Abstract
Background
Intervertebral disc degeneration is a common condition with few inexpensive and effective modes of treatment, but current investigations seek to clarify the underlying process and offer new treatment options. It will be important for physicians to understand the molecular basis for the pathology and how it translates to developing clinical treatments for disc degeneration. In this review, we sought to summarize for clinicians what is known about the molecular processes that causes disc degeneration.
Results
A healthy disc requires maintenance of a homeostatic environment, and when disrupted, a catabolic cascade of events occurs on a molecular level resulting in upregulation of proinflammatory cytokines, increased degradative enzymes, and a loss of matrix proteins. This promotes degenerative changes and occasional neurovascular ingrowth potentially contributing to the development of pain. Research demonstrates the molecular changes underlying the harmful effects of aging, smoking, and obesity seen clinically while demonstrating the variable influence of exercise. Finally, oral medications, supplements, biologic treatments, gene therapy, and stem cells hold great promise but require cautious application until their safety profiles are better outlined.
Conclusions
Intervertebral disc degeneration occurs where there is a loss of homeostatic balance with a predominantly catabolic metabolic profile. A basic understanding of the molecular changes occurring in the degenerating disc is important for practicing clinicians because it may help them to inform patients to alter lifestyle choices, identify beneficial or harmful supplements, or offer new biologic, genetic, or stem cell therapies.
Introduction
Low back pain is a chronic, expensive, common medical problem. Over 80% of adults will report back pain at some point in their lives, and it is the most common cause of limited activity in people younger than 45 years of age [4]. Back pain is the second most frequent cause for visits to the hospital, fifth most common reason for admission to the hospital, and the third most common cause of surgical procedures [4, 91]. The economic effects of back pain are also measured in lost productivity because back pain is the most common cause of absence from work [22], and in the United States, total health expenditures by individuals with back pain is estimated to be approximately USD 91 billion [53]. Because of the high personal and economic burden of back pain, it is critical that treating physicians understand the basic physiology of the intervertebral disc and how the disruption in disc homeostatic balance can negatively affect patients during the course of intervertebral disc degeneration.
Physicians treating patients with spine-related disorders often face difficult questions from patients about the cause and anticipated course of pathology and treatment options. As such, it is important for physicians also to be able to understand the basic biology of intervertebral disc degeneration. Physicians who understand and successfully communicate the pathology behind changes in the disc may help patients experiencing pain and disability from herniated discs or severe disc degeneration. For example, knowledge of the associations between lifestyle habits and intervertebral disc degeneration can help physicians guide patients to make changes in social habits including diet, exercise, and substance use that could alter the course of spine pathology and prevent future disability. Additionally, many treatment plans are emerging that may alleviate back pain and spine pathology, including oral therapies, molecular protein-based therapies, and viral or stem cell use. Physicians will need to understand the scientific background of these studies to appropriately discuss their application as well as risk and benefits with patients.
We performed this review to provide answers from the basic science perspective to the questions we perceive to be most relevant to clinicians, because these topics are likely to become increasingly relevant in this era of novel biologic therapies.
How Is the Normal Disc Composed?
The intervertebral disc is primarily composed of cartilaginous vertebral endplates (EP), annulus fibrosus (AF), and nucleus pulposus (NP) [52]. The endplates are at the superior and inferior aspects of the disc. EPs are cartilaginous structures distinct from articular cartilage found elsewhere in the body [10, 42]. The AF is a thick, dense structure, which is divided into the outer and inner annulus. The outer annulus is composed of organized, collagenous concentric lamellae, which is primarily composed of fibroblast-like cells that produce mainly type I collagen. The inner annulus is more fibrocartilaginous and is composed of both type I and type II collagen. The AF experiences the tensile strain of the spine. Encased within the AF is the NP. Healthy NP is gelatinous and primarily made of proteoglycans in a loose network of type II collagen. Proteoglycans have a core protein with radiating glycosaminoglycan chains of keratin sulfate and chondroitin sulfate. The cumulative hydrophilic nature of these proteins provides the NP with hydrostatic properties allowing it to counteract compressive loading of the spine [94].
The NP is highly cellular with little proteoglycan during early development, but in the adult, the NP is rich in extracellular proteoglycans with few cells. In infancy, many of the cells in the NP are large vacuolated cells, which are believed to be of notochordal origin. The proportion of these notochordal cells is greatly diminished with the concomitant increase in the chondrocyte-like cell in adult NP tissue. Notochordal cells play an important role in stimulating glycosaminoglycan and proteoglycan synthesis by NP cells and may also serve as progenitor cells to preserve and control the number of NP cells [74]. As a result of these anabolic effects, loss of notochordal cells from aging or disease states has magnified effects with eventual changes overall disc function and composition [75].
How Are Changes of the Intervertebral Disc Classified?
Although various definitions of changes seen in the intervertebral disc exist, one commonly accepted classification of nomenclature is provided through collaborative efforts of a combined task force of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology [20].
This group has classified degenerative and/or traumatic changes of the disc into a broad category including herniation and degeneration. Degeneration is classified as any fibrosis, narrowing of the disc height, bulging of the annulus, fissuring, defects in the EP, mucinous changes in the annular fibers, and osteophytes at the facet joints. Herniation is specifically defined as a “localized displacement of disc material beyond the limits of the intervertebral disc space” [20]. This classification encompasses all of the components of the disc (NP, AF, cartilage). The disc space is defined by the EP of the vertebral bodies and peripherally by the outer edge of the vertebral ring apophyses except osteophytes. Herniations are further classified as localized (< 25% disc circumference), broad-based (25%–50%), and circumferential (50%–100%) [20].
