Abstract
Colorectal cancer is one of the most common causes of cancer morbidity both in men and in women. However, females over 65 years old show higher mortality and lower 5-year survival rate of colorectal cancer compared to their age-matched male counterparts. The objective of this review is to suggest gender-based innovations to improve colorectal cancer outcomes in females. Women have a higher risk of developing right-sided (proximal) colon cancer than men, which is associated with more aggressive form of neoplasia compared to left-sided (distal) colon cancer. Despite differences in tumor location between women and men, most of scientific researchers do not consider sex specificity for study design and interpretation. Also, colorectal cancer screening guidelines do not distinguish females from male, which may explain the higher frequency of more advanced neoplasia when tumors are first detected and false negative results in colonoscopy in females. Moreover, socio-cultural barriers within females are present to delay screening and diagnosis. Few studies, among studies that included both men and women, have reported sex-specific estimates of dietary risk factors which are crucial to establish cancer prevention guidelines despite sex- and gender-associated differences in nutrient metabolism and dietary practices. Furthermore, anti-cancer drug use for colorectal cancer treatment can cause toxicity to the reproductive system, and gender-specific recurrence and survival rates are reported. Therefore, by understanding sex- and gender-related biological and socio-cultural differences in colorectal cancer risk, gender-specific strategies for screening, treatment and prevention protocols can be established to reduce the mortality and improve the quality of life.
Keywords: Colorectal cancer, Sex, Gender, Screening, Treatment, Prevention
Core tip: The objective of this review is to suggest gendered innovations to improve colorectal cancer outcomes. Women are more prone to right-sided colon cancer than men, which is associated with more aggressive form of neoplasia compared to left-sided colon cancer. Genetic and epigenetic factors as well as dietary habits play roles in sex-specific differences in colorectal cancer risk. We also suggest that socio-cultural environments partly explain gender-specific differences in colorectal cancer risk. Therefore, sex- and gender-specific strategies for research methods as well as protocols for screening, treatment, and prevention should be established to reduce the morbidity and mortality of colorectal cancer in women.
INTRODUCTION
The Global Health Observatory of the World Health Organization reported that 13% of all deaths originate from cancer. Colorectal cancer is the third most common cancer in the world and is one of the most common causes of female cancer mortality followed by lung and/or breast cancer[1]. Statistics from Korea and Japan indicated that colorectal cancer ranks number one cause of cancer morbidity in women aged more than 65 years old[2,3]. Also, the incidence and mortality of colorectal cancer in populations over 65 years old are higher in women than those in men implying that colorectal cancer is a major health threat among older women[1]. Considering the longer life expectancy of women compared to that of men, gender-targeted strategies to prevent and treat colorectal cancer should be properly delivered to improve the quality of life especially in older women.
Colorectal cancer screening guideline in general does not apply gender-specific recommendations. However, scientists have suggested that right-sided (proximal) colon cancer is more aggressive type tumor compared to left-sided (distal) colon cancer[4], and patients with proximal colon cancer are more often females than males[5]. In advanced colonic neoplasia, proximal colonic tumors are more often flat, while distal colonic tumors are polypoid-type which is more distinguishable by colonoscopy[6] implying an alternative screening suggestion needs to be discussed. Also, women possess a longer transverse colon compared to men posing lower detection rate in colonoscopy[7]. The sensitivity of fecal occult blood test (iFOBT), a most commonly used colorectal cancer screening test, is found to differ by sex[8]. The decreased gender-specificity of screening tools therefore may explain a higher mortality and shorter 5-year survival rate of women in many regions of the world.
Another crucial point that requires gender-specificity is dietary recommendations for cancer prevention. Dietary factors have been suggested to account for 30% of cancer deaths, indicating that dietary guidelines for cancer prevention represent an essential strategy to lower the burden of cancer[9]. Dietary guidelines for cancer prevention are mostly derived from the summary findings of prospective cohort studies, which are less prone to selection or recall bias than case-control studies. Despite gender-specific differences in dietary risk factors associated with cancer risk, the evidences to generate sex-specific summary estimates are limited.
Also, cancer treatment plan needs to consider sex-specific responses towards anti-cancer drugs based on their biological and genetic characteristics. Possible association between socioeconomic circumstances of women and cancer treatment also requires attention. Therefore, in this paper, we present the biological and socio-cultural differences between genders to provide strategies for gender-targeted colorectal cancer screening, treatment, and prevention.
