Abstract
AIM: To investigate fiber and prebiotic supplementation of enteral nutrition (EN) for diarrhea, fecal microbiota and short-chain fatty acids (SCFAs).
METHODS: MEDLINE, EMBASE, Cochrane Library, CINAHL, Academic Search Premier, and Web of Science databases were searched for human experimental and observational cohort studies conducted between January 1990 and June 2014. The keywords used for the literature search were fiber, prebiotics and enteral nutrition. English language studies with adult patient populations on exclusive EN were selected. Abstracts and/or full texts of selected studies were reviewed and agreed upon by two independent researchers for inclusion in the meta-analysis. Tools used for the quality assessment were Jadad Scale and the Scottish Intercollegiate Guidelines Network Critical Appraisal of the Medical Literature.
RESULTS: A total of 456 possible articles were retrieved, and 430 were excluded due to lack of appropriate data. Of the 26 remaining studies, only eight investigated the effects of prebiotics. Results of the meta-analysis indicated that overall, fiber reduces diarrhea in patients receiving EN (OR = 0.47; 95%CI: 0.29-0.77; P = 0.02). Subgroup analysis revealed a positive effect of fiber supplementation in EN towards diarrhea in stable patients (OR = 0.31; 95%CI: 0.19-0.51; P < 0.01), but not in critically ill patients (OR = 0.89; 95%CI: 0.41-1.92; P = 0.77). Prebiotic supplementation in EN does not improve the incidence of diarrhea despite its manipulative effect on bifidobacteria concentrations and SCFA in healthy humans. In addition, the effect of fiber and/or prebiotic supplementation towards fecal microbiota and SCFA remain disputable.
CONCLUSION: Fiber helps minimize diarrhea in patients receiving EN, particularly in non-critically ill patients. However, the effect of prebiotics in moderating diarrhea is inconclusive.
Keywords: Bifidobacteria, Diarrhea, Enteral nutrition, Fiber, Prebiotics, Short-chain fatty acids
Core tip: Despite the importance of enteral nutrition (EN) for patients, diarrhea is a common complication in those receiving EN. Meta-analysis conducted in this review revealed that fiber supplementation in EN reduces diarrhea incidence. However, the positive effect is only seen in stable patients, and is not observed in critically ill patients. In addition, the effect of fiber and prebiotic supplementation towards fecal microbiota and short-chain fatty acids remain disputable due to the mixed findings. The heterogeneity of study populations, antibiotics therapies, and variation of the dosage for fiber and prebiotics likely explain such outcomes.
INTRODUCTION
Enteral nutrition (EN) is a beneficial support given to patients who are malnourished or at risk for malnutrition via oral nutritional supplements or tube feeding. Provision of nutrition through EN helps to maintain gut function by preventing mucosal atrophy[1], reducing endotoxin translocation[2], and preserving gut immunity[3]. However, despite its importance, diarrhea remains a common complication, affecting 2%-95% of patients who consume EN[4], with a higher incidence in critical care settings[5], depending on subjects and how diarrhea is defined[6]. Diarrhea not only inconveniences patients and their caretakers, but it also contributes to negative clinical consequences.
There are a number of factors involved in the pathogenesis of diarrhea during EN, including enteropathogenic infection, use of antibiotics, and altered physiologic response[4]. Enteral formulas used in EN are rich in nutrients and provide an excellent medium for bacteria proliferation, including pathogens. Poor handling during the preparation and administration of EN can contaminate the feed and cause infection[7]. Similarly, antibiotic treatment is strongly associated with diarrhea in patients receiving EN[8]. In fact, antibiotic use alters gut microbiota[9], which leads to increased risk of pathogen overgrowth[10]. In addition, the EN might also contribute to the occurrence of diarrhea by altering physiologic responses of the ascending colon where water is secreted into the lumen[11]. Traditionally, formulas used in EN were not comprised of a fiber component, thus allowing the gut to rest and preventing tube obstruction. However, fiber was gradually introduced in EN in response to accumulating evidence of its effects in modulating gut function and improving immune, blood glucose, and serum lipid regulation[12]. A meta-analysis showed that the introduction of fiber into the enteral formula was beneficial in reducing the incidence of diarrhea[13].
Physiologic effects exerted by the chemical composition of fiber are determined by its properties: viscosity, fermentability, and solubility. Fibers also include prebiotics that are fermentable, which lead to specific changes in the composition and/or activity of gut microbiota that benefit the well-being and health of the host[14]. For example, prebiotics that include fructo-oligosaccharides (FOS), oligofructose, and inulin were shown in multiple human studies to increase the concentrations of bifidobacteria[15]. A similar positive result was also demonstrated in healthy adults when fiber and oligosaccharides were added to the enteral formulas[16]. However, the effect was not clearly observed in patients receiving EN containing prebiotics[17].
Schneider et al[18] reported a favorable effect of fiber on short chain fatty acids (SCFAs). Bacterial fermentation of the ingested fiber in the colon produces SCFAs, primarily acetic, propionic and butyric acid. These SCFAs provide various health benefits to the host, such as supplying fuel to colonocytes, regulating proliferation and differentiation of epithelial cells, increasing colonic blood flow, reducing colonic pH, stimulating pancreatic secretions, other gut hormones and the autonomic nervous system, promoting sodium and water absorption, and possibly affecting gut motility[19].
Currently, there are three reviews investigating fiber in EN that have reported on the various types of fiber used, and the effect on healthy individuals and patients[13,20,21]. The aim of this review and meta-analysis was to evaluate recent evidence regarding the effect of dietary fiber and prebiotic supplementation in enteral formulas on diarrhea, fecal microbiota, and SCFAs.
