Abstract
Fluid homeostasis, blood pressure and redox balance in the kidney are regulated by an intricate interaction between local and systemic anti-natriuretic and natriuretic systems. Intrarenal dopamine plays a central role on this interactive network. By activating specific receptors, dopamine promotes sodium excretion and stimulates anti-oxidant and anti-inflammatory pathways. Different pathological scenarios where renal sodium excretion is dysregulated, as in nephrotic syndrome, hypertension and renal inflammation, can be associated with impaired action of renal dopamine including alteration in biosynthesis, dopamine receptor expression and signal transduction. Given its properties on the regulation of renal blood flow and sodium excretion, exogenous dopamine has been postulated as a potential therapeutic strategy to prevent renal failure in critically ill patients. The aim of this review is to update and discuss on the most recent findings about renal dopaminergic system and its role in several diseases involving the kidneys and the potential use of dopamine as a nephroprotective agent.
Keywords: Dopamine; Hypertension; kidney; Na+, K+-ATPase; Sodium; Oxidative stress; D1 receptors; D2 receptors; Renal failure; Edema
Core tip: Renal dopaminergic system is a local and independent natriuretic system necessary to maintain the normal balance of sodium and water, blood pressure and renal redox steady state. Different findings from experimental and clinical studies highlight the participation of renal dopamine in the pathophysiology of renal inflammation, hypertension, diabetic nephropathy and edema formation. Recent findings from experimental and clinical studies allow us to understand the complexity of this system as well as its possible contribution for future therapeutic strategies to prevent renal diseases.
INTRODUCTION
Kidney has all the bioenzymatic machinery necessary to possess a local dopaminergic system. Renal dopamine production depends on the precursor L-dihydroxyphenylalanine and dopa decarboxylase activity. Although dopa decarboxylase is present in high concentrations in the proximal tubular cells, L-dopa uptake by sodium dependent and independent transporters represents the limiting step for intrarenal dopamine synthesis[1-4]. Intrarenal dopamine can leave the cell through the apical border by a diffusional process, whereas plasma dopamine can be uptaken through the basal cell border by a saturable process[5]. The organic cation transporters (OCTs and OCTNs) have been postulated as potential carriers for dopamine through the tubular cells[5-7]. Finally, dopamine can be eliminated with urine flow or degraded by methylation (via catechol-O-methyl transferase or COMT) to 3-methoxytyramine, and by deamination (via monoamine oxidase or MAO) to 3,4-dihydroxyphenylacetic acid[8].
Several organs and systems are involved in the regulation of blood pressure. In particular, the kidney plays an essential role in the etiology of hypertension, but also represents a target organ vulnerable to hypertensive tissue damage. Alterations in renal tubule transport may be linked to the onset of hypertension in which dopamine could play an important role by affecting sodium handling on the proximal tubule[9]. Dopamine, as a major regulator of proximal tubule salt and water reabsorption, exerts its physiological actions through two families of receptors located in the tubular cell surface: D1-like receptors (D1 and D5) and D2-like receptors (D2, D3 and D4)[10-14]. Through activation of D1-like receptors, locally produced dopamine acts as an autocrine/paracrine natriuretic hormone by inhibiting the activity of both apical (e.g., Na+/H+ exchange, Cl-/HCO3- exchange and Na+/Pi cotransport) and basolateral (e.g., Na+, K+-ATPase and Na+/HCO3- cotransport) transporters[15-17]. The D1-like receptors, coupled to the stimulatory G proteins Gαs and Golf, are characterized by their capacity to activate adenylate cyclase, while D2-like receptors, coupled to the inhibitory G proteins Gαi and Go, are characterized by their capacity to inhibit adenylate cyclase and modulate ion channels[18,19]. The classical signaling pathway for D1-like receptors leads to activation of adenylate cyclase and increases cyclic adenosine 3′,5′-monophosphate (cAMP) levels and protein kinase A (PKA) activation. PKA may either directly phosphorylate a target protein, such as a sodium transporting protein, or initiate a cascade of phosphorylation events by phosphorylation and activation of dopamine and cAMP-regulated phosphoprotein DARPP32[1]. D1 receptor can also stimulate phospholipase Cβ1 in renal tubules[20]. On the other hand, D2-like receptors can suppress Akt (protein kinase B) signaling pathway[21]. Both types of dopamine receptors are also linked to mitogen-activated protein kinase activation through different pathways and can interact with each other, resulting in new signaling pathways. In renal cortical cells the interaction between D1 and D2 receptors increases phospholipase C stimulation[22].
At glomerular level, dopamine increases cAMP in mesangial and podocyte cells via D1-like receptor and inhibits angiotensin II-mediated contraction in mesangial cells[23,24]. Through this mechanism, dopamine induces depolarization of the podocyte that may lead to its relaxation[25]. These data suggest that dopamine can augment natriuresis and diuresis by increasing directly water and sodium filtration at glomerular level. Besides these effects on sodium and water homeostasis, it has been demonstrated that dopamine could exert anti-inflammatory and anti-oxidants properties by activation of D1-like and D2-like receptors[26-29].
To date, several studies reported that an intact dopaminergic system is required to maintain renal hemodynamic, fluid and electrolyte balance, redox steady state and blood pressure within a normal range and to antagonize the renin-angiotensin system[30,31]. In this way, alterations in dopamine production and its receptor number, function and/or post-translational modification are associated with different pathological scenarios like oxidative stress, genesis and progression of renal dysfunction, edema formation and genetic or essential hypertension. In clinical practice, dopamine is used as a first line vasoactive agent in patients with hemodynamic instability unresponsive to fluid therapy[32]. However, despite its diuretic and natriuretic properties, its clinical use in patients with renal failure remains controversial.
EFFECTS OF INTRARENAL DOPAMINE ON OXIDATIVE STRESS AND RENAL INFLAMMATION
The redox state of cells represents a balance between the generation of free radical/highly reactive species and the presence of antioxidant mechanisms. Acting as cellular messengers, reactive oxygen species (ROS) are implicated in the destruction of invading pathogens. Pathological situations involving overproduction of free radicals (e.g., atherosclerosis, hypertension, etc.) can lead to an increase in oxidative stress status, disrupting the normal cellular signaling mechanisms by alteration of the normal redox state of cells[33-36].
Oxidative stress and infiltration of inflammatory cells in the kidney are involved in the development of renal injury and hypertension[37]. Renal tubule cells produce both pro- and anti-inflammatory cytokines and chemokines, which are secreted across their apical and basolateral membranes, and are implicated in the development and progression of glomerular and tubular injury[38,39].
Several enzymes and receptors are involved in the regulation of the redox balance, including nicotinamide adenine dinucleotide reduced form (NADPH) oxidase and dopamine receptors (D1-like and D2-like receptors)[30]. Renal dopaminergic system represents a negative regulator of ROS. In this sense, D1, D2, and D5 receptors can exert antioxidant effects through direct and indirect inhibition of pro-oxidant enzymes, specifically, NADPH oxidase, and through stimulation of antioxidant enzymes such as superoxide dismutase (SOD) and heme-oxygenase (HO) among others, which can also indirectly inhibit NADPH oxidase activity[30,40]. Particularly, stimulation of D2 receptors in the kidney increases the expression of endogenous anti-oxidants, such as Parkinson protein 7 (PARK7 or DJ-1), paraoxonase 2, and HO-2, all of which can inhibit NADPH oxidase activity. By inhibition of phospholipase D2, the D5 receptor reduces NADPH oxidase activity. This receptor subtype also increases the expression of another antioxidant enzyme, HO-1. Finally, D1 receptor inhibits NADPH oxidase activity via PKA and PKC cross-talk and stimulates SOD, glutathione peroxidase, and glutamyl cysteine transferase activities (Figure 1)[30,40].
Figure 1.
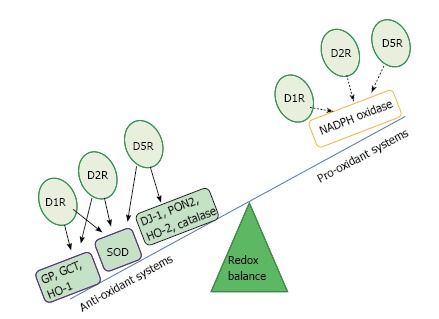
Dopamine receptors and regulation of redox state. Full line: Stimulation; Dotted line: Inhibition. D1R: Dopamine receptor subtype 1; D2R: Dopamine receptor subtype 2; D5R: Dopamine receptor subtype 5; NADPH: Nicotinamide adenine dinucleotide reduced form; SOD: Superoxide dismutase; HO-1: Heme oxygenase 1; HO-2: Heme oxygenase 2; PON2: Paraoxonase 2; DJ-1: Parkinson protein 7; GP: Glutathione peroxidase; GCT: Glutamyl cysteine transferase.
