Abstract
Primary biliary cirrhosis (PBC) is an autoimmune, slowly progressive, cholestatic, liver disease characterized by a triad of chronic cholestasis, circulating anti-mitochondrial antibodies (AMA), and characteristic liver biopsy findings of nonsuppurative destructive cholangitis and interlobular bile duct destruction. About 10% of PBC patients, however, lack AMA. A variant, called PBC-autoimmune hepatitis (AIH) overlap, is characterized by the above findings of PBC together with findings of elevated serum alanine aminotransferase, elevated serum immunoglobulin G, and circulating anti-smooth muscle antibodies, with liver biopsy demonstrating periportal or periseptal, lymphocytic, piecemeal necrosis. PBC is hypothesized to be related to environmental exposure in genetically vulnerable individuals. It typically occurs in middle-aged females. Prominent clinical features include fatigue, pruritis, jaundice, xanthomas, osteoporosis, and dyslipidemia. The Mayo Risk score is the most widely used and best prognostic system. Ursodeoxycholic acid is the primary therapy. It works partly by reducing the concentration and injury from relatively toxic bile acids. PBC-AIH overlap syndrome is treated with ursodeoxycholic acid and corticosteroids, especially budesonide. Obeticholic acid and fibrate are promising new, but incompletely tested, therapies. Liver transplantation is the definitive therapy for advanced disease, with about 70% 10-year survival after transplantation. Management of pruritis includes local skin care, dermatologist referral, avoiding potential pruritogens, cholestyramine, and possibly opioid antagonists, sertraline, or rifaximin. Management of osteoporosis includes life-style modifications, administration of calcium and vitamin D, and alendronate. Statins are relatively safe to treat the osteopenia associated with PBC. Associated Sjogren’s syndrome is treated by artificial tears, cyclosporine ophthalmic emulsion to stimulate tear production; and saliva substitutes, cholinergic agents, and scrupulous oral and dental care. Complications of cirrhosis from advanced PBC include esophageal varices, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatoma formation.
Keywords: Primary biliary cirrhosis, Ursodeoxycholic acid, Cirrhosis, Liver transplantation, Cholestatic liver disease
Core tip: Primary biliary cirrhosis (PBC) is an autoimmune, slowly progressive, cholestatic, liver disease characterized by a triad of chronic cholestasis, circulating anti-mitochondrial antibodies, and characteristic liver biopsy findings of nonsuppurative destructive cholangitis and interlobular bile duct destruction. Prominent clinical features include fatigue, pruritis, jaundice, xanthomas, osteoporosis, and dyslipidemia. Ursodeoxycholic acid is the primary therapy. Obtecholic acid and fibrate are promising new, but incompletely tested, therapies. Liver transplantation is the definitive therapy for advanced disease, with about 70% 10-year survival after transplantation. Complications of cirrhosis from advanced PBC include esophageal varices, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatoma formation.
INTRODUCTION
Primary biliary cirrhosis (PBC) is an autoimmune, slowly progressive, cholestatic, liver disease[1]. A triad of chronic biochemical cholestasis, circulating anti-mitochondrial antibodies (AMA), and characteristic liver biopsy findings, as described below, is diagnostic of PBC[2]. Development of PBC is hypothesized to be related to environmental exposure in genetically vulnerable individuals, but further studies are needed to understand its complex etiology[3]. This work reviews the epidemiology, pathophysiology, clinical presentation, treatment, and prognosis of PBC, with a focus on recent advances.
HISTORY
PBC, as it is now known, was first reported by Addison et al[4] in 1851. The term PBC was, however, coined by Ahrens et al[5] in 1950. Walker et al[6] first described the association between AMA seropositivity and PBC.
EPIDEMIOLOGY
PBC is ten-fold more common in women than men[1]. The reason for this difference is unknown, but a relatively recent study revealed that X chromosome monosomy was more common in women with PBC. This finding suggests that genes related to X-linked immunodeficiencies can lead to granuloma formation and elevated IgM levels, both of which occur in PBC[7].
The median age of diagnosis is about 50 years[8]. There is significant geographic variation, with a much higher prevalence in the United States, at 400 per million[8], or northern Europe, at 200-250 per million, than in Africa, Asia, or Australia, at 20 per million[9]. This difference, however, could largely arise from detection bias; far more epidemiologic studies have been conducted in North America and Europe than in Africa and Asia[10]. Though differences in case-finding methodology, disease awareness, and access to health care may have confounded these studies, the immense geographic disparities suggest that environmental and/or genetic factors affect PBC. Environmental agents, including exposure to sunlight, chemicals, toxins, bacteria and viruses, may differ across geographic regions, and such differences may play a role in pathogenesis[11]. The educational level of individuals with PBC is similar to that of controls, but PBC is apparently more prevalent in patients who belong to higher socioeconomic strata for unclear reasons[12].
PBC is strongly associated with recurrent urinary tract infections[10,12-14]. In an epidemiologic study, 48% of PBC patients had prior recurrent urinary tract infections vs 31% of controls; this difference was statistically significant[12]. Escherichia coli (E. coli), the most frequent cause of recurrent urinary tract infections in women, has been studied in relation with PBC. E. coli infections trigger autoimmune responses, perhaps by molecular mimicry of proteins in E. coli with the human pyruvate dehydrogenase complex (PDC-E2), which induces B- and T-cell cross-reactive responses that characteristically occur in patients with PBC[10,12,14,15]. Other microorganisms implicated in PBC include Novosphingovium aromaticivorans, Lactobacillus delbrueckii, Toxoplasma gondii, Mycobacteria and retroviruses, though these associations are weaker than that for E. coli[14]. Numerous studies in the United States and United Kingdom suggest a significant relationship between smoking tobacco and PBC[10,12,15-17]. Chemicals inhaled in tobacco smoke are postulated to decrease immunologic tolerance[11]. Some data suggest patients who use hair dyes are at increased risk of PBC[15], but the evidence is conflicting[10]. PBC is apparently negatively correlated with alcohol consumption[15].
Genetic and familial factors play a major role in PBC[18]. The concordance rate is 63% in monozygotic twins[19], and the concordance rate is 4% in first degree relatives of patients with PBC. Moreover, sisters of a woman with PBC have a 14-fold higher risk of PBC compared to the general population[12].
Autoimmune disorders occur more frequently in patients with PBC, including autoimmune thyroid disorders, Raynaud syndrome, and Sjogren syndrome[12]. These disorders generally precede the onset of PBC by about 4 years. Patients with thyroid disorders should be tested for the presence of AMA. A recent meta-analysis suggested that human leukocyte antigen (HLA)-DR 7 and -DR 8 are risk factors for PBC, whereas HLA-DR 11 and -DR 13 are protective factors[20]. Other genetic variants associated with PBC include polymorphisms of cytotoxic T-lymphocyte antigen[21,22], tumor necrosis factor (TNF)[23], vitamin D receptor[24], and HLA loci 11 and 12.
DIAGNOSIS
PBC is diagnosed provided two of the following three criteria are satisfied: (1) AMA titer > 1:40; (2) alkaline phosphatase (AP) > 1.5 times the upper limit of normal for > 24 wk; and (3) compatible liver histology, demonstrating nonsuppurative destructive cholangitis and interlobular bile duct destruction[25-27].
AMA
AMA is the most specific serological marker among > 60 autoantibodies analyzed in PBC patients[28,29]. It is detected in > 90% of patients with PBC, whereas it is detected in < 1% of the general population[1,29]. Patients with hepatitis C virus infection, however, have an 8% prevalence of AMA positivity. The antibody targets are members of a family of enzymes, the 2-oxo-acid dehydrogenase complexes, which include PDC-E2, branched chain 2-oxo-acid dehydrogenase complex, and 2-oxo-glutaric acid dehydrogenase complex. These enzymes catalyze oxidative decarboxylation of ketoacid substrates and are located on the inner mitochondrial membrane[27,30,31].
AMA is routinely detected in clinical laboratories by enzyme-linked immunoassay. Its titer does not correlate with PBC severity or activity. It is reasonable to determine serum liver function tests annually in seropositive individuals who initially have normal serum liver function tests[25]. When PBC is strongly suspected in AMA-negative patients, PBC-specific antinuclear antibodies (ANA), should be determined, including sp100 and gp210[7,32]. Elevated serum AP levels and characteristic liver histology, as aforementioned, also help in diagnosing PBC[25]. Occasionally, magnetic resonance cholangiopancreatography or endoscopic retrograde cholangiopancreatography may be necessary to exclude primary sclerosing cholangitis (PSC) or other alternative etiologies for cholestatic liver disease[33]. PSC is strongly suspected in a patient with elevated serum AP who has inflammatory bowel disease[34].
The time from detection of AMA to development of PBC is about 6 years (range 1-19 years)[35]. Only about 10% of patients who are AMA seropositive, but lack clinical features of PBC, subsequently develop PBC. A recent Greek study examined the significance of AMA, ANA-specific antibodies (anti-gp210 and anti-sp100), and antichromatin antibodies[36]. Autoantibody positivity, with increased autoantibody titers of anti-gp210 and anti-sp100 during follow-up, were related to advanced PBC. Mildly elevated anti-sp100 titers were associated with improved long-term prognosis (P = 0.025), and better response to ursodeoxycholic acid (UDCA) (P = 0.016)[35].
LIVER HISTOLOGY
Though liver biopsy is not mandatory for diagnosis, it helps stage the disease and differentiate PBC from other cholestatic liver disorders[27,37,38]. PBC has 4 histologic stages: (1) portal inflammation with or without florid bile duct lesions; (2) increase in size of periportal lesions with interface hepatitis; (3) distortion of hepatic architecture with numerous fibrous septa; and (4) cirrhosis. These stages occur sequentially with disease progression. The term “florid bile duct lesion” describes focal lesions that exhibit intense inflammatory infiltration and necrosis around bile ducts (Figure 1). The inflammatory infiltrate consists primarily of lymphocytes and mononuclear cells closely apposed with the basal membrane of cholangiocytes undergoing necrosis. The infiltrate may also contain macrophages and polymorphonuclear cells, and occasionally epithelioid granulomas, especially in early PBC[39]. The inflammatory infiltrates often compress and occlude portal venules. Generally, terminal hepatic venules are retained in their central location with progression to fibrosis and sometimes even to cirrhosis. Ductopenia, or bile duct paucity, is defined as presence of bile ducts in < 50% of portal tracts[27]. Interface hepatitis consists of lymphocytic piecemeal necrosis and biliary piecemeal necrosis which is associated with cholestasis[27]. Lymphocytic piecemeal necrosis consists of hepatocellular necrosis or apoptosis associated with lymphohistiocytic cells. This lesion is similar to that found in autoimmune hepatitis (AIH). Biliary piecemeal necrosis exhibits a striking reaction of ductular proliferation, accompanied by edema, neutrophil infiltration, periductular fibrosis, and necrotic hepatocytes.
Figure 1.
