Abstract
Background
Primary bone or soft tissue tumors of the femur sometimes present with severe and extensive bone destruction, leaving few limb-salvage options other than total femur replacement. However, there are few data available regarding total femur replacement and, in particular, regarding implant failures.
Questions/purposes
We asked: (1) What are the revision-free and overall implant survival rates of conventional total femur replacements in patients treated for sarcoma of the femur or soft tissues? (2) What are the revision-free and overall implant survival rates of expandable total femur replacements in skeletally immature patients? (3) Using the comprehensive International Society of Limb Salvage failure-mode classification, what types of complications occur with conventional and expandable total femur replacements?
Patients and Methods
Our retrospective, single-center cohort study was based on data prospectively collected for 50 patients who received a total femur replacement after tumor resection for indications other than carcinoma or metastatic disease. Of the 50 patients, six (12%) were lost to followup before 6 months. Ten of the remaining 44 patients received expandable implants. The mean followup was 57 months (range, 1–280 months) and 172 months (range, 43–289 months) for patients who underwent conventional and expandable total femur replacements, respectively. For implant survival, competing risk analyses were used.
Results
At 5 years, revision-free implant survival of conventional total femur replacements was 48% (95% CI, 0.37–0.73), and overall implant survival was 97% (95% CI, 0.004–0.20). Five-year revision-free implant survival of expandable total femur replacements was 30% (95% CI, 0.47–1.00) and overall implant survival was 100%. With conventional total femur replacements soft tissue failures occurred in 13 of 34 patients, structural failures in three, infection in six, and local tumor progression in one. No patient had aseptic loosening with conventional total femur replacements, but hip disarticulation occurred in two patients owing to extensive wound-healing problems and infection. With expandable total femur replacements soft tissue failure, aseptic loosening, and infection occurred in one patient each of 10, and structural failures in three of 10 (two periprosthetic fractures, one loosening of an enhanced tendon anchor). No hip disarticulations were performed. Additionally expandable total femur replacement-related failures included hip instability in eight of 10 patients, contractures attributable to massive scar tissue in six, and defect of the implant’s expansion mechanism in four patients.
Conclusions
Although the indications for total femoral resection are rare, we think that total femur replacement is a reasonable treatment option for reconstruction of massive femoral bone defects after tumor resection in adults and skeletally immature patients, and results in limb salvage in most patients.
Level of Evidence
Level IV, therapeutic study.
Introduction
Improvements in implant design and manufacturing and advances in surgical techniques [21] and adjuvant treatments for malignancies have resulted in limb-salvage surgery after tumor resection becoming a standard approach. Modular endoprosthetic reconstruction of single joints has become a well-established treatment option, especially for patients needing hip or knee reconstruction. However, there are instances where a tumor is so extensive in the femur or skip metastases are present that resection of the entire femur is necessary. The first total femur replacement was performed in 1952 and a second case reportedly was done in 1965 using a custom-made vitallium endoprosthesis, with a good functional result at 6 months [4]. The first reconstructions with total femur replacements after resection of malignant neoplasms were described by Marcove et al. [20]. Later, the first modular expandable prostheses were introduced for skeletally immature patients with sarcoma [18].
Since then, numerous studies of total femur replacements with small numbers of patients have been published [2, 6, 7, 12–15, 19, 22–26, 28, 30, 31], including one that focused on expandable total femur prostheses [29]. Some of the studies reported results for total femur replacements together with partial femur reconstructions, which makes it difficult to draw conclusions for total femur replacement-specific complications [1, 21]. Most of the studies emphasized oncologic and functional outcomes of the patients; only a few specifically addressed the modes by which total femur replacement failed. To facilitate clearer communication of megaendoprosthetic complications, the comprehensive International Society of Limb Salvage (ISOLS) failure-mode classification was published in 2011 [8], with modifications for expandable reconstructions in 2014 [9].
The aims of our study were to answer the following questions: (1) What are the revision-free and overall implant survival rates for conventional total femur replacements in patients treated for sarcoma of the femur or soft tissues? (2) What are the revision-free and overall implant survival rates of expandable total femur replacements in skeletally immature patients? (3) Using the comprehensive ISOLS failure-mode classification, what types of complications occurred in patients who underwent conventional and expandable total femur replacements?
Patients and Methods
From April 1983 to April 2012, 64 patients were treated with total femur replacements after tumor resections, including 10 who received expandable implants. Information for our retrospective cohort study was collected from our prospective database and from original medical records. Approval of the respective institutional review boards was obtained before beginning the study.
Fourteen patients with carcinoma and metastatic disease were excluded because their tumors and their life expectancy were different than those of patients with primary bone and soft tissue tumors, leaving 50 patients as our study cohort. Of the 50 remaining patients, six (12%) were lost to followup within 6 months after surgery. A total of 44 patients thus were included in our study (Table 1).
Table 1.