Why Do Discs Herniate?
Disc herniations can be a result of a sudden injury such as trauma or progressive degenerative changes as a result of the cumulative effect of repetitive stresses. Biomechanical studies have demonstrated that failure rarely occurs by axial compression alone and that flexion and torsion are also important contributors to the development of herniations in both acute trauma and degenerative changes [26, 40, 97].
In situations of chronic stress, a characteristic pattern of cellular changes occurs in the development of disc degeneration. The AF undergoes myxomatous degeneration and cyst formation, causing swelling of the AF fibers, leading to disruption and disorganization of fiber bundles [26, 40]. Similar to the AF, the NP also undergoes changes of dehydration, fibrosis, and necrosis. As a result of these degenerative changes, repetitive excessive loading can cause the NP material to herniate. Herniation of the NP content can occur through previously developed annular tears that have formed from the weakened AF, fissures in the EP, or disruptions at the EP junction [72]. Disc herniation can occur from both acute trauma and chronic degenerative changes with the latter as a result of catabolic changes over time, which leads to weakening of the disc structures.
If the Disc Is Aneural and Avascular, Why Does It Cause So Much Pain in Patients?
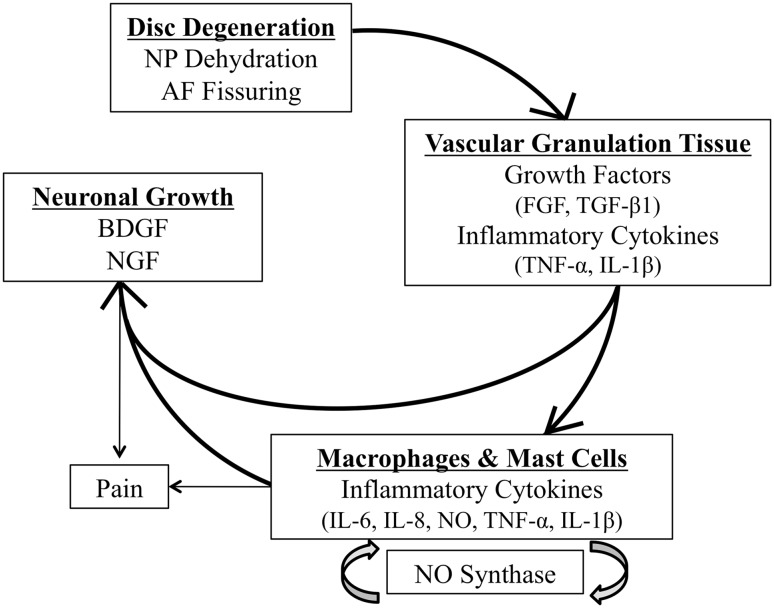
Studies disagree about whether and how the disc is able to cause pain, and as such, many investigations suggest possible connections. The young, healthy intervertebral disc is mainly avascular and aneural except for the outer one-third of the AF [56]. As aging occurs, disc matrix proteoglycan, a known inhibitor of vascular ingrowth, decreases and degenerative changes such as microfissures stimulate growth of innervated, vascularized granulation tissue, which propagates along fissures through the outer AF to the inner AF and NP [43, 70]. The vascularized granulation tissue permits the migration of macrophages and mast cells into the area as well. Collectively, the granulation tissue and the infiltrating cells express growth factors and proinflammatory cytokines, including fibroblast growth factor, transforming growth factor β1 (TGF-β1), interleukin (IL) 1β, and tumor necrosis factor α (TNF-α) [1, 43, 70].
The presence of macrophages and mast cells propagates the inflammatory cascade and may contribute to the development of pain. Macrophages increase the levels of multiple inflammatory mediators, especially IL-6 and IL-8, nitric oxide, TNF-α, and IL-1β [36]. The levels of these cytokines have correlated with pain intensity in patients, and persistent activation of sensory fibers upregulates nitric oxide synthase, thereby increasing the level of nitric oxide, suggesting a possible positive feedback loop of pain generation [39].
In addition to the development of vascularized granulation tissue, neuronal tissue develops. Degenerated disc cells secrete brain-derived growth factor, which promotes neuronal development [23]. The release of proinflammatory cytokines IL-1β and TNF-α from the surrounding tissues also upregulates nerve growth factor and expression of its receptors on the disc tissue [1]. Progressively small nerve fibers form along with the granulation tissue [70]. Nerve growth factor promotes the collateral sprouting of additional peripheral sensory nerves into the inner AF and the NP, increases nerve survival, and increases the action and sensitivity of nociceptive sensory neurons [1, 5, 15, 17, 24, 25, 46, 105].
Although no definitive mechanism has demonstrated a direct link between the intervertebral disc and pain, the changes in vascularization and neutralization of the disc that occur during the degenerative cascade suggest that there is a correlation between these changes and back pain (Fig. 1).
Fig. 1.
A series of events occur during disc degeneration that are proposed to cause discogenic pain. FGF = fibroblast growth factor; BDGF = brain-derived growth factor; NGF = nerve growth factor; NO = nitric oxide.
How Does Aging Have an Impact on Disc Degeneration and Herniations?
Aging and Oxidative Stress
With aging, there is progressive reduction in the number of vascular channels in the vertebral EP [9]. These channels are the primary source of diffusion of nutrients into and waste products out of the intervertebral disc. In addition to this loss of diffusion, aging increases the levels of proinflammatory cytokines such as IL-1, TNF-α, damaged proteins, mitochondrial dysfunction, DNA damage, and dysfunctional mechanisms of normal cell reparative mechanisms, all of which lead to disc matrix homeostatic imbalance [18, 21].
