Abstract
Background and Objectives
Pomegranate fruit is a rich source of bioactive compounds. The serious concern over unprocessed fruit juices is microbial contamination, which effectively inactivated by thermal processing, but it significantly affects juice functional compounds. Therefore, the effect of gamma irradiation and ultrasonic on inoculated microbial to pomegranate juices was studied.
Materials and Methods
Two pomegranate cultivars were purchased from the Agricultural Research Center of Saveh, and their juices were extracted by a manual device and immediately centrifuged. Then the studied microorganisms were re-suspended in sterile pomegranate juices. The juices were continuously sonicated at amplitude levels of 50, 75 and 100% and times of 0, 3, 6, 9, 12 and 15 min at temperature of 25 ± 1 °C. Irradiation treatment was also carried out at various doses of 0, 0.5, 1.0, 1.5, 2.0, 2.5 and 3 kGy.
Results
The results showed that lower amplitude levels (50 and 75%) did not inactivate E. coli and S. cerevisiae significantly (<1.5 log reduction), while at 100% amplitude level for 15 min, their population reduced by 3.47 and 1.86 log cfu/mL, respectively. Gamma irradiation treatment at 1 kGy also reduced E. coli by 6.66 log cfu/mL, whereas at 3 kGy it reduced S. cerevisiae by 5.08 log cfu/mL.
Conclusions
The low-dose gamma irradiation could potentially inactivate the studied microorganisms compared to the sonication, which had less destructive effects on their populations. Further research is needed to determine the effect of these methods on other fruit juices for industry purposes.
Keywords: Pomegranate juice, Ultrasound, Gamma irradiation, S. cerevisiae, E. coli
INTRODUCTION
Pomegranate fruit (Punica granatum L.) and its products have been used for centuries as a rich source of bioactive compounds (1), and it has recently been regarded as one of the new super-foods with health promoting effects (2). Total production of pomegranate in the world was approximately 1,500,000 tons in 2007 that Iran accounted for 47% of world production and the rest was mainly produced by other countries including Turkey, Afghanistan, India, Egypt, Spain, and the USA. Pomegranate is consumed as fresh fruit, juice, jam, jelly, and pomegranate supplements in the world (3). Traditionally, ready-to-drink pomegranate juice, which has a short shelf-life and is very similar to home-made pomegranate drink, is sold in grocery stores in Iran, without any specific processing and addition of preservatives.
Microbial contamination with acid-tolerant bacteria, fungi (yeasts and moulds), and pathogenic bacteria especially E. coli O157:H7 and Listeria monocytogenes is the serious concern over unpro-cessed fruit juices. These microorganisms can survive in acidic conditions during prolonged storage (4). Microbial growth in unpasteurized fruit juices causes deterioration of nutritional and sensorial properties such as functional ingredients, colour, flavor and odor as well as leading to food poisoning due to pathogenic bacteria or toxicogenic fungi (5). Consequently, in order to ensure the safety of juice products, reduction of 5 log in pathogen population was enacted by the United States Food and Drug Administration, FDA (6).
Thermal processing is the most effective method for inactivation of microorganisms and enzymes (7). However thermal pasteurization and sterilization techniques can reduce the organoleptic quality and freshness of foods (8-10). Non-thermal techniques compared with the thermal treatments have less destructive effects on nutritional and sensory properties of foods. Inactivation of spoilage and pathogenic microorganisms in food industry is the most important challenge of non-thermal technologies (11).
Some of non-thermal technologies as potential alternatives to thermal processing of foods include membrane filtration, osmotic dehydration, pulse electric field, ultrasound, ionizing radiation, high pressure, active packaging and ozone treatment (7, 11). Ultrasound technology has shown important advances in food industries in the last few years and its application in food processing has been reviewed by Knorr et al (12). The potential of ultrasound for inactivation of microorganisms and enzymes has been reported in various researches (12, 13). In addition, food irradiation which is often called cold pasteurization can be used to control and inactivate spoilage microorganisms and food-borne pathogens, such as Salmonella, E. coli, Listeria and Campylobacter (14). Irradiation of food products by gamma rays, X-rays, and electron beams has been used in 56 countries and their safety has been approved by the World Health Organization (WHO), the Center for Disease Control and Prevention (CDC), the United State Department of Agriculture (USDA) and FDA (14). The potential of gamma irradiation for inactivation of microorganisms such as L. monocytogenes, Salmonella enterica, Salmonella typhimurium, E. coli, coliform bacteria, total aerobic, yeast and moulds as well as enzymes has been reported in various researches (10, 15-17).
