Abstract
For human immunodeficiency virus (HIV)-infected patients, the 1990s were marked by the introduction of highly active antiretroviral therapy (HAART) representing a new perspective of life for these patients. The use of HAART was shown to effectively suppress the replication of HIV-1 and dramatically reduce mortality and morbidity, which led to a better and longer quality of life for HIV-1-infected patients. Apart from the substantial benefits that result from the use of various HAART regimens, laboratory and clinical experience has shown that HAART can induce severe and considerable adverse effects related to metabolic complications of lipid metabolism, characterized by signs of lipodystrophy, insulin resistance, central adiposity, dyslipidemia, increased risk of cardiovascular disease and even an increased risk of atherosclerosis. New drugs are being studied, new therapeutic strategies are being implemented, and the use of statins, fibrates, and inhibitors of intestinal cholesterol absorption have been effective alternatives. Changes in diet and lifestyle have also shown satisfactory results.
Keywords: Human immunodeficiency virus-1 infection, Highly active antiretroviral therapy, Protease inhibitors, Dyslipidemia, Atherosclerosis, Lipodystrophy, Statins, Fibrates, Diet, Lifestyle
Core tip: Antiretroviral therapy inhibits human immunodeficiency virus (HIV)-1 replication, reduces mortality and increases survival. On the other hand, HIV-1 infection and antiretroviral therapy affect lipid metabolism. In fact, lipodystrophy is a well-documented side effect of highly active antiretroviral therapy (HAART). Switching to a less metabolically active drug improve HAART-associated dyslipidemia. Other therapies may include statins, fibrates, inhibitors cholesterol absorption, fish oils, niacin. Moreover, changes in diet and lifestyle are needed to revert the dyslipidemia.
INTRODUCTION
The introduction of highly active antiretroviral therapy (HAART) for human immunodeficiency virus (HIV)-infected patients in the early nineties (1990) represented a new perspective on life for these patients[1]. The use of HAART was shown to effectively suppress the replication of HIV-1 and dramatically reduce mortality and morbidity, which has led to a better and longer quality of life for HIV-1 patients[2]. The different HAART regimens, all composed of at least three different antiretroviral drugs, are effective in reducing viral load (HIV-1-RNA) to undetectable levels after its inception[3]. HAART regimes inhibit viral replication by acting at different stages with their different combinations of drugs[4]. This allows them to reach the viral cycle and/or viral enzymes and causes them to be classified in different therapeutic groups according to their mechanisms of action: nucleoside reverse transcriptase inhibitors (NRTIs)[5], non-nucleoside reverse transcriptase inhibitors (NNRTIs)[6], protease inhibitors (PIs)[7], fusion inhibitors[8], entry inhibitors [CC chemokine receptor-5 antagonists][9] and integrase strand transfer inhibitors (InSTIs)[10] (Table 1). Apart from the substantial benefits that result from the use of various HAART regimens, laboratory and clinical experience has shown that HAART can induce severe and considerable adverse effects on metabolic complications of lipid metabolism, characterized by signs of lipodystrophy, insulin resistance, central adiposity, dyslipidemia, increased risk of cardiovascular disease and even an increased risk of atherosclerosis[11-14]. However, other factors, such as virological, genetic, and individual immunological features, may be involved in the metabolic and lipid alterations observed because not all of the patients exposed to the same HAART regimens are similarly affected[15-17]. All of these changes in the aspects of lipid metabolism during HIV-1 infection, specifically changes in high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), very low-density lipoprotein cholesterol (VLDL), triglycerides (TG), lipid peroxidation, and their relationship with atherosclerosis in HIV-1 patients, are a result of the critical role of cholesterol in the mechanism of HIV-1 replication[11,12,18,19]. HIV-1 decreases plasma HDL by impairing the cholesterol-dependent efflux transporter ATP-binding cassette protein A1 in human macrophages, which is a condition that has a high atherogenic risk[20,21]. The use of PI-based HAART currently constitutes a more potent option against HIV-1 infection, preventing the maturation of viral particles and effectively controlling the infection of new cells by HIV-1. However, observed changes in lipid metabolism in HIV-1 patients have been associated with this class of antiretroviral drugs[13,14,22,23]. There is significant support in the literature showing that the PIs are associated with increased hepatic triglycerides-synthesis, VLDL, and to a lesser extent, total cholesterol (TC)[11-14]. Moreover, it was observed that these drugs impair the hydrolysis of triglyceride-rich lipoproteins by lipase, which reduces the storage of free fatty acids and interferes with the normal postprandial metabolism of free fatty acids[23,24]. The PIs are analogous substrates of the aspartyl protease enzyme of HIV-1 that are involved in the cleavage process of viral proteins and form smaller functional viral particles with infective capacity. After the cleavage process, the newly formed infectious viral particles are released from infected cells in a mature form[7,25,26]. Once the PIs bind to the active site of the protease enzyme, and this process of cleavage is blocked, there is interference in the enzyme activity and inhibition in the process of viral maturation and the formation of infectious viral particles[25,26]. The different mechanisms by which PIs promote these changes remain unknown. However, the main effect of PIs seems to be suppressing the breakdown of the nuclear form of sterol-regulatory element binding protein-1 in the liver and adipose tissue. This regulator is a key element in the proteolytic pathway responsible for regulating cellular and plasma levels of fat and cholesterol[27]. Finally, other classes of antiretroviral drugs are available, including those with excellent activity against viral replication without having any apparent effect on lipid metabolism[12,23,28]. However, it is clear that the use and recommendation of PIs occurs in situations where other drugs and/or regimens have not achieved the desired effect, either by non-adherence to treatment, viral resistance or lack of an immune response[29,30]. Moreover, once the therapy with PIs is initiated, a change to a more conservative therapy without their use is not recommended nor used in clinical practice[31,32]. Thus, a continuous search that considers the individual characteristics of each PI available as a current therapy is needed to achieve alternative HAART regimens that can maintain a suppression of viremia with minor effects on the lipid metabolism of HIV-1 patients[32,33].
Table 1.
Antiretroviral drugs class
| Antiretroviral class | Generic name drug | Trade name/manufacturer/approval (yr) |
| Nucleos(t)ide reverse transcriptase inhibitors | Abacavir (ABC) | Ziagen® ViiV Healthcare (1998) |
| Didanozine (ddl) | Videx® Bristol-Myers Squibb Co. (1991) | |
| Emtricitabine (FTC) | Emtriva® Gilead Sci. (2003) | |
| Lamivudine (3TC) | Epivir® GlaxoSmithKline (1995) | |
| Stavudine (d4T) | Zerit® Bristol-Myers Squibb Co. (1994) | |
| Tenofovir (TDF) | Viread® Gilead Sci. (2001) | |
| Zidovudine (AZT) | Retrovir® ViiV Healthcare (1987) | |
| Zalcitabine (ddC) | Hivid® Roche (1992) | |
| Non-nucleoside reverse transcriptase inhibitors | Delavirdine (DLV) | Rescriptor® Pfizer (1997) |
| Efavirenz (EFV) | Sustiva® Bristol-Myers Squibb Co. (1998) | |
| Stocrin® Merck Sharp, Dohme (1998) | ||
| Nevirapine (NVP) | Viramune® Boehringer Ingelheim (1996) | |
| Etravirine (ETR) | Intelence® Janssen-Cilag (2008) | |
| Rilpivirine (RPV) | Edurant® Janssen-Cilag (2011) | |
| Protease inhibitors | Amprenavir | Agenerase® GlaxoSmithKline (1999) |
| Atazanavir | Reyataz® Bristol-Myers Squibb Co. (2003) | |
| Darunavir | Prezista® Janssen-Cilag (2006) | |
| Fosamprenavir | Lexiva® ViiV Healthcare (2003) | |
| Indinavir | Crixivan® Merck and Co. (1996) | |
| Lopinavir | Kaletra® Abbott (2000) | |
| Nelfinavir | Viracept® ViiV Healthcare (1997) | |
| Ritonavir | Norvir® AbbVie Inc. (1996) | |
| Saquinavir | Invirase® Roche (1995) | |
| Tipranavir | Aptivus® Boehringer Ingelheim (2005) | |
| Fusion inhibitors | Enfuvirtide, T-20 | Fuzeon® Hoffmann La Roche (2003) |
| Integrase strand transfer inhibitors | Dolutegravir (DTG) | Tivicay® GlaxoSmithKline (2013) |
| Elvitegravir (EVG) | Stribild® Gilead Sci. (2012) | |
| Raltegravir (RAL) | Isentress® Merck and Co. (2007) | |
| Entry inhibitors (CC chemokine receptor 5 antagonists) | Selzentry | Maraviroc® Pfizer (2007) |
HIV-ASSOCIATED LIPID DISORDERS
Lipid disorders during the course of HIV-1 infection and acquired immunodeficiency syndrome (AIDS) were observed long before the advent of antiretroviral regimens[34,35]. In the early phase of acute HIV-1 infection, patients display several varied clinical signs of immunosuppression such as fever, intestinal infections, weight loss and depletion of protein reserves[35,36]. The possibility of HIV-1 infection, by itself, causing changes in lipid metabolism was already postulated, because it is evident that plasma viremia may promote a decrease in the plasma concentrations of TC, HDL and LDL and, in later stages of infection, an elevation in the concentration of TG[35,36]. Specifically, the reduction of HDL likely occurs as a result of an activation of the immune system in early HIV-1 infection, which promotes an increase in lipid peroxidation, inflammatory cytokine production, and alterations in the reverse cholesterol transport. This process promotes an imbalance in the antioxidant system, a decrease in the production of anti-inflammatory cytokines and an elevation of pro-inflammatory cytokines, which increases the chance of developing atherosclerotic diseases[31-39]. As a result of the inflammatory process initiated by viral infection, the stimulation of endothelial lipase and phospholipase A2 occurs, which in turn can reduce HDL concentration[38-40]. The inflammatory process may also be characterized by an elevation of interferon-γ levels (IFNγ) originating from lymphocytes and macrophages. IFNγ levels are elevated at early stages of infection and are also correlated with the presence of hypertriglyceridemia[41,42]. Tumor necrosis factor-α (TNFα) is another potent pro-inflammatory mediator whose concentrations increase in HIV-1 infected ART-naïve patients. TNFα promotes lipid peroxidation and disturbances in the metabolism of free fatty acids and also acts on the suppression of lipolysis mediated by hormones[43] (Figure 1).
Figure 1.
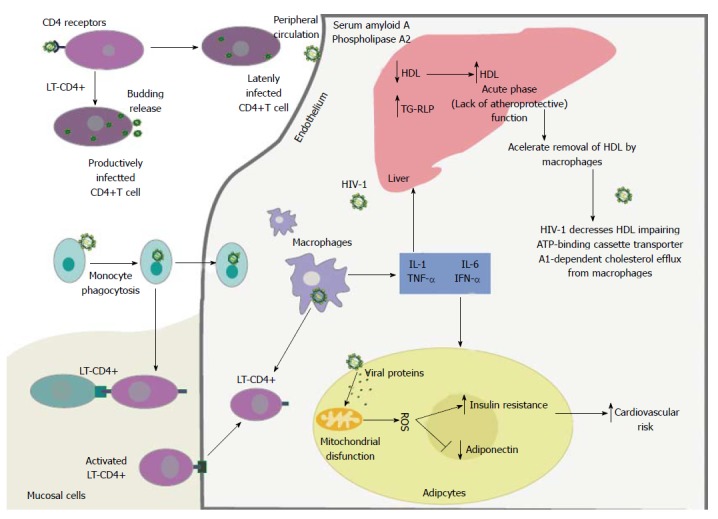
The human immunodeficiency virus type 1, upon entering peripheral circulation, will infect lymphocytes and macrophages. The viral proteins gp120 and gp41 of HIV-1 bind to the CD4+ receptor and coreceptors, C-C chemokine receptor type 5 and C-X-C chemokine receptor type 4, on the surface of these cells. The lymphocytes T-CD4 that are infected with HIV-1 produce viral particles and may remain in a latent form within circulation. Infected monocytes can directly present antigen to lymphocytes T-CD4, or transform into tissue macrophages. This process stimulates the host inflammatory response and amplifies the production of proinflammatory cytokines and promotes increased cellular oxidative stress. The production of proinflammatory cytokines by macrophages and lymphocytes promotes a decrease in plasma high-density lipoprotein cholesterol by impairing the cholesterol dependent efflux transporter ATP-binding cassette protein A1 in human macrophages. Additionally, viral proteins and proinflammatory cytokines including interleukin-1, interleukin-6, tumor necrosis factor α and interferon gamma stimulate endothelial lipase enzyme and different acute phase proteins, such as serum amyloid A. Viral proteins also exert effects on adipocytes resulting in mitochondrial dysfunction, production of reactive oxygen species, increased insulin resistance, decreased adiponectin, and change the clearance of triglyceride-rich lipoproteins and insulin resistance. Finally, all of the different cellular mechanisms involved and affected by HIV-1 infection promote an increased risk of cardiovascular disease. Source: de Almeida et al[211]. Gp120: Glycoprotein 120; gp41: Glycoprotein 41; CCR5: C-C chemokine receptor type 5; CXCR4: C-X-C chemokine receptor type 4; LT-CD4: Lymphocytes T-CD4; HDL: High-density lipoprotein; ABCA1: ATP-binding cassette protein A1; IL-1: Interleukin-1; IL-6: Interleukin-6; TNFα: Tumor necrosis factor α; IFN-γ: Interferon gamma; TG-RLP: Triglyceride-rich lipoproteins; ROS: Reactive oxygen species; HIV-1: Human immunodeficiency virus type 1.