There is growing evidence that aging of the disc is related to damage from oxidative stress. Oxidative stress is a known driver of cellular senescence and apoptosis. Higher levels of oxidized proteins and transcription factors activated by oxidative stresses have been found in older discs compared with young discs [64]. Other evidence of age-related oxidative damage in the disc is the presence of advanced glycalation endproducts, which are molecules produced by nonenzymatic glycosylation and oxidation of proteins and lipids [6, 83]. The most common advanced glycalation endproducts in the disc are pentosidine and carboxymethyl-lysine. Pentosidine crosslinks collagen molecules and increases collagen stiffness as well as decreasing the synthesis of matrix proteins and proteoglycans. [6, 16]. Additionally, notochordal cells, the cells that persist in the NP, which are of notochordal origin, are greatly affected by oxidative stressors and activate both intrinsic and extrinsic pathways of apoptosis [38]. Without aging, there is reduced catabolic activity of NP cells and decreased NP cell number. The influences of oxidative stress on the intervertebral disc remain an active area of research and the ability to control or eliminate oxidative stress may lead to possible therapeutic or preventive treatments in the future [38].
Aging and Mechanical Changes
Changes in the AF and NP including dehydration, loss of organization, and fibrosis occur with aging and can lead to lesions within the disc. Annular lesions are characterized by concentric tears, radial tears, and rim lesions. Concentric tears are crescent-shaped separations of the annular lamellae, whereas radial tears are irregular radial fissures that extend from the NP outward, and rim lesions are a separation of the outer annulus from the vertebral body [67]. Studies suggest different changes in mechanics from these individual lesions, which highlights the difficulty of testing because most spines have a combination of differing types of annular lesions [92, 93]. Despite the ongoing discussion about individual contributions of differing types of tears, the cumulative effect is a decreased ability of the disc to resist motion and an alteration in stress distribution in the disc, causing overloading on the surrounding structures. This alteration in disc mechanics has been demonstrated to have a substantial effect on disc mechanobiology with increased overall degenerative changes within the disc when these lesions occur, linking the alteration in mechanics and advancement of the disc degeneration [32].
How Do Environmental Factors Impact Disc Degeneration and Herniation?
Smoking
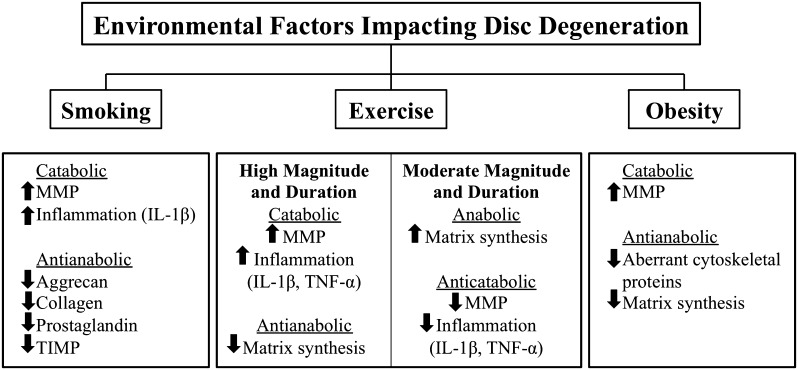
Smoking has also been demonstrated to have a profound impact on disc degeneration and herniation [8]. Smoking increases genes responsible for upregulation of proinflammatory stress responses in both the NP and AF [66]. Exposure to smoke extract also causes a dose-dependent toxicity in AF and NP cells. The disc is shifted to a catabolic profile with decreased expression of aggrecan, collagen, prostaglandin, and anticatabolic factors tissue inhibitor of matrix metalloproteinases and increasing activity of catabolic matrix metalloproteinases (MMPs) and the proinflammatory cytokine IL-1β [63, 66, 99, 102]. Smoking reduces production of aggrecan, a major proteoglycan component of disc matrix, and increases aggrecan cleavage, which diminishes the hydrostatic properties of aggrecan needed to counteract compressive forces [102]. These changes are demonstrated in overall histologic changes in the disc such as reduced cell proliferation, disruption in cell architecture, and disintegration of cells and matrix when compared with control cells [2].
Exercise
Maintenance of disc composition is a balance of catabolic and anabolic activity. In vitro, moderate physiologic loading of the spine produces a positive effect on the production of structural proteins and proteoglycans as well as slowing matrix degradation [28, 100]. The beneficial effects of moderate stresses associated with exercise appear to be related to magnitude and duration with the homeostatic effects being lost with long-duration and high-magnitude stresses that promote proinflammatory cytokines (IL-1β, TNF-α) and MMP production [85]. Similar findings have been shown clinically [54, 98]. Both laboratory and clinical evidence suggests that moderate activities promotes protective and reparative effects on the spine and may delay the development or progression of intervertebral disc disease.
Obesity
Obesity has wide-reaching effects on health, including disc degeneration and herniation. As body mass increases beyond a normal body mass index, disc degeneration increases linearly presumably as a result of the increased load on the intervertebral disc [50, 81, 90]. A peptide hormone, leptin, may be the link between disc degeneration and obesity because it is secreted primarily by adipose tissues and is a biomarker of obesity [14, 107]. Fibrocartilaginous tissues, including intervertebral disc cells, also secrete leptin and its functional receptor [27, 30]. Leptin increases MMP expression and promotes aberrant cell proliferation through the activation of multiple cytokine pathways and disruption of normal cytoskeletal organization by upregulating structural proteins F-actin, β-actin, and vimentin [48, 49, 108]. The resultant proliferation of abnormal NP cells is a possible mechanism for the underlying detrimental influence of obesity on the development of intervertebral disc disease (Fig. 2).