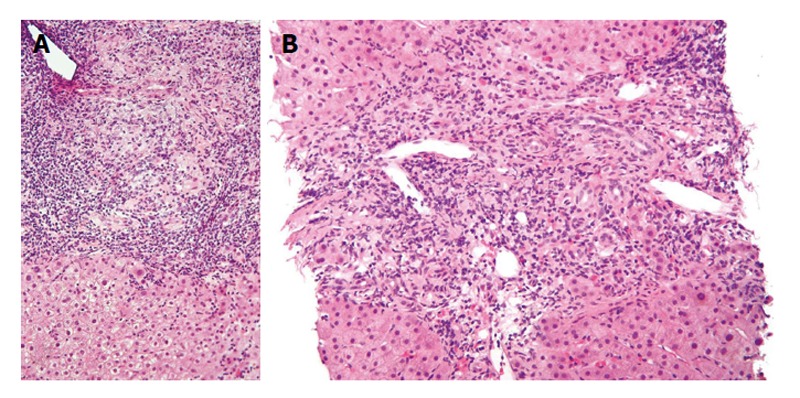
Lower (A) and higher (B) magnification photomicrographs of a liver biopsy sample in a patient with early primary biliary cirrhosis shows a moderately severe, mixed inflammatory infiltrate consisting mostly of lymphocytes and plasma cells concentrated around small bile ducts in the portal area. Note the absence of inflammation around hepatocytes (H and E stains). Both figures obtained with permission (granted under the GNU Free Documentation License, Version 1.2) from: http://commons.wikimedia.org/wiki/File:Primary_biliary_cirrhosis_intermed_mag_2.jpg. Accessed March 10, 2015.
PBC VARIANTS
PBC variants constitute about 5% of cases[40]. The five types of variants are listed in Table 1[33]. The two major variants are PBC-AIH overlap and AMA-negative PBC.
Table 1.
Primary biliary cirrhosis - autoimmune hepatitis overlap can present in the following ways
| Immunoserological overlap: e.g., positive ANA/anti-smooth muscle antibody titers and elevated IgG in conjunction with AMA-positive PBC; or AMA positivity in AIH |
| Biochemical overlap: AST/ALT > 5 times upper limit of normal in patients with PBC or PSC; or AP > 3 times upper limit of normal in patients with AIH (or GGT > 5 times upper limit of normal in children) |
| Radiological overlap: clinical features of AIH with cholangiographic abnormalities indicative of inflammatory cholangiopathy; cholangiographic features of primary sclerosing cholangitis are randomly distributed annular strictures out of proportion to upstream dilatation[33] |
| Histological overlap: lymphoplasmacytic infiltrate and interface hepatitis on liver biopsy with bile duct lesions indicative of either PBC or PSC |
| Varying combinations of the above, including sequential presentations |
ANA: Antinuclear antibodies; PBC: Primary biliary cirrhosis; AMA: Anti-mitochondrial antibodies; AIH: Autoimmune hepatitis; PSC: Primary sclerosing cholangitis; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma glutamyl transpeptidase.
PBC-AIH overlap
The PBC-AIH overlap syndrome consists of a range of clinical entities of AIH together with clinical, laboratory, or histological characteristics of PBC or PSC[41-43]. These two overlapping conditions rarely present simultaneously; PBC generally precedes AIH by 6 mo to several years[43]. No stringent criteria exist for this overlap syndrome. In the most widely accepted criteria, overlap syndrome is diagnosed when PBC is diagnosed by the aforementioned criteria and AIH by the presence of the following criteria: (1) serum alanine aminotransferase ≥ 5 × upper limit of normal, serum immunoglobulin G (IgG) levels ≥ 2 × upper limit of normal, or positive test for anti-smooth muscle anti-bodies; and (2) liver biopsy demonstrating moderate or severe periportal or periseptal, lymphocytic, piecemeal necrosis[44,45]. The simplified International Autoimmune Hepatitis Group criteria for diagnosis of overlap syndrome are not currently recommended[43]. The Paris criteria for diagnosis of PBC-AIH overlap include an AP-to-aminotransferase ratio < 1.5, elevated serum IgG level, and anti-smooth muscle antibody (anti-SMA) titer > 1:80[25,26,46].
AMA negative PBC
AMA-negative PBC and classic PBC have similar clinical findings and laboratory characteristics[40,47-49]. They have similar age and sex distributions, similar incidence of complications, and similar time-to-liver-transplantation and survival. The relationship between AMA-negative PBC and autoimmune cholangiopathy is controversial. Some authorities use these terms interchangeably[49,50], whereas other authorities view them as disparate entities, with autoimmune cholangiopathy representing PBC with negative AMA titers, but positive ANA or anti-SMA titers[40]. Before labeling individuals as AMA-negative PBC, methods to detect circulating AMA should be assiduously employed, including sensitive immunochemical testing with bead microassays, use of three mitochondrial autoantigens[51], and serial testing[52]. Liver biopsy is mandatory to diagnose PBC in the setting of AMA seronegativity[50]. Other etiologies of liver disease in the differential diagnosis should be excluded, including sclerosing cholangitis, IgG4 cholangitis, drug induced liver injury, etc.[50]. Additionally, other causes of elevated serum AP should be investigated, including hepatic causes such as infectious hepatitis, granulomatous liver disease, infiltrative liver disease, and idiopathic adult ductopenia; and non-hepatic causes such as pregnancy, bone disease, heart failure, renal failure, leukemia, lymphoma, etc.[53].
CLINICAL FEATURES
The clinical features and epidemiology of PBC are described in Table 2[54-60]. The most common clinical symptoms are fatigue and pruritus. Patients with fatigue and pruritus at onset are more likely to progress to cirrhosis and are less likely to respond to UDCA[61]. Pruritus is often severe and disabling, and associated with a poor quality of life. Both symptoms are described in detail below.
Table 2.
Clinical features of primary biliary cirrhosis
| Clinical features | Prevalence | Mechanism |
| Fatigue | 20%-85%[55,56,58] | Excessive manganese deposits in globus pallidum, elevated inflammatory cytokines |
| Pruritus | 20%-75%[35,58] | Cholestasis, increased opiodergic tone |
| Jaundice | 10%-60%[58] | Cholestasis |
| Xanthomas | 15%-50%[58] | Hypercholesterolemia and hyperlipidemia[56] |
| Osteoporosis | 35%[59] | Disturbances in bone remodeling due to metabolic changes in PBC |
| Dyslipidemia | > 75%[60] | Reduction in biliary secretion of cholesterol. Toxic effects of unconjugated bilirubin |
PBC: Primary biliary cirrhosis.
Most patients with PBC have mildly elevated serum aminotransferase levels[62,63], and increased level of immunoglobulins, especially IgM. A study of 25 patients with PBC vs age- and sex-matched controls revealed that IgM was uniformly elevated in patients with PBC[64]. The increase in aminotransferase levels primarily reflects the degree of periportal and lobular necrosis and inflammation, whereas hyperbilirubinemia reflects the degree of ductopenia and biliary piecemeal necrosis[27].
NATURAL HISTORY
The natural history of PBC, including the percentage of patients requiring liver transplantation or dying, has recently become less severe due to earlier diagnosis[25], and introduction of UDCA therapy[65,66].
PROGNOSTIC MODELS
The Mayo Risk score is the most widely used and best prognostic system[67]. It is superior to the Child-Pugh score in predicting prognosis[67]. It incorporates patient age, serum bilirubin concentration, albumin concentration, prothrombin time, and degree of edema. The Mayo Risk score = 0.04 (Age) + 10.87 Loge (Bilirubin) - 22.53 Loge (Albumin) + 12.38 Loge (Prothrombin time) + 10.86 (Edema score). An edema score of 0=no edema without diuretics, 1 = edema with diuretics, and 0.5 = otherwise. This risk score is used to calculate expected patient survival for up to 7 years of follow-up[68]. A Mayo Risk score of 7.8 is considered the optimal time to evaluate a patient for liver transplantation[69,70].
TREATMENT
The aim of PBC therapy is to reverse injury from bile duct inflammation to relieve symptoms, prevent disease progression, relieve laboratory abnormalities, and prevent the consequences of chronic cholestasis, including pruritus, fatigue, osteoporosis, and fat-soluble vitamin deficiencies[71]. The mechanism of action and therapeutic effects of drugs for PBC are summarized in Table 3[72,73].
Table 3.
Drug therapy for primary biliary cirrhosis
| Drug | Mechanism(s) of action | Adverse effects |
| Ursodeoxycholic acid | Protection of cholangiocytes, stimulation of biliary secretions of bile acids | Diarrhea, hepatic decompensation (rare) |
| Corticosteroids | Anti-inflammatory, especially useful for interface hepatitis | Cataracts, hyperglycemia, osteoporosis, immunosuppression, poor wound healing, weight gain |
| Budesonide | Anti-inflammatory, especially useful for interface hepatitis | Nausea, dyspepsia; systemic toxicity is much less than for other corticosteroids[73] |
| Obeticholic acid | Reduces bile acid synthesis, downregulates bile acid uptake proteins | Pruritus |
| Fibrates | Activates peroxisome proliferator-activated receptors | Myalgias, rhabdomyalysis, elevated liver enzymes[72] |
Ursodeoxycholic acid
Chronic cholestasis results in intrahepatic and systemic accumulation of potentially cytotoxic bile acids that initially promote hepatocyte proliferation, but subsequently cause liver damage, and ultimately cause hepatocyte apoptosis, biliary fibrosis, and cirrhosis[74]. UDCA has three mechanisms of action: (1) protection of cholangiocytes from cytotoxicity of hydrophobic bile acids by modulating the composition of mixed phospholipid-rich micelles, reduced bile acid cytotoxicity, and, possibly, reduced concentration of hydrophobic bile acids in cholangiocytes; (2) stimulation of biliary secretion of bile acids; and (3) protection of hepatocytes against bile acid-induced apoptosis, by inhibiting mitochondrial membrane permeability transition (MMPT)[75,76]. UDCA consists of water insoluble crystals that are absorbed by passive nonionic diffusion mostly in the small intestine with a small amount absorbed in the colon, reabsorbed from portal blood via first-pass metabolism, conjugated mainly with glycine and taurine, and actively secreted into bile. Administration of UDCA increases bile acid saturation in bile in a dose-dependent manner, resulting in increased clearance of bile acids from blood, and reduced symptoms from cholestasis, particularly pruritus. These beneficial effects occur with the recommended daily-administered dose of 13-15 mg/kg per day which increases the solubility of bile acids in bile by 40%-50%. However, efficacy is not established beyond this recommended dose[74].
UDCA is the only therapy approved by the United States Food and Drug Administration (FDA)[77]. It is well tolerated at the recommended dose. Diarrhea occurs in 2%-9% of cases[78]. Hispanics reportedly respond less well to UDCA than non-Hispanics[79]. UDCA can occasionally cause right upper quadrant pain, and rarely hepatic decompensation, when administered to patients with end-stage PBC[78]. A prospective study of 297 Dutch patients with PBC showed that UDCA, when administered to patients with early histologic disease, significantly improved transplant-free survival (1 year = 99.7%, 5 year = 87%, and 10 year = 71%) than that predicted by the Mayo model[80]. The AASLD recommends administration of UDCA in patients with PBC with abnormal liver function tests, regardless of histological stage[36]. A meta-analysis of 1038 patients in seven randomized clinical trials with long-term follow-up demonstrated UDCA decreased the incidence of liver transplantation [odds ratio (OR) = 0.65, P = 0.01], and decreased the combined rates of mortality or liver transplantation (OR = 0.76, P = 0.05). A meta-analysis of French, Canadian, and Mayo Clinic trials[81] demonstrated beneficial effects of UDCA on patient survival and time-to-liver transplantation. This benefit was observed in patients with moderate-to-severe disease, but not in patients with mild disease (serum bilirubin concentration < 1.4 mg/dL, stage I or II histological abnormalities)[81]. After liver transplantation, UDCA, compared to placebo, does not affect retransplantation rates, acute cellular rejection, or mortality related to allograft rejection[77].
