Abstract
Background
The aim of the present study was to evaluate the capability of helminths to absorb heavy metals in comparison with that of the host tissues.
Methods
We compared the concentration of cadmium (Cd) and chromium (Cr) in urban rats and in their harboring helminthes —Moniliformis moniliformis, Hymenolepis diminuta and larval stage of Taenia taenaeiformis (Cysticercus fasciolaris). The heavy metal absorption was evaluated in 1g wet weight of parasites and tissues digested in nitric acid, using Inductivity Coupled Plasma (ICP_OES).
Results
A higher concentration of heavy metals was revealed in the helminths than in the host tissues. Bioconcentration factor (BF= C in parasite/C in tissue) for both Cd and Cr absorption was more than 10-fold higher in M. moniliformis than in the three compared host tissues. The BF of Cd in M. moniliformis compared to the liver, kidney and muscle of the host was 9.16, 14.14 and 17.09, respectively. BF in Cr in the same parasite and the same host tissues ranged from 10.67, 7.06 and 4.6. High level of absorption in H. diminuta was significantly likewise; the individual BF of Cd and Cr in H. diminuta compared to the liver, kidney and muscle of the hosts was 4.95, 5.94 and 4.67 vs. 2.67, 11.56 and 5.59. The mean concentration of Cd and Cr in C. fasciolaris was also significantly higher than that in the rat livers (P<0.007 and P<0.004, respectively).
Conclusion
This study claims that parasites of terrestrial animals exposed to heavy metals can be more accurate indicators than the host tissues as new environmental monitoring agents.
Keywords: Helminths, Bioindicators, Heavy metals, Moniliformis moniliformis
Introduction
Bioaccumulation of both heavy metals and toxins by small living organisms may have practical uses; particularly as a means of detecting and/or sequestering harmful elements and/or toxins within the environment. Earth worms, for instance, have long been known as terrestrial bioindicators (1). Similarly, the sludge worm, Tubifex tubifex, has a capacity to remove (hence detoxify) cadmium (Cd) from the environment (2).
Since aquatic ecosystems are the terminus for all toxic metals in solution on the earth, the interaction of marine life and their parasites is of interest vis-à-vis the sequestration of toxins (3). Regarding the use of helminthes as biological indicators, the fish acanthocephalan can be considered more appropriate, due to its greater capacity to accumulate heavy metals; compared to their host and the ecosystem they reside in (4). The cestodes infesting fresh water fish also have a capacity to absorb Lead (Pb) and Cd compared to their host (5). The role of nematodes (i.e., Anguillicola crassus) for Pb bioaccumulation was recognized in eels, while the coexistent acanthocephalan, Paratenuisentis ambiguous, in the same hosts showed 100 times greater absorption capacity (6). Further experiments, however, did not confirm the role of another nematode, Contracaecum rodolphii, in the assessment of environmental heavy metal pollution (7).
In Iran, the ability of the cestodes, Anthobothrium sp. and Paraorigmatobothrium sp., regarding bioabsorption of heavy metals has been studied in the aquatic ecosystem of the Persian Gulf. Helminthes in the sharks showed higher bioaccumulation of Pb and Cd than the host tissues (8). The rationale of evaluating these two elements for the current study is heavy metal pollution in Tehran metropolis especially with Cadmium and Chromium with the value of public health (9, 10).
This study investigated the level of bioabsorption of Cd and Cr in (a) an acanthocephalan (Moniliformis moniliformis), (b) cestodes (Hymenolepis diminuta), (c) the larva of Taenia taeniaeformis and (d) the host tissues of Rattus sp. in Tehran. The significant role of these helminths as a biological means for assessing the occurrence and prevalence of heavy metals in terrestrial environments is described herein.
Materials & Methods
In 2010-2011 a total of 60 rats were caught alive during the pest control at several sites of Tehran, Iran. Rats were sacrificed in accordance with current accepted ether inhalation method in a cage with enough space and time to avoid putting distress on the animals. The study was approved by Ethical Committee of the Tehran University of Medical Sciences, Iran.
In order to harvest the parasites needed for this experiment; the rats were euthanized, dissected and carefully examined for acanthocephalan and cestodes in the Laboratory of Helminthology, Department of Parasitology and Mycology, Faculty of Public Health, Tehran University of Medical Sciences (TUMS), Tehran, Iran. Adult worms were precisely identified and counted. Interestingly a 141 high-burden of adult Moniliformis moniliformis was found in a single alive rat which has been reported with the concern of histopathological changes (11). M. moniliformis, H. diminuta and Cysticercus fasciolaris as well as host tissue samples (kidney, liver and muscle) were isolated for analysis. Samples were individually placed in acid washed containers, preventing any possible heavy metal contaminations, and preserved at -20 °C for future analysis (12). Tissue samples weighing between 0.03 and 1.4 g were digested in 7 ml of nitric acid and heated to 50 °C overnight (8). To extract the organic layer of solution, 5 ml of chloroform was used. The remaining yellow aqueous layer was diluted to 10 ml using distilled water. To avoid artificial chemical interference during the experiment, stainless steel instruments were used throughout. Metal analyses were performed using inductively coupled plasma - optical emission spectroscopy (ICP-OES, SPECTRO ARCOS). The analytical blanks with varied concentration of pure elements were prepared under the same condition. The bioconcentration factors in the three parasites examined were calculated as the ratio of the metal concentration in the specific parasites to that of each host tissue (13).