MATERIALS AND METHODS
Literature search
Literature published between January 1990 to June 2014 that described the effect of EN supplemented with fiber on diarrhea, fecal microbiota, and SCFAs were systematically identified by searching MEDLINE, EMBASE, The Cochrane Library, CINAHL, Academic Search Premier and Web of Science databases. The following keywords and MeSH terms were used: artificial nutrition/feeding, nutritional support, enteral alimentation/formula, tube feeding, chemically defined diets, sips feeds, oral nutritional supplements, nutrition therapy, and dietary supplements. Additionally, fiber/fibre and specific types of fiber terms were searched individually: roughage, wheat brans, oligosaccharides, oligofructose, inulin, fructo-oligosaccharides, non-starch polysaccharides, soy polysaccharides, lignin, resistant starch, pectin, arabic gum, pectin, guar gum acacia gum, cellulose, pea fiber, oat, inulin-type fructans, and prebiotics. Lastly, local journals, follow-up reference lists of key papers, and relevant reviews were also hand-searched to locate additional publications that were not accessible through electronic databases.
Study selection
Two reviewers independently assessed potentially relevant articles for eligibility after eliminating duplications. The selection of articles underwent three stages: selection based on titles, followed by abstract consideration, and finally by assessing the full text. Disagreements were resolved through discussion. Inclusion criteria for this review were: (1) primary research of randomized controlled trial (RCT), non-RCT studies, and observational cohort study designs; (2) studies conducted on adult patients of any health or nutritional status receiving EN; (3) studies assessing effects of fiber in EN on diarrhea and/or fecal microbiota and/or SCFAs; and (4) studies conducted from January 1990 to June 2014. Exclusion criteria included studies that: (1) did not use enteral formula as the sole or main source of nutrients, either orally or through a tube; (2) involved supplementation of synbiotics (prebiotics and probiotics) in the enteral formula; and (3) involved animal or in vitro experiments or were case control and cross-sectional studies, review articles or dissertations. This review also was limited to published and available full articles in the English language.
Data extraction and outcome measures
Following the initial search, reference lists were imported to reference manager software (EndNote version 7.1; Thomson Reuters Corp., New York, NY, United States). Two reviewers extracted the data from each selected study, including: population descriptions (location, inclusion and exclusion criteria, method of recruitment, and consent), methodology (aim, design, study duration, and ethical approval), risk of bias assessment, participants (number of randomized, withdrawals and exclusions, and characteristics of the study participants), interventions (timing and delivery of EN, formula used, and fiber dosage and type), and outcomes (diarrhea incidence, fecal microbiota, and SCFA concentrations).
Quality assessment
Two reviewers independently assessed the methodological quality of the included studies using the Jadad Scale for Reporting Randomized Controlled Trials and the Scottish Intercollegiate Guidelines Network (SIGN) Critical Appraisal of The Medical Literature; disputes were resolved by discussion. The Jadad Scale considers criteria relating to randomization, blinding, withdrawals, and dropouts[22]. Scores ranging from 0 to 5 were given based on fulfillment of criteria addressed, with a higher score representing studies of better quality. The SIGN Critical Appraisal of The Medical Literature implements the Grading of Recommendations Assessment, Development and Evaluation approach within its guideline development[23]. The quality assessment for controlled trials in SIGN incorporates ten items: focused research question, randomization, adequate concealment, blinding of subjects and investigators, similar group characteristics, methodology of measuring relevant outcomes, study drop out, intention to treat analysis, and comparable results for multicenter research. Studies were assigned to one of three groups (high quality, acceptable or unacceptable) based on the methodology quality to minimize bias.
Statistical analysis
Binary outcomes were combined using the Mantel-Haenszel method with results presented as odds ratios (ORs) and 95%CIs. An OR > 1 indicates that fiber supplementation in EN is associated with higher odds of outcome, i.e., diarrhea. Statistical heterogeneity was evaluated using the I2 statistic, an estimation of variation in the effect of treatment beyond chance. An I2 > 50% was regarded as substantial heterogeneity, in which case a random effects model was used with subgroup analysis, otherwise a fixed effects model was used. A visual appraisal of a Funnel plot was used to indicate the possibility of publication bias. A P < 0.05 was considered as statistically significant.
RESULTS
The literature search identified 538 records. Forty-one studies were retrieved after excluding duplicates and titles that were not relevant to the research questions. A flow diagram describing the selection of studies is shown in Figure 1. Twenty-two experimental studies and four observational cohort studies that met the inclusion criteria were used for this review[17,18,24-47]. Characteristics of these studies are presented in Table 1. Eight studies were conducted on critically ill patients, 16 in mixed wards inclusive of medical, surgical and geriatrics wards, two in outpatient clinics, and two included studies did not explicitly mention the departments/units where the patients were hospitalized. SIGN methodological assessment classified two studies as unacceptable, which were therefore not used in this review.
Figure 1.
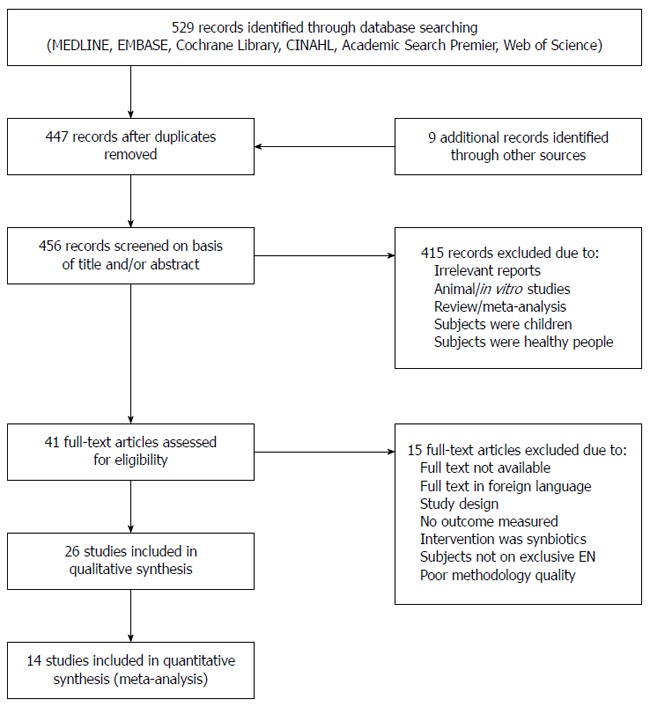
Flow diagram of included and excluded studies for the systematic review. EN: Enteral nutrition.
Table 1.