UDCA protects against hepatoma development in patients with PBC, as demonstrated in a study of 930 patients[82,83]. Patients with PBC treated with UDCA experience an approximately three-fold decline in risk of hepatoma as compared to untreated patients. However, failure to improve liver function test abnormalities after one year of UDCA therapy represents a risk factor for hepatoma development[84].
Biochemical response to UDCA
A biochemical response to UDCA has been variably described as: (1) a Mayo risk score < 4.5, or serum AP level < 2 × upper limit of normal after 6 mo of treatment[85]; (2) a reduction in serum AP level to < 40% of baseline or to within normal limits at one year[86]; or (3) serum AP < 3 × upper limit of normal, AST < 2 × upper limit of normal, and bilirubin < 1 mg/dL after 1 year of UDCA therapy. Patients satisfying criterion 3 had a 90% rate of transplant-free survival at 10 years[87].
Corticosteroids
UDCA alone may not produce a biochemical response in patients with features of AIH, and such patients often require concomitant immunosuppressive therapy. Meta-analysis of seven randomized, controlled trials of patients with PBC with features of AIH showed that UDCA combined with corticosteroids significantly improved serum parameters of liver function and histologic grades, but did not significantly improve mortality or liver transplantation rate[88]. Long term use of corticosteroids can cause adverse effects, including hyperglycemia, osteoporosis, cataracts, weight gain, increased risk of opportunistic infections, etc.[89].
Budesonide
Budesonide is a corticosteroid that demonstrates first-pass metabolism in the liver, decreases systemic exposure to corticosteroids by 90% and reduces systemic toxicity as compared to other corticosteroids[90]. Randomized clinical trials have shown that budesonide at 6-9 mg/kg per day in combination with UDCA can improve serum biochemical parameters of liver function and liver histology, especially in patients with grade I-III fibrosis[91,92]. One study, however, showed only marginally significant improvement in serum AP levels with budesonide at the cost of increased systemic toxicity. This study did not, however, assess the grade of fibrosis in study patients and the finding of marginal improvement might reflect poor prognosis of PBC patients with cirrhosis[93]. Patients with cirrhosis administered budesonide have worse side effects than those without cirrhosis, and have an increased risk of portal venous thrombosis. Hence, patients with grade IV fibrosis or cirrhosis are generally not administered budesonide[94].
Obetocholic acid
Obetocholic acid, a farnesoid-X-receptor (FXR) agonist, is present in liver, intestine, kidneys, and adrenals. It plays an important role in the enterohepatic circulation of bile acids. It reduces bile acid synthesis by its action on 7 alpha hydroxylase, down-regulates bile acid uptake proteins, and increases expression of bilirubin exporter pumps[95,96]. An international, double-blind, placebo-controlled clinical trial in patients with PBC showed substantial improvement in various liver enzyme levels including AP, gamma glutamyl transpeptidase, and alanine aminotransferase with various drug doses[97-99]. A long-term, phase III trial of obetocholic acid in UDCA-treated patients is currently in progress (EudraCT Number: 2011-004728-36)[71].
Fibrates
Fibrates can benefit patients who respond suboptimally to UDCA, as reflected by significant improvement in cholestasis, cytolysis, and pruritus after adding fibrates[71,100-105]. Its mechanisms of action are incompletely understood. Fibrates activate the peroxisome proliferator-activated receptors, and apparently stimulate multidrug resistance protein 3 located primarily in liver, which in turn protects the hepatobiliary system by inducing phosphatidylcholine transport across the bile canalicular membrane to render bile less toxic[100]. Fibrates substantially improve serum biochemical tests of liver function, especially serum AP, though improvement in survival is yet to be demonstrated.
Other therapies
As PBC presumably has an immunologic component, numerous immunosuppressants and immunomodulators other than corticosteroids have been tested for treating PBC. No evidence supports their efficacy (Table 4[106-120]). Similarly, numerous other drugs have failed to demonstrate efficacy in clinical trials and are not currently recommended[26,27].
Table 4.
Drugs without efficacy in primary biliary cirrhosis as demonstrated in clinical trials
| Drug | Ref. |
| Azathioprine | [106] |
| Chlorambucil | [107] |
| Methotrexate | [108-110] |
| Mycophenolate mofetil | [111] |
| Cyclosporine | [112] |
| Penicillamine | [113,114] |
| Colchicine | [115,116] |
| Malotilate | [117] |
| Thalidomide | [118] |
| Silymarin | [119] |
| Statins | [120] |
Rituximab, an anti-CD20 monoclonal antibody, produces selective B-cell depletion that could potentially ameliorate autoimmune disease by decreasing autoantibody production and antigen presentation by B cells[121]. However, this biologic therapy has little efficacy[121,122]. A human monoclonal antibody directed against interleukin 12 (IL-12)/IL-23 (ustekinumab) is currently being investigated in PBC in a phase II trial (EudraCT Number: 2011-000554-31)[71]. Definitive data about safety and efficacy of ustekinumab in PBC are currently lacking.
PBC-AIH overlap is treated with UDCA and immunosuppression using corticosteroids or azathioprine. These agents produce a favorable serum biochemical response[43,44,123-125]. In such patients, UDCA alone increases the rate of fibrosis[124].
Liver transplantation
Liver transplantation is the only definitive treatment for PBC. It improves survival[126]. As per the United Network for Organ Sharing (UNOS) database, between 1995 and 2006, the number of liver transplants increased by a mean of 249 per annum in the United States, but the number of liver transplants performed for PBC decreased steadily by a mean of 5.4 cases per annum[127]. In Europe, PBC is still the third most common reason for liver transplantation, with a relative rate of 9%[128]. One-, 5- and 10-year survival for PBC in Europe are 86%, 80% and 72%, respectively[128]. These rates are higher than those for patients transplanted for hepatitis B, hepatitis C, alcoholic cirrhosis, or other autoimmune liver diseases.
As per European Association for the Study of the Liver (EASL) guidelines, any PBC patient with a serum bilirubin > 5.9 mg/dL, a Mayo Risk score > 7.8, and/or a Model for End-Stage Liver Disease (MELD) score of > 12 should be evaluated for potential liver transplantation[129]. Potential liver transplant candidates should be referred early for evaluation at a liver transplantation center to determine eligibility and assure timely listing as a liver transplant candidate.
As for all patients with end stage liver disease, clinical indications for liver transplantation in PBC include refractory ascites, recurrent spontaneous bacterial peritonitis, recurrent variceal hemorrhage, hepatic encephalopathy, hepatorenal syndrome type I, and hepatocellular carcinoma, subject to the Milan criteria[130]. Indications specific to PBC include refractory pruritus and chronic fatigue[128]. After determining that a patient is a liver transplant candidate, patients are assigned to receive donor livers from UNOS in America according to the MELD score.
The incidence of recurrent PBC after liver transplantation is about 30% at 10 years and about 40% at 15 years[127,131-133]. Median time to recurrence is 3-5.5 years. Diagnosis of recurrent PBC after liver transplantation is often challenging because AMA is persistently positive in most patient after transplantation[134], and elevated serum AP can be due to various etiologies after transplantation including acute rejection, chronic rejection, viral infection, drug toxicity, graft vs host disease, bile duct pathology, or hepatic vein/artery pathology, in addition to recurrent PBC. Liver biopsy is essential for diagnosing recurrent PBC[128]. Diagnostic criteria for recurrent PBC include: (1) PBC was an indication for liver transplant (obligatory); (2) graft histopathology suggests recurrent PBC, including: epithelioid granulomas, mononuclear inflammatory infiltrate, formation of lymphoid aggregates, and bile duct damage; and (3) other causes of graft failure have been excluded. Recurrent PBC is definitively diagnosed when all 3 criteria are met, including the presence of at least 3 of the 4 histologic features in criterion 2. Recurrent PBC is likely diagnosed when only 2 histologic features in criterion 2 are present[128,132]. Anti-parietal cell antibodies may be a marker for recurrent PBC[135]. There are conflicting data about whether donor’s age, recipient’s age, cold ischemic time, warm ischemia time, number of HLA mismatches, and specific immunosuppressive regimens are risk factors for recurrence[136-140].
The appropriate therapy for recurrent PBC is unclear. UDCA has been advocated as a therapy[141,142]. Anecdotal evidence suggests improvement in serum biochemical parameters of liver function following UDCA administration. UDCA most likely acts in recurrent PBC just like it acts on the native liver before transplantation.
SYMPTOMATIC THERAPY
Fatigue
Fatigue occurs in up to 70% of patients[55]. It is associated with excessive daytime sleepiness and autonomic dysfunction. Cerebral structural abnormalities, related to excessive manganese deposits in the globus pallidum, may be a contributing factor[143-146]. Patients with fatigue had significantly higher serum levels of manganese[143], and increased manganese deposits in the brain, likely secondary to impaired biliary excretion. Other implicated factors include elevated inflammatory cytokines (IL-1, IL-6, TNF)[147], elevated progesterone levels[148,149], and impaired peripheral muscle function[150,151]. Fatigue at clinical presentation may be associated with an aggressive clinical course, rapid progression to cirrhosis, and poor response to UDCA[61].
Fatigue severity is measured according to the fatigue impact scale (FIS)[152] or PBC-40 (PBC-40 question profile)[153]. The FIS is a detailed and cumbersome tool, which takes approximately 3 min to complete in a non-fatigued person, but may take considerably more time in severely fatigued patients. There are 40 items on the scale, each of which is scored from 0 (no problem) to 4 (extreme problem), providing a continuous scale score of 0-160[154].
There is no specific treatment for the fatigue. Some data suggest that modafinil improves fatigue symptoms in PBC patients, without major side effects[155], but it is currently not approved by the FDA for this indication. Randomized, prospective studies are needed to establish efficacy. Some PBC patients experience a symptom complex of fatigue, symptoms of cognitive dysfunction, and social and emotional dysfunction[156]. Various agents such as ondansteron, fluoxetine, and antioxidants have been studied to ameliorate this fatigue, but none have demonstrated efficacy[147].
