Abstract
The optimal clinical management of aggressive/advanced lung neuroendocrine tumors (NETs) is still debated, due to their rarity and the lack of prospective randomized studies. Results derive from retrospective mono-Institutional series, and few dedicated prospective trials, recently designed, are still ongoing. In low-grade tumors [bronchial carcinoids (BCs)] surgery, whenever feasible, remains the mainstay of treatment, and chemo/radiotherapy (RT) should be reserved to progressive diseases (PD). In case of resected N1-N2 BCs, a “watch and see” policy associated with a close clinical/radiological follow-up is recommended. Somatostatin analogs (SSA) seem to be effective in controlling BCs associated endocrine syndromes, while SSA antiproliferative effect has also been reported in the past. Targeted therapy with new drugs (Everolimus) seems to be very promising, but further trials are needed. Surgery alone is not sufficient to treat high-grade NETs: adjuvant CT is required also in early stages. Platinum-Etoposide regimen demonstrated to be the most effective; irinotecan and other biological drugs are considered very promising. In conclusion, the management of advanced lung NETs should be individualized by multidisciplinary teams which include Medical and Radiation Oncologists, Surgeons, Pathologists, Pulmonologists, Endocrinologists, Interventional Radiologists, and the prognosis is mainly dependent on tumor grade and its anatomical extent.
Keywords: Lung, neuroendocrine tumors (NETs), surgery, chemotherapy, biological therapy, radiotherapy (RT), survival, metastases, recurrence
Introduction: the nature of the problem
Neuroendocrine tumors (NETs) of the Lung represent a distinct subgroup of primary lung neoplasms, characterized by particular morphologic, ultrastructural, immunohistochemical and molecular peculiarities and different biological behavior.
The 2004 World Health Organization (WHO) classification of tumors (1), combining architectural patterns (cell size, organoid palisading rosettes, distinct nuclear/cytoplasm ratio) with other parameters (different mitotic index, presence of necrosis and proliferation index), classifies NETs into four different groups, ranging from the low-grade typical carcinoid (TC) to high-grade, poorly differentiated large-cell neuroendocrine carcinoma (LCNC) and small-cell lung cancer (SCLC). Amid them, an intermediate-grade tumor [atypical carcinoid (AC)] is also recognized.
Recently, as reported by Modlin et al. and Yao et al. (2,3), NETs incidence has risen up to 1.57/1,000,000/year, likely due to an improvement in histological diagnostic tools, as well as to the dramatic diffusion of lung cancer screening programs worldwide. The increasing prevalence was about 6% per year, regardless of confounding demographic factors, such as age, gender, race, and stage distribution. SCLC is the most frequent lung NET, representing approximately 15% of invasive lung cancers (4), while LCNC accounts for about 3% of all surgically resected primary lung cancers (5). Carcinoid tumors are about 1% to 2% of invasive lung neoplasms, overall accounting for 0.1% to 0.2% of all lung cancers, with approximately 6,000 new cases per annum in USA (6), and represent 20% to 25% of all carcinoid tumors in the human body.
Smoking is strongly correlated with SCLC and LCNC; recent reports (7,8) confirm the role of smoking also in AC development.
In 2012, the European Society of Thoracic Surgeons (ESTS) has launched the NETs Working Group (NETs-WG), gathering a group of experts worldwide with the aim to accumulate knowledge on such rare neoplasms, disseminating it into the scientific community. After designing a specific dedicated database, a retrospective data collection started and up to June 2014, 2051 patients have been included from European and North American high-volume Institutions. Several scientific publications followed this project, along with two Satellite Sessions during the 2013 and 2014 ESTS Annual Meetings.
The present paper reports the latter, dedicated to the management of advanced lung bronchial carcinoids (BCs) and LCNCs. Due to the rarity of these tumors, and the lack of prospective randomized clinical trials, their clinical management is based on the experience of single Centres, and still remains anedoctical. The Surgeon’s and Oncologist’s perspectives are considered, with the belief that patients must be managed in a multidisciplinary approach.
Advanced neuroendocrine lung tumor: the surgeons’ perspective
Bronchial carcinoids (BCs)
BCs must be regarded as malignant tumors, since they can present with lymph-nodal involvement at the time of intervention, can locally recur or give distant metastases. As for other non-small cell lung cancers (NSCLCs), surgery represents the mainstay of treatment; anatomic resections should be performed, along with systematic hilar and mediastinal lymphadenectomy.
