Abstract
Background and Objectives
Acinetobacter causes a wide variety of illness in debilitated and hospitalized patients. Carbapenem resistance in Acinetobacter is an emerging problem and is a cause of concern as many nosocomial infections with Acinetobacter are resistant to most other antibiotics. The present study was aimed to study metallo-β-lactamase (MBL) production in Acinetobacter species.
Materials and Methods
During one year prospective study, all isolates of Acinetobacter obtained from various clinical samples like respiratory, pus, blood and others were included. Antimicrobial susceptibility testing was done by standard Kirby Bauer disk diffusion method. Metallo β-lactamase (MBL) detection was done by imipenem-EDTA combined disk method.
Results
Among 1017 isolates, 964 were A. baumannii, 48 were A. lwoffii and 5 were A. hemolyticus. Out of these, majority of the isolates were obtained from respiratory samples, followed by pus. A .baumannii showed high level of resistance to cephalosporins, cotrimoxazole and piperacillin. A .lwoffii and A. hemolyticus showed lesser resistance to all antibiotics. Imipenem resistance was observed in 389 (40.3 %) isolates of A.baumannii and MBL activity was seen in 80.3% of isolates. MBL positive isolates of A. baumannii showed higher resistance as compared to MBL negative isolates.
Conclusion
This study demonstrated that multidrug resistant strains of Acinetobacter are common in tertiary care hospitals. Unwarranted and unrestricted usage of antibiotics is associated with emergence of resistance in nosocomial pathogens. Regular monitoring and documentation of carbapenem resistant is crucial in developing strategies to control infection due to these bacteria.
Keywords: Carbapenem resistance, metallo ß-lactamase, A.baumannii
INTRODUCTION
Acinetobacter causes a wide variety of illness in debilitated and hospitalized patients. These bacteria survive for long period in hospital environment and thereby the opportunities for cross infection between patients are enhanced (1). Acinetobacter species play a significant role in the colonization and infection of patients admitted in hospitals. It has been implicated in variety of nosocomial infections. Acinetobacter baumannii is intrinsically less susceptible to antibiotics than Enterobacteriaceae; moreover, it has propensity to acquire resistance. Because of frequent resistance to aminoglycosides, flouroquinolones, ureidopenicillins and third –generation cephalosporins, carbapenems are important agents for managing Acinetobacter infections (2).
The resistance of Acinetobacter baumannii to carbapenem is now a major worldwide issue (3-6). The carbapenems are β-lactam antimicrobial agent with an exceptionally broad spectrum of activity. Carbapenem resistance in Acinetobacter is an emerging problem and is a cause of concern as many nosocomial Acinetobacter are resistant to most other antibiotics. Carbapenem resistance in Acinetobacter is attributed to various causes such as reduced expression of outer membrane proteins and carbapenamases β-lactamases (7). Some carbapenem resistant isolates produce either metallo beta-lactamases (Ambler class B β-lactamases ) or more commonly OXA type enzymes (Ambler class D β-lactamases or oxacillinases) having weak activity against carbapenems (8). Metallo-beta-lactamase (MBL) producing Acinetobacter baumannii has become a growing therapeutic concern worldwide. The rapid detection of MBL positive isolates is necessary to control infection and to prevent their dissemination. The aim of this study was to determine the prevalence of MBL among carbapenem resistant strains of Acinetobacter species in our hospital
MATERIAL AND METHODS
The one year prospective study was conducted in the department of microbiology. Various clinical samples like respiratory, pus, blood, urine and others were processed according to the standard procedures. The isolates were identified as non fermenting Gram negative bacilli (NFGNB) on the basis of colony characteristics, Gram’s staining, motility test, oxidase and alkaline reaction on Triple Sugar Iron agar. All oxidase negative and nonmotile NFGNB isolates were further identified by various tests like OF-glucose, arginine dihydrolase, growth at 44°C, citrate utilization and haemolysis on blood agar (9). The antimicrobial susceptibility testing was done by Kirby Bauer disc diffusion method using gentamicin (10 μg), amikacin (30 μg), netilmicin (30 μg), cotrimoxazole (1.25/23.75 μg), ciprofloxacin (5 μg), ceftazidime (30 μg), cefepime (30μg), piperacillin (100 μg), piperacillin/tazobactam (100/10 μg), and imipenem (10 μg) as per CLSI Guidelines (10). Imipenem resistant isolates were selected for the detection of MBL production by Imipenem-EDTA combined disc test (6).