SEX-RELATED BIOLOGICAL DIFFERENCES IN COLORECTAL CANCER RISK
The estimates of colorectal cancer morbidity greatly increase with older ages. The incidence and mortality of colorectal cancer in populations over 65 years old are higher in women than those in men[1]. Also, the 5-year survival rate of colorectal cancer among women is lower than among men, which is particularly noteworthy in women over 70 years old[10]. These evidences imply that colorectal cancer is a major health threat among older women. However, the need for scientific researches on sex- and gender-associated differences in colorectal cancer development has not been properly emphasized.
A recent systemic review reported that a higher proportion of women presents with right-sided colon cancer than men[4]. Right-sided colon cancer is often at a more advanced stage at diagnosis[4]. Therefore, the lower 5-year survival rate in women may be due to their increased incidence of right-sided cancer. A major cohort study involving 17641 patients compared left-sided colon cancer to right-sided colon cancer for clinical and histological characteristics, progress after the operation, and survival[11]. The results revealed a higher incidence of right-sided colon cancer in women and in older subjects. Also, the effect of age was more significant in women[11]. In the same study, patients with right-sided colon cancer exhibited vague symptoms and suffered from more associated diseases. Right-sided colon cancer was more advanced and less differentiated compared to left-sided colon cancer[11]. A recent cohort study has also indicated that the risk of proximal large polyps increased with age, female sex, and black race[12]. Although the effect of tumor location on survival remains uncertain, more information on pathophysiological differences in relation to gender is needed to plan strategies for screening and treatment of colorectal cancer.
Colorectal cancer exhibits different molecular and pathological characteristics depending on tumor location. Differences between right- and left-sided colon cancers are possibly due to differences in genetic makeup, life style, and/or dietary habits (Figure 1). Chromosomal instability, which is associated with 60%-70% of colorectal cancer, is more often observed in left-sided colon cancer, and defective genes include adenomatous polyposis coli, Kirsten-ras, deleted in colorectal cancer, and p53[13-15]. On the other hand, microsatellite instability (MSI)-high, CpG island methylator phenotype (CIMP)-high, and BRAF mutation are often observed in right-sided colon cancer[14,15]. Hereditary non-polyposis colorectal cancer is more likely to develop tumors on the right side of the colon, whereas familial adenomatous polyposis is associated with left-sided colon cancer[16,17]. The common clinical and molecular features of right- and left-sided colon cancers are presented in Figure 1.
Figure 1.
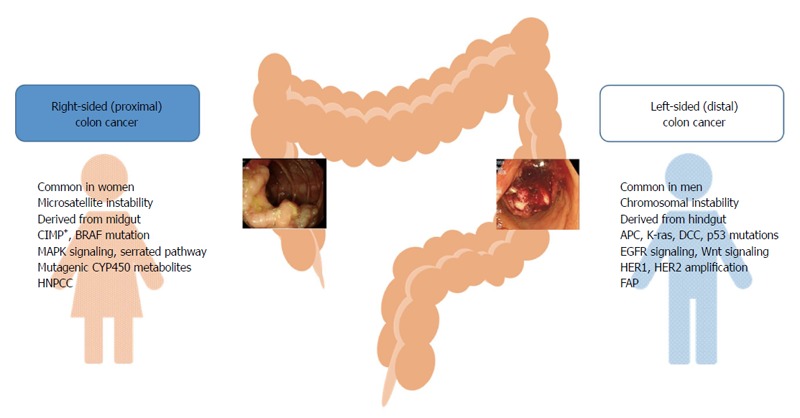
Common clinical and molecular characteristics of right- and left-sided colon tumors. APC: Adenomatous polyposis coli; CIMP: CpG island methylator phenotype; DCC: Deleted in colorectal cancer; FAP: Familial adenomatous polyposis; HNPCC: Hereditary non-polyposis colorectal cancer; K-ras: Kirsten-ras.
Hormonal factors may explain a large percentage of right-sided colorectal cancer in females. A population-based case-control study examining sex, reproductive factors, and hormone exposure related with MSI in colon cancer (n = 4246) suggested that estrogen exposure is a protective factor against MSI, while the lack of estrogen in older women increased the risk of MSI-high colon cancer[18]. In the same study, hormone replacement therapy (HRT) was associated with the reduced risk of unstable tumors[18]. The Women’s Health Initiative Clinical Trial reported that postmenopausal women undergoing HRT showed a 40% reduction in colorectal cancer risk, whereas women undergoing HRT whilst diagnosed with colorectal cancer exhibited a higher grade/stage of colorectal cancer[19]. These results indicate that HRT could have a detrimental effect on colorectal cancer risk after tumor has developed. Taken together, previous and current HRT is likely associated with the decreased risk of colorectal cancer, while chronic endogenous estrogen exposure may be linked to the increased risk of colorectal cancer in postmenopausal women[20,21].