Although the effects of ultrasonic and irradiation on some fruit and vegetables juices have been reported by several researches, no or a little information is available about pomegranate juice sonication and gamma irradiation. The objective of this study was to investigate the effects of ultrasonic and low dose gamma irradiation on the inactivation of S. cerevisiae and E. coli in pomegranate juices.
MATERIALS AND METHODS
Microbiological media
Malt extract agar and nutrient agar (Difco Laboratories, Detroit, USA), sorbitol MacConkey agar (Liofilchem, Teramo, Italy), dichloran rose bengal chloramphenicol agar (Merck, Darmstadt, Germany) were used in this study. KH2PO4, NaOH and NaCl were purchased from Merck (Darmstadt, Germany). Phosphate-buffered saline (PBS) was prepared based on the formulation recommended by Gabriel and Nakano (18).
Preparation of pomegranate juice
Fresh and commercially matured pomegranate cultivars of Malase Momtaze Saveh, MMS, and Alak Saveh, AS, were bought from the Agricultural Research Center of Saveh, Iran. Selection of pomegranate cultivars performed based on their physicochemical properties, higher total phenolic and anthocyanin contents and antioxidant activities, that published earlier (19). After deleting defective ones visually, each fruit was washed, drained, peeled, and then cut into pieces to separate the arils, edible parts of pomegranate, manually. Next, their juices were extracted by means of a manual device. The juices of Malase Momtaze Saveh arils,MMSA, and Alak Saveh arils, ASA were immediately centrifuged at 9500 g for 2 min at 4°C with a refrigerated centrifuge and subsequently refrigerated for further analysis.
Measurement of total titratable acidity (TA), pH and total soluble solids (TSS)
Titratable acidity, pH, and total soluble solids (°Brix) of treated and untreated pomegranate juices were measured using methods described by Martinez et al (20). Also, maturity index (MI) was calculated by dividing total soluble solids to titratable acidity (20).
Preparation of inoculums and inoculation of test samples
According to recommendation of previous studies due to acidic conditions of fruit juices, E. coli, considered as pathogenic microorganisms that survive at acidic condition, and S. cerevisiae were examined in this study as spoilage and human illness microorganisms (4, 5). S. cerevisiae (PTCC 5052) and E. coli (RITCC 1177) were obtained from the Iranian Research Organization for Science and Technology (IROST) and the Razi Institute Type Culture Collection (Tehran, Iran), respectively. Lyophilized S. cerevisiae and E. coli revived on malt extract agar and nutrient agar, and subsequently maintained at 4°C. The cells of E. coli and S. cerevisiae were loop-inoculated in nutrient broth (pH 7.0) and malt extract broth (pH 7.0), and then incubated at 35°C and 27°C, respectively for nearly 24 h without shaking in order to obtain cells in the early stationary phase (8, 18).
The resulting inoculated broth media were centrifuged at 6000 g for 5 min, and the precipitated cells were aseptically re-suspended in a 50-mL sterile phosphate-buffered saline (PBS, pH 7.2) solution and used as the inoculums. The acclimatization time of the re-suspended cells must be no longer than 20 min prior to inactivation (18). Then, 1 mL aliquot of E. coli and S. cerevisiae in PBS was re-suspended in 100 mL sterile pomegranate juice. The initial population of E. coli and S. cerevisiae in inoculated pomegranate juices was 4.5×106 and 1.1×105, respectively.
Ultrasonic treatment
An ultrasonic liquid processor (Misonix, Inc., New York, USA) supplied with a 19 mm diameter probe, amplitude levels of 24.4–61 µm, at constant frequency of 20 kHz was used to sonicate the 100 mL pomegranate juices. Ultrasonic treatment was carried out in a 150 mL double wall cylindrical vessel pyrex glass with 60 mm inner diameter, 80mm outer diameter, 65 mm outer height and 55 mm inner height connected to a recirculating refrigerated water bath (Cooling thermostat: Lauda Alpha RA 8, Lauda-Königshofen, Germany) to attain a constant temperature in the juice sample during sonication. Ethylene glycol (2°C according to amplitude levels) with flow rate of 0.5 L/min was used as the refrigerant to remove the heat generated during sonication to maintain sample temperature constant at 25 ± 1 °C. The ultrasound probe was submerged to a depth of 25 mm in the pomegranate juice to constantly sonicate at various wave amplitudes of 50, 75 and 100% and times of 0, 3, 6, 9, 12 and 15 min. For microbial studies, the inoculated pomegranate juices (100 mL) were sonicated in a 150 mL cylindrical vessel under the conditions described at the sub lethal temperature (25°C). The samples were recovered at the exit of the ultrasonic cell, poured into sterile glass tubes; the tubes were immediately immersed into and kept in an ice bath until survivor enumerations.
