Abstract
We previously found loss of forkhead box A1 (FOXA1) expression to be associated with aggressive urothelial carcinoma of the bladder, as well as increased tumor proliferation and invasion. These initial findings were substantiated by The Cancer Genome Atlas, which identified FOXA1 mutations in a subset of bladder cancers. However, the prognostic significance of FOXA1 inactivation and the effect of FOXA1 loss on urothelial differentiation remain unknown. Application of a univariate analysis (log-rank) and a multivariate Cox proportional hazards regression model revealed that loss of FOXA1 expression is an independent predictor of decreased overall survival. An ubiquitin Cre-driven system ablating Foxa1 expression in urothelium of adult mice resulted in sex-specific histologic alterations, with male mice developing urothelial hyperplasia and female mice developing keratinizing squamous metaplasia. Microarray analysis confirmed these findings and revealed a significant increase in cytokeratin 14 expression in the urothelium of the female Foxa1 knockout mouse and an increase in the expression of a number of genes normally associated with keratinocyte differentiation. IHC confirmed increased cytokeratin 14 expression in female bladders and additionally revealed enrichment of cytokeratin 14–positive basal cells in the hyperplastic urothelial mucosa in male Foxa1 knockout mice. Analysis of human tumor specimens confirmed a significant relationship between loss of FOXA1 and increased cytokeratin 14 expression.
In the United States, there are approximately 73,000 new cases of urinary bladder cancer (UBC) annually, accounting for approximately 15,000 deaths.1 Although the incidence is approximately four times higher in men than in women, women often present with more advanced disease and have worse clinical outcomes.2 Approximately 90% of UBCs are urothelial carcinomas (UCs), with squamous cell carcinomas (SCCs) and adenocarcinomas largely comprising the remainder. Management of non–muscle-invasive UBC [American Joint Committee on Cancer (AJCC) tumor stages pTa, pTis, and pT1] involves transurethral resection with or without adjuvant intravesical therapy followed by careful surveillance.3 For muscle-invasive UBC (AJCC tumor stage pT2 or higher), radical cystectomy with or without neoadjuvant chemotherapy is the cornerstone of management.4 Despite these aggressive measures, the disease recurs in approximately 50% of patients, and the 5-year overall survival rate is only 6% for patients who develop or present with distant metastatic disease.5 Therefore, there is a significant need for novel approaches geared toward identifying those patients most at risk for disease progression or death, as well as identifying pathways that can act as targets for novel therapeutics.
Interestingly, a significant subset (approximately 40%) of UC exhibits squamous differentiation, with some cases showing focal keratinizing squamous metaplasia (KSM) and others developing into predominant or pure SCC. Whereas pure SCC of the bladder has a worse prognosis than UC,6 whether the presence of squamous differentiation in the background of UC is indeed a poor prognostic indicator is still controversial. Both SCC and UC with squamous differentiation may be associated with resistance to systemic chemotherapy,7 which could account for poor overall outcomes. Currently, there is no consensus regarding the extent of squamous differentiation that confers increased clinical risk.
Forkhead box A1 (FOXA1) is a member of the forkhead box A subfamily of transcription factors, which includes genes encoding for FOXA1, FOXA2, and FOXA3. FOXA1 protein is expressed in the developing and adult urothelium, whereas FOXA2 protein expression is restricted to early bladder development, where it appears to play a significant role in urothelial development.8,9
We originally reported that loss of FOXA1 expression is associated with high-grade, late-stage UC, as well as increased tumor cell proliferation and invasion.10 Furthermore, we found that FOXA2 is expressed in a subset of UC, potentially recapitulating early bladder development. Recently, the Cancer Genome Atlas provided a comprehensive molecular characterization of muscle-invasive UBC.11 In addition to confirming our initial reports regarding the loss of FOXA1 in human UBC, this work extended our original analysis by revealing that FOXA1 is mutated in 5% of UBC. In addition, another recent report found that absence of FOXA1 expression was associated with a basal molecular phenotype that expresses markers associated with squamous differentiation and is more common in female UBC.12 Therefore, in addition to other molecular factors, both FOXA1 and FOXA2 appear to play a central role in bladder development, urothelial differentiation, and malignant progression.9–11 However, the prognostic and clinical significance of FOXA1 loss in human UC is currently unknown. To address this issue, we used a tissue microarray (TMA) consisting of >600 bladder tissue samples from 301 patients who underwent cystectomy for UBC to explore the association of FOXA1 loss with clinical outcome in these patients. Although FOXA1 loss is associated with both UC, UC with squamous differentiation, and pure SCC of the urinary bladder in humans, it is unclear whether inactivation of FOXA1 itself is sufficient to drive histologic alterations and/or tumor progression. Therefore, we used an inducible system to genetically ablate Foxa1 in the adult murine urothelium, which enabled us to directly address this question.