Surgery achieves 5- and 10-year survival rates higher than 95% for TC and 70% and 50% for AC, respectively, which is worse in case of lymph-nodal metastases.
Whereas the typical BC clinical presentation is characterized by two distinct forms (central and peripheral tumor), large metastatic N1-N2 nodes (Figure 1), as well as multiple diffuse bilateral lesions are sometimes observed (9-11).
Figure 1.
Advanced unresectable atypical carcinoid (CT scan).
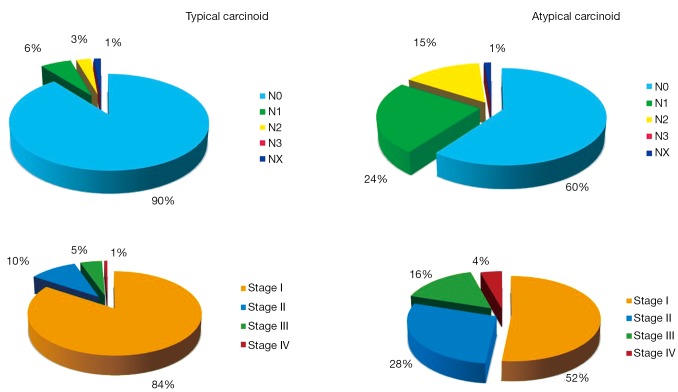
The lymph-nodal involvement percentage may vary according to BC’s histology: in a large multi-Institutional experience (12), node metastases were found in 9% TCs and in 36% ACs, respectively. Data arising from the ESTS NETs-WG (on 1,440 cases) are shown in Figure 2.
Figure 2.
Lymph-nodal involvement and stage distribution in bronchial carcinoids. Data arising from the ESTS NETs-WG on 1,440 cases (TCs: 1212; ACs: 228). ESTS, the European Society of Thoracic Surgeons; NETs-WG, Neuroendocrine Tumors Working Group; TCs, typical carcinoids; ACs, atypical carcinoids.
In case of single-station N2 disease, an upfront surgical approach is regarded feasible; when multiple lymph-nodal involvement is observed, a histological proven diagnosis [usually through mediastinoscopy or endobronchial ultrasound (EBUS)] is therefore mandatory. An induction chemotherapic treatment generally increases the chance of radical resection.
It is questionable the role of adjuvant treatment in low-grade N1-N2 tumors: a recent retrospective series reports that postoperative CT is associated with worse overall survival (OS) (13). According to the ESTS NETs-WG database, chemotherapy (CT) and/or biological therapy [long-acting somatostatin analogs (SSA), such as Octreotide or Lanreotide] and/or radiotherapy (RT) were proposed to 2% TCs, only. Adjuvant treatment (commonly Platin-Etoposide) CT or RT was administered in 20% N+ ACs.
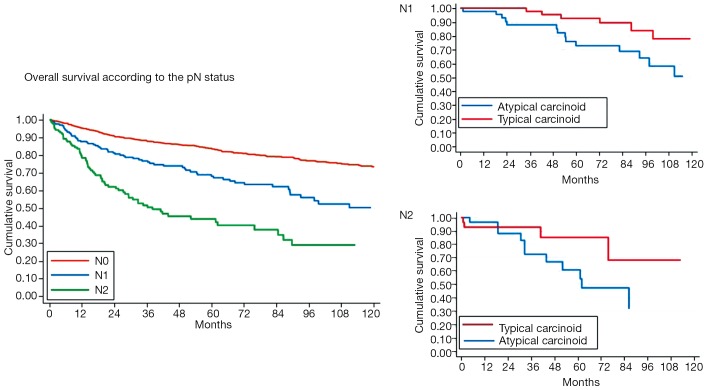
In accordance with other previous reports (8,12-17), we found that, at the multivariate analysis, survival was significantly influenced by the presence of lymph-nodal involvement (Figure 3) [hazard ratio (HR): 1.33; 95% CI: 1.17-1.53; P<0.001 for TCs; HR: 1.29; 95% CI: 1.04-1.60; P=0.019 for ACs], and tumor stage (Figure 4) (HR: 3.23; 95% CI: 1.95-5.36; P<0.001 for TCs; HR: 1.38; 95% CI: 1.05-1.82; P=0.020 for ACs). Local recurrences occurred in 2% TCs and 7% ACs; distant metastases were observed in 4% TCs and 26% ACs, respectively; both were more frequent in case of lymph-nodal involvement and in advanced Stages. A long lasting and accurate clinical/radiological follow-up is recommended in low-grade lung NETs, since metastases may develop several years after surgery. Resection of the recurrence, if feasible, may be a therapeutic option; systemic CT and/or RT are indicated in case of unresectable lesions.