It has been reported that certain genetic and epigenetic differences between sexes may determine colorectal cancer risk. CIMP-high was increased from the rectum to the cecum, with a higher percentage of females developing tumors in the cecum[22]. An earlier study reported that a methylated CpG island in the 5’ region of the p161NK4a tumor suppressor was positively associated with female gender[23]. Another study found that the vascular endothelial growth factor 936 polymorphism increased the risk of colon cancer in women only[24]. In addition, the PIK3CA mutation occurs more frequently in females and in proximal colon cancer, which is associated with poorer survival[25].
However, limited number of preclinical studies used animals of both sexes to investigate the molecular mechanisms of colon cancer development, the responses to environmental stresses, and the responses to treatment. In many cases, male animals were preferably used to eliminate possible interactions between estrogen and tumor formation. Given that right-sided colon cancer, which is associated with poor prognosis, is more common in women than men, it is important to understand sex-related biological factors which affect segment-specific colon tumor formation. To study sex differences, preclinical researches need to use animals from both sexes. Epidemiological studies need to consider biological variables in determining the incidence, mortality, and survival rate of colorectal cancer to produce better screening and treatment protocols.
GENDER-SPECIFIC SCREENING TOOLS AND GUIDELINES FOR COLORECTAL CANCER
Colorectal cancer screening provides effective opportunity to prevent the disease. However, there are no gender-specific screening tools or guidelines. A previous study showed that both black race and females tend to exhibit polyps greater than 9 mm while other races and males exhibit smaller polyps upon colonoscopy[26], suggesting possible sex- and race-specific delays in diagnosis. It has been also reported that screening via flexible sigmoidoscopy can detect polyps or tumors twice as frequently in men than in women[27]. Women felt less pain and discomfort when undergoing thinner flexible sigmoidoscopy[28], suggesting there are needs to consider sex differences in the sensitivity and feasibility towards colorectal cancer screening tools.
It is interesting to note that cumulative 10 year incidence and mortality of colorectal cancer in women at ages 55, 60, and 65 years follow almost identical rates of incidence and mortality of colorectal cancer in men at ages 50, 55, and 60[29], suggesting women exhibit delayed colorectal cancer development. Despite the seriousness of colon cancer in older women, sex-specific anatomical and physiological characteristics in women have made it difficult to detect tumors during screening processes. Women have the longer transverse colon and increased redundancy compare to men causing incomplete colonoscopy in women[7]. Preclinical studies suggested that dietary fiber consumption increases the length of the colon[30,31]. Therefore, higher dietary fiber consumption in women might be related to the longer colon length in women[32,33]. A large cross-sectional study (n = 4910) reported that a larger proportion of women exhibited flat- and depressed-type colorectal neoplasia while a higher percentage of men showed polypoid-type neoplasia which is more easily detectable[6]. Endoscopic observations clearly revealed morphological difference between right-sided colon cancer vs left-sided colon cancer (Figure 2).
Figure 2.
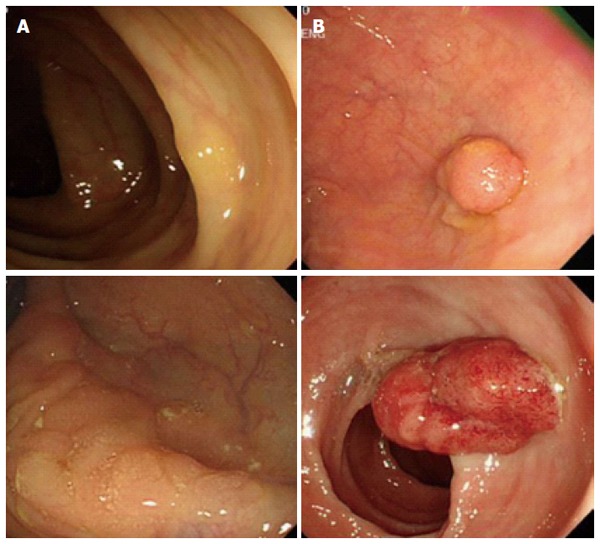
Different endoscopic appearances between right- (A) and left-sided (B) colon cancers.