Fig. 2.
Environmental factors impact disc degeneration by altering the homeostatic environment of the intervertebral disc. TIMP = tissue inhibitor of matrix metalloproteinases.
Disc degeneration describes the result of a loss of overall homeostasis within the intervertebral disc, although there are numerous individual changes that interact with each other that culminate in catabolism of the disc. Changing mechanics of the disc, reactive oxygen, smoking, obesity, or extremes of exercise appear detrimental to disc homeostasis through various mechanisms. We presented age-related changes as well as environmental factors, which may or may not be modifiable by patients, but are areas of current investigation that may lead to prevention or possible treatments for disc degeneration in the future.
Are There Any Therapies to Improve the Natural History of Disc Degeneration?
Oral Treatments
There is high interest in potential oral treatments to slow down the natural history of disc degeneration. Conventional treatments such as nonsteroidal antiinflammatory drugs (NSAIDs) provide effective short-term back pain relief but do not alter the progression of disc degeneration [55, 76]. There is conflicting evidence about the effects of NSAIDs on intervertebral disc tissues. Previous in vitro studies suggest a decrease in matrix protein production, including collagen and proteoglycans, on exposure to NSAIDs [77, 87]. Conversely, others show cyclooxygenease-2 inhibitors permit sustained collagen and glycosaminoglycan synthesis and prevent the activation of inflammatory pathways in vitro and in vivo [68, 84, 89]. Further evidence needs to sufficiently evaluate the impact of NSAID treatment on intervertebral disc disease.
Use of oral supplements, including glucosamine, has gained popularity over the past several years. Glucosamine specifically is the most common natural supplement used by patients with low back pain; however, the evidence for glucosamine is mixed [7]. In vitro studies on AF and NP cells demonstrated no change on proliferation rate, an increase in glycosaminoglycan content, and decreased inflammatory mediators but decreased viability of AF cells [59, 101]. In vivo studies suggest a more negative effect of oral glucosamine supplementation on disc degeneration with findings of overall negative effect on disc matrix, including decreased type II collagen in AF cells and decreased aggrecan expression in both AF and NP cells [33]. Oral glucosamine supplementation also caused decreased proteoglycan content, greater disc degenerative histologic features, and decreased MRI index and MRI disc area compared with controls [33].
Omega-3 fatty acids are another oral supplement that has been alleged to help with back pain and disc degeneration. Omega-3 fatty acids are polyunsaturated essential fatty acids and act as an antiinflammatory medication [13]. Omega-3 fatty acid molecules are naturally released from injured cell membranes and competitively inhibit proinflammatory cytokines, including IL-1, IL-6, IL-12, and TNF-α [13]. Given their antiinflammatory mechanism of action, studies have compared omega-3 fatty acids with NSAIDs and found equivalent pain relief for chronic back pain with less side effects, including decreased bleeding risk [13, 57]. This is an evolving area of investigation that will continue to develop as the market for supplements continues to grow.
Protein-based Therapies
Protein-based therapies target a broad variety of molecules with either anabolic or anticatabolic effects on the disc. TNF-α is a potent proinflammatory cytokine that promotes proteoglycan metabolism and stimulates catabolic enzymes such as MMPs and aggrecanese in addition to other antianabolic, procatabolic changes in the disc. Because of its broad influence, inhibition of TNF-α may aid in restoring a homeostatic balance [60, 103]. Another potent proinflammatory cytokine is IL-1. The importance of IL-1 receptor antagonists was demonstrated by knockout mice that lacked the natural inhibitor of IL-1. These mice developed spinal abnormalities similar to those found in human disc degeneration, including loss of proteoglycans, lack of normal collagen structure, and upregulation of MMPs [71]. This suggests that supplementing native IL-1 receptor antagonist molecules might help prevent or slow disc degeneration. Despite some in vitro evidence supporting both TNF-α inhibitors and IL-1 receptor antagonists, in vivo and clinical effectiveness still needs to be demonstrated for intervertebral disc degeneration [45, 82].
Growth factors and cytokines are highly involved in degenerative disc disease and therefore are primary targets for biologic treatments. Growth factors such as the bone morphogenetic proteins (BMPs) are potent mitogens and belong to the TGF-β superfamily, which activates intracellular signal transduction proteins in the nucleus to initiate gene transcription [11]. BMP-2 and BMP-7 (osteogenic protein 1) act to increase proteoglycan production, specifically aggrecan and collagen types I and II in AF and NP cells [11, 37, 47]. Local injection of degenerative discs with BMP-2 and BMP-7 has been reported to maintain disc height and increase production of proteoglycans in both rat and rabbit models [3, 31, 34, 41, 58]. Despite successful demonstration of increased proteoglycan and collagen content, BMPs also cause ossification of the annulus and aberrant bone formation [29, 35]. Although successful at restoring healthy disc characteristics, further investigations regarding the safe application of this potent treatment must be conducted.