Pruritus
Pruritus from cholestasis is mostly generalized, associated with scratching, sometimes violent, and sleep deprivation. It may even lead to suicidal ideation in extreme cases[157]. Intensity of pruritus is not correlated with PBC severity[157]. Treatment of this pruritus involves a multifaceted, individualized approach. Proper skin care is essential. Patients with pruritus from liver disease do not have primary pruritic skin lesions. However, lesions secondary to scratching, including excoriations, and sometimes prurigo nodularis (formation of localized or generalized, eroded, excoriated, and intensely pruritic cutaneous nodules from the scratching) can occur[158]. Patients have difficulty avoiding scratching because the pain induced by the scratching often relieves the pruritus. Patients should be referred to a dermatologist to exclude primary skin diseases that can contribute to the pruritus. All potential pruritogens should be removed from the body. Cholestyramine is commonly used to treat this type of pruritis[159]. The mechanism of action of cholestyramine to relieve pruritus is incompletely understood. It is a non-absorbable resin that binds anions, including bile acids and cholesterol, in the small intestine thereby promoting their fecal excretion. Its side effects, most commonly bloating, are generally minor. Cholestyramine should be administered immediately before and after breakfast to bind pruritogens that accumulate in the gallbladder during an overnight fast and that are otherwise poured into the small bowel after breaking the overnight fast. Physical removal or plasma separation of pruritogens have been attempted to treat extreme, refractory pruritus. A transient relief from pruritus has been reported with anion adsorption and plasma separation[160], using extracorporeal liver support systems such as Prometheus or MARS (molecular adsorbent recirculating system[160-165]). Analysis of plasma removed via MARS from patients suffering from refractory pruritus revealed 60 proteins, one of which, SLURP1, was three times higher in samples extracted from patients with cholestasis than in those extracted from controls. Nasobiliary drainage can help relieve the pruritis by removing bile salts[166].
Pruritus may have a central component, and is associated with increased opioidergic tone[167]. Opioid antagonists, including naloxone and naltrexone, have been used to relieve the pruritus[157]. Treatment with opiate antagonists is initiated by progressively increasing the dose of intravenous infusion for several hours before introducing it orally to reduce the risk of opiate withdrawal[168-170]. If a patient exhibits signs of withdrawal during dosage increases, the dose can be maintained at the prior dose for a day or two with subsequent increases, until the pruritus is relieved.
Review of patient diaries in a clinical trial of PBC therapy revealed that sertraline was associated with relief of pruritus[171]. In a subsequent randomized, placebo-controlled study, sertraline was again associated with relief of pruritus, as determined by a visual analogue scale, and associated with improved skin appearance, as determined by physical examination[172].
Antibiotics have been used to treat the pruritus. In clinical trials, rifampicin has relieved pruritus in some patients with liver disease[173-175]. Rifampicin stimulates PXR, which induces drug-metabolizing enzymes and transporters[176,177]. A meta-analysis of controlled, randomized clinical trials demonstrated that rifampicin was generally safe, but is occasionally hepatotoxic[178,179]. Thus, follow-up of serum tests of liver function is necessary when patients are initiated on this drug, and this drug should be stopped if drug hepatotoxicity is suspected. Metronidazole may ameliorate refractory pruritus in patients with PBC[180]. It is best used as short-term therapy because long-term administration can cause peripheral neuropathy[181].
Osteoporosis
Hepatic osteodystrophy refers to metabolic bone disease in patients with chronic liver disease[182]. PBC patients have a 20%-44% prevalence of osteoporosis. The prevalence increases with disease progression, and up to 80% of patients with cirrhosis have osteoporosis. As in the general population, risk factors for osteoporosis in PBC include old age, female gender, smoking, excessive alcohol consumption, underweight physique (body mass index < 19.0 kg/m2 in adults), early menopause (< 45 years of age), positive family history of osteoporosis, and corticosteroid therapy[182].
The mechanism of osteoporosis is unclear. PBC apparently produces metabolic changes that affect osteoprotegerin (OPG)-receptor activator of nuclear factor-κB - ligand (RANKL), the major mechanism of bone remodeling. Cirrhosis of any etiology, including PBC, impairs the function of osteoblasts, reduces the production of growth factors (especially insulin-like growth factor-1), and increases the synthesis of oncofoetal fibronectin. Unconjugated bilirubin and lithocholic acid, which accumulate in cirrhosis, may be directly toxic to bone precursors and osteoblasts. This subject has been extensively reviewed by Raszeja-Wyszomirska et al[182]. Malnutrition, vitamin deficiencies, especially of vitamins D and K, could also increase the risk of osteoporosis. Osteoporosis is diagnosed by dual energy X ray absorptiometry (DEXA) scans of lumbar spine and femur. These scans should be performed at disease diagnosis with surveillance annually thereafter according to EASL guidelines or surveillance every 2-3 years as per AASLD guidelines[27].
Treatment guidelines for the osteoporosis is not established. Treatment is begun at an early phase (DEXA score: -1 SD to -2.5 SD). Patients should be advised about lifestyle modifications[183], including regular weight-bearing exercises, avoidance of smoking, alcohol, etc. Patients should be prescribed calcium at 1000-1500 mg/d and vitamin D at 400-800 IU/d. Bisphosphonates, particularly alendronate, are commonly administered. Alendronate at 70 mg/wk significantly improves bone mineral density after 1 year of therapy[184]. Patients should not lie down for at least 30 min after ingesting alendronate to avoid esophageal reflux or ulcers induced by this medication. In a pilot study, 9 patients administered raloxifene exhibited a slight increase in bone mineral density in the lumbar spine[185]. Therapies including zoledronic, ibandronic acid, anabolic therapy with strontium ranelate or recombinant human parathormone 1-34, and denosumab-IgG2 monoclonal antibody against RANKL have not been studied for osteoporosis in PBC.
SPECIAL SITUATIONS
Associated autoimmune disorders
Prevalence of autoimmune disorders associated with PBC ranges from 2% to 20%[44,186-190]. Sjogren’s syndrome and Raynaud’s syndrome are strongly associated with PBC[27]. Clinical symptoms of Sjogren’s syndrome include ocular and oral dryness for > 3 mo, use of artificial tears > 3 times per day, and the need to drink liquids to swallow solid food. All PBC patients with suspicious symptoms should undergo Schirmer’s test, a highly specific test for Sjogren’s syndrome[191]. Patients with PBC should also undergo tests to exclude celiac disease, rheumatological disorders, and thyroid disease[192].
Management of Sjogren’s syndrome includes general measures to improve eye care, such as household humidification, use of artificial tears, including hydroxypropyl methylcellulose or carboxymethylcellulose, or cyclosporine ophthalmic emulsion to increase tear production[193]. General measures to improve oral health include regular visits to the dentist, mouth rinsing with water, use of fluoride-containing toothpaste, daily dental flossing, and avoidance of eating sugars between meals[27]. Patients with xerostomia are prescribed saliva substitutes. Cholinergic agents, such as pilocarpine and cevimeline, are empirically used in Sjogren’s syndrome[194]. Cevimeline, a cholinergic agent with a high affinity for M3 muscarinic receptors, relieves the perception of dry mouth and decreases the need for artificial saliva[194]. Oral candidiasis, a complication of dry mouth, requires specific antifungal therapy. Care must be exercised when swallowing esophagotoxic pills, such as potassium supplements, tetracycline, or alendronate, because of the sicca syndrome and occasional esophageal dysmotility. Drinking plenty of water while in the upright position is helpful[27]. Patients with Sjogren’s syndrome may experience vaginal dryness. Vaginal lubricants, such as K-Y jelly or vaginal inserts, are helpful. Cortisone creams should be avoided. Estrogen preparations are recommended in postmenopausal women.
Pregnancy
A case control study of 267 pregnant patients with PBC and 367 healthy pregnant controls revealed that most PBC patients have uneventful pregnancies[195]. Up to 60% of patients develop post-partum flares[196]. UDCA is safe to administer during pregnancy in patients with pruritus (FDA pregnancy category B).
Hyperlipidemia
Complications of chronic cholestasis include osteoporosis (described above) and hyperlipidemia. The hyperlipidemia in PBC is, however, apparently not associated with adverse cardiovascular effects[60,197-199]. It is unusual for cholesterol-lowering agents to be needed, but statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are a safe therapy, even when serum liver function tests are abnormal[200]. Fibrates have been used safely in some patients[201], but occasionally cause paradoxical elevations of serum cholesterol[202].
COMPLICATIONS FROM CIRRHOSIS FROM ADVANCED PBC
Patients with cirrhosis from advanced PBC are subject to all the usual complications of cirrhosis, including hepatoma development, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, and esophageal variceal bleeding. The diagnosis and treatment of these complications are briefly listed in Table 5[126,189,203-222], which includes references for further reading on these complications.
Table 5.
Complications of cirrhosis or portal hypertension in patients with primary biliary cirrhosis
| Complication | Special considerations in PBC | Ref. |
| Hepatoma | Like other cirrhotics, patients with PBC have increased risk of developing hepatomas | [189,203-205] |
| In patients with PBC who have not undergone a liver biopsy to document the diagnosis of cirrhosis, hepatoma screening should be initiated when the Mayo score > 4.1 | [126] | |
| Surveillance for hepatoma in patients with cirrhosis from PBC should be performed every six months by abdominal ultrasound or an alternative modality of abdominal imaging | [206] | |
| Spontaneous bacterial peritonitis | Diagnosed by abdominal paracentesis revealing > 250 polymorphonuclear leukocytes/mm3 in ascitic fluid | [207] |
| Treated with a short course of multiple antibiotics, generally including either a third-generation cephalosporin or flouroquinolones | [208] | |
| Hepatic encephalopathy | Diagnosed clinically by confusion, delirium, or stupor on physical examination, depending on degree of hepatic encephalopathy; possible presence of asterixis on physical examination; and elevated serum ammonia level in a cirrhotic patient | [209] |
| Treatment options include rifaximin, lactulose, supportive care, and reversal of underlying precipitating causes, such as dehydration, infection, or gastrointestinal bleeding | [209-211] | |
| HRS | Type 1 HRS defined as doubling of serum creatinine level, reaching a level > 2.5 mg/dL in < 2 wk. Type 2 HRS defined as a less severely elevated serum creatinine level. Must exclude other causes of renal failure, especially hypovolemia in both types of HRS | [212,213] |
| Treatment includes avoidance of nephrotoxic medications; short-term trial of volume expansion; and administration of vasopressin analogues, such as terlipressin, and α-adrenergic agonists, such as norepinephrine or midodrine. Ultimate treatment for type 1 HRS refractory to therapy is liver transplantation | [213-215] | |
| Esophageal varices | Usually occur only after Mayo score becomes > 4.1. Patients with advanced PBC can develop portal hypertension before developing established cirrhosis from nodular regenerative hyperplasia | [216-220] |
| Esophageal varices usually diagnosed and graded by esophagogastroduodenoscopy | ||
| Specific therapies for esophageal varices include: endoscopic banding, endoscopic injection therapy, and non-selective beta-blockers. Transjugular intrahepatic shunt is recommended for refractory variceal bleeding, especially when the MELD score < 18 | [221,222] |
PBC: Primary biliary cirrhosis; MELD: Model for end-stage liver disease; HRS: Hepatorenal syndrome.
FUTURE TRENDS
The future of PBC promises to be exciting. Genetic, immunologic, and epidemiologic data should further elucidate the pathogenesis of PBC, especially in the era of genome-wide association studies and epigenetics. Such advances may help hepatologists screen and diagnose PBC early, to improve survival, and institute preventive measures to reduce exposure to environmental factors that accelerate the disease. New drugs with molecular targets, such as obeticholic acid and ustekinimab, show considerable promise. Great advances have recently been made in improving transplant-free survival and this trend should continue for the next several years. Also, the prognosis after transplantation should continue to improve with improved immunosuppression and surgical techniques. Future agents might reverse advanced fibrosis in PBC, thereby reducing complications from portal hypertension and cirrhosis.