HAART-ASSOCIATED LIPID DISORDERS
HAART-associated dyslipidemia is complex and involves immunological, hormonal, and genetic predisposition aspects, as well as effects induced by various antiretroviral drugs[13,44]. The observed dyslipidemia is characterized by hypertriglyceridemia, hypercholesterolemia, and decreased serum levels of HDL, either accompanied or not by increased levels of LDL (Table 2)[44,45]. Other metabolic and/or clinical common disorders include insulin resistance with hyperinsulinemia, increased C-peptide levels, diabetes mellitus and lipodystrophy syndrome[44-48]. HAART also affects the hydrolysis of triglyceride-rich lipoproteins and tissue lipase, disrupts normal post-prandial free fatty acid and lipoprotein catabolism and interferes with peripheral fatty acid trapping; all of these effects could be due to the interaction of these fatty acids with the master transcriptional regulator sterol regulatory element binding protein 1[47-51]. Nevertheless, the presence of dyslipidemia in individuals who use HAART therapy is not necessarily accompanied by lipodystrophy and/or an evident insulin resistance, which suggests that the mechanisms involved in these disorders are independent[44,46,51,52]. The NNRTI-based HAART, zidovudine, stavudine or lamivudine, have become associated with the occurrence of dyslipidemia; however, lipid metabolism disorders are most evident in individuals who make use of PI-based therapy[44,45,52,53]. The mechanisms involved in PI-associated dyslipidemia are not fully understood; however, the prevailing hypothesis is based on the structural similarity between the catalytic region of the HIV-1 protease and two homologous human proteins involved in the metabolism of lipids, called cytoplasmic retinoic acid-binding protein type 1 (CRABP-1) and low-density lipoprotein-receptor-related protein type 1 (LRP1) (Figure 2).
Table 2.
Antiretroviral drugs: Impact on lipid and glucose metabolism
| Antiretroviral class | Drug | Effects on lipids | Effects on glucose |
| NRTIs | Abacavir (ABC) | ↑ Dyslipidemia | No effect |
| Didanozine (ddl) | ↑↑ Dyslipidemia | Insulin resistance | |
| Emtricitabine (FTC) | ↑ Dyslipidemia | No effect | |
| Lamivudine (3TC) | ↑ Dyslipidemia | No effect | |
| Stavudine (d4T) | ↑↑ Dyslipidemia | Insulin resistance | |
| Tenofovir (TDF) | ↑ Dyslipidemia | No effect | |
| Zidovudine (AZT) | ↑↑ Dyslipidemia | Insulin resistance | |
| NNRTIs | Efavirenz (EFV) | ↑↑ HDL, ↑ Dyslipidemia | No effect |
| Etravirine (ETR) | Neutral effects | No effect | |
| Nevirapine (NVP) | ↑↑ HDL, ↑LDL | ||
| Rilpivirine (RPV) | Neutral effect | ||
| PIs | Amprenavir/ritonavir | ↑↑↑ Dyslipidemia | Insulin resistance |
| Atazanavir/ritonavir | ↑ Dyslipidemia | Insulin resistance | |
| Darunavir/ritonavir | ↑ Dyslipidemia | Insulin resistance | |
| Fosamprenavir/ritonavir | ↑↑↑ Dyslipidemia | Insulin resistance | |
| Indinavir | ↑↑ Dyslipidemia | Insulin resistance | |
| Lopinavir/ritonavir | ↑↑↑ Dyslipidemia | Insulin resistance | |
| Nelfinavir | ↑↑ Dyslipidemia | Insulin resistance | |
| Saquinavir | ↑ Dyslipidemia | Insulin resistance | |
| Tipranavir/ritonavir | ↑↑↑ Dyslipidemia | Insulin resistance | |
| Fusion inhibitors | Enfuvirtide, T-20 | Neutral effect | No effect |
| InSTIs | Dolutegravir (DTG) | Neutral effect | No effect |
| Elvitegravir (EVG) | Neutral effect | No effect | |
| Raltegravir (RAL) | Neutral effect | No effect | |
| Entry inhibitors | Selzentry | Neutral effect | No effect |
NRTIs: Nucleos(t)ide reverse transcriptase inhibitors; NNRTIs: Non-nucleoside reverse transcriptase inhibitors; PIs: Protease inhibitors; InSTIs: Integrase strand transfer inhibitors.
Figure 2.
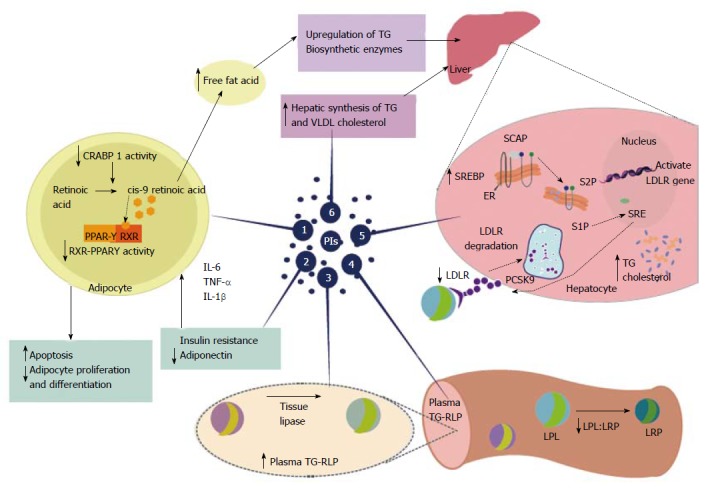
Highly active antiretroviral therapy-associated dyslipidemia is especially evident with the use of protease inhibitors. Protease inhibitors (PIs) promote a decrease in plasma high-density lipoprotein cholesterol and increased overall cholesterol, triglycerides(TG), and low-density lipoprotein cholesterol. These changes, induced by PIs, promote an increased risk of cardiovascular disease. Proposed mechanisms for PI-based dyslipidemia include the following: (1) There is structural similarity with the amino acid sequence of the C-terminal region of cytoplasmic retinoic acid-binding protein type 1 (CRABP1); thus, the PIs likely bind to CRABP-1, increasing apoptosis and diminishing the proliferation of peripheral adipocytes; (2) PI-mediated increases in the expression and secretion of proinflammatory cytokines, such as tumor necrosis factor alpha, interleukin 1 β and interleukin-6 are involved in altered adipocyte functions and decreased adiponectin; (3-4) PI-induced dyslipidemia is based on the structural similarity between the catalytic region of HIV-1 protease and the LDL-receptor-related protein that interferes with lipoprotein lipase complex formation (LRP-LPL). As a result the adipose storage capacity is reduced and plasma TG-rich lipoproteins are increased; (5) PI suppresses proteasome-mediated degradation of the sterol regulatory element binding proteins (SREBP) in the liver and adipocytes, which are transcription factors responsible for fatty acid and triglyceride synthesis in the liver and adipose tissue and control several steps of cholesterol synthesis. The suppression promotes nSREBP accumulation in the liver and an increase in the biosynthesis of total cholesterol and triglycerides, and adipose tissue, promoting increased insulin resistance, reduced expression of leptin and lipodystrophy; (6) PI-based therapy increases the hepatic synthesis of triglycerides, and to a lesser extent, very-low density lipoprotein cholesterol. Source: de Almeida et al[211]. PIs: Protease inhibitors; HDL: High-density lipoprotein; TG: Triglycerides; LDL: Low-density lipoprotein; CRABP1: C-terminal region of cytoplasmic retinoic acid-binding protein type 1; TNF-α: Tumor necrosis factor alpha; IL-1β: Interleukin 1β; IL-6: Interleukin-6; LRP: LDL-receptor-related protein; LPL: Lipoprotein lipase; TG-RLP: Triglyceride -rich lipoproteins are increased; SREBP: Sterol regulatory element binding proteins; VLDL: Very-low density lipoprotein; RXR-PPARγ: Retinoid X receptor-peroxisome proliferator-activated receptor γ; LDL-R: Low-density lipoprotein-receptor; PCSK9: Proprotein convertase subtilisin-kexin type 9; SCAP; Sterol regulatory element binding protein cleavage activating protein; S1P: Site 1 protease; S2P: Site 2 protease.
CRABP-1
CRABP-1 exhibits 58% homology in its amino acid sequence of the C-terminal region of the catalytic region of the HIV-1 protease. CRABP-1 usually binds intracellular retinoic acid and presents it to cytochrome P450 (CYP450) or 3A (CYP3A) enzymes, which convert retinoic acid to cis-9-retinoic acid and bind to the retinoid X receptor-peroxisome proliferator-activated receptor γ (RXR-PPARγ) heterodimer, stimulating adipocyte differentiation and inhibiting apoptosis[20,45,54]. The PIs likely bind to CRABP-1, which is homologous to the viral protease and erroneously inhibits the formation of cis-9-retinoic acid, leading to reduced RXR-PPARy activity, increased apoptosis and diminished proliferation of peripheral adipocytes. Such events cause peripheral lipoatrophy syndrome and hyperlipidemia due to adipocyte loss, decreased lipid storage and lipid release into the bloodstream. The inhibition of CYP3A by ritonavir is another possible mechanism involved in lipid abnormalities in HIV-1-infected patients and associated PI-based therapy and would promote a reduction in the formation of cis-9-retinoic acid and reduced enzymatic activity of RXR-PPARy. The decrease in RXR-PPARγ activity results in apoptosis within peripheral adipose stores, decreased adiponectin, and insulin resistance. However, central and visceral adipose stores are spared and expand with weight gain, contributing to insulin resistance[20,45,54].
LRP
LRP shares 63% homology with the catalytic region of HIV-1 protease. LRP binds to lipoprotein lipase (LPL) on the capillary endothelium, and the formation of this LRP-LPL complex promotes cleavage of fatty acids from TG, thereby promoting free fatty acid accumulation in peripheral adipocytes. A possible hypothesis is that the binding of PIs to LRP may inhibit the complex normal function of LRP-LPL and interfere with fatty acid storage, leading to hyperlipidemia. This hyperlipidemia is characterized by elevations in cholesterol levels, principally in the LDL and VLDL cholesterol fractions, because fatty acids released into the bloodstream subsequently reach the liver and promote a secondary hepatic synthesis of TG and VLDL[4,55].
Mitochondrial alterations
Another proposed mechanism for HAART-associated dyslipidemia is the mitochondrial alterations induced by HAART, especially with PI-based therapy. The hypothesis is that the HAART regimen will cause mitochondrial disturbances by inhibiting the mitochondrial DNA (mtDNA)-polymerase γ, leading to mitochondrial DNA depletion, respiratory chain dysfunction and reduced energy production by cells[56,57]. This disturbance in the mitochondrial respiratory chain may promote metabolic disorders in adipocytes, promote lipodystrophy syndrome and increase plasma lipid levels. Moreover, interference between PIs and cellular proteases could also trigger the development of metabolic alterations because some proteases are essential for mitochondrial biogenesis and metabolic function. Furthermore, functional changes of mitochondria in skeletal tissue promote insulin resistance and consequent dyslipidemia[56-58].
Genetic factors
HAART-associated lipodystrophy and dyslipidemia may be related to genetic predisposition, and studies with HIV-1 patients with hypertriglyceridemia and low HDL subjects were associated with different polymorphisms in the APOCIII gene. Promoter polymorphisms -455T > C and -482C > T in the APOCIII gene are both associated with increased levels of TG containing lipoproteins (VLDL) and low HDL values. Carriers of the -455T > C genetic variant had 30% lower levels of HDL cholesterol compared to those without this polymorphism, and plasma lipid concentrations increase according to the number of these variant alleles. Another variant nucleoside, the -1131T > C promoter polymorphism in the APOA5 gene, was associated with hypertriglyceridemia in PI-based patients[59-62].
Paraoxonases
Changes in antioxidant enzymes, such as the family of paraoxonases (PONs), may partially explain some of the mechanisms involved in HAART-associated dyslipidemia and consequently characterize a higher risk for cardiovascular diseases and atherosclerosis[63]. The hypothesis that the PIs can promote reductions in the activity of PONs and an increased risk for atherosclerotic disease in HIV-1 patients has been shown through previous evidence. PON1 is an antioxidant enzyme present in serum that is strongly associated with apolipoprotein-A1 (apoAl) from HDL and protects LDL against oxidative modifications[63,64]. The action of serum PON1 most likely occurs through the involvement of the enzyme in reverse cholesterol transport, a well-established anti-atherogenic propriety of HDL[65]. PON1 has the ability to inhibit LDL oxidation (oxLDL) and significantly reduce the lipid peroxidase enzyme, which decreases the accumulation of cholesterol in peripheral tissues[66]. The oxidative modification of LDL in the arterial wall plays a central role in the pathogenesis of atherosclerosis, which is characterized by the deposition of lipids and the formation of atherosclerotic plaques that cause narrowing of the blood vessels[67]. The inhibition of LDL oxidation by HDL is attributed to the high antioxidant content of this lipoprotein due to the antioxidant properties of apoA1 and by the presence of other different antioxidant enzymes, such as glutathione peroxidase and PON itself, which prevent the formation of or degrade bioactive products of LDL oxidation[68]. Some studies have shown that the activity of PON1 may be affected and/or inactivated by oxidative stress, which could explain its reduced activity during HIV-1 infection[63-65]. In HIV-1 patients and those who undergo HAART, there is a significant increase in oxidative stress. In turn, in asymptomatic individuals infected with HIV-1 and/or with AIDS, there is an increase in oxidative stress characterized by increased plasma metabolites of lipid peroxidation and/or a quantitative decrease in antioxidants compared to seronegative controls that are considered to be in a healthy condition. Therefore, possible reductions in the activity of PON1 and HDL concentrations may characterize an increased cardiovascular risk in individuals infected with HIV-1[64,65,69]. The PON1 activity that was reduced in ART-naïve patients, and restored in patients treated with HAART, suggested that the activity of PON1 is associated with the immune status in HIV-1 patients. However, in individuals treated with lopinavir/ritonavir, even with low plasma viremia, PON1 activity was reduced and a higher atherogenic risk was shown by the high TC:HDL ratio, suggesting that a PI-based regimen affects the mechanisms involved in the oxidation of LDL, thereby promoting greater atherogenic risk[63-68].
LDL oxidation
Oxidation is a common feature in lipid metabolism[70-72]. Oxidative modifications to LDL, which are considered the initial event in the pathogenesis of atherosclerosis, are attributed to oxidative stress mechanisms initiated by agents such as superoxide, nitric oxide and hydrogen peroxide that transform LDL into oxLDL[73,74]. The deposition of oxLDL in the arterial intimal layer promotes a cytotoxic effect on the vascular endothelium, followed by inflammation and modification of monocytes into macrophages that phagocytose oxLDL particles to form the foam cells that accumulate in the intima and lead to the development of atheromatous plaques[75]. The oxLDL particles are immunogenic, and serum levels of anti-oxLDL antibodies (Abs) can be used as indicators of oxidative stress[73-75]. The IgG anti-oxLDL Abs are pro-atherogenic and can predict the progression of coronary and carotid atherosclerosis, whereas IgM anti-oxLDL Abs appear to be associated with a possible protective role against the development of atheromatous plaques[76]. During the process of infection by HIV-1, the increase in atherogenic risk results from changes in lipid metabolism associated with the severity, duration, and stages of infection. Different degrees of lipodystrophy occur in patients along with a decrease in LDL receptor expression, which could lead to increased oxidation of LDL particles and the consequent development of atherosclerosis[77]. HIV-1 patients treated with lopinavir/ritonavir have shown higher levels of IgG anti-oxLDL Abs compared to patients treated with efavirenz or nevirapine regimens, and these levels were associated with an increase in the atherogenic indices[75-77].