Gene-based Therapies
Although injection of biologic molecules into the disc allows direct delivery of a protein product, the relatively short half-lives of these proteins prevents long-term maintenance of their action, which would be required for a chronic disease such as disc degeneration. Gene therapy has the potential to deliver a recombinant gene of interest to the cells with the option of incorporating it into the cell’s own DNA. This allows for long-term expression of a proanabolic or an anticatabolic therapeutic protein molecule. Many modalities of genetic transmission have been used, but as a result of their delivery efficiency, viral vectors are the modality most frequently used in intervertebral disc gene therapy research. Retroviruses, adenovirus, and adeno-associated viruses are a few of the different viruses used; each has its own risks and benefits, including the amount of genomic information that can be transmitted, immune response, and ability to integrate into the genome [40, 62, 69].
Gene therapies have been effective at slowing or reversing disc degeneration. In vivo transfection of intervertebral disc cells with an adenoviral vector containing the TGF-β1 gene resulted in increased TGF-β1 expression and proteoglycan synthesis [65]. Also, successful delivery of an adeno-associated virus encoded with genes for the proanabolic molecule BMP-2 and anticatabolic molecule tissue inhibitor of matrix metalloproteinases 1 showed decreased MRI, histologic, serum biochemical, and biomechanical evidence of disc degeneration over a 12-week period [41].
The ability to use a compound to induce or halt protein production from transduced genes offered a critical solution to control the potentially broad effects of overexpressed therapeutic gene products. Vectors can be developed containing a gene whose expression is initiated when the inducing agent such as tetracycline is delivered [96]. As such, with the removal of the induction agent, the protein production of the introduced gene ceases. The ability to control temporal expression of the therapeutic gene also allows for control and safe administration of these potentially powerful compounds [86].
Gene therapy is a promising field that would allow for long-term delivery of vital growth factors and cytokines to slow disc degeneration and potentially restore normal disc characteristics. While this technology is being refined, it will be critical to address the goals of identifying the optimal viral vector for delivery, minimizing inflammatory responses, maximizing gene transfection, and controlling subsequent gene expression to prevent complications.
Stem Cell Therapies
Stem cell therapies continue to develop in many fields including intervertebral disc degeneration. Several types of stem cells reported to have the potential to aid in repairing, delaying, or preventing intervertebral disc degeneration by repopulating the disc [19]. Stem cells differentiate into cells that create extracellular matrix and rebuild the disc and provide trophic effects such as upregulation of disc cell viability and inhibition of senescence and apoptosis [104].
Bone marrow-derived mesenchymal stem cells are relatively easy to obtain, are able to differentiate into intervertebral disc cells, provide trophic support, and modulate the immune system [51]. These cells require cell-to-cell contact on three-dimensional spheres and growth factors such as TGF-β1 for development into NP-like cells [73, 88]. After differentiation, these cells can be placed on a gel scaffold or directly injected into the disc [73, 88]. Use of bone marrow-derived mesenchymal stem cells has demonstrated the ability to slow degeneration by preserving disc health as evaluated by MRI, histology, immunohistochemistry, and gene expression [44, 78–80]. These cells also demonstrated similar phenotypic activity to NP cells, including upregulation of aggrecan, versican, and type II collagen with suppression of the proinflammatory cytokines TNF-α and IL-1β [44]. This suggests that bone marrow stem cells may also enhance matrix homeostasis in the disc in addition to increasing the number of NP-like cells present.
Synovial stem cells demonstrate the highest expansion potential, highest colony-forming efficiency, and fastest growth kinetics of the various types of mesenchymal stem cells tested in vitro [106]. Synovial stem cells have a large chondrogenic effect by inducing collagen and aggrecan production and maintain intervertebral disc height in vivo [61]. These studies demonstrate the potential of synovial stem cells to contribute to the progression of stem cell technologies for intervertebral disc therapies.
Adipose-derived stem cells are easy to obtain and could potentially eliminate the need for cell expansion. In addition to the ease of access, adipose-derived cells have stable growth kinetics, wide differentiation potential, faster doubling time, and greater cellular matrix production than bone marrow-derived mesenchymal stem cells [12]. Additional trials are needed to assess the effectiveness and stability of adipose-derived stem cells in vivo and the safety of this technology.
Questions remain about the safety and large-scale application of this technology. Concerns persist regarding potential oncogenic transformation of stem cells or cell spillage with differentiation in unanticipated tissue locations leading to complications; this technology requires further studies to define its safe application [95].
Discussion
Disc degeneration occurs with age and involves a shift in the metabolic productivity of the intervertebral disc. The loss of homeostasis with upregulation of catabolic and antianabolic gene expression leads to decreased matrix components, weaker AF, and a dehydrated NP. These degenerative changes appear to increase the likelihood of disc herniation. Additionally, the degenerative and inflammatory changes occurring as the disc degenerates promote increased neural and vascular ingrowth into the disc, potentially accounting for the painful discomfort patients experience with disc degeneration.
As outlined, several factors impact disc degeneration and disc herniation, including aging, environmental and lifestyle choices such as smoking, exercise, and obesity. Current oral medications target the symptoms of disc degeneration but do not alter the cause and evolving evidence suggests harmful effects of some oral supplements; thus, additional research is needed. Developing medications such as TNF-α inhibitors and IL-1 receptor antagonists have the potential to offer therapeutic effects on disc disease but have thus far been relatively unsuccessful. BMPs are potent mitogens proven to positively impact disc degeneration in vitro and in vivo but require cautious clinical application because aberrant placement could have devastating neurologic complications as a result of the close proximity to the spinal cord. Additionally, the use of gene and stem cell therapies to maintain and potentially rebuild intervertebral disc materials holds great promise. Targeting, slowing, or even potentially reversing intervertebral disc degeneration could potentially aid in preventing disc herniation in addition to slowing the chronic issues associated with disc degeneration. Although advances are continually being made, additional research is crucial for safe application of these emerging technologies.
