Abstract
A tremendous amount of data from research was published over the past decades concerning the roles of different vitamins in various gastrointestinal diseases. For instance, most vitamins showed an inverse relationship with the risk of colorectal carcinoma as well as other malignancies like gastric and esophageal cancer in observational trials, however interventional trials failed to prove a clear beneficial preventive role. On the other hand, more solid evidence was obtained from high quality studies for a role of certain vitamins in specific entities. Examples for this include the therapeutic role of vitamin E in patients with non-alcoholic steatohepatitis, the additive role of vitamins B12 and D to the standard therapy of chronic hepatitis C virus, the role of vitamin C in reducing the risk of gallstones, the positive outcome with vitamin B12 in patients with aphthous stomatitis, and the beneficial effect of vitamin D and B1 in patients with inflammatory bowel disease. Other potential uses are yet to be elaborated, like those on celiac disease, pancreatic cancer, pancreatitis, cholestasis and other potential fields. Data from several ongoing interventional trials are expected to add to the current knowledge over the coming few years. Given that vitamin supplementation is psychologically accepted by patients as a natural compound with relative safety and low cost, their use should be encouraged in the fields where positive data are available.
Keywords: Antioxidants, Colon cancer, Steatohepatitis, Alternative medicine, Supplements
Core tip: This extensive review provides a unique approach to the topic of vitamins and their use in gastrointestinal diseases. A lot of manuscripts exist about the subject, but an article specific to the practice of gastroenterology that summarises the use and benefits of vitamins is absent. While tremendous amounts of money (estimated to 13.1 billion USD in 2012) are being spent on these products, selecting the appropriate uses of these supplements remains an important issue to avoid unnecessary health expenditures.
INTRODUCTION
During the past four decades, emerging nutritional research on the efficacy of a complete diet and its role in disease prevention led to substantial pharmaceutical investments in the field of vitamins and dietary supplements[1]. Pharmaceutical companies focused on procuring nutrients that were deemed deficient in diets. Additionally, a number of reports mentioned lucrative numbers about the wider scale effect of those supplements in decreasing healthcare costs and expenses. A global, “perfect” diet with all the required components was estimated to save a yearly amount ranging between 21 and 43 billion USD of healthcare costs[2-5].
As a result of data encouraging the use of nutritional additives, there was a worldwide trend of increasing consumption of these supplements since their introduction to the market. For instance, between 1988 and 1994, 40% of the adult population in the United States used vitamins and supplements. A more recent report in 2011 from the CDC shows an increase in this rate to up to 50% with multivitamins/multi-minerals being the most commonly purchased category[6]. In 2012, a total of 13.1 billion USD was spent on multivitamin and mineral-containing supplements, of which an estimated 5.4 billion USD (40%) was spent on multi-vitamins alone[7].
Similar to its growth in the United States, the market of vitamins and dietary supplements was flourishing in Europe. The market value of the industry was of 7 billion Euros in 2009, 50% of which being the worth of vitamins and mineral products[8]. The global vitamin and supplement market was valued at 68 billion USD by Euromonitor in 2013. Inconsistent growth rates were observed among the fields’ categories, where certain subsidiaries like probiotics, fish-oils and anti-oxidants are witnessing more growth than others. Nonetheless, the vitamins and minerals market alone is expected to have a 4.5% growth in 2015[9]. The United States remains the world leader in the pharmaceutical field of vitamins and dietary supplements, having an estimated 30% market share worth 20 billion USD, followed by Japan with a 22% share. Europe and China follow with 14% and 12% respectively but it is expected that China will overtake both Europe and Japan in the coming years due to its expanding markets and unique under-demand products like Chinese herbal medicines[10].
A number of reasons lie behind the upsurge in usage of these vitamins. The notions of “gaining energy,” “improving,” and “maintaining” health were the most commonly reported motifs[11,12]. Though data from Europe are lacking, a study from the United States shows that increased age, higher economic status (reflected by insurance status), and the assumption of a healthy lifestyle, were all associated with a higher use of supplements. For instance, prevalence of supplement use shifts from 34% in people aged 20 to 39, to more than 65% in the elderly. A similar rise is observed in the insured (53% vs 31% in the non-insured), and in people who exercise frequently (56% vs 43% in individuals who do not exercise)[11]. Though no evidence fully supports the routine use of vitamins, a large percentage of people would regularly use those supplements despite their financial burden and the lack of guaranteed efficacy. In a study conducted on 900 US military subjects, two thirds of participants were confident about supplement efficacy and safety[13]. In another study, only 25% of participants said they would abandon the use of supplements if scientific data showed they had no significant benefit[12].
The vitamin market remains one of the quick growing pharmaceutical fields due to the ease of access to its products as most, if not all, can be obtained over the counter. In light of the controversies regarding the effectiveness of vitamins and supplements when used as daily additive doses, further investigations are needed to validate the value of the colossal amounts of money invested on such products.
Vitamins are chemically unrelated families of organic compounds that are essential as vital nutrients in limited amounts for normal metabolism. With the exception of vitamin D, these vitamins cannot be synthesized in humans and thus need to be taken through diet. Over the past decade, a remarkable amount of research has been dedicated to investigate the role of vitamins in various diseases including their potential use in the prevention or treatment of different malignant tumors, gastrointestinal inflammatory diseases, and hepatobiliary disorders. The majority of these trials were observational, however a respectable number of well-designed interventional trials have emerged recently.
Given the vast literature to be covered, this review will mainly focus on high-quality studies, concentrating in particular on interventional and randomized controlled trials (RCT) of clinical relevance that address the potential preventive and therapeutic role of dietary or supplementary vitamins in various gastrointestinal diseases. Table 1 summarizes the major characteristics of the known vitamins, including their recommended daily doses, major dietary sources and known deficiency syndromes.
Table 1.
Major characteristics of the known vitamins
| Vitamin | Recommended daily doses1 | Major dietary sources | Deficiency syndromes | Tolerable daily upper intake levels |
| A | M: 900 μg | Orange, ripe yellow fruits, leafy vegetables, carrots, pumpkin, liver, soy milk, milk, sweet potatoes, butter, egg yolk | Night-blindness | 3000 mcg |
| (Retinol, Carotenoids) | F: 700 μg | Hyperkeratosis, keratomalacia | ||
| B1 (Thiamine) | M: 1.2 mg | Whole grains, dried beans, oatmeal, brown rice, potatoes, pork, liver | Beriberi - dry (peripheral neuropathy) or wet (heart failure), Wernicke encephalopathy | ND |
| F: 1.1 mg | ||||
| B2 (Riboflavin) | M: 1.3 mg | Dairy products, bananas, popcorn, green beans, asparagus, dark green leafy vegetables | Rare | ND |
| F: 1.1 mg | Non-specific symptoms | |||
| B3 (Niacin, Nicotinic acid) | M: 16.0 mg | Meat, fish, eggs, mushrooms, tree nuts, peas, bran | Pellagra | 35 mg |
| F: 14.0 mg | ||||
| B5 (Pantothenic acid) | 5.0 mg | Fortified cereals, Salmon, Meat, broccoli, avocados | Paresthesia, “burning feet”, GI symptoms | ND |
| B6 (Pyridoxine) | 1.3-1.7 mg | Meat, liver, tree nuts, bananas, salmon, tuna, brown rice, potatoes | Anemia, peripheral neuropathy, insomnia, seborrheic dermatitis, stomatitis | 100 mg |
| B7 (Biotin) | 30.0 μg | Raw egg yolk, liver, peanuts, certain vegetables | Dermatitis, enteritis | ND |
| B9 (Folic acid) | 400 μg | Spinach, enriched rice, fortified cereal, liver, avocado, lentils | Megaloblastic anemia | 1000 mcg |
| Neural tube defects | ||||
| B12 (Cobolamine) | 2.4 μg | Meat, salmon, egg yolk, lentils, spinach | Megaloblastic/Pernicious anemia | ND |
| C (Ascorbic acid) | M: 90 mg | Citric fruits, papaya, broccoli, strawberries, paprika, liver | Scurvy | 2000 mg |
| F: 75 mg | ||||
| D (Cholecalciferol, Ergocalciferol) | 15 μg | liver, mushrooms, fatty fish, milk, egg yolk, fortified cereals | Rickets and osteomalacia | 100 mcg (4000 IU) |
| (> 70 yr: 20 μg) | 1 cup of milk = 50 IU; 30 min of sunlight = 10000 IU | |||
| E (Tocopherols) | 15.0 mg | Sunflower seeds, almonds, fortified cereals, spinach, turnip greens | Very rare; mild hemolytic anemia, ataxia, neural degeneration | 1000 mg |
| K (phylloquinone, menaquinones) | M: 120 μg | Leafy green vegetables, egg yolks, liver, mustard greens, asparagus, kiwi, dried prunes | Bleeding diathesis | ND |
| F: 90 μg |
In adults, not pregnant nor lactating. ND: Not determinable.
VITAMIN A
Vitamin A and inflammatory bowel disease
Vitamin A and retinoic acids are required for the development of proper immunity to pathogens by promoting immunoglobulin A response and phagocytic functions. Trials on animal models have shown a possible beneficial role of high vitamin A intake by inducing the highly suppressive FoxP3(+) regulatory T-cell subsets and thus ameliorating or even reversing intestinal inflammation[14]. Vitamin A levels were found to be low in most patients with inflammatory bowel disease (IBD), however no clear correlation with severity or activity could be identified[15]. A prospective European Cohort study involving 139 patients with ulcerative colitis (UC) found no association between diet, namely vitamins A, C, D and E intake and UC incidence or severity[16]. No therapeutic trials are available.
Antioxidants (vitamins A, C, E) and CRC
Antioxidants have been proposed as potential chemopreventive agents because of their roles in quenching free radicals and reducing oxidative damage to DNA[17]. The major antioxidants are vitamins A, C, E, Beta-carotene and Selenium. Data from retrospective and prospective cohort studies were highly inconsistent and controversial. A meta-analysis of 13 observational studies showed an inverse association (SRRs 0.47, 95%CI: 0.24-0.91) between the intake of beta-carotene in diet and the risk of colorectal adenomas. A similar but milder (22% SRR) beneficial role was noted with vitamin C, whereas no association was noted with the intake of vitamins A and E[18].
