Abstract
Background and objective
There is a concern on safety of human Fresh Frozen Plasma (FFP) as it is a source of some medicinal products. The possibility of transmission of blood-borne are reported often due to emerging viruses. There are some Pathogen Reduction Technologies (PRT) to inactivate viruses. Methylene Blue (MB) based method is one of them. The aim of this study was to examine new designated device to inactivate model viruses.
Materials and Methods
Four model viruses were used in this study:Vesicular stomatitis virus (VSV), Herpes Simplex Virus I (HSV-1), Bovine Viral DiarrheaVirus(BVDV) and Polio Virus.50% Tissue Culture Infective Dose (TCID 50) and Reed–Muench Methods were used to titer the viruses. MB in two final concentration of 0.1 μM and 1 μM and illumination in about 627nm with red LED (Lamp Emitting Diode) for 15, 30, 45 and 60 minutes were used. Three replicates employed for each experiments.
Results
1μMconcentration of MB showed more effective than 0.1μMin all designed illumination period for inactivation of HSV, VSV and BVDV. This method also demonstrated best results for enveloped model viruses. The most Log reduction for HSV, VSV and BVDV were6.28, 5.54 and 6.22, respectively. For HSV and BVDV inactivation, the best illumination period was 45 minutes.
Conclusion
Model viruses showed sensitivity combination of MB and illumination using red LEDs. As results show this device could inactivate model viruses and reduce their titer very close to approved commercial devices, in compare.
Keywords: Blood-borne Viruses, Methylene Blue, Red LED, Fresh Frozen Plasma
INTRODUCTION
Human Fresh Frozen Plasma (FFP) is a source of wide range of medicinal products and there is a concern on its safety (1). The risk of transfusion-transmitted blood- borne viruses today is lower than ever, due to donor selection criteria and blood-donor testing (2). However, the supply of blood products remain subject to contamination with known and yet to identify human pathogens (3).
Several different approaches have been undertaken to reduce or prevent transmitting blood-borne pathogens in Fresh Frozen Plasma (FFP).The most effective pathogen reduction methods that currently available for FFP involved the use of Solvent Detergent (SD), Methylene Blue (MB), amatosalen and riboflavin as additives (4).
The appropriate method should remove or inactivate pathogens, including emerging pathogens, without damaging the function or longevity of blood products. In addition, they should be free from toxic and immunogenic components (5).MB is a phenothiazine compound that in conjunction with photo could inactivate selective biologics (6). MB based Pathogen Reduction Technologies (PRT) have some advantages, particularly lack of plasma pooling, i.e. recipients would receive plasma from individual donations rather than from a plasma pool (7). Methylene blue in combination with light treatment FFP (MBLT- plasma) has been followed by at least three different protocols (8); Spring protocol (9), and two different protocols from Macopharma® (10).
The aim of this study was to evaluate the ability of novel device using MB in combination with red LED for inactivation of model viruses suspended in FFP.
MATERIAL AND METHODS
Device designation
143pcs (pieces) of 1WredLED (Lamp Emission Diodes) lamp have been used on the device to emit light with central wavelength of 627nmand 20 nm width (FWHM full width at half maximum). Two sides pouches irradiated simultaneously. The distance of the middle of the bag from LED arrays source was 4.5cm. This device was designed for light (LED) employing simulation software Wolfram Mathematica® considering the intensity profile. LED sand right number of used LEDs. Light emission to the FFP bag was adjusted and uniformed. A control Centre designed for temperature and exposure.
Fresh Frozen Plasma (FFP)
O+FFP were obtained from Iranian Blood Transfusion Centre (IBTO). These samples examined to be free from Hepatitis C Virus (HCV), Hepatitis B Virus (HBV) and Human Immunodeficiency Virus (HIV). They Aliquots into 50 ml plasma universal bags (JMS Co.,) and stored at -20°C.
Methylene blue
MB was obtained from Merck (Cat. No. 1592700010). Final concentration of MB in 50 ml of FFP adjusted to be 0.1 and 1 μM concentration. The dye solution was stored in dark at 4°C before use (11).