Acknowledgments
We thank the staff of Ferguson Laboratory for Orthopaedic and Spine Research for their support and guidance in selecting topics for discussion in this manuscript.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
References
- 1.Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976). 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 2.Akmal M, Kesani A, Anand B, Singh A, Wiseman M, Goodship A. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine (Phila Pa 1976). 2004;29:568–575. doi: 10.1097/01.brs.0000101422.36419.d8. [DOI] [PubMed] [Google Scholar]
- 3.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 4.Andersson G. Epidemiological features of chronic low back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74:2627–2642. doi: 10.1016/j.lfs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330:345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes P, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 8.Battie MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J, Koskenvuo M. 1991 Volvo Award in Clinical Sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976). 1991;16:1015–1021. [PubMed] [Google Scholar]
- 9.Boos N, Weissbach S, Rohrbach H. Classification of age related changes in lumber intervertebral discs. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Smith KC, Kazarien LE, Rosenberg LC, Ungar R. Articular cartilage and intervertebral disc proteoglycans differ in structure: an electron microscopic study. J Orthop Res. 1989;7:146–151. doi: 10.1002/jor.1100070121. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biologic functions of bone morphogenetic proteins. Front Biosci. 2004;9:349–358. doi: 10.2741/1090. [DOI] [PubMed] [Google Scholar]
- 12.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, Wang GJ, Kang L, Chang JK. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleland LG. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988;15:1471–1475. [PubMed] [Google Scholar]
- 14.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 15.Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of ‘painful’ lumbar discs. Spine. 1997;22:2342–2349. doi: 10.1097/00007632-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 16.DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijisma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004;10:1647–1652. doi: 10.2174/1381612043384727. [DOI] [PubMed] [Google Scholar]
- 19.Drazin D, Rosner J, Avalos P, Acosta F. Stem cell therapy for degenerative disc disease. Adv Orthop. 2012;2012:961052. doi: 10.1155/2012/961052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976). 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Finkel T, Holbrook JN. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 22.Frank A. Low back pain. BMJ. 1993;306:901–908. doi: 10.1136/bmj.306.6882.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman BJ, Fraser RD, Cain CM, Hall DJ, Chapple DC. A randomized, doubleblind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2005;30:2369–2377. doi: 10.1097/01.brs.0000186587.43373.f2. [DOI] [PubMed] [Google Scholar]
- 24.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 25.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland J. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 26.Gordon SJ, Yang KH, Mayer PJ, Mace AH, Kish VL, Radin EL. Mechanism of disc rupture: a preliminary report. Spine (Phila Pa 1976). 1991;16:450–456. doi: 10.1097/00007632-199104000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Gruber HE, Ingram JA, Hoelscher GL, Hanley EN., Jr Leptin expression by annulus cells in the human intervertebral disc. Spine J. 2007;7:437–443. doi: 10.1016/j.spinee.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976). 1997;22:1085–1091. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Haschtmann D, Ferguson SJ, Stoyanov JV. BMP-2 and TFG-β3 do not prevent spontaneous degeneration in rabbit disc explants but induce ossification of the annulus fibrosus. Eur Spine J. 2012;21:1724–1733. doi: 10.1007/s00586-012-2371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiu W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, Young DA. Leptin produced by joint white adipose tissue induces cartilage degradation and upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 31.Imai Y, Okuma M, An HS, Nakagawa K, Yamada M, Muehleman C, Thonar E, Masuda K. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine (Phila Pa 1976). 2007;32:1197–1205. doi: 10.1097/BRS.0b013e3180574d26. [DOI] [PubMed] [Google Scholar]
- 32.Kaigle AM, Holm SH, Hansson TH. Kinematic behavior of the porcine lumbar spine: a chronic lesion model. Spine. 1997;22:2796–2806. doi: 10.1097/00007632-199712150-00002. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs L, Vo N, Coehlo JP, Dong Q, Bechara B, Woods B, Hempen E, Hartman R, Preuss H, Balk J, Kang J, Sowa G. Glucosamine supplementation demonstrates a negative effect on intervertebral disc matrix in an animal model of disc degeneration. Spine. 2013 Jan 15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 34.Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine (Phila Pa 1976). 2005;30:1933–1939. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Lee JU, Moon SH, Kim HC, Kwon UH, Seol NH, Kim HJ, Park JO, Chun HJ, Kwon IK, Lee HM. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine. 2009;34:1834–1838. doi: 10.1097/BRS.0b013e3181ae18ba. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate annulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976). 2008;33:2253–2259. doi: 10.1097/BRS.0b013e318182c35f. [DOI] [PubMed] [Google Scholar]
- 37.Kim JS, Ellman MB, An HS, van Wijinen AJ, Borgia JA, Im JH. Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum. 2010;62:3706–3715. doi: 10.1002/art.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KW, Ha KY, Lee JS, Rhyu KW, An HS, Woo YK. The apoptotic effect of oxidative stress and antiapoptotic effect of caspase inhibitors on rat notochordal cells. Spine. 2007;32:2443–2448. doi: 10.1097/BRS.0b013e318157395a. [DOI] [PubMed] [Google Scholar]