Multiple large high quality RCTs[19-26] were conducted to examine the effectiveness of these elements for primary or secondary prevention of various gastrointestinal malignancies in the general population. Variable regimens were given for periods ranging between 4 and 12 years and patients were followed for up to 12 years; However the results were mostly disappointing[27-30]. A Cochrane systematic review[28] that included 20 RCTs addressing this specific relation showed that antioxidant supplements had no significant preventive effects on gastrointestinal cancers (RR = 0.94, 95%CI: 0.83-1.06). A similar conclusion was obtained by a more recent Cochrane review[29] regarding the effect of antioxidant supplementation on mortality with various diseases which included 78 RCTs with 296707 participants and a mean follow up duration of 3 years. Moreover, when considering the 56 trials with low risk of bias, the authors showed that the use of antioxidant supplementation not only was not protective, but also seemed to significantly increase mortality (12.9% vs 10.6%; RR = 1.04, 95%CI: 1.01-1.07). When using trial sequential analysis and after excluding factorial trials with potential confounders, this significant increase in mortality was specifically demonstrated with the supplementation of beta-carotene, vitamin E and higher doses of vitamin A. The potential effects of vitamin C and selenium supplementation on increased mortality need further study[30].
Vitamin A and non-alcoholic steatohepatitis
A clinical trial by Bahcecioglu et al[31] on 29 patients with biopsy-proven non-alcoholic steatohepatitis (NASH) found no association between serum levels of vitamin A and the histopathologic severity of the disease.
Vitamin A and hepatitis C virus
More than 90% of total body vitamin A is stored in the liver. Moreover, reactive oxygen species (ROS) have been reported to activate hepatic stellate cells, which then lead to hepatic fibrosis as well as disease progression in patients with hepatitis C virus (HCV).
Vitamin A deficiency was found to be common in patients with HCV compared to healthy controls (42%-54% vs 0%)[32-35]. Prevalence and severity of vitamin A deficiency were higher with liver disease progression. Epidemiological evidence and case-control studies showed that low serum retinol levels may correlate with the severity as well as the risk of developing cirrhosis or progressing to HCC in patients with HCV or other chronic liver diseases[33,36-39].
Recent data suggest that vitamin A modulates the expression of type-1 interferon-receptor, enhancing the anti-replication effect of interferon-α on HCV[40]. Bitetto et al[34] showed that on multivariate analysis, severe vitamin A deficiency (≤ 100 ng/mL) was one of the predictors for non-response to antiviral therapy.
In a pilot study of a cohort of 20 previous non-responders with HCV chronic infection, all-trans retinoic acid (ATRA), an analog of vitamin A, demonstrated a direct antiviral and a strong additive or synergistic effect with pegylated IFN. Monotherapy with ATRA for 12 wk induced a viral decay by > 1 log10 in 5 out of 10 patients, and the combination with peg-IFN after 12 wk of treatment led to a transient viral clearance in 3 out of 10 patients in this difficult-to-treat group[41]. This was contradicted recently by Schuchmann et al[42] in an open label randomized trial on 57 previously non-responders showing no clinical benefit of adding tretinoin (45 mg/m2 per day) to standard therapy.
Vitamin A and the pancreas
Patients with chronic pancreatitis are at risk of deficiencies in the fat-soluble vitamins due to the loss of pancreatic exocrine function. A prospective study showed that 3%, 53%, 10%, and 63% of patients with chronic pancreatitis were deficient in vitamin A, D, E and K respectively[43]. Multiple clinical trials suggested that the use of a combined preparation of antioxidants, (including beta carotene, vitamins C and K), in patients with painful chronic pancreatitis significantly reduced pain and improved quality of life when compared to placebo[44-48]. The largest of these trials was a RCT conducted in India including 127 patients receiving combined antioxidant therapy for 6 mo[48]. The active treatment group showed a significant decrease in the number of painful days (7.4 d vs 3.2 d) and the need for analgesics. However, a recent RCT using the same preparation of antioxidants for 6 mo in a population of 70 difficult-to-treat patients (failing traditional therapy, requiring high doses of narcotics and most continuing alcohol intake) failed to show any benefit despite the documented increase in serum levels of vitamin A, C and E[49].
No beneficial effect was noted in clinical trials when vitamin A or other antioxidants were given for treating acute pancreatitis[50], or when given to patients to reduce the risk of post-ERCP pancreatitis[51,52], or when given in combination with gemcitabine in patients with advanced pancreatic cancer[53].
VITAMIN B COMPLEX
Vitamin B12 and HCV
Liver is the physiological reservoir of cyanocobalamin in humans. Vitamin B12 deficiency was observed in several liver diseases like hepatitis, cirrhosis and HCC. In vitro studies in the early 2000s, reported that vitamin B12 inhibits HCV via internal ribosome entry-site inhibition[54,55].
A retrospective study by Rosenberg et al[56] showed that low pre-treatment serum B12 levels were associated with nonresponse to standard therapy in 99 treatment-naïve patients. Patients with pre-treatment B12 level < 360 pm had a null response rate of 31.5% and end-of-treatment response (ETR) rate of 68.5% as compared to 3.8% and 96% respectively in patients with levels above 360 pm. However the difference was no longer significant when the endpoint of sustained virologic response (SVR) was considered.
The only RCT to study the role of B12 supplementation on HCV treatment is a recent open label trial conducted in Italy that included 94 treatment-naive chronic HCV patients[57]. The majority of patients had genotype 1b (62%). Adding vitamin B12 (5000 mcg IM every 4 wk) to standard therapy (pegylated-INF and ribavirin) significantly increased the chance for complete EVR (64% vs 85%), ETR (63% vs 83%) and SVR (38% vs 72%, P < 0.001). This difference persisted when analyzing the subcategory of difficult-to-treat patients with genotype 1, where the SVR was 22% vs 63%[57].
Vitamin B12 and aphthous stomatitis
A possible association between recurrent aphthous stomatitis and vitamin B12 deficiency was suggested as early as the 1950s[58]. Multiple small trials[59-61] suggested a beneficial role of vitamin B12 in these patients, however the first double blind RCT was conducted in 2009 on 58 patients with recurrent aphthous stomatitis, randomized to receive 1000 mcg of sublingual vitamin B12 or placebo. After 6 mo of follow-up, 74.1% of participants in the intervention group reached “no aphthous ulcers status” as compared to 32% in the placebo group. The duration of outbreaks, the number of ulcers, and the level of pain were found to be significantly reduced[62].
Vitamin B and IBD
Vitamin B12: Crohn’s disease can commonly involve the terminal ileum, which is the site of B12 absorption. Data from observational studies are conflicting, however, a recent systematic review including 42 articles concluded that patients with IBD (UC or CD without ileal resection), regardless of disease location in the ileum, did not have an increased risk for Cobalamin deficiency. Only ileal resections greater than 20 cm predispose to deficiency and warrant treatment[63].
Vitamin B1: Thiamine supplementation was used in a small pilot study on 12 IBD patients with normal thiamine levels and severe fatigue as measured by chronic fatigue syndrome scale. At 20 d of therapy, all patients had complete or near complete regression of fatigue syndrome[64].
Folate: Folate deficiency was found to be associated with inflammatory bowel disease, however this did not correlate with disease extent or activity[65]. Two early case-control studies suggested that folate supplementation and a high red blood cell folate level significantly decrease the risk of dysplasia and neoplasia in patients with UC[66,67]. A later meta-analysis in 2003 showed that both sulfasalazine and folate supplementation have a protective effect on CRC development in patients with longstanding UC[68]. However, more recent studies have failed to find a chemopreventive role of folic acid supplementation[69,70]. It is important to remember that folate deficiency can occur as a consequence of IBD therapy, as with the use of methotrexate and sulfasalazine, and should be supplemented accordingly.
Vitamin B2: In a prospective trial including 24 patients with CD compared to healthy controls, the serum levels of several vitamins (including vitamins A, E, B1, B2, B6 and B9) were found to be significantly more depleted in the affected individuals. However, only vitamin B2 and nicotinic acid deficiencies were shown to have a negative correlation with the Crohn’s disease activity index[71]. No interventional trials are available.
Vitamin B5: Pantothenic acid (B3) rectal enemas have been tried as a local therapy for UC in a pilot study with no clear benefit[72].
Vitamin B6: Pyridoxine deficiency is relatively common in IBD affecting 10%-15% of patients in general and up to 25% of patients with active disease[73]. Low B6 plasma level may be considered a risk factor for thrombosis in patients with IBD due to its inverse relation with homocysteine.
Vitamin B and GI malignancies
B vitamins, including folate, riboflavin, pyridoxine, and cobalamin, are essential for methylation reactions, nucleotide synthesis, and DNA stability and repair[74]. In 1998, The FDA mandated folic acid fortification of enriched cereal-grain products, which led to a new dietary source of folate in addition to other available sources and resultant higher blood-folate concentrations.
Because folate has tumor growth-promoting effects, concerns regarding potential risks associated with high folate intake in the post-fortification era have been raised especially with the temporary increase in CRC incidence rates in the later 1990s. However this is unlikely due to folic acid fortification and, assuming a time lag of at least 10 years to have a benefit on CRC[75], folate appears to be one of the promising factors that could explain the current exceptional downward trend of CRC incidence in the United States[76]. Moreover it was suggested that folate might protect against CRC by preventing aberrations in DNA synthesis (DNA uracil misincorporation) and irregularities in DNA methylation (DNA hypomethylation)[77].
Vitamin B and colorectal adenomas
Three large RCTs[78-80] demonstrated no clear benefit of folate supplementation (0.5-1 mg daily) on the prevention of colorectal adenoma recurrence when followed for 3-6.5 years. Interestingly, in one of these studies[78], there was a statistically significant increased risk of having at least one advanced lesion (RR = 1.67, 95%CI: 1.00-2.80) or having 3 or more adenomas after 5 years of follow up. Two recent high quality meta-analyses confirmed these negative outcomes[81,82]. When a combination of folic acid (2.5 mg), vitamin B6 (50 mg), and vitamin B12 (1 mg) was used in another RCT on 1470 participants, the risk of colorectal adenoma was unchanged after a 9.3-year follow up period[83].