Characteristics of included studies (1990-2013)
| Ref. | Study design | Study population | Dose and type of fiber | Study duration | SIGN category | Jadad score |
| Dobb et al[24], 1990 | Double-blind RCT | 91 adult patients in ICU, The Royal Perth Hospital, Australia | Soy polysaccharide, 21 g/L | Max of 18 d/discharge ICU | High quality | 4 |
| Shankardass et al[25], 1990 | Double-blind, cross-over RCT | 28 long-term EN patients, Multicenter: Chedoke-McMaster Hospital, Queen Elizabeth Hospital, Riverdale Hospital, Sunnybrook Medical Centre, University of Toronto, Toronto, Ontario, Canada | Soy polysaccharide, 12.8 g/1000 kcal | 12 wk | Acceptable | 3 |
| Guenter et al[26], 1991 | Non-RCT | 100 ICU patients, Graduate Hospital Pennsylvania, United States | Soy polysaccharide, 14.4 g/L | Not mentioned | Acceptable | 0 |
| de Kruif et al[27], 1993 | RCT | 60 surgical patients, University Hospital, Netherlands | Soy polysaccharide, 20 g/L | 5 d | Acceptable | 3 |
| Collier et al[28], 1994 | Pre-post observational study | 57 surgical patients, Regional Medical Centre, Memphis Tennessee, United States | Soy polysaccharide, 21 g/L | Not mentioned | NA | 0 |
| Homann et al[29], 1994 | Double-blind RCT | 100 surgical and medical patients, Germany | Partially hydrolyzed guar gum, 20 g/L | 10 d | Acceptable | 2 |
| Zarling et al[30], 1994 | Cross-over RCT | 10 recovering stroke patients, Extended Care facilities, Hines VA Hospital, Illinois, United States | Oat and soy fiber, 14.4 g/L | 23 d | Acceptable | 2 |
| Reese et al[31], 1996 | Double-blind RCT | 80 surgical patients (head and neck cancer), University of Iowa Hospital, United States | Soy polysaccharide, 7 or 14 g/L | Until patient changed to oral/discharged | High quality | 5 |
| Heather et al[32], 1991 | RCT | 49 mixed wards patients, Portland Veterans Affairs Medical Centre, Portland, United States | Psyllium, 15 g/d | 6 d | Acceptable | 2 |
| Belknap et al[33], 1997 | RCT | 60 medical, surgical, and ICU patients, Department of Veterans Affairs Medical Center, Oklahoma, United States | Psyllium hydrophilic mucilloids, 14 g/d | 7 d | Acceptable | 3 |
| Sobotka et al[34], 1997 | Single-blind, pre-post single group trial | 9 patients, Charles University, Hradec Krdlove, Czech Republic | Inulin 15 g/L | 2 wk | Acceptable | 0 |
| Emery et al[35], 1997 | RCT | 31 ICU patients, Pennsylvania Hospital, United States | Banana flakes, 1.5 g/d | 7 d | Acceptable | 0 |
| Khalil et al[36], 1998 | Single-blind RCT | 16 surgical patients, National University Hospital, Singapore | Oat and soy polysaccharides, 14.4 g/L | 10 d | Acceptable | 2 |
| Cockram et al[37], 1998 | Single-blind RCT | 79 hemodialysis patients from three outpatients hemodialysis clinics, United States | FOS, 15.4 g/L | 3 wk | Acceptable | 2 |
| Schultz et al[38], 2000 | Double-blind, 2 × 2 factorial RCT | 44 critically ill patients, Maine Medical Center, Portland, United States | Mixed fiber1 and pectin, up to 17 g/d, inclusive of 10 g/L FOS | 9 d | High quality | 4 |
| Spapen et al[39], 2001 | Double-blind, RCT | 25 critically ill patients, Academic Hospital, Vrije, Brussels, Belgium | Partially hydrolyzed guar gum, 22 g/L | 21 d/withdrawal of EN | High quality | 4 |
| Nakao et al[40], 2002 | Pre-post single group trial | 20 geriatric patients, Nagoya University Hospital, Japan | Galactomannan, 7-28 g/d | 6 wk | Acceptable | 0 |
| Rushdi et al[41], 2004 | Double-blind RCT | 20 critically ill patients, Teaching Hospital, Cairo University, Egypt | Guar gum, 22 g/L | 4 d | High quality | 5 |
| Vandewoude et al[42], 2005 | RCT | 172 geriatric patients, Universitair Centrum Geriatrie, Belgium | Mixed fiber2, 30 g/d inclusive of inulin | Not mentioned, measured weekly | Acceptable | 1 |
| Schneider et al[18], 2006 | Double-blind, cross-over, RCT | 15 long-term EN patients, University Hospital, Nice, France | Mixed fiber3, 15 g/L inclusive of 3.45 g/L of FOS | 5 wk | High quality | 3 |
| Shimoni et al[43], 2007 | Non-RCT | 148 elderly patients from general internal medicine wards, Gastroenterology Laniado Hospital, Natanyia, Ramat Aviv, Israel | Soy polysaccharides, 13.6 g/1000 kcal | 5 d | Acceptable | 1 |
| Wierdsma et al[44], 2009 | Double-blind RCT | 19 patients, Outpatients Clinic of the VU University Medical Centre, Amsterdam, The Netherlands | Mixed fiber1, 17.6 g/L inclusive of 7 g of FOS | 8 wk | Acceptable | 3 |
| Chittawatanarat et al[45], 2010 | Double-blind RCT | 34 septic patients in ICU, Maharaj Nakorn Chiang Mai Hospital, Thailand | Mixed fiber2, 15.1 g/L inclusive of 5.3 g of FOS | 14 d, ≥ 5 d | High quality | 4 |
| Kato et al[46], 2012 | Pre-post single group trial | 15 patients from medical wards of Kameyama Kaisei Hospital, Japan | Psyllium, 5.2 g/d | 4 wk | Acceptable | 0 |
| Bittencourt et al[47], 2012 | Sequential and observational study | 110 adult patients, São Joaquim Hospital of Beneficência Portuguesa, Brazil | Soluble and insoluble fiber3, 15 g/L | ≥ 5 d | NA | 0 |
| Majid et al[17], 2013 | Double-blind RCT | 22 critically ill patients, Guy’s and St Thomas’ NHS Foundation Trust and King’s College Hospital NHS Foundation Trust, London, United Kingdom | Mixed fiber4, 15 g/L and additional 7 g/d oligofructose/inulin | 7-14 d | High quality | 5 |
Oat, soy polysaccharide, gum arabic, cellulose, and FOS;
Cellulose, hemicellulose A, pectin, hemicellulose B, and inulin;
Cellulose, lignin, hemicellulose, pectin, and FOS;
Soy polysaccharide, alpha-cellulose, arabic gum, inulin, oligofructose, and resistant starch. EN: Enteral nutrition; FOS: Fructo-oligosaccharides; ICU: Intensive care unit; RCT: Randomized controlled trial; SIGN: Scottish Intercollegiate Guidelines Network; NA: Not available.
