Abstract
Background
Prior studies of nonoperative treatment for lumbosacral radiculopathy have identified potential predictors of treatment failure, defined by persistent pain, persistent disability, lack of recovery, or subsequent surgery. However, few predictors have been replicated, with the exception of higher leg pain intensity, as a predictor of subsequent surgery.
Questions/purposes
We asked two research questions: (1) Does higher baseline leg pain intensity predict subsequent lumbar surgery? (2) Can other previously identified “candidate” predictors of nonoperative treatment failure be replicated?
Methods
Between January 2008 and March 2009, 154 participants with acute lumbosacral radicular pain were enrolled in a prospective database; 128 participants (83%) received nonoperative treatment and 26 (17%) received surgery over 2-year followup. Ninety-four nonoperative participants (73%) responded to followup questionnaires. We examined associations between previously identified “candidate” predictors and treatment failure defined as (1) subsequent surgery; (2) persistent leg pain on a visual analog scale; (3) persistent disability on the Oswestry Disability Index; or (4) participant-reported lack of recovery over 2-year followup. Confounding variables including sociodemographics, clinical factors, and imaging characteristics were evaluated using an exploratory bivariate analysis followed by a multivariate analysis.
Results
With the numbers available, higher baseline leg pain intensity was not an independent predictor of subsequent surgery (adjusted odds ratio [aOR], 1.22 per point of baseline leg pain; 95% confidence interval [CI], 0.98–1.53; p = 0.08). Prior low back pain (aOR, 4.79; 95% CI, 1.01–22.7; p = 0.05) and a positive straight leg raise test (aOR, 4.38; 95% CI, 1.60–11.9; p = 0.004) predicted subsequent surgery. Workers compensation claims predicted persistent leg pain (aOR, 9.04; 95% CI, 1.01–81; p = 0.05) and disability (aOR, 5.99; 95% CI, 1.09–32.7; p = 0.04). Female sex predicted persistent disability (aOR, 3.16; 95% CI, 1.03–9.69; p = 0.05) and perceived lack of recovery (aOR, 2.44; 95% CI, 1.02–5.84; p = 0.05).
Conclusions
Higher baseline leg pain intensity was not confirmed as a predictor of subsequent surgery. However, the directionality of the association seen was consistent with prior reports, suggesting Type II error as a possible explanation; larger studies are needed to further examine this relationship. Clinicians should be aware of potential factors that may predict nonoperative treatment failure, including prior low back pain or a positive straight leg raise test as predictors of subsequent surgery, workers compensation claims as predictors of persistent leg pain and disability, and female sex as a predictor of persistent disability and lack of recovery.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Lumbar disc herniation (LDH) is a common cause of acute lumbosacral radiculopathy or sciatica. Patient-reported outcomes with nonoperative treatment for lumbosacral radiculopathy are favorable for many individuals [25, 34, 35], but others fare poorly or may go on to decompression surgery. Early identification of those individuals with lumbosacral radiculopathy who are likely to fail nonoperative treatment is therefore a worthwhile goal, because these patients might be targeted for closer clinical observation and support.
Prior studies of nonoperative treatment for lumbosacral radiculopathy have identified potential predictors of treatment failure as defined by (1) subsequent surgery; (2) pain; (3) disability; or (4) perceived lack of recovery [2–4, 11, 20–22, 25, 27–30]. These studies took appropriate measures to account for confounding through multivariate analytic techniques. However, most of these studies examined unselected lists of potential predictor variables, identifying those variables found to be independently associated with nonoperative treatment failure as factors with likely prognostic value. This approach requires multiple statistical comparisons and raises the possibility that identified predictors are simply false-positives without true predictive value. Importantly, very few predictors have been subsequently tested and replicated in a separate cohort. A recent systematic review of prognostic factors for failure of nonoperative treatment for lumbosacral radiculopathy identified only a single factor (higher baseline leg pain intensity) that was independently predictive of nonoperative treatment failure (as defined by subsequent surgery) in at least two other samples [29]. This association was seen in two high-quality studies of acute/subacute lumbosacral radiculopathy with no studies showing conflicting results. Other potentially important predictor variables identified in this review showed only limited evidence such as an association with treatment failure in only a single study, conflicting evidence for an effect on treatment failure (one study demonstrating an effect and one or more studies demonstrating no effect), or limited to strong evidence of no association [29]. These findings highlight the need for studies whose primary aim is to confirm whether previously identified predictors of nonoperative treatment failure can be successfully replicated.
We therefore analyzed data from a prospective inception cohort study of individuals with acute lumbosacral radiculopathy attributed to LDH to replicate potential predictor variables identified in previous studies. In contrast to prior work, we considered only a highly selected list of candidate predictors in which at least one previous cohort study had demonstrated a statistically significant and multivariate-adjusted association with treatment failure. We asked two research questions related to predictors of treatment failure in nonoperative lumbar radiculopathy: (1) Does higher initial leg pain intensity predict subsequent lumbar surgery? (2) Can other previously identified candidate predictors of nonoperative treatment failure be replicated in our sample?