Vitamin B and colorectal cancer
Vitamins B6, B9, B12: Vitamin B6 may affect colorectal carcinogenesis via its role in DNA synthesis and methylation. Moreover, it was shown to inhibit angiogenesis, suppress nitric oxide, and reduce oxidative stress in animal models[84]. The risk of developing CRC was demonstrated in multiple prospective case-control studies to decrease by about 50% for every 100 pmol/L increase in the blood PLP levels (Pyridoxal 5’-phosphate, the active form of B6)[85]. However, data from observational studies were inconsistent with a general null or a modest inverse relation[86,87]. This was demonstrated in two high quality meta-analyses of case-control studies showing a significant inverse relation between B6 intake and CRC. The combined relative risk was found to be 0.80 in both meta-analyses (95%CI: 0.68-0.96)[85,88].
Data regarding the role of folate in CRC prevention is also controversial. In a recent meta-analysis including 11 cohort studies, the potential beneficial effects of higher dietary folate intake on CRC risk were not found to be statistically significant (HR = 0.92; 95%CI: 0.81-1.05)[89]. In contrast, another pooled analysis of 13 prospective cohort studies showed an inverse relation between folate intake and risk of CRC. The authors showed an estimated 2% risk reduction for every 100 μg/d increase in total folate intake[90]. Another recent meta-analysis by Liu et al[91] including 47 cohort studies, suggests an inverse association between the intake of vitamins B2, B6, B9, and D, and the risk of CRC. Other reports suggested a beneficial role for folate in reducing esophageal but not gastric cancer[92].
Three large RCTs originally designed to address the role of vitamin B in cardiovascular diseases[93-95], analyzed the role of vitamin B complex in the prevention of invasive cancers. These trials randomized patients to receive either daily vitamin B6 only, or combinations of folate (0.8-2.5 mg), vitamins B6 (40-50 mg) and B12 (0.4-1 mg) or placebo, with average treatment periods of 3.3 to 7.3 years. These studies failed to show an overall benefit of these interventions in preventing all types of cancers including CRC specifically.
Riboflavin: The Netherlands Cohort Study on diet and cancer (n = 120852) suggested that riboflavin tends to be associated with decreased proximal colon cancer risk among women (RR = 0.61; P-trend = 0.07)[96]. This finding was consolidated by the results from the Women’s Health Initiative Observational Study cohort that showed that higher total intakes of riboflavin were associated with a reduced risk of CRC (HR = 0.81; 95%CI: 0.66-0.99)[87]. Other smaller case-control studies reproduced similar conclusion of the protective effect of riboflavin intake on CRC[97,98].
Vitamin B and pancreatic cancer
The results from two recent meta-analyses regarding the role of folate supplementation in the prevention of pancreatic cancer were contradictory. Lin et al[99] concluded that individuals with a high dietary folate intake were about 34% less likely to develop pancreatic cancer compared with those with low intake. This conclusion was contradicted by another meta-analysis of 14 prospective cohort studies showing that the intake of folate - dietary and supplemental- was not related positively or negatively to the risk of pancreatic cancer[100].
Vitamin B and celiac disease
Patients with celiac disease have a higher total plasma homocysteine level than the general population, which is indicative of a poor vitamin status namely low serum levels vitamin B6, B9 and B12[101]. In a RCT of 65 patients with celiac disease on gluten-free diet, daily vitamin B supplementation (0.8 mg folic acid, 0.5 mg cyanocobalamin and 3 mg pyridoxine) for 6 mo was found to decrease the plasma homocysteine level by a median of 34% (P < 0.001), accompanied by significant improvement in well being, anxiety and depressed mood[102].
VITAMIN C
Vitamin C and GI malignancies (refer to antioxidants and CRC)
Data on the protective effect of vitamin C and other antioxidants on pancreatic cancer were conflicting with one of the largest cohorts from the Netherland of over 120000 participants and 16 years of follow up showing no benefit[103], as opposed to another large recent cohort study showing that patients eating a combination of the highest three quartiles of all of vitamins C, E and selenium had a decreased risk of pancreatic cancer (HR = 0.33, P < 0.05)[104]. Inconsistent data are also available regarding the protective role in esophageal and gastric cancer. A meta-analysis by Kubo et al[105] including 10 observational studies showed that vitamin C intake was inversely associated with the risk of esophageal adenocarcinoma (OR = 0.49, 95%CI: 0.39-0.62) but not with gastric cardia carcinoma.
Vitamin C and IBD
A case-control study including 239 patients with IBD showed that the intake of vitamin C (OR = 0.45; 95%CI: 0.21-0.99) was negatively related to UC risk[106]. One RCT including 57 patients with CD randomized to receive combined vitamin E (800 IU) and vitamin C (1000 mg) or placebo for 4 wk showed no effect of these supplements on the disease activity as measured by the Crohn’s disease activity complex (CDAI) despite the significant reduction in oxidative stress indices[107].
Vitamin C and gallstones
Clinical and experimental data reported in the 1970’s suggested a potential protective effect of vitamin C on the formation of gallstones[108]. This might be attributed to the reduction in bile acid biogenesis and the supersaturation of bile with cholesterol due to the deficiency in cholesterol 7α-hydroxylation in cases of ascorbic acid deficiency. Data from NHANES III study that included 996 cases of gallstones, showed that serum ascorbic acid level was inversely related to the prevalence of clinical and asymptomatic gallbladder disease among women, but not among men. Among women, each 27 micromol/L increase in serum level was independently associated with a 13% lower prevalence of gallstones (P < 0.06)[109]. In an observational study including 2129 participants, the subjects reporting regular vitamin C supplementation had significantly less prevalence of gallstone disease compared to those not taking vitamin C (4.7% vs 8.2%)[110].
Vitamin C and liver diseases
A meta-analysis and a Cochrane systematic review of RCTs addressing the role of antioxidant supplementation -including vitamins C and E - in the prognosis and mortality of liver diseases showed no convincing evidence of any beneficial effect[111,112].
VITAMIN D
Vitamin D3 is hydroxylated in the liver to produce 25(OH)D3, a reliable indicator of vitamin D status, and is further hydroxylated in the kidney to form the physiologically active hormone 1,25(OH)2D3. Circulating levels of 25(OH)D3 are a direct reflection of vitamin D status, which for any given individual depends on access to vitamin D either through exposure to sunlight or through dietary intake. Currently, the US Recommended Dietary Allowance (RDA) for vitamin D3 is 600 IU/d, and the tolerable upper intake level is 4000 IU/d. It has been estimated that an increase in vitamin D3 intake to 2000 IU/d in Americans would lead to a 27% decrease in the incidence of colorectal cancer[113].
The biological effects of vitamin D3 are mediated by the vitamin D receptor (VDR), which belongs to the superfamily of nuclear hormone receptors and is expressed in various organs and tissues of the human body, including the kidney and bone cells as well as the colonic mucosa. In the intestine, VDR plays an important role in regulating cell proliferation, differentiation, and the induction of apoptosis. In the US, 25% to 58% of adolescents and adults are deficient in vitamin D[114].
Vitamin D and CRC
The role of vitamin D in CRC prevention was first hypothesized in 1980 by Garland et al[115] based on ecological studies. They reported an inverse association between geographical latitude (solar radiation), vitamin D status and CRC incidence and mortality in United States[116]. Vitamin D has the ability to inhibit cell proliferation and increase apoptosis in vitro. Many cell types, including colorectal epithelial cells, contain vitamin D receptors (VDR). These cells are able to convert the circulating 25(OH)D into active 1,25(OH)D metabolites, which in turn bind to the cells’ own VDR to produce an autocrine effect by inducing cell differentiation and by inhibiting proliferation, invasiveness, angiogenesis, and metastatic potential (Figure 1)[117].
Figure 1.
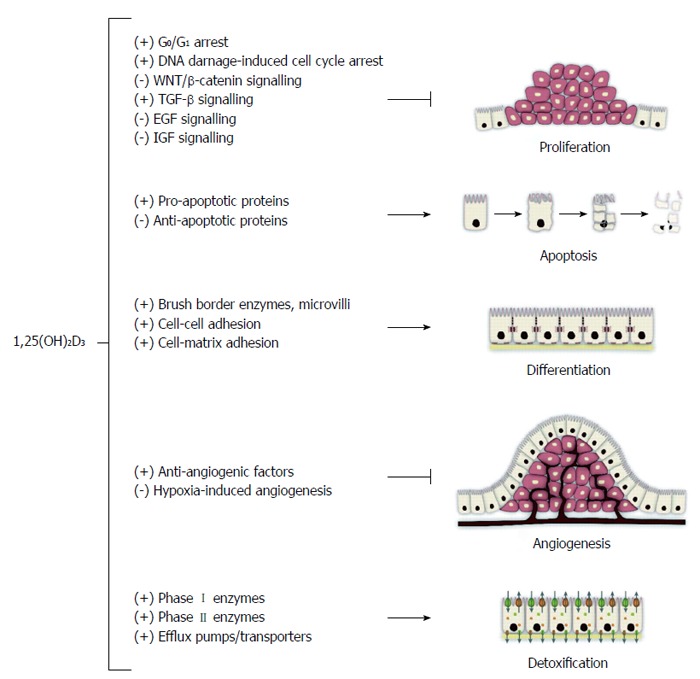
Summary of the effects of 1,25(OH)2D3 on colorectal cancer cells. With permission from Pereira et al[200].
Prospective observational studies suggest that higher vitamin D levels are associated with lower risk of incident CRC as well as improved survival in patients with established CRC. One of the largest observational nested case-control study (520000 participants) concluded that there is a strong inverse association between 25(OH)D concentration and CRC[118]. High vitamin D levels or vitamin D supplementation were also suggested to reduce mortality in patients diagnosed with CRC. Freedman et al[119] demonstrated in one of the largest prospective studies including 16818 participants that CRC mortality was inversely related to serum 25(OH)D level. Individuals with serum levels of 50-80 ng/mL and > 80 ng/mL had a relative risk of CRC mortality of 0.44 and 0.28, respectively. In another prospective trial of 257 CRC subjects, only 3% of patients had sufficient vitamin D levels (≥ 30 ng/mL). Higher 25(OH)D levels at surgery were associated with better overall survival under multivariate analysis[120].