Footnotes
P- Reviewer: Lau WY, Lian M, Sirin G S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: None for all authors. This paper does not discuss any confidential pharmaceutical data reviewed by Dr. Cappell as a consultant for the United States Food and Drug Administration (FDA) Advisory Committee on Gastrointestinal Drugs.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 11, 2014
First decision: December 26, 2014
Article in press: March 9, 2015
References
- 1.Kaplan MM. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303–330. doi: 10.1146/annurev-pathol-020712-164014. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DL, Juran BD, Lazaridis KN. Primary biliary cirrhosis. Best Pract Res Clin Gastroenterol. 2010;24:647–654. doi: 10.1016/j.bpg.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addison T, Gull W On a certain affection of the skin vitiligoidea- α plana, β tuberosa. Guy’s Hosp Rep. 1851;7:265–276. [Google Scholar]
- 5.Ahrens EH, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore) 1950;29:299–364. doi: 10.1097/00005792-195012000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Walker JG, Doniach D, Roitt IM, Sherlock S. Serological tests in diagnosis of primary biliary cirrhosis. Lancet. 1965;1:827–831. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- 7.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths L, Dyson JK, Jones DE. The new epidemiology of primary biliary cirrhosis. Semin Liver Dis. 2014;34:318–328. doi: 10.1055/s-0034-1383730. [DOI] [PubMed] [Google Scholar]
- 9.Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group. Gut. 1995;36:927–930. doi: 10.1136/gut.36.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juran BD, Lazaridis KN. Environmental factors in primary biliary cirrhosis. Semin Liver Dis. 2014;34:265–272. doi: 10.1055/s-0034-1383726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpechot C, Chrétien Y, Chazouillères O, Poupon R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53:162–169. doi: 10.1016/j.jhep.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Morreale M, Tsirigotis M, Hughes MD, Brumfitt W, McIntyre N, Burroughs AK. Significant bacteriuria has prognostic significance in primary biliary cirrhosis. J Hepatol. 1989;9:149–158. doi: 10.1016/0168-8278(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 14.Smyk DS, Rigopoulou EI, Bogdanos DP. Potential Roles for Infectious Agents in the Pathophysiology of Primary Biliary Cirrhosis: What's New? Curr Infect Dis Rep. 2013;15:14–24. doi: 10.1007/s11908-012-0304-2. [DOI] [PubMed] [Google Scholar]
- 15.Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59:508–512. doi: 10.1136/gut.2009.184218. [DOI] [PubMed] [Google Scholar]
- 16.Howel D, Fischbacher CM, Bhopal RS, Gray J, Metcalf JV, James OF. An exploratory population-based case-control study of primary biliary cirrhosis. Hepatology. 2000;31:1055–1060. doi: 10.1053/he.2000.7050. [DOI] [PubMed] [Google Scholar]
- 17.Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the United States. Hepatology. 2001;33:16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 18.Mantaka A, Koulentaki M, Chlouverakis G, Enele-Melono JM, Darivianaki A, Tzardi M, Kouroumalis EA. Primary biliary cirrhosis in a genetically homogeneous population: disease associations and familial occurrence rates. BMC Gastroenterol. 2012;12:110. doi: 10.1186/1471-230X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Zheng H, Tian QB, Rui MN, Liu DW. HLA-DR polymorphism and primary biliary cirrhosis: evidence from a meta-analysis. Arch Med Res. 2014;45:270–279. doi: 10.1016/j.arcmed.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zheng H, Li T, Gao P, Zhang XL, Liu DW. Cytotoxic T-lymphocyte associated antigen-4 gene polymorphisms and primary biliary cirrhosis: a systematic review. J Gastroenterol Hepatol. 2012;27:1159–1166. doi: 10.1111/j.1440-1746.2012.07118.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Wang B, Pan F, Zhang R, Xiao L, Guo H, Ma S, Zhou C. Association between cytotoxic T-lymphocyte antigen 4 gene polymorphisms and primary biliary cirrhosis in Chinese population: data from a multicenter study. J Gastroenterol Hepatol. 2013;28:1397–1402. doi: 10.1111/jgh.12165. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Cai X, Han Z, Shi Y, Zhou X, Han Y, Fan D. Meta-analysis: the relationship between TNF-alpha polymorphisms and susceptibility to primary biliary cirrhosis. Hepatogastroenterology. 2013;60:568–576. doi: 10.5754/hge12762. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Nezu S, Uegaki S, Kikuchi K, Shibuya A, Miyakawa H, Takahashi S, Bianchi I, Zermiani P, Podda M, et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol. 2009;50:1202–1209. doi: 10.1016/j.jhep.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev. 2014;13:441–444. doi: 10.1016/j.autrev.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 28.Agmon-Levin N, Shapira Y, Selmi C, Barzilai O, Ram M, Szyper-Kravitz M, Sella S, Katz BS, Youinou P, Renaudineau Y, et al. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010;34:55–58. doi: 10.1016/j.jaut.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Yamagiwa S, Kamimura H, Takamura M, Aoyagi Y. Autoantibodies in primary biliary cirrhosis: recent progress in research on the pathogenetic and clinical significance. World J Gastroenterol. 2014;20:2606–2612. doi: 10.3748/wjg.v20.i10.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha S, Leung PS, Gershwin ME, Fletcher MP, Ansari AA, Coppel RL. Combinatorial autoantibodies to dihydrolipoamide acetyltransferase, the major autoantigen of primary biliary cirrhosis. Proc Natl Acad Sci USA. 1993;90:2527–2531. doi: 10.1073/pnas.90.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutimer DJ, Fussey SP, Yeaman SJ, Kelly PJ, James OF, Bassendine MF. Frequency of IgG and IgM autoantibodies to four specific M2 mitochondrial autoantigens in primary biliary cirrhosis. Hepatology. 1989;10:403–407. doi: 10.1002/hep.1840100402. [DOI] [PubMed] [Google Scholar]
- 32.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, Zuin M, Podda M. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 33.Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, Nelson RC. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000;20:959–975; quiz 1108-1109, 1112. doi: 10.1148/radiographics.20.4.g00jl04959. [DOI] [PubMed] [Google Scholar]
- 34.Williamson KD, Chapman RW. Primary sclerosing cholangitis. Dig Dis. 2014;32:438–445. doi: 10.1159/000358150. [DOI] [PubMed] [Google Scholar]
- 35.Imam MH, Lindor KD. The natural history of primary biliary cirrhosis. Semin Liver Dis. 2014;34:329–333. doi: 10.1055/s-0034-1383731. [DOI] [PubMed] [Google Scholar]
- 36.Gatselis NK, Zachou K, Norman GL, Gabeta S, Papamichalis P, Koukoulis GK, Dalekos GN. Clinical significance of the fluctuation of primary biliary cirrhosis-related autoantibodies during the course of the disease. Autoimmunity. 2013;46:471–479. doi: 10.3109/08916934.2013.801461. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchows Arch A Pathol Anat Histol. 1978;379:103–112. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 38.Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257–1260. doi: 10.1177/003591576706001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi M, Kakuda Y, Harada K, Sato Y, Sasaki M, Ikeda H, Terada M, Mukai M, Kaneko S, Nakanuma Y. Clinicopathological study of primary biliary cirrhosis with interface hepatitis compared to autoimmune hepatitis. World J Gastroenterol. 2014;20:3597–3608. doi: 10.3748/wjg.v20.i13.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindgren S, Glaumann H, Almer S, Bergquist A, Björnsson E, Broomé U, Danielsson A, Lebrun B, Prytz H, Olsson R. Transitions between variant forms of primary biliary cirrhosis during long-term follow-up. Eur J Intern Med. 2009;20:398–402. doi: 10.1016/j.ejim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Floreani A, Franceschet I, Cazzagon N. Primary biliary cirrhosis: overlaps with other autoimmune disorders. Semin Liver Dis. 2014;34:352–360. doi: 10.1055/s-0034-1383734. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Arase Y, Ikeda K, Saitoh S, Tsubota A, Suzuki F, Kobayashi M, Akuta N, Someya T, Miyakawa Y, et al. Clinical and pathological characteristics of the autoimmune hepatitis and primary biliary cirrhosis overlap syndrome. J Gastroenterol Hepatol. 2004;19:699–706. doi: 10.1111/j.1440-1746.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther. 2012;36:517–533. doi: 10.1111/j.1365-2036.2012.05223.x. [DOI] [PubMed] [Google Scholar]
- 44.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 45.Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol. 2007;102:1244–1250. doi: 10.1111/j.1572-0241.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 46.Czaja AJ. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can J Gastroenterol. 2013;27:417–423. doi: 10.1155/2013/198070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bizzaro N, Covini G, Rosina F, Muratori P, Tonutti E, Villalta D, Pesente F, Alessio MG, Tampoia M, Antico A, et al. Overcoming a “probable” diagnosis in antimitochondrial antibody negative primary biliary cirrhosis: study of 100 sera and review of the literature. Clin Rev Allergy Immunol. 2012;42:288–297. doi: 10.1007/s12016-010-8234-y. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibody-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233–239. doi: 10.1111/j.1478-3231.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 49.Mendes F, Lindor KD. Antimitochondrial antibody-negative primary biliary cirrhosis. Gastroenterol Clin North Am. 2008;37:479–484, viii. doi: 10.1016/j.gtc.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Hirschfield GM, Heathcote EJ. Antimitochondrial antibody-negative primary biliary cirrhosis. Clin Liver Dis. 2008;12:323–331; viii-ix. doi: 10.1016/j.cld.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Gershwin ME. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 52.Miyakawa H, Tanaka A, Kikuchi K, Matsushita M, Kitazawa E, Kawaguchi N, Fujikawa H, Gershwin ME. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology. 2001;34:243–248. doi: 10.1053/jhep.2001.26514. [DOI] [PubMed] [Google Scholar]
- 53.Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clin Liver Dis. 2012;16:199–229. doi: 10.1016/j.cld.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Harthy N, Kumagi T, Coltescu C, Hirschfield GM. The specificity of fatigue in primary biliary cirrhosis: evaluation of a large clinic practice. Hepatology. 2010;52:562–570. doi: 10.1002/hep.23683. [DOI] [PubMed] [Google Scholar]
- 55.Cauch-Dudek K, Abbey S, Stewart DE, Heathcote EJ. Fatigue in primary biliary cirrhosis. Gut. 1998;43:705–710. doi: 10.1136/gut.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heathcote J. The clinical expression of primary biliary cirrhosis. Semin Liver Dis. 1997;17:23–33. doi: 10.1055/s-2007-1007180. [DOI] [PubMed] [Google Scholar]
- 57.Imam MH, Gossard AA, Sinakos E, Lindor KD. Pathogenesis and management of pruritus in cholestatic liver disease. J Gastroenterol Hepatol. 2012;27:1150–1158. doi: 10.1111/j.1440-1746.2012.07109.x. [DOI] [PubMed] [Google Scholar]
