Abstract
A 58-year-old man presented with the chief complaint of abdominal bloating and was incidentally found to have a liver tumor. As diagnostic imaging studies could not rule out malignancy, the patient underwent partial resection of segment 3 of the liver. The lesion pathologically showed eosinophilic proliferation, in addition to immunohistochemical positivity for human melanoma black 45 and Melan-A, thereby leading to the diagnosis of a hepatic perivascular epithelioid cell tumor (PEComa). A PEComa arising from the liver is relatively rare. Moreover, the name ‘PEComa’ has not yet been widely recognized, and the same disease entity has been called epithelioid angiomyolipoma (EAML), further diminishing the recognition of PEComa. In addition, PEComa imaging findings mimic those of malignant liver tumors, and clinically, this tumor tends to enlarge. Therefore, a PEComa is difficult to diagnose. We conducted a systematic review of PEComa and EAML cases and discuss the results, including findings useful for differentiating perivascular epithelioid cell tumors from malignant liver tumors.
Keywords: Angiomyolipoma, Tuberous sclerosis, Melan-A, Perivascular epithelioid cell tumor, Human melanoma black 45, Imaging
Core tip: Hepatic perivascular epithelioid cell tumors (PEComas) are very rare. This is the first review to investigate and compare the results of both PEComa and epithelioid angiomyolipoma patients. As PEComas are primarily benign tumors, surgical interventions may be avoidable. The key findings in the differential diagnosis of this tumor include a blotchy vascular pattern within the tumor and no hemorrhage within tumors less than 7 cm at the maximum diameter. Furthermore, if PEComas have hemodynamic features similar to those of hepatic angiomyolipomas, then the patterns of the drainage veins would very likely be useful for differentiating hepatocellular carcinomas from PEComas, as observed in our case.
INTRODUCTION
Hepatic perivascular epithelioid cell tumors (PEComas) are very rare. According to Bonetti et al[1], PEComas are perivascular epithelioid cell-derived tumors. PEComas of the lung also reportedly belong to the PEComa family, which includes clear-cell sugar tumors of the lung and lymphangioleiomyomatosis. In 2002, the World Health Organization (WHO) defined PEComas[2] as “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells”. However, PEComas and epithelioid angiomyolipomas (EAMLs) are still regarded by many experts as the same disease.
Hepatic PEComas are difficult to diagnose and a gold standard for identification using diagnostic imaging techniques is lacking. Instead, the diagnosis of hepatic PEComa is established on the basis of positive immunohistochemical staining results for human melanoma black 45 (HMB-45) and Melan-A[2-5]. Herein, we conducted a systematic review of hepatic PEComas, including our case, to examine potential key features that would allow diagnostic imaging. We also aimed to ascertain the degree of recognition of the PEComa disease entity.
CASE REPORT
A 58-year-old man presented with the chief complaint of abdominal bloating and was incidentally found to have a liver tumor. The patient’s medical history included a gastric ulcer, appendicitis, and intervertebral disc herniation. Tests for the hepatitis C virus antibody and hepatitis B virus surface antigen were negative, and no evidence of tuberous sclerosis was found.
An abdominal ultrasound showed an ill-defined mass approximately 4.5 cm in diameter that was hypoechoic internally with a partial hyperechoic area. Furthermore, no halos, posterior echo enhancement, or dilated peripheral bile ducts were observed. The entire mass was visualized as low density on non-contrast computed tomography (CT) (Figure 1A). Contrast-enhanced CT showed a strongly enhanced mass in the arterial phase with a weakly enhanced center; the internal component-like structure showed relatively strong enhancement (Figure 1B). The portal venous and late phase CT showed washout with weak enhancement of the partial area inside the mass (Figure 1C and D). Moreover, magnetic resonance imaging (MRI) of the mass showed ill-defined low signal intensity on the T1-weighted image (WI) (Figure 2A and B) and high signal intensity on the T2-WI. The weakly enhanced center of the mass, evident on CT, was visualized as high signal intensity on the T2-WI, very similar to that of the entire mass (Figure 2C). No decreases in signal intensities were observed in the out-of-phase and in-phase images, suggesting that no fatty components were present in the mass (Figure 2A and B). The mass was also visualized as high signal intensity on the T2-WI after administration of superparamagnetic iron oxide, suggesting the absence of Kupffer cells in the tumor (Figure 2D). In addition, the apparent diffusion coefficient (ADC) value was 1.491 × 10-3 mm2/s (Figure 2E). Celiac arteriography showed strong enhancement of the mass fed by thickened and distorted vessels from the left hepatic artery (Figure 3). These time-course findings raised suspicion of dilated drainage veins. Taken together, these imaging studies suggested that the patient likely had either a malignant hepatic tumor, such as a hepatocellular carcinoma (HCC) or hepatic metastasis, or a benign liver tumor. As a malignant tumor was possible, we considered establishing a diagnosis to be essential and thus decided that resection of the tumor was necessary. Even if the tumor was found to be benign, tumor resection would be appropriate due to the risk of rupture in the near future. The resected specimen showed no capsule formation, and the tumor was composed of pleomorphic round to polygonal epithelioid cells with a pale, clear, eosinophilic cytoplasm. The specimen also had pelitic changes and was highly vascular with small vessels (Figure 4A, C and D). On immunohistochemical staining, the tumor cells were positive for HMB-45 (Figure 4B), Melan A, vimentin and actin; partially positive for α-smooth muscle actin (SMA); and negative for hepatocyte markers, CK7, CK20, desmin, CD68 and CD117. Moreover, the walls of the vessels showing pelitic changes and the vascular endothelium were both positive for CD34 and factor VIII. Taking these findings together, the weakly enhanced area identified within the tumor on the CT and MR images was suspected to reflect the presence of inflammatory cells. Ultimately, partial resection of segment 3 of the liver was performed, and a diagnosis of hepatic PEComa was established. To date, there has been no evidence of recurrence or metastasis in the 5 years since the initiation of this treatment.