Irradiation treatments
The inoculated pomegranate juices with E. coli and S. cerevisiae were filled in sterilized amber Wheaton-320 glass vials (8 mL, Sigma, USA) under sterile conditions. Irradiation was carried out at various dosages of 0, 0.5, 1.0, 1.5, 2.0, 2.5 and 3 kGy using a Gamma cell-220 irradiator (Nordion, Canada). After gamma irradiation at ambient temperature, tubes were immediately immersed and kept in an ice bath until survivor enumerations.
Survivor enumeration and decimal reduction times calculations
Tubes containing the inactivated cells were serially diluted with PBS and then, the number of surviving E. coli were determined by surface-plating on sorbitol MacConkey agar and incubating at 35°C for 48 h. The number of surviving S. cerevisiae determined by surface-plating on dichloran rose bengal chloramphenicol gar and incubating at 25°C for 72h. Then, the log reduction and D-values (decimal reduction) of the sonicated and gamma irradiated juices were estimated. Survival curves were drawn on a semi log graph by plotting the logarithmic number of colony-forming units per milliliter against sonication times or irradiation doses. D-value is the negative inverse slope of the plot represented the irradiation dose or sonication time required to inactivate 90% of the microbial population (8). The duplicate samples of two independent trials were prepared for each treatment. The log counts of colony-forming units for each dose and sonication time were averaged, mean values plotted and curves were fitted by linear regression. All experiments were carried out in duplicate and mean was calculated to express the final result.
Statistical analysis
All analyses were performed by means of SAS statistical software, version 9.2 (SAS Institute, Inc). Values with different letters within a similar row are significantly different. A value of P < 0.05 was taken to be statistically significant.
RESULTS
The results of soluble solid content, pH, titratable acidity and maturity index of the studied pomegranate juice are presented in Table 1.
Table 1.
Some physicochemical quality parameters of untreated pomegranate juices.
| MMSA | ASA | |
|---|---|---|
| Total Soluble solids (°Brix) | 16.7 ± 0.2b* | 17.2 ± 0.1a |
| pH | 3.56 ± 0.01a | 3.09 ± 0.02b |
| Titratable acidity (g/100mL) | 0.81 ± 0.01b | 1.61 ± 0.00a |
| Maturity index | 20.70 ± 0.17 a | 10.72 ± 0.08 b |
Values with different letters (a, b) within a similar row are significantly different (P < 0.05).
Ultrasound inactivation of E. coli and S. cerevisiae in pomegranate juices
The pomegranate juice samples were inoculated by a suspension of E. coli and S. cerevisiae in phosphate buffer, and then they were subjected to sonication with varying amplitude levels and times.
D-values of ultrasonic treated samples of E. coli and S. cerevisiae are shown in Table 2. The D-values and log reduction of the studied microorganisms at lower amplitude levels were significantly (P < 0.05) less than higher amplitude levels. On the other hand, a significant difference in D-values and log reduction was observed among three amplitude levels. At higher amplitude levels, the inactivation rate of yeast and bacteria increased (Table 2).
Table 2.
Decimal reduction times of test microorganisms in sonicated pomegranate juices*.
| Microorganism | Pomegranate juice | Ultrasonic amplitude levels (%) |
||
|---|---|---|---|---|
| 50 | 75 | 100 | ||
| S. cerevisiae | MMSA | 16.94 ± 0.18ax | 14.28 ± 0.27bx | 8.33 ± 0.21cx |
| S. cerevisiae | ASA | 16.67 ± 0.47ax | 13.32 ± 0.23bx | 8.40 ± 0.15cx |
| E. coli | MMSA | 9.52 ± 0.14ax | 8.13 ± 0.17bx | 4.05 ± 0.15cx |
| E. coli | ASA | 10.20 ± 0.08ax | 9.52 ± 0.14by | 4.41 ± 0.09cx |
Values with different letters (a–c) within a similar row and (x-y) within a similar column associated with each microorganism are significantly different (P < 0.05).