The results were analyzed with the aid of t-test and Mann-Whitney U-Test in SPSS 17; 95% confidence interval was approved.
Results
Amongst 60 captured rats, 7 rats not infected with any parasitic worms were concerned as control group. Three parasitic worms, M. moniliformis, H. diminuta and C. fasciolaris, recovered from the intestine of 18 rats regarded as the test group (Table 1). All three parasites and the host tissues of both infected and uninfected rats were analyzed for the concentration of Cd and Cr. The parasites contained higher concentrations of metals than those of the host tissues (Fig. 1 and 2). The bioconcentration factor of Cd in M. moniliformis compared to the liver, kidney and muscle of the host was 9.16, 14.14 and 17.09, respectively. The Cr bioconcentration factor in the same parasite and the same host tissues was 10.67, 7.06 and 4.67, respectively. The bioconcentration factors of Cd and Cr in H. diminuta vs. host tissues revealed a high level of concentration in the worm. The respective bioconcentration factors of Cd and Cr in H. diminuta compared to the liver, kidney and muscle of the hosts was 4.95, 5.94 and 4.67 vs. 2.67, 11.56 and 5.59 (Table 2 and 3). The average concentration of Cd and Cr in C. fasciolaris was significantly higher than that in the host tissues, the rat livers (P<0.007 and P<0.004, respectively). The comparison of absorptive capacity in three species of parasites showed a noticeably high absorption of both heavy metals in acanthocephalan than the two cestodes (Fig. 1 and 2).
Table 1.
Species and numbers of hosts and parasites isolated in this study
| Host species | Infected hosts Parasites | |||
|---|---|---|---|---|
| M. moniliformis | H. diminuta | C. fasciolaris | ||
| Rattus rattus | 12 | 223 | 7 | 14 |
| Rattus norvegicus | 6 | - | 4 | 25 |
| Total | 18 | 223 | 11 | 39 |
Fig. 1.
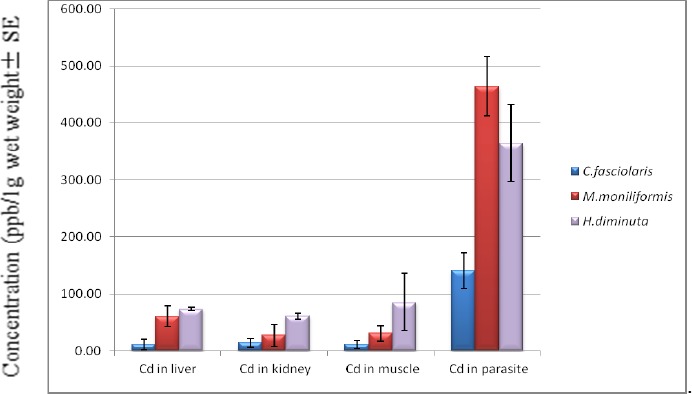
Mean concentrations of Cadmium in 3 parasite species and in the host tissues. This figure shows the significant higher absorption of Cd in Moniliformis moniliformis, Hymenolepis diminuta and Cysticercus fasciolaris versus the host tissues
Fig. 2.
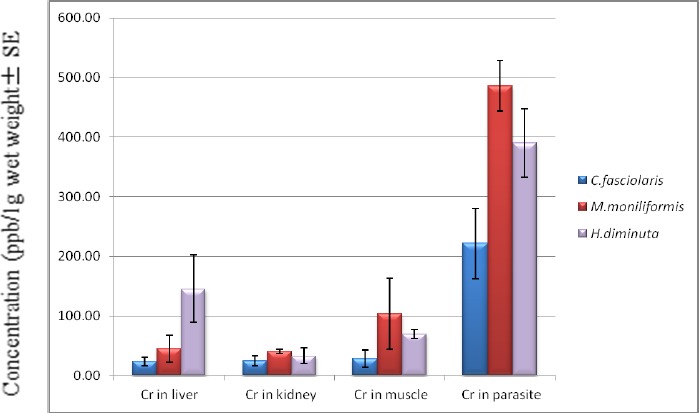
Mean concentrations of Chromium in 3 parasite species and in the host tissue. This figure shows the significant higher absorption of Cd in Moniliformis moniliformis, Hymenolepis diminuta and Cysticercus fasciolaris versus the host tissues
Table 2.