A recent systematic review by Ma et al[121] including 17 prospective trials with approximately 1 million participants assessed the association between vitamin D intake or serum levels of 25(OH)D and the risk of developing CRC. Results confirmed this inverse relation, with pooled RRs of CRC for the highest vs lowest categories of vitamin D intake and blood levels being 0.88 and 0.67, respectively. Moreover, a 10 ng/mL increment in blood 25(OH)D level conferred a RR of 0.74. This inverse relationship was again confirmed by several high quality meta-analyses[122-124]. As for the relation with colorectal adenomas, similar results were obtained in most prospective studies[125].
The relationship between VDR gene polymorphism and the risk of CRC was addressed by numerous studies. In 2001, Kim et al[126] reported for the first time an association between CRC and the VDR gene in a case-control study. Based on their analysis of 393 cases of colorectal adenomas, the BsmI BB genotype was found to be associated with a reduced risk of adenoma when intake of calcium and vitamin D was reduced. The results regarding other polymorphic sites in the VDR gene were inconsistent. A recent systematic meta-analysis suggested that only the BsmI polymorphism was related to the CRC risk. In particular, the BsmI B genotype was found to be related to an overall decrease in the risk for colorectal cancer (BB vs bb: OR = 0.87, 95%CI: 0.80-0.94, P < 0.01)[127].
Few RCTs addressing the role of vitamin D supplementation in prevention of CRC have been conducted. Trivedi et al[128] randomized 2686 individuals (65-85 years of age) to receive 100000 IU vitamin D3 or placebo every 4 mo (about 833 IU/d). No significant effect of vitamin D3 supplementation on CRC incidence or mortality was documented after 5 years. Similarly, another RCT including 36282 post-menopausal women who were treated with 400 IU/d vitamin D3 plus 1 g/d calcium vs placebo, failed to find any effect of the treatment on CRC incidence after 7 years of follow-up[129]. However in this study, the low dose of vitamin D3 used did not increase the circulating 25(OH)D3 levels and the degree of patient adherence to the treatment was low. Another RCT performed in Nebraska randomized 1179 post-menopausal women to either 1100 IU/d vitamin D3 plus 1.4-1.5 g/d calcium or calcium alone for 4 years[130]. Treatment with vitamin D3 plus calcium reduced total cancer incidence (RR = 0.40; 95%CI: 0.20-0.82), including CRC. However, the low number of CRC cases reduces the validity of this study.
A recent Cochrane review of RCTs concluded that currently there is no firm evidence that vitamin D supplementation decreases cancer occurrence (including CRC) in post-menopausal women[131]. Vitamin D3 supplementation decreased cancer mortality and vitamin D supplementation decreased all-cause mortality, but these estimates are at risk of type I errors due to the fact that too few participants were examined, and to the risk of attrition bias originating from substantial rates of participant dropout. Further studies with different population groups and longer follow up periods are needed.
Vitamin D and other GI malignancies
Results from retrospective and prospective cohorts linking vitamin D level or supplementation to a decreased risk of gastric or esophageal cancer were highly inconsistent and contradictory[132]. While some cohorts suggested a negative relation between vitamin D status and the risk of esophageal, gastric and pancreatic cancer, others showed a positive or a null relationship. One of the largest cohorts involving 1065 cases of upper GI cancers[133] and 942 pancreatic cancers[134] failed to prove a clear benefit from higher vitamin D concentrations. Instead, it suggested a 2-fold increase in the risk of pancreatic cancer with 25(OH)D concentrations of > 100 nmol/L.
Similarly, an inverse association between both HCC and cholangiocarcinoma and vitamin D has been reported[135-137]. However, evidence for a beneficial effect of vitamin D from prospective human interventional studies is still lacking.
Vitamin D and IBD
Vitamin D deficiency is common in patients with newly diagnosed as well as chronic IBD, however it is unclear if such a deficiency is a consequence or part of the pathogenesis of IBD. The link was initially suggested by epidemiologic and ecologic studies where a “north-south” gradient was noted with increased prevalence of IBD among populations in North America and Northern Europe or those living at higher altitudes, although other confounding factors may play major roles[138]. It is now widely accepted that a key pathogenic mechanism in the development of IBD is an inappropriate response of a defective mucosal immune system to unknown luminal antigens in a genetically susceptible host[139]. The role of vitamin D in regulation and homeostasis of the immune response has been well documented and demonstrated by several complex mechanisms[140-142]. Data about the association with VDR polymorphism were highly inconsistent[143-145]. However, the immunomodulatory effect of vitamin D was more pronounced in CD compared with UC probably due to the known differences in the underlying pathogenesis of these two entities[139,141].
A small RCT of 20 patients with CD showed that vitamin D3 treatment did not only significantly increase the IL-6 level, but also enhanced the CD4+ T cell proliferation[146]. Recent cross-sectional studies also associated vitamin D deficiency with increased activity of both CD and UC[147-149]. A large prospective cohort study included 72719 women who completed an assessment of diet and lifestyle, from which a 25(OH)D prediction score was developed and validated against directly measured levels of plasma 25(OH)[114]. They were followed-up over 22 years for the development of IBD. The results suggested that higher predicted plasma levels of 25(OH)D significantly reduce the risk for incident CD and non-significantly reduce the risk for UC in women.
In another prospective interventional study by Miheller et al[150] 37 inactive CD patients were divided to receive either vitamin D in its active form [1,25(OH)D] or plain vitamin D [25(OH)D]. At week 6, active vitamin D caused a significant decrease in the CDAI scores (69 vs 57) and CRP levels (15.8 mmol/L vs 7.81 mmol/L). However this superiority disappeared after 12 mo. In a pilot study of 18 patients with CD, vitamin D supplementation (up to 5000 IU/d) significantly decreased the CDAI score from a mean of 230 to 118 after 24 wk[151]. Recently, a RCT was conducted on 94 patients with CD in remission, randomized to receive either 1200 IU vitamin D3 or placebo daily for 12 mo[152]. Oral supplementation significantly increased serum vitamin D levels by 39% and insignificantly reduced the risk of relapse from 29% to 13% (P value of 0.06). In summary, cumulative data suggest a definite association between vitamin D deficiency and both IBD incidence as well as activity, with recent studies suggesting a clinical benefit from vitamin D replacement therapy. Further large interventional trials are needed.
Vitamin D and HCV
Vitamin D deficiency is very common (up to 92%) among patients with chronic liver disease, and at least one-third suffer from severe vitamin D deficiency (< 12 ng/mL)[153]. It was postulated that the known high prevalence of vitamin D deficiency in Hispanics and African American populations might be a contributing factor in their limited response to the standard antiviral regimens[154].
Petta et al[155] retrospectively analyzed a cohort of 167 patients treated with Peg-IFN/RBV for HCV, and detected an association between lower vitamin D serum levels and increased risk of severe fibrosis (F3 or F4) as well as decreased responsiveness to interferon-based therapy and failure to achieve SVR in genotype 1 patients. These results were confirmed by the same group in another prospective study on 117 patients with genotype 1 infection, where 25(OH)D serum levels and IL28B status were found to be independently associated with the likelihood to achieve RVR and SVR[156]. However, data from other trials were highly contradictory and inconsistent. A recent meta-analysis concluded that the baseline 25(OH)D level is not associated with SVR to PEG-IFN plus RBV therapy in chronic HCV infection, regardless of genotype[157].
The first randomized controlled open label trial to address the potential benefits of vitamin D supplementation was performed by Abu-Mouch et al[158] on 72 patients with genotype 1 infection. Results showed a significant benefit of adding vitamin D (2000 IU/d) to Peg-IFN/Ribavirin over the standard regimen alone. They reported a significant improvement in RVR (44% vs 17%), EVR (94% vs 48%) and SVR (86% vs 42%) as well as a marked decreased rate of relapse in the supplemented population (8% vs 36%). Another study conducted by the same group with the same design including 50 patients with chronic HCV genotypes 2 and 3, had the same trend of results where the SVR was 95% in the supplemented patients as compared to 77% with the standard regimen (P < 0.001)[154]. A recent meta-analysis by Villar et al[159] showed that overall, 71% of patients with chronic HCV infection had low vitamin D levels. Higher rates of SVR were observed in HCV individuals with vitamin D levels above 30 ng/mL (OR = 1.57) and those supplemented with vitamin D (OR = 4.59) regardless of genotype.
In conclusion, vitamin D levels should be routinely checked in all chronic HCV patients and can be used as a prognostic factor to predict response to PegIFN/RBV therapy. All patients with vitamin D deficiency should be supplemented with vitamin D in combination with that particular therapeutic regimen. Still, the role of vitamin D in the era of highly effective all oral direct acting antivirals is unclear and of likely diminished significance.
Vitamin D and the liver
Early small studies suggested that low vitamin D levels might be associated with the development of non-alcoholic fatty liver disease (NAFLD)[160-162]. However, a recent report by Katz et al[163] that included 1630 young adolescents did not find that vitamin D status was associated with suspected NAFLD after adjustment for obesity and metabolic syndrome. A recent article by Rhee et al[164] involving more than 6500 patients, showed that participants with higher serum 25(OH)D3 showed a significantly reduced risk for NAFLD compared with the low 25(OH)D3 groups, independent of obesity and metabolic syndrome.
Multiple studies have suggested that VDR polymorphisms may predispose to autoimmune liver diseases like primary biliary cirrhosis and autoimmune hepatitis and to correlate with disease severity[165-168].
Results from studies investigating the role of vitamin D plus calcium supplementation in preventing and treating hepatic osteodystrophy in patients with cholestatic liver disease were conflicting with heterogeneous designs and populations. However, The European Association for the Study of the Liver (EASL) has published guidelines for the management of cholestatic diseases in 2009, recommending that vitamin D (400-800 IU/d) supplementation should be considered in all patients with cholestatic liver diseases, despite the lack of firm evidence[169].
