Abstract
AIM: To study the clinical efficacy and safety of Fecal microbiota transplantation (FMT). We systematically reviewed FMT used as clinical therapy.
METHODS: We searched MEDLINE, EMBASE, the Cochrane Library and Conference proceedings from inception to July, 2013. Treatment effect of FMT was calculated as the percentage of patients who achieved clinical improvement per patient category, on an intention-to-treat basis.
RESULTS: We included 45 studies; 34 on Clostridium difficile-infection (CDI), 7 on inflammatory bowel disease, 1 on metabolic syndrome, 1 on constipation, 1 on pouchitis and 1 on irritable bowel syndrome (IBS). In CDI 90% resolution of diarrhea in 33 case series (n = 867) was reported, and 94% resolution of diarrhea after repeated FMT in a randomized controlled trial (RCT) (n = 16). In ulcerative colitis (UC) remission rates of 0% to 68% were found (n = 106). In Crohn’s disease (CD) (n = 6), no benefit was observed. In IBS, 70% improvement of symptoms was found (n = 13). 100% Reversal of symptoms was observed in constipation (n = 3). In pouchitis, none of the patients (n = 8) achieved remission. One RCT showed significant improvement of insulin sensitivity in metabolic syndrome (n = 10). Serious adverse events were rare.
CONCLUSION: FMT is highly effective in CDI, and holds promise in UC. As for CD, chronic constipation, pouchitis and IBS data are too limited to draw conclusions. FMT increases insulin sensitivity in metabolic syndrome.
Keywords: Fecal microbiota transplantation, Microbiota, Clostridium difficile infection, Inflammatory bowel disease, Metabolic syndrome
Core tip: Aberrancies in the host’s microbiota have been found in several diseases. The most radical way to modulate the microbiota is by fecal microbiota transplantation (FMT). FMT is already used for various diseases while evidence from randomized studies is only just emerging. We systematically reviewed the efficacy of FMT in Clostridium difficile infection (CDI), inflammatory bowel disease, constipation, irritable bowel syndrome, pouchitis, and metabolic syndrome. FMT could be incorporated in clinical practice for CDI; patients with other indications should currently only be treated in clinical trials. Upcoming randomized studies on the long-term efficacy and safety of FMT will be helpful in the implication of FMT in clinical practice.
INTRODUCTION
Interest is growing rapidly worldwide for fecal microbiota transplantation (FMT) as a ‘‘natural’’ therapy from both patients’- and physicians’ perspective. FMT is popular among some patients because it is not associated with adverse effects from regular medicinal therapy. Apart from offering a potentially efficacious therapy, FMT provides an ideal human model to study the influence of modulating the microbiota in various (pre-)disease states. The oldest account of FMT dates back to the 4th century, when a Chinese physician named Ge Hong produced a paper, in which he advised to consume fresh stool from a healthy neighbour when suffering from severe diarrhea[1]. The first report in the medical literature concerned four patients who were successfully treated with FMT for pseudomembranous colitis in 1958[2]. Since that time several case series on FMT have been published mainly on refractory and recurrent Clostridium difficile infection (CDI), but also for other intestinal diseases such as ulcerative colitis (UC) and irritable bowel syndrome (IBS)[3-6]. From the 1990’s FMT has been reported in chronic constipation, Crohn’s disease (CD), pouchitis, metabolic syndrome, chronic fatigue syndrome, idiopathic thrombocytopenic purpura and even in multiple sclerosis[7-13].
By performing a systematic review we aimed to provide a comprehensive assessment of the efficacy and safety of fecal microbiota transplantation used as clinical therapy for various diseases and pre-clinical conditions. Clinical efficacy of FMT was presented per indication. In addition, we described safety data, route of administration and criteria used for selection and screening of donors.
MATERIALS AND METHODS
This study was executed according to 27 items included in The PRISMA statement for reporting systematic reviews[14]. All available articles in the English language on clinical efficacy and safety of FMT used as clinical therapy in human subjects were included in this systematic review. These studies included randomized controlled trials (RCTs) that compared FMT with standard medical therapy or other active comparators, placebo or no intervention. Observational studies including case-control, cohort studies and case-series (number of patients treated greater than one) were also included. The search was not restricted to disease type, pre-clinical condition, year of publication, publication status or length of follow-up (FU). FMT was defined as administration of a suspension of donor feces (either fresh or frozen) into the gastrointestinal tract. If an unclear definition of treatment was given, studies were not included; bacteriotherapy with a suspension of specific bacterial groups was not regarded as FMT. This systematic review was not registered a priori nor was a protocol published prior to the start of the study. In the nature of this study, no request was performed for ethics committee approval.
Outcome measures
Efficacy of FMT was assessed by clinical improvement as defined by the authors in the included studies. Clinical improvement was defined as a resolution of diarrhea in CDI and, if available, the proportion of patients free from relapse during the follow-up period, clinical remission and/or clinical improvement in UC and CD, and clinical improvement in pouchitis, constipation and IBS. In metabolic syndrome, clinical improvement after FMT was defined as the effect on peripheral insulin sensitivity. Secondary outcomes included: the proportion of patients who experienced any adverse event (AE), withdrawal due to adverse events, serious adverse events (SAE’s) (deaths or hospitalization) and adverse events potentially associated with fecal transplantation including perforation, post-transplant sepsis or bacteremia, and transmission of communicable disease.
Search strategy and selection criteria
We searched MEDLINE, EMBASE, and the Cochrane Library from inception to July 2013 using the search terms “feces”, “faeces”, “stool”, or “microbiota” combined with, “donor”, “donation”, “transplantation”, “therapy”, “infusion” or “bacteriotherapy” with assistance of a clinical librarian. Conference proceedings: European Crohn’s and Colitis Organization (ECCO 2009 to 2013); the United European Gastroenterology Week (UEGW 2010 to 2013); the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2012 to 2013); the Infectious Diseases Society of America (IDSA 2003 to 2012); Digestive Disease Week (DDW 1979 to 2013); and the American College of Gastroenterology (ACG from 2010 to 2013) were searched to identify abstract publications. The search was limited to human subjects and English written articles. References from review articles were also searched to identify applicable studies that may have been missed by the database searches.
Data extraction
Records were imported into a bibliographic database and duplicates were removed manually. Where possible, those with potential overlaps in patient populations were identified before the analysis. In case of any uncertainty of duplicate data or where missing data were encountered, the author was contacted. Two authors (NGR and EMdV) independently assessed articles by title and abstract to determine eligibility. Full text articles were obtained for all studies deemed to be potentially eligible. Disagreements were resolved by discussion and consensus. The first author extracted data on the patient group (P), intervention (I), comparison (C) and outcome (O). Included studies were categorized according to indication for FMT. If patients received FMT for multiple indications [e.g., inflammatory bowel disease (IBD) and CDI] patients were categorized according to the condition for which the primary endpoint of the study was established.
Methodological quality of included studies
The Cochrane risk of bias tool was used to assess the methodological quality of the included RCT’s, each study was assessed for sequence generation, allocation concealment, blinding, handling of incomplete outcome data, selective outcome reporting and other sources of bias[15]. These items were rated as low (e.g., the study was double-blind and an identical placebo was used), high (e.g., study was open label), or unclear risk of bias (e.g., procedures for blinding were not adequately described). As no validated tool for the assessment of risk of bias in observational studies was available, we used the eight criteria for quality assessment of case series, published by Chambers et al[16]. These criteria address both quality of reporting as risk of bias. Each study was assessed for: adequate reporting of eligibility criteria, representative patient population, reporting measures of variability, reporting of loss to follow-up, follow-up of at least 90% of the included patients, prospective inclusion, consecutive recruiting of patients and relevant prognostic factors. These items were rated as “yes” or “no” resulting in an overall rating of “good”, if the answer was ‘‘yes’’ to all eight criteria; “satisfactory”, if the answer was ‘‘yes’’ to criteria 2, 4-7 and “poor”, if the answer was not ‘‘yes’’ to one or more of the criteria listed for ‘‘satisfactory.’’