Materials and Methods
Ethics Statement
Human UBC samples were collected by the Translational Pathology Shared Resource, a core facility of the Vanderbilt-Ingram Cancer Center, and later deidentified for testing and analysis. Clinical, pathological, and follow-up data were collected via review of medical records and extracted to the Research Electronic Data Capture database hosted at Vanderbilt University.13 This project was approved by the Institutional Review Board at Vanderbilt University. All animal work was approved by and performed in accordance with Institutional Animal Care and Use Committee guidelines.
Human Tissue Samples and Generation of TMAs
TMAs were generated using 657 bladder tissue cores from 301 patients who underwent cystectomy for UC or SCC of the bladder between January 2000 and May 2010. All patients underwent radical cystectomy, bilateral pelvic lymphadenectomy, and urinary diversion. Patients were subsequently followed up at 3 months, 6 months, and then at increasing intervals based on individual surgeons' practice patterns by physical examination, laboratory studies, and both chest and abdominal imaging. Clinicopathologic data were collected and included patient demographics, such as age at time of surgery, sex, and comorbidity recorded as age-adjusted Charlson comorbidity index,14 and tumor characteristics, such as AJCC tumor stage (seventh edition, 2010 guidelines15) and grade.
The original hematoxylin and eosin (H&E) slides were reviewed and diagnostic tissue was marked for construction of a TMA using a manual arrayer (Beecher Instruments, Sun Prairie, WI). One to three tissue cores (each 1.5 mm) of representative areas from each of the selected formalin-fixed, paraffin-embedded tissue blocks were used for the array. H&E slides from the TMAs were prepared, and the histopathological diagnosis of tissue samples represented on the TMA were identified for each patient (wherever available), including adjacent benign urothelium, noninvasive papillary urothelial carcinoma (pTa), urothelial carcinoma in situ (pTis), or invasive urothelial carcinoma (pT1-pT4), and were recorded in a Research Electronic Data Capture relational database.
IHC Staining and Evaluation
Immunohistochemistry (IHC) was performed on human and murine samples. Slides were deparaffinized and rehydrated through a series of graded alcohols and washed in deionized water for 5 minutes. Antigen retrieval was performed by placing slides in 1% antigen unmasking solution (Vector Labs, Burlingame, CA) and heating slides for 25 minutes on high power in a pressure cooker (Cook's Essentials CEPC800). Steam was released in short bursts to prevent boiling and preserve tissue integrity. Slides were cooled to room temperature and washed 3 times for 10 minutes in PBS (pH 7.4). All incubations were performed at room temperature unless otherwise noted. Endogenous peroxidases were blocked by incubation in 1% hydrogen peroxide in methanol for 20 minutes, and slides were again washed 3 times for 10 minutes in phosphate-buffered saline (PBS). Sections were incubated in PBS containing horse serum (Vector Labs) for 30 minutes to reduce nonspecific antibody binding and then incubated overnight with primary antibody at 4°C in a humidified chamber. Antibodies used include goat polyclonal FOXA1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), mouse polyclonal cytokeratin 10 (CK10) (1:200; Dako, Carpinteria, CA), mouse polyclonal cytokeratin 14 (CK14) (1:200; Dako), and mouse polyclonal uroplakin III (UPKIII) (1:5000; Fitzgerald, Acton, MA) all diluted in PBS containing horse serum.
Bromodeoxyuridine (BrdU) staining of mouse specimens was stained by the Vanderbilt Experimental Pathology Core. Slides were then washed 3 times for 10 minutes in PBS and sections were incubated in biotinylated secondary antibody diluted in PBS containing horse serum (1:200; Vector Labs) for 1 hour. Specific antibody binding was visualized using Vectastain Elite ABC Peroxidase kit (Vector Labs) according to the manufacturer protocol with diaminobenzidine substrate buffer as the chromogen (Thermo Scientific, Fremont, CA). The intensity and distribution of immunoreactivity for each TMA core were scored by a pathologist (J.M.C.) using the Allred scoring system, where scales of 0 to 3 were used for intensity and 0 to 5 for percentage of positive tumor cells.16 An IHC index was assigned to each sample by summation of the intensity score and distribution score.
Statistical Analysis of IHC Scores
For all analyses, replicate IHC scores from cores of the same pathological stage from the same patient were averaged. Correlations among IHC scores and tumor stage were assessed using Spearman rank coefficients. The extended Mantel-Haenszel test was used to assess correlations between IHC scores after adjusting for tumor stage. For survival analysis and correlation analysis comparing clinical tumor stage with IHC scores, the following exclusion criteria were applied to the original cohort of 301 tumor samples to reduce the potential influence of field effect phenomena. For patients with multiple specimens or different samples from a single specimen represented on the TMA, the sample of the highest pathological stage available was used, and the other samples were excluded. Patient samples recorded as adjacent benign urothelium (n = 25) and cores that could not be histologically assessed (n = 15) were also excluded.
