Abstract
Dengue is an arboviruses due to single-stranded enveloped ribonucleic acid viruses, named dengue viruses (DENV), that include four serotypes and are mainly transmitted via the bite of mosquitoes of the genus Aedes (A. aegypti and A. albopictus). The distribution of the disease was historically limited to intertropical areas; however, during the last thirty years, the perimeter of the disease extended considerably and temperate areas are now at risk of outbreaks. The present global burden of dengue is considerable: 2.5 billion people over more than 100 countries are concerned; 50 to 100 million infections occur every year, with a number of fatal cases of approximately 20000. Although frequently asymptomatic or limited to a mild fever, dengue is responsible for severe cases mainly consecutive to the occurrence of hemorrhagic complications that can lead to shock and death, notably in children from poor-resource settings. The place of DENV as a transfusion-transmitted pathogen has been recognized only in 2008. At the present time, only five cases of transfusion-transmitted dengue, including one case of dengue hemorrhagic fever, have been formerly documented. This review provides a general overview of dengue, its viruses and their vectors. It replaces the disease in the context of other viral diseases transmitted by arthropods. It discusses the threat of dengue on the supply of blood products in endemic and non endemic areas. Finally, it describes the specific and non specific measures available for improving the security of blood products with regards to this emerging risk. Interestingly, in 2009, the American Association of Blood Banks placed DENV in the highest category of emerging infectious agents for their potential impact on transfusion recipient safety for the next years in North America.
Keywords: Dengue, Dengue viruses, A. aegypti, A. albopictus, Transfusion-transmitted virus, Blood safety
Core tip: The place of dengue viruses as transfusion-transmitted pathogens has been recognized only in 2008. By now, only five cases of transfusion-transmitted dengue, including one case of dengue haemorrhagic fever, have been formerly documented. This review provides a general overview of dengue, its viruses and their vectors. It replaces the disease in the context of other viral diseases transmitted by arthropods. It discusses the threat of dengue on the supply of blood products in endemic and non-endemic areas. Finally, it describes the specific and non-specific measures available for improving the security of blood products concerning this emerging risk.
INTRODUCTION
Dengue is an arboviruses mainly transmitted by mosquito bite that constitutes a major public health concern: two-fifths of the world’s population, mainly located in the intertropical regions, are exposed to the risk of infection. According to the World Health Organization (WHO), an estimated 500000 people with severe dengue require hospitalization each year, a large proportion of whom are children; about 2.5% of those affected die[1]. Despite the large distribution of this “old” infection and the fact that the virus can be present for about one week in the blood of infected patients, the risk of dengue as a transfusion-transmitted disease emerged very recently (first publications in 2008). An attempt to explain this paradox is proposed later in this review. After a few recalls concerning dengue, its viruses and their vectors, the disease is replaced in the larger context of arboviruses associated to a demonstrated or possible risk of transmission via blood products. The third part of the manuscript intends to answer the question formulated in the title of the paper: “Is transfusion-transmitted dengue fever a potential public health threat?” The last part of the study describes the measures available for reducing this risk.
RECALLS ON DENGUE, ITS VIRUSES AND THEIR VECTORS
Dengue viruses
Dengue viruses (DENV) are single-stranded ribonucleic acid (RNA) viruses, 40 to 60 nm in size, belonging to the Flaviviridae family (Table 1) and exhibiting an icosahedral capsid and a lipid envelope. The viral genome codes for ten viral proteins: three structural (core, membrane-associated and envelope) and seven non structural ones. The envelope protein is responsible for the specific recognition of host cells and for the development of protective neutralizing antibodies. Non structural proteins have been associated with the pathogenesis of severe forms of the disease. Dengue viruses include four serotypes entitled DEN-1, DEN-2, DEN-3 and DEN-4. The infection by one serotype confers a strong protection against the corresponding serotype but only a partial immunity against the three other ones, which explains that an individual can be infected several times during life by DENV. It is worthwhile to note that a fifth dengue serotype has been identified on virus samples that were collected during an outbreak in Malaysia in 2007[2]. More data are awaited about the epidemiological significance of this observation.
Table 1.
Main arboviruses exhibiting a potential or demonstrated transfusion-associated risk
| Dengue virus | West Nile virus | Saint-Louis encephalitis virus | Tick-borne encephalitis virus | Chikungunya virus | Colorado tick fever virus | |
| Family | Flaviviridae | Flaviviridae | Flaviviridae | Flaviviridae | Togaviridae | Reoviridae |
| Virus characteristics | ||||||
| Nucleic acid Envelope | ssRNA Yes | ssRNA Yes | ssRNA Yes | ssRNA Yes | ssRNA Yes | dsRNA No |
| Vectors | Mosquitoes (Aedes aegypti and Aedes albopictus) | Mosquitoes (genus Culex but also Aedes albopictus) | Mosquitoes (genus Culex) | Ticks (genus Ixodes) | Mosquitoes (Aedes aegypti, Aedes albopictus) | Ticks (Dermacentor andersoni) |
| Usual vertebrate hosts | Humans | Birds | Birds | Rodents | Humans, primates | Humans |
| Geographical distribution | World (mainly intertropical regions) | Asia, Africa, Europe, Americas | Americas | Europe, Asia | Africa, Asia, West Pacific, Europe, | Western USA and Canada |
| Clinical features | ||||||
| Incubation period in days | 2-14 | 2-14 | 4-21 | 7-14 | 1-12 | 3-6 |
| Asymptomatic forms | 75% | 80% | > 99% | 80% | 15% | low% |
| Clinical manifestations | DF-DHF-DSS | Fever- encephalitis | Fever-encephalitis | Fever- encephalitis | Fever- joint pains | Fever-encephalitis |
| Vaccine | Phase III trials | No | No | Yes | No | No |
| Demonstrated transfusion-transmitted cases | Yes | Yes (high number) | No | Yes | No | Yes |
ssRNA: Single-stranded RNA; dsRNA: Double-stranded RNA; DF: Dengue fever; DHF: Dengue hemorrhagic fever; DSS: Dengue shock syndrome; CHIKV: Chikungunya virus.