Demographics, diagnoses, and surgical parameters
| Variable | Conventional total femur replacement n = 34 (77%) |
Expandable total femur replacement n = 10 (23%) |
|---|---|---|
| Patient age, mean years (range) | 34 (5–81) | 9 (4–13) |
| Sex (men/women) | 16/18 | 6/4 |
| Diagnosis | ||
| Osteosarcoma | 15 (44%) | 6 (60%) |
| Ewing’s sarcoma | 5 (15%) | 4 (40%) |
| Chondrosarcoma | 6 (18%) | 0 |
| Malignant fibrous histiocytoma | 3 (9%) | 0 |
| Other* | 5 (15%) | 0 |
| Chemotherapy (yes/no) | 26/8 | 10/0 |
| Radiotherapy (yes/no) | 8/26 | 1/9 |
| Surgical margins (wide/marginal/intralesional/resection in other institution) | 26/5/0/3 | 9/1/0/0 |
| Followup, mean months (range) | 57 (1–280) | 172 (43–289) |
| Implantation (primary/secondary) | 18/16 | 8/2 |
| Prostheses | ||
| Fixed/rotating hinge | 27/7 | 8/2 |
| Uncemented/cemented | 24/10 | 10/0 |
| Acetabular cup | 5 | 0 |
| Abductor reconstruction | ||
| Attached to fascia lata | 14 (41%) | 6 (60%) |
| Residual trochanteric bone | 3 (9%) | 0 |
| Enhanced tendon attachment | 8 (24%) | 3 (30%) |
| Ligament artificial reconstruction system | 9 (27%) | 1 (10%) |
* Spindle cell sarcoma, aneurysmal bone cyst, malignant Paget disease, soft tissue sarcoma, primitive neuroectodermal tumor.
We assigned patients who had one or more previous reconstructive operations to the proximal, diaphyseal, or distal part of the femoral bone to the secondary implantation group, and patients without such a surgical intervention to the primary implantation group. Indications in the primary group were extensive femoral tumor involvement. The primary implantation group included 26 patients with extensive femoral tumor involvement in 20, pathologic fracture in three, skip lesions in two, and inadequate previous tumor resection with iatrogenic contamination in one patient. The patients in the secondary implantation group had a mean of four previous operations (range, 1–22 operations). Indications were fractures after biologic reconstruction with plate fixation and local recurrence (five patients each), nonunion, cement spacer, and implant loosening in two patients each, and periprosthetic fracture and inadequate previous resection in one patient each.
Surgical Technique
All surgical procedures were performed by four orthopaedic surgeons (RW, PTF, RK, MD). In all total femur replacements we used a Watson-Jones approach to the hip with a long lateral incision that reached the anterolateral aspect of the patellar tendon and tibial tuberosity. After resection of the tumor including the total femoral bone and affected soft tissue compartments or removal of any previous reconstruction hardware, respectively, the total femur replacement was implanted.
Conventional total femur replacement was performed using a Howmedica Modular Replacement System (HMRS) (Howmedica Osteonics Corp, Mahwah, NJ, USA) with a fixed hinge knee mechanism in 23 patients until 2002; from then on the Global MRS (Stryker Corporation, Kalamazoo, MI, USA) with a rotating hinge mechanism was used in eight patients; a Kotz Modular Femur and Tibia Reconstruction system (KMFTR®; Stryker Corporation) in two patients, and, in one patient, a Pafford-Lewis prosthesis (Dow Corning Wright, Arlington TN, USA).
An expandable total femur replacement was performed in four patients using an HMRS with invasive expansion by a worm-driven manual device via a small skin incision and six noninvasive expansion mechanisms. The noninvasive expansion mechanisms consisted of one Modular Universal Tumor And Revision System (MUTARS®; Implantcast, Buxtehude, The Netherlands) expanded by electromagnetic induction and five HMRS subsequently changed to a noninvasive automatic elongation device after a mean of 2 years [17].
Cemented fixation of the tibial stem with the HMRS was performed in three of 32 patients owing to osteoporotic bone quality or an excavated metaphyseal tibial bone stock and in seven of eight patients with a Global MRS the tibial plateau was cemented, leaving the stem uncemented. With the expandable total femur replacement, the tibial plateaus with polished surfaces without flanges were used to enable future epiphyseal growth of the proximal tibia.
For acetabular reconstruction, three cementless cups and two pedestal cups were used, one after extraarticular hip resection and one after a partial pelvic resection (Enneking Types II/III).
Reconstruction methods of the gluteal abductor mechanism have varied influenced by endoprosthetic design (ie, the ability to reattach soft tissues to endoprosthetic components) and the amount of soft tissue resection dictated by tumor extent. When the joint capsule and acetabulum could be preserved we favored a purse string closure around a bipolar head. In cases of severe muscle loss of the abductors the remaining muscles were attached to the fascia lata. When the abductors could be detached from the greater trochanter without severe muscle loss or even a part of the greater trochanteric bone could be salvaged by osteotomy from the resected specimen, alternatively a custom-made enhanced tendon anchor (Stryker-Howmedica Osteonics Corp, Mahwah, NJ, USA) was available for soft tissue reattachment in combination with the HMRS. The Global MRS did not provide this form of soft tissue clamp, but allowed use of a proximal femoral component with and without a trochanteric hypomochlion. The latter was used for refixation of residual trochanteric bone to the prosthesis throughout sagittal holes by nonresorbable sutures, while the trochanteric design was used when only muscle or tendon was available for reattachment. With the availability of the Ligament Augmentation and Reconstruction System (LARS®) (LARS®, Surgical Implants and Devices, Arc-sur-Tille, France) in 2002, a LARS® tube or band was wrapped around the prosthesis in all cases of extensive periacetabular soft tissue loss to allow capsular repair and muscular reattachment of the residual abductors. Primary wound closure was possible in all patients without the use of additional muscle flaps.