In addition, among other screening tools, the sensitivity of iFOBT and guaiac FOBT was substantially higher among men than among women[8]. A previous study showed that women who experienced cancer in their reproductive tract tend to develop colorectal cancer more frequently[34]. Lastly, health care disparities between gender and ethnic groups have been presented[35]. Among 1071 surgical colon cancer patients, women (n = 521, 48.6%) had less screening diagnosis (overall: 17.8% vs 22.6%, P = 0.049) with subsequently higher rates of metastatic disease on pathology. These evidences suggest that colorectal cancer screening in women needs more attention in terms of their sensitivity, and gender-specific screening guidelines for colorectal cancer need to be deliberated.
Despite distinctive sexual differences in anatomy and physiology of the colon, gender-specific screening guidelines have not been emphasized. The longer average total and transverse colon length, more frequent occurrence of flat-type right-sided colon cancer and narrower colon diameter of women compared to those of men may cause technical limitation in endoscopic examinations. Therefore, it is necessary to customize endoscopic devices for women. There is no guideline regarding the age to stop colorectal cancer screening in many parts of the world. Asia-Pacific guideline stated that colorectal cancer screening should be continued until 75 years old for both men and women[36]. Due to longer life expectancy in women, colorectal cancer screening in older women needs to be emphasized.
GENDER-SPECIFIC ASSOCIATIONS BETWEEN DIETARY FACTORS AND COLORECTAL CANCER
The Expert Report, “Food, Nutrition, Physical Activity, and the Prevention of Cancer: a global perspective”[37] is the most comprehensive summary of the relationships between diet and cancer based on the analysis of over 7000 scientific studies. As a continuation, the World Cancer Research Fund (WCRF) also launched the Continuous Update Project to rigorously compile the most up-to-date evidence available[38]. We reviewed all of the prospective cohort studies that analyzed cancers of the colorectum, colon or rectum as the major endpoints included in the Continuous Update Project of the WCRF[38] and examined whether the associations between dietary factors and colorectal cancer risk were reported according to gender. When we calculated the proportion of sex-specific estimates among studies that included both women and men, an average of 51.02% of the studies reviewed in this report reported sex-specific estimates, ranging from 33.96% (red/processed, red, or processed meat) to 64.71% (total, dietary and serum/plasma folate) (Figure 3). For alcoholic beverages, 40.00% of the studies reported sex-specific estimates among studies which included both women and men. Given that sociological and cultural aspects of alcohol drinking vary by sex and that the toxic threshold of ethanol may differ by sex, it may be important to provide specific summaries for women and men.
Figure 3.
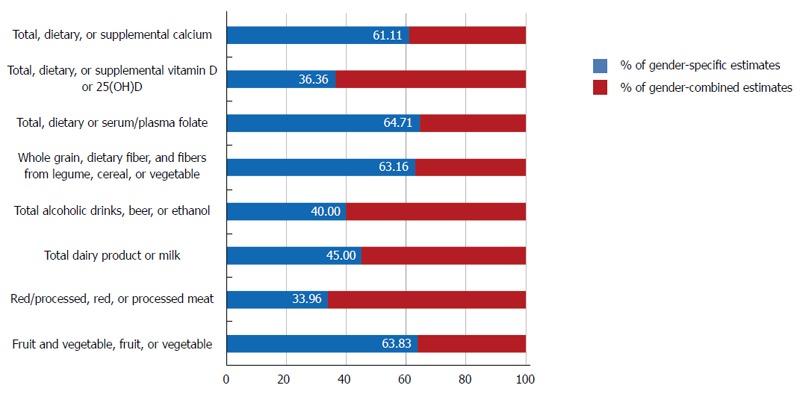
Proportion of sex-specific estimates reported in prospective studies that included both women and men.
Since the 1980s, many female-specific prospective cohort studies (e.g., the Nurses’ Health Study, the Iowa Women’s Health Study, the Black Women’s Health Study, the Breast Cancer Detection Demonstration Project Follow-up Cohort, the California Teachers Study, the New York University Women’s Health Study, the Shanghai Women’s Health Study, and the Sweden Mammography Cohort) have published their findings on diet and cancer prevention. These results have contributed to the accumulation of evidence regarding cancer prevention for women, particularly for cancers that occur primarily in women. However, large studies that included both women and men often did not report the sex-specific estimates. This lack of reported sex-specific estimates precludes meta-analyses of sex-specific estimates, which would have a greater statistic power and provide evidences for dietary guidelines for cancer prevention.