Vectors of DENV
The main vectors of DENV are mosquitoes of the Aedes genus (also called Stegomya).
The most common vector of dengue viruses is Aedes aegypti whose distribution is very large in intertropical regions of the world (Figure 1). In the Americas, discontinuation of Aedes aegypti control efforts in the mid-20th century has led to a resurgence of dengue throughout South and Central America, resulting in hundreds of thousands of dengue cases in these areas. In October 2012, an outbreak of DEN-1 infection was documented for the first time in the Portuguese island of Madeira[3]; the viral strain was shown to be very close to a virus strain originated from Venezuela[4].
Figure 1.
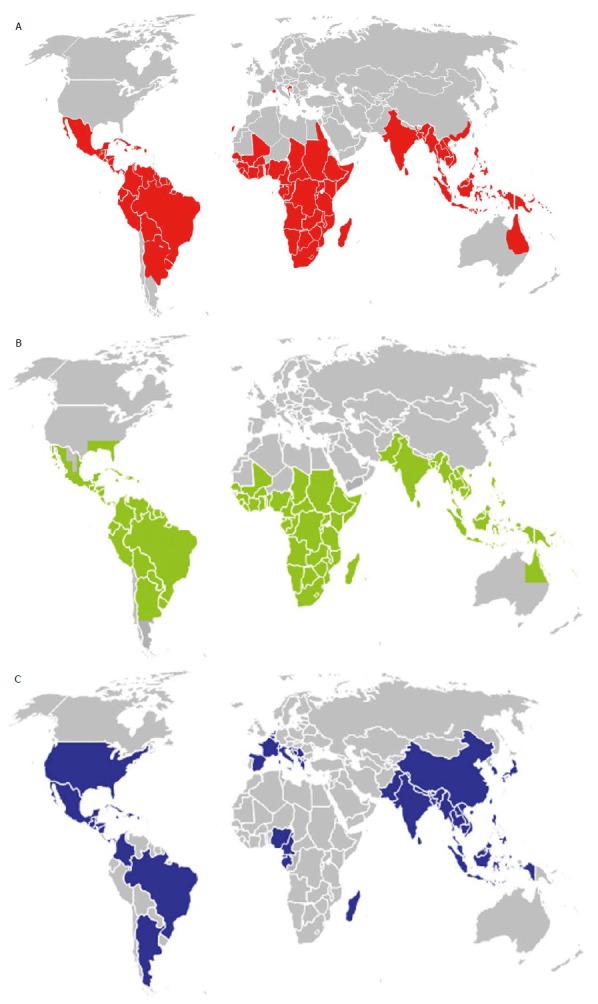
Overall distribution of dengue cases (endemic or epidemic) worldwide (A) and perimeter of expansion of the two main vectors of dengue viruses, Aedes aegypti (B) and Aedes albopictus (C).
Aedes albopictus (the tiger mosquito) is also involved in dengue outbreaks or isolated cases, notably in temperate regions as Europe where the mosquito is able to survive in cooler environment and expended very quickly (Figure 1) from Asia following the international trade in used tyres and other goods such as lucky bamboo. In 2010, an autochthonous outbreak of dengue was documented in Croatia[5] and two sporadic cases were identified in Nice city in the South-East of France[6].
A third species, Aedes polynesiensis, has been involved in rare cases. Aedes mosquitoes are highly domesticated mosquitoes that are able to grow in urban environment, notably in human-made containers filed with stagnant water (e.g., water storage tanks, subterranean pits, flowerpot trays). Interestingly, when both viruses are present in the same area, Aedes albopictus is able to displace Aedes aegypti from competing environment, which would facilitate the dissemination of DENV into temperate regions that are refractory to colonization by Aedes aegypti[7].
Routes of transmission of DENV
Dengue is mainly a mosquito-borne infectious disease. Besides the sylvatic reservoir that involves not human primates with occasional contamination of humans, the human cases are mostly related to the urban or peri-urban cycle where human beings are the main amplifying host for DENV (Figure 2). Female mosquitoes get infected by biting infected humans during their viremic phase; after 7 to 14 d of incubation, the mosquito is able to transmit the virus via blood feeding. Besides mosquito biting, DENV may be accidentally acquired after vertical transmission, especially in near-term pregnant women through the placenta[8], via the organ transplantation process[9,10], after needle-stick injury[11] and, as evidenced below, after transfusion of blood products.
Figure 2.
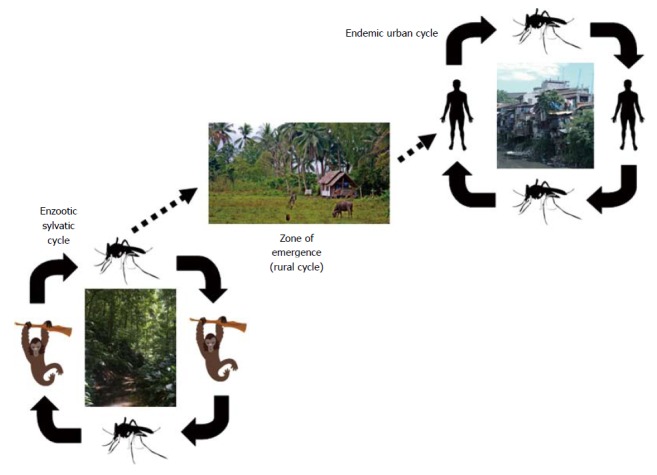
Simplified representation of the sylvatic and urban/peri-urban cycles of dengue that models the natural spread of dengue viruses through bites by infected mosquitoes.