Materials and Methods
We identified studies examining predictors of failure of nonoperative treatment for lumbosacral radiculopathy (subsequent surgery, persistent pain, persistent disability, and perceived lack of recovery) from the recent systematic review by Verwoerd et al. [29]. We updated the search methods from this review to include results up to December 2013, yielding two additional studies meeting the review inclusion criteria [11, 14]. Predictor variables with statistically significant, multivariate-adjusted associations with failure of nonoperative treatment for lumbosacral radiculopathy in at least one prior cohort study were included as candidate predictors in this analysis (Table 1 [2–4, 11, 12, 14, 17, 20, 21, 23, 27, 28, 30–32]). Some variables with limited or conflicting evidence for an association with treatment failure in prior studies were not available in our data set, including educational attainment, body mass index, speed of symptom onset, prior sciatica, physical activity exposures, work characteristics, kinesiophobia, nerve impingement, and genetic polymorphisms [29]. In contrast to the review by Verwoerd et al., in which predictors of treatment failure defined by a combination of two patient-reported outcomes were classified as predictors of “recovery,” we instead classified these predictors according to each of the constituent patient-reported outcomes (for example, where sex was predictive of treatment failure defined as persistent pain intensity and disability, we considered sex as a possible predictor of persistent pain and a possible predictor of persistent disability [31]).
Table 1.
Previously identified candidate predictors of failure of nonoperative treatment for lumbosacral radiculopathy and characteristics of the studies in which they were identified*
| Prognostic factor | Clinical setting | Country | Duration of symptoms† | Followup | Conflicting studies?ǂ | Supporting studies? |
|---|---|---|---|---|---|---|
| Predictors of treatment failure defined as subsequent surgery | ||||||
| Initial leg pain intensity [30] | Primary care | Netherlands | Acute | 6 months | No | Yes [20] |
| Initial disability [20] | Specialty care | Netherlands | Subacute | 1 year | No | No |
| Duration of symptoms [28] | Specialty care | France | Acute | 11–24 months | Yes [30] | No |
| Prior low back pain [30] | Primary care | Netherlands | Acute | 6 months | No | No |
| Positive straight leg raise test (SLR) [28] | Specialty care | France | Acute | 11–24 months | Yes [20, 30] | Yes [31]§ |
| Positive crossed SLR [30] | Primary care | Netherlands | Acute | 6 months | No | No |
| Positive femoral stretch test [30] | Primary care | Netherlands | Acute | 6 months | No | Yes [31]§ |
| Predictors of treatment failure defined as persistent leg pain at followup | ||||||
| Initial leg pain intensity [11] | Specialty care | Norway | Acute/chronic | 1–2 years | Yes [27] | No |
| Initial back pain intensity [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Female sex [12] | Specialty care | Denmark | Acute/chronic | 14 months | Yes [17, 27] | No |
| Duration of symptoms [23] | Specialty care | United States | Subacute/chronic | 2–4 years | Yes [11] | No |
| Smoking [11] | Specialty care | Norway | Acute/chronic | 1–2 years | Yes [17] | No |
| Medical comorbidities [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Workers compensation [3] | Specialty care | United States | Acute/chronic | 4 years | Yes [4] | Yes [2] |
| Muscle weakness (any) [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Herniation morphology [12]|| | Specialty care | Denmark | Acute/chronic | 14 months | No | No |
| Predictors of treatment failure defined as persistent disability at followup | ||||||
| Initial disability [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Initial back pain intensity [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Female sex [12] | Specialty care | Denmark | Acute/chronic | 2 years | No | No |
| Duration of symptoms [23] | Specialty care | United States | Subacute/ chronic | 2–4 years | No | Yes [11] |
| Smoking [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Medical comorbidities [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Prior low back pain [11] | Specialty care | Norway | Acute/chronic | 2–4 years | No | No |
| Workers compensation [3] | Specialty care | United States | Acute/chronic | 4 years, | Yes [4] | Yes [2] |
| Herniation morphology [12]|| | Specialty care | Denmark | Acute/chronic | 14 months | No | No |
| Abnormal tendon reflexes [11] | Specialty care | Norway | Acute/chronic | 1–2 years | No | No |
| Predictors of treatment failure defined as participant-reported lack of recovery at followup | ||||||
| Age [14] | Specialty care | Netherlands | Subacute | 5 year | Yes [21, 31] | No |
| Female sex [21] | Specialty care | Netherlands | Subacute | 1 year | Yes [31] | No |
| Duration of symptoms [31] | Primary care | Netherlands | Acute | 3 months | No | No |
| Smoking [21] | Specialty care | Netherlands | Subacute | 1 year | Yes [31] | No |
| Workers compensation [3] | Specialty care | United States | Acute/chronic | 5–10 years | No | No |
| Positive SLR [31] | Primary care | Netherlands | Acute | 3 months | Yes [21] | No |
| Positive femoral stretch test [31] | Primary care | Netherlands | Acute | 3 months | No | No |
| Foraminal herniation [33] | Primary care | Netherlands | Acute | 3 months | No | No |
* All associations between candidate predictors and outcomes refer to positive associations, except for those studies cited under Conflicting studies; †acute signifies duration of symptoms ≤ 12 weeks, whereas subacute signifies duration of 6–12 weeks; ǂConflicting studies refers to those in which no statistically significant association with an outcome was found or where a statistically significant association was found with a negative association (in the opposite direction than expected); §subsequent surgery was also included in a composite outcome; ||disc extrusions predicted lower pain intensity and disability; sequestrations and extrusions were collinear.