Figure 3.
Bronchial carcinoids overall survival (OS) according to the lymph-nodal involvement.
Figure 4.

Bronchial carcinoids overall survival (OS) according to tumor stages.
Neuroendocrine carcinomas
The typical radiological LCNC’s presentation is a spiculated peripheral pulmonary nodule; advanced lesions with hilar involvement are sometimes observed (Figure 5).
Figure 5.

Advanced right sided LCNC with lymph-nodal involvement (CT scan). LCNC, large-cell neuroendocrine carcinoma.
Five-year survival rate in resected LCNCs has been reported ranging from 20% to 55%, according to the different single-Institution series (18-21), very similar to SCLC. Tumor recurrences/distant metastases develop early, even after a complete resection as well as in early stage (22). Therefore, surgery alone is not sufficient even in stage I LCNC, and adjuvant treatments are required (23,24).
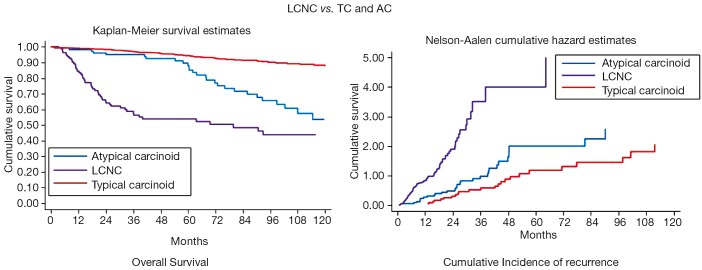
The N status and Stage distribution according to the ESTS NETs-WG database on 316 cases are shown in Figure 6. LCNC OS and Cumulative Incidence of Recurrence curves are reported in Figure 7. Local recurrences were observed in 19% of patients, while distant metastases developed in 37% of cases. At the multivariate analysis, survival was significantly influenced by tumor Stage (HR: 1.31; 95% CI: 1.10-1.55; P=0.002), only.
Figure 6.
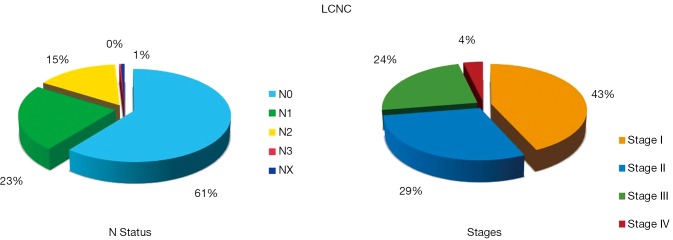
Lymph-nodal involvement distribution in LCNCs. Data arising from the ESTS NETs-WG on 316 cases. LCNC, large-cell neuroendocrine carcinoma; ESTS, the European Society of Thoracic Surgeons; NETs-WG, neuroendocrine tumors Working Group.
Figure 7.
LCNC: overall survival (OS) and cumulative incidence of recurrence curves compared to typical and atypical carcinoid. LCNC, large-cell neuroendocrine carcinoma; TC, typical carcinoids; AC, atypical carcinoids.
The importance of adjuvant CT has been recently highlighted. Veronesi (21) observed that Stage I LCNC treated with postoperative CT presented with a better survival than those who did not (P=0.07); Iyoda et al. (25) reported that Platinum-based adjuvant CT was effective to prevent tumor recurrences. Rossi et al. (26) observed how Platinum-Etoposide CT (typical SCLC’s regimen) was more effective than traditional NSCLC’s ones, after LCNC surgical resection.
The combination of Platinum-based induction CT, followed by surgery and postoperative CT/RT was recognized having a survival advantage in a large mono-Institutional series by series by Sarkaria et al. (27). The Authors suggested that this approach should be offered to all LCNC patients.
According to the ESTS NETs-WG database, induction CT/RT was offered to 17% of patients and adjuvant treatment to 42%. We also found a better survival in patients submitted to postoperative CT, even if data did not reach a statistical significance.