Clinical presentation
The infection occurs after an incubation period of 3-14 d (average 3-7 d). Approximately 75% of all DENV infections are asymptomatic, notably in adults. The common symptomatic infection, which appears as a mild febrile illness associated or not with more evocative symptoms, represent approximately 20% of DENV infections. In endemic areas, about 5% of all acute febrile illnesses can be related to DENV[12]. Severe forms may represent up to 5% of symptomatic infections; they are more frequent at the two extremes of life (very young children and elderly) and in patients with diabetes mellitus, hypertension and renal insufficiency[13]. As shown in Figure 3, the classification of dengue presentations evolved through time[14]. According to the WHO classifications of 1975 and 1997, symptomatic dengue was divided in undifferentiated fever, dengue fever (DF) and dengue hemorrhagic fever (DHF) ranging from mild hemorrhagic symptoms (grade I) to dengue shock syndrome (DSS) (grades III and IV). In 2009, WHO proposed a new simplified classification in two presentations: dengue (without or with warning signs) and severe dengue (Figure 3). The latter classification is more adapted to clinical evaluations in primary care or resource-limited settings; however, it does not differentiate hemorrhagic forms from other severe presentations. A trend to capillary fragility together with the risk of thrombocytopenia is common features of all dengue cases, even those without hemorrhagic complications. It can be searched for by the tourniquet test that consists in applying and inflating a blood pressure cuff to the midpoint between the systolic and diastolic blood pressures for five minutes. The test is positive if more than 10 to 20 petechiae per square inch develop.
Figure 3.
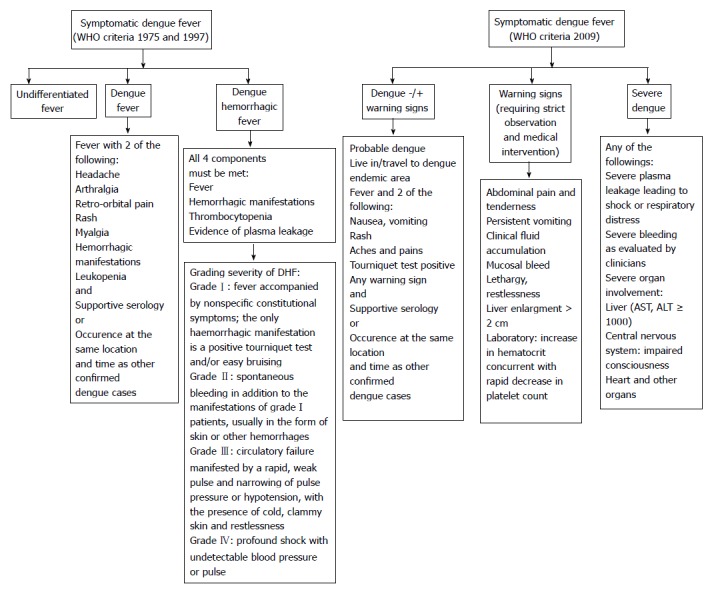
Successive classifications of dengue clinical presentations according to the World Health Organisation definitions. WHO: World Health Organisation. AST: Aspartate transaminase; ALT: Alanine transaminase.
Pathophysiology
From a pathophysiological point of view, many aspects of disease remain unsolved (for a review, see[15]). The fist targets of DENV after mosquito bite seems to be Langerhans cells, dermal cells and interstitial dendritic cells, but many other cells can replicate the virus, including hepatocytes, lymphocytes, endothelial cells, neuronal cells and muscle satellite cells[16]. Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)[17] and the mannose receptor (CD206)[18] have been described as potential host receptors for virus entry. As for other flaviviruses, both signal transducer and activator of transcription 1 and 2 possess the ability to independently limit the severity of DENV pathogenesis. When these signalling pathways are inactivated, notably within the hepatosplenic compartment, the deregulation of cell-mediated immunity may lead to the activation of CD4+ and CD8+ T cells, which results in a “cytokine/chemokine storm” that plays an important role in the vascular permeability leading to leakage of plasma into the extravascular compartment seen in DHF. The resulting hemoconcentration and decreased blood pressure may result in DSS.
More severe infections are known to occur after secondary infection than after primary infection. It has been suggested that facilitating antibodies against the envelope glycoprotein[19] and the “original antibody sin” theory[20] are involved in this observation.
Laboratory diagnosis
The virological diagnosis of dengue is required in case of severe infection and for confirming an outbreak. Different tools of direct diagnosis including cell culture, antigen detection and nucleic acid technologies (NAT), and of indirect diagnosis (serological tests) are available for documenting a recent infection.
Virus isolation from blood or tissues is possible by inoculation to mosquitoes or cell culture but these techniques are fastidious and limited to specialised laboratories.
In contrast, serological tests are very useful because they are relatively simple to implement, even in the absence of a laboratory of virology. They consists in microplate immunoassays that can measure IgM-specific antibodies, positive as soon as 4-5 d after the beginning of symptoms and lasting for up to 6 mo[21] (with a peak at week 2), and IgG-specific antibodies that become positive a few days after IgM and are a long-lasting marker of past infection. In patients infected at any time by other flaviviruses, which is relatively common in endemic areas, cross-reactive antibodies may interact with dengue serology and lead to false-positive results. The measure of neutralizing antibodies on a late serum specimen, a technique that requires cell culture within a specialised laboratory, may be useful to distinguish specific from unspecific IgM response.