Figure 1.
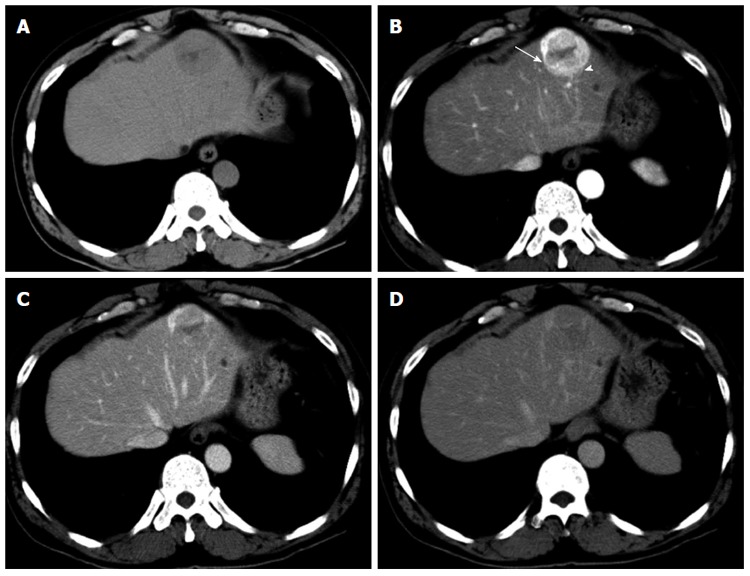
Computed tomography of the liver. A: The entire mass was visualized as low density on non-contrast computed tomography (CT); B: Contrast-enhanced CT showed a strongly enhanced mass in the arterial phase, and the center of the mass was weakly enhanced; the internal component-like structure showed relatively strong enhancement with a blotchy vascular pattern within the tumor (arrowhead) and distorted vessels (arrow); C and D: The portal (C) and late (D) phase CT scans showed washout of the contrast medium, and a portion of the internal area was weakly enhanced.
Figure 2.
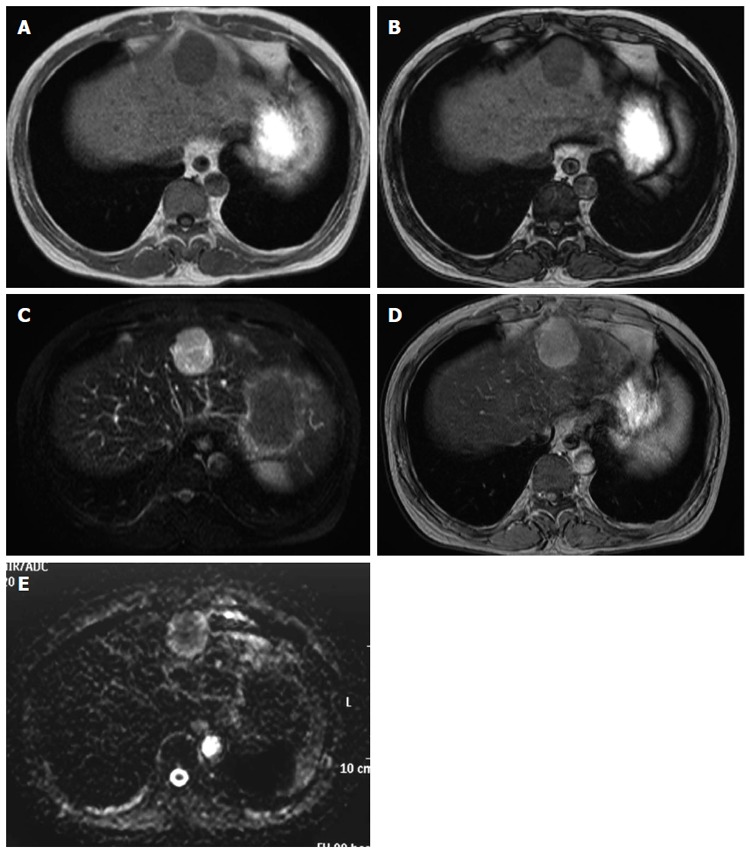
Magnetic resonance imaging of the liver. A magnetic resonance imaging (MRI) examination was performed on a 1.5 T Achieva Philips device. T1-weighted image (WI): FOV 350, matrix 256 × 204, slice thickness 8 mm, TR 147 ms, TE 2.3/4.6 ms, flip angle 80 degrees; T2-WI: FOV 350, matrix 240 × 196, slice thickness 8 mm, TR 3300 ms, TE 90 ms; T2*-WI: FOV 350, matrix 432 × 346, slice thickness 8 mm, TR 186 ms, TE 8.7 ms, flip angle 60 degrees; Diffusion-WI: FOV 350, matrix 128 × 102, slice thickness 8 mm, TR 1385 ms, TE 70 ms. A: The mass showed ill-defined low signal intensity on the T1-WI; B: There were no decreases in signal intensities in the out-of-phase (B) and in-phase (A) images; C: The mass showed high signal intensity on the T2-WI. The weakly enhanced center of the mass, as shown on computed tomography, was visualized as high signal intensity on the T2-WI, very similar to that observed in the entire mass; D: The mass was also visualized as high signal intensity on the T2*-WI after administration of superparamagnetic iron oxide; E: The mass showed high signal intensity on the diffusion-WI. The apparent diffusion coefficient was 1.491 × 10-3 mm2/s.
Figure 3.
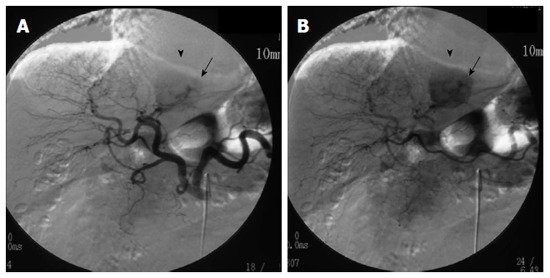
Digital subtraction angiography. Arteriography of the celiac artery showed strong enhancement (arrowheads) with thickened and distorted vessels (arrows) fed by the left hepatic artery on A and B.