Patients with overall tumor stages pT2 or higher were excluded if invasive tumor was not represented on the TMA (n = 17). Because of the low numbers, patients with tumors of low histological grade (n = 8) were also excluded from survival analysis. Two patients had low-grade disease at the time of cystectomy, but we were not able to assess the tissue cores included in the TMAs. This resulted in the use of 234 patients available for survival analysis. Overall survival was defined as the interval from the date of cystectomy to the date of death from any cause, or patients were censored at the date of last follow-up.
Univariate analyses were performed using log rank tests and visualized using Kaplan-Meier survival curves. For univariate log rank analyses, age was grouped into quartile distributions, and comorbidity index was grouped into tertile distributions. Tumor stage was grouped by organ-confined disease (AJCC tumor stages pTa, pTis, pT1, and pT2), extravesical disease (pT3 to pT4), and node-positive disease (any pT or N1 to N2). Variables associated with adverse prognosis based on previous studies17 and variables with P < 0.1018 on univariate analysis were included in a multivariate analysis using a Cox proportional hazards model.
The extended Mantel-Haenszel test was used to analyze the correlation between FOXA1 and CK14 expression in human tissue, independent of tumor stage. Fisher's exact test was applied to determine significance of observed phenotypes after Foxa1 knockout in male and female mice. For all tests, P < 0.05 was considered statistically significant. Stata version 12.0 (Stata Corp., College Station, TX) was used for analysis of human clinical data and mouse knockout data.
Mouse Breeding Experiments
Foxa1loxp mice have been previously described.19 Globally inducible Foxa1 knockout was achieved by breeding B6.Cg-Tg(UBC-Cre/ERT2)1Ejb/j mice (hereafter simply UBC-Cre/ERT2) to Foxa1loxp/loxp mice, resulting in UBC-Cre/ERT2/Foxa1loxp/loxp mice, respectively. Once sexually mature, male and female UBC-Cre/ERT2/Foxa1loxp/loxp and littermate Foxa1loxp/loxp control mice were injected i.p. with 1 mg/d of tamoxifen for 5 days and separated into three groups. The first group was euthanized immediately after 5 days to determine the efficacy of UBC-Cre/ERT2 to induce ablation of Foxa1 expression. The second and third groups of mice were euthanized 3 and 6 months after tamoxifen injection, respectively, to determine the effect of long-term Foxa1 knockout. All mice were injected with BrdU before euthanasia. A subset of individual bladders were dissected and bisected, with one bladder half being dedicated to formalin fixation and paraffin embedding by standard procedures and the other half being used for microarray studies (see below).
Gene Expression Studies
Dissected tissue was placed in RNAlater (Qiagen, Venlo, the Netherlands) to stabilize RNA, and RNA was extracted using RNEasy (Qiagen) according to the manufacturer's instructions. Microarray analysis on RNA extracted from bladder tissue dissected from control and experimental mice (four control Foxa1loxp/loxp and three Foxa1 knockout male mice and four control Foxa1loxp/loxp and six Foxa1 knockout female mice) was performed using the Mouse Gene 1.0 ST Array (Affymetrix Corp, Santa Clara, CA) by the Vanderbilt Technologies for Advanced Genomics core. The Mouse Gene 1.0 ST Array contains probes for >28,000 coding transcripts and approximately 7000 noncoding transcripts, including 2000 long intergenic noncoding transcripts. Instrument control and data acquisition were performed using the Affymetrix GeneChip Command Console. Fold change was calculated in a log base 2 space, using the formula followed by inverting and negating ratios <1.0.
To analyze data, we first normalized the microarray gene-expression data and analyzed the RNA abundance between the Foxa1 knockout mice and the control mice within each sex using the limma package provided by BioConductor version 2.14 (Fred Hutchinson Cancer Research Center, Seattle, WA). We then compared the effect of Foxa1 knockout on gene expression between female and male mice and identified differentially expressed genes by sex, as well as those shared by both sexes. The Benjamini-Hochberg procedure was used to control the false discovery rate in multiple comparisons. Differentially expressed genes were prioritized based on the following criteria: |logFC|>2 and false discovery rate–adjusted P < 0.01. All statistical analyses were performed in R version 3.0.2 (http://www.r-project.org). Data were analyzed through the use of the Ingenuity Pathway Analysis (IPA; Qiagen, Redwood City, CA) and were used with permission.