An antigen test detecting the DENV NS1 protein in blood by immunoassay is now available. It is positive during the first 5 d following the initial symptoms. The sensitivity of the test is optimal during primary infection[22]. A negative test does not exclude the diagnosis in case of secondary infection[23,24].
The detection of DENV genome in blood or tissues by NAT has become the gold standard for the diagnosis of recent infection. It is positive within the first 5 d of disease. NAT tests are very sensitive and specific. Different molecular technologies are used for the diagnosis of DENV infection, including realtime polymerase chain reaction (RT-PCR), transcription-mediated amplification (TMA) and other isothermal amplification assays. The choice of primers may apply either on highly conserved parts of RNA genome within the 4 serotypes or on a combination of sequences specific of each of the 4 serotypes.
Prevention
At the individual level, the vaccinal approach is certainly the more suitable way to control dengue durably. The existence of at least four serotypes that are sufficiently antigenically different necessitates the use of four monovalent vaccines. However, as mentioned above, there is safety concern about a possible increase of virus infectivity via antibody dependent enhancement when a vaccinated subject is exposed to a wild virus. Although no vaccine against dengue is presently available, several approaches have been proposed for controlling the spread of disease (for review, see[25,26]). The most advanced solution is a live-attenuated tetravalent vaccine based on chimeric yellow fever dengue virus that is produced by Sanofi and could be commercially-available before the end of this year.
At the vector level, the eradication of susceptible mosquitoes is the more effective way to contain the epidemic. However, the large use of insecticides has shown its limits in terms of toxicity for the environment together with the rapid development of cross-resistances. A vector control program has been launched by the WHO[27]. It is based on actions combining the elimination of containers harbouring larval and adult mosquitoes (plastic cups, broken bottles, used tyres, flowerpots), the use of insect repellents, mosquito traps and mosquito net in the home. Future strategies are in progress to modify the vectors by biological interventions including transgenic mosquitoes or their infection by the intracellular bacterium Wolbachia that reduces the replication of arboviruses in susceptible vectors[28,29].
Curative treatment
The curative treatment is mainly symptomatic. No antiviral drug has yet demonstrated any effect against DENV. DF resolves spontaneously within a few days; analgesics containing ibuprofen and aspirin must be avoided to prevent hemorrhagic complications. Cases of DHF must be hospitalised; with replacement of fluid leakage and intensive monitoring; the mortality can be reduced under 1% when adequate cares are given but may reach up to 20% in case of poor medical intake. DSS and severe forms of dengue involving organ failure constitute a critical medical issue that needs urgent hospitalisation in an emergency unit.
DENGUE IN THE LARGER CONTEXT OF ARBOVIRAL DISEASES ASSOCIATED TO A DEMONSTRATED OR POSSIBLE RISK OF TRANSMISSION VIA BLOOD PRODUCTS
A total of approximately 130 arboviruses are known to cause disease in humans. Since they are transmitted via arthropod bite, these viruses are present in the bloodstream for a few days, which imply an at least theoretical risk of transmission via blood products if the patients are sampled during the viremic stage. The arboviruses known or suspected to be transmitted to recipients via blood products are presented in Table 1.
As reviewed by Petersen et al[30], the emergence of West Nile virus (WNV) in New York City in 1999 ant its rapid dissemination through Northern America during the following years is a good illustration of the sudden recognition of the role of transfusions in the spread of the virus, a fact that had been completely occulted before, despite many decades of circulation of WNV in the Ancient world. At the early phase of the USA outbreak, it was relatively difficult to establish a relationship between WNV infection and blood products[31,32], mainly due to the limits of contemporary diagnostic tools (IgM serology and NAT) that were insufficiently sensitive to identify infected donors, even retrospectively[30]. Another lesson driven from the WNV outbreak in USA was the decreased sensitivity of NAT when tested on minipools, a measure intended to decrease the costs and delay of WNV screening in blood donors.
The very successful emergence of Chikungunya virus (CHIKV) in the Indian Ocean and notably in the French Reunion Island is another illustration of the recent recognition of a new transfusion-transmitted risk. Even if no positive case was documented by NAT, probably for the same reasons as those evoked just before, it was modelled that, given a mean duration of 7.5 d for viremia and an exposition rate to CHIKV of 38% in inhabitants of the island, the prevention measures taken (eviction of autochthonous donors for red blood cells and systematic treatment of platelets by the Intercept®technology) had prevented the use of approximately 40 infected gifts during the whole epidemic period[33].
Concerning arboviral diseases in general, these examples illustrate that “planning efforts are hindered by the notoriously unpredictable nature of outbreaks and that importations of exotic arboviruses are random events with uncertain consequences”[30]. The rapid extension of dengue suggests that the subsequent transfusion transmission risk can be partly anticipated.
EVIDENCE FOR THE TRANSMISSION OF DENV BY BLOOD PRODUCTS AND ITS IMPACT ON PUBLIC HEALTH
As stated above, the global burden of dengue is considerable: according to the WHO, 2.5 billion people over more than 100 countries are concerned; 50 to 100 million infections occur every year, with a number of fatal cases of approximately 20000. A recent study[34] estimated that these figures could be increased by a factor of 3 to 4 to reflect the real load of dengue.