Advanced neuroendocrine lung tumor: the oncologist’s perspective
Clinical management of advanced/aggressive lung NETs requires a multidisciplinary approach; several figures should be involved: the Surgeon, the Medical and Radiation Oncologist, the Pulmonologist, the Pathologist, the Endocrinologist, the Interventional Radiologist and, sometimes, the Gastroenterologist. Every decision and strategy should be decided in the course of specific tumor boards, which allow the identification of different treatment possibilities, including, whenever feasible, surgery.
The main aims of the lung NETs’ medical management are the control of tumor’s growth and of endocrinopathies and endocrine secretory activity in general, whenever paraneoplastic syndromes are present. The absence of a curative option in case of metastatic disease, as well as the prognostic heterogeneity of lung NETs, make the quality of life a predominant objective.
Lung NETs rarity and the consequent difficulty to enroll patients with advanced diseases, may explain the present lack of randomized clinical trial, which are contrariwise, urgently needed.
Hormonal secretion’s control
Lung NETs (especially BCs) have associated endocrine syndromes in a lower percentage compared to gastro-entero-pancreatic ones (10-12% vs. 30%) (28).
SSA constitutes the gold standard for a symptomatic control. Flushing and diarrhea improved in more than 50% of cases in specific series; SSA also showed an antiproliferative effect when used in metastatic ACs with carcinoid syndrome (29).
When Cushing’s syndrome is present, it can be treated with commonly available drugs, such as Ketoconazole, Metyrapone, Etomidate or Mifepristone (30). In the absence of hormonal control, many patients may benefit of SSA or Interferon, associated with loco-regional therapies (liver palliative surgery, radiofrequency metastasis ablation or trans arterial chemoembolization).
Low-grade NETs
There are no consolidated prospective clinical trials that guide the treatment of advanced BCs, and the only available results arise from small retrospective mono-Institutional series as well as from subgroup analysis of few multicentric studies which also include gastro-entero-pancreatic NETs. The first multicentric randomized prospective trial completely dedicated to thoracic (Pulmonary and Thymic) NETs (Luna trial) started at the end of 2013 and is still ongoing.
In asymptomatic patients with advanced BCs and a low proliferative index, a “watch and see” policy might be considered, if clearly explained to the patient, on the basis of a strict and regular clinical/radiological follow-up.
The SSA antiproliferative effect also in non-secreting tumors has been reported in the past, and supported by small clinical series (29,31-33). SSA can produce tumor stabilization in about 30-70% of patients with low-grade NETs, as reported by prospective and retrospective studies that also included lung NETs (34,35). The most reasonable first-line treatment by SSA in terms of progression-free survival (PFS) has been highlighted by the results of two International studies (PROMID, using Octreotide, and CLARINET trial, using Lanreotide) (36).
Systemic CT should be used in case of advanced unresectable BCs. In general, its results (in terms of mono or polychemotherapy) are disappointing; data arise from small retrospective mono-Institutional series, with several limitations due to different inclusion criteria and some other biases.
Cisplatin-Etoposide scheme is the classic regimen reserved to BCs who rapidly became progressive or when the associated endocrine syndromes must be clinically controlled. The overall response rate (ORR) unfortunately demonstrated limited effect of these drugs.
Temozolomide (alone or in association) showed promising clinical benefits, even if data come from small retrospective series (37,38). Temozolomide has been also proposed in case of unresectable brain metastases (39).
Mammalian target of rapamycin (mTOR) has been identified as a kinase activated in the PI3K pathway of lung NETs (40). Recently, mutations in PI3K have been also described in BCs.
The role of mTOR Inhibitors has rapidly become of clinical interest in Thoracic Oncology. Everolimus (alone or in combination with SSA) was effective, according to the lung NETs subgroup analysis of the large multicentric RADIANT II study (41).
The PFS in the Everolimus arm was >8 months higher than that in the placebo one. However, problems in correct patients’ subdivision between the two arms were emphasized and did not allow to achieve correct statistical analyses, even if a 2.4-fold PFS improvement has been observed (42).
The RADIANT IV, a large prospective phase III multicentric study, which explore the efficacy of Everolimus vs. placebo in progressive lung NETs, has recently completed patients recruitment and results are expected by the end of the year. Another study (the LUNA trial), entirely designed for thoracic NETs, is actually ongoing, and will investigate the efficacy of Everolimus as single agent vs. its association with Pasireotide (a pan-Somatostatin receptor analogue) or Pasireotide alone in progressive diseases (PD). The results of these studies will confirm the role of Everolimus for patients with PD BCs.
Loco-regional therapies (radiofrequency or cryoablation) are also available, especially in case of liver, bone or lung metastases.