Figure 4.
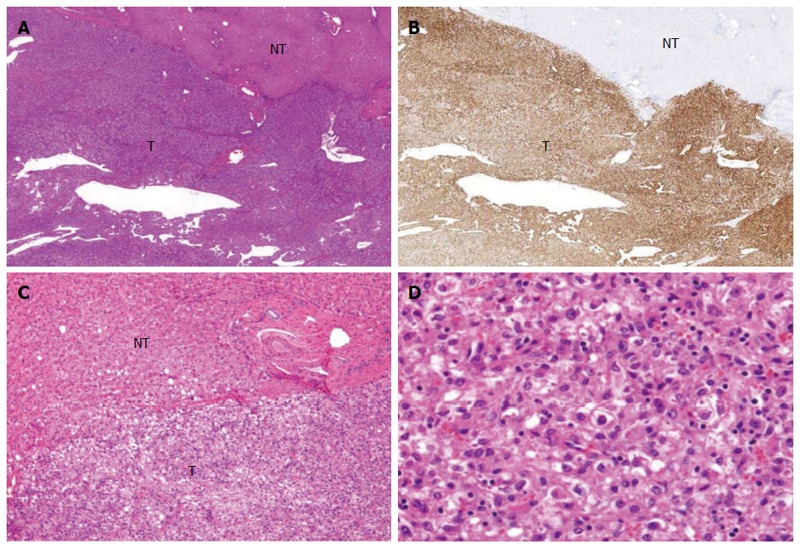
Microscopic features of the operative specimen. A and C: Show low and high power views, respectively, of the borderline area of the tumor (T) and non-tumor (NT) areas (magnification × 20 and × 200, respectively, hematoxylin eosin staining); B: Shows immunostaining features using an anti-HMB45 antibody (magnification × 20); D: Shows the tumor including fat (magnification × 200; hematoxylin eosin staining).
DISCUSSION
Definition of PEComa
Bonetti et al[1] were the first group to propose the concept of a PEComa family of tumors derived from PECs, which include clear-cell sugar tumors and lymphangioleiomyomatosis of the lung. In 2002, the WHO defined PEComas as “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells”[2].
Characteristics of the pathological diagnosis
The normal tissue counterpart of PECs is unknown. Immunohistochemical markers related to malignant melanomas, such as HMB-45, Melan A, and microphthalmia transcription factor, are quite useful for definitively diagnosing PEComa[3]. Typically, PECs are characterized by positive staining for myogenic markers, such as SMA, pan muscle actin, and calponin, and negative staining for cytokeratin and S100 protein[2-5].
Literature review
Our search of PubMed and Scopus identified 33 cases with primary hepatic PEComas, including our present patient, from 25 articles[6-30] and 40 cases with EAML from 17 articles[31-47]. Furthermore, a search limited to Japanese language publications yielded 3 articles on primary hepatic PEComas. These limited results reflect the poor recognition of PEComas. Given the almost equal number of articles concerning PEComas and EAMLs, it appears necessary to further promote the recognition of PEComas. Interestingly, according to the WHO classification[2], an angiomyolipoma is recognized as a PEComa. However, in clinical practice, an angiomyolipoma that has no or only a minimal fatty component is typically categorized as a PEComa. In fact, the concept of “PEComa” was not recognized by doctors (not even by radiologists) until recently, despite representing the highest number of operations for HCC in Japan.
This is the first review to investigate the results of both PEComa and EAML patients, with a total of 9 men and 64 women with a median age of 46 years (range, 17-75 years). The peak incidence was in the age range of 30-50 years, which accounted for 78% of all affected patients. A report on PEComa patients included 5 men and 28 women with a median age of 51 years (range, 18-75 years) and showed an age distribution that was bimodal, exhibiting an early peak in the 30 s and a second peak in the 50 s (Tables 1 and 2). Among all reported cases, 91% were from Asian and European countries; reports from Asian countries accounted for 66% and European countries accounted for 23% of the cases (Tables 1 and 2). A review of the annual number of reported PEComa and EAML cases in Asia revealed the incidence of PEComa to be increasing; thus, the number of reported PEComa cases is higher than that of EAML cases. In contrast, we did not identify a difference in the annual number of reported PEComa and EAML cases in European countries (Figure 5).
Table 1.