Despite the fact that dengue is the leading arboviruses in the world, there are only three reported observations of DENV transmission via blood products in the literature. The first report concerned a 76 year-old woman who received a blood transfusion in 2002 in a Hong-Kong hospital following a severe anaemia; two days later, she developed low-grade fever that resolved spontaneously (she received antibiotics for a suspicion of urinary infection). The case was secondarily related to dengue because the donor presented a typical dengue infection documented by serology. Molecular testing performed on the donated blood product was positive for DEN-1. Two months after transfusion, the recipient exhibited IgM antibodies confirmed by seroneutralisation assay. The case was published only six years later[35]. The second study, also published in 2008[36], involved a cluster of three cases contaminated in Singapore by the same donor who developed fever and myalgia after blood donation. Two days after transfusion, 2 of the 3 recipients developed a symptomatic infection that resolved spontaneously. The 3 recipients demonstrated serological evidence of acute dengue infection. A PCR assay performed on blood specimens from the donor and the 2 symptomatic recipients was positive for DEN-2.
The third observation, published in 2012[37] was documented from the outbreak of dengue that occurred in Puerto-Rico in 2007. Of 15350 donation samples tested retrospectively, 29 were found positive for DENV genome by TMA assay. Three of the recipients of these contaminated samples could be tested by NAT and one of them, who received red blood cells containing 108 copies/mL DEN-2, was found positive. Three days after transfusion, he developed DHF. Both donor and recipient were shown to harbour viruses with the same envelope sequence. This is the first case of severe dengue infection transmitted by blood products.
One may wonder about the gap between the important role played by dengue in Public Health worldwide and the limited number of transfusion-transmitted documented cases reported so far. Different arguments can be advanced for explaining such a paradox: (1) in the absence of documented inquiry between donor and recipient, it is often difficult to differentiate infection transmitted by mosquitoes and blood products; (2) the disease is frequently asymptomatic or mild in donor, recipient or both, with spontaneous resolution within a few days; (3) most of transfusion-transmitted cases are intended to occur in areas where dengue is endemic, which contributes to minimize the risk, especially in low-income countries where the virological documentation of dengue cases is not available easily, and, last but not least, and (4) most recipients of blood products have been already exposed to mosquito-transmitted DENV early in their life, which prevents them from being infected again via infected blood products.
In 2009, the American Association of Blood Banks stratified in four levels (red, orange, yellow and green) the emergent or re-emergent infectious agents that could represent a potential threat to transfusion in North America for the next years[38]. Besides epidemiological considerations and subjective assessment of public perception, the following scientific criteria were taken into consideration: (1) the agent must be present in blood at least for a few hours or days; (2) this blood phase must be at least in part asymptomatic for allowing the blood donor to pass through the filter of clinical selection; (3) the infectious agent must be able to induce, at least in some cases, a severe disease; and (4) finally, the blood pathogen must resist to inactivation by the innate or adaptative immunity of the donor (i.e., bacterical power of serum). According to these criteria, DENV was classified in the upper red level, together with Babesia sp and the human variant of Creutzfeldt-Jakob disease. These agents were considered as low to high scientific/epidemiologic evidence of risk regarding blood safety with the potential for severe clinical outcomes.
The arguments that pleaded for the upper-level classification of DENV with regard to blood safety in North America were as follows[38]: (1) the viremia is frequently asymptomatic and usually lasts for 2 to 7 d; (2) the viral load may be relatively high (from 104 to 108 copies/mL by NAT) in blood with the four serotypes of DENV, as exemplified by retrospective studies conducted in blood donors from Honduras, Brazil[39] and Puerto-Rico[37], with recovery of live virus from PCR-positive products in a few cases; (3) the disease can occur as important outbreaks; (4) the competent mosquitoes have a large distribution in the considered area (here United States); (5) the viral infection has a high seroprevalence in populations boarding the considered area; and (6) infected blood products could be imported from epidemic or endemic areas. At the opposite, the prevalence of positive samples was relatively low in the retrospective studies cited above (0.07% in 16521 blood gifts from Puerto-Rico[40], 0.30% in 2994 blood gifts from Honduras[39] and 0.06% in 4858 blood gifts from Brazil[39]).
The potential threat of dengue to transfusion safety is majored by the rapid spread of the disease worldwide whose incidence has increased 30-fold in the past 50 years[41]. Half of the planet is already exposed (Figure 1A) and the distribution of competent vectors (Figures 1B and C) is progressing very rapidly, notably with the climate changes[42] and the development of transcontinental travels. Regions with temperate climate as Europe or North America[43] can be the target of future outbreaks as illustrated by the recent cases observed in Croatia[5], Nice[6] or Florida[44]. In non-dengue endemic areas, asymptomatic infection is primarily associated with travellers returning from dengue-endemic areas. A few years ago, the recovery of areas endemic for malaria and dengue favoured the selection of blood donors returning from these countries. By now, dengue, as well as other arbovirosis, constitutes a risk that needs to be taken into consideration specifically.
MEASURES AVAILABLE FOR REDUCING THE RISK OF TRANSFUSION-TRANSMITTED DENGUE
Until a vaccine is widely used for preventing the expansion of dengue through the world population, it will be necessary to implement measures able to reduce the risk of transfusion-transmitted dengue. These measures include (1) the clinical selection of donors; (2) the implementation of screening tests specific for dengue; and (3) the non-specific reduction or inactivation of pathogens by the use of physical or chemical treatments applied to blood products. Their indications may differ in endemic and non-endemic areas[45].
Clinical selection of donors
In endemic areas, this measure would consist in excluding donors who may be at higher risk of infection. Given the fact that the exposition to mosquito bite is rather unpredictable, such a measure is not realistic. On the other hand, the presence of fever in donors of blood products is a general contra-indication of blood gift.