Finally, peptide receptor radiotargeted therapy (PRRT) is now used to treat unresectable distant metastases, using 90Yttium-DOTA Octreotide or 177Lutetium-DOTA Octreotide. Grade 3-4 toxicity (especially renal) is reported in about 30% of cases, and may limit the use of these techniques (43).
High-grade NETs
Surgery alone is not sufficient to treat LCNC, even in Stage I. Local recurrences and/or distant metastases are, in fact, extremely frequent and very early, also after complete tumor resection. Since a preoperative cyto-histological diagnosis is often impossible, induction CT is performed in very few cases.
Many recent reports revealed that Platinum and SCLC-based CT regimens are effective in LCNC, especially in advanced disease; therefore, Platinum-Etoposide actually represent the gold standard in the treatment of such tumors (26,44,45).
New chemotherapic agents have been recently evaluated: amid them, irinotecan (associated with Cis or Nedaplatin) seems to be very promising, especially in Stage I patients and in adjuvant setting (46).
In conclusion, the management of advanced lung NETs should be individualized by multidisciplinary teams which include Medical and Radiation Oncologists, Surgeons, Pathologists, Pulmonologists, Endocrinologists and Interventional Radiologists. The prognosis is mainly dependent on tumor grade and its anatomical extent. Surgery, whenever feasible, remains the mainstay of treatment even in case of advanced disease; other therapeutic options are mainly addressed to palliate symptoms, since the results of systemic chemotherapy are still limited. New antineoplastic biological drugs seem encouraging, but their real effectiveness must be proven in ongoing clinical trials.
Acknowledgements
The European Society of Thoracic Surgeons (ESTS) Neuroendocrine Tumors of the Lung Working-Group Steering Committee is also composed by Hisao Asamura (Japan), Alessandro Brunelli (UK), Mariano Garcia-Yuste (Spain), Eric Lim (UK), Konstantinos Papagiannopoulos (UK), Interpal Sarkaria (USA) and Pascal Thomas (France).
Disclosure: The authors declare no conflict of interest.
References
- 1.Travis WD, Brambilla E, Müller-Hermelink HK, et al. eds. Pathology & Genetics, tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004. [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results Program: Lung Cancer histologically confirmed SEER 18 registries research data plus Hurricane Katrina impacted Louisiana cases 1973-2010, November 2012. Available online: www.seer,cancer.gov
- 5.Sakurai H, Asamura H.Large-cell neuroendocrine carcinoma of the lung: surgical management. Thorac Surg Clin 2014;24:305-11. [DOI] [PubMed] [Google Scholar]
- 6.Oberg K, Hellman P, Kwekkeboom D, et al. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21Suppl 5:v220-2. [DOI] [PubMed] [Google Scholar]
- 7.Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [DOI] [PubMed] [Google Scholar]
- 8.Filosso PL, Oliaro A, Ruffini E, et al. Outcome and prognostic factors in bronchial carcinoids: a single-center experience. J Thorac Oncol 2013;8:1282-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Choi YD, Kim BJ, et al. Multiple peripheral typical carcinoid tumors of the lung: associated with sclerosing hemangiomas. Diagn Pathol 2013;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazici U, Gulhan E, Agackiran Y, et al. Synchronous bilateral multiple typical pulmonary carcinoid tumors. Ann Thorac Surg 2010;89:1278-80. [DOI] [PubMed] [Google Scholar]
- 11.Beshay M, Roth T, Stein R, et al. Synchronous bilateral typical pulmonary carcinoid tumors. Eur J Cardiothorac Surg 2003;23:251-3. [DOI] [PubMed] [Google Scholar]
- 12.García-Yuste M, Matilla JM, Cueto A, et al. Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg 2007;31:192-7. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum DP, Speicher PJ, Gulack BC, et al. Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors. Ann Thorac Surg 2015;99:428-34. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CF, Jr, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest 2001;119:1143-50. [DOI] [PubMed] [Google Scholar]
- 15.Soga J, Yakuwa Y.Bronchopulmonary carcinoids: An analysis of 1,875 reported cases with special reference to a comparison between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg 1999;5:211-9. [PubMed] [Google Scholar]
- 16.Filosso PL, Ruffini E, Di Gangi S, et al. Prognostic factors in neuroendocrine tumours of the lung: a single-centre experience. Eur J Cardiothorac Surg 2014;45:521-6; discussion 526. [DOI] [PubMed] [Google Scholar]