Revision-free implant survival was defined as the time from implantation of the total femur replacement to the first revision with involvement of the prosthesis, excluding planned revision for prosthetic expansion in patients who underwent expandable total femur replacement. Overall implant survival was defined as the time from implantation until hip disarticulation. Complications were classified according to a comprehensive ISOLS classification system [8]. Additionally, we assigned bushing wear to structural failures. For expandable-specific complications, we referred to the modified classification for expandable implants [9]. Defects of the implant’s expansion mechanism accounted for Type 3A complications; a scar tissue contracture resulting in longitudinal growth arrest and limited ROM was classified as Type 6A. Hip instability (Type 6B) was defined as being caused by growth of the acetabulum in younger children, which when not followed by growth of the femoral endoprosthetic head, led to secondary dysplasia. Furthermore, loss of the greater trochanter led to gluteal insufficiency, while hip adductors contracted owing to elongation of the implant with resultant muscular imbalance. Consequently, the femoral head moved cranially and laterally and increased dysplasia of the acetabular roof (Fig. 1).
Fig. 1A–E.
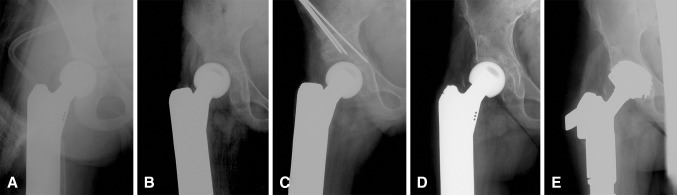
Radiologic examples of subsequent hip instability in expandable total femur replacement are shown. (A) A postoperative radiograph after initial reconstruction with an expandable implant is shown. (B) At 15-months postoperatively, the femoral head has moved cranially and laterally, leading to relative hip dysplasia. Consequently, a Salter osteotomy was performed to establish acetabular congruency. (C) A postoperative radiograph after the Salter pelvic osteotomy is shown. (D) Twenty-nine months later, progression of acetabular dysplasia with arthritis was seen. (E) This postoperative radiograph was obtained after implantation of an acetabular cup (Zimmer Alloclassic; Zimmer Gmbh, Winterthur, Switzerland).
Our standard followup protocol includes clinical and radiographic examinations of the tumor site every 4 months for 3 years, every 6 months for the following 3 years, and yearly thereafter. Physical functioning was assessed in all but seven conventional total femur replacements using the Musculoskeletal Tumor Society functional evaluation system (MSTS) [5]. All scores were evaluated at the time of the most recent followup.
Our statistical analyses focused on implant survival. Primary endpoints were first revision and removal of the implant. Survival analyses were performed using a competing-risk model with patient mortality as the competing event [16]. Descriptive summary statistics included means and frequencies. All calculations were made using SPSS Statistics V21 (IBM, Armonk, NY, USA) and R (R Foundation for Statistical Computing, Vienna, Austria), an open-source statistical software project [27].
Results
Survival of Conventional Total Femur Replacement
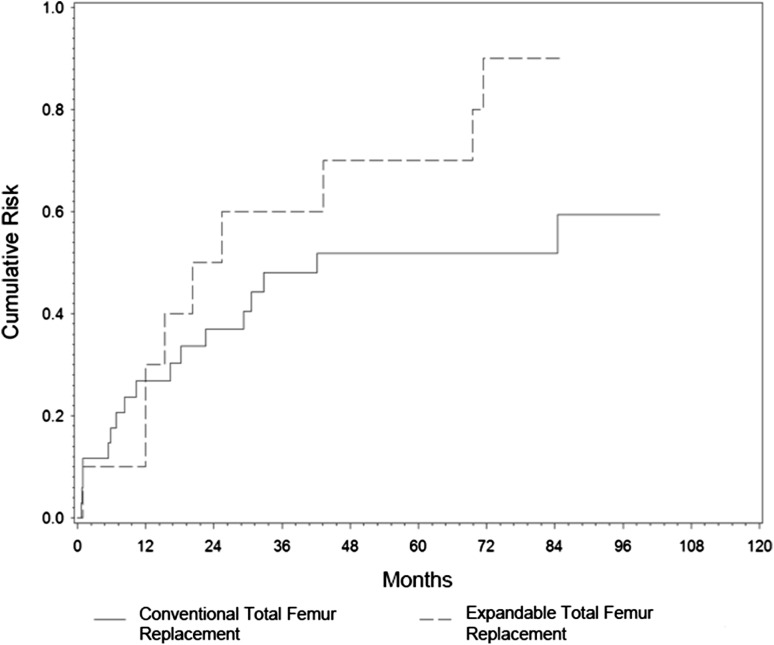
Median revision-free implant survival after conventional total femur replacement was 3.5 years (95% CI, 0.37–0.73), with 48% (95% CI, 0.37–0.73) and 41% (95% CI, 0.43–0.82) at 5 and 8.5 years, respectively (Fig. 2). Correspondingly, overall implant survival was 97% (95% CI, 0.004–0.20) after 5 years and 91% (95% CI, 0.02–0.38) after 10 and 20 years. Overall implant removal by hip disarticulation was performed in two patients.