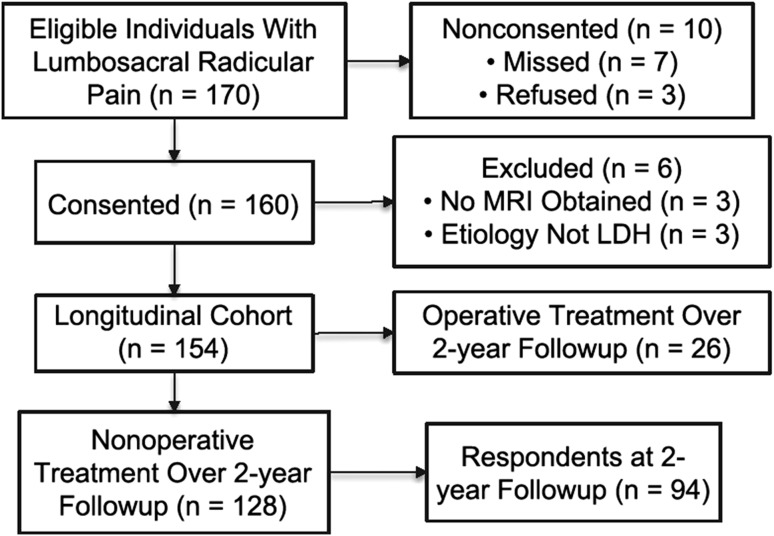
Inclusion criteria considered for study entry were a clinical presentation of lumbosacral radicular pain in a L2, L3, L4, L5, or S1 dermatome; symptom duration ≤ 12 weeks; and age ≥ 18 years. Exclusion criteria included known pregnancy or severe and active medical or psychiatric comorbidities that would limit study participation; infectious, inflammatory, or neoplastic causes of radiculopathy; significant deformity or spondylolisthesis; and prior lumbar spine surgery at the affected level. To be retained in the prospective cohort, participants needed to have an available MRI confirming LDH corresponding with the clinical presentation. Further details of inclusion and exclusion criteria are provided elsewhere [25]. Between January 2008 and March 2009, 170 individuals met initial study criteria, of which 163 were approached to participate in this observational study. Three individuals declined to participate, and six did not have an available MRI confirming LDH corresponding with the clinical presentation, leaving 154 participants who met all study criteria and consented to participate in this longitudinal study (Fig. 1).
Fig. 1.
A flowchart of study participation is shown.
Participant-reported information was collected at the baseline clinic visit and by mailed questionnaires at 1-month, 3-month, 6-month, 1-year, and 2-year followup. Participants reported on lumbar spine surgery performed since the time of study recruitment at all followup time points. To ensure accuracy with respect to ascertainment of subsequent surgery, a medical record review was performed at 2-year followup to capture any missing data related to lumbar spine surgery; where necessary, participants were contacted by telephone to determine their surgical status. Of 154 study participants, 26 (17%) received surgery, and 128 (83%) received nonoperative treatment only, over the 2-year followup (Table 2). Of the 128 participants who received nonoperative treatment, 94 (73%) responded to mailed followup questionnaires including patient-reported outcomes and were available for analysis 2 years later (Fig. 1). Forty-two participants (46%) had persistent leg pain, 19 (20%) had persistent disability, and 50 (53%) reported lack of recovery. Respondents were not different from nonrespondents with respect to sociodemographics and baseline clinical measures (data not shown).
Table 2.
Characteristics of the study sample
| Participant characteristics | All participants (n = 154) | Nonoperative participants only (n = 128) |
|---|---|---|
| Sociodemographics and medical history | ||
| Age (years) | 52.9 (13.4) | 53.7 (13.3) |
| Male sex | 105 (68.2%) | 83 (64.8%) |
| Duration of symptoms (weeks) | 4.9 (3.0) | 5.0 (3.1) |
| Prior low back pain history | 116 (75.3%) | 92 (71.9%) |
| Workers compensation | 10 (6.5%) | 10 (7.8%) |
| Cigarette smoking | 30 (19.5%) | 26 (20.3%) |
| Medical comorbidities*,† | 2.8 (3.3) | 2.8 (3.3) |
| Clinical factors at baseline | ||
| Visual analog scale leg painǂ (0–10) | 7.0 (2.4) | 6.8 (2.5) |
| Visual analog scale back painǂ (0–10) | 5.1 (3.3) | 4.9 (3.2) |
| Oswestry Disability Index† (0–100) | 51 (21) | 50 (20) |
| Positive straight leg raise | 66 (42.9%) | 47 (36.7%) |
| Positive crossed straight leg raise§ | 11 (7.1%) | 7 (5.5%) |
| Positive femoral stretch test | 33 (21.4%) | 28 (21.9%) |
| Any muscle weakness | 95 (61.7%) | 81 (63.3%) |
| Any impaired reflexes | 55 (35.7%) | 45 (35.2%) |
| Herniation characteristics on MRI | ||
| Foraminal or extraforaminal location | 53 (34.4%) | 48 (37.5%) |
| Herniation morphology | ||
| Extrusion | 98 (63.6%) | 82 (64.1%) |
| Sequestration | 14 (9.1%) | 11 (8.6%) |
* Self-Acquired Comorbidity Questionnaire (total score) [13]; †4 participants with missing data; ǂ1 participant with missing data; §2 participants with missing data.