The year reported, location, size, age, sex, clinical symptoms, and imaging findings of 33 cases of hepatic perivascular epithelioid cell tumor
| Year reported | Reference | Authors | Age (yr) | Sex | Size (cm) | Location | Clinical symptoms | Detail of imaging findings | Country | Vessels in lesion |
| 2014 | our case | 58 | M | 4.5 | Lateral segment | abdominal bloating | HCC pattern | Asian | Yes | |
| 2014 | [6] | Bergamo et al | 31 | F | 10.0 | Right lobe | vomiting and gastric reflux | early enhancement only | Asian | |
| 2014 | [7] | Zhang et al | 63 | F | 3.5 | S5 | None | HCC pattern | European | |
| 2013 | [8] | Khaja et al | 51 | F | 4.0 | S4 | None | HCC pattern | Other | |
| 2013 | [9] | Tay et al | 51 | F | 9.0 | S2/3 | appetite loss | HCC pattern | Asian | Yes |
| 2013 | [10] | Liu et al | 25 | F | 5.5 | S7 | None | early enhancement only | Asian | |
| 2013 | [11] | Shen et al | 55 | M | 1.5 | S6 | None | HCC pattern | Asian | |
| 2013 | [12] | Jafari et al | 53 | F | 7.5 | Lateral segment | epigastralgia | HCC pattern | European | |
| 2013 | [13] | Cheung et al | 53 | F | 10.0 | Right lobe | abdominal discomfort | HCC pattern | Asian | |
| 2013 | [14] | Zhao et al | 58 | M | 7.6 | S6 | abdominal distention | HCC pattern | Asian | |
| 2013 | [15] | Yu et al | 41 | F | 2.2 | S6 | abdominal pain and vomiting | HCC pattern | Asian | |
| 2012 | [16] | Tan et al | 38 | F | 4.0 | Right lobe(S8) | 3 patients; colic pain or abdominal discomfort | HCC pattern | Asian | |
| 2012 | 34 | F | 4.3 | Right lobe(S8) | None | Asian | ||||
| 2012 | 49 | F | 2.5 | Left lobe | None | Asian | ||||
| 2012 | 75 | F | 8.0 | Right lobe | None | Asian | ||||
| 2012 | 33 | F | 2.5 | Right lobe | None | Asian | ||||
| 2012 | 71 | M | None | Right lobe | None | Asian | ||||
| 2012 | 41 | F | multiple | Left lobe | non early enhancement-gradual enhancement | Asian | ||||
| 2012 | [17] | Durczyński, et al | 18 | F | 15.0 | Left lobe | Unknown | None | European | |
| 2011 | [18] | Ahn et al | 36 | F | 7.0 | Left lobe | (tumor palpable) | HCC pattern | Asian | |
| 2009 | [19] | Akitake et al | 36 | F | 3.5 | S2 | None | HCC pattern | Asian | |
| 2009 | [20] | Strzelczyk et al | 57 | F | 20.0 | Right lobe | abdominal pain and vomiting | non-contrast CT | European | |
| 2009 | [21] | Sánchez Pérez et al | 32 | F | 5.5 | S7 | None | no early enhancement | European | |
| 2009 | [22] | Priola et al | 36 | F | 11.0 | Left lobe | abdominal pain | hemorrhage | European | |
| 2009 | [23] | Patra et al | 50 | F | 15.0 | Right lobe | abdominal pain | early-gradual enhancement | Asian | |
| 2008 | [24] | Della Vigna et al | 46 | F | 3.5 | S3 | None | HCC pattern | European | |
| 2008 | [25] | Paiva et al | 51 | F | 0.8 | Left lobe | None | None | Other | |
| 2008 | [26] | Zimmermann et al | 53 | M | 8.6 | Right lobe | abdominal pain | None | Other | |
| 2007 | [27] | Larbcharoensub et al | 31 | F | 1.8 | S8 | abdominal pain | HCC pattern | Asian | |
| 2007 | [28] | Fang et al | 56 | F | 5.1 | S4 | abdominal distention | enhancement in the portal phase | Asian | |
| 2007 | 63 | F | 8.0 | S1 | None | HCC pattern and late enhancement | Asian | |||
| 2007 | [29] | Svajdler et al | 55 | F | 3.5 | S4 | None | HCC pattern | European | |
| 2006 | [30] | Parfitt et al | 60 | F | 14.0 | Right lobe | abdominal discomfort and pain | None | Other |
HCC: Hepatocellular carcinoma; CT: Computed tomography.
Table 2.
The year reported, location, size, age, sex, clinical symptoms, enhanced images and vessels within the lesions of 40 cases with hepatic epithelioid angiomyolipoma
| Year reported | Reference | Authors | Age (yr) | Sex | Size (cm) | Location | Clinical symptoms | Detail of imaging findings | Vessels in lesion | Country |
| 2013 | [31] | Occhionorelli et al | 25 | F | 8.0 | Left lobe | Abdominal pain | Early-gradual Enhancement | European | |
| 2013 | [32] | Saito et al | 46 | M | 1.2 | S2 | None | Early-gradual Enhancement | Asian | |
| 2013 | [33] | Zhao et al | 54 | F | 0.6 | S4 | None | HCC pattern | Asian | |
| 30 | F | 3.7 | S7 | None | Non early enhancement-gradual Enhancement | Asian | ||||
| 49 | N | 9.7 | S4 | None | Early-gradual Enhancement | Asian | ||||
| 51 | F | 3.0 | S5 | None | HCC pattern | Asian | ||||
| 52 | M | 3.2 | S6 | None | HCC pattern and late Enhancement | Asian | ||||
| 2013 | [34] | Lo et al | 70 | F | 24 | Right lobe | Abdominal distention | None | Asian | |
| 47 | F | 0.2 | Right lobe | None | Early-gradual Enhancement | Asian | ||||
| 41 | F | 10.0 | Left lobe | Abdominal discomfort | HCC pattern | Asian | ||||
| 40 | F | 9.0 | Right lobe | Epigastralgia | HCC pattern | Asian | ||||
| 36 | F | 7.5 | Right lobe | None | HCC pattern | Asian | ||||
| 2012 | [35] | Agaimy et al | 63 | F | 1.0 | S2/3 | Nausea | HCC pattern | European | |
| 2013 | [36] | Ji et al | 64 | F | 5.0 | S1 | None | HCC pattern | Yes | Asian |
| 43 | F | 6.0 | S2/3 | None | Early-gradual Enhancement | Yes | Asian | |||
| 56 | F | 4.0 | S4 | None | Early-gradual Enhancement | Asian | ||||
| 40 | F | 8.5 | S2/3 | None | HCC pattern | Yes | Asian | |||
| 46 | F | 4.2 | S7/8 | None | HCC pattern | Yes | Asian | |||
| 32 | F | 6.0 | S2/3 | None | HCC pattern | Yes | Asian | |||
| 2012 | [37] | Xie et al | 32 | F | 3.4 | S7 | Dyspnea | HCC pattern | Other | |