Statistical analysis
The efficacy of treatment was compared across studies per treatment category. If more than one RCT was available per indication, a meta-analysis on efficacy of treatment was performed as appropriate. We intended to pool the data for meta-analyses if the patient groups, outcomes and interventions were sufficiently similar. This was determined by consensus. For case series, a summary of efficacy of treatment was reported. The overall treatment effect of FMT was calculated as the percentage of patients who received FMT and achieved clinical improvement per treatment category. All analyses were carried out on an intention-to-treat (ITT) basis. As such, dropouts or withdrawals before the completion of the studies were considered to be treatment failures. If possible, the presence of heterogeneity among studies was assessed using the χ2 test, the I2 statistic was used to assess the degree of inconsistency between the trials[17]. Sensitivity analyses were performed to investigate statistically significant heterogeneity. A sensitivity analysis was conducted to determine the impact of trial quality on the overall results. Trials deemed to be at high risk of bias were excluded from the analysis to see if the results changed. Efficacy of FMT was compared per route of administration (nasogastric or nasoduodenal tube infusion vs infusion into the colon vs retention enema). Data were analyzed using the SPSS statistics 20 software.
RESULTS
Study selection
After duplicate removal, the search yielded 2029 records. Based on screening of title and abstract 1817 records were excluded, mainly because the topic did not pertain to FMT. For the remaining 212 records, reasons for exclusion are shown in Figure 1. Forty-five studies met the inclusion criteria and were included in the review. Only two RCTs were found, all other studies were retrospective series or pilot studies.
Figure 1.
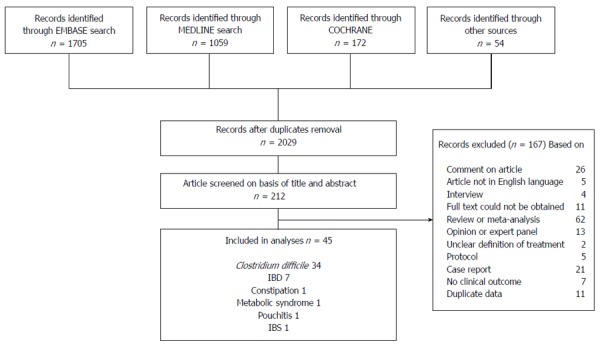
Identification, screening, eligibility and inclusion of studies. IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome.
Risk of bias within studies
A quality assessment of included case series is presented in Table 1. Forty-two case series were rated as “poor”, only one of the included case series was rated as “satisfactory”. None of the case series was considered to be of “good” quality. In 15 of the 43 case series, patients were prospectively included. Quality assessment of two included randomised studies is shown in Table 2. The study performed by Vrieze et al[10] was rated as high methodological quality on four out of five items, the study by van Nood et al[18] was rated as “high” methodological quality on three items. A sensitivity analysis to determine the impact of trial quality on the overall results could not be properly executed, due to the overall “poor” assessed quality of the included case series. Excluding the 32 case-series deemed to be at high risk of bias for sub-analyses of efficacy of fecal transplantation in CDI would result in determination of treatment effect in only one case series qualified as “satisfactory” compared to the only RCT included for this indication. In IBD, all included 7 studies were assessed equally as “poor” quality, which made further comparison between studies impossible.
Table 1.
Quality assessment of selected case series according to the Chambers criteria
| Indication for FMT | Author | Year | Publication type (J, CA) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Case series quality rating |
| CDI | Aas | 2003 | J | + | + | + | + | + | - | - | - | Poor |
| Arkkila | 2010 | J | - | + | + | + | + | + | - | - | Poor | |
| Aroniadis | 2013 | J | + | - | + | + | + | - | - | - | Poor | |
| Bansal | 2013 | J | - | + | - | - | ? | - | - | - | Poor | |
| Bobo | 2013 | CA | + | - | + | + | + | + | - | - | Poor | |
| Borody | 2013 | CA | - | + | + | - | ? | - | - | - | Poor | |
| Bowden | 1981 | J | - | - | + | + | + | - | - | - | Poor | |
| Brandt | 2012 | J | + | + | + | + | + | - | - | - | Poor | |
| Byrne | 2008 | CA | + | + | - | - | + | + | - | - | Poor | |
| Eisman | 1958 | J | - | - | - | - | - | - | - | - | Poor | |
| Elopre | 2013 | J | - | - | - | - | + | - | - | - | Poor | |
| Fischer | 2013 | CA | - | - | + | + | + | - | - | + | Poor | |
| Garborg | 2010 | J | + | + | - | + | + | - | - | - | Poor | |
| Hamilton | 2012 | J | + | + | + | + | + | + | + | + | Good | |
| Ihunnah | 2013 | CA | - | - | + | + | - | - | - | - | Poor | |
| Jorup-Rönström | 2012 | J | + | + | + | + | + | - | - | - | Poor | |
| Kassam | 2010 | CA | - | + | + | + | + | + | + | - | Satisfactory | |
| Kelly | 2012 | J | - | + | + | + | + | - | - | - | Poor | |
| Khanna | 2013 | CA | + | + | + | - | + | + | - | - | Poor | |
| Louie | 2013 | CA | + | - | - | - | - | - | - | - | Poor | |
| MacConnachie | 2009 | J | + | + | - | - | + | - | - | - | Poor | |
| Mattila | 2012 | J | + | + | + | + | + | - | - | + | Poor | |
| Mellow | 2010 | J | - | + | + | + | + | - | - | - | Poor | |
| Miller | 2010 | J | - | + | - | - | + | - | - | - | Poor | |
| Neelakanta | 2011 | J | - | - | - | + | + | - | - | - | Poor | |
| Newton | 2013 | CA | - | - | - | - | - | - | + | - | Poor | |
| Potakamuri | 2013 | CA | - | + | + | - | ? | - | - | - | Poor | |
| Rohlke | 2010 | J | - | + | - | + | + | - | - | - | Poor | |
| Rubin | 2013 | J | + | + | + | + | + | - | - | - | Poor | |
| Shiekh Sroujieh | 2012 | CA | + | + | + | + | + | + | - | - | Poor | |
| Silverman | 2010 | J | - | + | - | - | + | - | - | - | Poor | |
| Yoon | 2010 | J | + | + | + | + | + | - | - | - | Poor | |
| Youngster | 2013 | CA | + | + | - | + | + | + | - | - | Poor | |
| IBD | Angelberger | 2012 | J | - | + | - | + | + | + | - | - | Poor |
| Borody | 2012 | CA | - | + | + | +2 | +2 | - | - | - | Poor | |
| Greenberg | 2013 | CA | - | + | + | -1 | -1 | - | - | + | Poor | |
| Kump | 2013 | CA | - | + | - | + | + | + | - | - | Poor | |
| Kump | 2013 | J | + | + | + | + | + | + | - | + | Poor | |
| Kunde | 2013 | J | + | + | + | + | + | + | - | +/- | Poor | |
| Vermeire | 2012 | CA | - | + | + | + | + | + | - | - | Poor | |
| IBS | Pinn | 2013 | CA | - | - | + | - | + | - | - | - | Poor |
| Pouchitis | Landy | 2013 | CA | + | - | + | + | + | + | - | - | Poor |
| Constipation | Borody | 2001 | J | - | - | - | - | + | + | - | - | Poor |
Sixteen out of 21 treated patients were successfully contacted for FU;
62 patients with FU results were included. Chambers criteria: (1) were selection/eligibility criteria adequately reported? (2) was the selected population representative of that seen in normal practice? (3) was an appropriate measure of variability reported? (4) was loss to follow-up reported or explained? (5) were at least 90% of those included at baseline followed up? (6) were patients recruited prospectively? (7) were patients recruited consecutively? and (8) did the study report relevant prognostic factors? J: Journal article; CA: Conference abstract. ?: Unknown; CDI: Clostridium difficile-infection; IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome.