Most studies that investigated fiber supplementation in EN used soy polysaccharide (n = 7), followed by mixed fiber (n = 6), partially hydrolyzed guar gum (n = 3), psyllium (n = 3), oat and soy fiber (n = 2), FOS (n = 1), inulin (n = 1), banana flakes (n = 1), and galactomannan (n = 1); one study did not mention the type of fiber used. Fiber was administered as an integrated component of the enteral formula in 14 studies, and added as supplementation in 10 studies. Two studies used fiber containing enteral formula with additional fiber supplementation as part of the intervention[17,28], for which diarrhea incidence among adult patients receiving EN ranged from 10.5 to 90.0%. There was variability in the definition of diarrhea among studies, taking into account partly or all of the stool properties: volume, consistency, and frequency. Diarrhea definitions were based on diarrhea score, number of liquid stools per day and/or volume, number of loose or watery stools, with a scale based on consistency and frequency, and use of stool chart, i.e., Bristol and King’s stool chart.
Fourteen experimental studies with data on diarrhea incidence during EN (fiber-containing EN vs fiber-free EN) were included for meta-analysis. As shown in Figure 2, pooling of the studies under a random effects model confirmed the protective effect of fiber in reducing incidence of diarrhea among adult patients requiring EN (P < 0.01). Subgroup analysis was conducted due to statistically significant heterogeneity of the data (I2 = 54%). The analysis revealed homogeneity among studies conducted in non-critically ill patients (I2 = 28%), but studies conducted among critically ill patients were heterogeneous (I2 = 52%). Additionally, a positive effect of fiber supplementation during EN on reducing the incidence of diarrhea was not seen in the critically ill patients, but was significant in the non-critically ill patients (P < 0.01).
Figure 2.
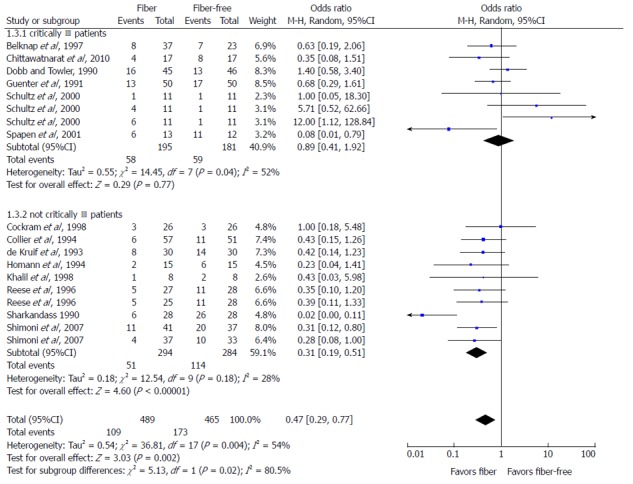
Meta-analysis of the effect of fiber supplementation in enteral nutrition on incidence of diarrhea.
Asymmetry presentation of the funnel plot in Figure 3 revealed that inter-study heterogeneity existed and this may be an indication of potential publication bias. Of the 26 studies investigating the effect of fiber in EN on the three main outcomes, only eight studies had prebiotics in the intervention. An additional meta-analysis failed to indicate any protective effect of prebiotic supplementation in EN against the incidence of diarrhea (Figure 4).
Figure 3.
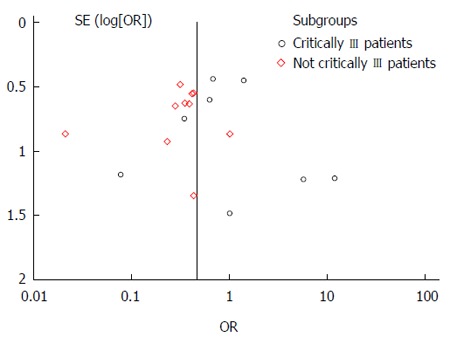
Funnel plot for the effect of fiber supplementation in enteral nutrition on incidence of diarrhea.
Figure 4.
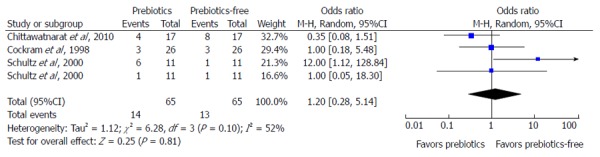
Meta-analysis of the effect of prebiotics supplementation in enteral nutrition on incidence of diarrhea.
The effect of fiber supplementation in EN towards fecal microbiota was only investigated in four studies[17,18,40,44] (Table 2). Only one study had shown a significant increase in total bacteria when patients were given fiber (mixed fiber with prebiotics)-supplemented EN[18]. Likewise, the same study found no changes in regard to the composition of the dominant bacteria group (gram positive/negative, aerobic and anaerobic). On the contrary, the study by Nakao et al[40] reported a significant decrease in aerobic bacteria with galactomannan supplementation. There were no reports of significant changes in fecal bifidobacteria concentrations in patients receiving fiber-supplemented EN[17,18,44].
Table 2.