Results
FOXA1 Loss Is an Independent Predictor of Decreased Overall Survival in Humans with Bladder Cancer
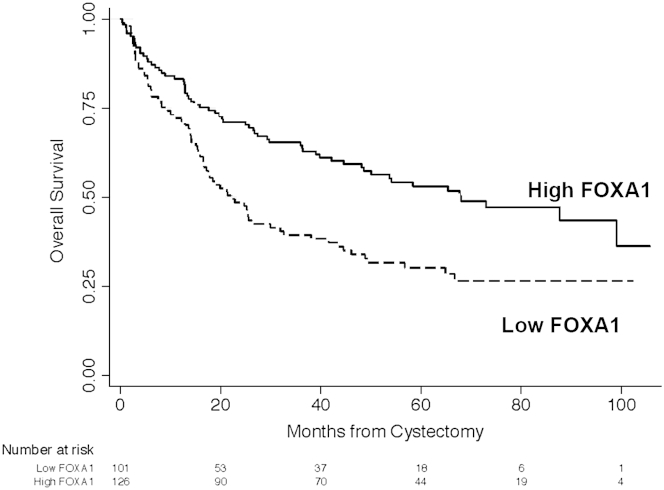
Clinicopathologic characteristics of the tumors represented on the TMA are listed in Table 1. Primary histological tumor types for the 301 patients in our study included 278 pure UCs (92%) and 23 UCs with squamous differentiation (8%). Four patients (1%) were treated with neoadjuvant chemotherapy. At the time of last follow-up, there were 139 deaths (59%). Median follow-up was 15.1 months (interquartile range, 5.6 to 29.7) for censored patients. Median overall survival duration was 42.1 months (95% CI, 27.4–58.4 months), with a 5-year actuarial estimated overall survival rate of 42.3% (95% CI, 35.6%–48.8%). Confirming our previous finding in an independent cohort,10 FOXA1 expression was inversely correlated with increasing tumor stage by Spearman rank correlation (P < 0.001; ρ = −0.38; n = 234). We compared the overall survival of patients with low versus high FOXA1 expression. For survival analysis, IHC scores were dichotomized as low or high expression using the median (5.5) as a cutoff value, with values at the median grouped into the high expression category. Low FOXA1 expression was associated with shorter overall survival on univariate log rank analysis (P < 0.001) (Figure 1). Median overall survival for low versus high FOXA1 expression was 22.1 months (95% CI, 16.5–31.9 months) and 68 months (95% CI, 48.2 months to not reached), respectively. Other variables associated with overall survival on univariate analysis include patient age (P < 0.001), Charlson comorbidity index (P < 0.001), and tumor stage (P < 0.001). Sex (P = 0.061) was also included in the multivariate model. After controlling for patient age, tumor stage, sex, and Charlson comorbidity index score, high FOXA1 expression is an independent predictor of higher overall survival (Table 2).
Table 1.
Patient and Tumor Characteristics Included in Tissue Microarray
| Characteristic | Finding |
|---|---|
| Age, years, median (IQR) | 67 (59–73) |
| Sex, no. (%) | |
| Male | 239 (79) |
| Female | 62 (21) |
| Race, no. (%)∗ | |
| White | 279 (93) |
| Nonwhite | 20 (6) |
| Not reported | 2 (1) |
| Charlson Comorbidity Index, no. (%) | |
| 0–1 | 51 (17) |
| 2–3 | 124 (41) |
| >4 | 126 (42) |
| T stage, no. (%) | |
| Ta | 7 (2) |
| Tis | 38 (13) |
| T1 | 41 (14) |
| T2 | 98 (32) |
| T3 | 83 (28) |
| T4 | 34 (11) |
| N stage, no. (%) | |
| N0 | 231 (77) |
| N1 or greater | 70 (23) |
| Grade, no. (%) | |
| Low grade | 8 (3) |
| High grade | 293 (97) |
IQR, interquartile range.
Data on race was self-reported by patients.
Figure 1.
Loss of FOXA1 expression is significantly associated with decreased overall survival in human bladder cancer. Kaplan-Meier survival curves by FOXA1 expression. Univariate analysis shows loss of FOXA1 is significantly associated with reduced overall survival (P < 0.001). See Table 2 for multivariate analysis.
Table 2.