Studies have reported that dietary factors are associated differently with colorectal cancer depending on the location of tumors. High carbohydrate intake increased right-sided colon cancer in women, but increased rectal cancer in men[39]. High fat and protein intakes increased risks of right- and left-sided colon cancers, respectively[40,41]. Recent evidence from a large Canadian population-based case-control study suggested that high intake of polyunsaturated fat, trans-fat, cholesterol, sucrose, and lactose was associated with the increased risk of right-sided colon cancer[42]. In addition, meat consumption increased the risk of left-sided colon cancer compared to right-sided colon cancer[43-45], whereas total iron and iron from supplements were inversely associated with distal colon cancer[45]. Also, high calcium intake[46-48] and serum/plasma 25-hydroxyvitamin D level[49] were inversely associated with distal colon cancer.
Consumption of soy products containing phytoestrogens has been shown to be inversely associated with the risk of colorectal cancer[50-53]. A recent meta-analysis found that soy consumption was associated with an approximately 21% reduction in colorectal cancer risk only in women, presumably due to the structural and metabolic similarities of soy isoflavones to estrogen[50]. This result supports a previous report suggesting soy consumption differentially affects estrogen metabolism depending on the endogenous estrogen level[54]. Indeed, an experimental study reported that high phytoestrogens intake increases ER-α expression, decreases apoptosis, and induces inflammation markers in colonic mucosa of female mice possibly due to the high estrogenic background[55].
The International Agency for Research on Cancer (IARC), based on the IARC online database GLOBOCAN 2012, estimated a substantial increase to 19.3 million new cancer cases per year by 2025[1]. Given the biological and socio-cultural differences between genders, gender-specific analyses should be conducted to provide optimal cancer prevention strategies and to reduce the number of new colorectal cancer cases both in men and women. Large population-based cohort studies need to report sex-specific estimates of dietary risk factors to provide better guidelines for cancer preventive dietary intake.
SEX- AND GENDER-SPECIFIC DIFFERENCES IN COLORECTAL CANCER TREATMENT
Clinical studies have suggested that colorectal cancer treatment in premenopausal women needs attention due to its possible effects on female fertility. A retrospective study reported that 41% of women receiving adjuvant 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX) chemotherapy experienced amenorrhea during chemotherapy, and 16% exhibited persistent amenorrhea 1 year after the completion of chemotherapy, which may affect early menopause and fertility[56]. In addition to chemotherapy, surgical and radiation therapies also need to be considered for female fertility preservation[57].
Furthermore, gender-specific recurrence and survival rates were detected. The genotype of the TP53 tumor suppressor gene was predictive of survival following adjuvant chemotherapy in women with stage III colon cancer[58]. In stage II and III colorectal cancer patients, polymorphisms in PLS3 and LCP1 were associated with tumor recurrence in women with proximal colorectal cancer[59]. Therefore, it is necessary to use a distinct evaluation protocol for the response of women to colorectal cancer treatment.
Taken together, research efforts towards the development of anti-cancer drugs displaying less toxicity to the reproductive system are required. Treatment protocols specifically recommended for women of child-bearing age should be suggested based on sound scientific evidence. A sex-specific decrease in the survival rate of women subjected to a specific anti-cancer drug may be associated with the genetic background. Thus, a long-term follow-up study of cancer survivors needs to be considered to ensure the safety of specific anti-cancer drug use with respect to not only reproductive function but also possible genetic effects on subsequent generations. Also, possible gender-specific barriers to cancer treatment need to be studied. This is specifically important because optimal anti-cancer drug regimen for colorectal cancer should be required based on the effect of sex on drug efficacy and toxicity. Among 1785 colon cancer patients aged more than 65 years old, women were less likely to receive 5-fluorouracil treatment and showed a shorter duration of treatment compared to men, which were possibly associated with the observation that women were more prone to dehydration than men[60]. Indeed, women experienced more severe toxicity including stomatitis, leukopenia, alopecia, and diarrhea compared to men when receiving 5-fluorouracil-based treatment[61].
It is recommended to complete all planned chemotherapy cycles to improve disease-free survival. However, recent studies reported that stage III female colon cancer patients tend to omit adjunctive chemotherapy sessions compared to male counterparts[62-64]. Also, a greater percentage of elderly female patients and female patients with a prolonged hospital stay exhibited a higher rate of discontinuation[62]. Therefore, further researches are needed to provide evidence on gender-specific barriers to colorectal cancer treatment and establish optimal anti-cancer drug regimen by gender.