In non endemic areas, the clinical selection of donors consists in excluding travellers returning from endemic regions for a period of 4 wk. For instance, the latter measure was adapted in Europe towards tourists returning from Madeira during the recent 2012-2013 outbreak. The main limit of this strategy is the need for continuous adaptation of these exclusion measures to various epidemiological situations, which may lead to complicate the work of personnel in charge of this selection and to discourage donors from coming again for blood gift.
Screening tests specific for dengue
This strategy is useful in endemic areas or during an outbreak. Serology is not adapted for screening purpose because the viremia precedes of a few days the antibody answer. Only NAT could allow detecting the presence of viral genome in blood from infected donors. Such a strategy was applied in the Puerto-Rico outbreak in 2005[40] and 2007[37]. During the Madeira outbreak, an in-house RT-PCR assay was implemented for screening blood products; 43 of 1948 donations tested positive for DENV genome (further identified as DEN-1) between 9 September 2012 and 11 March 2013[46]. For large-scale screening purpose as in blood donors, Gen-Probe Inc. (San Diego, CA, United States) developed a prototype TMA assay using highly conserved primers; the analytical sensitivity of the test was of approximately 15 copies/mL for each serotype[39]. The low levels of viremia in many donors with dengue justify the individual testing of blood products, which limit this strategy to countries with high-income economy. By contrast to West Nile virus, no automated molecular screening test is currently commercially available.
In the future, the development of fully automated multiplexing assays detecting simultaneously several blood-transmitted pathogens in microarray plates or using nanotechnology would be very useful for areas where multiple infectious agents at risk for blood safety may circulate at the same time (i.e., in the Caribbean or in South-East Asia)[47].
Non specific reduction or inactivation of pathogens
Many systems are now available for treating blood products in order to inactivate some pathogens (for reviews see[38,48-50]). Most of these techniques are able to inactivate bacteria and lipid-enveloped viruses as DENV. Due to technical purposes, they can be applied to plasma, platelets or red blood cells. The main techniques that are efficient on DENV are briefly describer thereafter.
Some techniques are exclusively dedicated to plasma. Solvent-detergent treatment is able to disrupt viral envelopes. Dyes containing phenothiazine like methylene blue, when activated by visible light, are responsible for an oxidation of guanine present in viral genomes. Nanofiltration is able to retain viral particles whose size is over that of the pores of the nanofilter.
Other techniques based on photoactivation by ultra-violet (UV) rays may be applied to both plasma and platelet concentrates. The Intercept® system from Cerus Corporation (Concord, CA, United States) uses a psoralen derivative, amotosalen, as active compound. The Mirasol® system from Terumo BCT (Lakewood, CO, United States) use riboflavin (vitamin B2) as active compound. The Theraflex UV® system from MacoPharma (Tourcoing, France), by combining an exposition to UV light and strong shaking, induces the formation of cyclobutyl rings. Using those different technologies, a small proportion of platelets may be lost but the properties of activation, adhesion and aggregation of the cells resisting to the treatment are sufficiently well conserved to warrant their clinical use.
For red blood concentrates, some processes are in experimentation, including riboflavin (Caridian), Inactine® (PEN110 from the Vitex Company, Prestons, NSW, Australia) and an alkylating agent, Amustaline, from Cerus Corporation, whose activation occurs through exposition to acidic pH.
The main advantage of these strategies is the inactivation or reduction of a wide range of pathogens, including those that are still unidentified. However, the benefit-risk of each treatment needs a careful evaluation.
Economic considerations
The measures listed above regarding the prevention of transfusion-transmitted dengue represent an extra-cost for the Health system, especially those involving screening molecular tests specific for dengue that would be dedicated to the transmission of a single pathogen. No cost-effectiveness study has already been conducted to evaluate the economic burden of the implementation of a molecular screening targeting DENV neither in endemic or non endemic areas.
Lessons can be drawn from the experience acquired with the systematic screening of blood products for the presence of WNV in the United States during the epidemic period. Two studies were published on this topic in 2005[51] and 2006[52]. They demonstrated that the optimal cost-effectiveness strategy for WNV screening in blood products depends on different factors, including mainly the prevalence of the agent in the considered population, but also the ability to pool or not the samples before screening (i.e., mean viral load), the seasonal period concerned by the screening and the consequences for the recipients. Globally, these studies demonstrated that targeted donor screening seems to be more cost-effective than mass donor screening.
It is too early to consider whether these conclusions regarding WNV in a developed country may be applied to DENV in endemic and non endemic area. In dengue non endemic countries that correspond mostly to places with high living standards, it is likely that the emergence of a dengue outbreak will conduct to the set-up of a molecular screening, as it was done in Madeira recently[46]. In the epidemic of DENV that occurred in northern Queensland, Australia, in 2008-2009, the risk for a dengue-infectious blood donation was estimated as 1 in 7146[53]. Although the temporary exclusion of potentially infected donors was chosen to limit transfusion-transmitted dengue during these outbreaks, the authors raised the question of the better cost-effectiveness of a strategy involving the use of a suitable screening test or of a pathogen reduction technology[53].
In dengue-endemic areas, the risk may be higher, as shown during the 2005 outbreak in Singapore through a mathematical modelling, with an estimated risk for a dengue-infectious blood donation of 1 in 1667 to 6154[54]. The implementation of a screening test would be probably cost-effective as compared to the exclusion of blood donors but it is likely that neither of these two strategies could be implemented in low income countries where the disease is the more prevalent, at least in a near future.