Additionally, it has been demonstrated that dopamine regulates the immune response and the inflammatory reaction by inhibiting the release of interferon γ (IFNγ), interleukin 2 (IL-2), and IL-4 and the lipopolysaccharide-stimulated production of IL-12p40 in immune cells[29,41]. Other authors showed that mice with intrarenal dopamine deficiency have increased oxidative stress and inflammatory cells infiltration; and that reduction in intrarenal dopamine synthesis is associated with increased detrimental effects of angiotensin II on renal injury[42,43].
Experimental studies demonstrated that mice lacking D2 receptor (-/-) have increased levels of blood pressure as well as renal expression of inflammatory factors and renal injury[44,45]. To clarify if decreased D2 receptor function increases the vulnerability to renal inflammation, independently of blood pressure, Zhang et al[46] carried out experiments with D2 receptor (-/-) mice, and demonstrated that the treatment with apocynin (an inhibitor of NADPH oxidase) normalized blood pressure levels and decreased oxidative stress, without affecting the expression of inflammatory factors. In support of this evidence, it was reported that short-term D2 receptor silencing in one kidney (leaving the other kidney intact) in mice, induced the overexpression of inflammatory factors and markers of renal injury in the treated kidney, without increasing blood pressure levels[46]. Altogether, these studies indicate that D2 receptor impairment may cause renal inflammation as a primary effect, contributing to the subsequent development of hypertension. Polymorphisms of the human D2 receptor gene may be of clinical relevance since reduction in D2 receptor expression and function may lead to renal damage and oxidative stress[45,46].
Although these evidences indicate that alteration of the renal dopaminergic system may be associated with increased blood pressure via oxidative stress, this seems not to be the only mechanism by which impairment of renal dopaminergic system could lead to hypertension.
ROLE OF RENAL DOPAMINERGIC SYSTEM IN THE PATHOPHYSIOLOGY OF HYPERTENSION
Essential hypertension affects 25% of the adult population and constitutes a major risk factor for stroke, myocardial infarction, and heart and kidney failure[47-50]. The etiology of hypertension is complex and involves both genetic and environmental factors[51]. Genome-wide association studies have been able to identify 2% of genetic factors believed to influence blood pressure[52-58]. However, the studies were not designed to identify predisposing genes engaged in a complex network of gene-gene and gene-environment interactions[59]. Many genes have been proposed to cause hypertension and more than one gene is undoubtedly involved. On the other hand, the impairment of renal dopaminergic system functionality in hypertension has been extensively studied in patients as well in experimental animals, and strong evidences indicate that the alteration of this system plays a pathophysiological role in the development of different types of hypertension[30,60] (Figure 2). Several animal experimental models of hypertension exhibit one or more defects related to the renal dopaminergic system, and vice versa, different models with impairment of this system are associated with the development of hypertension (Table 1).
Figure 2.
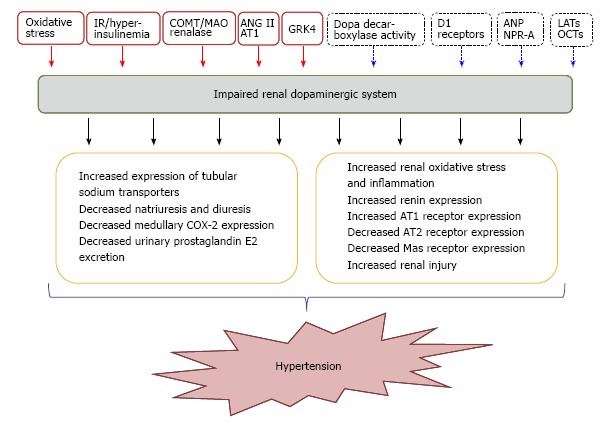
Impaired renal dopaminergic system and its association with hypertension. Red full squares and arrows indicate those factors that promote the impairment of renal dopamine; blue dotted squares and arrows indicate those factors that enhance renal dopaminergic system. IR: Insulin-resistance; COMT: Catechol-O-methyl-transferase; MAO: Monoamine-oxidase: AT1: Angiotensin II receptor subtype 1; AT2: Angiotensin II receptor subtype 2; COX-2: Cyclooxygenase type 2; ANP: Atrial natriuretic peptide; NPR-A: Natriuretic peptide receptor type A; LATs: L-aminoacids transporters; OCTs: Organic cationic transporters; GRK4: G-protein receptor kinase 4.
Table 1.
Renal dopaminergic system impairment in experimental hypertension
| Animal experimental model | Renal dopaminergic system impairment | Principal findings | Ref. |
| Spontaneously hypertensive rats | D1-like receptor function impairment caused by a defective coupling of the receptor with AC | Increased sodium reabsorption as a mechanism of hypertension | Ohbu et al[61] |
| Dahl salt-sensitive rats | D1-like receptor function impairment caused by a defective coupling of the receptor with AC | Prehypertensive Dahl salt-sensitive rats exhibit a blunted natriuretic response to dopamine compared with Dahl salt-resistant rats | Nishi et al[62] |
| DOCA salt-sensitive rats | Decreased renal dopamine production | Renal dopaminergic system is dominantly supressed in this model of hypertension | Iimura et al[63] |
| Dopamine receptor knockout mice | Defective D1-D2 like receptor/signal transduction | Impaired D1 and D2-like receptor signal pathway associated with development of hypertension | Banday et al[64] Zeng et al[65] Albrecht et al[66] |
| C57BL/6 mice | D1-like receptor function impairment associated with increased expression of GRK4 upon salt loading | Impaired ability to excrete a salt load with a resultant increase in blood pressure levels | Escano et al[67] |
| Mice with selective proximal tubule AADC deletion | Deletion of the kidney’s ability to generate dopamine is associated with unbuffered response to angiotensin II that leads to hypertension and decreased longevity in mice | Increased expression of tubular sodium transporters, decreased natriuresis and diuresis in response to L-Dopa, decreased medullary COX-2 expression and urinary prostaglandin E2 excretion, increased renin and AT1 receptor expression, decreased AT2 and Mas receptor expression, and finally salt-sensitive hypertension. | Zhang et al[42] |
| Old FBN rats | Reduction of G-protein coupling in response to D1R activation associated with exaggerated AT1 receptor activity | Increase of oxidative stress | Chugh et al[68] |
| Renalase knockout mice | Alteration of urinary dopamine concentration in luminal fluid and proximal tubular transport | Impaired sodium excretion with increased blood pressure | Desir[18] |
| 3/4 nephrectomized (3/4nx) rats | Decrease in urinary levels of dopamine and in renal AADC activity | A reduction in the natriuretic response to volume expansion with a time-dependent increase in both systolic and diastolic blood pressure | Moreira-Rodrigues et al[69] |
| Obese Zucker rats | Decrease in D1-like dopamine receptor binding sites and diminished activation of G proteins | Overproduction of ROS | Hussain et al[70] |
GRK4: G protein receptor kinase 4; D1R: Dopamine receptor subtype 1; COX-2: Cyclooxygenase type 2; AT1: Angiotensin II receptor subtype 1; AT2: Angiotensin II receptor subtype 2; ROS: Reactive oxygen species; AC: Adenylate cyclase; FBN: Fischer 344 x Brown Norway F1.
The interaction between renal dopamine and angiotensin II can take place at receptors level[18]. In this way, AT2 and D1 receptors cooperatively oppose the vasoconstrictor and antinatriuretic functions elicited by angiotensin II at AT1 receptor. It has been demonstrated that in vivo administration of fenoldopam (a highly selective D1-like receptor agonist) in sodium loaded Sprague Dawley rats induces the translocation of AT2 receptors from intracellular compartment to the apical plasma membranes[71]. This effect was confirmed by the fact that fenoldopam-induced natriuretic response was completely inhibited by the intrarenal co-infusion of the AT2 receptor antagonist PD123390[72]. Therefore, the alterations in D1 receptor-dependent translocation of AT2 receptor must be considered as a contributor factor for the initiation and progression of disease processes including hypertension. Blood pressure levels increase with aging, and alterations in both D1 and AT1 receptor functions are closely associated with the development of age-related hypertension[68,73-75]. Aging is a process associated with increase in oxidative stress and dysfunction of renal D1 and AT1 receptors[76-79]. Both receptors influence the activity of tubular Na+, K+-ATPase and contribute to maintain sodium homeostasis and blood pressure[77,79]. It has been reported in spontaneously hypertensive and obese Zucker rats an increase in oxidative stress and altered renal D1 and AT1 receptor functions[80-83].
Another possible mechanism involved in the impaired natriuretic effect in spontaneously hypertensive rats (SHRs) could be related to impaired endothelin B and D3 receptor interaction[84]. The endothelin B and dopamine receptors can interact to regulate renal function and blood pressure[85]. It has been demonstrated that activation of renal D3 receptor induces natriuresis and diuresis, but this effect is reduced in the presence of an endothelin B receptor antagonist, demonstrating that dopamine effects depends partially on endothelin B receptors. Moreover, stimulation of endothelin B receptor increases D3 receptor protein expression and vice versa in renal proximal tubule from Wistar Kyoto rats but not from SHRs[84,86]. Another study indicates that D3 receptors physically interact with proximal tubule endothelin B receptors and that the blunted natriuretic effect of dopamine in SHRs may be explained, in part, by abnormal D3/endothelin B receptor heterodimerization[85].