CONCLUSION
Clinical and preclinical studies have indicated that there are sex- and gender-associated differences in colorectal cancer development. Both genetic and environmental factors are believed to play roles in sex and gender differences in right- vs left-sided colon cancers. Therefore, biological and pathophysiological differences in colorectal cancer development between men and women need to be clearly addressed. Despite higher incidence of right-sided colon cancer in women, a substantially higher number of preclinical studies use only male animals in colorectal cancer research. Researchers should be aware of sex-specific pathophysiological differences in colorectal cancer development and use both male and female animals for their research. In addition, there is a great deal of needs for developing gender-specific endoscopy devices with higher sensitivity due to sex-specific differences in biological and anatomic characteristics of the colon. Colorectal cancer screening guidelines may need to emphasize gender-specific points for colorectal cancer screening.
Diet is one of the most closely associated environmental factors in colorectal cancer development. Dietary factors to increase or decrease the risk of developing colorectal cancer are continuously updated based on large scale cohort studies. However, only a half of studies reported sex-specific risk estimates despite potential sex-associated differences between dietary factors and colorectal cancer risk. Given that there are sex- and gender-specific differences in the biological responses to dietary components, it is necessary to analyze and report gender-specific risk estimates to provide better guidelines for cancer prevention strategies. Furthermore, researches addressing sex-specific differences in responses to anti-cancer drugs for colorectal cancer are required to reduce side-effects on reproductive system and to investigate genetic effects on drug efficacy. By understanding sex- and gender-related biological and socio-cultural differences in colorectal cancer risk, gender-specific strategies for screening, treatment, and prevention protocols for colorectal cancer can be established to reduce the mortality and increase the quality of life.
Footnotes
Supported by Mid-Career Research Program, No. 2012R1A2A2A01046228 of the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science, and Technology as well as the Gendered Innovations in Science and Engineering; No. 350-20130047 of the Center for Women in Science, Engineering and Technology (WISET) of Korea funded by the Seoul National University Research and Development Business Foundation.
Conflict-of-interest: The authors have no conflict-of-interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 6, 2015
First decision: February 10, 2015
Article in press: March 31, 2015
P- Reviewer: Chiacchiera F, Divella R, Syed V S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray , F . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388–396. doi: 10.1093/jjco/hyu003. [DOI] [PubMed] [Google Scholar]
- 4.Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59:A4444. [PubMed] [Google Scholar]
- 5.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 6.Kaku E, Oda Y, Murakami Y, Goto H, Tanaka T, Hasuda K, Yasunaga M, Ito K, Sakurai K, Fujimori T, et al. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2011;9:503–508. doi: 10.1016/j.cgh.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Saunders BP, Fukumoto M, Halligan S, Jobling C, Moussa ME, Bartram CI, Williams CB. Why is colonoscopy more difficult in women? Gastrointest Endosc. 1996;43:124–126. doi: 10.1016/s0016-5107(06)80113-6. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. Am J Gastroenterol. 2010;105:2457–2464. doi: 10.1038/ajg.2010.301. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 10.Park HC, Shin A, Kim BW, Jung KW, Won YJ, Oh JH, Jeong SY, Yu CS, Lee BH. Data on the characteristics and the survival of korean patients with colorectal cancer from the Korea central cancer registry. Ann Coloproctol. 2013;29:144–149. doi: 10.3393/ac.2013.29.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, Carney P. Race, ethnicity, and sex affect risk for polyps & gt; 9 mm in average-risk individuals. Gastroenterology. 2014;147:351–358; quiz e14-15. doi: 10.1053/j.gastro.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs ET, Thompson PA, Martínez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731–746. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 15.Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 16.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 17.Lynch HT, Watson P, Lanspa SJ, Marcus J, Smyrk T, Fitzgibbons RJ, Kriegler M, Lynch JF. Natural history of colorectal cancer in hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II) Dis Colon Rectum. 1988;31:439–444. doi: 10.1007/BF02552613. [DOI] [PubMed] [Google Scholar]