Table 2.
Methodological quality of included randomised trials
| Ref. | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting |
| Vrieze et al[10] | Low1 | Unclear2 | Low3 | Low4 | Low |
| van Nood et al[18] | Low1 | Unclear2 | High3 | Low | Low |
Automated biased coin minimization, computer generated randomisation not in the paper but verified by the first author;
Not described; rated as unclear for this item;
Patients were randomised to either allogenic or autologous feces a, open label design;
All patients completed the study; two subjects were excluded from analyses because of antibiotic use during the trial unrelated to the microbial infusion, all except one patient (due to a clinically driven protocol deviation) were taken into the intention to treat analyses.
Patients, treatment information, and donor screening
The studies were published between 1958 and 2013. A total number of 1029 patients underwent FMT. The clinical efficacy of FMT was assessed in patients with: CDI[2,19-50] (n = 883), IBD[5,51-56] (n = 112), IBS[57] (n = 13), pouchitis[9] (n = 8), constipation[58] (n = 3) and metabolic syndrome[10] (n = 10 randomised to the donor feces arm). Age of the included patients varied widely from 6 to 94 years. Two studies on fecal transplantation in pseudomembranous colitis published in 1958 and 1981 were regarded as fitting the diagnoses of CDI although determination of Clostridium toxin was not available in the first study and not routinely used in 1981[2,50]. Assessment of Clostridium toxin in the stool was not performed in all studies to confirm the diagnose CDI before treatment, nor to assess whether there was adequate clearance of CDI after treatment. Most of the studies measured clinical response with regard to patients’ symptoms. The diagnoses of IBD was confirmed by pathology in three studies[5,53,55], the other four studies did not confirm the diagnoses of IBD beyond clinical diagnoses by the treating physician[51,52,54,56]. Pinn et al[57] did not describe criteria for the diagnoses of IBS and included patients with diarrhea-predominant, constipation-predominant and IBS patients with alternating stool pattern. Landy at al[9] confirmed chronic refractory pouchitis clinically, endoscopically and histologically. Borody et al[58] defined chronic constipation as a stool frequency of once every four to seven days associated with symptoms. Vrieze et al[10] used the following criteria for recruiting patients with a metabolic syndrome: a body mass index > 30 kg/m2 or waist circumference > 102 cm and a fasting plasma glucose level > 5.6 mmol/L.
Follow-up varied between ten days to eight years in CDI, 12 wk to 16.5 years in IBD, six to 18 mo in IBS, four weeks in pouchitis, one to 28 mo in constipation and six weeks in metabolic syndrome. Of the 45 included studies, two were randomised trials of FMT for CDI and metabolic syndrome, in which FMT was compared with active comparators or placebo respectively. van Nood et al[18] conducted an open-label, RCT in patients with CDI in which the infusion of donor feces was preceded by a short regimen of vancomycin and bowel lavage, a standard vancomycin regimen, or a standard vancomycin regimen with bowel lavage. Vrieze et al[10] conducted a double-blind placebo controlled trial, which compared the infusion of fecal intestinal microbiota from lean donors to autologous microbiota infusion in male recipients with metabolic syndrome. The remaining 43 included studies were uncontrolled case series, in which patients were treated with FMT via the upper gastrointestinal tract (tube infusion via the stomach, duodenum or jejunum or oral ingestion of gelatin coated capsules containing microbes after centrifugation of a suspension of donor feces) or via the lower gastrointestinal tract or colon (infusion via the endoscope channel into the terminal ileum, coecum or sigmoid or rectal infusion by enema’s). Infusion via the upper GI route supposedly leads to more profound replacement of the microbiota in the small bowel and proximal colon. The mode of infusion for each study was categorized into administration via the upper GI tract (U), colon (C) or retention enema (Ce) Table 3. The amount of fresh feces prepared for infusion or the amount of infused fecal suspension was reported in 23 studies and varied from 30 to 250 g of fresh stool, 20 mL to 350 mL of fresh stool, 6 to 8 tablespoons of fresh stool in studies in which the amount of prepared feces per treatment was reported and 30 to 700 mL fecal infusion if the amount of infused suspension after adding saline solution was reported. FMT regiments varied between single treatments to 14-d regiments (Table 3). Different donors were used among studies; donors could be family friends, partners, relatives, friends or unrelated healthy subjects. Relation of the donor to the patient was expressed in 3 categories: “genetically related” (e.g., 1st or 2nd degree relative), “sharing the same household”; (e.g., partner) or “other” (e.g., healthy volunteer) (Table 3). Table 4 shows the protocol for screening of fecal donors as used in the two RCT’s[10,18]. In 2013, already an optimized screening protocol for fecal donors was published by the same authors[59], which concerns not only the risk for transmission of infectious diseases, but also to the risk of transmitting other (autoimmune) diseases with regard to several conditions that may be transferred through feces.
Table 3.