HAART-ASSOCIATED LIPODYSTROPHY
Lipodystrophy is a syndrome that includes peripheral fat wasting and central obesity and is a well-documented side effect of HAART (Table 3)[16,48,78]. In addition to the decrease in the expression of LDL receptors, and a consequent increase in serum concentrations of LDL, the most obvious mechanisms involved in HAART-associated lipodystrophy and dyslipidemia are the mitochondrial changes induced by HAART[13,56-58]. The inhibition of mtDNA-polymerase γ, which leads to mitochondrial DNA depletion in respiratory chain dysfunction and a reduced energy production in cells, may promote metabolic disorders in adipocytes and promote increased lipodystrophy syndrome and plasma lipid levels[56-58,79,80]. Both therapies, PIs- and NRTIs-based, are associated with the inhibition of mtDNA-polymerase γ[79-81]. The abnormalities observed in lipodystrophy syndrome include lipoatrophy, lipohypertrophy, and metabolic disturbances. Lipoatrophy is associated with the loss of subcutaneous fat, usually in the lower limbs, face and buttocks. The observation of lipoatrophy in HIV patients has been demonstrated in therapy with both PIs- and NRTIs-based therapies. Several studies initially suggested that lipoatrophy in HIV-1 patients is primarily associated with the use of PI-based therapies; however, more recent reports show that the incidence of lipoatrophy was significantly higher in the efavirenz plus two NRTIs group than in the lopinavir or efavirenz plus two NRTIs plus lopinavir groups[82-84]. The association of lipoatrophy with efavirenz use was mainly in combination with either stavudine or zidovudine but not with tenofovir/lamivudine.
Table 3.
Clinical diagnosis and treatment to human immunodeficiency virus-associated lipodystrophy syndrome
| Clinical diagnosis | Treatment options |
| Lipoatrophy | |
| Sunken eyes, sunken cheeks, prominent zygomatic arch, prominent veins, skinny or muscular appearance, loose skin folds loss of contour | Switching antiviral therapies: stavudine or zidovudineto abacavir or tenofovir, other switch, and/or reconstructive procedures |
| Lipohypertrophy | |
| Increased abdominal girth with visceral fat accumulation, dorsocervical or supraclavicular fat pad | Diet, exercise, liposuction |
| Related findings | |
| Hypertriglyceridemia, usually with depressed HDL, hypercholesterolemia, insulin resistance, glucose intolerance | Statins, fibrates, inhibits intestinal cholesterol absorption, fish oils, diet, exercise, drugs (metformin, acarbose, sulfonylureas, glinides or leptin) |
Lipohypertrophy is the result of a metabolic disorder in which there is excess fat accumulation in the adipose tissue, resulting in a central obesity process. The most affected regions are the intra-abdominal, trunk and/or breast, anterior neck, and dorsocervical region (i.e., buffalo hump)[14,85]. There may be co-existing fat deposition in the liver, muscle, myocardium, and epicardium[86,87]. The most accepted hypothesis regarding the development of lipohypertrophy suggests that a defect in peripheral adipocytes promotes increased availability of fatty acids in the general circulation. The available fatty acids are then selectively deposited in visceral adipose tissue owing to the higher rate of lipid turnover and uptake in visceral adipocytes[88]. This disruption in the metabolism of fatty acids characterized by increased uptake in the visceral adipose tissue could be related to the effects of HIV itself via the HIV-1 accessory protein Vpr or to the effects of HAART[89]. In patients infected with HIV and treated with HAART, especially with PIs, there seems to be an association between HIV treatment and the development of lipohypertrophy[84,90,91]. However, various longitudinal studies have failed to demonstrate that HAART is the main cause of lipohypertrophy in HIV-1 patients[92-95]. The contribution of PIs to lipohypertrophy is based on several hypothetical mechanisms. PIs impair adipocyte differentiation through interactions with adipocyte proteasomal gene expression systems, down-regulation of cellular retinoic acid binding protein (CRABP), sterol regulatory binding protein levels with resultant dysregulation of gene expression stimulated by cortisol, activation of the adipocyte renin-angiotensin system and adipokine effects (including adiponectin and leptin), and decrease in peroxisomal proliferator-activator receptors α and γ[96-98]. This metabolic disorder results in the hypertrophy of adipose tissue, particularly in visceral tissues, resulting in increased TG levels, lowered HDL cholesterol levels, hypertension, increased propensity for type 2 diabetes, and increased insulin resistance[98-100]. This metabolic disorder results in hypertrophy of adipose tissue, particularly in visceral tissues, with the consequent increase of TG and reduced HDL cholesterol, and hypertension, increased propensity for type 2 diabetes, and an increased insulin resistance in adipocytes[98-100]. Insulin resistance is a common metabolic disorder that can accompany lipodystrophy (i.e., lipohypertrophy) and is associated with an increased cardiovascular risk, especially among HIV-infected individuals with lipodystrophy[101]. As described in the literature, HIV-infected individuals exhibit a higher prevalence of dyslipidemia, including both abnormal distribution of fatty acids and altered glucose homeostasis, compared to HIV seronegative individuals after adjustment for age and body mass index[102]. The disturbance in glucose metabolism appears to be closely linked to abnormal fat distribution, particularly visceral adiposity and lower extremity lipoatrophy. Lipodystrophy promotes accumulation of intramuscular lipids, which is associated with a reduction of insulin action in this tissue[103]. Importantly, in addition to the lipohypertrophy observed in HIV-infected individuals taking HAART, there appears to be an increase in fat distribution and deposition in places such as the liver and muscles regardless of the use of HAART[86,104]. The mechanisms involved in HIV-associated lipodystrophy are diverse, but it is suggested that HAART plays an important role[105], as well as the endothelial dysfunction associated with the HIV infection itself[106], vascular endothelial injury[107], and inflammation with elevated serum levels of C-reactive protein[108], TNF-α, IL-6, and adiponectin[102,109-111].
SWITCHING ANTIVIRAL THERAPIES
The search for different therapeutic strategies to reverse HAART-associated dyslipidemia has led to the use of less metabolically active antiretroviral drugs without compromising antiretroviral efficacy. Ritonavir is the most representative drug in HAART-associated dyslipidemia, and in combination with lopinavir confers higher risks for cardiovascular disease in HIV-1 patients. Amprenavir and nelfinavir promote lower impacts compared to the therapy with lopinavir/ritonavir[29,45,64,77,112]. In turn, the use of indinavir and saquinavir shows even less of an effect on lipid metabolism in HIV-1 patients receiving HAART. Currently, atazanavir has the least impact on lipid metabolism[113,114]. In contrast, nelfinavir promotes the elevation of TC, TG and LDL levels, and its replacement by atazanavir permits the reduction of the concentrations of these parameters without affecting antiretroviral activity[115]. A more recent alternative is tipranavir, a non-peptide PI prescribed for patients with multidrug resistance. However, it has shown deleterious effects that promote atherogenic risk by increasing the levels of TC and TG[116]. Another strategy to control dyslipidemia has been the discontinuation of the PI-based regimens and a switch to a NRTI- or NNRTI-based protocol. For ART-naïve patients, HAART regimens that include at least one NNRTI, or abacavir and two NRTIs, might be as efficient as PI-based therapy, although they are not the standard choice. This exchange of HAART in patients with viral suppression did not reduce antiretroviral efficacy during long-term use[116,117]. A strategy that must be better evaluated is the long-term use of the NRTI/NNRTI class of drugs before the use of PI-based therapy. The use of NRTI-associated nevirapine reduces levels of TC and TG promotes an increase in HDL and a decrease in atherogenic risk. The use of NNRTIs may also alter the lipid profile due mostly to the use of efavirenz. Using this medication, TG levels were higher in comparison to the use of nevirapine. However, in studies with a large number of HIV-1 patients, accompanied at intervals of ninety days and with undetectable HIV-1-RNA, the levels of TC, LDL and TG were kept within the desirable limit in the groups treated with nevirapine and efavirenz, including HDL levels within the reference values[116-118]. Only the HIV-1 patients treated with a PI-based regimen showed lipid abnormalities and increased risks for cardiovascular disease[13,22,117]. In addition, possible alterations in lipid metabolism resulting from the use of NNRTI-based therapy are easier and faster to reverse with the use of statins, fibrates, diet and lifestyle. Although the individual effects of NRTIs remain unclear, stavudine was associated with TC and TG elevations greater than zidovudine and tenofovir. The addition of fusion inhibitors to the existing therapies, such as enfuvirtide/T-20, had little effect on plasma lipids. The possibility of different HAART strategies eliminating or reducing the dyslipidemia in HIV-1 patients must be evaluated, and the risk of development of variants of the virus with multi-drug resistance must be taken into account[119]. In HIV-1 patients with favorable historical responses to HAART and accompanied by a physician experienced in HIV-infection, the transition from a PI-based to a therapy with nevirapine, abacavir, or even atazanavir may be preferable to the use of a hypolipidemic. In practice, many patients will show pre-existing resistance to the drugs, limiting options for the exchange of the treatment[77,113-115]. Experts must assess the risks of toxicity of the new treatment and the possibility of virologic relapse when switching HAART regimens.
OTHER THERAPIES FOR HAART-ASSOCIATED DYSLIPIDEMIA
The use of hypolipidemic drug therapy becomes necessary when HAART-associated dyslipidemia occurs or persists for a long period and when alterations in diet, exercise and other HAART strategies are ineffective. Difficulties in the treatment of dyslipidemia in HIV-1 patients involve potential interaction between drugs, toxicity, intolerance, and low patient adherence to multiple drug regimens. Several alternatives are available, which, when adequately monitored, may be beneficial in reducing HAART-associated dyslipidemia.
Statins
Statins are a group of drugs that inhibit the enzyme HMG-CoA reductase (3-hydroxy-3-methylglutaryl coenzyme A reductase) and are considered the primary drugs for the treatment of primary hypercholesterolemia[120] in addition to others effects[121,122]. In clinical practice, the use of statins has achieved excellent results in reducing TC and LDL, leading to a decreased risk of coronary artery events and in the primary and secondary prevention of heart diseases[123,124]. Statins inhibit the key rate-controlling enzyme in the de novo synthesis of cholesterol, which is responsible for production of > 50% of total body cholesterol. Inhibition of HMG-CoA reductase also promotes an increase in the synthesis of hepatic LDL receptors and reduced VLDL production[123-125]. The most important drugs of this class are simvastatin, fluvastatin, atorvastatin, lovastatin, pravastatin and rosuvastatin. All of these drugs reduce LDL concentrations, although the use of simvastatin and atorvastatin has shown superior effects in HIV-1 seronegative patients[123-125]. In HIV-1-infected patients affected with dyslipidemia, the use of simvastatin, pravastatin, fluvastatin and rosuvastatin promotes reduction of dyslipidemia, but not is complete remission once other factors and elements are associated with the dyslipidemia in these patients[123-126]. The different drugs that compose HAART have metabolizing effects similar to statin (Table 4). Most of these compounds are metabolized by CYP3A4 and may cause clinically relevant interactions with other agents that are changed by this enzymatic complex, such as cyclosporine, erythromycin, itaconazole, ketoconazole, oral anticoagulants, PIs and NNRTIs[126-128]. An additional complicating feature is that individual statins are metabolized at differing degrees, in some cases producing active metabolites. They are also substrates for P-glycoprotein, a drug transporter present in the small intestine, which may influence their oral bioavailability[127-129]. The presence of elevated statin levels in plasma increases the risk of liver toxicity, promoting elevations of serum transaminases and possible toxic hepatitis as well as skeletal muscle toxicity and myalgia with elevations of serum creatine kinase elevations, especially in the case simvastatin and atorvastatin[127-131]. Fluvastatin is metabolized by CYP2C9 enzyme; pravastatin and rosuvastatin are not significantly metabolized by the CYP450 system and have a very low risk of drug interactions. Reductions in the levels of TC and TG were observed in patients with dyslipidemia associated with HIV-1 and treated with a PI and the use of rosuvastatin. Simvastatin, lovastatin and atorvastatin should be avoided because they present a high risk of pharmacological interactions with PIs. Moreover, in a recent study, pravastatin had the lowest binding to plasma proteins of the statin agents and dietary advice associated with this statin compound significantly reduced total cholesterol levels in HIV patients treated with HAART, without significant adverse events[126-130]. It is reasonable to recommend the use of pravastatin and/or rosuvastatin as a first-line treatment for hypercholesterolemia in PI-treated patients and the use of fluvastatin, characterized by a slightly lower efficacy, as a second-line regimen. Additional benefits are obtained in patients treated with indinavir or pravastatin and fluvastatin, which significantly reduces the levels of TC and LDL, while maintaining good tolerability. Different associations between statins and antiretrovirals present considerable tolerability but always require monitoring of serum transaminases and creatine kinase. Different clinical studies and the routine use of fluvastatin, pravastatin, or rosuvastatin have shown that they are most suitable and safe to reduce LDL cholesterol levels in HIV patients[126-132].
Table 4.