- 39.Koch A, Zacharowski K, Boehm O, Stevens M, Lipfert P, von Giesen HJ, Wolf A, Freynhagen R. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res. 2007;56:32–37. doi: 10.1007/s00011-007-6088-4. [DOI] [PubMed] [Google Scholar]
- 40.Kuga N, Kawabuchi M. Histology of intervertebral disc protrusion: an experimental study using an aged rat model. Spine (Phila Pa 1976). 2001;26:E379–E384. doi: 10.1097/00007632-200109010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Leckie SK, Bechara BP, Hartman RA, Sowa GA, Woods BI, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, Vo NV, Wang B, Kang JD. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JM. Interleukin-1B induces angiogenesis and innervations of human intervertebral disc degeneration. J Orthop Res. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 44.Le Maitre CL, Baird P, Freemont AJ, Hoyland JA. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther. 2009;11:R20. doi: 10.1186/ar2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther. 2007;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 48.Li S, Duance VC, Blain EJ. Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat. 2008;213:725–732. doi: 10.1111/j.1469-7580.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G, Liu J. Leptin induces cycle D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MER/ERK pathways. PLoS One. 2012;7:e53176. doi: 10.1371/journal.pone.0053176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, Riihimaki H. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond). 2005;29:903–908. doi: 10.1038/sj.ijo.0802974. [DOI] [PubMed] [Google Scholar]
- 51.Longo UG, Papapietro N, Petrillo S, Franceschetti E, Maffulli N, Denaro V. Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int. 2012;2012:921053. doi: 10.1155/2012/921053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotz JC, Hsieh AH, Walsh AL, Palmer EI, Chin JR. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30:853–858. doi: 10.1042/bst0300853. [DOI] [PubMed] [Google Scholar]
- 53.Luo X, Pietrobon R, Sun S, Liu G, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 54.Luoma K, Riihimaki H, Raininko R, Luukkonen R, Lamminen A, Viikari-Juntura E. Lumbar disc degeneration in relation to occupation. Scand J Work Environ Health. 1998;24:358–366. doi: 10.5271/sjweh.356. [DOI] [PubMed] [Google Scholar]
- 55.Madigan L, Vaccaro A, Spector L, Milam R. Management of symptomatic lumbar degenerative disk disease. J Am Acad Orthop Surg. 2009;17:102–111. doi: 10.5435/00124635-200902000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man: histology of intervertebral discs, 11th communication. Acta Anat (Basel). 1959;38:96–113. [PubMed] [Google Scholar]
- 57.Maroon JC, Bost JW. Omega-3 fatty acids (fish oil) as an anti-inflammatory: an alternative to nonsteroidal anti-inflammatory drugs for discogenic pain. Surg Neurol. 2006;65:326–331. doi: 10.1016/j.surneu.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit annular puncture model. Spine (Phila Pa 1976). 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 59.Mavrogonatou E, Kletsas D. The effect of glucosamine sulfate on the proliferative potential and glycosaminoglycan synthesis of nucleus pulposus intervertebral disc cells. Spine. 2013;38:308–314. doi: 10.1097/BRS.0b013e31826a0a8d. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto K, An H, Pichika R, Thonar E, Masuda K. Tumor necrosis factor α inhibits proteoglycan metabolism and stimulates matrix metalloproteinase and aggrecanese production by human intervertebral disc cells. Spine J. 2004;4(Suppl):S66. [Google Scholar]
- 61.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, Sekiya I. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 63.Nemoto Y, Matsuzaki H, Tokuhasi Y, Okawa A, Uematu Y, Nishimura T, Oda H. Histological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model. J Orthop Sci. 2006;11:191–197. doi: 10.1007/s00776-005-0987-4. [DOI] [PubMed] [Google Scholar]
- 64.Nerlich AG, Bachmeier BE, Schleicher E, Rohrbach H, Paesold G, Boos N. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci. 2007;1096:239–248. doi: 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- 65.Nishida K, Kang JD, Suh JK, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells: implications for the treatment of intervertebral disc degeneration. Spine (Phila Pa 1976). 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa T, Matsuzaki H, Uei H, Nakajima S, Tokuhashi Y, Esumi M. Alteration of gene expression in intervertebral disc degeneration of passive cigarette-smoking rats: separate quantitation in separated nucleus pulposus and annulus fibrosus. Pathobiology. 2005;72:146–151. doi: 10.1159/000084118. [DOI] [PubMed] [Google Scholar]
- 67.Osti O, Vernon-Roberts B, Fraser R. Anulus tears and intervertebral disc degeneration—an experimental study using an animal model. Spine. 1990;15:299–306. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 68.Ou YS, Tan C, An H, Jiang DM, Quan ZX, Tang K, Luo XJ. The effects of NSAIDs on types I, II, and III collagen metabolism in a rat osteoarthritis model. Rheumatol Int. 2012;32:2401–2405. doi: 10.1007/s00296-011-1978-8. [DOI] [PubMed] [Google Scholar]
- 69.Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Chérel Y, Chenuaud P, Schmidt M, von Kalle C, Rolling F, Moullier P, Snyder RO. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng B, Hao J, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 71.Phillips KL, Jordan-Mahy N, Nicklin MJ, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72:1860–1867. doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- 72.Rajasekaran S, Bajaj N, Tabaki V, Kanna R, Prasad Shetty A. The Anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine. 2013;38:1491–1500. doi: 10.1097/BRS.0b013e31829a6fa6. [DOI] [PubMed] [Google Scholar]