Treatment information on fecal microbiota transplantation, summarised for all studies
| Indication | First author | Year | Pre-treatment with bowel lavage? (Y/N) | Route of administration1 | Number of transplantations (n) | Amount of fresh stool per treatment (mL/g/tablespoons) | Suspension infused (cc or mL) | Donor2 | ae (n) | Withdrawal due to ae (n) | Ae potentially associated with fmt3 (n) | Sae (n) |
| CDI | Aas | 2003 | NM | U | 1 | 30 g | NM | G and O | 0 | 0 | 0 | 24 |
| Arkkila | 2010 | Y | C | 1-2 | 20-30 mL | NM | G or H? and O | NM | NM | 0 | 14 | |
| Aroniadis | 2013 | NM | NM | 1-2 | NM | NM | NM | NM | NM | 0 | 14 | |
| Bansal | 2013 | NM | U and C | NM | NM | NM | G or H? and O | NM | NM | NM | NM | |
| Bobo | 2013 | NM | U and Ce | 1-2 | NM | NM | NM | NM | NM | NM | NM | |
| Borody | 2013 | NM | NM | 1-42 | NM | NM | NM | 0 | 0 | 0 | 0 | |
| Bowden | 1981 | N | Ce and U | 1 | NM | NM | H and O | 0 | 0 | 0 | 34 | |
| Brandt | 2012 | NM | C | 1 | NM | 300-700 cc infused | G and H and O | 0 | 0 | 0 | 0 | |
| Byrne | 2008 | N | Ce | 1-3 | 300-500 g | NM | G and H and O | 4 | 0 | 0 | 0 | |
| Eisman | 1958 | N | Ce | 4 | NM | NM | O | 0 | 0 | 0 | 0 | |
| Elopre | 2013 | NM | U | 1 | NM | NM | G | 0 | 0 | 0 | 0 | |
| Fischer | 2013 | Y | C | 1-2 | NM | NM | O | 0 | 0 | 0 | 0 | |
| Garborg | 2010 | N | U or C | 1 | 50-100 g | NM | G and H and O | 0 | 0 | 0 | 54 | |
| Hamilton | 2012 | Y | C | 1-2 | 50 g | NM | G and H and O | 155 | 0 | 0 | 0 | |
| Ihunnah | 2013 | NM | NM | 1-2 | NM | NM | NM | 5 | NM | 0 | 24 , 86 | |
| Jorup-Rönström | 2012 | NM | Ce | 1-3 | 30 cc suspension | 30 cc suspension | O | 0 | 0 | 0 | 0 | |
| Kassam | 2010 | N | Ce | 1-2 | NM | NM | NM | 0 | 0 | 0 | 0 | |
| Kelly | 2012 | Y | C | 1 | 6-8 tablespoons | NM | R | 0 | 0 | 0 | 0 | |
| Khanna | 2013 | NM | C | 1 | 50 g | NM | NM | 0 | 0 | 0 | 0 | |
| Louie | 2013 | Y | U | 24-34 caps | 50 g | NM | R | NM | NM | NM | NM | |
| MacConnachie | 2009 | N | U | 1 | 30 g | NM | O | 0 | 0 | 0 | NM | |
| Mattila | 2012 | Y | C | 1 | 20-30 mL | 100 mL suspension | G and H and O | 0 | 0 | 0 | 144 | |
| Mellow | 2010 | NM | C | 1 | NM | NM | NM | 0 | 0 | 0 | 34 | |
| Miller | 2010 | NM | C | 1 | NM | NM | G and H | NM | 0 | NM | NM | |
| Neelakanta | 2011 | NM | C | 1 | pt 1: 250 g, pt2: NM | NM | G or H | NM | NM | NM | NM | |
| Newton | 2013 | NM | NM | NM | NM | NM | NM | NM | NM | NM | 34 | |
| Potakamuri | 2013 | NM | NM | 1-5 | NM | NM | NM | NM | NM | NM | 2 (14, 16) | |
| Rohlke | 2010 | Y | C | 1 | NM | 200-350 mL infused | G and H and O | NM | NM | NM | NM | |
| Rubin | 2013 | N | U | 1 | 25 mL | NM | O | 0 | 0 | 0 | 0 | |
| Shiekh Sroujieh | 2012 | NM | U or C | 1 | 30-50 g | NM | NM | 0 | 0 | 0 | 0 | |
| Silverman | 2010 | N | Ce | 1-2 | 50 mL | NM | G and H | 47 | 0 | 0 | 0 | |
| CDI | Van Nood | 2013 | Y | U | 1-2 | > 50 g | NM | G and H and O | 15' | 0 | 158 | 16 |
| Yoon | 2010 | NM | C | 1 | NM | NM | G and H | 0 | 0 | 0 | 0 | |
| Youngster | 2013 | NM | U or C | 1-2 | NM | NM | NM | 0 | NM | 0 | NM | |
| IBD | Angelberger | 2013 | Y | U and Ce | 3 | NM | 23.8 g (16.7-25)U, 20 g (6-21.7)C | O | 5 | 0 | 29 | 0 |
| Greenberg | 2013 | NM | U or C + Ce | > 1 | NM | NM | NM | 3 | 0 | 0 | 0 | |
| Borody | 2012 | NM | NM | NM | NM | NM | NM | NM | NM | NM | 0 | |
| Kump | 2013 | NM | C | 2-5 | NM | NM | O | NM | 0 | NM | 0 | |
| Kump | 2013 | Y | C | 1 | 100-150 g | NM | O | 1 | 0 | 110 | 0 | |
| Kunde | 2013 | N | Ce | 511 | 90 g (70-113) | NM | G and O | 9 | 112 | 210 | 0 | |
| Vermeire | 2012 | Y | U | 3 | 200 g | NM | NM | 3 | 0 | 310 | 0 | |
| IBS | Pinn | 2013 | NM | NM | 1-3 | NM | NM | NM | NM | NM | NM | NM |
| Pouchitis | Landy | 2013 | N | U | 1 | 30 g | NM | NM | NM | NM | NM | NM |
| Constipation | Borody | 2001 | Y | Ce | 5-14 | NM | NM | NM | NM | NM | NM | NM |
| Metabolic syndrome | Vrieze | 2012 | Y | U | 1 | NM | NM | O | NM | NM | NM | NM |
Route of administration: Upper GI tract (U), colon (C) or colon per enema (Ce);
Genetic related (G), sharing the same household (H) or other (O);
Perforation, post-transplant spesis/ bacteriemia/ transmission of communicable diseases;
Death, both unrelated and related;
Some irregularity of bowel movements and excessive flatulence during the first couple of weeks following the procedure;
Hospitalised;
Post infectious IBS (1). antibiotic treatment for urinary tract infections (2). Antibiotics for perioperative prophylaxis of a hip replacement (1). None of these 3 patients relapsed with CDI despite the antibiotic therapy;
(94%) had diarrhea, cramping (31%) and belching (19%): symptoms resolved within 3 h. During FU 3 patients who were treated with donor feces (19%) had constipation; 9Fever: blood cultures were taken, but no bacterial pathogen was detectable (2);
Fever;
Intolerance with immediate leaking of enemas for 3 consecutive days;
Daily for 5 d. NM: Not mentioned; IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome.
Table 4.
Donor screening for fecal microbiota transplantation
| Screening questionnaire1 |
| A questionnaire addressing risk factors for potentially transmissible diseases |
| Fecal test |
| Parasites, including Blastocystis hominis and Dientamoeba fragilis |
| Clostridium difficile, and enteropathogenic bacteria |
| Serology |
| Antibodies to HIV, human T-cell lymphotropic virus types 1 and 2, hepatitis A, B, and C, Cytomegalovirus, Epstein-Barr virus |
| Treponema pallidum, Strongyloides stercoralis, and Entamoeba histolytica |
Efficacy of FMT in CDI and IBD
CDI: In 33 case series published on CDI, the efficacy of FMT (defined as “resolution of diarrhea”) ranged from 87.8% to 90.0% in repeated FMT’s, comparable to a treatment effect of 81% to 94% in repeated FMT's in the single published RCT. Treatment efficacy > 80% was achieved in severe and complicated CDI[47], hospitalized patients[45], immunocompromised patients[26,41], patients with > 3 episodes of CDI in their medical history[32] and patients with underlying IBD[38,44]. Resolution of diarrhea and relapse-free FU (reported in 21 out of 34 studies) was 80.9% (range 46% to 100%). Number, age and gender of patients enrolled, additional clinical data on patient group, duration of follow-up, primary outcome and the percentage of included patients free from relapse during follow-up are shown in Table 5.
Table 5.