Baseline data were collected at the time of recruitment on a range of sociodemographic and clinical factors, including participant-reported sex, duration of acute radicular pain, current or past significant cigarette smoking, having an active workers compensation claim, and a history of significant low back pain (LBP). Medical comorbidity burden was measured using the Self-Administered Comorbidity Questionnaire (SACQ) [13]. The SACQ produces a continuous score reflecting comorbidity burden ranging from 0 (no comorbidities) to 45 (highest comorbidity burden). Lumbar spine MRI features were recorded by the recruiting physician according to their review of available images and radiologist diagnostic impressions. Herniation morphology was classified as protrusion, extrusion, or sequestration [10]. Herniation location was classified as central (central/paracentral/lateral recess location) or foraminal (foraminal/extraforaminal location) [10]. Straight leg raise (SLR) testing, femoral stretch testing, crossed SLR testing, lower extremity strength, and deep tendon reflex testing were evaluated at the baseline clinical examination using standardized methods that have been described elsewhere [26]. All participants received education on the natural history of new lumbosacral radiculopathy and were encouraged to gradually normalize activities of daily living. Other treatments varied depending on the individual and included oral medications, physical therapy, and/or epidural corticosteroid injections; some participants were referred for surgical consultation in cases of poor response to treatment, progressive sensorimotor impairments, or by patient request.
The four measures representing treatment failure included (1) subsequent surgery; (2) persistent leg pain; (3) persistent disability; and (4) participant-reported lack of recovery over 2-year followup. Leg and back pain intensity were measured using a 0 to 10 visual analog scale (VAS) [6] with higher scores representing more severe pain. The VAS is a validated, reliable, and responsive scale that is widely used in back pain research [8]. Back-related disability was measured using the Oswestry Disability Index (ODI) [9]. The ODI has demonstrated validity and reliability in prior back pain studies [9]. ODI scores range from 0 to 100 with higher scores representing greater disability. Self-reported recovery was measured according to satisfaction with the current status of back and leg symptoms using the question “If you were to spend the rest of your life with your back or leg symptoms just the way they have been in the last 24 hours, how would you feel?” Responses included a seven-grade Likert scale ranging from “delighted” (1) to “terrible” (7). Although there are no widely accepted measures for global-perceived recovery, we defined clinically relevant recovery as a score of 1 (delighted) or 2 (pleased) as has been recommended previously [18]; all other scores constituted a lack of recovery. For the patient-reported outcomes of leg pain, disability, and lack of recovery, only data from the 1- and 2-year followup time points were used for this analysis.
Statistical Analysis
We used descriptive statistics to characterize the sample. We screened for collinearity between predictor variables using correlation matrices. We then conducted bivariate and multivariate regression analyses for the association of these predictor variables with each of the four measures representing treatment failure (subsequent surgery, leg pain intensity on the VAS, disability on the ODI, and participant-reported lack of recovery) as dependent variables. For those dependent variables with continuous scores (VAS leg pain and ODI disability), we assessed the relevant statistical assumptions required for use of linear regression and detected violations of linearity and normality of error distributions. Therefore, VAS leg pain and ODI disability were dichotomized. In the absence of widely accepted criteria for defining absent/minimal sciatic pain, we dichotomized VAS leg pain as absent (< 1) versus persistent (≥ 1), a threshold applied previously [12]. ODI disability scores were dichotomized at a cutoff point of 20, which defines the lowest stratum of disability originally proposed with the ODI [9] and has been used in prior studies [5, 15, 16]. Associations of candidate predictor variables with each dependent variable representing treatment failure were first examined in bivariate logistic regression models including one predictor at a time and subsequently in multivariate logistic regression models including multiple predictors simultaneously. Analyses for the dependent variable of subsequent surgery included the entire study sample, whereas analyses for the dependent variables leg pain, disability, and perceived lack of recovery were restricted to those participants receiving nonoperative treatment over the entire 2-year followup. Accounting for a commonly used rule of thumb requiring 10 events per predictor variable [19], we expected to have limited statistical efficiency to adjust for all covariates simultaneously in the multivariate models. Therefore, only predictor variables with p values ≤ 0.15 in the bivariate models were included in the final multivariate models. We defined statistical significance as a p value ≤ 0.05. We used the method of last value carried forward to account for missing data. Sample size was determined according to power calculations for a separate study unrelated to the hypotheses of this ancillary study [25]. All analyses were performed using SPSS software, Version 20.0.0 (IBM Corporation, Armonk, NY, USA).
Results
With the numbers available, higher baseline leg pain intensity was not associated with subsequent surgery (adjusted odds ratio [aOR], 1.22 per VAS point; 95% CI, 0.98–1.53; p = 0.08) after adjusting for prior LBP, a positive SLR at baseline, and a positive crossed SLR at baseline (Table 3). However, a history of prior LBP (aOR, 4.79; 95% CI, 1.01–22.7; p = 0.05) and a positive SLR at baseline (aOR, 4.38; 95% CI, 1.60–11.9; p = 0.004) were each independently associated with a greater odds of subsequent surgery.
Table 3.