- 17.Schrevens L, Vansteenkiste J, Deneffe G, et al. Clinical-radiological presentation and outcome of surgically treated pulmonary carcinoid tumours: a long-term single institution experience. Lung Cancer 2004;43:39-45. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorn F, Dienemann H, Muley T, et al. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg 2015;99:983-9. [DOI] [PubMed] [Google Scholar]
- 19.Filosso PL, Rena O, Guerrera F, et al. Clinical management of atypical carcinoid and large-cell neuroendocrine carcinoma: a multicentre study on behalf of the European Society of Thoracic Surgeons (ESTS) Neuroendocrine Tumours of the Lung Working Group. Eur J Cardiothorac Surg 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Asamura H.Large-cell neuroendocrine carcinoma of the lung: surgical management. Thorac Surg Clin 2014;24:305-11. [DOI] [PubMed] [Google Scholar]
- 21.Veronesi G, Morandi U, Alloisio M, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer 2006;53:111-5. [DOI] [PubMed] [Google Scholar]
- 22.Iyoda A, Hiroshima K, Toyozaki T, et al. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001;91:1992-2000. [DOI] [PubMed] [Google Scholar]
- 23.Iyoda A, Hiroshima K, Nakatani Y, et al. Pulmonary large cell neuroendocrine carcinoma: its place in the spectrum of pulmonary carcinoma. Ann Thorac Surg 2007;84:702-7. [DOI] [PubMed] [Google Scholar]
- 24.Kozuki T, Fujimoto N, Ueoka H, et al. Complexity in the treatment of pulmonary large cell neuroendocrine carcinoma. J Cancer Res Clin Oncol 2005;131:147-51. [DOI] [PubMed] [Google Scholar]
- 25.Iyoda A, Hiroshima K, Moriya Y, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg 2009;138:446-53. [DOI] [PubMed] [Google Scholar]
- 26.Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [DOI] [PubMed] [Google Scholar]
- 27.Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg 2011;92:1180-6; discussion 1186-7. [DOI] [PubMed] [Google Scholar]
- 28.Ferolla P.Medical treatment of advanced thoracic neuroendocrine tumors. Thorac Surg Clin 2014;24:351-5. [DOI] [PubMed] [Google Scholar]
- 29.Filosso PL, Ruffini E, Oliaro A, et al. Long-term survival of atypical bronchial carcinoids with liver metastases, treated with octreotide. Eur J Cardiothorac Surg 2002;21:913-7. [DOI] [PubMed] [Google Scholar]
- 30.Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Ferolla P, Faggiano A, Grimaldi F, et al. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest 2012;35:326-31. [DOI] [PubMed] [Google Scholar]
- 32.Shen C, Shih YC, Xu Y, et al. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: a population-based analysis. Cancer 2014;120:2039-49. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri G, Lastoria S, Montella L, et al. Role of somatostatin analogue-based therapy in unresponsive malignant thymomas. Ann Med 1999;31Suppl 2:80-5. [PubMed] [Google Scholar]
- 34.Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001;37:1014-9. [DOI] [PubMed] [Google Scholar]
- 35.Faiss S, Pape UF, Böhmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003;21:2689-96. [DOI] [PubMed] [Google Scholar]
- 36.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [DOI] [PubMed] [Google Scholar]
- 37.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986-91. [DOI] [PubMed] [Google Scholar]
- 38.Crona J, Fanola I, Lindholm DP, et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013;98:151-5. [DOI] [PubMed] [Google Scholar]
- 39.Kulke MH, Scherübl H. Accomplishments in 2008 in the management of gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res 2009;3:S62-6. [PMC free article] [PubMed] [Google Scholar]
- 40.Hay N.The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005;8:179-83. [DOI] [PubMed] [Google Scholar]
- 41.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12. [DOI] [PubMed] [Google Scholar]
- 42.Fazio N, Granberg D, Grossman A, et al. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest 2013;143:955-62. [DOI] [PubMed] [Google Scholar]
- 43.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [DOI] [PubMed] [Google Scholar]
- 44.Iyoda A, Makino T, Koezuka S, et al. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014;62:351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. [DOI] [PubMed] [Google Scholar]
- 46.Kenmotsu Y, Oshita F, Sugiura M, et al. Nedaplatin and irinotecan in patients with large-cell neuroendocrine carcinoma of the lung. Anticancer Res 2012;32:1453-6. [PubMed] [Google Scholar]