Fig. 2.
A comparison of the cumulative risk of revision-free implant survival in conventional and expandable total femur replacements is shown.
Survival of Expandable Total Femur Replacement
Median revision-free implant survival after expandable total femur replacement was approximately 2 years (95% CI, 0.36–1.0), with 30% (95% CI, 0.47–1.0) and 10% (95% CI, 0.73–1.0) surviving after 5 and 7 years, respectively (Fig. 2). No expandable total femur replacements had to be removed leading to an overall implant survival of 100%.
Frequencies and Types of Complications After Total Femur Replacement
With conventional total femur replacements, the mean time to soft tissue failure was 14 months (range, 1–40 months) and included 12 dislocations in eight of 34 patients. Three patients had severe gluteal insufficiencies, two required implantation of an acetabular component owing to secondary arthritis, and one had an extensive wound-healing disorder resulting in hip disarticulation 1 month after conventional total femur replacement reconstruction. There was no occurrence of aseptic loosening with conventional total femur replacement. The mean time to structural failure in three patients with conventional total femur replacements was 76 months (range, 42–103 months) and included one dislocation of the mechanical axis and three bushing wears. Six patients treated with conventional total femur replacement sustained an infection after a mean of 50 months (range, 5–155 months), which led to surgical revision in five and nonsurgical treatment in one patient with an existing fistula attributable to a reduced general state of health. The other five patients had a mean of 2.4 septic revisions (range, 1–4 revisions) including eight one-stage and four two-stage revisions. One of these patients required hip disarticulation 80 months after implantation. One patient with osteosarcoma had initial tumor resection at another institution, received conventional total femur replacement secondarily, and had local and distant recurrence develop after 7 months. The patient received local irradiation and died 3 months later. Neither administration of chemotherapy, radiation, nor design of hinge mechanism in the knee was associated with an increased risk of any type of complication in conventional total femur replacements (Table 2).
Table 2.
Implant failure, implant removal, and functional outcomes
| Variable | Conventional total femur replacement (n = 34; 77%) | Expandable total femur replacement (n = 10; 23%) |
|---|---|---|
| Failure type | ||
| Type 1 soft tissue failure | 13 (38%) | 1 (10%) |
| Type 2 aseptic loosening | 0 | 1 (10%) |
| Type 3 structural failure* | 3 (9%) | 6 (60%) |
| Defect of growing mechanism | N/A | 4 (40%) |
| Bushing wear | 2 (6%) | 0 |
| Type 4 Infection | 6 (18%) | 1 (10%) |
| Type 5 Tumor progression | 1 (2%) | 0 |
| Type 6 Pediatric failure | ||
| Scar tissue contracture | N/A | 6 (60%) |
| Hip instability | N/A | 8 (80%) |
| Unplanned revision | ||
| Total | 17 (50%) | 9 (90%) |
| Single revision | 9 (27%) | 2 (20%) |
| Multiple revision | 8 (24%) | 7 (70%) |
| Minor revision | ||
| Wound-healing disturbance | 5 (15%) | 3 (30%) |
| Temporary peroneal palsy | 5 (15%) | 0 |
| Planned revision | ||
| Manual expansion† | N/A | 7 (70%) |
| Expansion by exchange‡ | N/A | 9 (90%) |
| Total number of revisions per patient (mean, range) | 1.3 (0–9) | 7.2 (2–20) |
| 2-year revision-free implant survival | 63% | 50% |
| 5-year revision-free implant survival | 48% | 30% |
| Musculoskeletal Tumor Society score (mean, range) | 70 (27–97) | 88 (60–97) |
| Implant removal (amputation) | 2 (6%) | 0 |
| 5-year overall implant survival | 97% | 100% |
| 10-year overall implant survival | 91% | 100% |
* Including defect of growing mechanism and bushing wear; †manual expansion = elongation by a worm-drive mechanism with screw driver; ‡expansion by exchange = elongation by exchange of a modular part of the prosthesis; N/A = not applicable.