Studies investigating the effect of fiber supplementation in enteral nutrition on fecal microbiota
| Ref. | Total microbiota count | Dominant group | Bifidobacteria | Others |
| Nakao et al[40], 2002 | No change | ↓ in aerobic bacteria | Not measured | Not measured |
| Schneider et al[18], 2006 | ↑ | No change in composition of aerobic and anaerobic, gram positive and gram negative bacteria | No change | ↑ in the numbers of enterococci at the end of the fiber-free EN |
| ↑ in the numbers of bacteroides at the end of the mixed fiber EN | ||||
| Wierdsma et al[44], 2009 | Not measured | Not measured | ↓ In patients compared to healthy controls | Not measured |
| Concentration remained stable in the FOS group but ↓ in the non-FOS group during intervention | ||||
| Majid et al[17], 2013 | No change | No change | No change | ↓ Faecalibacterium prausnitzii and Bacteroides-Prevotella in the prebiotics group |
EN: Enteral nutrition; FOS: Fructo-oligosaccharides.
Five studies investigated the effect of fiber supplementation in EN on SCFAs[17,18,34,40,46] (Table 3). Fiber supplementation increased total SCFA in two studies[18,46], whereas three studies found no changes in the SCFA concentration[17,34,40]. In addition, prebiotic supplementation in EN did not increase the concentration of SCFA[17,34], with the exception of one positive result[18].
Table 3.
Studies investigating the effect of fiber supplementation in enteral nutrition towards short-chain fatty acids
| Ref. | Total SCFA | Acetate | Propionate | Butyrate |
| Sobotka et al[34], 1997 | No change | No change | No change | No change |
| Nakao et al[40], 2002 | No change | ↑ | ↑ | No change |
| Schneider et al[18], 2006 | ↑ | ↑ | No change | ↑ |
| Kato et al[46], 2012 | ↑ | ↑ | No significant amount detected | No significant amount detected |
| Majid et al[17], 2013 | No change | No change | No change | No change |
EN: Enteral nutrition; SCFA: Short-chain fatty acid.
DISCUSSION
The results of this updated meta-analysis of prospective studies confirm previous evidence showing that fiber supplementation decreases diarrhea incidence for adult patients requiring EN[13]. The dosage of fiber used in the included studies ranged from 5.2 to 39.0 g/d[41,46]. In this review, soy polysaccharide emerged as the most extensively studied fiber in EN for patients. Likewise, it is also the most common fiber added in the enteral formula. The mechanisms of action for minimizing diarrhea incidence include the ability of fiber to hold water[48], increase bulk[49], and improve gut barrier function[12]. However, this effect of fiber varies based on the type of patients studied[13]. Subgroup analysis conducted in this current review shows that the incidence of diarrhea was only reduced in non-critically ill patients, consistent with previous reviews[13,21]. It is possible that the severity of illness and the antibiotics therapy undertaken by the critically ill patients counters the beneficial effect of fiber supplementation. The use of antibiotics or antifungal drugs is an independent factor that contributes to higher prevalence of diarrhea in critically ill patients[50]. Moreover, critically ill patients often suffer from gastrointestinal dysfunction with abnormal motility patterns and impaired barrier integrity[51]. Although the potential benefits are not clearly observed in the critically ill patients, the main finding of this review has significant implications for health care professionals, and advocates the use of fiber-containing over fiber-free enteral formulas to other groups of patients.
Prebiotic components of fiber meet three distinct criteria: (1) are resistant to gastric acidity, hydrolysis by mammalian enzymes, and gastrointestinal absorption; (2) can by fermented by intestinal microbiota; and (3) selectively stimulate the growth and/or activity of intestinal bacteria associated with health and well-being[52]. Our results show that prebiotic supplementation in enteral formulas did not minimize the incidence of diarrhea in adult patients receiving EN. The prebiotic dosage reported by studies included in this meta-analysis ranged from 5.3 to 15.4 g/L of FOS[37,45]. Two of the three studies included in the meta-analysis were conducted in an intensive care unit setting, which may explain the lack of a significant benefit from prebiotic supplementation in EN for diarrhea.
Ingested fiber influences the intestinal microbiota by providing the required substrate for colonic fermentation, and consequently assists in microbiota proliferation. Over the years, reports revealed that the introduction of prebiotics in healthy humans increases the concentrations of bifidobacteria when EN is given as the sole source of nutrition[16]. However, the effect of fiber and prebiotic supplementation on fecal microbiota in adult patients receiving EN could not be concluded due to conflicting findings[17,18,40,44]. These inconsistent results might be due to the heterogeneity of study populations, e.g. the inclusion of stable and critically ill patients. Additionally, concurrent use of antibiotics with EN might alter the colonic microbiota composition. Most antibiotics alter the bacterial composition of gut microbiota as reflected by the suppression of anaerobic bacteria and an increased incidence of Clostridium difficile-associated diseases[10]. However, by controlling the confounding factor, antibiotics therapy remains difficult, as it is part of a medical treatment received by patients who are critically ill. The range of prebiotics dosages (5.20-13.75 g/d) may also have contributed to the lacking of a bifidogenic effect. Healthy people require 10 g of prebiotics to increase fecal bifidobacteria concentration[53], thus patients might require a higher dosage to exert such an effect. Most studies that investigated prebiotics were conducted in patients receiving EN supplemented with a mixture of various types of fiber (inclusive of prebiotics) instead of a single source of fiber; only two studies used FOS as its sole source of fiber in the intervention[34,37]. Due to the limited numbers of RCTs, a meta-analysis investigating the role of fiber, specifically prebiotics on fecal bifidobacteria, other microbiota, and SCFAs, could not be conducted.
Fermentation of fiber yields SCFAs, which are a source of nutrients for colonic mucosal cells. Therefore, the luminal acidity produced by the increased concentrations of SCFAs helps maintain an environment with a low pH for the colonic microbiota, subsequently preventing an enteropathogenic infection[19]. While provision of fermentable fiber increases SCFAs in healthy humans[54], mixed results emerged in this review for studies investigating the effect of fiber supplementation in EN in patients. Despite two studies displaying no changes in SCFA concentrations, a significant increase in SCFA concentrations was observed in studies conducted on stable patients (geriatrics, long-term EN, and medical patients)[18,40,46]. Similarly, supplementation of fiber in EN given to the critically ill patients did not cause an increase in the SCFA concentration[17]. According to a recent study, critically ill patients suffered from a low SCFA concentration as compared to healthy individuals, possibly due to the reduction in total obligate anaerobes throughout the intensive care unit admission[55]. Moreover, fiber fermentation varies depending on the source of fibers used in the studies[56]; different fibers yield different concentrations of total SCFA.