Multivariate Cox Proportional Hazards Model Predicting Overall Survival after Radical Cystectomy
| Variable | Overall survival |
|
|---|---|---|
| HR (95% CI) | P value | |
| Age | 0.99 (0.97–1.02) | 0.526 |
| Charlson Comorbidity Index | 1.25 (1.11–1.40) | <0.001 |
| Sex | ||
| Male | Referent | |
| Female | 0.63 (0.41–0.97) | 0.037 |
| Tumor stage | ||
| Organ-confined disease | Referent | |
| Extra-vesical disease | 2.00 (1.27–3.15) | 0.003 |
| Node-positive disease | 3.49 (2.31–5.28) | <0.001 |
| Low FOXA1 | 1.49 (1.04–2.13) | 0.028 |
UBC-Cre/ERT2 System Is Sufficient to Induce Foxa1 Knockout in Murine Urothelium
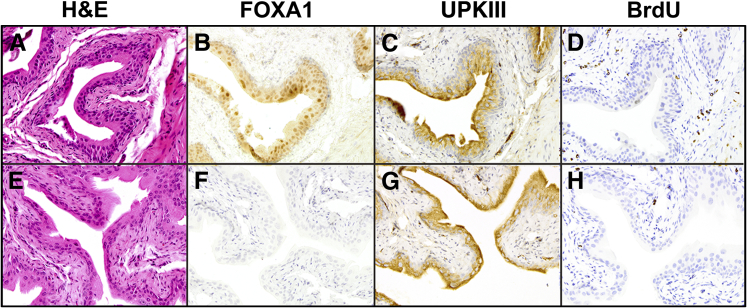
To determine the phenotypic effect of genetically ablating Foxa1 expression in murine urothelium, we initiated a breeding program consisting of a tamoxifen-inducible Cre recombinase driven by the ubiquitin promoter (UBC-Cre/ERT2) and our Foxa1loxp/loxp mice, resulting in the creation of UBC-Cre/ERT2/Foxa1loxp/loxp mice. Littermate control Foxa1loxp/loxp mice and experimental UBC-Cre/ERT2/Foxa1loxp/loxp mice were injected with tamoxifen for 5 days and then separated into 3 different groups (Materials and Methods). Histomorphometric analysis of control Foxa1loxp/loxp mice (Figure 2A) and experimental UBC-Cre/ERT2/Foxa1loxp/loxp mice (Figure 2E) after tamoxifen injection for 5 days (Figure 2E) revealed no obvious differences in tissue architecture, whereas IHC analysis of UBC-Cre/ERT2/Foxa1loxp/loxp mice revealed efficient and widespread ablation of urothelial Foxa1 expression; however, control Foxa1loxp/loxp mice retained Foxa1 expression (Figure 2, B and F). Both tamoxifen-treated Foxa1loxp/loxp mice and tamoxifen-treated experimental UBC-Cre/ERT2/Foxa1loxp/loxp expressed UpkIII (Figure 2, C and G), a marker of urothelial differentiation, and were quiescent in regard to proliferation (Figure 2, D and H). These results indicate that the UBC-Cre/ERT2 system efficiently ablates Foxa1 expression in the urothelium of adult mice.
Figure 2.
UBC-Cre/ERT2 efficiently ablates Foxa1 expression in the urothelium after tamoxifen injection. Control Foxa1loxp/loxp mice and experimental UBC-Cre/ERT2/Foxa1loxp/loxp mice were injected with 1 mg per day of tamoxifen for 5 days and euthanized. Tissue was then prepared as described in Materials and Methods for histological analysis to assess the effect of short-term Foxa1 knockout. Hematoxylin and eosin (H&E), FOXA1, uroplakin III (UPKIII), and bromodeoxyuridine (BrdU) staining of bladders dissected from tamoxifen-injected Foxa1loxp/loxp (A–D) and UBC-Cre/ERT2/Foxa1loxp/loxp (E–H) mice. There are no significant histological changes after Foxa1 knockout (A and E). Although Foxa1 expression is detected in the urothelium of control Foxa1flox/flox mice (B), it is absent in the urothelium of tamoxifen-injected UBC-Cre/ERT2/Foxa1loxp/loxp bladders (F). Expression of the terminal marker of urothelial differentiation UPKIII is detected in both tamoxifen-injected control Foxa1loxp/loxp bladders (C), as well as in tamoxifen-injected UBC-Cre/ERT2/Foxa1loxp/loxp urothelium (G). Both Foxa1loxp/loxp mice and UBC-Cre/ERT2/Foxa1loxp/loxp mice bladders do not exhibit urothelial proliferation as shown by BrdU staining (D and H, respectively).