- 73.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, Hoyland JA. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 74.Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 75.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roelofs P, Deyo R, Koes B, Scholten R, van Tulder M. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766–1774. doi: 10.1097/BRS.0b013e31817e69d3. [DOI] [PubMed] [Google Scholar]
- 77.Sadowski T, Steinmeyer J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase -3, and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthritis Cartilage. 2001;9:407–415. doi: 10.1053/joca.2000.0406. [DOI] [PubMed] [Google Scholar]
- 78.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, Nakai T, Ando K, Hotta T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 79.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976). 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 80.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 81.Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum. 2012;64:1488–1496. doi: 10.1002/art.33462. [DOI] [PubMed] [Google Scholar]
- 82.Sinclair SM, Shamji MF, Chen J, Jing L, Richardson WJ, Brown CR, Fitch RD, Setton LA. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine. 2011;36:1190–1196. doi: 10.1097/BRS.0b013e3181ebdb43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivan SS, Tsitron E, Wachtel E, Roughley P, Sakkee N, van der Ham F, Degroot J, Maroudas A. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith RL, Kajiyama G, Lane NE. Nonsteroidal anti-inflammatory drugs: effects on normal and interleukin 1 treated human articular chondrocyte metabolism in vitro. J Rheumatol. 1995;22:1130–1137. [PubMed] [Google Scholar]
- 85.Sowa G, Coelho P, Vo N, Bedison R, Chiao A, Davies C, Studer R, Kang J. Determination of annulus fibrosus cell response to tensile strain as a function of duration, magnitude, and frequency. J Orthop Res. 2011;29:1275–1283. doi: 10.1002/jor.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sowa G, Westrick E, Pacek C, Coelho P, Patel D, Vadala G, Georgescu H, Vo N, Studer R, Kang J. In vitro and in vivo testing of a novel regulatory system for gene therapy for intervertebral disc degeneration. Spine (Phila Pa 1976). 2011;36:E623–E628. doi: 10.1097/BRS.0b013e3181ed11c1. [DOI] [PubMed] [Google Scholar]
- 87.Srinivas GR, Chichester CO, Barrach HJ, Matoney AL. Effects of certain antiarthritic agents on the synthesis of type II collagen and glycosaminoglycans in rat chondrosarcoma cultures. Agents Actions. 1994;41:193–199. doi: 10.1007/BF02001916. [DOI] [PubMed] [Google Scholar]
- 88.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi T, Uemura Y, Taguchi H, Ogawa Y, Yoshida S, Toda M, Kobayashi T, Seguchi H, Tani T. Cross talk between COX-2 inhibitor and hyaluronic acid in osteoarthritic chondrocytes. Int J Mol Med. 2004;14:139–144. [PubMed] [Google Scholar]
- 90.Takatalo J, Karppinen J, Taimela S, Niinimaki J, Laitinen J, Sequeiros RB, Samartzis D, Korpelainen R, Nayha S, Remes J, Tervonen O. Association of abdominal obesity with lumbar disc degeneration—a magnetic resonance imaging study. PLoS One. 2013;8:e56244. doi: 10.1371/journal.pone.0056244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low-back pain hospitalization: recent United States trends and regional variations. Spine. 1994;19:1207–1213. doi: 10.1097/00007632-199405310-00002. [DOI] [PubMed] [Google Scholar]
- 92.Thompson RE, Pearcy MJ, Barker TM. The mechanical effect of intervertebral disc lesions. Clin Biomech. 2004;19:448–455. doi: 10.1016/j.clinbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Thompson RE, Pearcy MJ, Downing JK, Manthey BA, Parkinson IH, Fazzalari NL. Disc lesions and the mechanics of the intervertebral joint complex. Spine. 2000;20:3026–3035. doi: 10.1097/00007632-200012010-00010. [DOI] [PubMed] [Google Scholar]
- 94.Urban JP, Maroudas A. Swelling of the intervertebral disc in vitro. Connect Tissue Res. 1981;9:1–10. doi: 10.3109/03008208109160234. [DOI] [PubMed] [Google Scholar]
- 95.Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 96.Vadalà G, Sowa GA, Smith L, Hubert MG, Levicoff EA, Denaro V, Gilbertson LG, Kang JD. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine (Phila Pa 1976). 2007;32:1381–1387. doi: 10.1097/BRS.0b013e3180601215. [DOI] [PubMed] [Google Scholar]
- 97.Veres SP, Robertson PA, Broom ND. ISSLS Prize Winner. How loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine (Phila Pa 1976). 2010;35:1897–1908. doi: 10.1097/BRS.0b013e3181d9b69e. [DOI] [PubMed] [Google Scholar]
- 98.Videman T, Nurminen M, Troup JD. 1990 Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine. 1990;15:28–40. [PubMed] [Google Scholar]
- 99.Vo N, Wang D, Sowa G, Witt W, Ngo K, Coelho P, Bedison R, Byer B, Studer R, Lee J, Di YP, Kang J. Differential effects of nicotine and tobacco smoke condensate on human annulus fibrosus cell metabolism. J Orthop Res. 2011;29:1585–1591. doi: 10.1002/jor.21417. [DOI] [PubMed] [Google Scholar]
- 100.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–337. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 101.Walsh AJ, O’Neill CW, Lotz JC. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J. 2007;7:601–608. doi: 10.1016/j.spinee.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Wang D, Nasto LA, Roughley P, Leme AS, Houghton AM, Usas A, Sowa G, Lee J, Niederhofer L, Shapiro S, Kang JD, Vo N. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang YT, Wu XT, Wang F. Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration. J Orthop Sci. 2010;15:707–719. doi: 10.1007/s00776-010-1536-3. [DOI] [PubMed] [Google Scholar]
- 105.Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells de-rived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 108.Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. Expression of leptin and its functional receptor on disc cells: contribution to cell proliferation. Spine (Phila Pa 1976). 2008;33:E858–E864. doi: 10.1097/BRS.0b013e31818338e5. [DOI] [PubMed] [Google Scholar]