| 2010 | [38] | Wen et al | 25 | M | 4.1 | S4 | None | HCC pattern | Asian | |
| 2009 | [39] | Leenman et al | 29 | F | 5.0 | S3 | Abdominal pain | None | Other | |
| 2009 | [40] | Xu et al | 51 | F | 6.5/4 | S6/7, S4 | Unknown | No early enhancement | Yes | Asian |
| 42 | F | 3.2 | S1 | Unknown | No early enhancement | Asian | ||||
| 35 | F | 6.5 | S6 | Unknown | Early enhancement only | Yes | Asian | |||
| 36 | F | 0.5 | S4 | Unknown | Early enhancement only | Yes | Asian | |||
| 17 | F | 9.0 | S578 | Unknown | Early enhancement only | Yes | Asian | |||
| 55 | F | 4.0 | S2/3 | Unknown | Early enhancement only | Yes | Asian | |||
| 36 | F | 2.0 | S4 | Unknown | Early enhancement only | Yes | Asian | |||
| 46 | F | 3.0 | S2/3 | Unknown | Early enhancement only | Yes | Asian | |||
| 47 | F | 1.5 | S6 | Unknown | Early enhancement only | Yes | Asian | |||
| 2009 | [41] | Alatassi et al | 26 | F | 11 (multiple) | Multiple | None | None | Other | |
| 2007 | [42] | Khalbuss et al | 39 | F | 12.0 | S2/3 | Abdominal pain | None | Other | |
| 2006 | [43] | Rouquie et al | 67 | F | 7.0 | S4 | None | Early enhancement only | Yes | European |
| 2004 | [44] | Tryggvason et al | 42 | F | 6.0 | S2/3 | Abdominal pain | HCC pattern | European | |
| 2000 | [45] | Savastano et al | 39 | F | 1.2 | S2/3 | Unknown | HCC pattern | Yes | European |
| 2000 | [46] | Flemming et al | 46 | F | 3.5/1 | S6, S3 | Unknown | Early enhancement only | European | |
| 50 | F | 6.0 | S1 | Unknown | HCC pattern | European | ||||
| 46 | F | 19.0 | S4-8 | Unknown | HCC pattern | European | ||||
| 2000 | [47] | Yamasaki et al | 30 | F | 2.0 | Right lobe | None | Early enhancement only | Asian |
Figure 5.
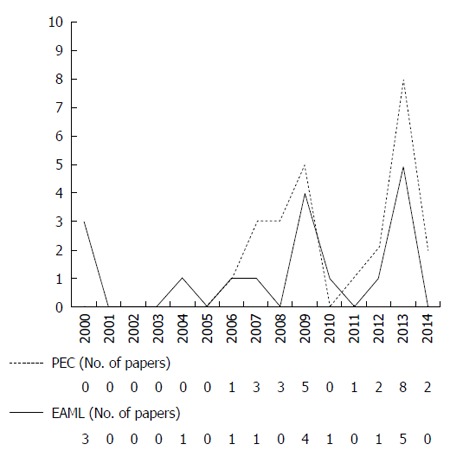
The annual numbers of reported perivascular epithelioid cell tumor and epithelioid angiomyolipoma cases. PEC: Perivascular epithelioid cell tumor; EAML: Epithelioid angiomyolipoma.
Clinical information
Clinical symptoms were reported in 55 of the 73 patients (no description in the other 18). Of these 55 patients, 29 (53%) had gastrointestinal symptoms, such as abdominal pain, abdominal discomfort, and vomiting. The symptoms were evaluated to determine whether they were correlated with tumor size[3]. Even patients with small tumors had symptoms (Tables 1 and 2). In addition, our present patient may have had inflammatory features because inflammatory cells were present within the tumor.
For half of the patients reported, there was no description of infection, while 4 patients reportedly had hepatitis B viral infection[14,31,38], which was considered to be unrelated to their PEComas. Of note, only 2 patients had tuberous sclerosis[37,41], and 5 had been diagnosed with PEComas involving multiple sites[10,16,40,41,46].
Diagnostic imaging
With regard to diagnostic imaging, we reviewed 74 lesions for which the tumor size and location were described; the tumor sizes ranged from 1.2 to 25 cm (median, 5.2 cm). There were 38 right lobe tumor sites (16 in the right lobe only, 4 in segment 1, 11 in the posterior segment, and 7 in the anterior segment) and 38 left lobe sites (7 in the left lobe only, 18 in the lateral segment, and 11 in the medial segment). These results indicate that the ratio of right to left lobe lesions is approximately 1 to 1, reducing the potential for predicting sites of development. A study investigating only PEComas found that tumor sizes ranged from 0.8 to 20 cm (median, 5.3 cm) and that 19 lesions were located in the right lobe (9 in the right lobe only, 1 in segment 1, 5 in the posterior segment, and 4 in the anterior segment) and 14 lesions were located in the left lobe (6 in the left lobe only, 5 in the lateral segment, and 3 in the medial segment). These data suggest that PEComas are more likely to develop in the right lobe of the liver. However, on the basis of the combined results of the reported PEComa and EAML cases, the possibility that PEComas develop relatively equally among regions of the liver must be considered (Tables 1 and 2).
The patterns of contrast enhancement were also evaluated in 58 cases. HCC patterns were observed in 30 cases: enhancement only in the arterial phase was observed in 12 cases; enhancement in the arterial phase without washout was observed in 8 cases; persistent enhancement after visualization of HCC patterns was observed in 2 cases; insufficient enhancement in the arterial phase and gradual enhancement in the late phase were observed in 6 cases; no definitive findings or unspecified patterns were noted in 14 cases; and hemorrhage was observed in 1 case. Collectively, 86% of the cases exhibited enhancement in the arterial phase, while 17% had a unique pattern, and 10% showed delayed enhancement (Tables 1 and 2). Moreover, all reported cases with hemorrhage within the tumor were also evaluated. All tumors with interior hemorrhage were at least 70 mm at the maximum diameter, with the exception of one that was 30 mm and showed hemorrhagic necrosis[47]. A blotchy vascular pattern, which appears to be useful for differentiating benign from malignant lesions, was observed in 17 patients. This pattern was considered to reflect abnormal vessels of leiomyoma lesions and to be a characteristic finding of PEComas.