VITAMIN E
Vitamin E and IBD
With its high antioxidant capacity and anti-inflammatory activity, vitamin E would be expected to reduce injury and/or improve tissue after injury from IBD. The effect of vitamin E on colitis was mainly studied on animal models with experimentally induced colitis with modest benefit and conflicting results[170-175].
In a case series of 15 patients with mild to moderate active UC, the addition of an alpha-tocopherol enema, 8000 U daily to the standard 5-ASA and/or immunomodulator medications significantly decreased the average disease activity index score after 12 wk of intervention from 8 to 2.3 (P < 0.0001)[176]. No other large interventional trials were conducted afterwards.
Vitamin E and non-alcoholic steatohepatitis
In addition to insulin resistance and features of metabolic syndrome, oxidative stress has been implicated as a key factor contributing to hepatic injury in patients with NASH[177]. Studies have demonstrated lower levels of plasma a-tocopherol in NASH patients compared to healthy controls[178]. Early pilot studies and small RCTs suggested a beneficial role, biochemically and histologically, of vitamin E in patients with NASH[179]. Sanyal et al[180] conducted a large RCT, the PIVENS study that randomized 247 adult non-diabetic patients with NASH to receive vitamin E supplementation (800 IU daily for 96 wk), or pioglitazone (30 mg daily), or placebo. Both pioglitazone and vitamin E were associated with significant reductions in liver enzymes, NAFLD score, hepatic steatosis and lobular inflammation but not in the fibrosis scores[180,181]. Vitamin E therapy, as compared to placebo, was associated with a significantly higher rate of improvement on liver biopsy (43% vs 19%, P = 0.001). Another large RCT, The TONIC trial, addressed the efficacy of vitamin E (400 IU twice daily) for the treatment of NASH in 173 non-diabetic children and adolescents[182]. Vitamin E was not superior to placebo in attaining the primary outcome, a reduction in ALT levels of 50% or less from the baseline, or 40 U/L or less at each visit from 48 to 96 wk. Resolution of NASH was significantly greater for the vitamin E treatment group that was attributed mainly by significant improvement in hepatocellular ballooning. No improvement in fibrosis or a significant reduction in steatosis and inflammation were observed.
Vitamin E was also shown to be beneficial in several interventional trials when used in combination with other agents like pioglitazone itself [183], atorvastatin and vitamin C[184], and UDCA[185]. These interventions led to a significant decrease in the histologic signs of steatosis by up to 70%, a reduction in fibrosis in many patients as well as the reduction of serum liver enzymes by up to 80%. A joint guideline issued in 2012 by the AASLD, the AGA, and the ACG, recommends the use of vitamin E at a dose of 800 mg daily, as first-line pharmacotherapy for non-diabetic patients with biopsy-proven NASH (with or without advanced fibrosis, but without cirrhosis).
However, data from recent observational studies raised the concerns of a possible increase in all-cause mortality in patients taking doses of vitamin E higher than 400 IU/d. Moreover a meta-analysis that included 9 trials suggested that vitamin E might increase the risk of hemorrhagic stroke[186]. More recently, an extended follow-up of a large RCT observed a significant increase in prostate cancer incidence (HR = 1.17) in healthy men taking vitamin E 400 IU daily for over 7 years[187].
Vitamin E and acute pancreatitis
It has been postulated that oxidative stress plays a role in the pathogenesis of acute pancreatitis (AP). Experimental models suggested some beneficial effects of using antioxidants supplementation in AP in animals[188]. Few small RCTs are available most of which combining vitamin E with other antioxidants, failed to prove any benefit (end-organ dysfunction, hospital stay, mortality) of this intervention in patients with severe AP[50,189]. Two recent systematic reviews including 12 RCTs investigating the use of antioxidant supplementation for the prevention of post-ERCP pancreatitis (PEP) showed no beneficial effect on the incidence and the severity of PEP[52,190].
VITAMIN K
Vitamin K and IBD
Few observational studies correlated low vitamin K levels with IBD and IBD activity[191] as well as its involvement in the pathogenesis of osteoporosis in these patients[192]. No interventional trials are available.
Vitamin K and CRC
Subclasses of vitamin K include vitamins K1 (phylloquinone, found in green leafy vegetables), K2 (menaquinone, produced by intestinal bacteria), K3 (menadione, a synthetic form and a provitamin of Vit K) and K5. Animal models have demonstrated in vivo and in vitro anti-tumor effects of theses subclasses (namely K2, K3 and K5) on CRC by inducing caspase-dependent apoptotic death of tumor cells[193]. In the only available Phase II clinical trial to date, Tetef et al[194] failed to prove an added benefit of vitamin K2 when given in combination with mitomycin C in 43 patients with advanced GI malignancies.
Vitamin K and HCC
Vitamin K2 was found to induce elevation of serum levels of des-γ-carboxy prothrombin (DCP), a serum protein that increases at a notably high level in patients with HCC, and that was shown to have mitogenic effect on human HCC cell lines[195]. The antitumor activity of vitamin K on HCC has been extensively investigated. Habu et al[196] have reported that vitamin K2 has a preventive effect on the development of HCC in women with viral liver cirrhosis. Small interventional trials suggested that vitamin K2 might prevent HCC recurrence after 3 years post-therapy but without a clear survival benefit[197]. However, the largest RCT to investigate the preventive role of vitamin K2 on HCC recurrence and survival after curative surgical or ablative therapy was conducted by Yoshida et al[198]. This study randomized 548 patients to receive placebo, 45 mg/d, or 90 mg/d vitamin K2 in a double-blind fashion. None of these interventions proved effective in preventing recurrence or improving survival at 1 year post-therapy.
Vitamin K for UGI bleeding in patients with liver diseases
A recent Cochrane review failed to find any randomized trials or observational studies of any kind investigating the potential benefit or harm of using vitamin K in patients with acute or chronic liver disease and presenting with UGI bleeding[199].
CONCLUSION
Table 2 summarizes the available evidence regarding the therapeutic roles of different vitamins in GI diseases. Vitamin supplementation is an attractive therapeutic option as it is relatively cheap, generally safe with a wide therapeutic window, and well perceived by the patients as “natural” or “organic” remedies. Recent data from RCTs provide promising results regarding the role of vitamins - namely vitamin D and B12 - in combination with IFN-based therapy in improving the response and most of the major outcomes in patients with HCV infection. Solid evidence has also emerged on the role of vitamin E in the treatment of NASH. In IBD patients with low vitamin D levels, supplementation with the vitamin was found in many RCTs to improve the outcome and decrease the risk of flares and complications. Moreover, a remarkable preventive role of many vitamins like B6, B9, B12 and D on the risk of developing CRC was suggested by a large number of observational studies and consolidated by few well designed interventional trials. Other implications of the use of vitamins in GI diseases have been reported in small trials and need to be further investigated.
Table 2.
Summary of the role of vitamins in gastrointestinal and liver diseases based on published intervention trials
| Vitamin | Colorectal cancer | Other gastrointestinal malignancies | Inflammatory bowel disease | Chronic hepatitis C | Other |
| A | No role | No role | No role | Controversial (not enough data) | May improve chronic pancreatitis pain |
| B1 | No data | No data | Improved IBD fatigue syndrome | No data | No data |
| B2 | Probably protective (non-randomized trials) | No role | No data | No data | None |
| B6 | Probably protective (non-randomized trials) | No role | No data | No data | Possible benefit in celiac disease |
| B9 | Probably protective (non-randomized trials) | Probably protective for pancreatic cancer | No data | No data | Possible benefit in celiac disease |
| B12 | No role | No role | No role | Probable adjunctive effect (one RCT) | Aphtous stomatitis |
| Possible benefit in celiac disease | |||||
| C | No role | Probable protective role in esophageal and pancreatic cancer | No role | No role | Prevention of gallstones |
| D | No role | Not protective against esophageal or pancreatic cancer | Inverse relation probable preventive and therapeutic effects | Additive effect to standard therapy (two RCTs) | Beneficial role in cholestatic liver diseases |
| E | No role | No role | No role | No data | Therapeutic role in non-alcoholic steatohepatitis |
| K | No role | Probably protective in HCC | No role | No role | None |
No role: No evidence by currently available interventional trials; No data: No good quality interventional trials available; Most vitamins had an inverse relationship with the risk of CRC, however interventional trials failed to prove a beneficial preventive role. Probable role: Data from observational studies or inconsistent RCTs results.