Preparation and quantification of model viruses
4 model viruses were used to evaluate new designated device, including enveloped viruses; Bovine Viral Diarrhea Virus (BVDV; Flaviviridae, Pestivirus, Nadal strain) a surrogate for HCV, Herpes Simplex Virus (HSV; Herpesviridae, Alpha herpes virinae, Herpes Simplex Virus, Type I) and Vesicular Stomatitis Virus (VSV; Rhabdoviridae, Vesiculovirus, Indian strain) models for HBV and HIV, respectively, and also one non- Enveloped virus, Polio (Picornaviridae, Enterovirus, Sabin vaccine). Model viruses’ selection wasbased on WHO guideline (12) and obtained from Research Centre of Iranian Blood Transfusion Organization (IBTO), Department of Virology (12).
Model viruses were propagated in different cell lines; BVDV in Bovine Kidney (BK),HSV and VSV in Vero (African Green Monkey Kidney) and Polio in HeLa (Human Carcinoma Cervix) cell lines (National Cell Bank of Iran, Iran Pasteur Institute, NCBI Nos. C500, C101 and C115, respectively).
All cell lines were grown in Dulbecco Modified Eagle ?s Medium (DMEM) with 10% fetal calf serum with penicillin- streptomycin antibiotics (Gibco, UK) as antibiotics. The antibiotics were used in 1% of final concentration. They incubated at 37°C with5% CO2. Confluent monolayers of each cell type were grown in sterile T25 flasks (SPL, Korea) and inoculated with 0.5 ml of the model viruses. Upon Cytopathic Effect (CPE) completion, they harvested and then storedat -80 °C for 24 h followed by filtration of supernatant through 0.45 μ nitrocellulose filter (Biofil). Filtered solution stored at -80 °C for further virus titration in 96-well flat-bottom sterile plate using 50% Tissue Culture Infectivity Dose (TCID 50) method. Reed-Muench method used for calculation of virus titer.
Inactivation methods
Each bag that contains 50 ml FFP was spiked with, 5 ml of prepared stock virus. Two different concentration of MB (0.1 and 1 μM) and 4 different illumination periods for each model virus were used: 15, 30, 45 and 60 minutes. Each assay repeated 3 times. Several controls were examined that including FFP with model virus; FFP with model virus & MB without illumination; and FFP with model virus& Illumination without MB. 1 ml of each sample was used to calculate virus titer using TCID50 and Reed and Muench calculation method. All cell cultures in flat-bottom sterile 96 well-titer plates were studied microscopically for the presence of CPE. Quantitative infectivity assays were performed using the end-point titration methods, TCID50.Each well observed daily for positive or negative for CPE. The final results were converted to aTCID50 / ml(13).
FFP proteins quality
4 FFP proteins selected to measure their concentration in FFP sample before and after pathogen inactivation to determine FFP quality. Factors V(STA®-Deficient V REF 00744), and VIII (STA®-Deficient VIII REF 00725),also Fibrinogen (STA®-Fibrinogen REF 00674)and Antithromb inactivity (STA®-Stachrom® AT III REF 00672)(Stago©, France) were measured.
Statistics
SPSS 16.00 and ANOVA were employed for data analysis.
RESULTS
MB without illumination and1h illumination without MB showed no significant effects on virus titers (p>0.05). 1μM concentration of MB had significantly more effective than 0.1μM in each illumination exposure time in HSV, VSV and BVDV.
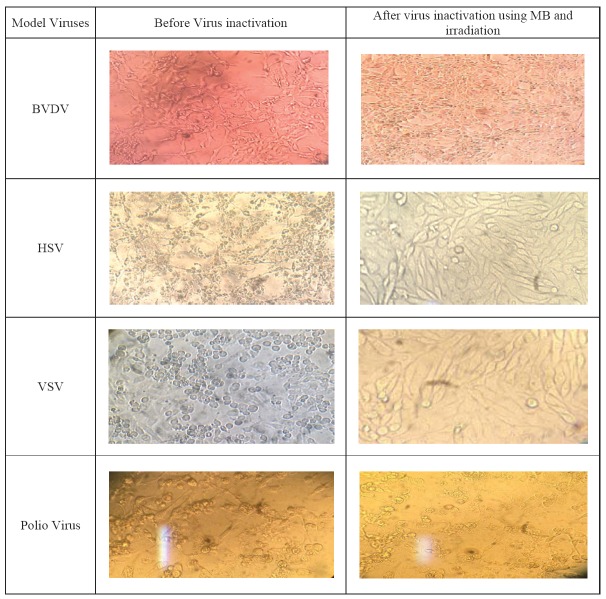
This method demonstrated the best results for enveloped viruses and most Log reduction for HSV, VSV and BVDV viruses; 6.28, 5.54 and 6.22, respectively. For HSV and BVDV inactivation, the best illumination periods was45 minutes (Table 1& 2). CPE of model viruses before and after of using MB and irradiation is shown in Fig. 1.