The interaction between prostanoids and renal dopamine on sodium and water excretion must also be considered. It has been demonstrated that the natriuretic response to dopamine was lower in Dahl salt-sensitive rats but this effect was reversed when chromosome 5 was transfered into these rats, leading to an increase of the renal expression of CYP4A protein and the production of 20-HETE[87]. Moreover, the inhibition of Na+, K+-ATPase activity by dopamine in the proximal tubule may be the result of the synergism between 20-HETE and the D1 signaling pathway[88]. In addition, other metabolites of arachidonic acid produced in the proximal tubule are epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids. As 20-HETE, epoxyeicosatrienoic acids can also regulate Na+, K+-ATPase activity and serve as second messengers for the natriuretic effects of dopamine. Since renal production of cytochrome P450 metabolites of arachidonic acid is altered in hypertension, a lower prostanoid synthesis may be involved in the impaired response to dopamine in this context[89].
Another protein that could be involved in the pathophysiology of hypertension is the novel amine oxidase, renalase[18]. Renalase is synthesized in the kidney with high expression in the proximal tubule, and then secreted into plasma and urine[19]. Renalase specifically degrades catecholamines, including dopamine. Recent findings indicate that renalase deficiency is associated with increased blood pressure and elevated circulating catecholamines[90,91]. Renalase expression depends on salt intake, and recombinant renalase exhibits a potent and prolonged hypotensive effect on blood pressure in Dahl salt-sensitive rats and rats with chronic kidney disease[92,93]. Urinary renalase metabolizes urinary catecholamines and it has been hypothesized that it might regulate dopamine concentration in the luminal fluid. However, the mechanisms of hypertension in animals with renalase deficiency and its relationship with the renal dopaminergic system are still unclear and deserve to be investigated in more detail.
Given its participation on sodium and water excretion and blood pressure regulation as well as its antioxidant properties in the kidney, alteration in renal dopaminergic system should also be considered in the pathophysiology of other diseases associated with kidney damage such as diabetic nephropathy.
RENAL DOPAMINE, HYPERINSULINEMIA AND PATHOPHYSIOLOGY OF DIABETIC NEPHROPATHY
Diabetes in cursive is the most prevalent cause of end-stage kidney disease and its incidence has increased by more than 50% in the last 10 years[94]. Diabetic nephropathy is associated with elevated glomerular filtration rate, enhanced tubular sodium reabsorption, reduced sodium delivery to the macula densa and also with progressive glomerular and tubulointerstitial injuries[95,96]. Diabetic nephropathy is a major cause of mortality in both types diabetes. Adults with type 1 and 2 diabetes demonstrate insulin resistance, which is associated with diabetic nephropathy[97,98]. Experimental studies of diabetic nephropathy helped to understand the pathophysiological mechanisms underlying the disease process and allowed to identify potential molecular targets for future pharmacological treatment. In this way, it has been demonstrated a decrease in endothelial nitric oxide synthase (eNOS) activity and renal dopamine production and an increase in Nrf-2, JAK/STAT and mTORC1 signaling as contributor factors in the development of diabetic nephropathy[99]. Alteration of mechanisms involving dopamine handling and signaling by the proximal tubule cells could lead to a progressive damage in diabetic nephropathy. In addition to the renin angiotensin system, alterations of renal cyclooxygenase-2 (COX-2) function are also involved in renal hemodynamic changes and structural abnormalities observed in diabetic nephropathy[100-102]. Previous findings showed that renal dopamine inhibits COX-2 expression in the macula densa, suggesting that the impairment of intrarenal dopaminergic system observed in diabetes, may contribute to reduce the luminal offer of sodium to this area, resulting in an elevated macula densa COX-2 expression[17,103,104] (Figure 3). Another experimental study carried out in mouse models of type 1 diabetes demonstrated that enhanced proximal tubule dopamine levels by deletion of COMT-/- gen was associated with substantial amelioration of early hyperfiltration, decreased macula densa COX-2 expression, decreased albuminuria and glomerulopathy, and inhibition of inflammation markers, oxidative stress, and fibrosis[31,99]. Conversely, depletion of proximal tubule dopamine levels by deletion of dopa decarboxylase gen in diabetic mice developed a marked increase in albuminuria as well as increment of mesangial expansion, renal macrophage infiltration, and renal nitrotyrosine levels[31]. These findings contribute to confirm the major role played by the intrarenal dopaminergic system on the development and progression of kidney injury caused by diabetes mellitus.
Figure 3.
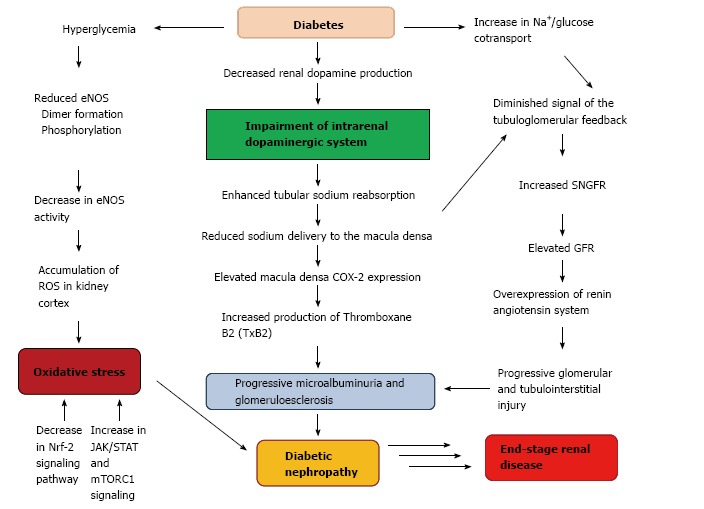
Association between diabetes and renal dopaminergic system in the pathophysiology of diabetic nephropathy. COX-2: Cyclooxygenase-2; GFR: Glomerular filtration rate; SNGFR: Single nephron glomerular filtration; eNOS: Endothelial nitric oxide synthase; ROS: Reactive oxygen species.
In renal proximal tubule cells, insulin and dopamine counter-regulate each other by opposing their effects on Na+, K+-ATPase activity[70]. Although insulin has been reported to enhance renal proximal tubule uptake of L-dopa in normal fed control rats, this regulatory mechanism is absent in cells isolated from animals fed with fructose, a model of insulin resistance. In addition, the chronic exposure of renal proximal tubule cells to insulin causes a reduction in D1 receptor abundance and uncoupling from G-proteins, which results in the impairment of the inhibitory effects of dopamine on Na+, K+-ATPase[70,105]. Hyperinsulinemic animals and patients with type 2 diabetes present a defective renal dopaminergic system[106]. In obese Zucker rats, a model of type 2 diabetes or in insulin-induced hypertension, renal D1 receptors are down-regulated and dopamine fails to produce diuresis and natriuresis[70]. Moreover, when these animals were treated with an insulin sensitizer (rosiglitazone), plasma insulin levels decreased and D1 receptor function were restored[107,108] (Figure 4). Based on these evidences, it is possible that the regulatory mechanisms of the renal dopaminergic system are impaired in diabetic nephropathy due to insulin resistance.
Figure 4.

Association between insulin resistance and impairment of renal dopaminergic system. Full lines: Stimulation; stripped lines: Inhibition.
Previous studies have shown that in both type 1 and type 2 diabetes, expression of renal D1 receptor gene was reduced above 50% through a down-regulation mechanism that involves the extracellular cAMP-adenosine signaling pathway[70,109]. An increase in intrarenal dopamine synthesis and the subsequent stimulation of vascular D1 receptors appear to prevent early glomerular hyperfiltration in diabetic rats[110]. Conversely to the D1 like receptors, selective antagonism of D2 like receptors was demonstrated to reverse glomerular hyperfiltration induced by experimental diabetic hyperglycemia[111]. Additionally, activation of the D3 receptor in rats caused diuresis, natriuresis and increased glomerular filtration[112]. To demonstrate the renoprotective effect of a D3 receptor antagonist (A-437203), hypertensive type 2 diabetic (SHR/N-cp) rats were used to evaluate the renal effects of D3 antagonism on glomerulosclerosis damage index, glomerular volume, desmin expression as marker of podocyte damage, and urinary albumin excretion[113]. The results of this study suggest that D3 receptor antagonism has a beneficial effect on renal morphology and albuminuria, which is comparable in magnitude to that of angiotensin-converting enzyme inhibitor treatment as the gold standard[113].
Despite its role in the pathophysiology of diabetic nephropathy, the potential clinical use of dopamine in this context is still matter of basic research. Nonetheless, the therapeutic use of dopamine is restricted to its dose-dependent actions on the cardiovascular system.