Sexually Dimorphic Histological Phenotypes of Foxa1 Knockout Murine Urothelium
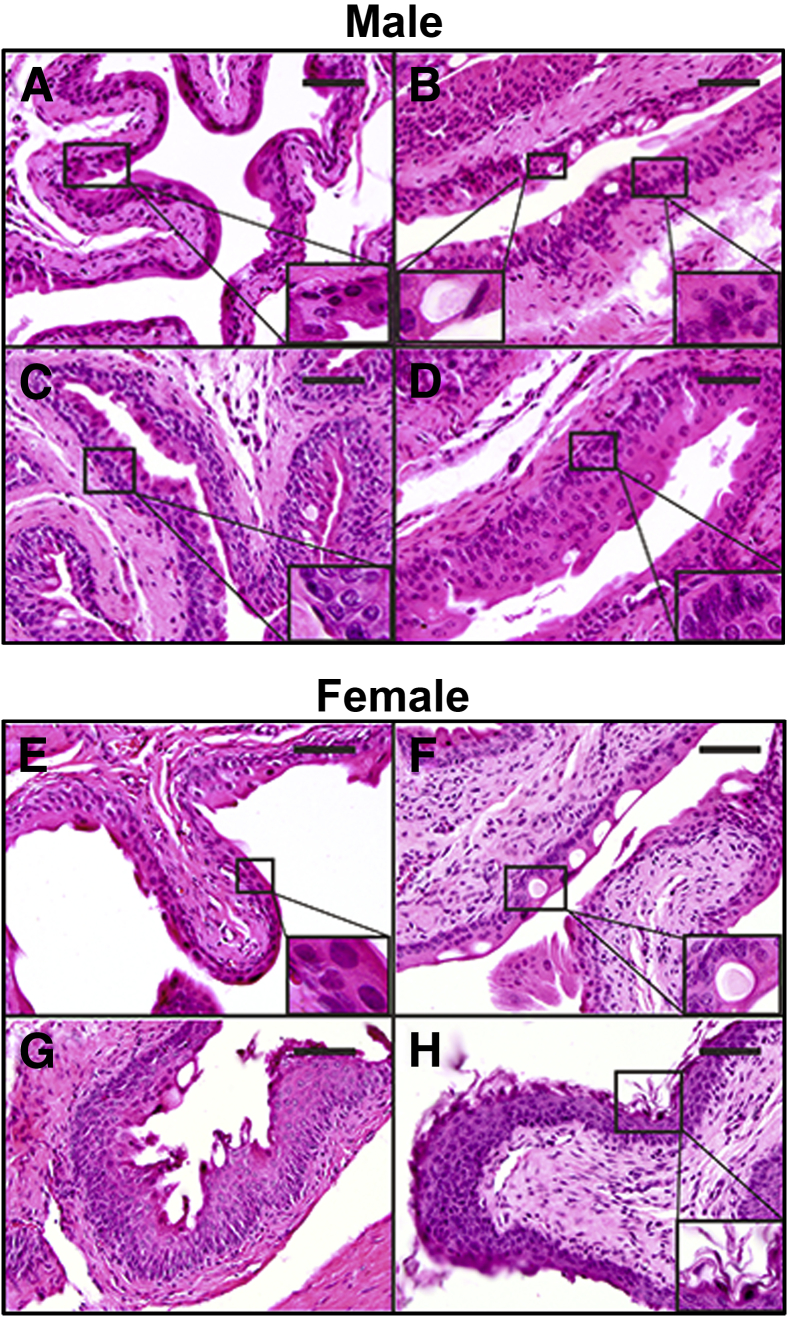
Bladder tissue dissected from tamoxifen-injected, genetic control Foxa1loxp/loxp mice exhibited typical murine urothelial morphology, consisting of a superficial umbrella cell layer and a single layer each of intermediate and basal cells (Figure 3A). Bladder tissue dissected from male UBC-Cre/ERT2/Foxa1loxp/loxp mice obtained 3 months after tamoxifen injection revealed basal cell hyperplasia (Figure 3B) and degenerative vacuoles within the umbrella cell layer (Figure 3B). The presence of urothelial hyperplasia was also detected 6 months after tamoxifen-induced Foxa1 knockout (Figure 3, C and D). Nine of the 10 male Foxa1 knockout mice developed urothelial hyperplasia, and the other mouse developed squamous metaplasia. Importantly, urothelial hyperplasia was significantly associated with Foxa1 knockout in male mice (P < 0.0005; Fisher's exact test). We did not detect hyperplasia in any Foxa1loxp/loxp control male mice after tamoxifen injection.
Figure 3.