Table 1.
Inactivation of Model viruses using 1 μM concentration of MB and different illumination period (n=3).
| Model Virus (Mean titer) | HSV | VSV | BVDV | Polio | |
|---|---|---|---|---|---|
| Exposure Time (minutes) | Energy J/cm2 | ||||
| Initial titer | 0 | 107.28 | 107.76 | 107.22 | 107.22 |
| FFP sample: | |||||
| 0 min | 0 | 106.28 | 106.76 | 106.22 | 106.22 |
| 15 min | 27 | 101.22 | 103.76 | 101.76 | 106.28 |
| 30 min | 54 | 10.28 | 102.49 | 101.21 | 105.48 |
| 45 min | 81 | 0.0* | 101.76 | 0.0* | 105.28 |
| 60 min | 108 | 0.0* | 101.22 | 0.0* | 105.22 |
NO CPE observed in any well
Table 2.
Inactivation of Model viruses using 0.1 μM concentration of MB and different illumination time (n=3).
| Model Virus (Mean titer) | HSV | VSV | BVDV | Polio | |
|---|---|---|---|---|---|
| Exposure Time (minutes) | Energy J/cm2 | ||||
| Initial titer | 0.0* | 107.28 | 107.76 | 107.22 | 107.22 |
| FFP sample: o min | 0.0* | 106.28 | 106.76 | 106.76 | 106.22 |
| 15 min | 27 | 105.48 | 105.48 | 105.78 | 106.28 |
| 30 min | 54 | 10.3.49 | 104.42 | 104.32 | 106.22 |
| 45 min | 81 | 102.49 | 104.24 | 103.41 | 106.22 |
| 60 min | 108 | 102.22 | 104.22 | 103.22 | 105.22 |
NO CPE observed in any well
Fig 1.
CPE of model viruses before and after inactivation using combination of MB and irradiation method.
DISCUSSION
Donor FFP are tested in some countries for the presence of some pathogens prior to administration, nevertheless, small finite risk of transmission of infectious agent’s majority due to window period and emerging viruses still bearing potential risk factor (14-16). Several new technologies have been developed to reduce blood transfusion risks. MB based technologies are categorized in 3 main protocols. In this study, a new pathogen device was designed to reduce viruses. These new device used red LEDs from two sides to inactivate pathogens, so its exposure time reduced. It caused cost and time benefits and led to maintain FFP proteins in acceptable range.
As results showed, this new device is effective for enveloped viruses (HSV, VSV, and BVDV) and could reduce virus titer near to WHO recommendations but it is not appropriate for non-enveloped viruses (such as poliovirus with < Log 102 reduction). HSV and BVDV inactivation was demonstrated to be more sensitive to this protocol.
Illumination for 30-45 minutes and in 1μMconcentration of MB was strong enough to inactivate most viruses. However, illumination for 60 minutes had no significant difference in pathogen reduction in compare with 45 minutes (p<0.05). MB in 0.1 μM was not efficient, and so MB concentration is an important point.
Fibrinogen had most reduction in this protocol that shows its sensitivity to MB and illumination but antithrombin is the most resistance one. Factor V and VIII activity reduction was near to Theraflex Methods (17). In spite of reduction in these factors, they maintained in normal range.
Spring protocol use fluorescent lamps illumination from one side and has no temperature control. In Theraflex Macotronic V® method sodium lamps from two sides are used. And also in the latest technology employed by Theraflex MacotronicB® LED employed in two sides. Our new device shows the outcome similar to the latest technology of MB based inactivation methods. In Both Theraflex and our new device temperature is monitored, and all three commercial protocols used MB in 1μM as best final concentration (18, 19).
Achieving zero risk in chemotherapy remains the ultimate goal. This approach has not been met yet, but there are some promising results observed. Pathogen reduction by TMB, plasma is one additional critical factor to fulfill this aim. Eventually it is expected that with increasing clinical data and further methodological improvement, acceptance measure will rise, making pathogen reduction to become the standard routine procedure in many countries (19). This new designated and device needs more developments for commercialization.