Results
Among 1017 isolates, 515(50.6%) isolates were from respiratory samples, 222 (21.8%) from pus, 159 (15.6%) from blood, 88 (8.6%) from other clinical samples and 33 (3.2%) from urine samples (Fig. 1).
Fig. 1.
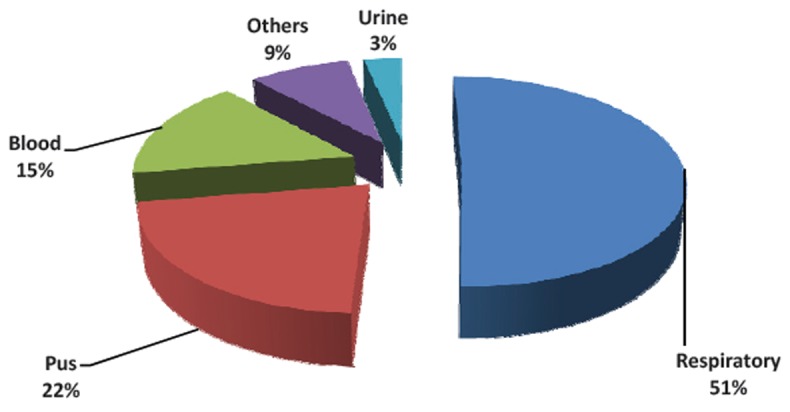
Distribution of Acinetobacter spp. in various samples.
Out of total isolates, 964 (94.7%) were identified as A. baumannii, 48(4.7%) A. lwoffii and 5 (0.4%) A. hemolyticus. A. baumannii showed high level of resistance to cephalosporins, cotrimoxazole and piperacillin. Majority of A. baumannii (71%) were sensitive to piperacillin-tazobactam. Among aminoglycosides, netilmicin showed lesser resistance (46.9%) than amikacin (64.9%) and gentamicin (88.1%). A. lwoffii and A. hemolyticus showed lesser resistance to all antibiotics as compared to A. baumannii. All isolates of A. lwoffii and A. hemolyticus were sensitive to imipenem whereas 389 (40.3 %) isolates of A. baumannii were found to be imipenem resistant (Fig. 2).
Fig. 2.
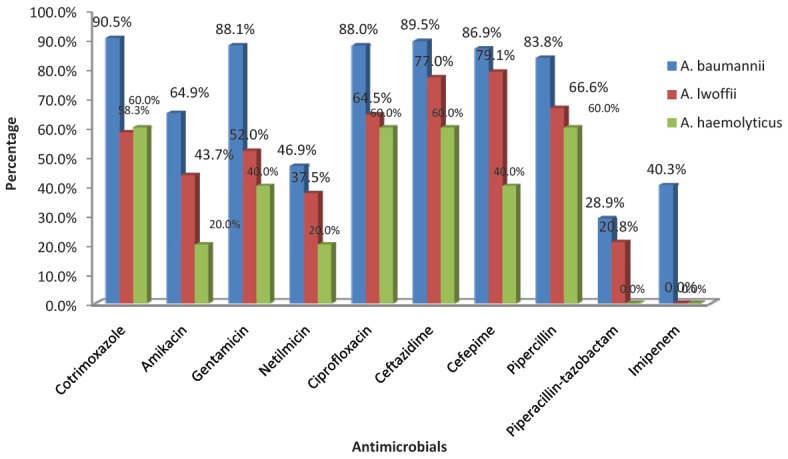
Antimicrobial resistance profile of Acinetobacter spp. (n = 1017).
MBL activity was seen in 313 (80.3%) of imipenem resistant A. baumannii isolates. MBL positive isolates of A. baumannii were showing significantly higher resistance to all antimicrobials tested except cotrimoxazole as compared to MBL negative isolates and it was statistically significant (P < 0.05) (Table 1).
Table 1.