Foxa1 knockout results in hyperplasia in male mice and keratinizing squamous metaplasia in female mice. A: Hematoxylin and eosin (H&E) of control Foxa1loxp/loxp male mice (6 months after tamoxifen injection) reveals a single layer of superficial umbrella cells and a single layer of intermediate and basal urothelium (inset). B: Analysis of bladder tissue dissected from male UBC-Cre/ERT2/Foxa1loxp/loxp mice 3 months after tamoxifen injection reveals the presence of degenerative vacuoles (inset, bottom left) and basal hyperplasia (inset, bottom right) of urothelium. C and D: Basal cell hyperplasia is also present in bladder tissue dissected from male UBC-Cre/ERT2/Foxa1loxp/loxp mice 6 months after tamoxifen injection. The insets show basal cell hyperplasia. E: Histological analysis of female control Foxa1loxp/loxp male mice (6 months after tamoxifen-injection) reveals urothelial morphologic findings identical to those of male mice, typical of normal bladder tissue. Inset shows normal bladder urothelium. F: Analysis of bladder tissue dissected from female UBC-Cre/ERT2/Foxa1loxp/loxp mice 3 months after tamoxifen injection reveals presence of degenerative vacuoles (inset) similar to those observed in male UBC-Cre/ERT2/Foxa1loxp/loxp mice. G and H: However, we also detected keratinizing squamous metaplasia in female UBC-Cre/ERT2/Foxa1loxp/loxp mice 3 months and 6 months after tamoxifen injection, with hyperkeratinization (inset and H). Scale bars: 25 μm.
Bladder tissue dissected from female Foxa1loxp/loxp control mice (Figure 3E) 6 months after tamoxifen injection appeared identical to that dissected from male Foxa1loxp/loxp control mice. Moreover, analysis of bladder tissue dissected from female UBC-Cre/ERT2/Foxa1loxp/loxp mice obtained 3 months after tamoxifen injection revealed the appearance of degenerative vacuoles within the umbrella cell layer (Figure 3F) similar to those observed in the male mice at 3 months (Figure 3B).
Phenotypic alterations consistent with KSM were observed in female UBC-Cre/ERT2/Foxa1loxp/loxp mice after FOXA1 knockout 3 and 6 months after tamoxifen injection (Figure 3, G and H). Seven of 10 female Foxa1 knockout mice developed squamous metaplasia, and one other mouse exhibited areas of cellular hyperplasia. As was the case with urothelial hyperplasia in male Foxa1 knockout mice, Foxa1 knockout in female mice was significantly associated with squamous differentiation (P = 0.001; Fisher's exact test). These results indicate that Foxa1 expression is required to maintain normal differentiation of urothelium in male and female mice, and genetic ablation of Foxa1 in the urothelium has sex-specific effects on urothelial differentiation.
Microarray Analysis Reveals Sex-Specific Alterations in Gene Expression after Foxa1 Knockout
We performed microarray analysis to confirm the sex-dependent nature of the histological phenotypes observed after Foxa1 knockout in UBC-Cre/ERT2/Foxa1loxp/loxp mice. In addition, we used this approach to identify the most significantly up-regulated genes after Foxa1 ablation in male and female mice. Microarray analysis and separation of data by sex (Supplemental Tables S1–S3) revealed a heterogeneous list of up-regulated genes in male UBC-Cre/ERT2/Foxa1loxp/loxp mice, including alterations in the expression of tumor suppressors and various transcription factors (Table 3). On the other hand, 6 of the 10 most overexpressed genes in female UBC-Cre/ERT2/Foxa1loxp/loxp mice compared with control Foxa1loxp/loxp mice have been previously associated with keratinocyte differentiation, including Ck14,20 Krt6a/Ck6a,21 Lipk,22 Sprr2F,23 Dsg,24 and Krt10/Ck1025 (Table 3). These findings are consistent with histological evidence of KSM (Figure 3, G and H) and previous reports of an association between FOXA1 loss and squamous differentiation in humans.10,12,13
Table 3.