DOPAMINE AS NEPHROPROTECTIVE AGENT? EXPERIMENTAL AND CLINICAL EVIDENCES IN RENAL DYSFUNCTION
Dopamine represents an essential drug in intensive care units and is still used as a first line vasopressor agent especially in hypotensive adult patients refractory to fluid resuscitation[32]. Because of its interaction with different catecholamine receptors, the pharmacological profile of dopamine is dose dependent[114]. At low doses (0.3-5 μg/kg per minute), dopamine stimulates D1 and D2 receptors inducing natriuresis, diuresis and enhances renal blood flow by renal vasodilation. At higher doses, when adrenergic stimulation prevails, dopamine increases renal blood flow through stimulation of cardiac output[32,114,115]. In healthy adult volunteers, the administration of a low dose dopamine increases renal blood flow and induces natriuresis and diuresis[116,117]. For these reasons, a low-dose dopamine represents a therapeutic option to limit or prevent renal failure in critically ill patients by increasing renal blood flow[118]. Although a low dose dopamine appears to be able to increase urinary output in critically ill adult patients at risk of renal failure, a high number of clinical studies indicate that the administration of a low dose dopamine might not be able to exert any protective effect to prevent the onset or improve the course of an established acute renal failure, but on the contrary, its use may increase its risk[32,114,119-122]. Taken all together, the nephroprotective action of a low dose dopamine in critical ill patients remains to date controversial (Table 2).
Table 2.
Clinical studies providing evidence against/in support of clinical use of low dose dopamine
| Study design | Results | Ref. | |
| Against clinical use of low dose dopamine | The Australian and New Zealand Intensive Care Society (ANZICS): multicenter, randomized, double-blind, placebo-controlled | 324 patients with at least two criteria for the systemic inflammatory response syndrome and clinical evidence of early renal dysfunction: continuous intravenous infusion of low-dose dopamine (2 µg/kg per minute) did not attenuate the peak serum creatinine compared with placebo. There was no statistical difference in mortality between dopamine and placebo arms | Bellomo et al[119] |
| Meta-analysis study: 17 studies were randomized clinical trials (n = 854) | Low dose dopamine administration did not prevent mortality or the onset of acute renal failure, or the need for haemodialysis in clinically ill patients | Kellum and M Decker[120] | |
| Meta-analysis study: 15 randomized controlled studies | Dopamine administration did not present beneficial results in terms of serum creatinine changes and incidence of acute renal failure in clinically ill patients | Marik[121] | |
| Sepsis Occurrence in Acutely Ill Patients (SOAP): Cohort, multiple-center, observational study | Dopamine administration in shock patients, compared to patients who did not receive it, was associated with 20% increase in ICU and hospital mortality rates | Sakr et al[122] | |
| Renal Optimization Strategies Evaluation (ROSE) study: multicenter, double-blind, placebo-controlled randomized clinical trial | Low dose dopamine (2 µg/kg per minute) did not enhance decongestion or improved renal function when added to diuretic therapy in 360 patients with acute heart failure and renal dysfunction | Chen et al[123] | |
| In support of clinical use of low dose dopamine | Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial: randomized clinical trial | The addition of low-dose dopamine (5 μg/kg per minute) to low-dose furosemide (5 mg/h) was associated with improvement in renal function profile and potassium homeostasis at 24 h and it was equally effective as high-dose furosemide (20 mg/h) alone on subjective perception of dyspnoea in 60 patients with acute decompensated heart failure | Giamouzis et al[124] |
| Retrospective clinical study | Continuous infusion of furosemide in addition to low-dose dopamine compared to intermittent boluses of furosemide was less nephrotoxic and carried a lower readmission rate at 30 d in 116 patients with acute decompensated heart failure | Aziz et al[125] | |
| A prospective single-center randomized double-blind placebo controlled trial | The treatment with high-dose fenoldopam at 1 μg/kg per minute (short-acting D1 agonist) during cardiopulmonary bypass in 80 pediatric patients undergoing cardiac surgery for congenital heart disease significantly decreased urinary biomarkers of acute kidney injury (urinary neutrophil gelatinase-associated lipocaline and cystatin C levels) and also reduced the incidence of acute kidney injury in the postoperative period and the use of diuretics and vasodilators | Ricci et al[126] | |
| Clinical case finding | Low doses of ANP (0.0125 μg/kg per minute) with low dose dopamine (1.0 μg/kg per minute) in acute decompensated heart failure increased urine output, decreased heart rate, improved congestion with a reduced brain natriuretic peptide level, reduced serum creatinine and the levels of urinary liver-type fatty acid binding protein -a novel reno-tubular stress marker- and 8-hydroxydeoxyguanosine -an oxidative stress marker | Kamiya[127] | |
| Prospective randomized clinical study | Low dose dopamine infusion reduces renal tubular injury following cardiopulmonary bypass in 48 patients with normal or near normal baseline renal function | Sumeray et al[128] |
Although these evidences attempt to the clinical use of dopamine, some other findings support the clinical benefit of its use in different scenarios like cardio-renal syndrome, cardiopulmonary bypass and acute decompensated heart failure under treatment with atrial natriuretic peptide (ANP)[119,128,129]. Renal dysfunction is one of the most important co-morbidities in heart failure, being a potent predictor of cardiovascular complications and mortality[130]. This relationship is commonly termed cardio-renal syndrome, where both, cardiac and renal dysfunctions, share similar pathophysiology such as activation of the renin-angiotensin-aldosterone and sympathetic nervous systems, imbalance between nitric oxide and reactive oxygen species, and inflammation[131]. Clinical guidelines recommend the treatment of heart failure or renal failure separately without consensus about how managing patients with cardio-renal syndromes[129]. Its specific treatment points out to ameliorate decreased urine output and glomerular filtration rate, increased serum creatinine, and to prevent weight loss[132]. Recent studies in this clinical setting have focused on newer therapies, including renal protective dopamine[124,125,129] (Table 2).
Acute kidney injury after cardiac operations with cardiopulmonary bypass is a life-threatening complication, with a reported incidence of up to 36%[133]. To prevent this situation diuretics have been the mainstay to promote renal function and urine flow after pediatric cardiopulmonary bypass[134,135]. Fenoldopam mesylate is a short-acting D1 agonist that appears to improve renal function in clinical situations of reduced blood flow by enhancing renal blood flow[126,136]. Then, co-administration of both agents could be a reasonable therapeutic strategy to preserve renal function in this context (Table 2).
A primary therapeutic goal for acute heart failure is to achieve decongestion to relief symptoms without inducing renal dysfunction[137,138]. However, adult patients with acute heart failure and moderate or severe renal dysfunction are at risk for inadequate decongestion and enhanced renal dysfunction, both condition associated with worse prognosis[139]. Renal adjuvant therapies like dopamine could enhance decongestion and preserve renal function during treatment of acute heart failure. In this setting, combined therapy with ANP and dopamine might be useful to improve the management of acute decompensated heart failure without renal injury in patients who do not respond to ANP alone[127]. This beneficial interaction between both agents could be related to previous experimental findings that demonstrated that ANP stimulates dopamine uptake by tubular cells, reduces its catabolism and diminishes the turnover[140,141]. These effects may favor renal biodisponibility of dopamine in tubular cells and enhance overinhibition of renal Na+, K+-ATPase activity[141]. Nonetheless, future prospective studies are needed to affirm this suggestion (Table 2).
As it has been demonstrated in patients with heart failure, the interaction between two natriuretic hormones, such as ANP and dopamine, can also be present in other situations with increased extracellular fluid, such as nephrotic syndrome.
RENAL DOPAMINE IN THE PATHOGENESIS OF EDEMA FORMATION
Nephrotic syndrome exhibits increased proteinuria and enhanced sodium retention that contribute to edema and ascites formation[142]. Although reduced plasma volume and serum albumin concentration contribute to sodium retention in nephrotic syndrome, a primary intrarenal sodium handling abnormality could also be implicated in this clinical scenario[143]. In this way, experiments carried out in rats with puromycin aminonucleoside (PAN) induced nephrotic syndrome showed a blunted activity of the renal dopaminergic system evidenced by decreased urine dopamine, decreased availability of D1 receptor in renal proximal tubules and reduced dopa decarboxylase activity[144]. These findings were associated with an increase in Na+, K+-ATPase activity in renal proximal tubules[144]. On the other hand, renal dopamine and ANP are known to interact with each other in order to regulate sodium homeostasis[145]. The complex interaction between these two natriuretic systems is evidenced by the fact that ANP stimulates proximal tubular dopamine uptake through natriuretic peptide receptor type A (NPR-A), guanylate cyclase stimulation and protein kinase G (PKG) activation[4]. ANP also recruits silent D1 receptor from the interior of the renal tubular cells towards the plasma membrane where they become functionally active[146]. A recent work of Fernandes Cerqueira et al[147] demonstrated in rats with PAN-nephrotic syndrome that the increase of natriuresis and urinary cGMP excretion evoked by an acute volume expansion were blunted, despite the increased levels of circulating ANP, suggesting the unresponsiveness to ANP in this pathology. Treatment with a phosphodiesterase type 5 inhibitor (zaprinast) restored the excretion of cGMP and the natriuresis to similar levels of control rats, and increased the expression of D1 receptors in tubular cells[147]. This evidence indicates that D1 receptors are involved in ANP unresponsiveness observed in PAN-nephrotic syndrome, where the alteration of renal dopaminergic system represents a contributor factor for the edema and ascites formation (Figure 5).