The inactivation of both microorganisms at lower amplitude levels of 50 and 75% were not considerable (< 2log reduction), while a log cfu/mL reduction was achieved at the lethal amplitude level (100%) and 15 min that reduced E. coli and S. cerevisiae populations by 3.37-3.56 log cfu/mL and 1.84-1.88 log cfu/mL, respectively (Figs. 1 & 2). There was a noticeable and similar reduction trend among pomegranate juices, especially in the E. coli population, whereas the reduction rate of S. cerevisiae population in the treated samples was different, so that the D-values of these microorganisms in MMSA and ASA samples at 75% intensity were significantly different. In addition, the results showed that E. coli compared with S. cerevisiae in the sonicated pomegranate juice had a greater log reduction indicating its lower resistance to ultrasonic when suspended in pomegranate juices. The results demonstrated that the pH of pomegranate juices had no significant effect on E. coli and S. cerevisiae inactivation.
Fig. 1.
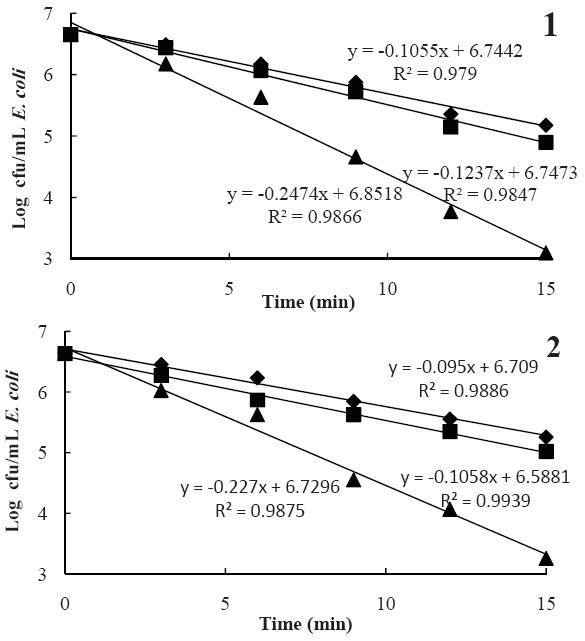
Survival curve of E. coli at ultrasound amplitude levels of 50 (●), 75 (■) and 100 (▲) in Malase Momtaze Saveh arils (MMSA, 1) and Alak Saveh arils (ASA, 2) juices.
Fig. 2.
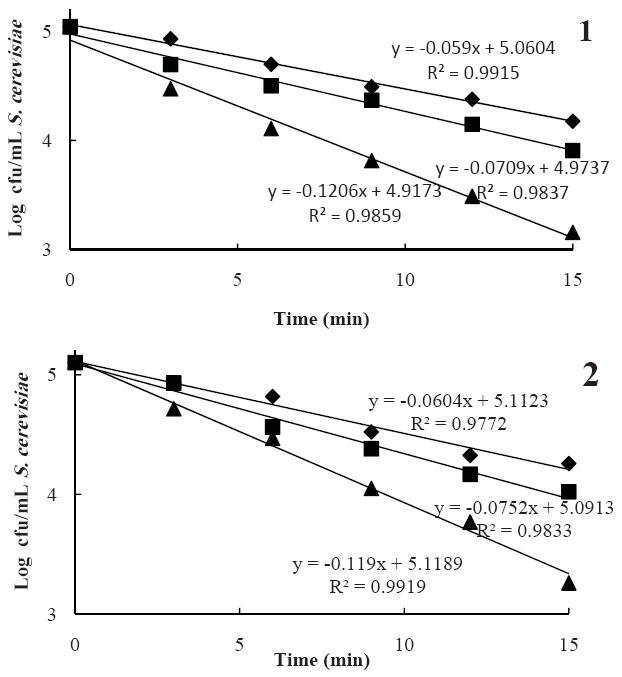
Survival curve of S. cerevisiae at ultrasound amplitude levels of 50 (●), 75 (■) and 100 (▲) in Malase Momtaze Saveh arils (MMSA, 1) and Alak Saveh arils (ASA, 2) juices.