Associations between candidate predictors and nonoperative lumbosacral radiculopathy treatment failure at 2-year followup*
| Prognostic factor | Bivariate associations | Multivariate associations | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Predictors of treatment failure defined as subsequent surgery (n = 154; 26 participants underwent surgery) | ||||
| Initial leg pain intensity (VAS) [30] | 1.25 (1.02–1.53) | 0.03 | 1.22 (0.98–1.53) | 0.08 |
| Initial disability (ODI) [20] | 1.01 (0.99–1.03) | 0.40 | – | – |
| Duration of symptoms (weeks) [28] | 0.95 (0.82–1.10) | 0.53 | – | – |
| Prior low back pain [30] | 4.70 (1.06–20.9) | 0.04 | 4.79 (1.01–22.7) | 0.05 |
| Straight leg raise test [28] | 4.68 (1.83–12.0) | 0.001 | 4.38 (1.60–11.9) | 0.004 |
| Crossed straight leg raise test [30] | 3.09 (0.83–11.5) | 0.09 | 1.25 (0.29–5.37) | 0.77 |
| Femoral stretch test [30] | 0.85 (0.29–2.46) | 0.77 | – | – |
| Predictors of treatment failure defined as persistent leg pain after nonoperative treatment† (n = 92; 42 participants with persistent leg pain) | ||||
| Initial leg pain intensity (VAS) [11] | 1.07 (0.90–1.27) | 0.43 | – | – |
| Initial back pain intensity (VAS) [11] | 0.91 (0.79–1.04) | 0.16 | – | – |
| Female sex [12] | 0.92 (0.40–2.14) | 0.85 | – | – |
| Duration of symptoms (weeks) [23] | 0.95 (0.83–1.09) | 0.45 | – | – |
| Smoking [11] | 0.80(0.28–2.33) | 0.68 | – | – |
| Medical comorbidities (SACQ) [11] | 1.11 (0.97–1.27) | 0.12 | 1.13 (0.98–1.30) | 0.10 |
| Workers compensation [3] | 8.17 (0.94–71) | 0.06 | 9.04 (1.01–81) | 0.05 |
| Muscle weakness (any) [11] | 0.98 (0.43–2.26) | 0.96 | – | – |
| Disc extrusion [12] | 1.15 (0.48–2.76) | 0.76 | – | – |
| Disc sequestration [12] | 0.18 (0.02–1.55) | 0.12 | 0.30 (0.03–2.73) | 0.29 |
| Predictors of treatment failure defined as persistent disability after nonoperative treatmentǂ (n = 94; 19 participants with persistent disability) | ||||
| Initial disability (ODI) [11] | 1.02 (1.00–1.05) | 0.11 | 1.03 (0.99–1.06) | 0.11 |
| Initial back pain intensity (VAS) [11] | 1.01 (0.85–1.19) | 0.94 | – | – |
| Female sex [12] | 3.64 (1.27–10.4) | 0.02 | 3.16 (1.03–9.69) | 0.05 |
| Duration of symptoms (weeks) [23] | 1.16 (0.99–1.35) | 0.06 | 1.16 (0.98–1.37) | 0.08 |
| Smoking [11] | 1.88 (0.57–6.18) | 0.30 | – | – |
| Medical comorbidities (SACQ) [11] | 1.09 (0.95–1.25) | 0.24 | – | – |
| Prior low back pain [11] | 0.67 (0.23–1.92) | 0.45 | – | – |
| Workers compensation [3] | 6.40 (1.29– 31.6) | 0.02 | 5.99 (1.09–32.7) | 0.04 |
| Disc extrusion [12] | 1.08 (0.37–3.19) | 0.88 | – | – |
| Disc sequestration [12] | NA§ | – | – | |
| Abnormal deep tendon reflex [11] | 0.87 (0.30–2.56) | 0.80 | – | – |
| Predictors of treatment failure defined as participant-reported lack of recovery after nonoperative treatment (n = 94; 44 participants with recovery) | ||||
| Age (per year) [14] | 1.00 (0.98–1.03) | 0.80 | – | – |
| Female sex [21] | 2.20 (0.94–5.16) | 0.07 | 2.44 (1.02–5.84) | 0.05 |
| Duration of symptoms (weeks) [31] | 0.97 (0.85–1.11) | 0.69 | – | – |
| Smoking [21] | 2.46 (0.79–7.66) | 0.12 | 2.84 (0.89–9.12) | 0.08 |
| Workers compensation [3] | 2.33 (0.43–12.7) | 0.33 | – | – |
| Straight leg raise test [31] | 1.78 (0.74–4.25) | 0.20 | – | – |
| Femoral stretch test [31] | 1.58 (0.59–4.27) | 0.37 | – | – |
| Herniation location [32] (foraminal) | 1.73 (0.73–4.07) | 0.21 | – | – |
* All predictor variables with bivariate associations with p ≤ 0.15 were included simultaneously in the multivariable models; statistically significant p values (≤ 0.05) in bold; †pain intensity ≥ 1 on VAS [12]; ǂdisability < 20 on ODI [9]; §odds ratio not calculable as a result of all participants with disc sequestrations reporting no/mild disability at followup; this association was not statistically significant when tested using Fisher’s exact test (data not shown); CI = confidence interval; VAS = visual analog scale; ODI = Oswestry Disability Index; SACQ = Self-Acquired Comorbidity Questionnaire [13].