Figure 5.
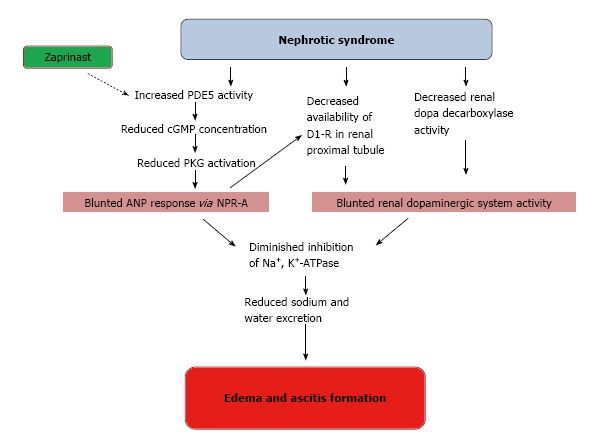
Impaired interactions between ANP and renal dopamine in nephrotic syndrome. Full lines: Stimulation; stripped lines: Inhibition. PDE5: Phosphodiesterase type 5.
Beyond its renal actions, dopamine effects on fluid homeostasis can also be exerted in other tissues. In this way, recent findings support a possible use of dopamine in edema resolution of pulmonary pathologies[148,149]. Acute lung injury and its severe form, the acute respiratory distress syndrome are prevalent causes of morbidity and mortality[150]. The outcome of patients with acute hypoxemic respiratory failure improves when lung epithelial function is restored and pulmonary edema resolves[151,152]. Pulmonary edema is cleared from the alveoli by active sodium transport, in which sodium enters into the cell via apical amiloride-sensitive sodium channels and pumped out from the cell via the basolaterally located Na+, K+-ATPase. Water follows the sodium gradients, resulting in alveolar fluid reabsorption[153]. Although dopamine inhibits Na+, K+-ATPase in the kidney and promotes natriuresis and diuresis, in alveolar cells dopamine increases, in a dose-dependent manner, lung edema clearance in rats by 40%-70% above the control clearance levels[154-156]. Experimental studies using models of lung injury have demonstrated that alveolar fluid clearance is impaired in parallel with decreased Na+, K+-ATPase function. In these lung injury models, dopamine (10-5 M) instilled into airspace increased alveolar fluid reabsorption by translocating preformed Na+, K+-ATPase pumps from intracellular pools (i.e., late endosomal compartment) to the cell plasma membrane in alveolar epithelial-type II cells[150,157,158]. This effect is produced through short-term and long-term fashion mechanisms. The short-term mechanism depends on the activation of D1 receptors since fenoldopam reproduces dopamine actions, meanwhile the long-term mechanism implies D2 receptors[159,160]. Accumulation of protein-rich alveolar edema fluid in acute lung injury is the result of an increased microvascular permeability[161-164]. Many experimental and human studies support the hypothesis that vascular endothelial growth factor (VEGF) plays a critical role in shaping the vascular barrier function in acute lung injury[165-169]. Although, D1 and D2 receptors are implicated in the synthesis and trafficking of Na+, K+-ATPase, the D2 receptor is also implicated in the regulation of VEGF-induced vascular permeability as well as angiogenesis[159,160,170-172]. This was confirmed by the fact that a D2 receptor agonist failed to reduce pulmonary edema in D2 receptor (-/-) mice, suggesting that dopamine acts through D2 receptor to inhibit pulmonary edema-associated vascular permeability, which is mediated through VEGF-VEGFR2 signaling and conveys protective effects in an acute lung injury model[148]. Although D1 and D2 receptors subtypes seem to be beneficial to reduce edema formation, D3 receptor appears to exert an opposite effect. Several clinical findings reported that pramipexole, a potent non ergoline agent with high affinity to D3 receptors and used for Parkinson’s disease, restless legs syndrome, resistant depression and bipolar depression, is associated with the development of pedal and chronic lower limb edema (with a frequency that ranges from 5% to 22.5%)[173-176]. This adverse effect disappears after the discontinuation of the drug[173-176]. Since dopamine is an important regulator of the sympathetic nervous system, aldosterone secretion, as well as adenosine triphosphate-mediated sodium/potassium channels, the peripheral effects of pramipexole at these levels could have a role[177,178]. However the relationship between D3 receptor agonism and edema formation remains unclear.
FUTURE PERSPECTIVES
An intact renal dopaminergic tonus is required for the maintenance of sodium homeostasis and normal blood pressure. By its anti-oxidative and anti-inflammatory properties, intrarenal dopamine plays a major role as a nephroprotective agent to prevent or ameliorate renal dysfunction. Oxidative stress or hyperinsulinemic states may decrease the number of functional dopaminergic receptors in the proximal tubules. In this way, it is worthwhile to test the effect of antioxidant drugs to enhance or restore the biodisponibility of these receptors. A recent observation that dopamine receptors availability in the plasma membrane may be regulated by other hormones, like ANP, could open up a possible therapeutic approach[179].
It has been emphasized the importance of endogenous dopamine and renal D1 receptor on the regulation of sodium and body fluid homeostasis. Although there is evidence that a defective renal dopaminergic system contributes to the development and maintenance of hypertension, it is still not clear what triggering factors cause the selective defects in the renal dopaminergic system. Some of these triggering factors could be an excess of sodium intake that could lead to an activation of intrarenal angiotensin II and increase in ROS, an increase in carbohydrate intake and a high fat diet, both factors that promotes an insulin resistance state. Furthermore, the renal dopaminergic system is sensitized by a high salt intake and volume expansion, which opens the question about how intrarenal sodium sensors may influence on renal dopamine bioavailability. This approach may lead to the development of new pharmacological strategies in conditions of salt retention and hypertension. Moreover, identification of abnormalities in different steps of crucial importance for the regulation of the renal dopaminergic tonus should provide additional molecular biological tools for the early diagnosis and treatment of pre-hypertensive patients.
The fact that dopamine exerts nonselective actions upon multiple dopaminergic and adrenergic receptors must be considered, and this, can limit its therapeutic use in renal diseases. The potential therapeutic use of exogenous dopamine and D1-like receptor agonists is limited to special conditions like critical ill patients who are at risk of kidney failure. However, given the controversial results from clinical studies the use of dopamine in this context must be examined more closely.
At last, further clinical studies must be carried out to confirm the participation of renal dopaminergic system in pathological contexts involving impaired sodium excretion as nephrotic syndrome or insulin resistance states.
CONCLUSION
Intrarenal dopamine represents a local natriuretic system with beneficial actions on blood pressure, oxidative stress and inflammation. Dopamine secreted into the tubular lumen acts via D1-like and D2-like receptors in an autocrine/paracrine manner to inhibit different tubular ion transporters and to regulate the production of reactive oxygen species and the inflammatory response. These renoprotective effects can be affected by situations that impair its integrity and functionality. The comprehension of the mechanisms by which renal dopaminergic system is involved in the pathogenesis and development of renal diseases may contribute to improve the diagnosis, evolution, prognosis and treatment of renal pathologies.
ACKNOWLEDGMENTS
We would like to thank Dr. Silvana L Della Penna, for her assistance in improving the manuscript writing.
Footnotes
P- Reviewer: Bjornstad P, Nihalani D, Riutta A S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
Supported by The ANPCYT, No. PICT 2012-1775, Universidad de Buenos Aires, Nos. UBACYT 20020110200048 and 20020130200105BA; and Sociedad Argentina de Hipertensión Arterial (Stimulus Grant for Reasearch on Hypertension 2014-2015).
