This hypothetical organization is depicted in figure 7. Lesioning of substance P receptors greatly diminished nociceptive responses to all the nicotinic agonists tested; however, our results suggest for two excitatory pathways mediating the nociceptive response; one, nonselective to the nicotinic agonist and involving contributions from excitatory interneurons containing both nicotinic and substance P receptors, while the other would involve primary afferent terminals and subsequent release of both substance P and excitatory amino acids. The blood pressure and heart rate response data from the present study suggest that stimulation of this latter circuit by IT nicotinic receptors also activates a subset of inhibitory neurons (composites containing α3, α5, β2 and/or β4 combinations) that possess substance P receptors (see figure 7). Therefore, the present results point to the likely involvement of spinal inhibitory pathways which modulate the cardiovascular and autonomic responses to painful stimuli thereby exerting a negative modulatory feedback, as depicted in figure 7. The potential importance of such circuits to hyper-reactive autonomic responses following severe spinal cord injury warrants further investigation.
Acknowledgments
Grants: Supported by USPHS Grant 5 PO1 HL- 35018.
Abbreviations Used
- IT
Intrathecal
- SP
systolic pressure
- HR
heart rate
- NeuN B
neuronal nuclei B
- IB4
isolectin B4
- CGRP
calcitonin gene related peptide
- EAA
excitatory amino acids
- sP
substance P
- NK1-r
neurokinin 1 receptor
- sP-SAP
saporin substance P conjugate
- nAChr
nicotinic acetylcholine receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11:343–349. doi: 10.1007/BF02292765. [DOI] [PubMed] [Google Scholar]
- Benoliel R, Tanaka M, Caudle RM, Iadarola MJ. Co-localization of N-methyl-D-aspartate receptors and substance P (neurokinin-1) receptors in rat spinal cord. Neurosci Lett. 2000;291:61–64. doi: 10.1016/s0304-3940(00)01337-9. [DOI] [PubMed] [Google Scholar]
- Bradaia A, Seddik R, Schlichter R, Trouslard J. The rat spinal cord slice: Its use in generating pharmacological evidence for cholinergic transmission using the alpha7 subtype of nicotinic receptors in the central autonomic nucleus. J Pharmacol Toxicol Methods. 2005;51:243–252. doi: 10.1016/j.vascn.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Burman KJ, McKitrick DJ, Minson JB, West A, Arnolda LF, Llewellyn-Smith IJ. Neurokinin-1 receptor immunoreactivity in hypotension sensitive sympathetic preganglionic neurons. Brain Res. 2001;915:238–243. doi: 10.1016/s0006-8993(01)02907-9. [DOI] [PubMed] [Google Scholar]
- Chen HK, Su CK. Endogenous activation of nicotinic receptors underlies sympathetic tone generation in neonatal rat spinal cord in vitro. Neuropharmacology. 2006;51:1120–1128. doi: 10.1016/j.neuropharm.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Cortelli P, Pierangeli G. Chronic pain-autonomic interactions. Neurol Sci. 2003;24(Suppl 2):S68–70. doi: 10.1007/s100720300045. [DOI] [PubMed] [Google Scholar]
- Dussor GO, Helesic G, Hargreaves KM, Flores CM. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain. 2004;107:22–32. doi: 10.1016/j.pain.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Dussor GO, Jones DJ, Hulsebosch CE, Edell TA, Flores CM. The effects of chemical or surgical deafferentation on [3H]-acetylcholine release from rat spinal cord. Neuroscience. 2005;135:1269–1276. doi: 10.1016/j.neuroscience.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Osborne MG, Henry JL, Coderre TJ, Dray A. Antisense oligonucleotide knockdown of mGluR1 alleviates hyperalgesia and allodynia associated with chronic inflammation. Pharmacol Biochem Behav. 2002;73:401–410. doi: 10.1016/s0091-3057(02)00831-6. [DOI] [PubMed] [Google Scholar]
- Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Osaka H, Stanislaus S, Calvo RM, Deerinck T, Yaksh TL, Taylor P. Nicotinic acetylcholine receptor distribution in relation to spinal neurotransmission pathways. J Comp Neurol. 2003;467:44–59. doi: 10.1002/cne.10913. [DOI] [PubMed] [Google Scholar]
- Khan IM, Marsala M, Printz MP, Taylor P, Yaksh TL. Intrathecal nicotinic agonist-elicited release of excitatory amino acids as measured by in vivo spinal microdialysis in rats. J Pharmacol Exp Ther. 1996;278:97–106. [PubMed] [Google Scholar]
- Khan IM, Stanislaus S, Zhang L, Taylor P, Yaksh TL. A-85380 and epibatidine each interact with disparate spinal nicotinic receptor subtypes to achieve analgesia and nociception. J Pharmacol Exp Ther. 2001;297:230–239. [PubMed] [Google Scholar]
- Khan IM, Taylor P, Yaksh TL. Cardiovascular and behavioral responses to nicotinic agents administered intrathecally. J Pharmacol Exp Ther. 1994a;270:150–158. [PubMed] [Google Scholar]
- Khan IM, Taylor P, Yaksh TL. Stimulatory pathways and sites of action of intrathecally administered nicotinic agents. J Pharmacol Exp Ther. 1994b;271:1550–1557. [PubMed] [Google Scholar]
- Khan IM, Wennerholm M, Singletary E, Polston K, Zhang L, Deerinck T, Yaksh TL, Taylor P. Ablation of primary afferent terminals reduces nicotinic receptor expression and the nociceptive responses to nicotinic agonists in the spinal cord. J Neurocytol. 2004;33:543–556. doi: 10.1007/s11068-004-0516-6. [DOI] [PubMed] [Google Scholar]
- Li JL, Kaneko T, Shigemoto R, Mizuno N. Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. J Comp Neurol. 1997;378:508–521. doi: 10.1002/(sici)1096-9861(19970224)378:4<508::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Manelli AM, Arneric SP, Donnelly-Roberts DL. Evidence for nicotinic receptors potentially modulating nociceptive transmission at the level of the primary sensory neuron: studies with F11 cells. J Neurochem. 1997;69:930–938. doi: 10.1046/j.1471-4159.1997.69030930.x. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Llewellyn-Smith IJ, Minson JB, Arnolda LF, Chalmers JP, Pilowsky PM. Neurokinin-1 receptors and spinal cord control of blood pressure in spontaneously hypertensive rats. Brain Res. 1999;815:116–120. doi: 10.1016/s0006-8993(98)01107-x. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Porreca F, Dickenson AH. Evidence for spinal dorsal horn hyperexcitability in rats following sustained morphine exposure. Neurosci Lett. 2006;407:156–161. doi: 10.1016/j.neulet.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Tang X, Neckel ND, Schramm LP. Locations and morphologies of sympathetically correlated neurons in the T(10) spinal segment of the rat. Brain Res. 2003;976:185–193. doi: 10.1016/s0006-8993(03)02601-5. [DOI] [PubMed] [Google Scholar]
- Tang X, Neckel ND, Schramm LP. Spinal interneurons infected by renal injection of pseudorabies virus in the rat. Brain Res. 2004;1004:1–7. doi: 10.1016/j.brainres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977;202:411–428. [PubMed] [Google Scholar]
- Yang Z, Coote JH. Role of GABA and NO in the paraventricular nucleus-mediated reflex inhibition of renal sympathetic nerve activity following stimulation of right atrial receptors in the rat. Exp Physiol. 2003.;88:335–342. doi: 10.1113/eph8802561. [DOI] [PubMed] [Google Scholar]