Footnotes
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 9, 2015
First decision: February 10, 2015
Article in press: March 31, 2015
P- Reviewer: Afzal NA, Feuerstadt P, Massironi S, Patanè S, Thompson JR S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Yetley EA. Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J Clin Nutr. 2007;85:269S–276S. doi: 10.1093/ajcn/85.1.269S. [DOI] [PubMed] [Google Scholar]
- 2.Fraza OE. High Costs Of Poor Eating Patterns in the United States. Accessed on August 20, 2010. U.S. Department of Agriculture. Washington, DC: Economic Research Service; 1999. pp. AIB–750. [Google Scholar]
- 3.Kim SY, Nayga RM, Capps O. Food Label Use, Self-Selectivity, and Diet Quality. J Consumer Affairs. 2001;35:346–363. [Google Scholar]
- 4.DeVol R, Bedroussian A. An Unhealthy America: The Economic Burden of Chronic Disease. Santa Monica, CA: Milken Institute; 2007. [Google Scholar]
- 5.Drichoutis AC, Lazaridis P, Nayga Jr. RM. Nutrition Knowledge and Consumer Use of Nutritional Food Labels. Eur Review Agricult Econom. 2005;32:93–118. [Google Scholar]
- 6.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994) NCHS Data Brief. 2011;(61):1–8. [PubMed] [Google Scholar]
- 7.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getman S. EU Regulations on Food Supplements, Health foods, herbal medicines. USA: United States Commercial Service, United States of America Department of Commerce; 2011. [Google Scholar]
- 9.Future Trends and Growth Opportunities in Vitamins and Minerals. Business Insights. 2010 [Google Scholar]
- 10.Vitamin and Supplement Industry Market Research and Statistics. Report Linker. 2013 [Google Scholar]
- 11.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 12.Blendon RJ, Benson JM, Botta MD, Weldon KJ. Users’ views of dietary supplements. JAMA Intern Med. 2013;173:74–76. doi: 10.1001/2013.jamainternmed.311. [DOI] [PubMed] [Google Scholar]
- 13.Carvey CE, Farina EK, Lieberman HR. Confidence in the efficacy and safety of dietary supplements among United States active duty army personnel. BMC Complement Altern Med. 2012;12:182. doi: 10.1186/1472-6882-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–1402.e1-6. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousvaros A, Zurakowski D, Duggan C, Law T, Rifai N, Goldberg NE, Leichtner AM. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: effect of disease activity. J Pediatr Gastroenterol Nutr. 1998;26:129–135. doi: 10.1097/00005176-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57–64. doi: 10.1159/000121412. [DOI] [PubMed] [Google Scholar]
- 17.Kirkali G, Keles D, Canda AE, Terzi C, Reddy PT, Jaruga P, Dizdaroglu M, Oktay G. Evidence for upregulated repair of oxidatively induced DNA damage in human colorectal cancer. DNA Repair (Amst) 2011;10:1114–1120. doi: 10.1016/j.dnarep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Yu E, Liu L, Zhang W, Wei X, Gao X, Song N, Fu C. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22:529–539. doi: 10.1097/CEJ.0b013e328364f1eb. [DOI] [PubMed] [Google Scholar]
- 19.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 25.Malila N, Virtamo J, Virtanen M, Albanes D, Tangrea JA, Huttunen JK. The effect of alpha-tocopherol and beta-carotene supplementation on colorectal adenomas in middle-aged male smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:489–493. [PubMed] [Google Scholar]
- 26.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 27.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 28.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev. 2008;16:CD004183. doi: 10.1002/14651858.CD004183.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 31.Bahcecioglu IH, Yalniz M, Ilhan N, Ataseven H, Ozercan IH. Levels of serum vitamin A, alpha-tocopherol and malondialdehyde in patients with non-alcoholic steatohepatitis: relationship with histopathologic severity. Int J Clin Pract. 2005;59:318–323. doi: 10.1111/j.1742-1241.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 32.Forrester JE, Wang XD, Knox TA, Borek CG, Tang AM, Johnson EJ. Factors associated with serum retinol, alpha-tocopherol, carotenoids, and selenium in Hispanics with problems of HIV, chronic hepatitis C, and drug use. J Public Health Policy. 2009;30:285–299. doi: 10.1057/jphp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peres WA, Chaves GV, Gonçalves JC, Ramalho A, Coelho HS. Vitamin A deficiency in patients with hepatitis C virus-related chronic liver disease. Br J Nutr. 2011;106:1724–1731. doi: 10.1017/S0007114511002145. [DOI] [PubMed] [Google Scholar]
- 34.Bitetto D, Bortolotti N, Falleti E, Vescovo S, Fabris C, Fattovich G, Cussigh A, Cmet S, Fornasiere E, Ceriani E, et al. Vitamin A deficiency is associated with hepatitis C virus chronic infection and with unresponsiveness to interferon-based antiviral therapy. Hepatology. 2013;57:925–933. doi: 10.1002/hep.26186. [DOI] [PubMed] [Google Scholar]
- 35.Arantes Ferreira Peres W, Villaça Chaves G, Saraiva Gonçalves JC, Ramalho A, Moraes Coelho HS. Assessment of the relative dose-response test as indicators of hepatic vitamin A stores in various stages of chronic liver disease. Nutr Clin Pract. 2013;28:95–100. doi: 10.1177/0884533612455827. [DOI] [PubMed] [Google Scholar]
- 36.Rocchi E, Seium Y, Camellini L, Casalgrandi G, Borghi A, D’Alimonte P, Cioni G. Hepatic tocopherol content in primary hepatocellular carcinoma and liver metastases. Hepatology. 1997;26:67–72. doi: 10.1053/jhep.1997.v26.pm0009214453. [DOI] [PubMed] [Google Scholar]
- 37.Newsome PN, Beldon I, Moussa Y, Delahooke TE, Poulopoulos G, Hayes PC, Plevris JN. Low serum retinol levels are associated with hepatocellular carcinoma in patients with chronic liver disease. Aliment Pharmacol Ther. 2000;14:1295–1301. doi: 10.1046/j.1365-2036.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 38.Clemente C, Elba S, Buongiorno G, Berloco P, Guerra V, Di Leo A. Serum retinol and risk of hepatocellular carcinoma in patients with child-Pugh class A cirrhosis. Cancer Lett. 2002;178:123–129. doi: 10.1016/s0304-3835(01)00843-6. [DOI] [PubMed] [Google Scholar]
- 39.Yu MW, Hsieh HH, Pan WH, Yang CS, CHen CJ. Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Res. 1995;55:1301–1305. [PubMed] [Google Scholar]
- 40.Hamamoto S, Fukuda R, Ishimura N, Rumi MA, Kazumori H, Uchida Y, Kadowaki Y, Ishihara S, Kinoshita Y. 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor. J Lab Clin Med. 2003;141:58–66. doi: 10.1067/mlc.2003.8. [DOI] [PubMed] [Google Scholar]
- 41.Böcher WO, Wallasch C, Höhler T, Galle PR. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int. 2008;28:347–354. doi: 10.1111/j.1478-3231.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuchmann M, Kittner JM, Schlaak JF, Klass DM, Eisenbach C, Berg T, Trautwein C, Günther R, Zeuzem S, Gösseringer R, et al. No beneficial effect of all-trans retinoic acid in previous non-responder patients with chronic hepatitis C: the ATRACTION study, a phase II randomised trial. Dig Liver Dis. 2013;45:323–329. doi: 10.1016/j.dld.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Sikkens EC, Cahen DL, Koch AD, Braat H, Poley JW, Kuipers EJ, Bruno MJ. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13:238–242. doi: 10.1016/j.pan.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499–503. doi: 10.1016/j.gassur.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990;4:357–371. doi: 10.1111/j.1365-2036.1990.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 46.De las Heras Castaño G, García de la Paz A, Fernández MD, Fernández Forcelledo JL. Use of antioxidants to treat pain in chronic pancreatitis. Rev Esp Enferm Dig. 2000;92:375–385. [PubMed] [Google Scholar]
- 47.Shah NS, Makin AJ, Sheen AJ, Siriwardena AK. Quality of life assessment in patients with chronic pancreatitis receiving antioxidant therapy. World J Gastroenterol. 2010;16:4066–4071. doi: 10.3748/wjg.v16.i32.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149–159.e2. doi: 10.1053/j.gastro.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology. 2012;143:655–663.e1. doi: 10.1053/j.gastro.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 50.Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439–1444. doi: 10.1136/gut.2006.115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavy A, Karban A, Suissa A, Yassin K, Hermesh I, Ben-Amotz A. Natural beta-carotene for the prevention of post-ERCP pancreatitis. Pancreas. 2004;29:e45–e50. doi: 10.1097/00006676-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Gu WJ, Wei CY, Yin RX. Antioxidant supplementation for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. Nutr J. 2013;12:23. doi: 10.1186/1475-2891-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michael A, Hill M, Maraveyas A, Dalgleish A, Lofts F. 13-cis-Retinoic acid in combination with gemcitabine in the treatment of locally advanced and metastatic pancreatic cancer--report of a pilot phase II study. Clin Oncol (R Coll Radiol) 2007;19:150–153. doi: 10.1016/j.clon.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Takyar SS, Gowans EJ, Lott WB. Vitamin B12 stalls the 80 S ribosomal complex on the hepatitis C internal ribosome entry site. J Mol Biol. 2002;319:1–8. doi: 10.1016/S0022-2836(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 55.Li D, Lott WB, Martyn J, Haqshenas G, Gowans EJ. Differential effects on the hepatitis C virus (HCV) internal ribosome entry site by vitamin B12 and the HCV core protein. J Virol. 2004;78:12075–12081. doi: 10.1128/JVI.78.21.12075-12081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg P, Hagen K. Serum B12 levels predict response to treatment with interferon and ribavirin in patients with chronic HCV infection. J Viral Hepat. 2011;18:129–134. doi: 10.1111/j.1365-2893.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 57.Rocco A, Compare D, Coccoli P, Esposito C, Di Spirito A, Barbato A, Strazzullo P, Nardone G. Vitamin B12 supplementation improves rates of sustained viral response in patients chronically infected with hepatitis C virus. Gut. 2013;62:766–773. doi: 10.1136/gutjnl-2012-302344. [DOI] [PubMed] [Google Scholar]
- 58.BRACHMANN F. [Treatment of chronically recurrent aphthae with vitamin B12] Zahnarztl Welt. 1954;9:3/58–3/59. [PubMed] [Google Scholar]
- 59.Wray D, Ferguson MM, Mason DK, Hutcheon AW, Dagg JH. Recurrent aphthae: treatment with vitamin B12, folic acid, and iron. Br Med J. 1975;2:490–493. doi: 10.1136/bmj.2.5969.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulcan E, Toker S, Hatipoğlu H, Gulcan A, Toker A. Cyanocobalamin may be beneficial in the treatment of recurrent aphthous ulcers even when vitamin B12 levels are normal. Am J Med Sci. 2008;336:379–382. doi: 10.1097/MAJ.0b013e31816a05f2. [DOI] [PubMed] [Google Scholar]
- 61.Volkov I, Rudoy I, Abu-Rabia U, Masalha T, Masalha R. Case report: Recurrent aphthous stomatitis responds to vitamin B12 treatment. Can Fam Physician. 2005;51:844–845. [PMC free article] [PubMed] [Google Scholar]
- 62.Volkov I, Rudoy I, Freud T, Sardal G, Naimer S, Peleg R, Press Y. Effectiveness of vitamin B12 in treating recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Med. 2009;22:9–16. doi: 10.3122/jabfm.2009.01.080113. [DOI] [PubMed] [Google Scholar]