Conflict-of-interest: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 30, 2014
First decision: July 18, 2014
Article in press: February 9, 2015
References
- 1.Silva E, Gomes P, Soares-da-Silva P. Increases in transepithelial vectorial Na+ transport facilitates Na+-dependent L-DOPA transport in renal OK cells. Life Sci. 2006;79:723–729. doi: 10.1016/j.lfs.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Bröer A, Klingel K, Kowalczuk S, Rasko JE, Cavanaugh J, Bröer S. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem. 2004;279:24467–24476. doi: 10.1074/jbc.M400904200. [DOI] [PubMed] [Google Scholar]
- 3.Pinho MJ, Serrão MP, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int. 2004;66:216–226. doi: 10.1111/j.1523-1755.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 4.Correa AH, Choi MR, Gironacci M, Valera MS, Fernández BE. Signaling pathways involved in atrial natriuretic factor and dopamine regulation of renal Na+, K+ -ATPase activity. Regul Pept. 2007;138:26–31. doi: 10.1016/j.regpep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 6.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 7.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 8.Armando I, Konkalmatt P, Felder RA, Jose PA. The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl Res. 2014 doi: 10.1016/j.trsl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Roles of renal proximal tubule transport in the pathogenesis of hypertension. Curr Hypertens Rev. 2013;9:148–155. doi: 10.2174/15734021113099990009. [DOI] [PubMed] [Google Scholar]
- 10.Sanada H, Xu J, Watanabe H, Jose PA, Felder RA. Differential expression and regulation of dopamine-1 (D-1) and dopamine-5 (D-5) receptor function in human kidney. Am J Hypertens. 2000;13:156. [Google Scholar]
- 11.Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: control of renal sodium excretion and blood pressure. Hypertension. 2013;61:673–680. doi: 10.1161/HYPERTENSIONAHA.111.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 13.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 14.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 15.Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3- exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–F1126. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- 16.Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desir GV. Role of renalase in the regulation of blood pressure and the renal dopamine system. Curr Opin Nephrol Hypertens. 2011;20:31–36. doi: 10.1097/MNH.0b013e3283412721. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–1282. doi: 10.1161/CIRCULATIONAHA.107.732032. [DOI] [PubMed] [Google Scholar]
- 20.Yu PY, Asico LD, Eisner GM, Jose PA. Differential regulation of renal phospholipase C isoforms by catecholamines. J Clin Invest. 1995;95:304–308. doi: 10.1172/JCI117656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza BR, Romano-Silva MA, Tropepe V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci. 2011;31:5512–5525. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack A. Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE. 2004;2004:pe50. doi: 10.1126/stke.2552004pe50. [DOI] [PubMed] [Google Scholar]
- 23.Bek M, Fischer KG, Greiber S, Hupfer C, Mundel P, Pavenstädt H. Dopamine depolarizes podocytes via a D1-like receptor. Nephrol Dial Transplant. 1999;14:581–587. doi: 10.1093/ndt/14.3.581. [DOI] [PubMed] [Google Scholar]
- 24.Barnett R, Singhal PC, Scharschmidt LA, Schlondorff D. Dopamine attenuates the contractile response to angiotensin II in isolated rat glomeruli and cultured mesangial cells. Circ Res. 1986;59:529–533. doi: 10.1161/01.res.59.5.529. [DOI] [PubMed] [Google Scholar]
- 25.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 27.Yu P, Han W, Villar VA, Li H, Arnaldo FB, Concepcion GP, Felder RA, Quinn MT, Jose PA. Dopamine D1 receptor-mediated inhibition of NADPH oxidase activity in human kidney cells occurs via protein kinase A-protein kinase C cross talk. Free Radic Biol Med. 2011;50:832–840. doi: 10.1016/j.freeradbiomed.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N, Tanaka S, Katsuki I, Matsushita S, Tanaka Y. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2011;186:3745–3752. doi: 10.4049/jimmunol.1002475. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–1026. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 30.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang MZ, Yao B, Yang S, Yang H, Wang S, Fan X, Yin H, Fogo AB, Moeckel GW, Harris RC. Intrarenal dopamine inhibits progression of diabetic nephropathy. Diabetes. 2012;61:2575–2584. doi: 10.2337/db12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marinosci GZ, De Robertis E, De Benedictis G, Piazza O. Dopamine Use in Intensive Care: Are We Ready to Turn it Down? Transl Med UniSa. 2012;4:90–94. [PMC free article] [PubMed] [Google Scholar]
- 33.Yu P, Han W, Villar VA, Yang Y, Lu Q, Lee H, Li F, Quinn MT, Gildea JJ, Felder RA, et al. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol. 2014;2:570–579. doi: 10.1016/j.redox.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bretón-Romero R, Lamas S. Hydrogen peroxide signaling mediator in the activation of p38 MAPK in vascular endothelial cells. Methods Enzymol. 2013;528:49–59. doi: 10.1016/B978-0-12-405881-1.00003-3. [DOI] [PubMed] [Google Scholar]
- 35.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res. 2013;112:382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segerer S, Schlöndorff D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol. 2007;27:260–274. doi: 10.1016/j.semnephrol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Tay YC, Harris DC. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 2004;66:655–662. doi: 10.1111/j.1523-1755.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, D Asico L, Yu P, Grandy DK, Felder RA, Armando I, et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med. 2012;53:437–446. doi: 10.1016/j.freeradbiomed.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haskó G, Szabó C, Németh ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. J Neuroimmunol. 2002;122:34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol. 2012;302:F742–F749. doi: 10.1152/ajprenal.00583.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 45.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, et al. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One. 2012;7:e38745. doi: 10.1371/journal.pone.0038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruilope L, Kjeldsen SE, de la Sierra A, Mancia G, Ruggenenti P, Stergiou GS, Bakris GL, Giles TD. The kidney and cardiovascular risk--implications for management: a consensus statement from the European Society of Hypertension. Blood Press. 2007;16:72–79. doi: 10.1080/08037050701338985. [DOI] [PubMed] [Google Scholar]
- 48.Erdine S, Aran SN. Current status of hypertension control around the world. Clin Exp Hypertens. 2004;26:731–738. doi: 10.1081/ceh-200032144. [DOI] [PubMed] [Google Scholar]
- 49.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 50.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 51.Zeng C, Villar VA, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens. 2009;31:156–178. doi: 10.1080/10641960802621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrap SB. Blood pressure genetics: time to focus. J Am Soc Hypertens. 2009;3:231–237. doi: 10.1016/j.jash.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, Zhou J, Lashley K, Chen Y, Christman M, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5:e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 55.Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, Rice K, Verwoert GC, Launer LJ, Gudnason V, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, Shi X, Pan L, Rampersaud E, Shen H, et al. From the Cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore JH, Williams SM. New strategies for identifying gene-gene interactions in hypertension. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol. 2008;23:2131–2146. doi: 10.1007/s00467-008-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohbu K, Kaskel FJ, Kinoshita S, Felder RA. Dopamine-1 receptors in the proximal convoluted tubule of Dahl rats: defective coupling to adenylate cyclase. Am J Physiol. 1995;268:R231–R235. doi: 10.1152/ajpregu.1995.268.1.R231. [DOI] [PubMed] [Google Scholar]
- 62.Nishi A, Eklöf AC, Bertorello AM, Aperia A. Dopamine regulation of renal Na+,K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21:767–771. doi: 10.1161/01.hyp.21.6.767. [DOI] [PubMed] [Google Scholar]
- 63.Iimura O, Ura N, Nakagawa M. Comparative hypertensionology-renal dopaminergic activity in experimental hypertensive rats. Clin Exp Hypertens. 1997;19:117–130. doi: 10.3109/10641969709080809. [DOI] [PubMed] [Google Scholar]
- 64.Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep. 2008;10:268–275. doi: 10.1007/s11906-008-0051-9. [DOI] [PubMed] [Google Scholar]
- 65.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest. 1996;97:2283–2288. doi: 10.1172/JCI118670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660–R1669. doi: 10.1152/ajpregu.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–1036. doi: 10.1161/HYPERTENSIONAHA.112.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreira-Rodrigues M, Sampaio-Maia B, Pestana M. Renal dopaminergic system activity in rat remnant kidney up to twenty-six weeks after surgery. Life Sci. 2009;84:409–414. doi: 10.1016/j.lfs.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 70.Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese zucker rats. Hypertension. 1999;34:1091–1096. doi: 10.1161/01.hyp.34.5.1091. [DOI] [PubMed] [Google Scholar]