With expandable total femur replacements, one patient sustained a soft tissue failure (hip dislocation) 1 month postoperatively and one had aseptic loosening of an uncemented tibial stem after 101 months. Structural failures included two periprosthetic fractures after 86 and 214 months, and one loosening of an enhanced tendon anchor after 70 months. One patient sustained an infection after 63 months, which resolved after one-stage revision. There was no local recurrence in patients who had an expandable femur replacement. Referring to expandable-specific failures, hip instability occurred in eight of 10 patients at a mean of 57 months (range, 12–233 months). Six of the patients were treated by pelvic osteotomy after a mean of 33 months (range, 12–72 months) and two received an acetabular cup after 25 and 233 months. All six patients with a previous pelvic osteotomy received a cup after a mean of 35 months (range, 4–113 months). Contracture by scar tissue occurred in six patients (60%) after a mean of 41 months (range, 25–62 months), mainly in noninvasive elongation mechanisms (five of six), and resection of the scar tissue sleeve was required for each of the patients. Defects of the expansion mechanism occurred in one invasive and in three noninvasive implants after a mean of 32 months (range, 21–49 months). The invasive mechanism had a spontaneous shortening and the patient underwent exchange of the prosthesis. Three noninvasive mechanisms failed owing to ingrowth of soft tissues into the gearbox requiring cleaning of the gearbox twice in two patients and subsequent invasive manual lengthening in one patient.
Patients with expandable total femur replacements underwent a mean of 4.6 operative limb-lengthening procedures (range, 0–14 procedures) with a mean total expansion of 95 mm (range, 20–224 mm) per patient (Table 3). The mean expansion per expanding procedure was 21 mm for all expandable total femur replacements and 23 mm and 16 mm for noninvasive and invasive expandable total femur replacements, respectively. The largest expansion per lengthening procedure was achieved using the electromagnetic MUTARS® (123 mm without an operative expansion procedure). Four patients who received noninvasive expansion mechanisms did not benefit from the noninvasive design in terms of undergoing fewer expanding operations. Two of the patients had the noninvasive mechanism only for a limited time; one patient achieved skeletal maturity earlier than estimated and one had the device removed after 3 years owing to infection. The other two patients with noninvasive implants had revision for scar tissue contractures and defects of the expansion mechanism. The mean limb-length discrepancy at the end of elongation was −35 mm (range, −180 mm to 25 mm) in all patients who underwent expandable total femur replacement. The patient with the greatest limb-length discrepancy (−180 mm) initially underwent reconstruction using a cement spacer at another institution and subsequently received an invasive expandable total femur replacement 3 years later, at age 8 years. Owing to a soft tissue contracture, equal limb length could not be achieved.
Table 3.
Details of expandable total femur replacement focusing on limb lengthening
| Patient | Type of expansion mechanism and prosthesis | Age at surgery/last followup (years) | Total lengthening | Number of expansions | Limb-length discrepancy |
|---|---|---|---|---|---|
| Noninvasive* | |||||
| 1 | HMRS (11–15 years) | 7/25 | 145 mm | 2 | 0 mm |
| 2 | HMRS (15–18 years) | 12/32 | 60 mm | 6 | −20 mm |
| 3 | HMRS (8–16 years) | 7/26 | 89 mm | 4 | −23 mm |
| 4 | HMRS (6–9 years) | 4/20 | 224 mm | 14 | −62 mm |
| 5 | HMRS (9–18 years) | 8/19 | 87 mm | 6 | −30 mm |
| 6 | MUTARS® (12–15 years) | 12/15 | 123 mm | 0 | −70 mm |
| Mean | 121 mm | 5.3 | −34 mm | ||
| Invasive | |||||
| 7 | HMRS | 13/37 | 20 mm | 1 | +5 mm |
| 8 | HMRS | 10/30 | 129 mm | 8 | +25 mm |
| 9 | HMRS | 13/20 | 47 mm | 3 | +5 mm |
| 10 | HMRS | 8/12 | 28 mm | 2 | −180 mm |
| Mean | 56 mm | 3.5 | −36 mm | ||
| All mean | 95 mm | 4.6 | −35 mm | ||
HMRS = Howmedica Modular Replacement System (Howmedica Osteonics Corp, Mahwah, NJ, USA); MUTARS® = Modular universal tumor and revision system (Implantcast, Buxtehude, The Netherlands); *noninvasive lengthening was performed by electromagnetic induction in the MUTARS® and with automatic elongation device in the HMRS, age of patient in years in which noninvasive device was used in parentheses.
Discussion
In rare cases tumor involvement of the femur is so extensive that reconstruction can be done only with a total femur replacement. However, to our knowledge, there are no published reports regarding total femur replacement implant failures using a standardized failure-mode classification; therefore we investigated our total femur replacements for implant survival and different types of failures using the comprehensive ISOLS failure-mode classification [8, 9]. Our study revealed a median revision-free implant survival of 42 and 25 months, with a revision rate of 17 of 34 and nine of 10 conventional and expandable total femur replacements, respectively. However, long-term limb survival (91% and 100% for conventional and expandable total femur replacements, respectively) was not compromised. Soft tissue failures, including dislocations and expandable-specific failures, were the most frequent type of complication after conventional and expandable total femur replacements, respectively.
Our study has numerous limitations. First, the cohort was small. Second, patients with several types of tumors were included, each with their own general prognoses owing to the rare indications for conventional and expandable total femur replacements. Third, during the 30-year interval of our study, three different total femur replacement models were implanted, with heterogeneity in abductor reconstruction and tibial fixation leading to small numbers of different reconstruction techniques, making it impossible to draw conclusions regarding which reconstruction has fewer complications. In addition, the high percentage of patient mortality attributable to underlying oncologic diseases considerably reduced followup periods.