Statins to highly active antiretroviral therapy-associated dyslipidemia
| Drug | Metabolism and interactions |
| Simvastatin | Considerable CYP3A4 metabolism. ↑ simvastatin levels with PIs and ↓↓ levels with efavirenz. Not recommended with atazanavir, atazanavir/ritonavir, fosamprenavir/ritonavir, saquinavir/ritonavir, tipranavir/ritonavir, lopinavir/ritonavir, indinavir/ritonavir, darunavir/ritonavir and nelfinavir. Doses of 80 mg/d with NNRTIs, raltegravir and selzentry |
| Lovastatin | Not recommended with atazanavir, atazanavir/ritonavir, fosamprenavir/ritonavir, saquinavir/ritonavir, tipranavir/ritonavir, lopinavir/ritonavir, indinavir/ritonavir, darunavir/ritonavir and nelfinavir. Doses of 80 mg/d with NNRTIs, raltegravir and selzentry |
| Atorvastatin | Somewhat CYP3A4 metabolism, ↑ levels with PIs darunavir, lopinavir, saquinavir/ritonavir, fosamprenavir. ↓levels with efavirenz. Doses of 20 mg/d with PIs, 80 mg/d with NNRTIs, raltegravir and selzentry |
| Pravastatin | Reduced interaction with CYP450 metabolism, primarily renal excretion but 50% ↓ with lopinavir/ritonavir, 45% ↓with nelfinavir, 80% ↑with darunavir/ritonavir, and 40% ↓ with efavirenz. Doses of 80 mg/d with PIs, NNRTIs, raltegravir and selzentry |
| Fluvastatin | Metabolized by CYP2C9, and occasional interactions with nelfinavir and efavirenz. Doses of 80 mg/d with PIs, NNRTIs, raltegravir and selzentry |
| Rosuvastatin | Not CYP3A4 metabolized but 5 × ↑ levels with lopinavir/ritonavir and darunavir/ritonavir (uncertain). Low starting doses (5-10 mg) recommended with PIs. Doses of 20 mg/d with PIs, 40 mg/d with NNRTIs, raltegravir and selzentry |
NNRTIs: Non-nucleoside reverse transcriptase inhibitors.
Fibrates
Fibrates represent the cornerstone of drug therapy for hypertriglyceridemia and mixed hyperlipidemia. These compounds are characterized by an extended activity on the hepatic synthesis of both TC and TG, LPL and acetyl-CoA-carboxylase, and the favorable effects on peripheral lipolysis inhibition and glycemic control[133]. Fibrates are also metabolized by CYP450 system, but they appear to affect only CYP4A enzymes and do not show clinically relevant interactions with PIs. However, concomitant use of both fibrates and statins can increase the risk of skeletal muscle toxicity and should be avoided[134-136]. In HIV-1 seronegative individuals, the use of a fibrate and a statin in a monotherapy regimen exhibits moderate lipid-lowering effects and good tolerability[136-138]. In HIV-1 patients, fibrates do not have the same efficacy of statins in preventing cardiovascular disease. Studies with HIV-1 patients treated with PI-based therapy and fibrates, including gemfibrozil, bezafibrate or fenofibrate, showed a significant reduction in the concentration of TC, TG and hypertriglyceridemia[135,137,138]. Fibrates appear as a suitable alternative for the treatment of dyslipidemia associated with HIV, especially in the presence of hypertriglyceridemia. Periodic monitoring of serum creatinine, creatine kinase, and transaminases should be performed for the use of fibrates[137-139]. The association between fibrates and statins has been used with relative safety and demonstrated in different studies with large numbers of HIV-1 seronegative volunteers, except for the use of the combination of statins and gemfibrozil, which is not recommended[138-140]. The use of statins, fibrates, or associates has shown positive results in HIV-associated dyslipidemia, and the pravastatin/fenofibrate combination has promoted an improvement in lipid parameters and is safe and efficacious[141,142]. However, as already described, there is a need for clinical and laboratory monitoring, with careful evaluations of possible clinical symptoms, such as myalgia, and laboratory symptoms such as serum transaminases, creatine kinase and creatinine.
Inhibitors of intestinal cholesterol absorption
Ezetimibe is effective at lowering lipid levels because it has the ability to inhibit the intestinal cholesterol absorption, and it shows good tolerability because it does not interact with the metabolism of CYPA4 enzymes[143,144]. In non-HIV-1-infected patients who have dyslipidemia, the monotherapy with ezetimibe or when combined with statins or fenofibrate has shown considerable efficacy and safety[145,146]. In HIV-1 patients with high serum levels of LDL, the use of ezetimibe has also been considered an effective alternative[144]. Monotherapy using 10 mg/d of ezetimibe has promoted reductions of more than 20% of serum LDL and, in addition, reduces the concentrations of TC and TG and increases HDL concentrations[143-146]. Studies have shown that in individuals with HIV that is beyond effective treatments, ezetimibe has no interaction with HAART, and those receiving a PI-based association of fenofibrate/ezetimibe showed greater efficacy compared with pravastatin in monotherapy resolution of dyslipidemia[147-149].
Fish oil
The ability of fish oil, commonly known as omega-3 fatty acids [namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], to reduce elevated TG concentrations has been observed in different studies[150,151]. HIV-1 patients using both HAART and fish oil showed an effective reduction in the concentration of TG[152]. This ability to reduce TG levels promotes a direct benefit in risk reduction of atherogenic cardiovascular disease through a combination of anti-inflammatory and anti-platelet actions[152-154]. For HIV-1 patients, the use of fish oil associated with fenofibrate showed additive effects in reducing TG. Given these considerable results, the American Heart Association’s (AHA) dietary guidelines, recommends that healthy adults have a minimum of two portions of fish per week, and those who have elevated TG should consume 2-4 g of EPA and DHA daily as a dietary supplement[152-155].
Niacin
Niacin (water-soluble vitamin B3), or nicotinic acid, is a powerful reducing agent of serum lipids when administered at pharmacological doses. Its ability to reduce the levels of lipoproteins and apolipoprotein-B-containing lipoproteins and to raise HDL levels has been shown, characterizing it as an atheroprotective drug[156,157]. Niacin has beneficial effects on other cardiovascular risk factors, including lipoprotein (a), C-reactive protein, platelet-activating factor acety lhydrolase, plasminogen activator inhibitor 1 and fibrinogen[158,159]. The molecular mechanisms involving the action of niacin are not fully understood, but its effect on hypertriglyceridemia in uninfected individuals is recognized[157-159]. In HIV-1 patients, the use of niacin in an extended release formulation significantly reduced the levels of TC, TG and HDL. However, the use of niacin in HIV-1 patients with dyslipidemia need to be carefully monitored because the presence of adverse events have been commonly shown, including headache, flushing, pruritus, rash, hyperuricemia, and exacerbation of insulin resistance[160,161].
OTHER AGENTS
Other agents may contribute to HIV-associated dyslipidemia. The use of recombinant methionyl human leptin was associated with reduced insulin resistance and increased HDL levels[162]. Tetradecylthio acetic acid, an agent whose mechanism is still unknown, promotes a reduction in levels of plasma lipoproteins[163]. Additionally, Acipimox, a drug with sustained action and a structure similar to niacin, has been associated with decreased insulin resistance and significantly reduced levels of TG in HIV-1 adults[164]. In a double-blind study, the use of cholestin was able to reduce the levels of TC and LDL cholesterol without modifying HDL and TG, and without showing adverse effects[165]. The use of L-carnitine (3 g/d) resulted in a significant reduction in serum triglycerides in patients with HIV-associated dyslipidemia[166]. These and other drugs studied aimed to revert the HIV-associated dyslipidemia but require more control to be considered appropriate for the treatment of dyslipidemia.
NEW DRUGS TO TREATMENT HIV INFECTION
Since the introduction of zidovudine (1987) for the treatment of HIV-1 infection, followed by the emergence of fusion inhibitors, such as enfuvirtide/T-20 (2003), and more recently the approval by the Food and Drug Administration (FDA) of raltegravir (2007) and dolutegravir (2013), both InSTIs drugs, HIV-1 treatments have been adapting to new challenges. Once the inability of different HAART regimens to cure infection was recognized, new drugs, strategies and therapeutic regimens were developed with the goal of greater efficiency associated with safety and fewer adverse effects. The common adverse effects observed by the use of the first class of drugs such as zidovudine, and the dyslipidemia caused by the use of PIs, are obstacles that are being minimized in newer experimental drugs. Currently, more than 30 drugs are approved and available in various forms (the different classes of antiretroviral drugs), and many others are in experimental stages.
NRTIs
Festinavir (BMS986001) is a thymidine analogue drug, derived from stavudine, but with less potential toxicity[167]. It has been used in cases where there is HIV-1 resistance to abacavir and tenofovir and is an oral drug recommended for HIV-1 patients with multi-drug resistance. The compound has a 50% effective concentration (EC50) for the inhibition of mtDNA-polymerase γ and is 100 times less toxic to the mtDNA-polymerase γ in renal proximal tubular cells, muscle cells, and adipocytes and on the cellular levels of adenosine triphosphate and/or lactate production (ATP) than stavudine. The mitochondrial toxic effects of stavudine are the main cause of the adverse effects associated with lipodystrophy and peripheral neuropathy, which has led to the decline in its use and indicated that festinavir has a minor impact on lipid metabolism[167-169]. Apricitabine (AVX754, formerly SPD754) is a drug for oral administration and is currently in the experimental phase (Phase IIB clinical trial). It is structurally related to lamivudine and emtricitabine and, as such, is an analog of cytidine[170]. This drug is well tolerated, and its most common side effects include headache, nausea, muscle aches and diarrhea. The use of apricitabine in HIV-1 infected patients had no effect on bone marrow, liver or kidney toxicity, and lipase. However, its use caused changes in lipid metabolism, most noticeably elevated serum TG, indicating that its use should be evaluated in patients who initiated therapy with apricitabine or who already have a dyslipidemic profile[170-172]. GS-7340 is a prodrug of tenofovir called tenofovir disoproxil fumarate. Unlike tenofovir, GS-7340 is stable in plasma and is then converted to tenofovir inside of cells by the cellular enzyme cathepsin, which is highly expressed in lymphoid tissue[173]. Within the cell, the drug is transformed into the active metabolite tenofovir diphosphate, an inhibitor of HIV-1 reverse transcriptase. Phase III studies are underway to better define the safety profile and efficacy, and initially, the drug does not show effects on lipid metabolism. However, formulations containing 300 mg of the drug promoted adverse effects on the kidneys and bone marrow toxicity[173-175]. Other drugs of the NRTI class are in the experimental phase, such as racivir (an enantiomer of emtricitabine), elvucitabine (Phase II clinical trial), and amdoxovir (AMDX or DAPD). For these drugs, current data about the adverse effects are insufficient to characterize their impact on lipid metabolism[174-178].
NNRTIs
Etravirine (ETR, Intelence®) is a drug from the second generation of NNRTIs and shows efficacy, safety and good tolerability in HIV-1 patients[179]. One of the primary advantages of etravirine is as a replacement for other NNRTIs to which the HIV-1 virus is resistant, mainly due to the presence of the K103N and Y181C mutations in the case of efavirenz and nevirapine, respectively. The FDA approved the drug in 2008 for use in patients with multiple drug resistance. However, the drug is a substrate and an inhibitor of different CYP3A4 enzymes, which in turn are contraindicated in the use with antimicrobial and anticonvulsant drugs metabolized by the CYP450 system. In patients receiving HAART who have alterations in lipid metabolism, the switch to a therapy containing etravirine has shown satisfactory results and a reversal of dyslipidemia[179-182]. Rilpivirine (Edurant®) is a second-generation NNRTI class drug. It is more potent than diarylpyrimidine (DAPY), and adverse effects are considerably reduced compared to older NNRTIs such as efavirenz. After clinical trials, rilpivirine was approved by the FDA in 2011, and its use is often combined with emtricitabine and tenofovir. Rilpivirine produces few changes in TC, LDL, HDL and TG in HIV-1 patients. In comparison to treatment with efavirenz, this drug promotes an increase in lipids and in the TC:HDL ratio, which is characterized by an increased risk of cardiovascular diseases in these patients[183,184]. MK-1439 is a new and effective drug against a variety of HIV-1 mutants that are resistant to NNRTIs[185]. Preclinical studies (Phase l clinical trial) that are currently in progress show that this drug has a good pharmacokinetic profile with the possibility of a low concentration daily dose needed to obtain an optimal effect. Additionally, it has good absorption, low potential for toxicity and the ability to be used with other antiretroviral agents. MK-1439 showed good results in cases where the K103N mutation of HIV-1 led to resistance against nevirapine and efavirenz, as well as in the presence of the Y1818C mutation, which leads to a lower susceptibility in treatment with nevirapine, rilpivirine and etravirine. In vitro data suggest that MK-1439 has beneficial properties that warrant additional development as a new antiviral drug; however, no data are available about its potential impact on lipid metabolism[185-187]. New drugs of the third generation of NNRTIs are in various experimental stages such as BILR 355 BS (Phase IIa), (+)-Calanolide A (Phase I), GSK 2248761 (Phase IIb), MK-4965 (Phase I), MK-6186 (Phase I), RDEA806 (Phase IIa), and UK-453061 (Phase IIb). These new drugs have not been approved by the FDA and still require different clinical trials prior to their release as drugs available for the treatment of HIV-1 infection, and they currently have no scientific information regarding their possible effects on lipid metabolism.
Fusion/entry inhibitors
The HIV-1 envelope glycoprotein (Env) complex, which is composed of three receptor-binding gp120 subunits and three fusion protein gp41 subunits, mediates virus entry by fusing viral and cellular membranes and offers an attractive target for developing antiviral agents[188]. In succession with enfuvirtide/T20, a number of design strategies have been applied to develop new peptide-based fusion inhibitors with improved stability, bioavailability and potency[188,189]. There are several drug classes that are in two experimental phases. Albuvirtide (FB006M), T649, T2634, T2544, T1249, SC34EK, and SC29EK are in the class of fusion inhibitors. BMS 663068, BMS 626529, vicriviroc (SCH 417690), and cenicriviroc (TAK-652, TBR-652) are in the class of entry inhibitors. These and other drugs are in experimental stages or have been suspended, and there are no initial and/or conclusive data about their potential toxic effects and the impact on lipid metabolism.