The main limitation of this review is the heterogeneity of the patients, such as the inclusion of patients with varying severity of illness, mainly the critically ill and the non-critically ill patients, and the use of antibiotics, which could confound the results. However, these factors are inevitable when conducting research in patients. Secondly, this review also lacks uniformity with regard to the definition of diarrhea[57,58], for which objective and subjective considerations might influence the results of the studies in terms of the incidence of diarrhea. As such, the use of a validated tool in defining diarrhea should be considered in future research. Some studies were excluded from the meta-analysis, as the definition of diarrhea was not mentioned explicitly despite indicating a measurable outcome. Lastly, this review only incorporates publications written in English, and therefore may introduce language bias to the review.
This systematic review demonstrates that fiber assists in minimizing diarrhea in adult patients receiving EN, particularly those who are not critically ill. However, prebiotics (part of the fiber component) may not provide the same impact as fiber based on current evidence. Therefore, the alteration of microbiota and SCFAs using fiber and/or prebiotics in minimizing diarrhea remains inconclusive.
COMMENTS
Background
Enteral nutrition (EN) provides nutrients crucial for patient recovery. However, diarrhea is one of the common complications of EN. Fiber supplementation in EN has been shown to regulate bowel function. The undigested fiber increases proliferation of colonic microbiota, including bifidobacteria, and its fermentation yields short chain fatty acids (SCFAs), which enhance the absorption of water and sodium in the colon.
Research frontiers
Even though fiber is considered to improve diarrhea during EN, the effect of prebiotics remains unknown. To the best of our knowledge, this is the first systematic review to investigate the effect of both fiber and prebiotics in minimizing diarrhea in EN. Additionally, the impact of fiber supplementation in EN on fecal microbiota and SCFA was also explored.
Innovations and breakthroughs
Fiber added in EN has been shown to reduce the incidence of diarrhea in non-critically ill, tube-fed patients. This effect can be due to alterations in SCFAs and colonic microbiota. However, such an effect was not seen if prebiotics are added in EN, despite the fact that prebiotics increase bifidobacteria concentrations in healthy human subjects who consumed at least 10 g/d. Thus, the evidence to support the use of fiber and/or prebiotics in the manipulation of fecal microbiota and SCFA in patients receiving EN is limited.
Applications
Fiber-containing enteral formulas should be considered for patients receiving EN to help minimize diarrhea, and the use of prebiotics as first-line feeding management requires further scientific evidence.
Terminology
Prebiotics are selectively fermented ingredients that allow specific changes, both in the composition and/or activity of the gut microbiota, thereby conferring benefits upon host well-being and health.
Peer-review
This is a good review in which the authors conducted meta-analyses of the effects of fiber or prebiotic supplementation on diarrhea observed in patients receiving EN. It was concluded that fiber alleviates diarrhea in non-critically ill patients, and prebiotics may not provide the same impact as fiber, based on the current evidence. The results are interesting and suggest that the alteration of microbiota and SCFAs using fiber and/or prebiotics in minimizing diarrhea remains inconclusive.
Footnotes
Supported by The University of Malaya Research Grant (No. PG127-2013A, No. UMRP022A-14HTM and No. UMRG 388-11HTM).
Conflict-of-interest: The authors declare that they have no conflicts of interest.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 25, 2014
First decision: November 14, 2014
Article in press: January 30, 2015
P- Reviewer: Ohshima Y, Park YK, Suchodolski JS, Touil-Boukoffa C S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Alpers DH. Enteral feeding and gut atrophy. Curr Opin Clin Nutr Metab Care. 2002;5:679–683. doi: 10.1097/00075197-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Luyer MD, Jacobs JA, Vreugdenhil AC, Hadfoune M, Dejong CH, Buurman WA, Greve JW. Enteral administration of high-fat nutrition before and directly after hemorrhagic shock reduces endotoxemia and bacterial translocation. Ann Surg. 2004;239:257–264. doi: 10.1097/01.sla.0000108695.60059.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47:109–116. [PMC free article] [PubMed] [Google Scholar]
- 4.Whelan K. Enteral-tube-feeding diarrhoea: manipulating the colonic microbiota with probiotics and prebiotics. Proc Nutr Soc. 2007;66:299–306. doi: 10.1017/S0029665107005551. [DOI] [PubMed] [Google Scholar]
- 5.Wiesen P, Van Gossum A, Preiser JC. Diarrhoea in the critically ill. Curr Opin Crit Care. 2006;12:149–154. doi: 10.1097/01.ccx.0000216583.64804.46. [DOI] [PubMed] [Google Scholar]
- 6.Majid HA, Bin Sidek MA, Chinna K. Psychometric properties of DAPonDEN: definitions, attitudes and practices in relation to diarrhea during enteral nutrition questionnaire. Prev Med. 2013;57 Suppl:S64–S66. doi: 10.1016/j.ypmed.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Levy J, Van Laethem Y, Verhaegen G, Perpête C, Butzler JP, Wenzel RP. Contaminated enteral nutrition solutions as a cause of nosocomial bloodstream infection: a study using plasmid fingerprinting. JPEN J Parenter Enteral Nutr. 1989;13:228–234. doi: 10.1177/0148607189013003228. [DOI] [PubMed] [Google Scholar]