Therefore, it is difficult to provide universal surgical guidelines for issues to be addressed with conventional and expandable total femur replacements, replacing the hip and knee in one stage frequently in combination with extensive soft tissue loss around the thigh. These may include management of knee kinematics (rotating versus fixed hinge prostheses), abductor reconstruction, and expandable-specific complications. The use of fixed or rotating hinge designs in this series was dictated basically by endoprosthetic design rather than kinematic observations, as newer implant designs mostly no longer provide fixed hinges. We found that with total femur replacement, a concomitant soft tissue loss around the thigh may put patients at risk for inability to stabilize a rotating hinge knee mechanism resulting in discomfort or subjective instability. Based on this we tried to continue using early-generation implants (in this case the HMRS) in cases of extensive tumor masses and vast resections. Likewise, the mode of abductor reconstruction may be subject to the actual amount of resected musculature. In cases of residual trochanteric bone or viable tendinous abductor structures we preferred direct attachment to the endoprosthetic implant, while lesser residual muscle structures seem to be more easily attached to the fascia lata. Alternatively, the LARS® may be a helpful tool for more stable soft tissue repair in cases of extensive loss, but simultaneously may work as an additional foreign body elevating the risk of deep prosthetic infection. Our best results regarding hip stability were obtained when we could retain the acetabulum and capsule in combination with a bipolar head, while acetabular components seem to have an elevated risk of dislocation, especially when abductors are diminished. In this context the use of tripolar cups may be a helpful alternative to reduce dislocation rates. Finally, expandable total femur replacements may result in additional revisions requiring exact timing. In most cases of expandable total femur replacement with bipolar heads, the resulting hip dysplasia indicated pelvic osteotomies throughout the lengthening procedures, as increasing soft tissue tension enforced the pressure on the femoral head to subside laterally. In these cases we performed pelvic osteotomies often at the beginning of the lengthening to avoid additional instability of the hip. Implantation of acetabular components was indicated mostly with respect to clinical symptoms of arthritic pain. Finally, resection of scar tissue in response to total femur replacement along the entire thigh frequently is limited by the reduced soft tissue mantle, therefore partly explaining cases with considerable residual leg-length discrepancy (three patients with more than 3 cm in this series) attributable to soft tissue contractures.
The difference in reported revision-free and overall implant survival after conventional total femur replacement in patients with tumors is striking. This is the first study, to our knowledge, that shows competing risk analysis of conventional total femur replacement for revision-free and overall implant survival, with 48% and 41% after 5 and 8.5 years, respectively. We had a high failure rate of 50% of conventional total femur replacements. Reported rates range from 23% [14] to 48% [8]. Comparing failure rates in the literature is challenging owing to different failure mode classifications and different lengths of followup. We used the comprehensive ISOLS classification system published in 2011 [8] and modified in 2014 [9]. Sewell et al. [31] used a similar classification system and reported implant failure in 33% of patients and revision-free implant survival of 56% at 5 years. Our study showed a patient survival rate of 59% at 10 years compared with 16% in the series of Sewell et al. [31]. Thus, there is a life-long risk for revision and because our study patients had prolonged survival, it may be an explanation for our higher failure rate. However, overall implant survival rates of 91% with conventional total femur replacement were not compromised and were similar to results from studies with lower revision rates [14, 24, 28].
Revision-free implant survival of expandable total femur replacements of 30% at 5 years and 10% at 7 years was relatively poor. To the best of our knowledge there are no published data regarding revision-free implant survival of expandable total femur replacements. Failure rates between 60% [30] and 66% [10] were reported compared with 90% in our series. However overall implant survival in those two studies was equal to our overall implant survival rate. Hwang et al. [11] reported one of five patients with an expandable total femur replacement had to undergo amputation owing to prolonged prosthetic infection.
Similar to other studies, ISOLS Type I failures were the most common, including hip dislocation in 24% of patients who underwent conventional total femur replacement [3, 30]. Bickels et al. [3] reported that acetabular preservation, capsular repair, and reconstruction of the abductor mechanism can decrease hip dislocation. Ruggieri et al. [28] reported no hip dislocations in a series of 23 patients with osteosarcoma who underwent total femur replacement. They stressed the importance of surgical care in capsule closure and soft tissue reinforcement to avoid the need for acetabular resurfacing [28]. Owing to the small sample size of different types of abductor reconstruction techniques we were not able to draw conclusions regarding hip dislocation for the different techniques. The infection rate for conventional total femur replacements ranges from 3% [30] to 22% [2] and was 18% in our study. Infection is the most common cause leading to implant removal [28, 32]. Jeon et al. [12] reported on total femur resection with extracorporal radiation and finally replantation of the autologous femoral bone-prosthesis composite. Infection occurred in 38% of these patients [12]. Our local recurrence rate of 2% with conventional total femur replacements is in accordance with published ranges of 0% to 9% [13, 28, 30, 31]. Studies including only patients with sarcoma [28, 30] reported lower local recurrence rates than studies including patients with metastatic disease [13, 31].