- 18.Slattery ML, Potter JD, Curtin K, Edwards S, Ma KN, Anderson K, Schaffer D, Samowitz WS. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61:126–130. [PubMed] [Google Scholar]
- 19.Ritenbaugh C, Stanford JL, Wu L, Shikany JM, Schoen RE, Stefanick ML, Taylor V, Garland C, Frank G, Lane D, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: the Women’s Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2008;17:2609–2618. doi: 10.1158/1055-9965.EPI-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster PA. Oestrogen and colorectal cancer: mechanisms and controversies. Int J Colorectal Dis. 2013;28:737–749. doi: 10.1007/s00384-012-1628-y. [DOI] [PubMed] [Google Scholar]
- 21.Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130:419–430. doi: 10.1002/ijc.26026. [DOI] [PubMed] [Google Scholar]
- 22.Bae JM, Kim JH, Cho NY, Kim TY, Kang GH. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109:1004–1012. doi: 10.1038/bjc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiencke JK, Zheng S, Lafuente A, Lafuente MJ, Grudzen C, Wrensch MR, Miike R, Ballesta A, Trias M. Aberrant methylation of p16INK4a in anatomic and gender-specific subtypes of sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:501–506. [PubMed] [Google Scholar]
- 24.Bae SJ, Kim JW, Kang H, Hwang SG, Oh D, Kim NK. Gender-specific association between polymorphism of vascular endothelial growth factor (VEGF 936 C>T) gene and colon cancer in Korea. Anticancer Res. 2008;28:1271–1276. [PubMed] [Google Scholar]
- 25.Phipps AI, Makar KW, Newcomb PA. Descriptive profile of PIK3CA-mutated colorectal cancer in postmenopausal women. Int J Colorectal Dis. 2013;28:1637–1642. doi: 10.1007/s00384-013-1715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3:798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 27.Eloubeidi MA, Wallace MB, Desmond R, Farraye FA. Female gender and other factors predictive of a limited screening flexible sigmoidoscopy examination for colorectal cancer. Am J Gastroenterol. 2003;98:1634–1639. doi: 10.1111/j.1572-0241.2003.07480.x. [DOI] [PubMed] [Google Scholar]
- 28.Farraye FA, Horton K, Hersey H, Trnka Y, Heeren T, Provenzale D. Screening flexible sigmoidoscopy using an upper endoscope is better tolerated by women. Am J Gastroenterol. 2004;99:1074–1080. doi: 10.1111/j.1572-0241.2004.30215.x. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–831. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullogh JS, Ratcliffe B, Mandir N, Carr KE, Goodlad RA. Dietary fibre and intestinal microflora: effects on intestinal morphometry and crypt branching. Gut. 1998;42:799–806. doi: 10.1136/gut.42.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark A, Nyska A, Madar Z. Metabolic and morphometric changes in small and large intestine in rats fed high-fiber diets. Toxicol Pathol. 1996;24:166–171. doi: 10.1177/019262339602400204. [DOI] [PubMed] [Google Scholar]
- 32.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27:107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- 33.Westenhoefer J. Age and gender dependent profile of food choice. Forum Nutr. 2005;(57):44–51. doi: 10.1159/000083753. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg DS, Newschaffer CJ, Topham A. Risk for colorectal cancer after gynecologic cancer. Ann Intern Med. 1999;131:189–193. doi: 10.7326/0003-4819-131-3-199908030-00005. [DOI] [PubMed] [Google Scholar]
- 35.Amri R, Stronks K, Bordeianou LG, Sylla P, Berger DL. Gender and ethnic disparities in colon cancer presentation and outcomes in a US universal health care setting. J Surg Oncol. 2014;109:645–651. doi: 10.1002/jso.23567. [DOI] [PubMed] [Google Scholar]
- 36.Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T, Ng SS, Lau JY, Zheng S, Adler S, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64:121–132. doi: 10.1136/gutjnl-2013-306503. [DOI] [PubMed] [Google Scholar]
- 37.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 38.World Cancer Research Fund, American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. Washington, DC: American Institute for Cancer Research; 2011. [Google Scholar]
- 39.Borugian MJ, Sheps SB, Whittemore AS, Wu AH, Potter JD, Gallagher RP. Carbohydrates and colorectal cancer risk among Chinese in North America. Cancer Epidemiol Biomarkers Prev. 2002;11:187–193. [PubMed] [Google Scholar]
- 40.McMichael AJ, Potter JD. Diet and colon cancer: integration of the descriptive, analytic, and metabolic epidemiology. Natl Cancer Inst Monogr. 1985;69:223–228. [PubMed] [Google Scholar]