- 8.Guenter P. Safe practices for enteral nutrition in critically ill patients. Crit Care Nurs Clin North Am. 2010;22:197–208. doi: 10.1016/j.ccell.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2013;62:1591–1601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 11.Bowling TE, Raimundo AH, Grimble GK, Silk DB. Reversal by short-chain fatty acids of colonic fluid secretion induced by enteral feeding. Lancet. 1993;342:1266–1268. doi: 10.1016/0140-6736(93)92360-6. [DOI] [PubMed] [Google Scholar]
- 12.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia M, Engfer MB, Green CJ, Silk DB. Systematic review and meta-analysis: the clinical and physiological effects of fibre-containing enteral formulae. Aliment Pharmacol Ther. 2008;27:120–145. doi: 10.1111/j.1365-2036.2007.03544.x. [DOI] [PubMed] [Google Scholar]
- 14.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 Suppl 2:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 15.Kolida S, Gibson GR. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137:2503S–2506S. doi: 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- 16.Whelan K, Judd PA, Preedy VR, Simmering R, Jann A, Taylor MA. Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr. 2005;135:1896–1902. doi: 10.1093/jn/135.8.1896. [DOI] [PubMed] [Google Scholar]
- 17.Majid HA, Cole J, Emery PW, Whelan K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: a randomised controlled trial. Clin Nutr. 2014;33:966–972. doi: 10.1016/j.clnu.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Schneider SM, Girard-Pipau F, Anty R, van der Linde EG, Philipsen-Geerling BJ, Knol J, Filippi J, Arab K, Hébuterne X. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin Nutr. 2006;25:82–90. doi: 10.1016/j.clnu.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Green CJ. Fibre in enteral nutrition. Clin Nutr. 2001;20:23–39. [Google Scholar]
- 20.del Olmo D, López del Val T, Martínez de Icaya P, de Juana P, Alcázar V, Koning A, Vázquez C. [Fiber in enteral nutrition: systematic review of the literature] Nutr Hosp. 2004;19:167–174. [PubMed] [Google Scholar]
- 21.Yang G, Wu XT, Zhou Y, Wang YL. Application of dietary fiber in clinical enteral nutrition: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2005;11:3935–3938. doi: 10.3748/wjg.v11.i25.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Methodology checklist. SIGN. 2014. Available from: http://www.sign.ac.uk/methodology/checklists.html.
- 24.Dobb GJ, Towler SC. Diarrhoea during enteral feeding in the critically ill: a comparison of feeds with and without fibre. Intensive Care Med. 1990;16:252–255. doi: 10.1007/BF01705161. [DOI] [PubMed] [Google Scholar]
- 25.Shankardass K, Chuchmach S, Chelswick K, Stefanovich C, Spurr S, Brooks J, Tsai M, Saibil FG, Cohen LB, Edington JD. Bowel function of long-term tube-fed patients consuming formulae with and without dietary fiber. JPEN J Parenter Enteral Nutr. 1990;14:508–512. doi: 10.1177/0148607190014005508. [DOI] [PubMed] [Google Scholar]
- 26.Guenter PA, Settle RG, Perlmutter S, Marino PL, DeSimone GA, Rolandelli RH. Tube feeding-related diarrhea in acutely Ill patients. JPEN J Parenter Enteral Nutr. 1991;15:277–280. doi: 10.1177/0148607191015003277. [DOI] [PubMed] [Google Scholar]
- 27.de Kruif JT, Vos A. The influence of soyfibre supplemented tube feeding on the occurrence of diarrhoea in postoperative patients. Clin Nutr. 1993;12:360–364. doi: 10.1016/0261-5614(93)90033-z. [DOI] [PubMed] [Google Scholar]
- 28.Collier P, Kudsk KA, Glezer J, Brown RO. Fiber-containing formula and needle catheter jejunostomies: a clinical evaluation. Nutr Clin Pract. 1994;9:101–103. doi: 10.1177/0115426594009003101. [DOI] [PubMed] [Google Scholar]
- 29.Homann HH, Kemen M, Fuessenich C, Senkal M, Zumtobel V. Reduction in diarrhea incidence by soluble fiber in patients receiving total or supplemental enteral nutrition. JPEN J Parenter Enteral Nutr. 1994;18:486–490. doi: 10.1177/0148607194018006486. [DOI] [PubMed] [Google Scholar]
- 30.Zarling EJ, Edison T, Berger S, Leya J, DeMeo M. Effect of dietary oat and soy fiber on bowel function and clinical tolerance in a tube feeding dependent population. J Am Coll Nutr. 1994;13:565–568. doi: 10.1080/07315724.1994.10718448. [DOI] [PubMed] [Google Scholar]
- 31.Reese JL, Means ME, Hanrahan K, Clearman B, Colwill M, Dawson C. Diarrhea associated with nasogastric feedings. Oncol Nurs Forum. 1996;23:59–66; discussion 66-68. [PubMed] [Google Scholar]
- 32.Heather DJ, Howell L, Montana M, Howell M, Hill R. Effect of a bulk-forming cathartic on diarrhea in tube-fed patients. Heart Lung. 1991;20:409–413. [PubMed] [Google Scholar]
- 33.Belknap D, Davidson LJ, Smith CR. The effects of psyllium hydrophilic mucilloid on diarrhea in enterally fed patients. Heart Lung. 1997;26:229–237. doi: 10.1016/s0147-9563(97)90060-1. [DOI] [PubMed] [Google Scholar]
- 34.Sobotka L, Brátova M, Slemrová M, Manák J, Vizd’a J, Zadák Z. Inulin as the soluble fiber in liquid enteral nutrition. Nutrition. 1997;13:21–25. doi: 10.1016/s0899-9007(97)90874-1. [DOI] [PubMed] [Google Scholar]
- 35.Emery EA, Ahmad S, Koethe JD, Skipper A, Perlmutter S, Paskin DL. Banana flakes control diarrhea in enterally fed patients. Nutr Clin Pract. 1997;12:72–75. doi: 10.1177/011542659701200272. [DOI] [PubMed] [Google Scholar]