Effect of gamma irradiation on inoculated juices
The effect of gamma irradiation on the survival of inoculated E. coli and S. cerevisiae (log reduction cycle) in MMSA and ASA pomegranate juices are shown in Fig. 3. The R2 values of regression lines ranged from 0.94 to 0.98. The studied microorganisms including E. coli and S. cerevisiae in the fresh pomegranate juices inactivated by irradiation at 1 kGy and 3 kGy, respectively. The D-values of E. coli and S. cerevisiae in the juices were approximately calculated by 0.15 kGy and 0.58 kGy, respectively. The D-values varied depending on the type of microorganism used. It should be considered that irradiation dose up to 1 kGy could not completely inactivate S. cerevisiae in the fresh pomegranate juices. A decreasing trend in the studied microbial population was observed by increasing the irradiation dosage. Gamma irradiation significantly reduced the microbial contamination level with a dose dependant tendency (P < 0.05). The microbial population reduced to the below detection limit at 3 kGy for S. cerevisiae and at 1 kGy for E. coli. According to Fig. 3, the log reduction of E. coli cells at 1 kGy was 6.64-6.67, whereas the S. cerevisiae counts reduced at 3 kGy by 5.06-5.09 log cfu/mL. The log reduction of the studied microorganisms at various levels of gamma ray was significantly different, but the type of pomegranate juice (Fig. 3) showed no significant effect on the inactivation of microorganisms. Also, the D-value of S. cerevisiae was higher than E. coli indicating its higher resistance to gamma ray.
Fig. 3.
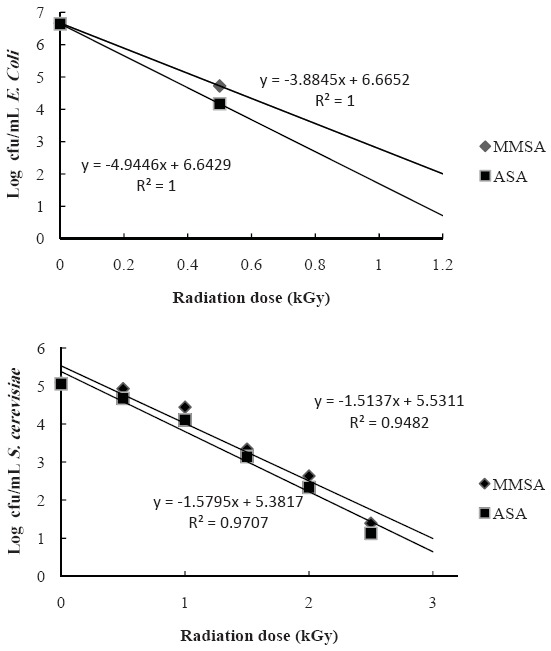
Survival curve for E. coli and S. cerevisiae strains in irradiated pomegranate juice (Malase Momtaze Saveh arils (MMSA); Alak Saveh arils (ASA)) with gamma rays.
DISCUSSION
The maturity index (TSS/TA) is responsible for the taste and flavor of pomegranate, which some author used for classifying the pomegranate cultivars (20). For example, this classification has been optimized for Spanish cultivars: maturity index (MI) = 5–7 for sour, MI = 17–24 for sour–sweet and MI – 31–98 for sweet cultivars (20).
Important spoilage microorganisms found on fruit surfaces are yeasts, moulds and acid-tolerant bacteria. pH influences the growth rate of microorganisms and its shift from 3.4 to 4.0 can significantly increase the growth rate of some acid-tolerant bacteria (17). The studied microorganisms were selected based on the previous studies on spoilage and human pathogens micro-organisms in fruit juices. Some researchers reported that yeasts are the principal fungi spoiling fruit juices (5).
The pomegranate juice samples were inoculated by a suspension E. coli and S. cerevisiae in phosphate buffer, and then they were subjected to sonication with varying amplitude levels and times. Ultrasound has been identified as a potential technology to meet the FDA requirement of 5 log reduction in pertinent microorganisms found in fruit juices (6, 21).