- 41.West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW, Lyon JL, Sorensen AW. Dietary intake and colon cancer: sex- and anatomic site-specific associations. Am J Epidemiol. 1989;130:883–894. doi: 10.1093/oxfordjournals.aje.a115421. [DOI] [PubMed] [Google Scholar]
- 42.Hu J, La Vecchia C, Negri E, Mery L. Nutrients and risk of colon cancer. Cancers (Basel) 2010;2:51–67. doi: 10.3390/cancers2010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hjartåker A, Aagnes B, Robsahm TE, Langseth H, Bray F, Larsen IK. Subsite-specific dietary risk factors for colorectal cancer: a review of cohort studies. J Oncol. 2013;2013:703854. doi: 10.1155/2013/703854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. Int J Cancer. 2005;113:829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 45.Ferrucci LM, Sinha R, Huang WY, Berndt SI, Katki HA, Schoen RE, Hayes RB, Cross AJ. Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer. 2012;106:608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stemmermann GN, Nomura A, Chyou PH. The influence of dairy and nondairy calcium on subsite large-bowel cancer risk. Dis Colon Rectum. 1990;33:190–194. doi: 10.1007/BF02134177. [DOI] [PubMed] [Google Scholar]
- 47.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94:437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 48.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165:1178–1186. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 49.Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Riboli E, Hercberg S, Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 50.Yan L, Spitznagel EL, Bosland MC. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:148–158. doi: 10.1158/1055-9965.EPI-09-0856. [DOI] [PubMed] [Google Scholar]
- 51.Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, Gao YT, Zheng W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89:577–583. doi: 10.3945/ajcn.2008.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–3053. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budhathoki S, Joshi AM, Ohnaka K, Yin G, Toyomura K, Kono S, Mibu R, Tanaka M, Kakeji Y, Maehara Y, et al. Soy food and isoflavone intake and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Scand J Gastroenterol. 2011;46:165–172. doi: 10.3109/00365521.2010.522720. [DOI] [PubMed] [Google Scholar]
- 54.Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:781–786. [PubMed] [Google Scholar]
- 55.Bises G, Bajna E, Manhardt T, Gerdenitsch W, Kallay E, Cross HS. Gender-specific modulation of markers for premalignancy by nutritional soy and calcium in the mouse colon. J Nutr. 2007;137:211S–215S. doi: 10.1093/jn/137.1.211S. [DOI] [PubMed] [Google Scholar]
- 56.Cercek A, Siegel CL, Capanu M, Reidy-Lagunes D, Saltz LB. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer. 2013;12:163–167. doi: 10.1016/j.clcc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 57.O'Neill MT, Ni Dhonnchu T, Brannigan AE. Topic update: effects of colorectal cancer treatments on female fertility and potential methods for fertility preservation. Dis Colon Rectum. 2011;54:363–369. doi: 10.1007/DCR.0b013e31820240b3. [DOI] [PubMed] [Google Scholar]
- 58.Warren RS, Atreya CE, Niedzwiecki D, Weinberg VK, Donner DB, Mayer RJ, Goldberg RM, Compton CC, Zuraek MB, Ye C, et al. Association of TP53 mutational status and gender with survival after adjuvant treatment for stage III colon cancer: results of CALGB 89803. Clin Cancer Res. 2013;19:5777–5787. doi: 10.1158/1078-0432.CCR-13-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ning Y, Gerger A, Zhang W, Hanna DL, Yang D, Winder T, Wakatsuki T, Labonte MJ, Stintzing S, Volz N, et al. Plastin polymorphisms predict gender- and stage-specific colon cancer recurrence after adjuvant chemotherapy. Mol Cancer Ther. 2014;13:528–539. doi: 10.1158/1535-7163.MCT-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliver JS, Martin MY, Richardson L, Kim Y, Pisu M. Gender differences in colon cancer treatment. J Womens Health (Larchmt) 2013;22:344–351. doi: 10.1089/jwh.2012.3988. [DOI] [PubMed] [Google Scholar]
- 61.Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, Novotny PJ, Poon MA, O’Connell MJ, Loprinzi CL. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20:1491–1498. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 62.van der Geest LG, Portielje JE, Wouters MW, Weijl NI, Tanis BC, Tollenaar RA, Struikmans H, Nortier JW. Complicated postoperative recovery increases omission, delay and discontinuation of adjuvant chemotherapy in patients with Stage III colon cancer. Colorectal Dis. 2013;15:e582–e591. doi: 10.1111/codi.12288. [DOI] [PubMed] [Google Scholar]
- 63.Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–1991. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 64.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]