After controlling for medical comorbidities and disk sequestrations, workers compensation claims were associated with persistent leg pain after 2 years of nonoperative treatment (aOR, 9.04; 95% CI, 1.01–81; p = 0.05). After adjustment for initial disability and duration of symptoms, female sex (aOR, 3.16; 95% CI, 1.03–9.69; p = 0.05) and workers compensation claims (aOR, 5.99; 95% CI, 1.09–32.7; p = 0.04) were associated with persistent disability after 2 years of nonoperative treatment. After adjustment for cigarette smoking, female sex was associated with lack of recovery (aOR, 2.44; 95% CI, 1.02–5.84; p = 0.05).
Discussion
The identification of prognostic factors for nonoperative treatment failure is an important area in clinical research, because patients presenting with such factors might be targeted for closer observation or be directed earlier toward more invasive treatment options such as surgery. However, in situations in which a prognostic factor is identified according to its predictive value in only a single study, there is a possibility for false-positive results occurring as a result of multiple statistical comparisons. Therefore, replication must be performed to validate factors as true predictors of treatment failure. In this validation study of previously identified prognostic factors in nonoperative lumbosacral radiculopathy, we were unable to confirm an association between higher initial leg pain intensity and subsequent lumbar decompression surgery. Several other candidate predictor variables were confirmed as predictors of treatment failure, including prior LBP and positive SLR as predictors of subsequent surgery, workers compensation claims as a predictor of persistent leg pain and persistent disability, and female sex as a predictor of persistent disability and lack of recovery. However, the majority of previously identified prognostic factors did not show large-magnitude associations with nonoperative treatment failure.
There are some limitations to this study. First, our relatively modest sample size may have resulted in insufficient statistical power to detect some important relationships between predictor variables and treatment failure (ie, Type II error). This limitation was especially relevant to our first research question, which examined whether higher leg pain intensity was a predictor of subsequent surgery, a relationship seen in two prior high-quality longitudinal studies [20, 30]. Although we did not find this relationship to be statistically significant, we detected an effect of leg pain intensity on subsequent surgery with a magnitude and directionality that was quite consistent with that seen in these earlier studies. As an example, in our study, a 2-point increase in baseline VAS leg pain intensity corresponded to 1.5 times the odds of subsequent surgery. In the earlier study by Peul et al., a 2-point increase VAS leg pain intensity was associated with 1.7 times the odds of subsequent surgery [20]. Indeed, our results are suggestive that an effect of baseline leg pain intensity on subsequent surgery exists, although we failed to confirm it. Future longitudinal studies may require large sample sizes to replicate effects seen in prior studies.
Second, our study was not able to test all potential predictor variables that had been identified in prior studies, because we did not collect some of these variables. This was the case for several factors, including the important psychological factors of fear avoidance and kinesiophobia, and important comorbidities such as obesity [24]. Third, some predictors of possible importance were not tested in our study because we limited statistical testing only to those factors that had been previously identified in an earlier study. An example of this is the presence of radiculopathy-related motor or sensory deficits, which are considered to be clinically important predictors of subsequent surgery (because progression of neurologic deficits in lumbosacral radiculopathy is itself an indication for surgery) but have not been found to be predictors of treatment failure in prior cohort studies examining this relationship [20, 28, 30]. These prior studies (and ours as well) did not differentiate minor from marked degrees of motor or sensory loss, and this omission might explain the null findings previously seen. As a result of these limitations, future large-scale studies are still needed to identify (and replicate) candidate predictor variables for nonoperative treatment failure in lumbosacral radiculopathy. Such studies may have a greater chance to replicate potential predictors when conducted in samples with similar characteristics to the original cohorts that identified the predictors, especially with respect to important study features such as the duration of radiculopathy-related symptoms and the clinical setting. Because virtually all previous studies have involved specialty care samples, studies within primary care should be a priority for future work.
Although many prior studies have examined multivariate-adjusted predictors of treatment failure in lumbosacral radiculopathy, few have examined side by side these four different definitions representing nonoperative treatment failure (subsequent surgery, persistent leg pain, persistent disability, and lack of recovery). No single factor emerged as a predictor of all or most of these definitions for treatment failure. This is likely attributable in part to important qualitative differences between these definitions. In particular, treatment failure defined as “subsequent surgery” is quite different from treatment failure defined by patient-reported outcome scores such as leg pain intensity, disability, and perceived recovery; the former depends not only on the clinical course of symptom improvement, but also on the complex decision of whether a patient is suitable for decompression surgery. This decision involves not only the impression of the patient regarding their radiculopathy status, but also the impression of the spine surgeon. This decision therefore has the potential to be strongly influenced by factors wholly unrelated to the biology of radiculopathy recovery such as patient perceptions of lumbar decompression and idiosyncratic surgical practice patterns that are specific to geographic regions and clinical settings [7]. Our study confirmed the earlier finding of Vroomen et al. that a history of prior LBP episodes is a strong predictor of subsequent surgery [30]. Vroomen et al. proposed that for patients with a history of LBP, developing new lower extremity radicular pain might be perceived as a disconcerting indication of deterioration and incline the patient toward a more aggressive treatment approach. This is a credible explanation, but an alternative might be that any degree of preexisting pain could lead a patient to pursue options that are perceived as having a greater chance of definitively resolving pain and preventing future episodes. It seems unlikely however that a history of LBP would prompt a surgeon to view a patient as a better surgical candidate given that lumbar decompression surgery for symptomatic lumbosacral radiculopathy resulting from LDH is generally viewed as producing more reliable improvements for leg pain than for LBP [1]. In contrast, the association we detected of a positive SLR test with a higher odds of subsequent surgery seems less likely to be driven by patient-related factors (because a positive SLR test has no intrinsic meaning to patients) and more likely to be driven by surgeon-related factors (because a positive SLR might indicate a more clearly defined or “classic” radiculopathy to the surgeon). Variability in surgeons’ views regarding the clinical use of the SLR might explain the conflicting results seen in different samples regarding the SLR as a predictor of subsequent surgery [20, 28, 30].