At higher amplitude levels, the inactivation rate of yeast and bacteria increased (Table 2), which is in accordance with previous studies (8, 22). Also, the results demonstrated that the source of pomegranate juices and consequently pH of these juices had no significant effect on E. coli and S. cerevisiae inactivation, which are in agreement with findings of Guerrero et al. (8). They reported that the inactivation rate of yeasts exponentially increased with increases in amplitude, but the reduction of pH (pH 5.6 and 3.0) did not significantly affect ultrasound yeast sensitivity except for experiments performed at high amplitude level and temperature of 45°C.
Ultrasound has been recognized as one of the non-thermal technologies that significantly reduces the microbial load, but preserving the organoleptic properties of fresh food products. The potential of ultrasound for inactivation of microorganisms and enzymes in food and model systems has been reported in various researches. The inactivation of E. coli in apple cider (23), Streptococcus mutans in physiological salt solution (24), Alicyclobacillus acidoterrestris in apple juice (25), L. monocytogenes in apple cider (13) and orange juice (26), Pichia fermentans in tomato juice (27), and S. cerevisiae in model system (8) has been investigated.
The inactivation mechanism of microorganisms is mainly based on physical and chemical factors resulting from the influence of ultrasound on liquid foods. Among the physical effects, thinning of cell membranes, localized heating and pressure change (5500°C and 50,000 kPa); and chemical effects, production of free radicals such as OH°, H° due to sonochemical reactions are presented (24, 28). Effectiveness and efficiency of ultrasound in microorganism inactivation depend on the physicochemical properties of food, volume of food being processed, types of microorganisms, treatment temperature, power level and duration of the ultrasound treatment (9, 11).
The 5 log and 2.7 log reductions of Shigella boydii and L. monocytogenes populations in a sterilized saline solution were achieved after about 12.75 min and 20 min, respectively at temperatures below 37°C during sonication (29). The effect of sonication on Pichia fermentans in tomato juice at different times (2 to 10 min) was investigated (30) and concluded that using ultrasound in mild temperatures (40°C) for 7.5 min to reach the desired 5 log reduction in yeast cells is possible. In another study, Patil et al (22) reported that 5 log reduction of E. coli strains by ultrasound with increasing the amplitude level at 15 min or less can be achieved. Also, using an ultrasonic process at 40°C for 20 min could reduce the microbial population of E. coli by 5.3 log cycles (23).
However, based on scientific reports, direct comparison of these results is difficult since different nature of microbial strains has a significant effect on inactivation rate in sonication (13). Ultrasound treatment conditions, such as frequency, amplitude, position of ultrasonic probe in the reactor, geometry of the reactor and probe, sampling method and location, acoustic energy density (AED), and properties of the medium also play an important role in determining the rate of inactivation (9). In this study, using the circulation system and double glass vessel with internal coil prevented the increase of temperature during the sonication process in order to evaluate the inactivation effects of ultrasound alone.
As expected, low dose gamma irradiation was even less effective in reducing yeasts and moulds populations (17). Various environmental factors, for example chemical composition of foods may affect the radiation resistance and sensitivity (10, 31). Therefore, the radiation sensitivity of microorganisms should be considered in radiation sterilization (31).
The key organisms in food irradiation are yeasts and moulds, which have a higher D-value than pathogenic bacteria. In fruit juices, D-values reported for yeasts and moulds are from 1 to 3 kGy (32) and from 0.3 to 0.7 kGy for pathogenic bacteria (33). Buchanan et al. (33) showed that, a dose of 1.8 kGy should be sufficient to achieve the 5D inactivation of E. coli which recommended by the National Advisory Committee for Microbiological Criteria for Foods Irradiation.
The effectiveness of gamma irradiation in inactivating L. monocytogenes and S. enterica in fresh orange juice at 0-4 kGy (17), E. coli 0157:H7 and aerobic mesophiles, and yeasts and moulds in shredded iceberg lettuce at 0-0.55 kGy (34), S. typhimurium and E. coli in carrot and kale juice at 0-3 kGy (10) and total aerobic and coliform bacteria in carrot and kale juice at 0-5 kGy (31), two typical radiation-resistant bacteria, Bacillus megaterium and Exiguobacterium acetylicum and polyphenol oxidase activity in fresh kale juice at 0-5 kGy (31), total bacteria and fungi in pomegranate juice at 0-10 kGy (15), total aerobic, yeast and mould in tamarind juice at 0-5 kGy (16) have been reported.