Studies on fecal microbiota transplantation in Clostridium difficile-infection, outcome data
| First author | Year | Patients enrolled (n) | Age (mean ± SD or median, range/IQR) | Male sex (n) | FU | Primary endpoint | Resolution of diarrhea | Resolution of diarrhea + free from relapse during FU |
| Aas | 2003 | 181 | 73 ± 9 | 5 | 3 mo | 90 d | 94% | \ |
| Arkkila | 2010 | 37 | 69 (24-90) | 12 mo | 92% | 86% | ||
| Aroniadis | 2013 | 132 | 70 (38-89) | 3 | 15 mo (1-42) | 1-7 d | 84%, 92%3 | 50% |
| Bansal | 2013 | 12 | 70 (31-96) | 4 | 3 mo | > 90 d | 92% | \ |
| Bobo | 2013 | 214 | 70.9 ± 11.9 | 10 | 1 mo | 30 d | 95% | \ |
| Borody | 2013 | 285 | F:36 ± 18.1 M: 31 ± 16 | 17 | 86% | |||
| Bowden | 1981 | 16 | 56 (14-85) | 7 | 12 d | 12 d | 81% | \ |
| Brandt | 2012 | 77 | 65 ± 17 | 21 | 17 mo (3-68) | 90 d | 91% | 81% |
| Byrne | 2008 | 45 | 62 (30-91) | 12 | 12 mo | 96% | ||
| Eisman | 1958 | 4 | 45-68 | 3 | < 10 d | 24-48 h | 100% | 100% |
| Elopre | 2013 | 26 | 48, 48 | 1 | 5 yr and 6 wk | 1 d | 100% | 100% |
| Fischer | 2013 | 127 | 46 ± 17 | 7 | 30 d | 7 d | 75%/, 92%/3 | 75% |
| Garborg | 2010 | 39 | 75 (53-94) | 18 | 3 mo | 80 d | 73%, 83%3 | \ |
| Hamilton | 2012 | 435 | 69 ± 21 | 12 | 2 mo | 1-2 mo | 86%, 95%3 | |
| Ihunnah | 2013 | 668 | 12 mo (3-51) | 78%, 89%3 | 78% after 12 wk | |||
| Jorup-Rönström | 2012 | 32 | 75 (27–94) | 12 | 26 mo (1-68) | 69% | ||
| Kassam | 2010 | 141 | 65.3 (26-87) | 7 | 7 mo | 24 h | 100% | |
| Kelly | 2012 | 26 | 59 (19-86) | 2 | 11 mo (2-30) | post FMT | 92% | 85% |
| Khanna | 2013 | 135 | 27 (21-48) | 8 | 1-14 d | 50% | ||
| Louie | 2013 | 259 | 6 mo | 100% | 100% | |||
| MacConnachie | 2009 | 151 | 81.5 (68-95) | 14 | 4 mo (1-6) | 5-24 wk | 73%, 80%3 | 67% |
| Mattila | 2012 | 701 | 70 (22-90) | 28 | 12 mo | 12 wk | 94% | 89% |
| Mellow | 2010 | 131 | 67 (32-87) | 7 | 5 mo (1-10) | 30 d | 92% | 85% |
| Miller | 2010 | 2 | 34-50 | 0 | 9 mo, 1 mo | 9 mo, 1 mo | 100% | \ |
| Neelakanta | 2011 | 25 | 27-39 | 1 | 12 mo, 5 mo | 2 wk, post FMT | 50% | 50% |
| Newton | 2013 | 176 | 90 d | post FMT | 94% | 76% | ||
| Potakamuri | 2013 | 13 | 73.8 ± 18.8 | 2 | 5 wk- 18 mo | > 1 mo | 92% | 46% |
| Rohlke | 2010 | 19 | 49 (29-82) | 2 | 27.2 yr (6-65) | 6 mo | 95%, 100%3 | 79% |
| Rubin | 2013 | 7418 | 63 (6-94) | 26 | 2 mo | 60 d | 79% | 58% |
| Shiekh Sroujieh | 2012 | 68 | 66 (16-93) | 100 d | 1-4 d | 100% | 100% | |
| Silverman | 2010 | 7 | 72 (30-88) | 4 | 4- 14 mo | post FMT | 100% | 100% |
| Van Nood | 2013 | 16110 | 73 ± 13 | 8 | 2.5- 5 mo | 10 wk | 81%, 94%3 | 81% |
| Yoon | 2010 | 12 | 66 (30-86) | 3 | 3 wk- 8 yr | 3-5 d | 100% | 100% |
| Youngster | 2013 | 12 | 2 mo | 8 wk | 92% | \ |
Recurrent/refractory CDI. Aas et al (18), Kassam et al (7), MacConnachie et al (15), Mattila et al (70), Mellow et al (13), Rubin et al (74), van Nood et al (16);
Severe CDI 84%, complicated CDI 92%;
Resolution of diarrhea (% of the patients) after 2 FMT's;
All patients were hospitalised at inclusion;
IBD. Hamilt et al (14): CD (6), UC (4), lymphocytic colitis (4). Neelak et al (1 UC, 1 CD). Khanna et al CD (7), UC (6). Borody et al CD (14), UC (14);
Patients were immunocompromised: upon review of their medical history, Newton et al (7) based on HIV Elopre et al (2);
Other diagnoses (12): UC (3), UC and livertransplant for PSC (1), CD(3), multivisceral transplant (2), multiple myeloma (1), lung transplant (1), renal transplant (1);
Cases included 5 pediatric (Ihunnah et al) and 2 pediatric patients (Rubin et al);
> 3 episodes (25);
Amount of patients randomised to intervention (FMT) arm. /: Outcome defined as negative stool test (PCR) after FMT only; \: No further follow up after archivieving the primary endpoint. FMT: Fecal microbiota transplantation; FU: Follow-up.
IBD: Of patients treated with FMT for IBD, six patients were treated for CD and 106 for UC; four UC patients treated by Greenberg et al[56]. had concomitant CDI. All patients had active disease at inclusion varying from mild disease activity to therapy refractory disease. Location of IBD was reported in three out of seven studies. CD was located ileocolonic (n = 3) and restricted to the colon (n = 1) in the series published by Vermeire et al[55]. Extent of disease in UC was mostly a pancolitis[52,53]. Response to therapy was measured by five different assessments in UC: patient reporting of symptoms on a questionnaire comparing pre- and post-FMT data[56]; (clinical) Mayo score[53,60]; the total Mayo score[51]; the Paediatric UC Activity Index in children[52]; and the modified Powell-Tuck index[5]. In CD, two different clinical evaluation tools were used: “patient reporting of symptoms on a questionnaire comparing pre- and post-FMT data”[56] and the Crohn’s Disease Activity Index[55]. Five of the included studies used endoscopy for evaluation of mucosal response. Patients underwent endoscopy shortly after treatment (range 1 d to 90 d)[51,53,54], or on the longer term (1-198 mo, 34% of the patients were evaluated by endoscopy) in UC[5]. CD patients were evaluated by endoscopy eight weeks after FMT[55].
Clinical outcome data (measured by different standards) for FMT in IBD are shown in Table 6. In three of six studies on UC in which clinical remission was reported the percentage of patients who achieved clinical remission varied between 0% and 68%[5,52,53]. Clinical improvement was reported in six studies and varied between 20% and 92%[5,51-54,56].
Table 6.