- 63.Battat R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, Bessissow T, Seidman E, Bitton A. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20:1120–1128. doi: 10.1097/MIB.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 64.Costantini A, Pala MI. Thiamine and fatigue in inflammatory bowel diseases: an open-label pilot study. J Altern Complement Med. 2013;19:704–708. doi: 10.1089/acm.2011.0840. [DOI] [PubMed] [Google Scholar]
- 65.Steger GG, Mader RM, Vogelsang H, Schöfl R, Lochs H, Ferenci P. Folate absorption in Crohn’s disease. Digestion. 1994;55:234–238. doi: 10.1159/000201153. [DOI] [PubMed] [Google Scholar]
- 66.Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology. 1989;97:255–259. doi: 10.1016/0016-5085(89)90058-9. [DOI] [PubMed] [Google Scholar]
- 67.Lashner BA. Red blood cell folate is associated with the development of dysplasia and cancer in ulcerative colitis. J Cancer Res Clin Oncol. 1993;119:549–554. doi: 10.1007/BF01686465. [DOI] [PubMed] [Google Scholar]
- 68.Diculescu M, Ciocîrlan M, Ciocîrlan M, Piţigoi D, Becheanu G, Croitoru A, Spanache S. Folic acid and sulfasalazine for colorectal carcinoma chemoprevention in patients with ulcerative colitis: the old and new evidence. Rom J Gastroenterol. 2003;12:283–286. [PubMed] [Google Scholar]
- 69.Subramanian V, Logan RF. Chemoprevention of colorectal cancer in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2011;25:593–606. doi: 10.1016/j.bpg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 70.van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235–240. doi: 10.1136/gut.2011.237412. [DOI] [PubMed] [Google Scholar]
- 71.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S, Fujishima M. Multiple vitamin status in Crohn’s disease. Correlation with disease activity. Dig Dis Sci. 1993;38:1614–1618. doi: 10.1007/BF01303168. [DOI] [PubMed] [Google Scholar]
- 72.Loftus EV, Tremaine WJ, Nelson RA, Shoemaker JD, Sandborn WJ, Phillips SF, Hasan Y. Dexpanthenol enemas in ulcerative colitis: a pilot study. Mayo Clin Proc. 1997;72:616–620. doi: 10.1016/S0025-6196(11)63566-0. [DOI] [PubMed] [Google Scholar]
- 73.Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, Lecchi A, Lombardi R, Meucci G, Spina L, de Franchis R. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112–117. doi: 10.1111/j.1572-0241.2003.07160.x. [DOI] [PubMed] [Google Scholar]
- 74.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:511–519. [PubMed] [Google Scholar]
- 75.Keum N, Giovannucci EL. Folic acid fortification and colorectal cancer risk. Am J Prev Med. 2014;46:S65–S72. doi: 10.1016/j.amepre.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 76.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 77.Giovannucci E, Wu K. Cancers of the colon and rectum. Cancer epidemiology and prevention. 3rd ed. New York: Oxford University Press; 2006. pp. 809–829. [Google Scholar]
- 78.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 79.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 80.Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, Hunter DJ, Giovannucci E. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. 2009;90:1623–1631. doi: 10.3945/ajcn.2009.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carroll C, Cooper K, Papaioannou D, Hind D, Tappenden P, Pilgrim H, Booth A. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther. 2010;31:708–718. doi: 10.1111/j.1365-2036.2010.04238.x. [DOI] [PubMed] [Google Scholar]
- 82.Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, Logan RF, Baron JA. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer. 2011;129:192–203. doi: 10.1002/ijc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song Y, Manson JE, Lee IM, Cook NR, Paul L, Selhub J, Giovannucci E, Zhang SM. Effect of combined folic acid, vitamin B(6), and vitamin B(12) on colorectal adenoma. J Natl Cancer Inst. 2012;104:1562–1575. doi: 10.1093/jnci/djs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang XH, Ma J, Smith-Warner SA, Lee JE, Giovannucci E. Vitamin B6 and colorectal cancer: current evidence and future directions. World J Gastroenterol. 2013;19:1005–1010. doi: 10.3748/wjg.v19.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077–1083. doi: 10.1001/jama.2010.263. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X, Lee JE, Ma J, Je Y, Wu K, Willett WC, Fuchs CS, Giovannucci EL. Prospective cohort studies of vitamin B-6 intake and colorectal cancer incidence: modification by time? Am J Clin Nutr. 2012;96:874–881. doi: 10.3945/ajcn.112.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zschäbitz S, Cheng TY, Neuhouser ML, Zheng Y, Ray RM, Miller JW, Song X, Maneval DR, Beresford SA, Lane D, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332–343. doi: 10.3945/ajcn.112.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Theodoratou E, Farrington SM, Tenesa A, McNeill G, Cetnarskyj R, Barnetson RA, Porteous ME, Dunlop MG, Campbell H. Dietary vitamin B6 intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:171–182. doi: 10.1158/1055-9965.EPI-07-0621. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, Koren G. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. 2011;35:2–10. doi: 10.1016/j.canep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010;21:1919–1930. doi: 10.1007/s10552-010-9620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y, Yu Q, Zhu Z, Zhang J, Chen M, Tang P, Li K. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol. 2015;32:434. doi: 10.1007/s12032-014-0434-5. [DOI] [PubMed] [Google Scholar]
- 92.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131:1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 94.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300:2012–2021. doi: 10.1001/jama.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 96.de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372–2378. doi: 10.3945/jn.108.091157. [DOI] [PubMed] [Google Scholar]
- 97.Eussen SJ, Vollset SE, Hustad S, Midttun Ø, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, et al. Plasma vitamins B2, B6, and B12, and related genetic variants as predictors of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:2549–2561. doi: 10.1158/1055-9965.EPI-10-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van den Donk M, Buijsse B, van den Berg SW, Ocké MC, Harryvan JL, Nagengast FM, Kok FJ, Kampman E. Dietary intake of folate and riboflavin, MTHFR C677T genotype, and colorectal adenoma risk: a Dutch case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1562–1566. doi: 10.1158/1055-9965.EPI-04-0419. [DOI] [PubMed] [Google Scholar]
- 99.Lin HL, An QZ, Wang QZ, Liu CX. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health. 2013;127:607–613. doi: 10.1016/j.puhe.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Bao Y, Michaud DS, Spiegelman D, Albanes D, Anderson KE, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, et al. Folate intake and risk of pancreatic cancer: pooled analysis of prospective cohort studies. J Natl Cancer Inst. 2011;103:1840–1850. doi: 10.1093/jnci/djr431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hallert C, Grant C, Grehn S, Grännö C, Hultén S, Midhagen G, Ström M, Svensson H, Valdimarsson T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–1339. doi: 10.1046/j.1365-2036.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 102.Hallert C, Svensson M, Tholstrup J, Hultberg B. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment Pharmacol Ther. 2009;29:811–816. doi: 10.1111/j.1365-2036.2009.03945.x. [DOI] [PubMed] [Google Scholar]
- 103.Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int J Cancer. 2012;130:147–158. doi: 10.1002/ijc.25989. [DOI] [PubMed] [Google Scholar]
- 104.Banim PJ, Luben R, McTaggart A, Welch A, Wareham N, Khaw KT, Hart AR. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013;62:1489–1496. doi: 10.1136/gutjnl-2011-301908. [DOI] [PubMed] [Google Scholar]
- 105.Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102:2323–2330; quiz 2331. doi: 10.1111/j.1572-0241.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 106.Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K, et al. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11:154–163. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Aghdassi E, Wendland BE, Steinhart AH, Wolman SL, Jeejeebhoy K, Allard JP. Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress. a randomized controlled trial. Am J Gastroenterol. 2003;98:348–353. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 108.Ginter E. Chenodeoxycholic acid, gallstones and vitamin C. N Engl J Med. 1976;295:1260–1261. doi: 10.1056/NEJM197611252952218. [DOI] [PubMed] [Google Scholar]
- 109.Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III) Arch Intern Med. 2000;160:931–936. doi: 10.1001/archinte.160.7.931. [DOI] [PubMed] [Google Scholar]
- 110.Walcher T, Haenle MM, Kron M, Hay B, Mason RA, Walcher D, Steinbach G, Kern P, Piechotowski I, Adler G, et al. Vitamin C supplement use may protect against gallstones: an observational study on a randomly selected population. BMC Gastroenterol. 2009;9:74. doi: 10.1186/1471-230X-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C. Antioxidant supplements for liver diseases. Cochrane Database Syst Rev. 2011;(3):CD007749. doi: 10.1002/14651858.CD007749.pub2. [DOI] [PubMed] [Google Scholar]
- 112.Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C. Meta-analysis: antioxidant supplements for liver diseases - the Cochrane Hepato-Biliary Group. Aliment Pharmacol Ther. 2010;32:356–367. doi: 10.1111/j.1365-2036.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 113.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 114.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 116.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 117.Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, Daniel CR, Cohen V, Dash C. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila) 2009;2:213–223. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 120.Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, Tamez S, Tsutsumi Y, Yanaga K, Urashima M. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 122.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 123.Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Riboli E, Hercberg S, Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 124.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 125.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53:10–16. doi: 10.1016/j.ypmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 126.Kim HS, Newcomb PA, Ulrich CM, Keener CL, Bigler J, Farin FM, Bostick RM, Potter JD. Vitamin D receptor polymorphism and the risk of colorectal adenomas: evidence of interaction with dietary vitamin D and calcium. Cancer Epidemiol Biomarkers Prev. 2001;10:869–874. [PubMed] [Google Scholar]