Mean concentrations of Cadmium in various tissues of helminth-positive and negative rats
| Cd concentration in host tissues (ppb/1g wet weight ±SE) | |||
|---|---|---|---|
| Group | Liver | Kidney | Muscle |
| Infected | 22.90 | 21.13 | 19.20 |
| ±6.77 | ±5.66 | ±7.62 | |
| Uninfected | 76.21 | 75.06 | 45.26 |
| ±10.89 | ±7.35 | ±9.45 | |
| Pvalue | 0.002 | 0.001 | 0.015 |
Table 3.
Mean concentrations of Chromium in various tissues of helminth-positive and negative rats
| Group | Cr concentration in host tissues (ppb/1g wet weight ±SE) | ||
|---|---|---|---|
| Liver | Kidney | Muscle | |
| Infected | 37.29 | 33.93 | 42.26 |
| ±11.30 | ±9.58 | ±10.17 | |
| Uninfected | 84.97 | 67.25 | 104.26 |
| ±36.02 | ±12.74 | ±25.67 | |
| Pvalue | 0.046 | 0.009 | 0.008 |
Discussion
In the present study the bioabsorption level of heavy metals were evaluated in helminths and their host tissues. The role of certain helminth parasites—mainly acanthocephalan and cestodes—as bioindicators in aquatic ecosystems has previously been confirmed (4, 5, 14). The use of this biological trait is considered a valuable tool for assessing environmental pollution by heavy metals. The intestinal cestodes (viz., Skrjabinotaenia lobata and Gallegoides arfaai) of the wood mouse (Apodemus sylvaticus), for instance, have shown a greater bioaccumulation capacity of Pb and Cd than various tissues of their host. The A. sylvaticus/G. arfaai bioindication model has been introduced for evaluation of Pb exposure in the environment (12, 15).
In this study, the concentration of Cd and Cr in non-parasitized rat tissues was higher than those of the tissues of rats infected with helminthes. This has important implications for the use of parasites as bioindicators of toxic environmental pollutants (16). Among the helminthes examined from different aquatic and terrestrial ecosystems, acanthocephalan has always ranked high vis-à-vis its potential for heavy metal bioaccumulation (6, 7, 17, 18). Our results also revealed that M. moniliformis (an acanthocephalan) had two- and four-fold higher Cd and Cr accumulation capacity than H. diminuta and C. fasciolaris (Fig. 2). As with previous studies on acanthocephalans and cestodes (19), H. diminuta—a prevalent tapeworm among the rats—could be an accessible and cost-effective bioindicator for environmental pollution. The comparative accumulation capacity of these two cestodes as well as M. moniliformis—which exhibits the higher role of acanthocephalan in heavy metal absorption—is remarkable (Fig. 1 and 2).
A lower capacity of heavy metal bioaccumulation in adult cestodes compared to their larval stage has been documented for T. taeniaeformis and its larval stage in rats (20). To contrast, whole body analysis of C. fasciolaris compared with H. diminuta revealed results similar to our own (Fig. 2).
Conclusion
We confirm a trend to higher bioaccumulation (of Cd and Cr) by parasites over against the host tissues. As with previous research, the acanthocephalan M. moniliformis showed a roughly 10-fold greater bioaccumulation of Cd and Cr than the host tissues or H. diminuta. This capability can build an opportunity of more accurate heavy metals assessment in both terrestrial and aquatic environments as well as noticing the beneficial roles of helminths.
The host-parasite relationship in the terrestrial ecosystem vis-à-vis heavy metal bioaccumulation suggested a quasi-protective role of parasites in our ecosystem.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported by School of Public Health, Tehran University of Medical Sciences. We thank the laboratory staff of the Environmental Engineering Department at the School of Public Health. Cordial collaboration of Miss Neda Mirsepahi and Mr. Ali Rahimi for assistance during field activities and Mr. Bryan Roderick Hamman for editing the manuscript is highly appreciated. The authors would like to warmly thank Professor Yukifumi Nawa for his valuable comments and review. The authors declare that there is no conflict of interest.