To our knowledge, this is the first report to provide ADC values for PEComas. Our results are equivalent to the values in HCC patients and similar to those in leiomyoma patients[48]. Additional ADC data are awaited from future case series studies. As an added note, we do not expect ADC values of patients with PEComa to be different from those of HCC patients[49].
Clinical treatment and prognosis
We identified treatment approaches in 51 reported cases; 45 patients underwent surgery (1 underwent surgery due to an enlarged tumor after biopsy[24]), 6 received a biopsy only[6,8,16,37,41,46] and 1 was given chemotherapy with an mTOR (mammalian target of rapamycin) inhibitor[6].
The first line of treatment for primary hepatic PEComa is short-term observation or surgery because these tumors tend to enlarge over time. The genes responsible for the pathogenesis of tuberous sclerosis have been identified as those affected by loss of function mutations (TSC1 or TSC2, i.e., a tumor suppressor gene), and these genes induce changes that have been shown to be related to the etiology of PEComas[50]. Thus, everolimus has also been used as a novel therapy for this tumor type[6].
One of the patients reviewed herein had a poor outcome[30]. Studies of hepatic angiomyolipoma have found that approximately 3% of all patients have poor outcomes[51]. However, PEComas are rare, and criteria for malignancy have not yet been fully established, although Folpe et al[5] suggested that tumors with diameters exceeding 5.0 cm, infiltrative margins, high-grade nuclear atypia, a mitotic count of more than 1 per 50 high power fields, vascular invasion, or/and necrosis should be regarded as malignant. They further proposed that PEComas with none of the above findings are benign, while PEComas with one of these findings are of uncertain malignant potential, and PEComas with more than one finding are clearly malignant.
We described the detailed differential diagnoses between benign patterns and malignant patterns from the literature. The criteria for malignancy of hepatic PEComas have not yet been fully established. Our search of PubMed identified 5 cases with malignant hepatic angiomyolipoma[30,51-54]. An invasive growth pattern was found in 62% of the cases. Although these histological features suggest malignancy, distant metastases were not found[4]. No data suggested malignancy other than the tumor size. The median diameter of malignant hepatic angiomyolipoma was estimated to be 15 cm (range, 11-26 cm).
Summary
As PEComas are primarily benign tumors, surgical interventions might not be necessary. The key findings for making an accurate differential diagnosis include a blotchy vascular pattern within the tumor and no signs of hemorrhage within tumors with a diameter less than 7 cm. Furthermore, the portal vein serves as the drainage system for most HCCs; if PEComas have hemodynamic features similar to those of hepatic angiomyolipomas, the patterns of the drainage veins are then highly likely to be useful for differentiating HCC from PEComas, as observed in our case. However, the hepatic vein provides drainage for some HCCs, and as such, HCC cannot always be ruled out on the basis of drainage patterns alone[55].
We conclude that even if enhanced imaging patterns present findings similar to those of HCC, PEComas should still be considered when no abnormalities are found in the background parenchyma and the results of testing for hepatitis virus markers are negative. Finally, when the lesion is easily accessible, a biopsy is strongly recommended so that histopathological examinations can be performed.
COMMENTS
Case characteristics
A 58-year-old man presented with the chief complaint of abdominal bloating and was incidentally found to have a liver tumor.
Clinical diagnosis
The present study suggested that the patient likely had either a malignant hepatic tumor, such as a hepatocellular carcinoma or hepatic metastasis, or a benign liver tumor.
Differential diagnosis
Hepatocellular carcinoma, hepatic metastasis and benign hepatic tumor.
Laboratory diagnosis
Tests for hepatitis C virus antibody and hepatitis B virus surface antigen were negative, and routine blood tests were within normal limits.
Imaging diagnosis
A computed tomography scan and magnetic resonance imaging suggested that the patient likely had either hepatocellular carcinoma, hepatic metastasis, or a benign liver tumor.
Pathological diagnosis
On immunohistochemical staining, tumor cells were positive for HMB-45, Melan A, vimentin and actin; partially positive for α-smooth muscle actin; and negative for hepatocyte markers, CK7, CK20, desmin, CD68 and CD117.
Treatment
Partial resection of segment 3 of the liver was performed.
Term explanation
In 2002, the World Health Organization defined perivascular epithelioid cell tumors (PEComas) as “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells”. However, PEComa and epithelioid angiomyolipoma (EAML) are still regarded by many experts as the same disease.
Experiences and lessons
A PEComa arising from the liver is relatively rare. A diagnosis of hepatic PEComa is established on the basis of positive immunohistochemical staining results for human melanoma black 45 (HMB-45) and Melan-A. Patterns of the drainage veins would very likely be useful for differentiating hepatocellular carcinomas from PEComas.
Peer-review
The article is well written, the diagnosis is confirmed by radiography and pathology with immunohistochemistry of the key factors for PEComas, and it has a five year follow up.