The mechanism of microbial inactivation by gamma ray is damage to DNA and somewhat protein denaturation (35). Ionizing radiations damage microbial DNA either by direct (single and double strand breaks of nucleic acids) or indirect reaction (hydroxyl radicals originating from radiolysis of water) (36).
In conclusion, low-dose gamma irradiation could potentially inactivate the studied microorganisms compared to the ultrasonic process, which had less destructive effects on their populations. Further study is needed to determine the effect of these methods on other fruit juices in order to apply them in juice industry.
Acknowledgments
The financial support of Tarbiat Modares University Research Council is gratefully acknowledged.
REFERENCES
- 1.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 2.Johanningsmeier SD, Harris GK. Pomegranate as a functional food and nutraceutical source. Annu Rev Food Sci Technol. 2011;2:181–201. doi: 10.1146/annurev-food-030810-153709. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Project document for a regional standard for pomegranate prepared by the Islamic Republic of Iran. FAO/WHO; Tunisia: 2009. [Google Scholar]
- 4.Uljas HE, Ingham SC. Combinations of intervention treatments resulting in 5-log10-unit reductions in numbers of Escherichia coli O157: H7 and Salmonella typhimurium DT104 organisms in apple cider. Appl Environ Microbiol. 1999;65:1924–1929. doi: 10.1128/aem.65.5.1924-1929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tournas VH, Heeres J, Burgess L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006;23:684–688. doi: 10.1016/j.fm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Food and Drug Administration Report. Kinetics of microbial inactivation for alternative food processing technologies: ultrasound. Published June 2, 2000. [Google Scholar]
- 7.Ohlsson T, Bengtsson N. Minimal Processing Technologies in the Food Industry. Boca Raton, FLorida, USA: 2002. [Google Scholar]
- 8.Guerrero S, López-Malo A, Alzamora SM. Effect of ultrasound on the survival of Saccharomyces cerevisiae: influence of temperature, pH and amplitude. Innovative Food Sci Emerg Technol. 2001;2:31–39. [Google Scholar]
- 9.Lee H, Zhou B, Liang W, Feng H, Martin SE. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: microbial responses and kinetics modeling. J Food Eng. 2009;93:354–364. doi: 10.1111/j.1750-3841.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 10.Song HP, Kim DH, Jo C, Lee CH, Kim KS, Byun MW. Effect of gamma irradiation on the microbiological quality and antioxidant activity of fresh vegetable juice. Food Microbiol. 2006;23:372–378. doi: 10.1016/j.fm.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Piyasena P, Mohareb E, McKellar RC. Inactivation of microbes using ultrasound: a review. Int J Food Microbiol. 2003;87:207–216. doi: 10.1016/s0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 12.Knorr D, Zenker M, Heinz V, Lee DU. Applications and potential of ultrasonics in food processing. Trends Food Sci Technol. 2004;15:261–266. [Google Scholar]
- 13.Baumann AR, Martin SE, Feng H. Power ultrasound treatment of Listeria monocytogenes in apple cider. J Food Prot. 2005;68:2333–2340. doi: 10.4315/0362-028x-68.11.2333. [DOI] [PubMed] [Google Scholar]
- 14.GAO. Food irradiation: FDA could improve its documentation and communication of key decisions on food irradiation petitions. Washington, D C: GAO-10-309R; 2010. [Google Scholar]
- 15.Alighourchi H, Barzegar M, Abbasi S. Effect of gamma irradiation on the stability of anthocyanins and shelf-life of various pomegranate juices. Food Chem. 2008;110(4):1036–1040. doi: 10.1016/j.foodchem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Kim JK, Srinivasan P, Choi J, Kim JH, Han SB, et al. Effect of gamma irradiation on microbial analysis, antioxidant activity, sugar content and color of ready-to-use tamarind juice during storage. LWT-Food Sci Technol. 2009;42:101–105. [Google Scholar]
- 17.Foley D, Pickett K, Varon J, Lee J, Mln D, Caporaso R, et al. Pasteurization of fresh orange juice using gamma irradiation: microbiological, flavor, and sensory analyses. J Food Sci. 2002;67:1495–1501. [Google Scholar]