CONCLUSION
Dengue provides an excellent model of transfusion-transmitted disease. Despite the large distribution of the disease worldwide, the risk with blood products from infected donors was only recognized recently. Except for one case of DHF[37], the disease, when transmitted by blood, does not seem to be more severe than after mosquito bite. However, the area of dengue extended considerably during the last 50 years; after having been limited to intertropical regions for a long time, the disease is now reaching temperate areas because of the worldwide distribution of its two main vectors (Figure 1) and of the climate change[42]. Considering these emerging risks, there is an urgent need for mathematical models able to predict the spread of DENV and its consequence on the supply of blood products. While waiting for an efficient prophylactic vaccine that could be able to reduce the burden of the disease, it is important to develop efficient measures for securing blood products in endemic and non endemic areas. The attention paid to DENV as a transfusion-transmitted pathogen could help to prevent the emergence of other more harmful known or unknown viruses.
ACKNOWLEDGEMENTS
The authors wish to thank Mohammed Jeraiby for his careful rereading of the English style of the manuscript.
Footnotes
P- Reviewer: Juan Ernesto L, Krishnan T S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
Conflict-of-interest: The authors declared no conflict of interest with regard to the subject of this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 24, 2014
First decision: September 16, 2014
Article in press: Janurary 20, 2015
References
- 1.World Health Organization. Dengue and severe dengue [updated March 2014] Available from: http: //www.who.int/mediacentre/factsheets/fs117/en/
- 2.Normile D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- 3.Alves MJ, Fernandes PL, Amaro F, Osório H, Luz T, Parreira P, Andrade G, Zé-Zé L, Zeller H. Clinical presentation and laboratory findings for the first autochthonous cases of dengue fever in Madeira island, Portugal, October 2012. Euro Surveill. 2013;18 [PubMed] [Google Scholar]
- 4.Wilder-Smith A, Quam M, Sessions O, Rocklov J, Liu-Helmersson J, Franco L, Khan K. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill. 2014;19:20718. doi: 10.2807/1560-7917.es2014.19.8.20718. [DOI] [PubMed] [Google Scholar]
- 5.Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobučar A, Pem-Novosel I, Kurečić-Filipović S, Komparak S, Martić R, Duričić S, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 6.Gould EA, Gallian P, De Lamballerie X, Charrel RN. First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality! Clin Microbiol Infect. 2010;16:1702–1704. doi: 10.1111/j.1469-0691.2010.03386.x. [DOI] [PubMed] [Google Scholar]
- 7.Conway MJ, Colpitts TM, Fikrig E. Role of the vector in arbovirus transmission. Annu Rev Virol. 2014;1:71–88. doi: 10.1146/annurev-virology-031413-085513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouliot SH, Xiong X, Harville E, Paz-Soldan V, Tomashek KM, Breart G, Buekens P. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv. 2010;65:107–118. doi: 10.1097/OGX.0b013e3181cb8fbc. [DOI] [PubMed] [Google Scholar]
- 9.Tan FL, Loh DL, Prabhakaran K, Tambyah PA, Yap HK. Dengue haemorrhagic fever after living donor renal transplantation. Nephrol Dial Transplant. 2005;20:447–448. doi: 10.1093/ndt/gfh601. [DOI] [PubMed] [Google Scholar]
- 10.Rigau-Pérez JG, Laufer MK. Dengue-related deaths in Puerto Rico, 1992-1996: diagnosis and clinical alarm signals. Clin Infect Dis. 2006;42:1241–1246. doi: 10.1086/501355. [DOI] [PubMed] [Google Scholar]
- 11.Chen LH, Wilson ME. Nosocomial dengue by mucocutaneous transmission. Emerg Infect Dis. 2005;11:775. doi: 10.3201/eid1105.040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomashek KM, Margolis HS. Dengue: a potential transfusion-transmitted disease. Transfusion. 2011;51:1654–1660. doi: 10.1111/j.1537-2995.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP. Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J Microbiol Immunol Infect. 2006;39:121–129. [PubMed] [Google Scholar]
- 14.Srikiatkhachorn A, Rothman AL, Gibbons RV, Sittisombut N, Malasit P, Ennis FA, Nimmannitya S, Kalayanarooj S. Dengue--how best to classify it. Clin Infect Dis. 2011;53:563–567. doi: 10.1093/cid/cir451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guabiraba R, Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141:143–156. doi: 10.1111/imm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warke RV, Becerra A, Zawadzka A, Schmidt DJ, Martin KJ, Giaya K, Dinsmore JH, Woda M, Hendricks G, Levine T, et al. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J Gen Virol. 2008;89:1605–1615. doi: 10.1099/vir.0.2008/000968-0. [DOI] [PubMed] [Google Scholar]
- 17.Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Desprès P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva Voorham JM, Rodenhuis-Zybert IA, Ayala Nuñez NV, Colpitts TM, van der Ende-Metselaar H, Fikrig E, Diamond MS, Wilschut J, Smit JM. Antibodies against the envelope glycoprotein promote infectivity of immature dengue virus serotype 2. PLoS One. 2012;7:e29957. doi: 10.1371/journal.pone.0029957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 21.Prince HE, Matud JL. Estimation of dengue virus IgM persistence using regression analysis. Clin Vaccine Immunol. 2011;18:2183–2185. doi: 10.1128/CVI.05425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarasamy V, Chua SK, Hassan Z, Wahab AH, Chem YK, Mohamad M, Chua KB. Evaluating the sensitivity of a commercial dengue NS1 antigen-capture ELISA for early diagnosis of acute dengue virus infection. Singapore Med J. 2007;48:669–673. [PubMed] [Google Scholar]