References
- Suthar S, Singh S, Dahawan S (2008). Earthworms as bioindicator of metals (Zn, Fe, Mn, Cu, Pb and Cd) in soils: Is metal bioaccumulation affected by their ecological category? ECOL ENG, 32 (2): p. 99–107. [Google Scholar]
- Bouché ML, Habets F, Biagianti-Risbourg S, et al. (2000). Toxic Effects and Bioaccumulation of Cadmium in the Aquatic Oligochaete Tubifex tubifex. Ecotox Environ Safe, 46 (3): 246–251. [DOI] [PubMed] [Google Scholar]
- Ciutat A, Gerino M, Mesmer-Dudons N, et al. (2005). Cadmium bioaccumulation in Tubificidae from the overlying water source and effects on bioturbation. Ecotoc Environ Safe, 60 (3): 237–246. [DOI] [PubMed] [Google Scholar]
- Tekin-Ozan S (2008). Determination of heavy metal levels in water, sediment and tissues of tench (Tinca tinca L., 1758) from Beyşehir Lake (Turkey). Environ Monit Assess, 145 (1-3): 295–302. [DOI] [PubMed] [Google Scholar]
- Sures B, Taraschewski H, Rokicki J (1997). Lead and cadmium content of two cestodes, Monobothrium wageneri and Bothriocephalus scorpii, and their fish hosts. Parasitol Res, 83 (6): 618–623. [DOI] [PubMed] [Google Scholar]
- Sures B, Taraschewski H, Jackwerth E (1994). Lead content of Paratenuisentis ambiguus (Acanthocephala), Anguillicola crassus (Nematodes) and their host Anguilla anguilla. Dis Aquat Organ, 19 (2): 105–107. [Google Scholar]
- Vlastimil B, Frantisek T (2001). Cadmium and Lead concentrations in Contracaecum rudolphii (Nematoda) and its host, the cormorant Phalacrocorax carbo (Aves). Folia Parasit, 48: 77–78. [DOI] [PubMed] [Google Scholar]
- Malek M, Haseli M, Mobedi I, et al. (2007). Parasites as heavy metal bioindicators in the shark Carcharhinus dussumieri from the Persian Gulf. Parasitology, 134 (07): 1053–1056. [DOI] [PubMed] [Google Scholar]
- Farzin L, Amiri M, Shmas H, et al. (2008). Blood levels of lead, cadmium, and mercury in residents of Tehran. Biological Trace Element Research, 123 (1-3): 14–26. [DOI] [PubMed] [Google Scholar]
- Kumar Dey S, Roy S (2009). Effect of chromium on certain aspects of cellular toxicity. Iran J Toxicol, 2 (4): 260–267. [Google Scholar]
- Teimoori S, Gharaguzlu MJ, Makki MS, et al. (2011). Heavy Worm Burden of Moniliformis moniliformis in Urban Rats with Histopathological Description. Iran J Parasitol, 6 (3): 107–12. [PMC free article] [PubMed] [Google Scholar]
- Torres J, de Lapuente J, Eira C, et al. (2004). Cadmium and Lead concentration in Galleogides arfaai (Cestoda: Anoplocephalidae) and Apodemus sylvaticus (Rodentia : Muridae) from Spain. Parasitol Res, 94: 468–470. [DOI] [PubMed] [Google Scholar]
- Sures B, Siddall R, Taraschewski H (1999). Parasites as accumulation indicators of heavy metal pollution. Parasitol Today, 15 (1): 16–21. [DOI] [PubMed] [Google Scholar]
- Sures B (2001). The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquat Ecol, 35: 245–255. [Google Scholar]
- Torres J, Peig J, Eira C, Borras M (2006). Cadmium and lead concentration in Skrjabinotaenia lobata (Cestoda : Catentaeniidae) and its host, Apodemus sylvaticus (Rodentia: Muridae) in the urban dumping site of Garraf (spain). Enviroment Pollut, 143: 4–8. [DOI] [PubMed] [Google Scholar]
- Vidal-Martinez VM, Pech D, Sures B, et al. (2009). Can parasites really reveal environmental impact? Trends Parasitol, 26 (1): 44–51. [DOI] [PubMed] [Google Scholar]
- Sures B (2003). Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology Cambridge, 126: S53–S60. [DOI] [PubMed] [Google Scholar]
- Scheef G, Sures B, Taraschewski H (2000). Cadmium accumulation in Moniliformis moniliformis (Acanthocephala) from experimentally infected rats. Parasitol Res, 86: 688–691. [DOI] [PubMed] [Google Scholar]
- Sures B, Grube K, Taraschewski H (2002). Experimental Studies on the Lead Accumulation in the Cestode Hymenolepis diminuta and its final host, Rattus norvegicus. Ecotoxicology, 11: 365–368. [DOI] [PubMed] [Google Scholar]
- Sures B, Scheible T, Bashtar AR, Taraschewski H (2003). Lead concentration in Hymenolepis diminuta adults and Taenia taeniaformis Larvae compared to their rat hosts (Rattus norvegicus) sampled from the city Cairo, Egypt. Parasitology, 127: 483–487. [DOI] [PubMed] [Google Scholar]