- 127.Bai YH, Lu H, Hong D, Lin CC, Yu Z, Chen BC. Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol. 2012;18:1672–1679. doi: 10.3748/wjg.v18.i14.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 130.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 131.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;6:CD007469. doi: 10.1002/14651858.CD007469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trowbridge R, Mittal SK, Agrawal DK. Vitamin D and the epidemiology of upper gastrointestinal cancers: a critical analysis of the current evidence. Cancer Epidemiol Biomarkers Prev. 2013;22:1007–1014. doi: 10.1158/1055-9965.EPI-13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abnet CC, Chen Y, Chow WH, Gao YT, Helzlsouer KJ, Le Marchand L, McCullough ML, Shikany JM, Virtamo J, Weinstein SJ, et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:94–106. doi: 10.1093/aje/kwq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stolzenberg-Solomon RZ. Vitamin D and pancreatic cancer. Ann Epidemiol. 2009;19:89–95. doi: 10.1016/j.annepidem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, Fumolo E, Bignulin S, Cmet S, Minisini R, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16:3016–3024. doi: 10.3748/wjg.v16.i24.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pourgholami MH, Akhter J, Lu Y, Morris DL. In vitro and in vivo inhibition of liver cancer cells by 1,25-dihydroxyvitamin D3. Cancer Lett. 2000;151:97–102. doi: 10.1016/s0304-3835(99)00416-4. [DOI] [PubMed] [Google Scholar]
- 137.Seubwai W, Wongkham C, Puapairoj A, Khuntikeo N, Wongkham S. Overexpression of vitamin D receptor indicates a good prognosis for cholangiocarcinoma: implications for therapeutics. Cancer. 2007;109:2497–2505. doi: 10.1002/cncr.22716. [DOI] [PubMed] [Google Scholar]
- 138.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315. doi: 10.1038/ncpgasthep0215. [DOI] [PubMed] [Google Scholar]
- 139.Ardizzone S, Bianchi Porro G. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475–496. doi: 10.1046/j.1365-2796.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 140.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waterman M, Xu W, Stempak JM, Milgrom R, Bernstein CN, Griffiths AM, Greenberg GR, Steinhart AH, Silverberg MS. Distinct and overlapping genetic loci in Crohn‘s disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17:1936–1942. doi: 10.1002/ibd.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hughes DJ, McManus R, Neary P, O’morain C, O’sullivan M. Common variation in the vitamin D receptor gene and risk of inflammatory bowel disease in an Irish case-control study. Eur J Gastroenterol Hepatol. 2011;23:807–812. doi: 10.1097/MEG.0b013e328349283e. [DOI] [PubMed] [Google Scholar]
- 143.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Naderi N, Farnood A, Habibi M, Derakhshan F, Balaii H, Motahari Z, Agah MR, Firouzi F, Rad MG, Aghazadeh R, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008;23:1816–1822. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 145.Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8:417–420. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- 146.Bendix-Struve M, Bartels LE, Agnholt J, Dige A, Jørgensen SP, Dahlerup JF. Vitamin D3 treatment of Crohn’s disease patients increases stimulated T cell IL-6 production and proliferation. Aliment Pharmacol Ther. 2010;32:1364–1372. doi: 10.1111/j.1365-2036.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- 147.Blanck S, Aberra F. Vitamin d deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58:1698–1702. doi: 10.1007/s10620-012-2531-7. [DOI] [PubMed] [Google Scholar]
- 148.Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407–e413. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 149.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 150.Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszényi L, Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis. 2009;15:1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 151.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin Transl Gastroenterol. 2013;4:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 153.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 154.Nimer A, Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18:800–805. doi: 10.3748/wjg.v18.i8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 156.Petta S, Ferraro D, Cammà C, Cabibi D, Di Cristina A, Di Marco V, Di Stefano R, Grimaudo S, Mazzola A, Levrero M, et al. Vitamin D levels and IL28B polymorphisms are related to rapid virological response to standard of care in genotype 1 chronic hepatitis C. Antivir Ther. 2012;17:823–831. doi: 10.3851/IMP2100. [DOI] [PubMed] [Google Scholar]
- 157.Kitson MT, Sarrazin C, Toniutto P, Eslick GD, Roberts SK. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: a systematic review and meta-analysis. J Hepatol. 2014;61:1247–1252. doi: 10.1016/j.jhep.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 158.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Villar LM, Del Campo JA, Ranchal I, Lampe E, Romero-Gomez M. Association between vitamin D and hepatitis C virus infection: a meta-analysis. World J Gastroenterol. 2013;19:5917–5924. doi: 10.3748/wjg.v19.i35.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manco M, Ciampalini P, Nobili V. Low levels of 25-hydroxyvitamin D(3) in children with biopsy-proven nonalcoholic fatty liver disease. Hepatology. 2010;51:2229; author reply 2230. doi: 10.1002/hep.23724. [DOI] [PubMed] [Google Scholar]
- 161.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Katz K, Brar PC, Parekh N, Liu YH, Weitzman M. Suspected nonalcoholic Fatty liver disease is not associated with vitamin d status in adolescents after adjustment for obesity. J Obes. 2010;2010:496829. doi: 10.1155/2010/496829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, Kang MI, Park SW, Kim SW, Oh KW. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60:743–752. doi: 10.1507/endocrj.ej12-0387. [DOI] [PubMed] [Google Scholar]
- 165.Kempińska-Podhorecka A, Wunsch E, Jarowicz T, Raszeja-Wyszomirska J, Loniewska B, Kaczmarczyk M, Milkiewicz M, Milkiewicz P. Vitamin d receptor polymorphisms predispose to primary biliary cirrhosis and severity of the disease in polish population. Gastroenterol Res Pract. 2012;2012:408723. doi: 10.1155/2012/408723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tanaka A, Nezu S, Uegaki S, Kikuchi K, Shibuya A, Miyakawa H, Takahashi S, Bianchi I, Zermiani P, Podda M, et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol. 2009;50:1202–1209. doi: 10.1016/j.jhep.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 167.Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35:126–131. doi: 10.1053/jhep.2002.30084. [DOI] [PubMed] [Google Scholar]
- 168.Fan L, Tu X, Zhu Y, Zhou L, Pfeiffer T, Feltens R, Stoecker W, Zhong R. Genetic association of vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J Gastroenterol Hepatol. 2005;20:249–255. doi: 10.1111/j.1440-1746.2005.03532.x. [DOI] [PubMed] [Google Scholar]
- 169.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 170.Minaiyan M, Mostaghel E, Mahzouni P. Preventive Therapy of Experimental Colitis with Selected iron Chelators and Anti-oxidants. Int J Prev Med. 2012;3:S162–S169. [PMC free article] [PubMed] [Google Scholar]
- 171.Carrier J, Aghdassi E, Cullen J, Allard JP. Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr. 2002;132:3146–3150. doi: 10.1093/jn/131.10.3146. [DOI] [PubMed] [Google Scholar]
- 172.Isozaki Y, Yoshida N, Kuroda M, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 173.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, Tahan V, Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bitiren M, Karakilcik AZ, Zerin M, Ozardali I, Selek S, Nazligül Y, Ozgonul A, Musa D, Uzunkoy A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 175.Ademoglu E, Erbil Y, Tam B, Barbaros U, Ilhan E, Olgac V, Mutlu-Turkoglu U. Do vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–108. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 176.Mirbagheri SA, Nezami BG, Assa S, Hajimahmoodi M. Rectal administration of d-alpha tocopherol for active ulcerative colitis: a preliminary report. World J Gastroenterol. 2008;14:5990–5995. doi: 10.3748/wjg.14.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 178.Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis (NASH) Eur J Med Res. 2011;16:76–78. doi: 10.1186/2047-783X-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 180.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 184.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 185.Pietu F, Guillaud O, Walter T, Vallin M, Hervieu V, Scoazec JY, Dumortier J. Ursodeoxycholic acid with vitamin E in patients with nonalcoholic steatohepatitis: long-term results. Clin Res Hepatol Gastroenterol. 2012;36:146–155. doi: 10.1016/j.clinre.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 186.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11. doi: 10.1016/s0014-2999(99)00421-5. [DOI] [PubMed] [Google Scholar]
- 189.Bansal D, Bhalla A, Bhasin DK, Pandhi P, Sharma N, Rana S, Malhotra S. Safety and efficacy of vitamin-based antioxidant therapy in patients with severe acute pancreatitis: a randomized controlled trial. Saudi J Gastroenterol. 2011;17:174–179. doi: 10.4103/1319-3767.80379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mohseni Salehi Monfared SS, Vahidi H, Abdolghaffari AH, Nikfar S, Abdollahi M. Antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis: a systematic review. World J Gastroenterol. 2009;15:4481–4490. doi: 10.3748/wjg.15.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nakajima S, Iijima H, Egawa S, Shinzaki S, Kondo J, Inoue T, Hayashi Y, Ying J, Mukai A, Akasaka T, et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition. 2011;27:1023–1028. doi: 10.1016/j.nut.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 192.Kuwabara A, Tanaka K, Tsugawa N, Nakase H, Tsuji H, Shide K, Kamao M, Chiba T, Inagaki N, Okano T, et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int. 2009;20:935–942. doi: 10.1007/s00198-008-0764-2. [DOI] [PubMed] [Google Scholar]
- 193.Ogawa M, Nakai S, Deguchi A, Nonomura T, Masaki T, Uchida N, Yoshiji H, Kuriyama S. Vitamins K2, K3 and K5 exert antitumor effects on established colorectal cancer in mice by inducing apoptotic death of tumor cells. Int J Oncol. 2007;31:323–331. [PubMed] [Google Scholar]
- 194.Tetef M, Margolin K, Ahn C, Akman S, Chow W, Coluzzi P, Leong L, Morgan RJ, Raschko J, Shibata S. Mitomycin C and menadione for the treatment of advanced gastrointestinal cancers: a phase II trial. J Cancer Res Clin Oncol. 1995;121:103–106. doi: 10.1007/BF01202221. [DOI] [PubMed] [Google Scholar]
- 195.Suzuki M, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Nakanishi Y, Koike K, Takaki A, Shiratori Y. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem. 2005;280:6409–6415. doi: 10.1074/jbc.M406714200. [DOI] [PubMed] [Google Scholar]
- 196.Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358–361. doi: 10.1001/jama.292.3.358. [DOI] [PubMed] [Google Scholar]
- 197.Riaz IB, Riaz H, Riaz T, Rahman S, Amir M, Badshah MB, Kazi AN. Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2012;12:170. doi: 10.1186/1471-230X-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532–540. doi: 10.1002/hep.24430. [DOI] [PubMed] [Google Scholar]
- 199.Kakizaki S, Sohara N, Sato K, Suzuki H, Yanagisawa M, Nakajima H, Takagi H, Naganuma A, Otsuka T, Takahashi H, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol. 2007;22:518–522. doi: 10.1111/j.1440-1746.2007.04844.x. [DOI] [PubMed] [Google Scholar]
- 200.Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–R71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]