Even among patient-reported outcome scores in nonoperative lumbosacral radiculopathy (pain, disability, and recovery), prognostic factors appear to vary depending on the outcome score. Of the five factors we examined that had previously been identified as predictors of more than one patient-reported outcome (sex, duration of symptoms, smoking, medical comorbidities, and workers compensation), only sex and workers compensation claims were independently predictive of more than one patient-reported outcome score in our analysis. Female sex was predictive of persistent disability and lack of perceived recovery after 2 years of nonoperative treatment. The lack of an association between sex and persistent leg pain in our study suggests against sex-specific differences in pain as an explanation for why females have greater disability and less perceived recovery. On the other hand, the strong association of workers compensation claims with persistent leg pain suggests pain as one likely reason why patients with workers compensation claims are much more likely to have persistent disability after 2 years of nonoperative treatment. Taken together, these results are a reminder of the complexities involved when examining disability and perceived recovery as patient-reported outcome scores; these constructs involve much more than pain intensity alone and are strongly influenced by an individual’s expectations and beliefs regarding pain and function as well as other factors.
It is of note that the largest magnitude effects seen in our study (OR > 5.0) were for the association of workers compensation claims with persistent leg pain and disability. Although patient-reported outcomes are often perceived to be poor in workers compensation patients, in the specific context of lumbosacral radiculopathy, prior findings have been mixed: workers compensation claims showed associations with persistent leg pain and disability in the Maine Lumbar Spine Study (MLSS), but no such associations were seen in the Spine Patient Outcomes Research Trial (SPORT) [2–4]. Future studies of the effects of workers compensation claims in lumbosacral radiculopathy are needed; because existing compensation systems vary in different countries, studies conducted in the United States will be most useful for replication of the results seen in the MLSS and SPORT.
In summary, this study failed to confirm the results of earlier work demonstrating an association between higher initial leg pain intensity and subsequent lumbar decompression surgery. However, the magnitude and directionality of the effect seen were consistent with the results of earlier work and do not rule out the possibility that such a relationship exists. Other previously identified prognostic factors were successfully replicated for the association of prior LBP or positive SLR with subsequent surgery, the association of workers compensation claims with persistent leg pain and disability, and the association of female sex with persistent disability and perceived lack of recovery. Clinicians should be aware of these baseline factors that may predict individuals who are more likely to experience nonoperative treatment failure and who may be candidates for closer clinical observation or support.
Acknowledgments
We thank the study participants for their time and effort.
Footnotes
VA Puget Sound provided support for one of the author’s (PS) participation in this research. A portion of this research was conducted while this author was funded by the Rehabilitation Medicine Scientist Training K12 Program (RMSTP) and the National Institutes of Health (K12 HD 01097).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
This research was approved by the New England Baptist Hospital Institutional Review Board. Each author certifies that the New England Baptist Hospital Institutional Review Board approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at New England Baptist Hospital, Boston, MA, USA.
References
- 1.AAOS. Herniated Disk in the Lower Back (Outcomes). 2012. American Academy of Orthopaedic Surgeons. Available at: http://orthoinfo.aaos.org/topic.cfm?topic=a00534. Accessed April 19, 2004.