With expandable total femur replacements we observed infection, hip disarticulation, and aseptic loosening in only one of 10 patients. Reported infection rates range from 20% [11, 30] to 33% [10] and aseptic loosening in 20% [30]. No dislocations of the hip with extendable total femur replacements have been reported to our knowledge. Furthermore, we focused on secondary hip instability in skeletally immature patients. Schindler et al. [30] reported secondary hip instability occurred in 20% of pediatric patients. They believed the imbalance between the acetabulum and the bipolar head explained the dislocations in their patients and treated them with a larger bipolar head size. Additionally, we hypothesized that there is an underlying imbalance between detached abductors and contracted adductors in growing children, which may lead to dislocation. Among young patients treated with expandable total femur replacement, 80% experienced dislocation and the majority were treated by pelvic osteotomy followed by acetabular resurfacing. Scar tissue contractures in growing patients was described by Schiller et al. [29] as occurring in 15% of patients, compared with 60% in our study. Five of six of our patients with noninvasive expansion mechanisms had this complication. Modifications for the comprehensive failure mode system for expandable reconstructions included defects of the expansion mechanism. In our study, failure occurred in 40% of the expansion mechanisms. Hwang et al. [11] reported a defect of electromagnetic induction in 3% of mechanisms.
The observed 21-mm mean expansion per lengthening procedure was greater than the 12 mm reported by Schindler et al. [30]. In contrast, all patients who had expandable total femur replacements in the study by Schindler et al. [30] had an invasive expansion mechanism, which might be an explanation. With the numbers of patients in our study, it appeared that we were able to achieve more expansion per procedure with noninvasive compared with invasive expansion mechanisms, although we cannot conclude this definitively. Other changes such as implant design and surgeon experience may explain this observation. However, the limb-length discrepancy was greater in our study patients (mean, 35 mm) compared with 12 mm reported by Schiller et al. [29]. This might be because there is more scarring with total femur replacements, also described by Hwang et al. [11], than with partial femur reconstructions.
Soft tissue failures with conventional total femur replacements, including dislocation and hip instability in growing patients, accounted for most revisions in our study. Despite high failure rates of 50% with conventional and 90% with expandable total femur replacements, long-term implant survival was 91% with conventional and 100% with expandable replacements. With the number of patients in our study, the number of revisions did not appear to affect infection and overall implant survival. Although the indications for total femoral resection are rare, we believe total femur replacement offers a reasonable treatment option for reconstruction of massive femoral bone defects after tumor resection in adults and growing patients and avoids the necessity for amputation in most patients.
Acknowledgments
We thank M. Dominkus MD (Orthopaedic Hospital Speising, Vienna, Austria) for maintenance of the local tumor registry, and A. Kaider MSc (Center for Medical Statistics, Medical University of Vienna, Vienna, Austria) for calculating the competing risk analysis. In addition, we acknowledge R. Kotz MD (Wiener Privatklinik, Vienna, Austria) and M. Dominkus MD for performing some of the surgical procedures.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AR. Total femur replacement. Arch Orthop Trauma Surg. 2010;130:171–176. doi: 10.1007/s00402-009-0945-2. [DOI] [PubMed] [Google Scholar]
- 3.Bickels J, Meller I, Henshaw RM, Malawer MM. Reconstruction of hip stability after proximal and total femur resections. Clin Orthop Relat Res. 2000;375:218–230. doi: 10.1097/00003086-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Buchman J. Total femur and knee joint replacement with a vitallium endoprosthesis. Bull Hosp Joint Dis. 1965;26:21–34. [PubMed] [Google Scholar]
- 5.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 6.Erler K, Demiralp B, Ozdemir MT, Basbozkurt M. [Successful results of total femoral resection and prosthetic replacement in two patients][in Turkish] Acta Orthop Traumatol Turc. 2004;38:79–84. [PubMed] [Google Scholar]
- 7.Faisham WI, Zulmi W, Halim AS. Modular endoprosthetic replacement after total femur resection for malignant bone tumor. Med J Malaysia. 2005;60(suppl C):45–48. [PubMed] [Google Scholar]
- 8.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 9.Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system Including biological and expandable reconstructions. Bone Joint J. 2014;96:1436–1440. doi: 10.1302/0301-620X.96B11.34747. [DOI] [PubMed] [Google Scholar]