- 18.Gabriel AA, Nakano H. Effects of culture conditions on the subsequent heat inactivation of E. coli O157: H7 in apple juice. Food Control. 2011;22:1456–1460. [Google Scholar]
- 19.Alighourchi HR, Barzegar M, Sahari MA, Abbasi S. Effect of sonication on anthocyanins, total phenolic content, and antioxidant capacity of pomegranate juices. Int Food Res J. 2013;20:1703–1709. [Google Scholar]
- 20.Martinez JJ, Melgarejo P, Hernandez F, Salazar DM, Martinez R. Seed characterisation of five new pomegranate (Punica granatum L.) varieties. Sci Hortic. 2006;110:241–246. [Google Scholar]
- 21.Salleh-Mack SZ, Roberts JS. Ultrasound pasteurization: the effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrason Sonochem. 2007;14:323–329. doi: 10.1016/j.ultsonch.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Patil S, Bourke P, Kelly B, Frías JM, Cullen PJ. The effects of acid adaptation on Escherichia coli inactivation using power ultrasound. Innovative Food Sci Emerg Technol. 2009;10:486–490. [Google Scholar]
- 23.Ugarte-Romero E, Feng H, Martin SE, Cadwallader KR, Robinson SJ. Inactivation of Escherichia coli with power ultrasound in apple cider. J Food Sci. 2006;71:E102–E108. [Google Scholar]
- 24.Koda S, Miyamoto M, Toma M, Matsuoka T, Maebayashi M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500 kHz. Ultrason Sonochem. 2009;16:655–659. doi: 10.1016/j.ultsonch.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y, Hu Y, Yue T, Chen T, Lo Y. Effect of ultrasonic treatments on thermoacidophilic Alicyclobacillus acidoterrestris in apple juice. J Food Process Pres. 2009;33:370–383. [Google Scholar]
- 26.Ferrante S, Guerrero S, Alzamora SM. Combined use of ultrasound and natural antimicrobials to inactivate Listeria monocytogenes in orange juice. J Food Prot. 2007;70:1850–1856. doi: 10.4315/0362-028x-70.8.1850. [DOI] [PubMed] [Google Scholar]
- 27.Adekunte AO, Tiwari BK, Cullen PJ, Scannell AGM, O’Donnell CP. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500–507. [Google Scholar]
- 28.Valdramidis VP, Cullen PJ, Tiwari BK, O’Donnell CP. Quantitative modelling approaches for ascorbic acid degradation and non-enzymatic browning of orange juice during ultrasound processing. J Food Eng. 2010;96:449–454. [Google Scholar]
- 29.Ugarte-Romero E, Feng H, Martin SE. Inactivation of Shigella boydii 18 IDPH and Listeria monocytogenes Scott A with power ultrasound at different acoustic energy densities and temperatures. J Food Sci. 2007;72:M103–M107. doi: 10.1111/j.1750-3841.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 30.Adekunte A, Tiwari BK, Scannell A, Cullen PJ, O’Donnell C. Modelling of yeast inactivation in sonicated tomato juice. Int J Food Microbiol. 2010;137:116–120. doi: 10.1016/j.ijfoodmicro.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Song H, Lim S, Yun H, Chung J. Effects of gamma irradiation on the radiation-resistant bacteria and polyphenol oxidase activity in fresh kale juice. Radiat Phys Chem. 2007;76:1213–1217. [Google Scholar]
- 32.Narvaiz P, Lescano G, Kairiyama E. Irradiation of almonds and cashew nuts. LWT-Food Sci Technol. 1992;25:232–235. [Google Scholar]
- 33.Buchanan R, Edelson S, Snipes K, Boyd G. Inactivation of Escherichia coli O157: H7 in apple juice by irradiation. Appl Environ Microbiol. 1998;64:4533–4535. doi: 10.1128/aem.64.11.4533-4535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley DM, Dufour A, Rodriguez L, Caporaso F, Prakash A. Reduction of Escherichia coli 0157:H7 in shredded iceberg lettuce by chlorination and gamma irradiation. Radiat Phys Chem. 2002b;63:391–396. [Google Scholar]
- 35.Lado BH, Yousef AE. Alternative food-preservation technologies: efficacy and mechanisms. Microbes Infect. 2002;4:433–440. doi: 10.1016/s1286-4579(02)01557-5. [DOI] [PubMed] [Google Scholar]
- 36.Sommer R, Pribil W, Appelt S, Gehringer P, Eschweiler H, Leth H, et al. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res. 2001;35:3109–3116. doi: 10.1016/s0043-1354(01)00030-6. [DOI] [PubMed] [Google Scholar]