Comparison of Antibiotic resistance profile of MBL+ve and MBL−ve A. baumannii
| Antibiotic | MBL +ve A. baumannii (313) | MBL −ve A. baumannii (76) |
|---|---|---|
| Amikacin | 273 (87.22%) | 48 (63.16%) |
| Gentamicin | 295 (94.25%) | 63 (82.89%) |
| Netilmicin | 209 (66.77%) | 41 (53.95%) |
| Ciprofloxacin | 307 (98.08%) | 61 (80.26%) |
| Ceftazidime | 309 (98.72%) | 61 (80.26%) |
| Cefepime | 304 (97.12%) | 60 (78.95%) |
| Piperacillin | 285 (91.05%) | 62 (81.58%) |
| Piperacillin-Tazobactam | 179 (57.19%) | 32 (42.10%) |
| Cotrimoxazole | 305 (97.44%) | 75 (97.40%) |
DISCUSSION
In our study, the most common Acinetobacter species identified from various samples was A. baumannii followed by A. lwoffii and A. haemolyticus. Similar results had been reported in literature (11, 12). Most of the nosocomial infections are caused by A. baumannii, whereas other species are considered less virulent. A. baumannii isolates were resistant to most of the antibiotics used. Resistance to cephalosporins was observed in > 80% isolates and among aminoglycosides, resistance to amikacin was seen in about 65% and resistance to gentamicin was seen in about 89% of isolates similar to the reports in literature (13-15). Netilmicin showed higher sensitivity as compared to gentamicin and amikacin in Acinetobacter spp.
All Acinetobacter isolates were sensitive to imipenem in a study by Malini et al.(13). Resistance to imipenem was observed in 40.3% of A .baumannii isolates in our study whereas 14.2%, (16) 23% (17) and 57.4% (18) of Acinetobacter spp were resistant to imipenem as reported in literature. Among imipenem resistant isolates, 80.3% of A. baumannii showed MBL production whereas higher (96.6%) MBL production (17) and lower MBL production 7.5%, 14.8%, 49%, 56%, 74% in Acinetobacter spp.(5, 19, 20, 4, 18) as compared to our study was reported by various authors. In our study, statistically significant difference was found in the resistance profile of MBL positive and negative isolates for cephalosporins, aminogly-cosides, quinolones, piperacillin, piperacillin-tazobactam which is consistent with the other studies.(21, 22). This study demonstrated that multidrug resistant Acinetobacters are common in hospitals. Unwarranted and unrestricted usage of antibiotics is associated with emergence of resistance in common nosocomial pathogens like Acinetobacter species. Use of third generation cephalosporins has been shown to increase carbapenem resistance in Acinetobacter strains (2). Production of MBL has tremendous therapeutic consequences since these organisms also carry multidrug resistance genes and the only viable option remains the potentially toxic polymyxin B and colistin (23).
In conclusion, the present study revealed high proportion of MBL producing Acinetobacter isolates. Respiratory, pus and blood samples collected from patients were found to be the main sources of MBL producing isolates. Early detection and infection control practices are the best defenses against these organisms; therefore, systematic surveillance to detect MBL producers is necessary. It is important to follow antibiotic restriction policies to avoid excessive use of carbapenem and other broad spectrum antibiotics.