- 10.Henderson ER, Pepper AM, Marulanda G, Binitie OT, Cheong D, Letson GD. Outcome of lower-limb preservation with an expandable endoprosthesis after bone tumor resection in children. J Bone Joint Surg Am. 2012;94:537–547. doi: 10.2106/JBJS.I.01575. [DOI] [PubMed] [Google Scholar]
- 11.Hwang N, Grimer RJ, Carter SR, Tillman RM, Abudu A, Jeys LM. Early results of a non-invasive extendible prosthesis for limb-salvage surgery in children with bone tumours. J Bone Joint Surg Br. 2012;94:265–269. doi: 10.1302/0301-620X.94B2.27536. [DOI] [PubMed] [Google Scholar]
- 12.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Clinical outcome of osteosarcoma with primary total femoral resection. Clin Orthop Relat Res. 2007;457:176–182. doi: 10.1097/BLO.0b013e31802ba4af. [DOI] [PubMed] [Google Scholar]
- 13.Jones KB, Griffin AM, Chandrasekar CR, Biau D, Babinet A, Deheshi B, Bell RS, Grimer RJ, Wunder JS, Ferguson PC. Patient-oriented functional results of total femoral endoprosthetic reconstruction following oncologic resection. J Surg Oncol. 2011;104:561–565. doi: 10.1002/jso.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra S, Abudu A, Murata H, Grimer RJ, Tillman RM, Carter SR. Total femur replacement: primary procedure for treatment of malignant tumours of the femur. Eur J Surg Oncol. 2010;36:378–383. doi: 10.1016/j.ejso.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Katznelson A, Nerubay J. Total femur replacement in sarcoma of the distal end of the femur. Acta Orthop Scand. 1980;51:845–851. doi: 10.3109/17453678008990883. [DOI] [PubMed] [Google Scholar]
- 16.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 17.Kotz RI, Windhager R, Dominkus M, Robioneck B, Muller-Daniels H. A self-extending paediatric leg implant. Nature. 2000;406:143–144. doi: 10.1038/35018155. [DOI] [PubMed] [Google Scholar]
- 18.Lewis MM. The use of an expandable and adjustable prosthesis in the treatment of childhood malignant bone tumors of the extremity. Cancer. 1986;57:499–502. doi: 10.1002/1097-0142(19860201)57:3<499::AID-CNCR2820570316>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Mankin HJ, Hornicek FJ, Harris M. Total femur replacement procedures in tumor treatment. Clin Orthop Relat Res. 2005;438:60–64. doi: 10.1097/00003086-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Marcove RC, Lewis MM, Rosen G, Huvos AG. Total femur replacement. Comprehensive Therapy. 1977;3:13–19. [PubMed] [Google Scholar]
- 21.Mittermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Revision of the Kotz type of tumour endoprosthesis for the lower limb. J Bone Joint Surg Br. 2002;84:401–406. doi: 10.1302/0301-620X.84B3.12204. [DOI] [PubMed] [Google Scholar]
- 22.Morris HG, Capanna R, Campanacci D, Del Ben M, Gasbarrini A. Modular endoprosthetic replacement after total resection of the femur for malignant tumour. Int Orthop. 1994;18:90–95. doi: 10.1007/BF02484417. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura S, Kusuzaki K, Murata H, Takeshita H, Hirata M, Hashiguchi S, Hirasawa Y. More than 10 years of follow-up of two patients after total femur replacement for malignant bone tumor. Int Orthop. 2000;24:176–178. doi: 10.1007/s002640000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natarajan MV, Balasubramanian N, Jayasankar V, Sameer M. Endoprosthetic reconstruction using total femoral custom mega prosthesis in malignant bone tumours. Int Orthop. 2009;33:1359–1363. doi: 10.1007/s00264-009-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nerubay J, Katznelson A, Tichler T, Rubinstein Z, Morag B, Bubis JJ. Total femoral replacement. Clin Orthop Relat Res. 1988;229:143–148. [PubMed] [Google Scholar]
- 26.Puri A, Gulia A, Chan WH. Functional and oncologic outcomes after excision of the total femur in primary bone tumors: results with a low cost total femur prosthesis. Indian J Orthop. 2012;46:470–474. doi: 10.4103/0019-5413.98834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available at: http://www.R-project.org/. Accessed July 25, 2014.
- 28.Ruggieri P, Bosco G, Pala E, Errani C, Mercuri M. Local recurrence, survival and function after total femur resection and megaprosthetic reconstruction for bone sarcomas. Clin Orthop Relat Res. 2010;468:2860–2866. doi: 10.1007/s11999-010-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiller C, Windhager R, Fellinger EJ, Salzer-Kuntschik M, Kaider A, Kotz R. Extendable tumour endoprostheses for the leg in children. J Bone Joint Surg Br. 1995;77:608–614. [PubMed] [Google Scholar]
- 30.Schindler OS, Cannon SR, Briggs TW, Blunn GW, Grimer RJ, Walker PS. Use of extendable total femoral replacements in children with malignant bone tumors. Clin Orthop Relat Res. 1998;357:157–170. doi: 10.1097/00003086-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Sewell MD, Spiegelberg BG, Hanna SA, Aston WJ, Bartlett W, Blunn GW, David LA, Cannon SR, Briggs TW. Total femoral endoprosthetic replacement following excision of bone tumours. J Bone Joint Surg Br. 2009;91:1513–1520. doi: 10.1302/0301-620X.91B11.21996. [DOI] [PubMed] [Google Scholar]
- 32.Ward WG, Dorey F, Eckardt JJ. Total femoral endoprosthetic reconstruction. Clin Orthop Relat Res. 1995;316:195–206. [PubMed] [Google Scholar]