- 58.Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis. 2003;7:741–758. doi: 10.1016/s1089-3261(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 59.Mounach A, Ouzzif Z, Wariaghli G, Achemlal L, Benbaghdadi I, Aouragh A, Bezza A, El Maghraoui A. Primary biliary cirrhosis and osteoporosis: a case-control study. J Bone Miner Metab. 2008;26:379–384. doi: 10.1007/s00774-007-0833-1. [DOI] [PubMed] [Google Scholar]
- 60.Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265–269. doi: 10.1136/gut.51.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quarneti C, Muratori P, Lalanne C, Fabbri A, Menichella R, Granito A, Masi C, Lenzi M, Cassani F, Pappas G, et al. Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver Int. 2015;35:636–641. doi: 10.1111/liv.12560. [DOI] [PubMed] [Google Scholar]
- 62.Corpechot C, Poujol-Robert A, Wendum D, Galotte M, Chrétien Y, Poupon RE, Poupon R. Biochemical markers of liver fibrosis and lymphocytic piecemeal necrosis in UDCA-treated patients with primary biliary cirrhosis. Liver Int. 2004;24:187–193. doi: 10.1111/j.1478-3231.2004.0918.x. [DOI] [PubMed] [Google Scholar]
- 63.Poupon R, Chazouillères O, Balkau B, Poupon RE. Clinical and biochemical expression of the histopathological lesions of primary biliary cirrhosis. UDCA-PBC Group. J Hepatol. 1999;30:408–412. doi: 10.1016/s0168-8278(99)80098-1. [DOI] [PubMed] [Google Scholar]
- 64.Taal BG, Schalm SW, de Bruyn AM, de Rooy FW, Klein F. Serum IgM in primary biliary cirrhosis. Clin Chim Acta. 1980;108:457–463. doi: 10.1016/0009-8981(80)90353-8. [DOI] [PubMed] [Google Scholar]
- 65.Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:1313–1315. doi: 10.1016/j.cgh.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Liermann Garcia RF, Evangelista Garcia C, McMaster P, Neuberger J. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology. 2001;33:22–27. doi: 10.1053/jhep.2001.20894. [DOI] [PubMed] [Google Scholar]
- 67.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 68.Kim WR, Wiesner RH, Poterucha JJ, Therneau TM, Benson JT, Krom RA, Dickson ER. Adaptation of the Mayo primary biliary cirrhosis natural history model for application in liver transplant candidates. Liver Transpl. 2000;6:489–494. doi: 10.1053/jlts.2000.6503. [DOI] [PubMed] [Google Scholar]
- 69.Jacob DA, Bahra M, Schmidt SC, Schumacher G, Weimann A, Neuhaus P, Neumann UP. Mayo risk score for primary biliary cirrhosis: a useful tool for the prediction of course after liver transplantation? Ann Transplant. 2008;13:35–42. [PubMed] [Google Scholar]
- 70.Kim WR, Wiesner RH, Therneau TM, Poterucha JJ, Porayko MK, Evans RW, Klintmalm GB, Crippin JS, Krom RA, Dickson ER. Optimal timing of liver transplantation for primary biliary cirrhosis. Hepatology. 1998;28:33–38. doi: 10.1002/hep.510280106. [DOI] [PubMed] [Google Scholar]
- 71.Parés A. Old and novel therapies for primary biliary cirrhosis. Semin Liver Dis. 2014;34:341–351. doi: 10.1055/s-0034-1383733. [DOI] [PubMed] [Google Scholar]
- 72.Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935–938. doi: 10.1016/j.amjcard.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 73.Spencer CM, McTavish D. Budesonide. A review of its pharmacological properties and therapeutic efficacy in inflammatory bowel disease. Drugs. 1995;50:854–872. doi: 10.2165/00003495-199550050-00006. [DOI] [PubMed] [Google Scholar]
- 74.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S3–12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 75.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 76.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 77.Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;12:CD000551. doi: 10.1002/14651858.CD000551.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hempfling W, Dilger K, Beuers U. Systematic review: ursodeoxycholic acid--adverse effects and drug interactions. Aliment Pharmacol Ther. 2003;18:963–972. doi: 10.1046/j.1365-2036.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- 79.Levy C, Naik J, Giordano C, Mandalia A, O’Brien C, Bhamidimarri KR, Schiff ER, Martin P. Hispanics with primary biliary cirrhosis are more likely to have features of autoimmune hepatitis and reduced response to ursodeoxycholic acid than non-Hispanics. Clin Gastroenterol Hepatol. 2014;12:1398–1405. doi: 10.1016/j.cgh.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 80.ter Borg PC, Schalm SW, Hansen BE, van Buuren HR. Prognosis of ursodeoxycholic acid-treated patients with primary biliary cirrhosis. Results of a 10-yr cohort study involving 297 patients. Am J Gastroenterol. 2006;101:2044–2050. doi: 10.1111/j.1572-0241.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 81.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 82.Abbas G, Lindor KD. Pharmacological treatment of biliary cirrhosis with ursodeoxycholic acid. Expert Opin Pharmacother. 2010;11:387–392. doi: 10.1517/14656560903493460. [DOI] [PubMed] [Google Scholar]
- 83.Jackson H, Solaymani-Dodaran M, Card TR, Aithal GP, Logan R, West J. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology. 2007;46:1131–1137. doi: 10.1002/hep.21795. [DOI] [PubMed] [Google Scholar]
- 84.Kuiper EM, Hansen BE, Adang RP, van Nieuwkerk CM, Timmer R, Drenth JP, Spoelstra P, Brouwer HT, Kuyvenhoven JP, van Buuren HR. Relatively high risk for hepatocellular carcinoma in patients with primary biliary cirrhosis not responding to ursodeoxycholic acid. Eur J Gastroenterol Hepatol. 2010;22:1495–1502. doi: 10.1097/MEG.0b013e32834059e7. [DOI] [PubMed] [Google Scholar]
- 85.Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19:115–121. doi: 10.1111/j.1478-3231.1999.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 86.Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 87.Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, Chazouillères O, Poupon R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Lu J, Dai W, Wang F, Shen M, Yang J, Zhu R, Zhang H, Chen K, Cheng P, et al. Combination therapy of ursodeoxycholic acid and corticosteroids for primary biliary cirrhosis with features of autoimmune hepatitis: a meta-analysis. Gastroenterol Res Pract. 2013;2013:490731. doi: 10.1155/2013/490731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gallant C, Kenny P. Oral glucocorticoids and their complications. A review. J Am Acad Dermatol. 1986;14:161–177. doi: 10.1016/s0190-9622(86)70018-2. [DOI] [PubMed] [Google Scholar]
- 90.Clissold SP, Heel RC. Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis. Drugs. 1984;28:485–518. doi: 10.2165/00003495-198428060-00001. [DOI] [PubMed] [Google Scholar]
- 91.Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH, Ackermann H, Happ J, Leuschner U. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918–925. doi: 10.1016/s0016-5085(99)70351-3. [DOI] [PubMed] [Google Scholar]
- 92.Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747–752. doi: 10.1002/hep.20646. [DOI] [PubMed] [Google Scholar]
- 93.Angulo P, Jorgensen RA, Keach JC, Dickson ER, Smith C, Lindor KD. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology. 2000;31:318–323. doi: 10.1002/hep.510310209. [DOI] [PubMed] [Google Scholar]
- 94.Hempfling W, Grunhage F, Dilger K, Reichel C, Beuers U, Sauerbruch T. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology. 2003;38:196–202. doi: 10.1053/jhep.2003.50266. [DOI] [PubMed] [Google Scholar]
- 95.Marschall HU, Luketic V, Lovgren-Sandblom A, Benthin L, Kowdley K, Hirschfield G, Mason A, Lindor K, Gordon S, Vincent C, et al. The farnesoid X receptor (FXR) agonist obeticholic acid (OCA) increases plasma FGF-19 concentrations and decreases bile acid synthesis in primary biliary cirrhosis (PBC) (Abstract) J Hepatol. 2012;5(Suppl 2):S377. [Google Scholar]
- 96.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 97.Mason ALV, Lindor K, Hirschfield G. Farnesoid-X receptor agonists: a new class of drugs for the treatment of PBC? An international study evaluating the addition of INT-747 to ursodeoxycholic acid. J Hepatol. 2010;52(Suppl 1):S1–S2. [Google Scholar]
- 98.Lindor KD. Farnesoid X receptor agonists for primary biliary cirrhosis. Curr Opin Gastroenterol. 2011;27:285–288. doi: 10.1097/MOG.0b013e32834452c8. [DOI] [PubMed] [Google Scholar]
- 99.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC, Parés A, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761.e8. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Cuperus FJ, Halilbasic E, Trauner M. Fibrate treatment for primary biliary cirrhosis. Curr Opin Gastroenterol. 2014;30:279–286. doi: 10.1097/MOG.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 101.Hazzan R, Tur-Kaspa R. Bezafibrate treatment of primary biliary cirrhosis following incomplete response to ursodeoxycholic acid. J Clin Gastroenterol. 2010;44:371–373. doi: 10.1097/MCG.0b013e3181c115b3. [DOI] [PubMed] [Google Scholar]
- 102.Honda A, Ikegami T, Nakamuta M, Miyazaki T, Iwamoto J, Hirayama T, Saito Y, Takikawa H, Imawari M, Matsuzaki Y. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology. 2013;57:1931–1941. doi: 10.1002/hep.26018. [DOI] [PubMed] [Google Scholar]
- 103.Lens S, Leoz M, Nazal L, Bruguera M, Parés A. Bezafibrate normalizes alkaline phosphatase in primary biliary cirrhosis patients with incomplete response to ursodeoxycholic acid. Liver Int. 2014;34:197–203. doi: 10.1111/liv.12290. [DOI] [PubMed] [Google Scholar]
- 104.Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, Clark V, Firpi RJ, Morelli G, Soldevila-Pico C, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33:235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 105.Liberopoulos EN, Florentin M, Elisaf MS, Mikhailidis DP, Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010;4:120–126. doi: 10.2174/1874192401004010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, Doniach D, Ranek L, Tygstrup N, Williams R. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology. 1985;89:1084–1091. doi: 10.1016/0016-5085(85)90213-6. [DOI] [PubMed] [Google Scholar]
- 107.Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, Hanson RG, Minuk GY, Dusheiko GM, Campbell G. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology. 1986;91:1327–1334. doi: 10.1016/0016-5085(86)90183-6. [DOI] [PubMed] [Google Scholar]
- 108.Hendrickse MT, Rigney E, Giaffer MH, Soomro I, Triger DR, Underwood JC, Gleeson D. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial. Gastroenterology. 1999;117:400–407. doi: 10.1053/gast.1999.0029900400. [DOI] [PubMed] [Google Scholar]
- 109.González-Koch A, Brahm J, Antezana C, Smok G, Cumsille MA. The combination of ursodeoxycholic acid and methotrexate for primary biliary cirrhosis is not better than ursodeoxycholic acid alone. J Hepatol. 1997;27:143–149. doi: 10.1016/s0168-8278(97)80294-2. [DOI] [PubMed] [Google Scholar]
- 110.Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, McCashland TM, Zetterman RK, Peters MG, Di Bisceglie AM, et al. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology. 2005;42:1184–1193. doi: 10.1002/hep.20897. [DOI] [PubMed] [Google Scholar]