References
- 1.Bergogne–Berezin E, Towner KJ. Acinetobacter species as nosocomial pathogens: Microbiological, clinical and epidemiological features. Clin Micro Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manikal VM, Landman D, Saurina G, Oydna E, Lal H, Quale J. Endemic carbapenem resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, inter institutional spread and relation to antibiotic usage. Clin Infect Dis. 2000;31:101–106. doi: 10.1086/313902. [DOI] [PubMed] [Google Scholar]
- 3.Maltezou HC. Metallo-β-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? International Journal of Antimicrobial Agents. 2009;33:405 e1–405 e7. doi: 10.1016/j.ijantimicag.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Peymani A, Nahaei MR, Farajnia S, Hasani A, Mirsalehian A, Sohrabi N, et al. High prevalence of metallo-beta-lactamase-producing Acinetobacter baumannii in a teaching hospital in Tabriz, Iran. Jpn J Infect Dis. 2011;64:69–71. [PubMed] [Google Scholar]
- 5.Gupta V, Datta P, Chander J, et al. Prevalence of metallo-β lactamase (MBL) producing Pseudomonas spp. and Acinetobacter spp. in a tertiary care hospital in India. J Infect. 2006;52:311–314. doi: 10.1016/j.jinf.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quale J, Bratu S, Landman D, Heddurshetti R. Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin Infect Dis. 2003;37:214–220. doi: 10.1086/375821. [DOI] [PubMed] [Google Scholar]
- 8.Urban C, Segal-Maurer S, Rahal JJ. Consideration in control and treatment of nosocomial infections due to multidrug resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 9.Winn W Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, et al., editors. Koneman’s Color Atlas and textbook of Diagnostic Microbiology. 6. USA: Lippincott Williams and Wilkins Company; 2006. Nonfermenting Gram negative bacilli; pp. 305–391. [Google Scholar]
- 10.Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement CLSI document. 2007 Jan;70(1) M-100-S17. [Google Scholar]
- 11.Kumar V, Neelagund YF. Acinetobacter septicemia in neonates. Ind J Med Microbiol. 2004;22:71. [PubMed] [Google Scholar]
- 12.Shete VB, Ghadage DP, Muley VA, Bhore AV, et al. Acinetobacter septicemia in neonates admitted to intensive care units. J Lab Physicians. 2009;1:73–76. doi: 10.4103/0974-2727.59704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malini A, Deepa EK, Gokul BN, Prasad SR. Nonfermenting gram-negative bacilli infections in a tertiary care hospital in Kolar, Karnataka. Journal of laboratory physicians. 2009;1:62–66. doi: 10.4103/0974-2727.59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meharwal SK, Taneja N, Sharma SK, Sharma M. Complicated nosocomial UTI caused by nonfermenters. Indian J Urol. 2002;18:123–128. [Google Scholar]
- 15.Maria CB, Andrade SS, Silbert S, Gales AC, Jones RN, Sader HS. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis. 2004;8:284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone P, Rajendran P, Brahmadathan KN. Incidence of carbapenem resistant nonfermenting gram negative bacilli from patients with respiratory infections in the intensive care units. Indian J Med Microbiol. 2005;23:189–191. doi: 10.4103/0255-0857.16593. [DOI] [PubMed] [Google Scholar]
- 17.Hodiwala (Bhesania) A, Dhoke R, Urhekar AD. Incidence of metallo-beta-lactamase producing pseudomonas, Acinetobacter & enterobacterial isolates in hospitalised patients. Int JPharmBioSci. 2013;3:79–83. [Google Scholar]
- 18.Kabbaj H, Seffar M, Belefquih B, Akka D, Handor N, Amor M et al. Prevalence of Metallo-β-Lactamases Producing Acinetobacter baumannii in a moroccan Hospital. ISRN Infectious Diseases. 2013 Article ID 154921,3 pages www.hindawi.com/isrn/id/2013/154921.
- 19.John S, Balagurunathan R. Metallo beta lactamase producing Pseudomonas aeruginosa and Acinetobacter baumannii. Indian J Med Microbiol. 2011;29:302–304. doi: 10.4103/0255-0857.83918. [DOI] [PubMed] [Google Scholar]
- 20.Irfan S., Zafar A., Guhar D., Ahsan T., Hasan R. Metallo-β-lactamase producing clinical isolates of Acinetobacter species and Pseudomonas aeruginosa from intensive care unit patients of a tertiary care hospital. Indian J Med Microbiol. 2008;26:243–245. doi: 10.4103/0255-0857.42035. [DOI] [PubMed] [Google Scholar]
- 21.Pitout J., Gregson D., Poirel L., McClure J., Le P., Church D. Detection of Pseudomonas aeruginosa producing metallo-β-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43:3129–3135. doi: 10.1128/JCM.43.7.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varaiya A., Kulkarni N., Kulkarni M., Bhalekar P., Dogra J. Incidence of metallo-β-lactamase producing Pseudomonas aeruginosa in ICU patients. Indian J Med Res. 2008;127:398–402. [PubMed] [Google Scholar]
- 23.Livermore DM, Woodford N. Carbapenemse : A problem in waiting? Curr Opin Microbiol. 2000;3:489–495. doi: 10.1016/s1369-5274(00)00128-4. [DOI] [PubMed] [Google Scholar]