Table 3.
FFP proteins activity evaluation after pathogen reduction with 1 μM MB and Illumination in 30 minutes at 627 nm (n=3).
| Before illumination | After 30 min illumination | Activity loss % | Normal range | |
|---|---|---|---|---|
| Factor V (%) | 91.5 | 82.5 | 11.0 | 60-130 |
| Factor VIII (%) | 97.7 | 85.2 | 11.4 | 60-150 |
| Fibrinogen (mg/ml) | 287.5 | 237.5 | 12.1 | 200-400 |
| Antithrombin (%) | 108 | 104 | 10.3 | 80-120 |
References
- 1.World Health Organization (WHO) technical Report, Series NO.924. 2004 [PubMed] [Google Scholar]
- 2.Au Buchon JP. Update on the statue of pathogen inactivation methods. ISBT Science series. 2011;6:181–188. [Google Scholar]
- 3.Bihl F, Castelli D, Marincola F, Dodd RY, Brander C. Transfusion- transmitted infections. J Transl Medi. 2007:5–25. doi: 10.1186/1479-5876-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock G. A comparison of methods of pathogen inactivation of FFP. Vox Sang. 2011;100:169–178. doi: 10.1111/j.1423-0410.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier JPR, Transue S, Snyder EL. Pathogen inactivation techniques. Best Pract Res Clin Haematol. 2006;19:205–242. doi: 10.1016/j.beha.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallis C, Melnick JL. Photodynamic inactivation of animal viruses: a review. Photochemphotobiol. 1965;4:159–170. doi: 10.1111/j.1751-1097.1965.tb05733.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Guideline on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. Geneva; 26-30 November 2001. [Google Scholar]
- 8.Seghatchian J, Krailadsiri P. the quality of Methylene blue light trated FFP and cryopororecipitate. Transfuse Aphersis Sci. 2001;25:227–31. doi: 10.1016/s1473-0502(01)00109-4. [DOI] [PubMed] [Google Scholar]
- 9.Mohr H, Lambrecht B, Seltz A. a photodynamic virus inactivation of blood components. Immunol Invest. 1995;24:73–85. doi: 10.3109/08820139509062763. [DOI] [PubMed] [Google Scholar]
- 10.Solheim BG, Seghatchian J. update on pathogen reduction technology for therapeutic plasma: an overview. Transfus Apher Sci. 2006;35:83–90. doi: 10.1016/j.transci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lambrecht B, Mohr H, Knuver-Hope FJ, Shmith H. Photoinactivation of viruses in human fresh frozen plasma by henothiazine dyes in combination with visible light. Vox Sang. 1991;60:207–213. doi: 10.1111/j.1423-0410.1991.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 12.Aghaei A, Pourfatollah A, et al. inactivation of virus in intravenous immunoglobulin G using solvent/ detergent treatment and pasteurization. Human Antibodies. 2008;17:79–84. [PubMed] [Google Scholar]
- 13.Mahy BWJ, Kangro HO. Academic Press; 1996. Virology methods manual, quantitation of virus. [Google Scholar]
- 14.Busch M, Kleinman S, Neo G. current and emerge in infectious risks of blood transfusion. JAMA. 2003;289:959–962. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 15.Devta VT Jr., Hellman S, Rosenberg SA, editors. AIDS: Etiology, diagnosis, treatment and prevention. 3. Philadelphia, PA: Lippincot; 1992. [Google Scholar]
- 16.Schochet main G, AIDS testing. 2. New York, NY: springer verlag; 1994. [Google Scholar]
- 17.Williamson LM, Cardigan R, Prowse CV. Methylene blue-treated fresh-frozen plasma: what is its contribution to blood safety? Transfusion. 2003;43:1322–1329. doi: 10.1046/j.1537-2995.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 18.Rock G. A comparision of methods of pathogen inactivation of FFP. VOX Sang. 2010;100:169–178. doi: 10.1111/j.1423-0410.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 19.Seghatchian J, Sturff WG, Reichenberg S. Main properties of the THERAFLEX MB-system for pathogen reduction. Transfusion Med and Hemotherapy. 2011;38:000–000. doi: 10.1159/000323786. pulished on line. [DOI] [PMC free article] [PubMed] [Google Scholar]