InSTIs
Cobicistat (GS-9350) is a new InSTIs drug recently approved by the FDA (2012). This drug, similar to ritonavir, has the ability to inhibit hepatic enzymes that metabolize other drugs used to treat HIV-1 infection, such as raltegravir[190]. Cobicistat has become increasingly important, and its use has been associated with elvitegravir, permitting it to have higher blood concentrations with the use of smaller doses, which theoretically allows for greater suppression of viral replication when used with elvitegravir, and with fewer adverse effects. Cobicistat has been employed in combination with elvitegravir/emtricitabine/tenofovir (Stribild®)[190,191]. Cobicistat is a potent inhibitor of CYP3A enzymes that concurrently affect administered medications metabolized by this pathway. It also inhibits intestinal transport proteins, increasing the overall absorption of several drugs including atazanavir, darunavir, and tenofovir alafenamide fumarate. Phase III trials of the cobicistat-containing combination antiretroviral therapy regimens in ART-naïve patients have shown a small elevation of serum fasting lipid, with a relative increase in the levels of TC and TG, in addition to bilirubin elevations, jaundice, nausea and diarrhea[190-192]. Other drugs of the InSTI class are experimental, such as MK2048. MK-2048 represents a prototype second-generation InSTIs developed with the goal of retaining activity against viruses containing mutations associated with resistance to first-generation InSTIs (raltegravir and elvitegravir)[193]. It is a drug that acts by inhibiting the integrase enzyme four times longer and shows superior efficacy to raltegravir. Additionally, it is being investigated for use as part of a pre-exposure prophylaxis[193,194]. BI 224436 is the first non-catalytic site integrase inhibitor. It inhibits HIV replication via binding to a conserved allosteric pocket of the HIV integrase enzyme. This makes the drug distinct in its mechanism of action compared to raltegravir and elvitegravir, which bind at the catalytic site[195,196]. Another experimental drug is GSK744 (S/GSK1265744, Cabotegravir®), which has a structure similar to that of carbamoyl pyridone and dolutegravir. In investigational studies, the therapeutic agent has been packaged into nanoparticles (GSK744LAP), which confer an exceptionally long half-life of 21–50 d following a single dose. In theory, this would make suppression of HIV possible when dosing as infrequently as once every three months. These drugs do not have sufficient data on their toxicity profile and/or on lipid metabolism; however, they have been previously considered to have low metabolic toxicity[197,198].
DIET AND LIFESTYLE
Changes in diet and lifestyle, and the adequacy of a hypocaloric diet, are recommendations that seek to reduce the concentrations of TC and its fractions, especially LDL[199-201]. These changes bring benefits over short periods of time and reduce the risk for cardiovascular and atherosclerotic diseases. These recommendations are addressed to the entire population, as well as HIV-1 infected patients, and are measures that should be applied to delay the need for lipid-lowering drugs, even before the treatment of dyslipidemia is needed[199-202]. Changes in diet can directly alter the levels of circulating LDL including saturated fats, cholesterol, and trans-unsaturated fats. The biggest impact comes from saturated fats, which are generally those that have a solid state at room temperature or under refrigeration. The major sources of saturated fats are meat and meat products (poultry, pork, beef, lard, and sausages), dairy (milk and cheeses), and vegetable oils (derived from palm or coconut). For an adequate daily diet, the recommended consumption is equal or < 7% of saturated fats, for the total daily caloric intake. Dietary cholesterol is exclusively found in animal products such as meats (particularly organ meats and tissues such as brain, kidney, and liver), egg yolks, and dairy products[203,204]. It is recommended to keep dietary cholesterol consumption to < 200 mg/d. Trans fats and unsaturated fats are found in breads and cookies, doughnuts, stick margarine, and fried foods. This type of fat is added to foods to enhance the substance or texture of the product, to replace some of the animal fats, and even to increase the shelf life of certain products. The recommendation is to keep the consumption of trans fats as low as possible. The amount of trans fat is not included in the < 7% of calories/day allowed from saturated fats[203-205]. The consumption of unsaturated fats is preferred; sources include fish such as salmon, mackerel, tuna, and vegetables such as avocado, olives and olive oil and vegetable oils[206]. Other foods that are recommended for their maintenance and/or lipid-lowering effects are the omega-3 fats, which are polyunsaturated fats that can lower TG levels. Omega-3 fats are frequently referred to as fish oils because the most common sources are fatty fishes such as salmon, tuna, mackerel, and halibut. However, they are also found in krill and flax seed oil. The current recommendation is that 25%-35% of daily calories can come from fat sources, including saturated fats, which should be < 7%[206]. In addition, physical activity improves cardiorespiratory function, promotes the reduction of LDL and TG, and decreases insulin resistance (in both uninfected and HIV-1 patients)[207,208]. Physical exercise is effective in reducing TC and TG, reducing total fat mass and increasing muscle mass in HIV-1 infected patients with hypertriglyceridemia[45,119,208]. Additionally, physical exercise is associated with greater cardiovascular fitness, improved muscle strength and endurance, and the reduction of depression and anxiety. In addition, exercise lessens problems resulting from lipodystrophy (dyslipidemia, insulin resistance, and osteoporosis) and cardiovascular disease[208-210]. However, there are several factors that can directly influence the reduction of metabolic disorders observed in seropositive patients. The common observation of gastrointestinal diseases in patients in advanced stages of infection may offset the positive effects of a balanced dietary regimen[209,210].
PERSPECTIVES
The advances in antiretroviral therapy are clear, and practical results are observed in clinical practice where HIV-1 infected patients enjoy a better quality of life and a higher rate of survival, something unthinkable upon the discovery of HIV/AIDS in the early 1980s. New challenges for curing HIV-1 infection continue, and different approaches are the focus of several studies. The development of vaccines, the use of cell therapy, and the continuous development of new drugs that are more effective and have fewer side effects are obstacles that persist. Recently, approaches that target the intracellular trafficking of viral proteins and post-translational modifications of viral proteins have been considered as promising new treatments. Knowledge of the intracellular trafficking of viral proteins and the role of the polyprotein Gag of HIV-1 suggests that this process, once locked, would change the viral replication cycle by preventing formation of mature forms of the virus. Therefore, inhibitors could block viral maturation by interrupting the final stage of processing the Gag protein or by inhibiting intermolecular bond to the capsid protein immature.
This immature form, when connected to a new host cell, would suffer a disruption of the protein structure by the action of potent intracellular factors that restrict the subsequent phase of viral replication[211-213]. Additionally, viral PIs for HIV-1 that block viral maturation have become a therapeutic target. In addition to the maturation inhibitors that inhibit the formation of viral capsids, another issue of interest is that the cells themselves have intrinsic antiviral factors that may inhibit or restrict viral replication. One of the major families of cell restriction factors is tripartite motif 5 (TRIM5) composed of proteins that block retroviral infection, represented by two distinct forms of TRIM: TRIM5, which is expressed in most primates[214,215], and TRIM-Cyp, which is expressed in monkeys[216]. Both recognize the viral capsid but by different routes. The TRIM5 proteins are trimeric structures bind to one or two sites on the surface of the viral capsid and prevent the accumulation of reverse transcriptase. However, in late stages of viral replication, blocking is observed under some conditions of viral restriction[217]. The TRIM5α is associated with an accelerated degree of dissociation of the viral capsid, suggesting that this protein and its cofactors destabilize the structure of the capsid[218,219].
Other therapeutic factors that restrict the cellular antiviral protein APOBEC (apolipoprotein B mRNA-editing catalytic polypeptide) are the group of cytidine deaminases, which include APOBEC1 (A1), AID, APOBEC2 (A2), a subgroup of APOBEC3 (A3) proteins in humans and recently a protein, APOBEC4 (A4), expressed in some humans. These proteins have been presented as intracellular antiviral factors capable of blocking viral replication[220,221]. The function of the A3 gene remains unknown, but it has been reported that human A3G has the ability to block viral replication[222]. Similarly, A3G, A3B and A3F are also able to inhibit viral replication of HIV-1 and of other viruses, such as simian immunodeficiency virus and Hepatitis B virus[221-223]. Additionally, the tetherin protein, originally described as BST-2 (CD137/HM1.24), was identified as a new surface marker of malignant B cells and characterized as an antiviral intrinsic factor with the ability to restrict the exit of viral capsids from the membrane surface[224,225]. The same protein was also recognized as a target of the Vpu protein of HIV-1, a potential antagonist against tetherin[226,227]. Further studies on TRIM5, tetherin and APOBEC proteins, as well as potential inhibitors of the viral capsid maturation acting on the Gag polyprotein are necessary; however, the information obtained so far allow us to suggest that understanding the intracellular trafficking of viral proteins and mechanisms for post-translational modification of viral proteins could turn out to elucidate the complex replication cycle of HIV-1 from HIV-1 fusion in the host cell until the final stage of release of mature, infectious viral particles.
ACKNOWLEDGMENTS
We wish to thank Ana Stern, from the Laboratory of Genetics and Molecular Hematology, Laboratory of Genetics and Molecular Hematology (LIM31), University of São Paulo Medical School, for her contributions to our drawings graphics. Even, for all studies financially supported in the our laboratory with resources from The National Council of Technological and Scientific Development, the State of São Paulo Research Foundation and the National Institute of Science and Technology of Complex Fluids.
Footnotes
P- Reviewer: Ayieko J S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
Conflict-of-interest: The authors state that there are no conflicts of interest regarding the publication of this work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 28, 2014
First decision: November 27, 2014
Article in press: March 9, 2015
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passaes CP, Sáez-Cirión A. HIV cure research: advances and prospects. Virology. 2014;454-455:340–352. doi: 10.1016/j.virol.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Calvo KR, Daar ES. Antiretroviral therapy: treatment-experienced individuals. Infect Dis Clin North Am. 2014;28:439–456. doi: 10.1016/j.idc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Sobieszczyk ME, Talley AK, Wilkin T, Hammer SM. Advances in antiretroviral therapy. Top HIV Med. 2005;13:24–44. [PubMed] [Google Scholar]
- 5.Rigourd M, Lanchy JM, Le Grice SF, Ehresmann B, Ehresmann C, Marquet R. Inhibition of the initiation of HIV-1 reverse transcription by 3’-azido-3’-deoxythymidine. Comparison with elongation. J Biol Chem. 2000;275:26944–26951. doi: 10.1074/jbc.M003262200. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini J. Current status of the non-nucleoside reverse transcriptase inhibitors of human immunodeficiency virus type 1. Curr Top Med Chem. 2004;4:921–944. doi: 10.2174/1568026043388420. [DOI] [PubMed] [Google Scholar]
- 7.Randolph JT, DeGoey DA. Peptidomimetic inhibitors of HIV protease. Curr Top Med Chem. 2004;4:1079–1095. doi: 10.2174/1568026043388330. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto F, Kodama EN. Development of small molecule HIV-1 fusion inhibitors: linking biology to chemistry. Curr Pharm Des. 2013;19:1827–1834. doi: 10.2174/1381612811319100007. [DOI] [PubMed] [Google Scholar]
- 9.Boesecke C, Pett SL. Clinical studies with chemokine receptor-5 (CCR5)-inhibitors. Curr Opin HIV AIDS. 2012;7:456–462. doi: 10.1097/COH.0b013e328356e933. [DOI] [PubMed] [Google Scholar]
- 10.Arribas JR, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, Nguyen T, Ebrahimi R, White K, Piontkowsky D. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14:581–589. doi: 10.1016/S1473-3099(14)70782-0. [DOI] [PubMed] [Google Scholar]
- 11.Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003;361:726–735. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 12.Wohl DA, McComsey G, Tebas P, Brown TT, Glesby MJ, Reeds D, Shikuma C, Mulligan K, Dube M, Wininger D, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis. 2006;43:645–653. doi: 10.1086/507333. [DOI] [PubMed] [Google Scholar]
- 13.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sprinz E, Lazzaretti RK, Kuhmmer R, Ribeiro JP. Dyslipidemia in HIV-infected individuals. Braz J Infect Dis. 2010;14:575–588. doi: 10.1016/s1413-8670(10)70115-x. [DOI] [PubMed] [Google Scholar]
- 15.Grunfeld C. Dyslipidemia and its Treatment in HIV Infection. Top HIV Med. 2010;18:112–118. [PMC free article] [PubMed] [Google Scholar]
- 16.Freitas P, Carvalho D. Lipodystrophy: beyond generalization? Panminerva Med. 2013;55:253–268. [PubMed] [Google Scholar]
- 17.Behrens GM, Stoll M, Schmidt RE. Lipodystrophy syndrome in HIV infection: what is it, what causes it and how can it be managed? Drug Saf. 2000;23:57–76. doi: 10.2165/00002018-200023010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Campbell SM, Crowe SM, Mak J. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS. 2002;16:2253–2261. doi: 10.1097/00002030-200211220-00004. [DOI] [PubMed] [Google Scholar]