Studies on fecal microbiota transplantation in inflammatory bowel disease, treatment and outcome data
| Author | Publication year | Patients enrolled (n) | Diagnose, disease activity | Age (mean ± SD or median, range/ IQR) | Male sex (n) | FU (mo) | Medication use during study (n) | Timepoint primary endpoint (mo) | Clinical improvement | Clinical remission | Cessation of medication during FU (n/total number of patients on the drug) |
| Angelberger | 2013 | 5 | Refractory UC | 27 (22-51) | 3 | 7 | 5-asa (3), Immunosuppressive therapy stopped prior to FMT | 3 | 20% | 0 | |
| Borody | 2012 | 62 | Active UC | M: 42.3 ± 11.5 F: 48.45 ± 16.49 | 40 | 1-1981 | NM | 92% | 68% | ||
| Greenberg | 2013 | 16 | Refractory CD (2)/UC (14)2 | 39 (20-75) | 9 | 4.5-30 | Steroids (10), antitnf (4), 6MP (1) | After FMT | 63% | Steroids: stopped (4/10), decreased dose (3/10). anti-TNF stopped (1/4) | |
| Kump | 2013 | 9 | Refractory UC | NM | NM | 3 | 3 | 56% | |||
| Kump | 2013 | 6 | Refractory UC | 36 (17-52) | 3 | 12 | 3 | 33% | 0% | ||
| Kunde | 2013 | 103 | Active UC4 | (7-20) | 6 | 3 | 5-asa (7), 6MP (4), steroids (3) | 0.25 | 70% | 30% | 0 |
| Vermeire | 2012 | 4 | Refractory CD | 37.5 (29-50) | 1 | 2 | 2 | 0% | 0% |
Endoscopic follow-up (n = 21);
Concomitant CDI was present in 4 UC patients;
Ref Kunde: 1 subject did not tollerate treatment, this subject was considered to be a treatment failure. Endpoint data of the study was adjusted in this table;
Active disease diagnosed by colonoscopy < 6 mo before the enrollment. UC: Ulcerative colitis; FMT: Fecal microbiota transplantation; CD: Crohn’s disease; FU: Follow-up; NM: Not mentioned.
In CD, the four patients treated by Vermeire et al[55] did not experience clinical improvement after FMT. Greenberg et al[56] reported “improved frequency of disease flares” in 63% of the patients; combined for both UC and CD, results for “improvement of diarrhea” were reported separately, and one out of two treated CD patients reported a decrease in diarrhea frequency. In the four CD patients in whom an endoscopy was performed 8 wk after treatment, no endoscopic benefit was observed[55].
FMT in other indications
In total, three patients were treated for chronic constipation as part of a case series on FMT in both chronic constipation and UC[58]. In 100% of the patients there was complete reversal of constipation; defecation occurred one to two times per day without laxatives with an accompanying resolution of most associated symptoms such as episodic nausea and vomiting, bloating and abdominal pain, after FMT. Pinn et al[57] treated 13 IBS patients, resolution or improvement of symptoms was reported in 70%, including abdominal pain (72%), bowel habit (69%), dyspepsia (67%), bloating (50%), and flatus (42%). Eight pouchitis patients were treated by Landy et al[9], none of these patients achieved clinical remission after FMT measured by the pouch disease activity index, but two patients demonstrated a change to a ciprofloxacin sensitive bacteria following FMT. In a series of 18 male subjects diagnosed with a metabolic syndrome there was a statistically significant increase in peripheral insulin sensitivity measured by Hyperinsulinemic-Euglycemic Clamp (median rate of glucose disappearance changed from 26.2 to 45.3 μmol/kg per minute; P < 0.05) of recipients treated with donor feces compared to placebo[10].
Safety of fecal transplantation
SAE’s were reported in 34 out of 45 studies. In total, 35 (3.4%, all CDI cases) of 1029 patients, were reported to have died and 10 (0.97%) (out-)patients were hospitalised during FU. The number of AE’s, withdrawal due to AE’s, AE’s potentially associated with FMT and SAE’s are reported in (Table 3). One patient died from aspiration during sedation for FMT administered via colonoscopy, which was considered to be related to the FMT procedure[37]. Four patients were reported to have died from complicated CDI with small bowel involvement confirmed at autopsy (n = 1), a toxic megacolon due to persistent CDI one month after FMT (n = 1), and complicated CDI not further specified (n = 2)[39,48,50]. A severely ill patient treated with FMT for CDI, died of a peritonitis which could be related to treatment[49]. In the other 29 patients the cause of death was not related to CDI illness or of unknown cause. Reasons for hospitalisation included: cecal perforation during FMT treated with colectomy (n = 1), symptomatic choledocholithiasis (n = 1) and not further specified in eight patients[18,25,37]. Reported AE’s associated with FMT (Table 3) were mostly self-limiting and occurred frequently within hours after infusion. Intestinal reported symptoms were: bloating, flatulence, belching and abdominal cramps, remaining IBS-like symptoms after CDI clearance post FMT, abdominal discomfort, irregularity of bowel movements and vomiting. In 11 patients (all treated for IBD; three for CD and eight for UC) fever, without other clinical symptoms or signs of sepsis, was reported during and up to one day after FMT[51-53,55]. No causative agents were identified by blood culture, but a rise in CRP was measured in some of these patients. Fever disappeared within three days in all patients. Withdrawal due to treatment intolerance (leaking of enemas for three days) occurred in one adolescent[52]. Less likely related reported AE’s were: fatigue, itchiness, erythema, paraesthesia on the hip, collapse, and blisters on the tongue. A superficial mucosal tear caused by the FMT via colonoscopy was reported. Transmission of communicable diseases due to FMT did not occur in any patient.
Additional analyses
In CDI patients, the proportion of patients who achieved resolution of diarrhea after administration of FMT via the upper GI tract: 84.2% (n = 150), into the colon: 89.4% (n = 326) and per retention enema: 88.5% (n = 102) was comparable, P = 0.26. In the majority of UC patients (72%), mode of administration of FMT was not reported. In CD, not from all six patients the route of administration was reported and a comparison of efficacy of treatment according to infusion manner could not be made. A comparison of efficacy in age groups < 65 and > 65 years could not be executed due to a wide range and large overlap in age of the patients in the included case series.
DISCUSSION
The results from 33 case series suggest that fecal transplantation is a highly effective therapy for CDI with response rates up to 90% resolution of diarrhea. This is corroborated with a treatment effect of 81% to 94% in repeated FMT in the only randomized trial to date. All included studies reported > 50% efficacy, even in immunocompromised, severely ill and elderly patients, which was much higher than the 31% efficacy reported in patients treated with a standard regimen of vancomycin for CDI in the comparative arm of the randomized trial[18]. Comparable results were achieved for infusion of fecal content into either the upper GI tract, the colon or per retention enema. Studies on FMT in UC reported remission rates between 0% and 68%. Clinical improvement varied between 20% and 92%, but was measured using five different scales in six studies. The high response rate of 92% reported by Borody et al[5] is exceptional. This was a retrospective study, which is prone to inherent selection bias. Based on only six patients reported in the literature from two series, no clinical benefit of FMT has been observed in CD. In the single randomized trial to date on FMT in male subjects diagnosed with metabolic syndrome there was a statistically significant increase in insulin sensitivity of recipients treated with donor feces compared to placebo[10]. Positive results were achieved in small case series on chronic constipation (complete reversal of the disease in all three treated patients) and IBS (resolution or improvement of symptoms in 70% of 13 patients). FMT did not result in clinical remission in eight chronic refractory pouchitis cases, but in two patients a change in ciprofloxacin sensitivity was observed in cultures from coliform bacteria after FMT.
FMT was accompanied by mild, self-limiting gastrointestinal symptoms in the majority of patients. Transient fever was reported in 11 patients, with the striking finding that this occurred only in CD and UC patients. In 1029 treated patients, two possible related deaths occurred: one patient died from aspiration during sedation for FMT administered via colonoscopy and a severely ill CDI patient died of a peritonitis which could be possibly related to treatment. Transmission of communicable diseases due to FMT was not observed in any patient. In our own experience with more than 120 FMT’s via the nasojejunal route, as described in literature, transient fever only occurred in two UC patients and not occur in patients with CDI or metabolic syndrome. As long as the tip of the nasojejunal catheter is checked prior to infusion for adequate position in the duodenum, aspiration of fecal contents does not constitute a problem. Furthermore, we have not encountered transmission of microbial pathogens. In our opinion, FMT could be incorporated in clinical practice for CDI if there is adequate in house facilities. Currently, patients with IBD should only be treated in clinical trials, since there is a paucity of evidence in these patients.