- 2.Atlas SJ, Chang Y, Kammann E, Keller RB, Deyo RA, Singer DE. Long-term disability and return to work among patients who have a herniated lumbar disc: the effect of disability compensation. J Bone Joint Surg Am. 2000;82:4–15. doi: 10.2106/00004623-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SJ, Chang Y, Keller RB, Singer DE, Wu YA, Deyo RA. The impact of disability compensation on long-term treatment outcomes of patients with sciatica due to a lumbar disc herniation. Spine (Phila Pa 1976). 2006;31:3061–3069. [DOI] [PubMed]
- 4.Atlas SJ, Tosteson TD, Blood EA, Skinner JS, Pransky GS, Weinstein JN. The impact of workers’ compensation on outcomes of surgical and nonoperative therapy for patients with a lumbar disc herniation: SPORT. Spine (Phila Pa 1976). 2010;35:89–97. [DOI] [PMC free article] [PubMed]
- 5.Breitenseher MJ, Eyb RP, Matzner MP, Trattnig S, Kainberger FM, Imhof H. MRI of unfused lumbar segments after spondylodesis. J Comput Assist Tomogr. 1996;20:583–587. doi: 10.1097/00004728-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–97. doi: 10.1016/S0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res. 2006;443:139–146. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 8.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, Hartvigsen J, Leino-Arjas P, Latza U, Reis S, Gil Del Real MT, Kovacs FM, Oberg B, Cedraschi C, Bouter LM, Koes BW, Picavet HS, van Tulder MW, Burton K, Foster NE, Macfarlane GJ, Thomas E, Underwood M, Waddell G, Shekelle P, Volinn E, Von Korff M. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976). 2008;33:95–103. [DOI] [PubMed]
- 9.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940–2952; discussion 2952. [DOI] [PubMed]
- 10.Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine (Phila Pa 1976). 2001;26:461–462. [DOI] [PubMed]
- 11.Haugen AJ, Brox JI, Grovle L, Keller A, Natvig B, Soldal D, Grotle M. Prognostic factors for non-success in patients with sciatica and disc herniation. BMC Musculoskelet Disord. 2012;13:183. doi: 10.1186/1471-2474-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen TS, Albert HB, Sorensen JS, Manniche C, Leboeuf-Yde C. Magnetic resonance imaging findings as predictors of clinical outcome in patients with sciatica receiving active conservative treatment. J Manipulative Physiol Ther. 2007;30:98–108. doi: 10.1016/j.jmpt.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lequin MB, Verbaan D, Jacobs WC, Brand R, Bouma GJ, Vandertop WP, Peul WC. Surgery versus prolonged conservative treatment for sciatica: 5-year results of a randomised controlled trial. BMJ Open. 2013;3. [DOI] [PMC free article] [PubMed]
- 15.Luoto S, Hupli M, Alaranta H, Hurri H. Isokinetic performance capacity of trunk muscles. Part II: Coefficient of variation in isokinetic measurement in maximal effort and in submaximal effort. Scand J Rehabil Med. 1996;28:207–210. [PubMed] [Google Scholar]
- 16.Luoto S, Taimela S, Hurri H, Aalto H, Pyykko I, Alaranta H. Psychomotor speed and postural control in chronic low back pain patients: a controlled follow-up study. Spine. 1996;21:2621–2627. doi: 10.1097/00007632-199611150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Miranda H, Viikari-Juntura E, Martikainen R, Takala EP, Riihimaki H. Individual factors, occupational loading, and physical exercise as predictors of sciatic pain. Spine. 2002;27:1102–1109. doi: 10.1097/00007632-200205150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19:593–607. doi: 10.1016/j.berh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 20.Peul WC, Brand R, Thomeer RT, Koes BW. Improving prediction of ‘inevitable’ surgery during non-surgical treatment of sciatica. Pain. 2008;138:571–576. doi: 10.1016/j.pain.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Peul WC, Brand R, Thomeer RT, Koes BW. Influence of gender and other prognostic factors on outcome of sciatica. Pain. 2008;138:180–191. doi: 10.1016/j.pain.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, Thomeer RT, Koes BW. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 23.Rihn JA, Hilibrand AS, Radcliff K, Kurd M, Lurie J, Blood E, Albert TJ, Weinstein JN. Duration of symptoms resulting from lumbar disc herniation: effect on treatment outcomes: analysis of the Spine Patient Outcomes Research Trial (SPORT) J Bone Joint Surg Am. 2011;93:1906–1914. doi: 10.2106/JBJS.J.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rihn JA, Kurd M, Hilibrand AS, Lurie J, Zhao W, Albert T, Weinstein J. The influence of obesity on the outcome of treatment of lumbar disc herniation: analysis of the Spine Patient Outcomes Research Trial (SPORT) J Bone Joint Surg Am. 2013;95:1–8. doi: 10.2106/JBJS.K.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suri P, Hunter DJ, Jouve C, Hartigan C, Limke J, Pena E, Li L, Luz J, Rainville J. Nonsurgical treatment of lumbar disk herniation: are outcomes different in older adults? J Am Geriatr Soc. 2011;59:423–429. doi: 10.1111/j.1532-5415.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suri P, Rainville J, Katz JN, Jouve C, Hartigan C, Limke J, Pena E, Li L, Swaim B, Hunter DJ. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine (Phila Pa 1976). 2011;36:63–73. [DOI] [PMC free article] [PubMed]
- 27.Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol. 2004;57:174–179. doi: 10.1016/S0895-4356(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 28.Valls I, Saraux A, Goupille P, Khoreichi A, Baron D, Le Goff P. Factors predicting radical treatment after in-hospital conservative management of disk-related sciatica. Joint Bone Spine. 2001;68:50–58. doi: 10.1016/S1297-319X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 29.Verwoerd AJ, Luijsterburg PA, Lin CW, Jacobs WC, Koes BW, Verhagen AP. Systematic review of prognostic factors predicting outcome in non-surgically treated patients with sciatica. Eur J Pain. 2013;17:1126–1137. doi: 10.1002/j.1532-2149.2013.00301.x. [DOI] [PubMed] [Google Scholar]
- 30.Vroomen PC, de Krom MC, Knottnerus JA. When does the patient with a disc herniation undergo lumbosacral discectomy? J Neurol Neurosurg Psychiatry. 2000;68:75–79. doi: 10.1136/jnnp.68.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119–123. [PMC free article] [PubMed] [Google Scholar]
- 32.Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of lumbosacral nerve root compression. J Neurol Neurosurg Psychiatry. 2002;72:630–634. doi: 10.1136/jnnp.72.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vroomen PC, Wilmink JT, de KM. Prognostic value of MRI findings in sciatica. Neuroradiology. 2002;44:59–63. [DOI] [PubMed]
- 34.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine (Phila Pa 1976). 1983;8:131–140. [PubMed]
- 35.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]