- 19.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley TM, Blay Puryear W, Gummuluru S, Viglianti GA. PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog. 2010;6:e1000981. doi: 10.1371/journal.ppat.1000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley TM, Viglianti GA. Nuclear receptor signaling inhibits HIV-1 replication in macrophages through multiple trans-repression mechanisms. J Virol. 2011;85:10834–10850. doi: 10.1128/JVI.00789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 23.Sankatsing RR, Wit FW, Vogel M, de Groot E, Brinkman K, Rockstroh JK, Kastelein JJ, Stroes ES, Reiss P. Increased carotid intima-media thickness in HIV patients treated with protease inhibitors as compared to non-nucleoside reverse transcriptase inhibitors. Atherosclerosis. 2009;202:589–595. doi: 10.1016/j.atherosclerosis.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Riddler SA, Li X, Otvos J, Post W, Palella F, Kingsley L, Visscher B, Jacobson LP, Sharrett AR. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 25.Qiu X, Liu ZP. Recent developments of peptidomimetic HIV-1 protease inhibitors. Curr Med Chem. 2011;18:4513–4537. doi: 10.2174/092986711797287566. [DOI] [PubMed] [Google Scholar]
- 26.Rawlings ND, Tolle DP, Barrett AJ. Evolutionary families of peptidase inhibitors. Biochem J. 2004;378:705–716. doi: 10.1042/BJ20031825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui DY. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42:81–92. doi: 10.1016/s0163-7827(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 28.Riddler SA, Li X, Chu H, Kingsley LA, Dobs A, Evans R, Palella F, Visscher B, Chmiel JS, Sharrett A. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med. 2007;8:280–287. doi: 10.1111/j.1468-1293.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 29.Arribas JR, Pulido F, Delgado R, Lorenzo A, Miralles P, Arranz A, González-García JJ, Cepeda C, Hervás R, Paño JR, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study) J Acquir Immune Defic Syndr. 2005;40:280–287. doi: 10.1097/01.qai.0000180077.59159.f4. [DOI] [PubMed] [Google Scholar]
- 30.Patick AK, Potts KE. Protease inhibitors as antiviral agents. Clin Microbiol Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helleberg M, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Nielsen L, Laursen A, Obel N, Gerstoft J. Decreasing rate of multiple treatment modifications among individuals who initiated antiretroviral therapy in 1997-2009 in the Danish HIV Cohort Study. Antivir Ther. 2012;Epub ahead of print doi: 10.3851/IMP2436. [DOI] [PubMed] [Google Scholar]
- 32.Carr A, Hudson J, Chuah J, Mallal S, Law M, Hoy J, Doong N, French M, Smith D, Cooper DA. HIV protease inhibitor substitution in patients with lipodystrophy: a randomized, controlled, open-label, multicentre study. AIDS. 2001;15:1811–1822. doi: 10.1097/00002030-200109280-00010. [DOI] [PubMed] [Google Scholar]
- 33.Prosperi MC, Fabbiani M, Fanti I, Zaccarelli M, Colafigli M, Mondi A, D’Avino A, Borghetti A, Cauda R, Di Giambenedetto S. Predictors of first-line antiretroviral therapy discontinuation due to drug-related adverse events in HIV-infected patients: a retrospective cohort study. BMC Infect Dis. 2012;12:296. doi: 10.1186/1471-2334-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 35.Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996;17:518–532. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen C, Lindhardt BO, Jensen BL, Lauritzen E, Gerstoft J, Dickmeiss E, Gaub J, Scheibel E, Karlsmark T. Clinical course of primary HIV infection: consequences for subsequent course of infection. BMJ. 1989;299:154–157. doi: 10.1136/bmj.299.6692.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose H, Woolley I, Hoy J, Dart A, Bryant B, Mijch A, Sviridov D. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism. 2006;55:90–95. doi: 10.1016/j.metabol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, Duprez D, Neaton JD. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201:285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zangerle R, Sarcletti M, Gallati H, Reibnegger G, Wachter H, Fuchs D. Decreased plasma concentrations of HDL cholesterol in HIV-infected individuals are associated with immune activation. J Acquir Immune Defic Syndr. 1994;7:1149–1156. [PubMed] [Google Scholar]
- 40.Chi D, Henry J, Kelley J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium. 2000;7:223–242. doi: 10.3109/10623320009072210. [DOI] [PubMed] [Google Scholar]
- 41.Schroecksnadel K, Frick B, Winkler C, Fuchs D. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr Vasc Pharmacol. 2006;4:205–213. doi: 10.2174/157016106777698379. [DOI] [PubMed] [Google Scholar]
- 42.Cobos Jiménez V, Booiman T, de Taeye SW, van Dort KA, Rits MA, Hamann J, Kootstra NA. Differential expression of HIV-1 interfering factors in monocyte-derived macrophages stimulated with polarizing cytokines or interferons. Sci Rep. 2012;2:763. doi: 10.1038/srep00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaidya SA, Korner C, Sirignano MN, Amero M, Bazner S, Rychert J, Allen TM, Rosenberg ES, Bosch RJ, Altfeld M. Tumor necrosis factor α is associated with viral control and early disease progression in patients with HIV type 1 infection. J Infect Dis. 2014;210:1042–1046. doi: 10.1093/infdis/jiu206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185:1–11. doi: 10.1016/j.atherosclerosis.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Penzak SR, Chuck SK. Hyperlipidemia associated with HIV protease inhibitor use: pathophysiology, prevalence, risk factors and treatment. Scand J Infect Dis. 2000;32:111–123. doi: 10.1080/003655400750045196. [DOI] [PubMed] [Google Scholar]
- 46.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Körner T, Stoll M, Schmidt RE. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 47.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, Lo JC, Schambelan M. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Savès M, Raffi F, Capeau J, Rozenbaum W, Ragnaud JM, Perronne C, Basdevant A, Leport C, Chêne G. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1396–1405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 49.Sekhar RV, Jahoor F, Pownall HJ, Rehman K, Gaubatz J, Iyer D, Balasubramanyam A. Severely dysregulated disposal of postprandial triacylglycerols exacerbates hypertriacylglycerolemia in HIV lipodystrophy syndrome. Am J Clin Nutr. 2005;81:1405–1410. doi: 10.1093/ajcn/81.6.1405. [DOI] [PubMed] [Google Scholar]
- 50.van Wijk JP, Cabezas MC, de Koning EJ, Rabelink TJ, van der Geest R, Hoepelman IM. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J Clin Endocrinol Metab. 2005;90:3575–3582. doi: 10.1210/jc.2004-2343. [DOI] [PubMed] [Google Scholar]
- 51.Miserez AR, Muller PY, Spaniol V. Indinavir inhibits sterol-regulatory element-binding protein-1c-dependent lipoprotein lipase and fatty acid synthase gene activations. AIDS. 2002;16:1587–1594. doi: 10.1097/00002030-200208160-00003. [DOI] [PubMed] [Google Scholar]
- 52.Abebe M, Kinde S, Belay G, Gebreegziabxier A, Challa F, Gebeyehu T, Nigussie P, Tegbaru B. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross-sectional comparative study. BMC Res Notes. 2014;7:380. doi: 10.1186/1756-0500-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crane HM, Grunfeld C, Willig JH, Mugavero MJ, Van Rompaey S, Moore R, Rodriguez B, Feldman BJ, Lederman MM, Saag MS, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011;25:185–195. doi: 10.1097/QAD.0b013e328341f925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 55.Hu C, Oliver JA, Goldberg MR, Al-Awqati Q. LRP: a new adhesion molecule for endothelial and smooth muscle cells. Am J Physiol Renal Physiol. 2001;281:F739–F750. doi: 10.1152/ajprenal.2001.281.4.F739. [DOI] [PubMed] [Google Scholar]
- 56.Zaera MG, Miró O, Pedrol E, Soler A, Picón M, Cardellach F, Casademont J, Nunes V. Mitochondrial involvement in antiretroviral therapy-related lipodystrophy. AIDS. 2001;15:1643–1651. doi: 10.1097/00002030-200109070-00006. [DOI] [PubMed] [Google Scholar]
- 57.Chattopadhyay K, Aldous CA. A brief review on human mtDNA mutations and NRTI-associated mtDNA toxicity and mutations. Mitochondrial DNA. 2014;Epub ahead of print:1–3. doi: 10.3109/19401736.2014.958728. [DOI] [PubMed] [Google Scholar]
- 58.Apostolova N, Blas-García A, Esplugues JV. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-γ inhibition. Trends Pharmacol Sci. 2011;32:715–725. doi: 10.1016/j.tips.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Arnedo M, Taffé P, Sahli R, Furrer H, Hirschel B, Elzi L, Weber R, Vernazza P, Bernasconi E, Darioli R, et al. Contribution of 20 single nucleotide polymorphisms of 13 genes to dyslipidemia associated with antiretroviral therapy. Pharmacogenet Genomics. 2007;17:755–764. doi: 10.1097/FPC.0b013e32814db8b7. [DOI] [PubMed] [Google Scholar]
- 60.Egaña-Gorroño L, Martínez E, Cormand B, Escribà T, Gatell J, Arnedo M. Impact of genetic factors on dyslipidemia in HIV-infected patients starting antiretroviral therapy. AIDS. 2013;27:529–538. doi: 10.1097/QAD.0b013e32835d0da1. [DOI] [PubMed] [Google Scholar]
- 61.Fauvel J, Bonnet E, Ruidavets JB, Ferrières J, Toffoletti A, Massip P, Chap H, Perret B. An interaction between apo C-III variants and protease inhibitors contributes to high triglyceride/low HDL levels in treated HIV patients. AIDS. 2001;15:2397–2406. doi: 10.1097/00002030-200112070-00007. [DOI] [PubMed] [Google Scholar]
- 62.Foulkes AS, Wohl DA, Frank I, Puleo E, Restine S, Wolfe ML, Dube MP, Tebas P, Reilly MP. Associations among race/ethnicity, ApoC-III genotypes, and lipids in HIV-1-infected individuals on antiretroviral therapy. PLoS Med. 2006;3:e52. doi: 10.1371/journal.pmed.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parra S, Alonso-Villaverde C, Coll B, Ferré N, Marsillach J, Aragonès G, Mackness M, Mackness B, Masana L, Joven J, et al. Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis. 2007;194:175–181. doi: 10.1016/j.atherosclerosis.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Maselli LM, da Cunha J, Gutierrez EB, Maranhão RC, Spada C, Bydlowski SP. Human paraoxonase-1 activity is related to the number of CD4+ T-cells and is restored by antiretroviral therapy in HIV-1-infected individuals. Dis Markers. 2014;2014:480201. doi: 10.1155/2014/480201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aviram M, Rosenblat M. Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol. 2005;16:393–399. doi: 10.1097/01.mol.0000174398.84185.0f. [DOI] [PubMed] [Google Scholar]
- 66.Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993;104:129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- 67.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 68.Assmann G, Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 69.Ngondi JL, Oben J, Forkah DM, Etame LH, Mbanya D. The effect of different combination therapies on oxidative stress markers in HIV infected patients in Cameroon. AIDS Res Ther. 2006;3:19. doi: 10.1186/1742-6405-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bydlowski SP, Yunker RL, Rymaszewski Z, Subbiah MT. Coffee extracts inhibit platelet aggregation in vivo and in vitro. Int J Vitam Nutr Res. 1987;57:217–223. [PubMed] [Google Scholar]
- 71.Bydlowski SP, Yunker RL, Subbiah MT. Ontogeny of 6-keto-PGF1 alpha synthesis in rabbit aorta and the effect of premature weaning. Am J Physiol. 1987;252:H14–H21. doi: 10.1152/ajpheart.1987.252.1.H14. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz JL, Fernandes LR, Levy D, Bydlowski SP. Interrelationship between ATP-binding cassette transporters and oxysterols. Biochem Pharmacol. 2013;86:80–88. doi: 10.1016/j.bcp.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 73.Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Kádár A, Glasz T. Development of atherosclerosis and plaque biology. Cardiovasc Surg. 2001;9:109–121. doi: 10.1016/s0967-2109(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 75.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Faviou E, Vourli G, Nounopoulos C, Zachari A, Dionyssiou-Asteriou A. Circulating oxidized low density lipoprotein, autoantibodies against them and homocysteine serum levels in diagnosis and estimation of severity of coronary artery disease. Free Radic Res. 2005;39:419–429. doi: 10.1080/10715760500072156. [DOI] [PubMed] [Google Scholar]
- 77.da Cunha J, Ferreira Maselli LM, Treitinger A, Monteiro AM, Gidlund M, Maranhão RC, Spada C, Bydlowski SP. Serum levels of IgG antibodies against oxidized LDL and atherogenic indices in HIV-1-infected patients treated with protease inhibitors. Clin Chem Lab Med. 2013;51:371–378. doi: 10.1515/cclm-2012-0225. [DOI] [PubMed] [Google Scholar]
- 78.Monnerat BZ, Cerutti Junior C, Caniçali SC, Motta TR. Clinical and biochemical evaluation of HIV-related lipodystrophy in an ambulatory population from the Hospital Universitário Cassiano Antonio de Morais, Vitória, ES, Brazil. Braz J Infect Dis. 2008;12:364–368. doi: 10.1590/s1413-86702008000400002. [DOI] [PubMed] [Google Scholar]
- 79.Cossarizza A, Riva A, Pinti M, Ammannato S, Fedeli P, Mussini C, Esposito R, Galli M. Increased mitochondrial DNA content in peripheral blood lymphocytes from HIV-infected patients with lipodystrophy. Antivir Ther. 2003;8:315–321. [PubMed] [Google Scholar]
- 80.Pinti M, Salomoni P, Cossarizza A. Anti-HIV drugs and the mitochondria. Biochim Biophys Acta. 2006;1757:700–707. doi: 10.1016/j.bbabio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Joly V, Flandre P, Meiffredy V, Leturque N, Harel M, Aboulker JP, Yeni P. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;16:2447–2454. doi: 10.1097/00002030-200212060-00010. [DOI] [PubMed] [Google Scholar]
- 82.de Waal R, Cohen K, Maartens G. Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction. PLoS One. 2013;8:e63623. doi: 10.1371/journal.pone.0063623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, Garren KW, Butcher DL, Rooney JF, Haas DW, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotler DP. HIV lipodystrophy etiology and pathogenesis. Body composition and metabolic alterations: etiology and pathogenesis. AIDS Read. 2003;13:S5–S9. [PubMed] [Google Scholar]
- 85.Mulligan K, Parker RA, Komarow L, Grinspoon SK, Tebas P, Robbins GK, Roubenoff R, Dubé MP. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. J Acquir Immune Defic Syndr. 2006;41:590–597. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 86.Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol (1985) 2003;95:1005–1010. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]
- 87.Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24:2127–2130. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Harmelen V, Lönnqvist F, Thörne A, Wennlund A, Large V, Reynisdottir S, Arner P. Noradrenaline-induced lipolysis in isolated mesenteric, omental and subcutaneous adipocytes from obese subjects. Int J Obes Relat Metab Disord. 1997;21:972–979. doi: 10.1038/sj.ijo.0800504. [DOI] [PubMed] [Google Scholar]
- 89.Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sax PE, Kumar P. Tolerability and safety of HIV protease inhibitors in adults. J Acquir Immune Defic Syndr. 2004;37:1111–1124. doi: 10.1097/01.qai.0000138420.38995.86. [DOI] [PubMed] [Google Scholar]