Footnotes
Conflict-of-interest: The authors have no conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 18, 2014
First decision: December 2, 2014
Article in press: March 31, 2015
P- Reviewer: Chang ZG S- Editor: Yu J L- Editor: Webster JR E- Editor: Ma S
References
- 1.Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Soft Tissue and Bone. Lyon: IARC Press; 2002. pp. 221–222. [Google Scholar]
- 3.Nonomura A, Enomoto Y, Takeda M, Takano M, Morita K, Kasai T. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology. 2012;61:863–880. doi: 10.1111/j.1365-2559.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- 4.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, Cillo U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa) World J Surg Oncol. 2014;12:46. doi: 10.1186/1477-7819-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Wang L, Jiang Y, Wan Z, Li W, Yao C, Geng Z, Lv Y. Hepatic perivascular epithelioid cell tumors-not otherwise specified: a case report. Nan Fang Yi Ke Da Xue Xuebao. 2014;34:1–4. [PubMed] [Google Scholar]
- 8.Khaja F, Carilli A, Baidas S, Sriharan A, Norford S. PEComa: A Perivascular Epithelioid Cell Tumor in the Liver-A Case Report and Review of the Literature. Case Rep Med. 2013;2013:904126. doi: 10.1155/2013/904126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay ShY, Lao WT, Chen ChL, Chan WP. Contrast-enhanced ct and angiographic findings in hepatic perivascular epithelioid cell tumor. JBR-BTR. 2013;96:308–310. doi: 10.5334/jbr-btr.428. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Shi D, Xu Y, Cao L. Management of perivascular epithelioid cell tumor of the liver: A case report and review of the literature. Oncol Lett. 2014;7:148–152. doi: 10.3892/ol.2013.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen HQ, Chen DF, Sun XH, Li X, Xu J, Hu XB, Li MQ, Wu T, Zhang RY, Li KZ. MRI diagnosis of perivascular epithelioid cell tumor (PEComa) of the liver. Rom J Morphol Embryol. 2013;54:643–647. [PubMed] [Google Scholar]
- 12.Jafari A, Fischer HP, von Websky M, Hong GS, Kalff JC, Manekeller S. Primary perivascular epitheloid cell tumour (PEComa) of the liver: case report and review of the literature. Z Gastroenterol. 2013;51:1096–1100. doi: 10.1055/s-0033-1350123. [DOI] [PubMed] [Google Scholar]
- 13.Cheung TT, Trendell-Smith N, Poon RT. Primary perivascular epithelioid cell tumour (PEComa) of the liver. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao LJ, Yang YJ, Wu H, Huang SM, Liu K. Perivascular epithelioid cell tumor of the liver: a case report and literature review. Eur Rev Med Pharmacol Sci. 2013;17:1665–1668. [PubMed] [Google Scholar]
- 15.Yu D, Tang S. Hepatic perivascular epithelioid cell tumor: a case report and review of the literature. Intern Med. 2013;52:1333–1336. doi: 10.2169/internalmedicine.52.0144. [DOI] [PubMed] [Google Scholar]
- 16.Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom Imaging. 2012;37:781–787. doi: 10.1007/s00261-012-9850-1. [DOI] [PubMed] [Google Scholar]
- 17.Durczyński A, Hogendorf P, Szymański D, Sporny S, Strzelczyk J. Synchronous occurrence of multiple focal nodular hyperplasia and huge hepatic perivascular epithelioid cells tumor (PEComa) in young woman after oral contraceptive use--is there a common pathogenesis? Pol Przegl Chir. 2012;84:457–460. doi: 10.2478/v10035-012-0078-0. [DOI] [PubMed] [Google Scholar]
- 18.Ahn JH, Hur B. Primary Perivascular Epithelioid Cell Tumor (PEComa) of the Liver - A Case Report and Review of the Literature. Korean J Pathol. 2011;45:93–97. [Google Scholar]
- 19.Akitake R, Kimura H, Sekoguchi S, Nakamura H, Seno H, Chiba T, Fujimoto S. Perivascular epithelioid cell tumor (PEComa) of the liver diagnosed by contrast-enhanced ultrasonography. Intern Med. 2009;48:2083–2086. doi: 10.2169/internalmedicine.48.2133. [DOI] [PubMed] [Google Scholar]
- 20.Strzelczyk JM, Durczynski A, Szymanski D, Jablkowski M, Dworniak D, Sporny S. Primary perivascular epithelioid cell tumor (PEComa) of the liver: report of a case. Surg Today. 2009;39:916–921. doi: 10.1007/s00595-009-3945-5. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez Pérez B, Suárez Muñoz MA, Aranda Narváez JM, Fernández Aguilar JL, Santoyo Santoyo J. [Perivascular epithelioid cell tumour (PEComa) of the liver] Cir Esp. 2009;85:184–186. doi: 10.1016/j.ciresp.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Priola AM, Priola SM, Cataldi A, Marci V, Fava C. Acute abdomen as an unusual presentation of hepatic PEComa. A case report. Tumori. 2009;95:123–128. doi: 10.1177/030089160909500124. [DOI] [PubMed] [Google Scholar]
- 23.Patra S, Vij M, Kota V, Kancherla R, Rela M. Pigmented perivascular epithelioid cell tumor of the liver: report of a rare case with brief review of literature. J Cancer Res Ther. 2013;9:305–307. doi: 10.4103/0973-1482.113401. [DOI] [PubMed] [Google Scholar]
- 24.Della Vigna P, Preda L, Monfardini L, Gorone MS, Maffini FA, Bellomi M. Growing perivascular epithelioid cell tumor of the liver studied with contrast-enhanced ultrasonography and magnetic resonance imaging. J Ultrasound Med. 2008;27:1781–1785. doi: 10.7863/jum.2008.27.12.1781. [DOI] [PubMed] [Google Scholar]
- 25.Paiva CE, Moraes Neto FA, Agaimy A, Custodio Domingues MA, Rogatto SR. Perivascular epithelioid cell tumor of the liver coexisting with a gastrointestinal stromal tumor. World J Gastroenterol. 2008;14:800–802. doi: 10.3748/wjg.14.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann A, von der Brelie C, Berger B, Kappeler A, Candinas D. Primary perivascular epithelioid cell tumor of the liver not related to hepatic ligaments: hepatic PEComa as an emerging entity. Histol Histopathol. 2008;23:1185–1193. doi: 10.14670/HH-23.1185. [DOI] [PubMed] [Google Scholar]