- 71.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D(1) and angiotensin type 2 receptor interaction in natriuresis. Hypertension. 2012;59:437–445. doi: 10.1161/HYPERTENSIONAHA.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 73.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 74.Pestana M. Hypertension in the elderly. Int Urol Nephrol. 2001;33:563–569. doi: 10.1023/a:1019552602793. [DOI] [PubMed] [Google Scholar]
- 75.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 76.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int. 2000;58:712–720. doi: 10.1046/j.1523-1755.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 78.Zemel MB, Sowers JR. Salt sensitivity and systemic hypertension in the elderly. Am J Cardiol. 1988;61:7H–12H. doi: 10.1016/0002-9149(88)91098-3. [DOI] [PubMed] [Google Scholar]
- 79.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. 1993;22:842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 80.White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–1665. doi: 10.1097/00004872-199816110-00013. [DOI] [PubMed] [Google Scholar]
- 81.Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens. 2003;25:35–47. doi: 10.1081/ceh-120017739. [DOI] [PubMed] [Google Scholar]
- 82.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54:2219–2226. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- 83.Javkhedkar AA, Lokhandwala MF, Banday AA. Higher renal AT1 receptor affinity exaggerates Ang II induced Na/K-ATPase stimulation in spontaneously hypertensive rats. FASEB J. 2010;24:707. [Google Scholar]
- 84.Zhang Y, Fu C, Ren H, He D, Wang X, Asico LD, Jose PA, Zeng C. Impaired stimulatory effect of ETB receptor on D receptor in immortalized renal proximal tubule cells of spontaneously hypertensive rats. Kidney Blood Press Res. 2011;34:75–82. doi: 10.1159/000323135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, et al. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Wang X, Yang C, et al. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens. 2009;22:877–883. doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez MM, Gonzalez D, Williams JM, Roman RJ, Nowicki S. Inhibitors of 20-hydroxyeicosatetraenoic acid (20-HETE) formation attenuate the natriuretic effect of dopamine. Eur J Pharmacol. 2012;686:97–103. doi: 10.1016/j.ejphar.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirchheimer C, Mendez CF, Acquier A, Nowicki S. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+-K+-ATPase activity in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F1435–F1442. doi: 10.1152/ajprenal.00176.2006. [DOI] [PubMed] [Google Scholar]
- 89.Maier KG, Roman RJ. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens. 2001;10:81–87. doi: 10.1097/00041552-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 90.Schlaich M, Socratous F, Eikelis N. Renalase plasma levels are associated with systolic blood pressure in patients with resistant hypertension: 9C.01. J Hypertens. 2010;28:437. [Google Scholar]
- 91.Desir GV, Wu Y, Wang P. Renalase deficiency increases sympathetic tone and causes hypertension. J Am Soc Nephrol. 2008 Available from: http: //www.asn-online.org/education_and_meetings/renal_week/archives/ [Google Scholar]
- 92.Pestana M, Sampaio-Maia B, Moreira-Rodrigues M. Expression of renalase in a 3/4 nephrectomy rat model. NDT Plus. 2009;2:55. [Google Scholar]
- 93.Desir G, Tang L, Wang P, Li G, Velazquez H. Antihypertensive effect of recombinant renalase in Dahl salt sensitive (DSS) rats. Available from: http: //www.asn-online.org/education_and_meetings/renal_week/archives/
- 94.US Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 95.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 96.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 97.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, Nadeau KJ. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care. 2014;37:3033–3039. doi: 10.2337/dc14-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, Maahs DM. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013;36:3678–3683. doi: 10.2337/dc13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brosius FC, Alpers CE. New targets for treatment of diabetic nephropathy: what we have learned from animal models. Curr Opin Nephrol Hypertens. 2013;22:17–25. doi: 10.1097/MNH.0b013e32835b3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Komers R, Lindsley JN, Oyama TT, Anderson S. Cyclo-oxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clin Exp Pharmacol Physiol. 2007;34:36–41. doi: 10.1111/j.1440-1681.2007.04534.x. [DOI] [PubMed] [Google Scholar]
- 101.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001;107:889–898. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62:929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 103.Carranza A, Karabatas L, Barontini M, Armando I. Decreased tubular uptake of L-3,4-dihydroxyphenylalanine in streptozotocin-induced diabetic rats. Horm Res. 2001;55:282–287. doi: 10.1159/000050014. [DOI] [PubMed] [Google Scholar]
- 104.Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2004;286:F451–F457. doi: 10.1152/ajprenal.00227.2003. [DOI] [PubMed] [Google Scholar]
- 105.Carranza A, Musolino PL, Villar M, Nowicki S. Signaling cascade of insulin-induced stimulation of L-dopa uptake in renal proximal tubule cells. Am J Physiol Cell Physiol. 2008;295:C1602–C1609. doi: 10.1152/ajpcell.00090.2008. [DOI] [PubMed] [Google Scholar]
- 106.Tsuchida H, Imai G, Shima Y, Satoh T, Owada S. Mechanism of sodium load-induced hypertension in non-insulin dependent diabetes mellitus model rats: defective dopaminergic system to inhibit Na-K-ATPase activity in renal epithelial cells. Hypertens Res. 2001;24:127–135. doi: 10.1291/hypres.24.127. [DOI] [PubMed] [Google Scholar]
- 107.Umrani DN, Banday AA, Hussain T, Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese Zucker rats. Hypertension. 2002;40:880–885. doi: 10.1161/01.hyp.0000039963.01288.d3. [DOI] [PubMed] [Google Scholar]
- 108.Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–F304. doi: 10.1152/ajprenal.00362.2004. [DOI] [PubMed] [Google Scholar]
- 109.Kuzhikandathil EV, Clark L, Li Y. The extracellular cAMP-adenosine pathway regulates expression of renal D1 dopamine receptors in diabetic rats. J Biol Chem. 2011;286:32454–32463. doi: 10.1074/jbc.M111.268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barthelmebs M, Mayer P, Thomas A, Grima M, Imbs JL. Pathophysiological role of dopamine in the kidney: effects in diabetes mellitus and after contralateral nephrectomy. Hypertens Res. 1995;18 Suppl 1:S131–S136. doi: 10.1291/hypres.18.supplementi_s131. [DOI] [PubMed] [Google Scholar]
- 111.Luippold G, Beilharz M, Mühlbauer B. Reduction of glomerular hyperfiltration by dopamine D(2)-like receptor blockade in experimental diabetes mellitus. Nephrol Dial Transplant. 2001;16:1350–1356. doi: 10.1093/ndt/16.7.1350. [DOI] [PubMed] [Google Scholar]
- 112.Luippold G, Schneider S, Vallon V, Osswald H, Mühlbauer B. Postglomerular vasoconstriction induced by dopamine D(3) receptor activation in anesthetized rats. Am J Physiol Renal Physiol. 2000;278:F570–F575. doi: 10.1152/ajprenal.2000.278.4.F570. [DOI] [PubMed] [Google Scholar]
- 113.Gross ML, Koch A, Mühlbauer B, Adamczak M, Ziebart H, Drescher K, Gross G, Berger I, Amann KU, Ritz E. Renoprotective effect of a dopamine D3 receptor antagonist in experimental type II diabetes. Lab Invest. 2006;86:262–274. doi: 10.1038/labinvest.3700383. [DOI] [PubMed] [Google Scholar]
- 114.Chamorro C, Romera MA, Martinez-Melgar JL, Pardo C, Silva JA. Dopamine dose and renal damage. Lancet. 2001;357:1707–1708. doi: 10.1016/S0140-6736(00)04838-8. [DOI] [PubMed] [Google Scholar]
- 115.Lee MR. Dopamine and the kidney: ten years on. Clin Sci (Lond) 1993;84:357–375. doi: 10.1042/cs0840357. [DOI] [PubMed] [Google Scholar]
- 116.Mcdonald RH, Goldberg LI, Mcnay JL, Tuttle EP. Effect of dopamine in man: augmentation of sodium excretion, glomerular filtration rate, and renal plasma flow. J Clin Invest. 1964;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hollenberg NK, Adams DF, Mendell P, Abrams HL, Merrill JP. Renal vascular responses to dopamine: haemodynamic and angiographic observations in normal man. Clin Sci Mol Med. 1973;45:733–742. doi: 10.1042/cs0450733. [DOI] [PubMed] [Google Scholar]
- 118.Cuthbertson BH, Noble DW. Dopamine in oliguria. BMJ. 1997;314:690–691. doi: 10.1136/bmj.314.7082.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 120.Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526–1531. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Marik PE. Low-dose dopamine: a systematic review. Intensive Care Med. 2002;28:877–883. doi: 10.1007/s00134-002-1346-y. [DOI] [PubMed] [Google Scholar]
- 122.Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, De Backer D, Payen D. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med. 2006;34:589–597. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 123.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 125.Aziz EF, Alviar CL, Herzog E, Cordova JP, Bastawrose JH, Pamidimukala CK, Tojino A, Park TS, Musat D, Kukin M. Continuous infusion of furosemide combined with low-dose dopamine compared to intermittent boluses in acutely decompensated heart failure is less nephrotoxic and carries a lower readmission at thirty days. Hellenic J Cardiol. 2011;52:227–235. [PubMed] [Google Scholar]