- 91.Brown TT, Chu H, Wang Z, Palella FJ, Kingsley L, Witt MD, Dobs AS. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007;21:1731–1738. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 92.Alves MD, Brites C, Sprinz E. HIV-associated lipodystrophy: a review from a Brazilian perspective. Ther Clin Risk Manag. 2014;10:559–566. doi: 10.2147/TCRM.S35075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Justman JE, Hoover DR, Shi Q, Tan T, Anastos K, Tien PC, Cole SR, Hyman C, Karim R, Weber K, et al. Longitudinal anthropometric patterns among HIV-infected and HIV-uninfected women. J Acquir Immune Defic Syndr. 2008;47:312–319. doi: 10.1097/QAI.0b013e318162f597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ene L, Goetghebuer T, Hainaut M, Peltier A, Toppet V, Levy J. Prevalence of lipodystrophy in HIV-infected children: a cross-sectional study. Eur J Pediatr. 2007;166:13–21. doi: 10.1007/s00431-006-0193-1. [DOI] [PubMed] [Google Scholar]
- 97.Beregszaszi M, Dollfus C, Levine M, Faye A, Deghmoun S, Bellal N, Houang M, Chevenne D, Hankard R, Bresson JL, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 98.Desai N, Mullen P, Mathur M. Lipodystrophy in pediatric HIV. Indian J Pediatr. 2008;75:351–354. doi: 10.1007/s12098-008-0037-2. [DOI] [PubMed] [Google Scholar]
- 99.Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, Luboinski J, Laville M, Maachi M, Girard PM, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 100.Martinez E, Gatell J. Metabolic abnormalities and use of HIV-1 protease inhibitors. Lancet. 1998;352:821–822. doi: 10.1016/S0140-6736(05)60719-2. [DOI] [PubMed] [Google Scholar]
- 101.Gutierrez AD, Balasubramanyam A. Dysregulation of glucose metabolism in HIV patients: epidemiology, mechanisms, and management. Endocrine. 2012;41:1–10. doi: 10.1007/s12020-011-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Davis B, Sax P, Stanley T, Wilson PW, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 103.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–317. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 105.Shlay JC, Sharma S, Peng G, Gibert CL, Grunfeld C. The effect of individual antiretroviral drugs on body composition in HIV-infected persons initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:298–304. doi: 10.1097/QAI.0b013e3181aa1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seigneur M, Constans J, Blann A, Renard M, Pellegrin JL, Amiral J, Boisseau M, Conri C. Soluble adhesion molecules and endothelial cell damage in HIV infected patients. Thromb Haemost. 1997;77:646–649. [PubMed] [Google Scholar]
- 107.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009;17:53–59. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 110.Sankalé JL, Tong Q, Hadigan CM, Tan G, Grinspoon SK, Kanki PJ, Hotamisligil GS. Regulation of adiponectin in adipocytes upon exposure to HIV-1. HIV Med. 2006;7:268–274. doi: 10.1111/j.1468-1293.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 111.Jones SP, Qazi N, Morelese J, Lebrecht D, Sutinen J, Yki-Jărvinen H, Back DJ, Pirmohamed M, Gazzard BG, Walker UA, et al. Assessment of adipokine expression and mitochondrial toxicity in HIV patients with lipoatrophy on stavudine- and zidovudine-containing regimens. J Acquir Immune Defic Syndr. 2005;40:565–572. doi: 10.1097/01.qai.0000187443.30838.3e. [DOI] [PubMed] [Google Scholar]
- 112.Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte Ad, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 113.Young J, Weber R, Rickenbach M, Furrer H, Bernasconi E, Hirschel B, Tarr PE, Vernazza P, Battegay M, Bucher HC. Lipid profiles for antiretroviral-naive patients starting PI- and NNRTI-based therapy in the Swiss HIV cohort study. Antivir Ther. 2005;10:585–591. [PubMed] [Google Scholar]
- 114.Cahn PE, Gatell JM, Squires K, Percival LD, Piliero PJ, Sanne IA, Shelton S, Lazzarin A, Odeshoo L, Kelleher TD, et al. Atazanavir--a once-daily HIV protease inhibitor that does not cause dyslipidemia in newly treated patients: results from two randomized clinical trials. J Int Assoc Physicians AIDS Care (Chic) 2004;3:92–98. doi: 10.1177/154510970400300304. [DOI] [PubMed] [Google Scholar]
- 115.Grover SA, Coupal L, Gilmore N, Mukherjee J. Impact of dyslipidemia associated with Highly Active Antiretroviral Therapy (HAART) on cardiovascular risk and life expectancy. Am J Cardiol. 2005;95:586–591. doi: 10.1016/j.amjcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Hicks CB, Cahn P, Cooper DA, Walmsley SL, Katlama C, Clotet B, Lazzarin A, Johnson MA, Neubacher D, Mayers D, et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368:466–475. doi: 10.1016/S0140-6736(06)69154-X. [DOI] [PubMed] [Google Scholar]
- 117.Calza L, Manfredi R, Colangeli V, Tampellini L, Sebastiani T, Pocaterra D, Chiodo F. Substitution of nevirapine or efavirenz for protease inhibitor versus lipid-lowering therapy for the management of dyslipidaemia. AIDS. 2005;19:1051–1058. doi: 10.1097/01.aids.0000174451.78497.8f. [DOI] [PubMed] [Google Scholar]
- 118.Clotet B, van der Valk M, Negredo E, Reiss P. Impact of nevirapine on lipid metabolism. J Acquir Immune Defic Syndr. 2003;34 Suppl 1:S79–S84. doi: 10.1097/00126334-200309011-00012. [DOI] [PubMed] [Google Scholar]
- 119.Dubé MP, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, Henry WK, Currier JS, Sprecher D, Glesby MJ. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 120.Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. Cholesterol reduction yields clinical benefit: impact of statin trials. Circulation. 1998;97:946–952. doi: 10.1161/01.cir.97.10.946. [DOI] [PubMed] [Google Scholar]
- 121.Favero GM, F Otuki M, Oliveira KA, Bohatch MS, Borelli P, Barros FE, Maria DA, Fernandes D, Bydlowski SP. Simvastatin impairs murine melanoma growth. Lipids Health Dis. 2010;9:142. doi: 10.1186/1476-511X-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Lara Janz F, Favero GM, Bohatch MS, Aguiar Debes A, Bydlowski SP. Simvastatin induces osteogenic differentiation in human amniotic fluid mesenchymal stem cells (AFMSC) Fundam Clin Pharmacol. 2014;28:211–216. doi: 10.1111/fcp.12006. [DOI] [PubMed] [Google Scholar]
- 123.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 124.Boccara F, Simon T, Lacombe K, Cohen A, Laloux B, Bozec E, Durant S, Girard PM, Laurent S, Boutouyrie P. Influence of pravastatin on carotid artery structure and function in dyslipidemic HIV-infected patients receiving antiretroviral therapy. AIDS. 2006;20:2395–2398. doi: 10.1097/QAD.0b013e32801120e3. [DOI] [PubMed] [Google Scholar]
- 125.Stein JH, Merwood MA, Bellehumeur JL, Aeschlimann SE, Korcarz CE, Underbakke GL, Mays ME, Sosman JM. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147:E18. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 126.Calza L, Colangeli V, Manfredi R, Legnani G, Tampellini L, Pocaterra D, Chiodo F. Rosuvastatin for the treatment of hyperlipidaemia in HIV-infected patients receiving protease inhibitors: a pilot study. AIDS. 2005;19:1103–1105. doi: 10.1097/01.aids.0000174458.86121.43. [DOI] [PubMed] [Google Scholar]
- 127.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, Alston B, Fang F, Kosel B, Aweeka F. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 128.Fichtenbaum CJ, Gerber JG. Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet. 2002;41:1195–1211. doi: 10.2165/00003088-200241140-00004. [DOI] [PubMed] [Google Scholar]
- 129.Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–370. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 130.Hare CB, Vu MP, Grunfeld C, Lampiris HW. Simvastatin-nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis. 2002;35:e111–e112. doi: 10.1086/344179. [DOI] [PubMed] [Google Scholar]
- 131.Moro H, Tsukada H, Tanuma A, Shirasaki A, Iino N, Nishibori T, Nishi S, Gejyo F. Rhabdomyolysis after simvastatin therapy in an HIV-infected patient with chronic renal failure. AIDS Patient Care STDS. 2004;18:687–690. doi: 10.1089/apc.2004.18.687. [DOI] [PubMed] [Google Scholar]
- 132.Moyle GJ, Lloyd M, Reynolds B, Baldwin C, Mandalia S, Gazzard BG. Dietary advice with or without pravastatin for the management of hypercholesterolaemia associated with protease inhibitor therapy. AIDS. 2001;15:1503–1508. doi: 10.1097/00002030-200108170-00007. [DOI] [PubMed] [Google Scholar]
- 133.Calza L, Manfredi R, Chiodo F. Use of fibrates in the management of hyperlipidemia in HIV-infected patients receiving HAART. Infection. 2002;30:26–31. doi: 10.1007/s15010-001-2052-3. [DOI] [PubMed] [Google Scholar]
- 134.Mastroianni CM, d’Ettorre G, Forcina G, Lichtner M, Corpolongo A, Coletta S, Vullo V. Rhabdomyolysis after cerivastatin-gemfibrozil therapy in an HIV-infected patient with protease inhibitor-related hyperlipidemia. AIDS. 2001;15:820–821. doi: 10.1097/00002030-200104130-00029. [DOI] [PubMed] [Google Scholar]
- 135.Thomas JC, Lopes-Virella MF, Del Bene VE, Cerveny JD, Taylor KB, McWhorter LS, Bultemeier NC. Use of fenofibrate in the management of protease inhibitor-associated lipid abnormalities. Pharmacotherapy. 2000;20:727–734. doi: 10.1592/phco.20.7.727.35179. [DOI] [PubMed] [Google Scholar]
- 136.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 137.Calza L, Manfredi R, Chiodo F. Statins and fibrates for the treatment of hyperlipidaemia in HIV-infected patients receiving HAART. AIDS. 2003;17:851–859. doi: 10.1097/00002030-200304110-00010. [DOI] [PubMed] [Google Scholar]
- 138.Visnegarwala F, Maldonado M, Sajja P, Minihan JL, Rodriguez-Barradas MC, Ong O, Lahart CJ, Hasan MQ, Balasubramanyam A, White AC. Lipid lowering effects of statins and fibrates in the management of HIV dyslipidemias associated with antiretroviral therapy in HIV clinical practice. J Infect. 2004;49:283–290. doi: 10.1016/j.jinf.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Miller J, Brown D, Amin J, Kent-Hughes J, Law M, Kaldor J, Cooper DA, Carr A. A randomized, double-blind study of gemfibrozil for the treatment of protease inhibitor-associated hypertriglyceridaemia. AIDS. 2002;16:2195–2200. doi: 10.1097/00002030-200211080-00012. [DOI] [PubMed] [Google Scholar]
- 140.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 141.Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, Glesby MJ, Torriani FJ, Yang Y, Owens SI, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21:757–767. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 142.Nolan DP, O’Connor MB, O’Connor C, Moriarty M, O’Leary A, Bergin C. HIV-associated dyslipidaemia among HIV antibody-positive patients in Ireland: prevalence and management strategies. Int J STD AIDS. 2010;21:75–76. doi: 10.1258/ijsa.2009.009364. [DOI] [PubMed] [Google Scholar]
- 143.Negredo E, Moltó J, Puig J, Cinquegrana D, Bonjoch A, Pérez-Alvarez N, López-Blázquez R, Blanco A, Clotet B, Rey-Joly C. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159–2164. doi: 10.1097/01.aids.0000247573.95880.db. [DOI] [PubMed] [Google Scholar]
- 144.Coll B, Aragonés G, Parra S, Alonso-Villaverde C, Masana L. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS. 2006;20:1675–1677. doi: 10.1097/01.aids.0000238418.43937.3b. [DOI] [PubMed] [Google Scholar]
- 145.Patel SB. Ezetimibe: a novel cholesterol-lowering agent that highlights novel physiologic pathways. Curr Cardiol Rep. 2004;6:439–442. doi: 10.1007/s11886-004-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stein E. Results of phase I/II clinical trials with ezetimibe, a novel selective cholesterol absorption inhibitor. Eur Heart J. 2001;3:E11–E16. [Google Scholar]
- 147.Wohl DA, Waters D, Simpson RJ, Richard S, Schnell A, Napravnik S, Keys J, Eron JJ, Hsue P. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2008;47:1105–1108. doi: 10.1086/592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chastain LM, Bain AM, Edwards KL, Bedimo R, Busti AJ. A retrospective study of the lipid-lowering efficacy and safety of ezetimibe added to hydroxy methylglutaryl coenzyme A reductase therapy in HIV-infected patients with hyperlipidemia. J Clin Lipidol. 2007;1:634–639. doi: 10.1016/j.jacl.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 149.Grandi AM, Nicolini E, Rizzi L, Caputo S, Annoni F, Cremona AM, Marchesi C, Guasti L, Maresca AM, Grossi P. Dyslipidemia in HIV-positive patients: a randomized, controlled, prospective study on ezetimibe+fenofibrate versus pravastatin monotherapy. J Int AIDS Soc. 2014;17:19004. doi: 10.7448/IAS.17.1.19004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Phillipson BE, Rothrock DW, Connor WE, Harris WS, Illingworth DR. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985;312:1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- 151.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 152.Gerber JG, Kitch DW, Fichtenbaum CJ, Zackin RA, Charles S, Hogg E, Acosta EP, Connick E, Wohl D, Kojic EM, et al. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr. 2008;47:459–466. doi: 10.1097/QAI.0b013e31815bace2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Truchis P, Kirstetter M, Perier A, Meunier C, Zucman D, Force G, Doll J, Katlama C, Rozenbaum W, Masson H, et al. Reduction in triglyceride level with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J Acquir Immune Defic Syndr. 2007;44:278–285. doi: 10.1097/QAI.0b013e31802c2f3d. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt EB. Marine N-3 polyunsaturated fatty acids and coronary heart disease: come a long way but expect more. Cell Mol Biol (Noisy-le-grand) 2010;56:1–3. [PubMed] [Google Scholar]
- 155.Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 156.Farmer JA. Nicotinic acid: a new look at an old drug. Curr Atheroscler Rep. 2009;11:87–92. doi: 10.1007/s11883-009-0014-x. [DOI] [PubMed] [Google Scholar]
- 157.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 158.Meyers CD, Carr MC, Park S, Brunzell JD. Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemia. Ann Intern Med. 2003;139:996–1002. doi: 10.7326/0003-4819-139-12-200312160-00009. [DOI] [PubMed] [Google Scholar]
- 159.Digby JE, Lee JM, Choudhury RP. Nicotinic acid and the prevention of coronary artery disease. Curr Opin Lipidol. 2009;20:321–326. doi: 10.1097/MOL.0b013e32832d3b9d. [DOI] [PubMed] [Google Scholar]
- 160.Gerber MT, Mondy KE, Yarasheski KE, Drechsler H, Claxton S, Stoneman J, DeMarco D, Powderly WG, Tebas P. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clin Infect Dis. 2004;39:419–425. doi: 10.1086/422144. [DOI] [PubMed] [Google Scholar]
- 161.Rader DJ. Effects of nonstatin lipid drug therapy on high-density lipoprotein metabolism. Am J Cardiol. 2003;91:18E–23E. doi: 10.1016/s0002-9149(02)03384-2. [DOI] [PubMed] [Google Scholar]