- 27.Larbcharoensub N, Karnsombut P, Jatchavala J, Wasutit Y, Nitiyanant P. Primary hepatic clear cell myomelanocytic tumor. Case report and review of the literature. APMIS. 2007;115:1454–1459. doi: 10.1111/j.1600-0463.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- 28.Fang S, Zhou L, Jin M, Hu J. Perivascular epithelioid cell tumour of the liver. Liver Int. 2007;27:1293–1294. doi: 10.1111/j.1478-3231.2007.01561.x. [DOI] [PubMed] [Google Scholar]
- 29.Svajdler M, Bohus P, Goc V, Tkácová V. [Perivascular epithelioid cell tumor (PEComa) of the liver: a case report and review of the literature] Cesk Patol. 2007;43:18–22. [PubMed] [Google Scholar]
- 30.Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219–1222. doi: 10.5858/2006-130-1219-MNOPEC. [DOI] [PubMed] [Google Scholar]
- 31.Occhionorelli S, Dellachiesa L, Stano R, Cappellari L, Tartarini D, Severi S, Palini GM, Pansini GC, Vasquez G. Spontaneous rupture of a hepatic epithelioid angiomyolipoma: damage control surgery. A case report. G Chir. 2013;34:320–322. [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Sugimoto K, Iwahashi S, et al. Hepatic epithelioid angiomyolipoma with arterioportal venous shunting mimicking hepatocellular carcinoma: report of a case. J Med Invest. 2013;60:262–266. doi: 10.2152/jmi.60.262. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Ouyang H, Wang X, Ye F, Liang J. MRI manifestations of liver epithelioid and nonepithelioid angiomyolipoma. J Magn Reson Imaging. 2014;39:1502–1508. doi: 10.1002/jmri.24291. [DOI] [PubMed] [Google Scholar]
- 34.Lo RC. Epithelioid angiomyolipoma of the liver: a clinicopathologic study of 5 cases. Ann Diagn Pathol. 2013;17:412–415. doi: 10.1016/j.anndiagpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Agaimy A, Vassos N, Croner RS, Strobel D, Lell M. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int J Clin Exp Pathol. 2012;5:512–521. [PMC free article] [PubMed] [Google Scholar]
- 36.Ji JS, Lu CY, Wang ZF, Xu M, Song JJ. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309–314. doi: 10.1007/s00261-012-9911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie L, Jessurun J, Manivel JC, Pambuccian SE. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40:639–650. doi: 10.1002/dc.21703. [DOI] [PubMed] [Google Scholar]
- 38.Wen MC, Jan YJ, Li MC, Wang J, Lin A. Monotypic epithelioid angiomyolipoma of the liver with TFE3 expression. Pathology. 2010;42:300–302. doi: 10.3109/00313021003631254. [DOI] [PubMed] [Google Scholar]
- 39.Leenman EE, Mukhina MS, Nasyrov AR. [Monophasic angiomyolipoma (PEComa) of the liver] Arkh Patol. 2009;71:44–46. [PubMed] [Google Scholar]
- 40.Xu PJ, Shan Y, Yan FH, Ji Y, Ding Y, Zhou ML. Epithelioid angiomyolipoma of the liver: cross-sectional imaging findings of 10 immunohistochemically-verified cases. World J Gastroenterol. 2009;15:4576–4581. doi: 10.3748/wjg.15.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alatassi H, Sahoo S. Epithelioid angiomyolipoma of the liver with striking giant cell component: fine-needle aspiration biopsy findings of a rare neoplasm. Diagn Cytopathol. 2009;37:192–194. doi: 10.1002/dc.20979. [DOI] [PubMed] [Google Scholar]
- 42.Khalbuss WE, Fischer G, Bazooband A. Imprint cytology of epithelioid hepatic angiomyolipoma: mimicry of hepatocellular carcinoma. Acta Cytol. 2007;51:670–672. [PubMed] [Google Scholar]
- 43.Rouquie D, Eggenspieler P, Algayres JP, Béchade D, Camparo P, Baranger B. [Malignant-like angiomyolipoma of the liver: report of one case and review of the literature] Ann Chir. 2006;131:338–341. doi: 10.1016/j.anchir.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Tryggvason G, Blöndal S, Goldin RD, Albrechtsen J, Björnsson J, Jónasson JG. Epithelioid angiomyolipoma of the liver: case report and review of the literature. APMIS. 2004;112:612–616. doi: 10.1111/j.1600-0463.2004.apm1120909.x. [DOI] [PubMed] [Google Scholar]
- 45.Savastano S, Piotto M, Mencarelli R, Spanio P, Rubaltelli L. [A monotypic variant of hepatic angiomyolipoma completely composed of perivascular epithelioid cells. A case] Radiol Med. 2000;100:79–81. [PubMed] [Google Scholar]
- 46.Flemming P, Lehmann U, Becker T, Klempnauer J, Kreipe H. Common and epithelioid variants of hepatic angiomyolipoma exhibit clonal growth and share a distinctive immunophenotype. Hepatology. 2000;32:213–217. doi: 10.1053/jhep.2000.9142. [DOI] [PubMed] [Google Scholar]
- 47.Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451–456. doi: 10.1046/j.1365-2559.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014;210:368.e1–368.e8. doi: 10.1016/j.ajog.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, Koh YS, Cho CK, Kang HK. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010;11:295–303. doi: 10.3348/kjr.2010.11.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonomura A, Enomoto Y, Takeda M, Tamura T, Kasai T, Yosikawa T, Nakamime H. Invasive growth of hepatic angiomyolipoma; a hitherto unreported ominous histological feature. Histopathology. 2006;48:831–835. doi: 10.1111/j.1365-2559.2006.02427.x. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32:793–798. doi: 10.1097/PAS.0b013e3181607349. [DOI] [PubMed] [Google Scholar]
- 52.Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443–450. doi: 10.1046/j.1365-2559.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 53.Mizuguchi T, Katsuramaki T, Nobuoka T, Nishikage A, Oshima H, Kawasaki H, Kimura S, Satoh M, Hirata K. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol. 2004;19:1328–1330. doi: 10.1111/j.1440-1746.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 54.Deng YF, Lin Q, Zhang SH, Ling YM, He JK, Chen XF. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204:911–918. doi: 10.1016/j.prp.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology. 2009;252:605–614. doi: 10.1148/radiol.2522081414. [DOI] [PubMed] [Google Scholar]