- 126.Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S, Di Chiara L, Cogo P, Picardo S. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15:R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamiya M, Sato N, Akiya M, Okazaki H, Takahashi Y, Mizuno K. A case of marked diuresis by combined dopamine and atrial natriuretic peptide administration without renal injury in acute decompensated heart failure. Int Heart J. 2013;54:243–245. doi: 10.1536/ihj.54.243. [DOI] [PubMed] [Google Scholar]
- 128.Sumeray M, Robertson C, Lapsley M, Bomanji J, Norman AG, Woolfson RG. Low dose dopamine infusion reduces renal tubular injury following cardiopulmonary bypass surgery. J Nephrol. 2001;14:397–402. [PubMed] [Google Scholar]
- 129.Kim CS. Pharmacologic Management of the Cardio-renal Syndrome. Electrolyte Blood Press. 2013;11:17–23. doi: 10.5049/EBP.2013.11.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 131.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 132.Koniari K, Parissis J, Paraskevaidis I, Anastasiou-Nana M. Treating volume overload in acutely decompensated heart failure: established and novel therapeutic approaches. Eur Heart J Acute Cardiovasc Care. 2012;1:256–268. doi: 10.1177/2048872612457044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 134.Picca S, Ricci Z, Picardo S. Acute kidney injury in an infant after cardiopulmonary bypass. Semin Nephrol. 2008;28:470–476. doi: 10.1016/j.semnephrol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 135.van der Vorst MM, Ruys-Dudok van Heel I, Kist-van Holthe JE, den Hartigh J, Schoemaker RC, Cohen AF, Burggraaf J. Continuous intravenous furosemide in haemodynamically unstable children after cardiac surgery. Intensive Care Med. 2001;27:711–715. doi: 10.1007/s001340000819. [DOI] [PubMed] [Google Scholar]
- 136.Tumlin JA. Impaired blood flow in acute kidney injury: pathophysiology and potential efficacy of intrarenal vasodilator therapy. Curr Opin Crit Care. 2009;15:514–519. doi: 10.1097/MCC.0b013e328332f6f9. [DOI] [PubMed] [Google Scholar]
- 137.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 138.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 139.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fernández BE, Correa AH, Choi MR. Atrial natriuretic factor stimulates renal dopamine uptake mediated by natriuretic peptide-type A receptor. Regul Pept. 2005;124:137–144. doi: 10.1016/j.regpep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 141.Correa AH, Choi MR, Gironacci M, Aprile F, Fernández BE. Atrial natriuretic factor decreases renal dopamine turnover and catabolism without modifying its release. Regul Pept. 2008;146:238–242. doi: 10.1016/j.regpep.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 142.Humphreys MH. Mechanisms and management of nephrotic edema. Kidney Int. 1994;45:266–281. doi: 10.1038/ki.1994.33. [DOI] [PubMed] [Google Scholar]
- 143.Deschênes G, Wittner M, Stefano A, Jounier S, Doucet A. Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol. 2001;12:598–601. doi: 10.1681/ASN.V123598. [DOI] [PubMed] [Google Scholar]
- 144.Sampaio-Maia B, Moreira-Rodrigues M, Serrão P, Pestana M. Blunted renal dopaminergic system activity in puromycin aminonucleoside-induced nephrotic syndrome. Nephrol Dial Transplant. 2006;21:314–323. doi: 10.1093/ndt/gfi171. [DOI] [PubMed] [Google Scholar]
- 145.Marin-Grez M, Briggs JP, Schubert G, Schnermann J. Dopamine receptor antagonists inhibit the natriuretic response to atrial natriuretic factor (ANF) Life Sci. 1985;36:2171–2176. doi: 10.1016/0024-3205(85)90314-5. [DOI] [PubMed] [Google Scholar]
- 146.Trivedi M, Narkar VA, Hussain T, Lokhandwala MF. Dopamine recruits D1A receptors to Na-K-ATPase-rich caveolar plasma membranes in rat renal proximal tubules. Am J Physiol Renal Physiol. 2004;287:F921–F931. doi: 10.1152/ajprenal.00023.2004. [DOI] [PubMed] [Google Scholar]
- 147.Fernandes-Cerqueira C, Sampaio-Maia B, Quelhas-Santos J, Moreira-Rodrigues M, Simões-Silva L, Blazquez-Medela AM, Martinez-Salgado C, Lopez-Novoa JM, Pestana M. Concerted action of ANP and dopamine D1-receptor to regulate sodium homeostasis in nephrotic syndrome. Biomed Res Int. 2013;2013:397391. doi: 10.1155/2013/397391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vohra PK, Hoeppner LH, Sagar G, Dutta SK, Misra S, Hubmayr RD, Mukhopadhyay D. Dopamine inhibits pulmonary edema through the VEGF-VEGFR2 axis in a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L185–L192. doi: 10.1152/ajplung.00274.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adir Y, Sznajder JI. Regulation of lung edema clearance by dopamine. Isr Med Assoc J. 2003;5:47–50. [PubMed] [Google Scholar]
- 150.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 151.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 152.Sznajder JI. Strategies to increase alveolar epithelial fluid removal in the injured lung. Am J Respir Crit Care Med. 1999;160:1441–1442. doi: 10.1164/ajrccm.160.5.ed-13. [DOI] [PubMed] [Google Scholar]
- 153.Dematte JE, Sznajder JI. Mechanisms of pulmonary edema clearance: from basic research to clinical implication. Intensive Care Med. 2000;26:477–480. doi: 10.1007/s001340051186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goldberg LI. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972;24:1–29. [PubMed] [Google Scholar]
- 155.McGrath B, Bode K, Luxford A, Howden B, Jablonski P. Effects of dopamine on renal function in the rat isolated perfused kidney. Clin Exp Pharmacol Physiol. 1985;12:343–352. doi: 10.1111/j.1440-1681.1985.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 156.Barnard ML, Ridge KM, Saldias F, Friedman E, Gare M, Guerrero C, Lecuona E, Bertorello AM, Katz AI, Sznajder JI. Stimulation of the dopamine 1 receptor increases lung edema clearance. Am J Respir Crit Care Med. 1999;160:982–986. doi: 10.1164/ajrccm.160.3.9812003. [DOI] [PubMed] [Google Scholar]
- 157.Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol Biol Cell. 2002;13:1381–1389. doi: 10.1091/mbc.01-07-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lecuona E, Garcia A, Sznajder JI. A novel role for protein phosphatase 2A in the dopaminergic regulation of Na,K-ATPase. FEBS Lett. 2000;481:217–220. doi: 10.1016/s0014-5793(00)02009-3. [DOI] [PubMed] [Google Scholar]
- 159.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol. 2005;33:432–437. doi: 10.1165/rcmb.2005-0297TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281:L79–L85. doi: 10.1152/ajplung.2001.281.1.L79. [DOI] [PubMed] [Google Scholar]
- 161.Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ. 2007;335:389–394. doi: 10.1136/bmj.39293.624699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matthay MA, Calfee CS. Therapeutic value of a lung protective ventilation strategy in acute lung injury. Chest. 2005;128:3089–3091. doi: 10.1378/chest.128.5.3089. [DOI] [PubMed] [Google Scholar]
- 163.Ware LB, Kaner RJ, Crystal RG, Schane R, Trivedi NN, McAuley D, Matthay MA. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J. 2005;26:101–105. doi: 10.1183/09031936.05.00106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 165.Becker PM, Alcasabas A, Yu AY, Semenza GL, Bunton TE. Oxygen-independent upregulation of vascular endothelial growth factor and vascular barrier dysfunction during ventilated pulmonary ischemia in isolated ferret lungs. Am J Respir Cell Mol Biol. 2000;22:272–279. doi: 10.1165/ajrcmb.22.3.3814. [DOI] [PubMed] [Google Scholar]
- 166.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, Malkus H, Homer RJ, Elias JA. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol. 2008;39:420–430. doi: 10.1165/rcmb.2007-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. FASEB J. 2006;20:1519–1521. doi: 10.1096/fj.05-4708fje. [DOI] [PubMed] [Google Scholar]
- 168.Gurkan OU, O’Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L710–L718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- 169.Kaner RJ, Crystal RG. Pathogenesis of high altitude pulmonary edema: does alveolar epithelial lining fluid vascular endothelial growth factor exacerbate capillary leak? High Alt Med Biol. 2004;5:399–409. doi: 10.1089/ham.2004.5.399. [DOI] [PubMed] [Google Scholar]
- 170.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 171.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 172.Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1554–H1560. doi: 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- 173.Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161:564–566. doi: 10.1176/appi.ajp.161.3.564. [DOI] [PubMed] [Google Scholar]
- 174.Kleiner-Fisman G, Fisman DN. Risk factors for the development of pedal edema in patients using pramipexole. Arch Neurol. 2007;64:820–824. doi: 10.1001/archneur.64.6.noc60158. [DOI] [PubMed] [Google Scholar]
- 175.Tan EK, Ondo W. Clinical characteristics of pramipexole-induced peripheral edema. Arch Neurol. 2000;57:729–732. doi: 10.1001/archneur.57.5.729. [DOI] [PubMed] [Google Scholar]
- 176.Sato S, Hori T, Tsutsumi K, Asada T. Pramipexole-induced peripheral edema in a patient with bipolar depression. J Neuropsychiatry Clin Neurosci. 2011;23:E20. doi: 10.1176/jnp.23.4.jnpe20. [DOI] [PubMed] [Google Scholar]
- 177.Biglan KM, Holloway RG, McDermott MP, Richard IH. Risk factors for somnolence, edema, and hallucinations in early Parkinson disease. Neurology. 2007;69:187–195. doi: 10.1212/01.wnl.0000265593.34438.00. [DOI] [PubMed] [Google Scholar]
- 178.Zavala JA, Munhoz RP, Teive HA. Pramipexole-related chronic lower limb oedema in a patient with Parkinson’s disease. J Clin Neurosci. 2012;19:1298–1299. doi: 10.1016/j.jocn.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 179.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]