- 162.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 163.Fredriksen J, Ueland T, Dyrøy E, Halvorsen B, Melby K, Melbye L, Skalhegg BS, Bohov P, Skorve J, Berge RK, et al. Lipid-lowering and anti-inflammatory effects of tetradecylthioacetic acid in HIV-infected patients on highly active antiretroviral therapy. Eur J Clin Invest. 2004;34:709–715. doi: 10.1111/j.1365-2362.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 164.Hadigan C, Liebau J, Torriani M, Andersen R, Grinspoon S. Improved triglycerides and insulin sensitivity with 3 months of acipimox in human immunodeficiency virus-infected patients with hypertriglyceridemia. J Clin Endocrinol Metab. 2006;91:4438–4444. doi: 10.1210/jc.2006-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Keithley JK, Swanson B, Sha BE, Zeller JM, Kessler HA, Smith KY. A pilot study of the safety and efficacy of cholestin in treating HIV-related dyslipidemia. Nutrition. 2002;18:201–204. doi: 10.1016/s0899-9007(01)00688-8. [DOI] [PubMed] [Google Scholar]
- 166.Loignon M, Toma E. L-Carnitine for the treatment of highly active antiretroviral therapy-related hypertriglyceridemia in HIV-infected adults. AIDS. 2001;15:1194–1195. doi: 10.1097/00002030-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 167.Haraguchi K, Takeda S, Kubota Y, Kumamoto H, Tanaka H, Hamasaki T, Baba M, Paintsil E, Cheng YC. From the chemistry of epoxy-sugar nucleosides to the discovery of anti-HIV agent 4’-ethynylstavudine-Festinavir. Curr Pharm Des. 2013;19:1880–1897. doi: 10.2174/1381612811319100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Flexner C, Saag M. The antiretroviral drug pipeline: prospects and implications for future treatment research. Curr Opin HIV AIDS. 2013;8:572–578. doi: 10.1097/COH.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 169.Vere Hodge RA. Meeting report: 26th International Conference on Antiviral Research. Antiviral Res. 2013;100:276–285. doi: 10.1016/j.antiviral.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cahn P, Altclas J, Martins M, Losso M, Cassetti I, Cooper DA, Cox S. Antiviral activity of apricitabine in treatment-experienced HIV-1-infected patients with M184V who are failing combination therapy. HIV Med. 2011;12:334–342. doi: 10.1111/j.1468-1293.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 171.Cox S, Moore S, Southby J. Safety profile of apricitabine, a novel NRTI, during 24-week dosing in experienced HIV-1 infected patients. XVII International AIDS Conference (AIDS 2008); Mexico City. 2008. Abstract TUAB0106 pp. [Google Scholar]
- 172.Cahn P, Cassetti I, Wood R, Phanuphak P, Shiveley L, Bethell RC, Sawyer J. Efficacy and tolerability of 10-day monotherapy with apricitabine in antiretroviral-naive, HIV-infected patients. AIDS. 2006;20:1261–1268. doi: 10.1097/01.aids.0000232233.41877.63. [DOI] [PubMed] [Google Scholar]
- 173.Markowitz M, Zolopa A, Squires K, Ruane P, Coakley D, Kearney B, Zhong L, Wulfsohn M, Miller MD, Lee WA. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69:1362–1369. doi: 10.1093/jac/dkt532. [DOI] [PubMed] [Google Scholar]
- 174.Zolopa A, Ortiz R, Sax P. Comparative study of tenofovir alafenamide vs tenofovir disoproxil fumarate, each with elvitegravir, cobicistat, and emtricitabine, for HIV treatment. In: Abstracts of the Twentieth Conference on Retroviruses and Opportunistic Infections. Foundation for Retrovirology and Human Health, Alexandria, VA, USA. Atlanta, GA; 2013. p. Abstract 99LB. [Google Scholar]
- 175.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, Zhong L, Ramanathan S, Rhee MS, Fordyce MW, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 176.Ghosh RK, Ghosh SM, Chawla S. Recent advances in antiretroviral drugs. Expert Opin Pharmacother. 2011;12:31–46. doi: 10.1517/14656566.2010.509345. [DOI] [PubMed] [Google Scholar]
- 177.Desai M, Iyer G, Dikshit RK. Antiretroviral drugs: critical issues and recent advances. Indian J Pharmacol. 2012;44:288–298. doi: 10.4103/0253-7613.96296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Colucci P, Pottage JC, Robison H, Turgeon J, Ducharme MP. Effect of a single dose of ritonavir on the pharmacokinetic behavior of elvucitabine, a nucleoside reverse transcriptase inhibitor, administered in healthy volunteers. Antimicrob Agents Chemother. 2009;53:646–650. doi: 10.1128/AAC.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vingerhoets J, Azijn H, Fransen E, De Baere I, Smeulders L, Jochmans D, Andries K, Pauwels R, de Béthune MP. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol. 2005;79:12773–12782. doi: 10.1128/JVI.79.20.12773-12782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yanakakis LJ, Bumpus NN. Biotransformation of the antiretroviral drug etravirine: metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab Dispos. 2012;40:803–814. doi: 10.1124/dmd.111.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Casado JL, de Los Santos I, Del Palacio M, García-Fraile L, Pérez-Elías MJ, Sanz J, Moreno S. Lipid-lowering effect and efficacy after switching to etravirine in HIV-infected patients with intolerance to suppressive HAART. HIV Clin Trials. 2013;14:1–9. doi: 10.1310/hct1401-1. [DOI] [PubMed] [Google Scholar]
- 182.Poveda E, Garrido C, de Mendoza C, Corral A, Cobo J, González-Lahoz J, Soriano V. Prevalence of etravirine (TMC-125) resistance mutations in HIV-infected patients with prior experience of non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother. 2007;60:1409–1410. doi: 10.1093/jac/dkm372. [DOI] [PubMed] [Google Scholar]
- 183.Goebel F, Yakovlev A, Pozniak AL, Vinogradova E, Boogaerts G, Hoetelmans R, de Béthune MP, Peeters M, Woodfall B. Short-term antiviral activity of TMC278--a novel NNRTI--in treatment-naive HIV-1-infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- 184.Tebas P, Sension M, Arribas J, Duiculescu D, Florence E, Hung CC, Wilkin T, Vanveggel S, Stevens M, Deckx H. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-Infected adults treated with rilpivirine or Efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin Infect Dis. 2014;59:425–434. doi: 10.1093/cid/ciu234. [DOI] [PubMed] [Google Scholar]
- 185.Côté B, Burch JD, Asante-Appiah E, Bayly C, Bédard L, Blouin M, Campeau LC, Cauchon E, Chan M, Chefson A, et al. Discovery of MK-1439, an orally bioavailable non-nucleoside reverse transcriptase inhibitor potent against a wide range of resistant mutant HIV viruses. Bioorg Med Chem Lett. 2014;24:917–922. doi: 10.1016/j.bmcl.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 186.Saag MS. New and investigational antiretroviral drugs for HIV infection: mechanisms of action and early research findings. Top Antivir Med. 2012;20:162–167. [PMC free article] [PubMed] [Google Scholar]
- 187.Lai MT, Feng M, Falgueyret JP, Tawa P, Witmer M, DiStefano D, Li Y, Burch J, Sachs N, Lu M, et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014;58:1652–1663. doi: 10.1128/AAC.02403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chong H, Yao X, Zhang C, Cai L, Cui S, Wang Y, He Y. Biophysical property and broad anti-HIV activity of albuvirtide, a 3-maleimimidopropionic acid-modified peptide fusion inhibitor. PLoS One. 2012;7:e32599. doi: 10.1371/journal.pone.0032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He Y, Cheng J, Lu H, Li J, Hu J, Qi Z, Liu Z, Jiang S, Dai Q. Potent HIV fusion inhibitors against Enfuvirtide-resistant HIV-1 strains. Proc Natl Acad Sci USA. 2008;105:16332–16337. doi: 10.1073/pnas.0807335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nathan B, Bayley J, Waters L, Post FA. Cobicistat: a Novel Pharmacoenhancer for Co-Formulation with HIV Protease and Integrase Inhibitors. Infect Dis Ther. 2013;2:111–122. doi: 10.1007/s40121-013-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lepist EI, Phan TK, Roy A, Tong L, Maclennan K, Murray B, Ray AS. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56:5409–5413. doi: 10.1128/AAC.01089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, Liu HC, Zhong L, Yale K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 193.Bar-Magen T, Sloan RD, Donahue DA, Kuhl BD, Zabeida A, Xu H, Oliveira M, Hazuda DJ, Wainberg MA. Identification of novel mutations responsible for resistance to MK-2048, a second-generation HIV-1 integrase inhibitor. J Virol. 2010;84:9210–9216. doi: 10.1128/JVI.01164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bera S, Pandey KK, Vora AC, Grandgenett DP. HIV-1 integrase strand transfer inhibitors stabilize an integrase-single blunt-ended DNA complex. J Mol Biol. 2011;410:831–846. doi: 10.1016/j.jmb.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fader LD, Malenfant E, Parisien M, Carson R, Bilodeau F, Landry S, Pesant M, Brochu C, Morin S, Chabot C, et al. Discovery of BI 224436, a Noncatalytic Site Integrase Inhibitor (NCINI) of HIV-1. ACS Med Chem Lett. 2014;5:422–427. doi: 10.1021/ml500002n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fenwick C, Amad M, Bailey MD, Bethell R, Bös M, Bonneau P, Cordingley M, Coulombe R, Duan J, Edwards P, et al. Preclinical profile of BI 224436, a novel HIV-1 non-catalytic-site integrase inhibitor. Antimicrob Agents Chemother. 2014;58:3233–3244. doi: 10.1128/AAC.02719-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Taha H, Morgan J, Das A, Das S. Parenteral patent drug S/GSK1265744 has the potential to be an effective agent in pre-exposure prophylaxis against HIV infection. Recent Pat Antiinfect Drug Discov. 2013;8:213–218. doi: 10.2174/1574891x09666140417154727. [DOI] [PubMed] [Google Scholar]
- 198.Ford SL, Gould E, Chen S, Lou Y, Dumont E, Spreen W, Piscitelli S. Effects of etravirine on the pharmacokinetics of the integrase inhibitor S/GSK1265744. Antimicrob Agents Chemother. 2013;57:277–280. doi: 10.1128/AAC.01685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Loonam CR, Mullen A. Nutrition and the HIV-associated lipodystrophy syndrome. Nutr Res Rev. 2012;25:267–287. doi: 10.1017/S0954422411000138. [DOI] [PubMed] [Google Scholar]
- 200.Fields-Gardner C, Campa A. Position of the American Dietetic Association: Nutrition Intervention and Human Immunodeficiency Virus Infection. J Am Diet Assoc. 2010;110:1105–1119. doi: 10.1016/j.jada.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 201.Shah M, Tierney K, Adams-Huet B, Boonyavarakul A, Jacob K, Quittner C, Dinges W, Peterson D, Garg A. The role of diet, exercise and smoking in dyslipidaemia in HIV-infected patients with lipodystrophy. HIV Med. 2005;6:291–298. doi: 10.1111/j.1468-1293.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 202.Hadigan C, Jeste S, Anderson EJ, Tsay R, Cyr H, Grinspoon S. Modifiable dietary habits and their relation to metabolic abnormalities in men and women with human immunodeficiency virus infection and fat redistribution. Clin Infect Dis. 2001;33:710–717. doi: 10.1086/322680. [DOI] [PubMed] [Google Scholar]
- 203.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 204.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 205.Lazzaretti R. Nutritional intervention protects against the development of dyslipidemia in patients who start HAART: a randomized trial. XIV International AIDS Conference; Toronto, Canada. Aug 13–18, 2006. p. Abstract 2192713. [Google Scholar]
- 206.Schwellenbach LJ, Olson KL, McConnell KJ, Stolcpart RS, Nash JD, Merenich JA. The triglyceride-lowering effects of a modest dose of docosahexaenoic acid alone versus in combination with low dose eicosapentaenoic acid in patients with coronary artery disease and elevated triglycerides. J Am Coll Nutr. 2006;25:480–485. doi: 10.1080/07315724.2006.10719562. [DOI] [PubMed] [Google Scholar]
- 207.Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, Ordoñez-Llanos J, Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334. doi: 10.1016/s0021-9150(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 208.Wilson IB, Jacobson DL, Roubenoff R, Spiegelman D, Knox TA, Gorbach SL. Changes in lean body mass and total body weight are weakly associated with physical functioning in patients with HIV infection. HIV Med. 2002;3:263–270. doi: 10.1046/j.1468-1293.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 209.Lindegaard B, Hansen T, Hvid T, van Hall G, Plomgaard P, Ditlevsen S, Gerstoft J, Pedersen BK. The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. J Clin Endocrinol Metab. 2008;93:3860–3869. doi: 10.1210/jc.2007-2733. [DOI] [PubMed] [Google Scholar]
- 210.Roubenoff R, Wilson IB. Effect of resistance training on self-reported physical functioning in HIV infection. Med Sci Sports Exerc. 2001;33:1811–1817. doi: 10.1097/00005768-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 211.de Almeida ER, Reiche EM, Kallaur AP, Flauzino T, Watanabe MA. The roles of genetic polymorphisms and human immunodeficiency virus infection in lipid metabolism. Biomed Res Int. 2013;2013:836790. doi: 10.1155/2013/836790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Alcaraz LA, del Alamo M, Barrera FN, Mateu MG, Neira JL. Flexibility in HIV-1 assembly subunits: solution structure of the monomeric C-terminal domain of the capsid protein. Biophys J. 2007;93:1264–1276. doi: 10.1529/biophysj.106.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Forshey BM, von Schwedler U, Sundquist WI, Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J Virol. 2002;76:5667–5677. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Perron MJ, Stremlau M, Song B, Ulm W, Mulligan RC, Sodroski J. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci USA. 2004;101:11827–11832. doi: 10.1073/pnas.0403364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 216.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 217.Ohkura S, Yap MW, Sheldon T, Stoye JP. All three variable regions of the TRIM5alpha B30.2 domain can contribute to the specificity of retrovirus restriction. J Virol. 2006;80:8554–8565. doi: 10.1128/JVI.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nakayama EE, Shioda T. Role of Human TRIM5α in Intrinsic Immunity. Front Microbiol. 2012;3:97. doi: 10.3389/fmicb.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang J, Ge W, Zhan P, De Clercq E, Liu X. Retroviral restriction factors TRIM5α: therapeutic strategy to inhibit HIV-1 replication. Curr Med Chem. 2011;18:2649–2654. doi: 10.2174/092986711795933687. [DOI] [PubMed] [Google Scholar]
- 220.Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–296. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- 221.Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cullen BR. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J Virol. 2006;80:1067–1076. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazawa J, Oritani K, Itoh M, Ochi T, Ishihara K. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 225.Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Früh K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010;6:e1000913. doi: 10.1371/journal.ppat.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 227.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