- 111.Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. J Clin Gastroenterol. 2005;39:168–171. [PubMed] [Google Scholar]
- 112.Lombard M, Portmann B, Neuberger J, Williams R, Tygstrup N, Ranek L, Ring-Larsen H, Rodes J, Navasa M, Trepo C. Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology. 1993;104:519–526. doi: 10.1016/0016-5085(93)90422-9. [DOI] [PubMed] [Google Scholar]
- 113.Neuberger J, Christensen E, Portmann B, Caballeria J, Rodes J, Ranek L, Tygstrup N, Williams R. Double blind controlled trial of d-penicillamine in patients with primary biliary cirrhosis. Gut. 1985;26:114–119. doi: 10.1136/gut.26.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gong Y, Klingenberg SL, Gluud C. Systematic review and meta-analysis: D-Penicillamine vs. placebo/no intervention in patients with primary biliary cirrhosis--Cochrane Hepato-Biliary Group. Aliment Pharmacol Ther. 2006;24:1535–1544. doi: 10.1111/j.1365-2036.2006.03164.x. [DOI] [PubMed] [Google Scholar]
- 115.Vuoristo M, Färkkilä M, Karvonen AL, Leino R, Lehtola J, Mäkinen J, Mattila J, Friman C, Seppälä K, Tuominen J. A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology. 1995;108:1470–1478. doi: 10.1016/0016-5085(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 116.Poupon RE, Huet PM, Poupon R, Bonnand AM, Nhieu JT, Zafrani ES. A randomized trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. UDCA-PBC Study Group. Hepatology. 1996;24:1098–1103. doi: 10.1002/hep.510240520. [DOI] [PubMed] [Google Scholar]
- 117.The results of a randomized double blind controlled trial evaluating malotilate in primary biliary cirrhosis. A European multicentre study group. J Hepatol. 1993;17:227–235. doi: 10.1016/s0168-8278(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 118.McCormick PA, Scott F, Epstein O, Burroughs AK, Scheuer PJ, McIntyre N. Thalidomide as therapy for primary biliary cirrhosis: a double-blind placebo controlled pilot study. J Hepatol. 1994;21:496–499. doi: 10.1016/s0168-8278(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 119.Angulo P, Patel T, Jorgensen RA, Therneau TM, Lindor KD. Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology. 2000;32:897–900. doi: 10.1053/jhep.2000.18663. [DOI] [PubMed] [Google Scholar]
- 120.Stojakovic T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Stadlbauer V, Gurakuqi G, Stauber RE, März W, Trauner M. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology. 2007;46:776–784. doi: 10.1002/hep.21741. [DOI] [PubMed] [Google Scholar]
- 121.Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, Yang GX, Nakatani T, Vierling J, Lindor K, et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512–521. doi: 10.1002/hep.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933–941. doi: 10.1038/ajg.2013.51. [DOI] [PubMed] [Google Scholar]
- 123.Chazouillères O, Wendum D, Serfaty L, Rosmorduc O, Poupon R. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol. 2006;44:400–406. doi: 10.1016/j.jhep.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 124.Muratori P, Granito A, Pappas G, Pendino GM, Quarneti C, Cicola R, Menichella R, Ferri S, Cassani F, Bianchi FB, et al. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am J Gastroenterol. 2009;104:1420–1425. doi: 10.1038/ajg.2009.126. [DOI] [PubMed] [Google Scholar]
- 125.Ozaslan E, Efe C, Heurgué-Berlot A, Kav T, Masi C, Purnak T, Muratori L, Ustündag Y, Bresson-Hadni S, Thiéfin G, et al. Factors associated with response to therapy and outcome of patients with primary biliary cirrhosis with features of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2014;12:863–869. doi: 10.1016/j.cgh.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 126.Tinmouth J, Tomlinson G, Heathcote EJ, Lilly L. Benefit of transplantation in primary biliary cirrhosis between 1985-1997. Transplantation. 2002;73:224–227. doi: 10.1097/00007890-200201270-00012. [DOI] [PubMed] [Google Scholar]
- 127.Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol. 2011;35:446–454. doi: 10.1016/j.clinre.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 128.Raczyńska J, Habior A, Pączek L, Foroncewicz B, Pawełas A, Mucha K. Primary biliary cirrhosis in the era of liver transplantation. Ann Transplant. 2014;19:488–493. doi: 10.12659/AOT.890753. [DOI] [PubMed] [Google Scholar]
- 129.Taniguchi M. Liver transplantation in the MELD era--analysis of the OPTN/UNOS registry. Clin Transpl. 2012:41–65. [PubMed] [Google Scholar]
- 130.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 131.Gilroy RK, Lynch SV, Strong RW, Kerlin P, Balderson GA, Stuart KA, Crawford DH. Confirmation of the role of the Mayo Risk Score as a predictor of resource utilization after orthotopic liver transplantation for primary biliary cirrhosis. Liver Transpl. 2000;6:749–752. doi: 10.1053/jlts.2000.9746. [DOI] [PubMed] [Google Scholar]
- 132.Neuberger J, Portmann B, Macdougall BR, Calne RY, Williams R. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1–4. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 133.Schreibman I, Regev A. Recurrent primary biliary cirrhosis after liver transplantation--the disease and its management. MedGenMed. 2006;8:30. [PMC free article] [PubMed] [Google Scholar]
- 134.Dubel L, Farges O, Bismuth H, Sebagh M, Homberg JC, Johanet C. Kinetics of anti-M2 antibodies after liver transplantation for primary biliary cirrhosis. J Hepatol. 1995;23:674–680. doi: 10.1016/0168-8278(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 135.Ciesek S, Becker T, Manns MP, Strassburg CP. Anti-parietal cell autoantibodies (PCA) in primary biliary cirrhosis: a putative marker for recurrence after orthotopic liver transplantation? Ann Hepatol. 2010;9:181–185. [PubMed] [Google Scholar]
- 136.Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, Enders FT, Lindor KD, Krom RA, Wiesner RH. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13:1236–1245. doi: 10.1002/lt.21124. [DOI] [PubMed] [Google Scholar]
- 137.Hashimoto T, Sugawara Y, Makuuchi M. Impact of human leukocyte antigen mismatching on outcomes of living donor liver transplantation for primary biliary cirrhosis. Liver Transpl. 2007;13:938–939. doi: 10.1002/lt.21118. [DOI] [PubMed] [Google Scholar]
- 138.Manousou P, Arvaniti V, Tsochatzis E, Isgro G, Jones K, Shirling G, Dhillon AP, O’Beirne J, Patch D, Burroughs AK. Primary biliary cirrhosis after liver transplantation: influence of immunosuppression and human leukocyte antigen locus disparity. Liver Transpl. 2010;16:64–73. doi: 10.1002/lt.21960. [DOI] [PubMed] [Google Scholar]
- 139.Morioka D, Egawa H, Kasahara M, Jo T, Sakamoto S, Ogura Y, Haga H, Takada Y, Shimada H, Tanaka K. Impact of human leukocyte antigen mismatching on outcomes of living donor liver transplantation for primary biliary cirrhosis. Liver Transpl. 2007;13:80–90. doi: 10.1002/lt.20856. [DOI] [PubMed] [Google Scholar]
- 140.Neuberger J, Gunson B, Hubscher S, Nightingale P. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2004;10:488–491. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 141.Guy JE, Qian P, Lowell JA, Peters MG. Recurrent primary biliary cirrhosis: peritransplant factors and ursodeoxycholic acid treatment post-liver transplant. Liver Transpl. 2005;11:1252–1257. doi: 10.1002/lt.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levitsky J, Hart J, Cohen SM, Te HS. The effect of immunosuppressive regimens on the recurrence of primary biliary cirrhosis after liver transplantation. Liver Transpl. 2003;9:733–736. doi: 10.1053/jlts.2003.50132. [DOI] [PubMed] [Google Scholar]
- 143.Forton DM, Patel N, Prince M, Oatridge A, Hamilton G, Goldblatt J, Allsop JM, Hajnal JV, Thomas HC, Bassendine M, et al. Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut. 2004;53:587–592. doi: 10.1136/gut.2003.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Griffiths L, Jones DE. Pathogenesis of primary biliary cirrhosis and its fatigue. Dig Dis. 2014;32:615–625. doi: 10.1159/000360515. [DOI] [PubMed] [Google Scholar]
- 145.Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346:270–274. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- 146.Rose C, Butterworth RF, Zayed J, Normandin L, Todd K, Michalak A, Spahr L, Huet PM, Pomier-Layrargues G. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology. 1999;117:640–644. doi: 10.1016/s0016-5085(99)70457-9. [DOI] [PubMed] [Google Scholar]
- 147.Abbas G, Jorgensen RA, Lindor KD. Fatigue in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2010;7:313–319. doi: 10.1038/nrgastro.2010.62. [DOI] [PubMed] [Google Scholar]
- 148.Ahboucha S, Butterworth RF, Pomier-Layrargues G, Vincent C, Hassoun Z, Baker GB. Neuroactive steroids and fatigue severity in patients with primary biliary cirrhosis and hepatitis C. Neurogastroenterol Motil. 2008;20:671–679. doi: 10.1111/j.1365-2982.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 149.Söderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit de H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 150.Goldblatt J, James OF, Jones DE. Grip strength and subjective fatigue in patients with primary biliary cirrhosis. JAMA. 2001;285:2196–2197. doi: 10.1001/jama.285.17.2196. [DOI] [PubMed] [Google Scholar]
- 151.Hollingsworth KG, Newton JL, Taylor R, McDonald C, Palmer JM, Blamire AM, Jones DE. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol. 2008;6:1041–1048. doi: 10.1016/j.cgh.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 152.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18 Suppl 1:S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 153.Jacoby A, Rannard A, Buck D, Bhala N, Newton JL, James OF, Jones DE. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54:1622–1629. doi: 10.1136/gut.2005.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frith J, Newton J. Fatigue Impact Scale. Occup Med (Lond) 2010;60:159. doi: 10.1093/occmed/kqp180. [DOI] [PubMed] [Google Scholar]
- 155.Ian Gan S, de Jongh M, Kaplan MM. Modafinil in the treatment of debilitating fatigue in primary biliary cirrhosis: a clinical experience. Dig Dis Sci. 2009;54:2242–2246. doi: 10.1007/s10620-008-0613-3. [DOI] [PubMed] [Google Scholar]
- 156.Newton JL, Pairman J, Sutcliffe K, Wilton K, Jones DE. A predictive model for fatigue and its etiologic associations in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2008;6:228–233. doi: 10.1016/j.cgh.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 157.Bergasa NV. Pruritus of Cholestasis. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press; 2014. p. Chapter 6. [Google Scholar]
- 158.Kanavy H, Bahner J, Korman NJ. Treatment of refractory prurigo nodularis with lenalidomide. Arch Dermatol. 2012;148:794–796. doi: 10.1001/archdermatol.2011.2918. [DOI] [PubMed] [Google Scholar]
- 159.Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology. 1966;50:323–332. [PubMed] [Google Scholar]