- 23.Tricou V, Vu HT, Quynh NV, Nguyen CV, Tran HT, Farrar J, Wills B, Simmons CP. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC Infect Dis. 2010;10:142. doi: 10.1186/1471-2334-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaterji S, Allen JC, Chow A, Leo YS, Ooi EE. Evaluation of the NS1 rapid test and the WHO dengue classification schemes for use as bedside diagnosis of acute dengue fever in adults. Am J Trop Med Hyg. 2011;84:224–228. doi: 10.4269/ajtmh.2011.10-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thisyakorn U, Thisyakorn C. Latest developments and future directions in dengue vaccines. Ther Adv Vaccines. 2014;2:3–9. doi: 10.1177/2051013613507862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yauch LE, Shresta S. Dengue virus vaccine development. Adv Virus Res. 2014;88:315–372. doi: 10.1016/B978-0-12-800098-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization (WHO) Global strategy for dengue prevention and control, 2012-2020. Geneva: WHO Press; 2012. [Google Scholar]
- 28.Rodriguez-Roche R, Gould EA. Understanding the dengue viruses and progress towards their control. Biomed Res Int. 2013;2013:690835. doi: 10.1155/2013/690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98:495–503. doi: 10.1111/j.1423-0410.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 32.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 33.Brouard C, Bernillon P, Quatresous I, Pillonel J, Assal A, De Valk H, Desenclos JC. Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. 2008;48:1333–1341. doi: 10.1111/j.1537-2995.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang V, Wong TY, Leung YH, Ma E, Law YL, Tsang O, Chan KM, Tsang I, Que TL, Yung R, et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J. 2008;14:170–177. [PubMed] [Google Scholar]
- 36.Tambyah PA, Koay ES, Poon ML, Lin RV, Ong BK. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med. 2008;359:1526–1527. doi: 10.1056/NEJMc0708673. [DOI] [PubMed] [Google Scholar]
- 37.Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, Zou S, Dodd RY, Tirado-Marrero LM, Hunsperger E, Santiago GA, et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion. 2012;52:1657–1666. doi: 10.1111/j.1537-2995.2012.03566.x. [DOI] [PubMed] [Google Scholar]
- 38.Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49 Suppl 2:1S–29S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 39.Linnen JM, Vinelli E, Sabino EC, Tobler LH, Hyland C, Lee TH, Kolk DP, Broulik AS, Collins CS, Lanciotti RS, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48:1355–1362. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 40.Mohammed H, Linnen JM, Muñoz-Jordán JL, Tomashek K, Foster G, Broulik AS, Petersen L, Stramer SL. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48:1348–1354. doi: 10.1111/j.1537-2995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 41.Allain JP, Stramer SL, Carneiro-Proietti AB, Martins ML, Lopes da Silva SN, Ribeiro M, Proietti FA, Reesink HW. Transfusion-transmitted infectious diseases. Biologicals. 2009;37:71–77. doi: 10.1016/j.biologicals.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Naish S, Dale P, Mackenzie JS, McBride J, Mengersen K, Tong S. Climate change and dengue: a critical and systematic review of quantitative modelling approaches. BMC Infect Dis. 2014;14:167. doi: 10.1186/1471-2334-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Añez G, Rios M. Dengue in the United States of America: a worsening scenario? Biomed Res Int. 2013;2013:678645. doi: 10.1155/2013/678645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention (CDC) Locally acquired Dengue--Key West, Florida, 2009-2010. MMWR Morb Mortal Wkly Rep. 2010;59:577–581. [PubMed] [Google Scholar]
- 45.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19:66–77. doi: 10.1111/j.1365-3148.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ECDC Mission Report. Dengue outbreak in Madeira, Portugal, March 2013. Available from: http: //www.ecdc.europa.eu/en/publications/Publications/dengue-madeira-ECDC-mission-2013.pdf.
- 47.de Mendoza C, Altisent C, Aznar JA, Batlle J, Soriano V. Emerging viral infections--a potential threat for blood supply in the 21st century. AIDS Rev. 2012;14:279–289. [PubMed] [Google Scholar]
- 48.Luban NL. The spectrum of safety: a review of the safety of current hemophilia products. Semin Hematol. 2003;40:10–15. doi: 10.1016/s0037-1963(03)80740-0. [DOI] [PubMed] [Google Scholar]
- 49.Blajchman MA. Protecting the blood supply from emerging pathogens: the role of pathogen inactivation. Transfus Clin Biol. 2009;16:70–74. doi: 10.1016/j.tracli.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Epstein JS. Alternative strategies in assuring blood safety: An overview. Biologicals. 2010;38:31–35. doi: 10.1016/j.biologicals.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Custer B, Busch MP, Marfin AA, Petersen LR. The cost-effectiveness of screening the U.S. blood supply for West Nile virus. Ann Intern Med. 2005;143:486–492. doi: 10.7326/0003-4819-143-7-200510040-00007. [DOI] [PubMed] [Google Scholar]
- 52.Korves CT, Goldie SJ, Murray MB. Cost-effectiveness of alternative blood-screening strategies for West Nile Virus in the United States. PLoS Med. 2006;3:e21. doi: 10.1371/journal.pmed.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT, Van Der Merwe KL, Flower RL, McBride WJ. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013;19:787–789. doi: 10.3201/eid1905.121664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilder-Smith A, Chen LH, Massad E, Wilson ME. Threat of dengue to blood safety in dengue-endemic countries. Emerg Infect Dis. 2009;15:8–11. doi: 10.3201/eid1501.071097. [DOI] [PMC free article] [PubMed] [Google Scholar]