The evidence for FMT in this systematic review is mostly based on case series of poor quality, with the exception of two RCT’s in CDI and metabolic syndrome, both from our own institution. Worldwide, FMT became quickly part of clinical care rather than an experimental treatment in series on CDI and IBD. Follow-up data were retrospectively collected in a selection of patients up to 16.5 years after treatment in 65% of the included series, which could have resulted in publication and selection bias. After agreement with the authors, four studies were excluded because of duplicate data (overlap of conference proceedings and corresponding full publication or overlap between patient populations)[58,61-63]. In four articles, the first authors could not confirm overlap between patient populations and we choose not to exclude these studies, which could have led to over- or underestimation of primary and secondary outcome data presented in this review[24,29,37,43]. The strength of our study also harbors its limitation. By including conference proceedings we strived to collect all available data on this novel treatment modality. However these abstract reports were brief and lacked details on the methods used for screening and FMT treatment. This approach bears the risk of reporting on studies that have not gone through the process of peer review.
More robust data on FMT will become available in the next two to three years. Currently, there are 12 trials on IBD; seven on UC, two on CD and three on IBD in general, and ten trials on CDI registered on clinical trials.gov. Fifteen of these studies are randomized trials. Single studies are registered for metabolic syndrome, IBS, pouchitis and healthy volunteers examining the restoration of the patient’s fecal microbiota after antimicrobial exposure. All of these trials will give rise to new research questions including preferred route of administration, and the number of FMT’s needed to attain remission or cure. In addition, by using FMT as a highly informative human model of the interaction between the gut microbiome and the host, a wealth of data will be generated regarding the pathophysiology of several diseases.
In conclusion, FMT appears to be highly effective in Clostridium difficile-infection and may be a promising therapy in ulcerative colitis. Infusion of donor feces significantly increased insulin sensitivity in male patients with a metabolic syndrome. As for Crohn’s disease, chronic constipation, pouchtis and IBS data are still too limited to draw conclusions. FMT is performed according to not yet standardized treatment protocols and despite the absence of infectious complications in 1029 patients reported in this review, vigilant surveillance of adverse events is needed. More randomized controlled data on the long-term efficacy of FMT as well as translational data on the impact of modulating the patient’s microbiota by the infusion of donor feces and all its contents are still warranted.
COMMENTS
Background
Fecal Microbiota transplantation (FMT) was first reported in the literature in 1958. Since that time, approximately 500 patients who received FMT were reported in the literature for different indications: infectious diarrhea [Clostridium difficile-infection (CDI)], gastro-intestinal diseases [inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), pouchitis, constipation) and Metabolic Syndrome. The majority these patients received FMT for CDI, which was proven as a more effective therapy when compared to treatment with antibiotics in a randomized controlled trial. FMT is widely used as clinical therapy for a wide range of indications whereas the available evidence in the literature is scarce.
Research frontiers
In order to accurately assess the application of FMT, the authors systematically reviewed the clinical efficacy and safety of FMT in different indications.
Innovations and breakthroughs
FMT is highly effective in CDI, and holds promise in ulcerative colitis. As for Crohn’s disease, chronic constipation, pouchitis and IBS data are too limited to draw conclusions. FMT increases insulin sensitivity in metabolic syndrome. Based on the current results, FMT can be considered as a safe treatment in the studied population.
Applications
Vigilant surveillance of adverse events is needed, since FMT is performed according to not yet standardized treatment protocols. More randomized controlled data on the long-term efficacy of FMT as well as translational data on the impact of modulating the patient’s microbiota by the infusion of donor feces and all its contents are still warranted.
Peer-review
This paper reviewed the clinical efficacy and safety of FMT on CDI, inflammatory bowel disease, metabolic syndrome, constipation, pouchitis and IBS. Promising results were obtained and further studies are needed to elucidate the mechanisms of FMT and to guard the adverse effects in large population of the patients.
Footnotes
Supported by “Dutch Digestive Foundation” Grant 2011 (WO 11-17) (to Rossen NG).
Conflict-of-interest: The authors declare that they have no commercial, personal, political, intellectual, or religious conflict of interest with respect to this manuscript.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at Dryad repository, who will provide a permanent, citable and open-access home for the dataset.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 30, 2014
First decision: October 14, 2014
Article in press: February 11, 2015
P- Reviewer: Adler MG, Carter D, Li SD S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Ge H. Zhou Hou Bei Ji Fang. Tianjin: Tianjin Science and Technology; 2000. [Google Scholar]
- 2.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 3.Schwan A, Sjölin S, Trottestam U, Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of normal faeces. Scand J Infect Dis. 1984;16:211–215. doi: 10.3109/00365548409087145. [DOI] [PubMed] [Google Scholar]
- 4.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 5.Borody T, Wettstein A, Campbell J, Leis S, Torres M, Finlayson S, Nowak A. Fecal microbiota transplantation in ulcerative colitis: Review of 24 years experience. Am J Gastroenterol Conf 77th Annu Sci Meet Am Coll Gastroenterol. 2012;107:S665. [Google Scholar]
- 6.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 7.Andrews PJ, Barnes P, Borody TJ. Chronic constipation reversed by restoration of bowel flora: A case and a hypothesis. Eur J Gastroenterol Hepatol. 1992;4:245–247. [Google Scholar]
- 8.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44:551–561. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 9.Landy J, Omar Al-Hassi H, Ronde E, Mann E, Peake S, McLaughlin S, Perry-Woodford ZL, Ciclitira PJ, Nicholls RJ, Clark SK, et al. A prospective controlled pilot study of faecal microbiota transplantation for chronic refractory pouchitis. ECCO Conf Abstr. 2013:P591. [Google Scholar]
- 10.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Borody TJ, Nowak A, Torres M, Campbell J, Finlayson S LS. Bacteriotherapy in chronic fatigue syndrome (CFS): a retrospective review. Am J Gastroenterol. 2012;107:1481. [Google Scholar]
- 12.Borody TJ, Campbell J, Torres M, Nowak A, Leis S. Reversal of idiopathic thrombocytopenic purpura with Fecal Microbiota Transplantation (FMT) Am J Gastroenterol. 2011;106:941. [Google Scholar]
- 13.Borody TJ, Leis S, Campbell J, Torres M, Nowak A, Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) Am J Gastroenterol. 2011:942. [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Version 510 (updated March 2011), Cochrane Collab; 2011. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 16.Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62:1253–1260.e4. doi: 10.1016/j.jclinepi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 19.Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, Alm EJ, Gevers D, Russell GH, Hohmann EL. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515–1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44:562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 21.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Shiekh Sroujieh L, Hassan M, Zainah H, Alangaden G, Jeepalyam S, Morilla Holguin ME, Johnson L, Zervos M, Ramesh M. Intestinal Microbiota Transplantation (Fecal Transplantation) for Clostridium difficile Infection-A Single Center Experience. ID week 1223. 2012 [Google Scholar]