- 160.Pusl T, Denk GU, Parhofer KG, Beuers U. Plasma separation and anion adsorption transiently relieve intractable pruritus in primary biliary cirrhosis. J Hepatol. 2006;45:887–891. doi: 10.1016/j.jhep.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 161.Acevedo Ribó M, Moreno Planas JM, Sanz Moreno C, Rubio González EE, Rubio González E, Boullosa Graña E, Sanchez-Turrión V, Sanz Guajardo D, Cuervas-Mons V. Therapy of intractable pruritus with MARS. Transplant Proc. 2005;37:1480–1481. doi: 10.1016/j.transproceed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Doria C, Mandalá L, Smith J, Vitale CH, Lauro A, Gruttadauria S, Marino IR, Foglieni CS, Magnone M, Scott VL. Effect of molecular adsorbent recirculating system in hepatitis C virus-related intractable pruritus. Liver Transpl. 2003;9:437–443. doi: 10.1053/jlts.2003.50055. [DOI] [PubMed] [Google Scholar]
- 163.Parés A, Cisneros L, Salmerón JM, Caballería L, Mas A, Torras A, Rodés J. Extracorporeal albumin dialysis: a procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:1105–1110. doi: 10.1111/j.1572-0241.2004.30204.x. [DOI] [PubMed] [Google Scholar]
- 164.Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol. 2010;53:307–312. doi: 10.1016/j.jhep.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 165.Sturm E, Franssen CF, Gouw A, Staels B, Boverhof R, De Knegt RJ, Stellaard F, Bijleveld CM, Kuipers F. Extracorporal albumin dialysis (MARS) improves cholestasis and normalizes low apo A-I levels in a patient with benign recurrent intrahepatic cholestasis (BRIC) Liver. 2002;22 Suppl 2:72–75. doi: 10.1034/j.1600-0676.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 166.Beuers U, Gerken G, Pusl T. Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis. Hepatology. 2006;44:280–281. doi: 10.1002/hep.21271. [DOI] [PubMed] [Google Scholar]
- 167.Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- 168.Neuberger J, Jones EA. Liver transplantation for intractable pruritus is contraindicated before an adequate trial of opiate antagonist therapy. Eur J Gastroenterol Hepatol. 2001;13:1393–1394. doi: 10.1097/00042737-200111000-00022. [DOI] [PubMed] [Google Scholar]
- 169.Jones EA, Dekker LR. Florid opioid withdrawal-like reaction precipitated by naltrexone in a patient with chronic cholestasis. Gastroenterology. 2000;118:431–432. doi: 10.1016/s0016-5085(00)70225-3. [DOI] [PubMed] [Google Scholar]
- 170.Jones EA, Neuberger J, Bergasa NV. Opiate antagonist therapy for the pruritus of cholestasis: the avoidance of opioid withdrawal-like reactions. QJM. 2002;95:547–552. doi: 10.1093/qjmed/95.8.547. [DOI] [PubMed] [Google Scholar]
- 171.Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2736–2741. doi: 10.1111/j.1572-0241.2003.08662.x. [DOI] [PubMed] [Google Scholar]
- 172.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 173.Bachs L, Parés A, Elena M, Piera C, Rodés J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102:2077–2080. doi: 10.1016/0016-5085(92)90335-v. [DOI] [PubMed] [Google Scholar]
- 174.Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology. 1988;94:488–493. doi: 10.1016/0016-5085(88)90442-8. [DOI] [PubMed] [Google Scholar]
- 175.Hoensch HP, Balzer K, Dylewizc P, Kirch W, Goebell H, Ohnhaus EE. Effect of rifampicin treatment on hepatic drug metabolism and serum bile acids in patients with primary biliary cirrhosis. Eur J Clin Pharmacol. 1985;28:475–477. doi: 10.1007/BF00544371. [DOI] [PubMed] [Google Scholar]
- 176.Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci. 2005;94:1169–1186. doi: 10.1002/jps.20324. [DOI] [PubMed] [Google Scholar]
- 177.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 178.Heathcote EJ. Is rifampin a safe and effective treatment for pruritus caused by chronic cholestasis? Nat Clin Pract Gastroenterol Hepatol. 2007;4:200–201. doi: 10.1038/ncpgasthep0746. [DOI] [PubMed] [Google Scholar]
- 179.Prince MI, Burt AD, Jones DE. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut. 2002;50:436–439. doi: 10.1136/gut.50.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Berg CL, Gollan JL. Primary biliary cirrhosis: new therapeutic directions. Scand J Gastroenterol Suppl. 1992;192:43–49. doi: 10.3109/00365529209095978. [DOI] [PubMed] [Google Scholar]
- 181.Kusumi RK, Plouffe JF, Wyatt RH, Fass RJ. Central nervous system toxicity associated with metronidazole therapy. Ann Intern Med. 1980;93:59–60. doi: 10.7326/0003-4819-93-1-59. [DOI] [PubMed] [Google Scholar]
- 182.Raszeja-Wyszomirska J, Miazgowski T. Osteoporosis in primary biliary cirrhosis of the liver. Prz Gastroenterol. 2014;9:82–87. doi: 10.5114/pg.2014.42502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Christianson MS, Shen W. Osteoporosis prevention and management: nonpharmacologic and lifestyle options. Clin Obstet Gynecol. 2013;56:703–710. doi: 10.1097/GRF.0b013e3182a9d15a. [DOI] [PubMed] [Google Scholar]
- 184.Parés A, Guañabens N. Osteoporosis in primary biliary cirrhosis: pathogenesis and treatment. Clin Liver Dis. 2008;12:407–424; x. doi: 10.1016/j.cld.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 185.Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int. 2005;25:117–121. doi: 10.1111/j.1478-3231.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- 186.Czaja AJ. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology. 1998;28:360–365. doi: 10.1002/hep.510280210. [DOI] [PubMed] [Google Scholar]
- 187.Floreani A, Baragiotta A, Guido M. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: a cause of resistance to ursodeoxycholic treatment. Dig Liver Dis. 2003;35:128–129. doi: 10.1016/s1590-8658(03)00010-0. [DOI] [PubMed] [Google Scholar]
- 188.Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345–353. doi: 10.1038/ajg.2009.616. [DOI] [PubMed] [Google Scholar]
- 189.Suzuki A, Lymp J, Donlinger J, Mendes F, Angulo P, Lindor K. Clinical predictors for hepatocellular carcinoma in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:259–264. doi: 10.1016/j.cgh.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 190.Talwalkar JA, Keach JC, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: an evaluation of a modified scoring system. Am J Gastroenterol. 2002;97:1191–1197. doi: 10.1111/j.1572-0241.2002.05703.x. [DOI] [PubMed] [Google Scholar]
- 191.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 192.Floreani A, Franceschet I, Cazzagon N, Spinazzè A, Buja A, Furlan P, Baldo V, Gershwin ME. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:192–197. doi: 10.1007/s12016-014-8427-x. [DOI] [PubMed] [Google Scholar]
- 193.Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005;89:1363–1367. doi: 10.1136/bjo.2005.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mavragani CP, Moutsopoulos HM. Conventional therapy of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2007;32:284–291. doi: 10.1007/s12016-007-8008-3. [DOI] [PubMed] [Google Scholar]
- 195.Floreani A, Infantolino C, Franceschet I, Tene IM, Cazzagon N, Buja A, Baldo V, Gershwin ME, Gervasi MT. Pregnancy and primary biliary cirrhosis: a case-control study. Clin Rev Allergy Immunol. 2015;48:236–242. doi: 10.1007/s12016-014-8433-z. [DOI] [PubMed] [Google Scholar]
- 196.Efe C, Kahramanoğlu-Aksoy E, Yilmaz B, Ozseker B, Takci S, Roach EC, Purnak T, Kav T, Ozaslan E, Wahlin S. Pregnancy in women with primary biliary cirrhosis. Autoimmun Rev. 2014;13:931–935. doi: 10.1016/j.autrev.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 197.Allocca M, Crosignani A, Gritti A, Ghilardi G, Gobatti D, Caruso D, Zuin M, Podda M, Battezzati PM. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut. 2006;55:1795–1800. doi: 10.1136/gut.2005.079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ritzel U, Leonhardt U, Näther M, Schäfer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36:454–458. doi: 10.1016/s0168-8278(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 199.Sorokin A, Brown JL, Thompson PD. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis. 2007;194:293–299. doi: 10.1016/j.atherosclerosis.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 200.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690–695. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 201.Nakamuta M, Enjoji M, Kotoh K, Shimohashi N, Tanabe Y. Long-term fibrate treatment for PBC. J Gastroenterol. 2005;40:546–547. doi: 10.1007/s00535-004-1583-7. [DOI] [PubMed] [Google Scholar]
- 202.Schaffner F. Paradoxical elevation of serum cholesterol by clofibrate in patients with primary biliary cirrhosis. Gastroenterology. 1969;57:253–255. [PubMed] [Google Scholar]
- 203.Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138–1142. doi: 10.1002/hep.510260508. [DOI] [PubMed] [Google Scholar]
- 204.Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29:1396–1398. doi: 10.1002/hep.510290511. [DOI] [PubMed] [Google Scholar]
- 205.Silveira MG, Suzuki A, Lindor KD. Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology. 2008;48:1149–1156. doi: 10.1002/hep.22458. [DOI] [PubMed] [Google Scholar]
- 206.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 208.Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297–310. doi: 10.1136/gutjnl-2011-300779. [DOI] [PubMed] [Google Scholar]
- 209.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 210.Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123–132. doi: 10.1111/apt.12803. [DOI] [PubMed] [Google Scholar]
- 211.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 212.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84:662–670. doi: 10.1136/gut.2006.107789. [DOI] [PubMed] [Google Scholar]
- 213.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 214.Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125–135. doi: 10.2174/157016111201140327163930. [DOI] [PubMed] [Google Scholar]
- 215.Lata J. Hepatorenal syndrome. World J Gastroenterol. 2012;18:4978–4984. doi: 10.3748/wjg.v18.i36.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bressler B, Pinto R, El-Ashry D, Heathcote EJ. Which patients with primary biliary cirrhosis or primary sclerosing cholangitis should undergo endoscopic screening for oesophageal varices detection? Gut. 2005;54:407–410. doi: 10.1136/gut.2004.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Levy C, Zein CO, Gomez J, Soldevila-Pico C, Firpi R, Morelli G, Nelson D. Prevalence and predictors of esophageal varices in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:803–808. doi: 10.1016/j.cgh.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 218.Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840–845. doi: 10.1111/j.1478-3231.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 219.Abraham SC, Kamath PS, Eghtesad B, Demetris AJ, Krasinskas AM. Liver transplantation in precirrhotic biliary tract disease: Portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30:1454–1461. doi: 10.1097/01.pas.0000213286.65907.ea. [DOI] [PubMed] [Google Scholar]
- 220.Kew MC, Varma RR, Dos Santos HA, Scheuer PJ, Sherlock S. Portal hypertension in primary biliary cirrhosis. Gut. 1971;12:830–834. doi: 10.1136/gut.12.10.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764–1772. doi: 10.1002/hep.22273. [DOI] [PubMed] [Google Scholar]
- 222.Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345:669–681. doi: 10.1056/NEJMra003007. [DOI] [PubMed] [Google Scholar]