- 36.Khalil L, Ho KH, Png D, Ong CL. The effect of enteral fibre-containing feeds on stool parameters in the post-surgical period. Singapore Med J. 1998;39:156–159. [PubMed] [Google Scholar]
- 37.Cockram DB, Hensley MK, Rodriguez M, Agarwal G, Wennberg A, Ruey P, Ashbach D, Hebert L, Kunau R. Safety and tolerance of medical nutritional products as sole sources of nutrition in people on hemodialysis. J Ren Nutr. 1998;8:25–33. doi: 10.1016/s1051-2276(98)90034-6. [DOI] [PubMed] [Google Scholar]
- 38.Schultz AA, Ashby-Hughes B, Taylor R, Gillis DE, Wilkins M. Effects of pectin on diarrhea in critically ill tube-fed patients receiving antibiotics. Am J Crit Care. 2000;9:403–411. [PubMed] [Google Scholar]
- 39.Spapen H, Diltoer M, Van Malderen C, Opdenacker G, Suys E, Huyghens L. Soluble fiber reduces the incidence of diarrhea in septic patients receiving total enteral nutrition: a prospective, double-blind, randomized, and controlled trial. Clin Nutr. 2001;20:301–305. doi: 10.1054/clnu.2001.0399. [DOI] [PubMed] [Google Scholar]
- 40.Nakao M, Ogura Y, Satake S, Ito I, Iguchi A, Takagi K, Nabeshima T. Usefulness of soluble dietary fiber for the treatment of diarrhea during enteral nutrition in elderly patients. Nutrition. 2002;18:35–39. doi: 10.1016/s0899-9007(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 41.Rushdi TA, Pichard C, Khater YH. Control of diarrhea by fiber-enriched diet in ICU patients on enteral nutrition: a prospective randomized controlled trial. Clin Nutr. 2004;23:1344–1352. doi: 10.1016/j.clnu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Vandewoude MF, Paridaens KM, Suy RA, Boone MA, Strobbe H. Fibre-supplemented tube feeding in the hospitalised elderly. Age Ageing. 2005;34:120–124. doi: 10.1093/ageing/afh242. [DOI] [PubMed] [Google Scholar]
- 43.Shimoni Z, Averbuch Y, Shir E, Gottshalk T, Kfir D, Niven M, Moshkowitz M, Froom P. The addition of fiber and the use of continuous infusion decrease the incidence of diarrhea in elderly tube-fed patients in medical wards of a general regional hospital: a controlled clinical trial. J Clin Gastroenterol. 2007;41:901–905. doi: 10.1097/01.mcg.0000225662.23179.b6. [DOI] [PubMed] [Google Scholar]
- 44.Wierdsma NJ, van Bodegraven AA, Uitdehaag BM, Arjaans W, Savelkoul PH, Kruizenga HM, van Bokhorst-de van der Schueren MA. Fructo-oligosaccharides and fibre in enteral nutrition has a beneficial influence on microbiota and gastrointestinal quality of life. Scand J Gastroenterol. 2009;44:804–812. doi: 10.1080/00365520902839675. [DOI] [PubMed] [Google Scholar]
- 45.Chittawatanarat K, Pokawinpudisnun P, Polbhakdee Y. Mixed fibers diet in surgical ICU septic patients. Asia Pac J Clin Nutr. 2010;19:458–464. [PubMed] [Google Scholar]
- 46.Kato Y, Nakao M, Iwasa M, Hasegawa S, Yamada K. Soluble fiber improves management of diarrhea in elderly patients receiving enteral nutrition. Food Nutr Sci. 2012;3:1547–1552. [Google Scholar]
- 47.Bittencourt AF, Martins JR, Logullo L, Shiroma G, Horie L, Ortolani MC, Silva Mde L, Waitzberg DL. Constipation is more frequent than diarrhea in patients fed exclusively by enteral nutrition: results of an observational study. Nutr Clin Pract. 2012;27:533–539. doi: 10.1177/0884533612449488. [DOI] [PubMed] [Google Scholar]
- 48.Russell J, Bass P. Canine gastric emptying of fiber meals: influence of meal viscosity and antroduodenal motility. Am J Physiol. 1985;249:G662–G667. doi: 10.1152/ajpgi.1985.249.6.G662. [DOI] [PubMed] [Google Scholar]
- 49.Chen HL, Haack VS, Janecky CW, Vollendorf NW, Marlett JA. Mechanisms by which wheat bran and oat bran increase stool weight in humans. Am J Clin Nutr. 1998;68:711–719. doi: 10.1093/ajcn/68.3.711. [DOI] [PubMed] [Google Scholar]
- 50.Hill LT. Gut dysfunction in the critically ill - mechanisms and clinical implications. S Afr Med J. 2013;29:11–15. [Google Scholar]
- 51.Thibault R, Graf S, Clerc A, Delieuvin N, Heidegger CP, Pichard C. Diarrhoea in the ICU: respective contribution of feeding and antibiotics. Crit Care. 2013;17:R153. doi: 10.1186/cc12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 53.Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourié B, Bornet F, Rambaud JC. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J Nutr. 1999;129:113–116. doi: 10.1093/jn/129.1.113. [DOI] [PubMed] [Google Scholar]
- 54.Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 55.Yamada T, Shimizu K, Ogura H, Asahara T, Nomoto K, Yamakawa K, Hamasaki T, Nakahori Y, Ohnishi M, Kuwagata Y, et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J Parenter Enteral Nutr. 2014:Epub ahead of print. doi: 10.1177/0148607114529596. [DOI] [PubMed] [Google Scholar]
- 56.Titgemeyer EC, Bourquin LD, Fahey GC, Garleb KA. Fermentability of various fiber sources by human fecal bacteria in vitro. Am J Clin Nutr. 1991;53:1418–1424. doi: 10.1093/ajcn/53.6.1418. [DOI] [PubMed] [Google Scholar]
- 57.Lebak KJ, Bliss DZ, Savik K, Patten-Marsh KM. What’s new on defining diarrhea in tube-feeding studies? Clin Nurs Res. 2003;12:174–204. doi: 10.1177/1054773803012002005. [DOI] [PubMed] [Google Scholar]
- 58.Majid HA, Emery PW, Whelan K. Definitions, attitudes, and management practices in relation to diarrhea during enteral nutrition: a survey of patients, nurses, and dietitians. Nutr Clin Pract. 2012;27:252–260. doi: 10.1177/0884533611431986. [DOI] [PubMed] [Google Scholar]