- 23.Rubin TA, Gessert CE, Aas J, Bakken JS. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe. 2013;19:22–26. doi: 10.1016/j.anaerobe.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–570. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 25.Potakamuri Lakshmi N, Turnbough L, Maheshwari A, Kantsevoy S, Ofosu A, Thuluvath PJ. Effectiveness of Fecal Microbiota Transplantation for the treatment of recurrent Clostridium difficile infection: Community hospital experience. ACG Annu Sci Meet Abstr. 2013:P933. [Google Scholar]
- 26.Newton D. Fecal Biotherapy as treatment for recurrent Clostridium difficile infection in immunocompromised patients. ACG Annu Sci Meet Abstr. 2013:P925. [Google Scholar]
- 27.Neelakanta A, Moudgal V, Upadhyay N, Valenstein P, Gunaratnam NT. Successful Treatment of Refractory Clostridium difficile Infection(Cdi) With Intestinal Microbiota Transplant (IMT) in Two Patients With Inflammatory Bowel Disease (IBD) and Its Effects on IBD. Gastroenterology. 2011;142:S–395. [Google Scholar]
- 28.Miller CB, Dellon E, Isaacs K, Gangarosa L. Fecal bacteriotherapy via colonoscopy as rescue therapy for refractory and recurrent clostridium difficile - Associated diarrhea. Am J Gastroenterol. 2010;105:S323. [Google Scholar]
- 29.Mellow M. Colonoscopic fecal bacteriotherapy in the treatment of recurrent clostridium difficile infection-results and follow-up. Am J Gastroenterol. 2010;105:121–150. [PubMed] [Google Scholar]
- 30.Mattila E, Uusitalo-Seppälä R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, Moilanen V, Salminen K, Seppälä M, Mattila PS, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 31.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM. 2009;102:781–784. doi: 10.1093/qjmed/hcp118. [DOI] [PubMed] [Google Scholar]
- 32.Louie T, Cannon K, O’Grady H, Wu K, Ward L. Fecal microbiome transplantation (FMT) via oral fecal microbial capsules for recurrent Clostridium difficile infection (rCDI) ID week. 2013 [Google Scholar]
- 33.Khanna S, Kashyap PC, Rainey JF, Kammer PP, Loftus EV. Outcomes from Fecal Microbiota Trasplantation in adults with C. difficile Infection and Inflammatory Bowel Disease. ACG Annu Sci Meet Abstr. 2013:1657. [Google Scholar]
- 34.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 35.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal Transplantation via Retention Enema is Effective for Recurrent or Refractory Clostridium difficile-Associated Diarrhea. Gastroenterology. 2010;138:S207–S208. [Google Scholar]
- 36.Jorup-Rönström C, Håkanson A, Sandell S, Edvinsson O, Midtvedt T, Persson AK, Norin E. Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients. Scand J Gastroenterol. 2012;47:548–552. doi: 10.3109/00365521.2012.672587. [DOI] [PubMed] [Google Scholar]
- 37.Ihunnah C, Khoruts A, Fischer M, Afzali A, Aroniadis , Barto A, Borody TJ, Brandt LJ, Giovanelli A, Gordon S, et al. Fecal Microbiota Transplantation (FMT) for Treatment of Clostridium difficile Infection (CDI) in Immunocompromised Patients. ACG Annu Sci Meet Abstr. 2013:S179–S180. [Google Scholar]
- 38.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 39.Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42:857–861. doi: 10.3109/00365548.2010.499541. [DOI] [PubMed] [Google Scholar]
- 40.Fischer M. Fecal MIcrobiota Transplantation for recurrent Clostridium difficile in patients with prolonged immunosuppression. UEGW. 2013:P922. [Google Scholar]
- 41.Elopre L, Rodriguez M. Fecal microbiota therapy for recurrent Clostridium difficile infection in HIV-infected persons. Ann Intern Med. 2013;158:779–780. doi: 10.7326/0003-4819-158-10-201305210-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne B, Ward L, Louie M, Louie T, Krulicki WA, Louie TJ. Home-Based Fecal Flora Infusion to Arrest Multiply-Recurrent Clostridium difficile Infection(CDI). ID week k-4201 abstract 2008. ID week. 2008 [Google Scholar]
- 43.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 44.Borody TJ, Wettstein A, Nowak S, Finlayson SL. Fecal Microbiota Transplantation (FMT) eradicated clostridium difficile infection (CDI) in inflammatory bowel disease (IBD) UEGW. 2013 [Google Scholar]
- 45.Bobo L. Fecal Microbial Transplantation: Highly Effective Treatment for Severe Clostridium difficile Infection. ID week. 2013:281–284. [Google Scholar]
- 46.Siddharth B, Serban R, Kemal NR, Casey K, Dunnigan K. Fecal Microbiota Transplant for recurrent Clostridium difficile infection at a teaching hospital in upstate New York our experience. ACG Annu Sci Meet Abstr. 2013:P976. [Google Scholar]
- 47.Aroniadis OC, Brandt LJ, Greenberg A, Borody TJ, Mellow M, Surawicz C, Cagle LA, Neshatian L. Long-Term Follow-up Study of Fecal Microbiota Transplantation (FMT) for Severe or Complicated Clostridium difficile Infection (CDI) Gastroenterology. 2013;144:S185. doi: 10.1097/MCG.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 48.Arkkila PE, Uusitalo-Seppälä R, Lehtola L, Moilanen V, Ristikankare M, Mattila EJ. Fecal Bacteriotherapy for Recurrent Clostridium difficile Infection. Gastroenterology. 2010;138:S–5. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 49.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 50.Bowden TA, Mansberger AR, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178–183. [PubMed] [Google Scholar]
- 51.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 52.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 53.Kump PK, Gröchenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 54.Kump PK, Gröchenig HP, Spindelböck W, Gorkiewicz G, Wenzl H, Petritsch W, Reicht G. Preliminary clinivcal results of repeatedly fecal microbiota transplantation (FMT) in chronic activ ulcerative colitis. UEGW. 2013:OP187. [Google Scholar]
- 55.Vermeire S, Joossens M, Verbeke K, Hildebrand F, Machiels K, Van den Broeck K, Van Assche G, Paul J. Rutgeerts, Jeroen Raes2 3. Pilot Study on the Safety and Efficacy of Faecal Microbiota Transplantation in Refractory Crohn. Gastroenterology. 2012;142:P7160, S–360. [Google Scholar]
- 56.Greenberg A, Aroniadis O, Shelton C, Brandt LJ. Long-term follow-up study of fecal microbiota transplantation (FMT) for Inflammatory Bowel Disease (IBD) ACG Annu Sci Meet Abstr. 2013:P1629. [Google Scholar]
- 57.Pinn D, Aroniadis O, Brandt LJ. Follow-up study of fecal microbiota transplantation (FMT) for the treatment of refractory irritable bowel synrome (IBS) ACG Annu Sci Meet Abstr. 2013:P1688. [Google Scholar]
- 58.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 59.Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol. 2013;27:127–137. doi: 10.1016/j.bpg.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Kump PK, Gröchenig HP, Lackner S, Trajanovski S, Reicht G, Hoffmann , Wenzl HH, Petritsch W, Gorkiewicz G. Successful improvement of dysbiosis by fecal microbiota transplantation is not sufficient to induce clinical remission in chronic active ulcerative colitis. ECCO. 2013:P364. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 61.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Kelly C. Successful treatment of recurrent clostridium difficile infection with donor stool administered at colonoscopy: A case series. Am J Gastroenterol. 2010:S135. [Google Scholar]
- 63.Angelberger S, Lichtenberger C, Gratzer C, Papay P, Primas C, Eser A, Mikulits A, Dejaco C, Novacek G, Vogelsang H, et al. Fecal transplantation in patients with moderately to severely chronic active ulcerative colitis (UC) J Crohn’s Colitis. 2012;6:S159. [Google Scholar]