List of Significantly Up-Regulated Genes After Foxa1 Knockout in Male and Female Mice
| Male Foxa1 knockout |
Female Foxa1 knockout |
||||
|---|---|---|---|---|---|
| Gene | Log fold change | P value | Gene | Log fold change | P value |
| Ldoc1 | 3.95 | 0.003008 | Krt14 | 2.41 | 0.002304 |
| Lypd5 | 3.76 | 0.001831 | Krt6a | 2.11 | 0.071096 |
| Ighg3 | 2.09 | 0.005952 | Lipk | 2.09 | 0.001091 |
| Retnla | 2.06 | 0.092382 | Sprr2f | 2.05 | 0.018622 |
| Dmbt1 | 2.00 | 0.034411 | Dsg3 | 1.84 | 0.035792 |
| Kap | 1.96 | 0.000547 | Ppbp | 1.78 | 0.076981 |
| Sdr16c6 | 1.88 | 0.008045 | Krt10 | 1.58 | 0.00112 |
| Lipk | 1.82 | 0.095307 | Skint3 | 1.57 | 0.022924 |
| Mup4 | 1.53 | 0.000946 | Calcb | 1.49 | 0.074217 |
| Anxa9 | 1.51 | 0.037675 | Ephx3 | 2.76 | 0.000339 |
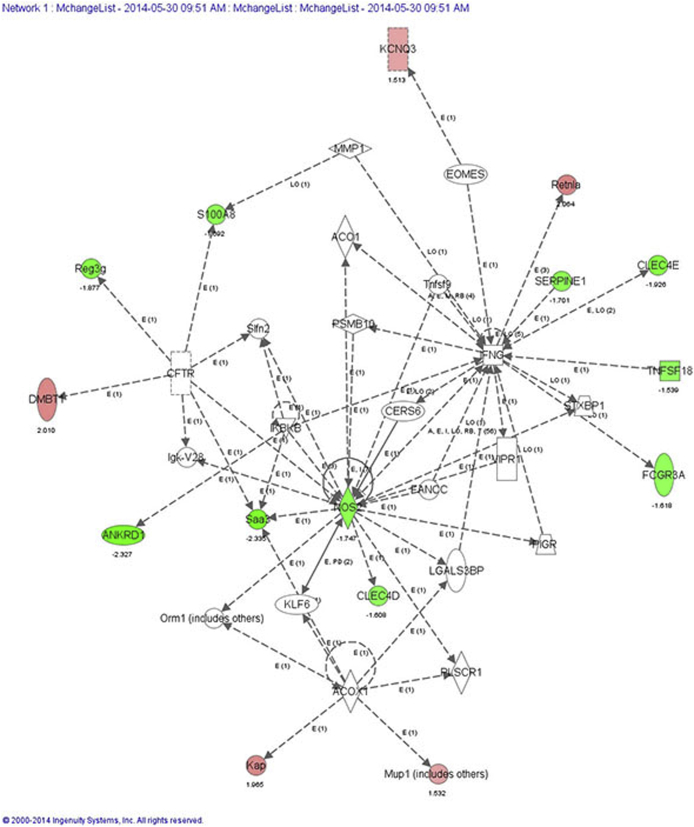
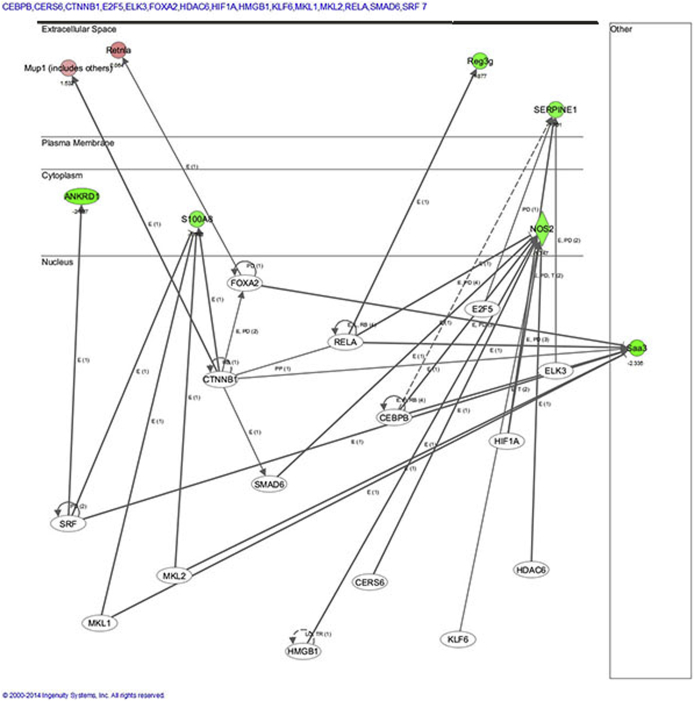
We next used IPA to further analyze the microarray data to identify networks and pathways specifically affected by Foxa1 knockout in male and female bladder tissue that may potentially be altered in human disease. In male Foxa1 knockout bladders, the top network identified was inflammatory disease, inflammatory response, and cell cycle. This pathway consists of several different genes that may play important roles in human bladder disease in the context of FOXA1 inactivation. For example, genes whose expression is associated with inflammation included Clec4d and Clec4e, Nos2, S100a8, Saa3, and Reg3g, a bactericidal lectin (Supplemental Figure S1). In addition, IPA identified a potential role for Dmbt1, Kap, Retnla/b, and Serpine1, which are all implicated in tumorigenesis. We then used the Upstream Analysis function in IPA to identify potential transcriptional regulators activated by Foxa1 knockout in male bladders (Supplemental Figure S2). Among these transcriptional regulators was CTNNB1/β-catenin, which has been recently linked to bladder tumorigenesis in male mice,26 suggesting a potential mechanism whereby loss of FOXA1 drives activation of β-catenin during male bladder tumorigenesis.
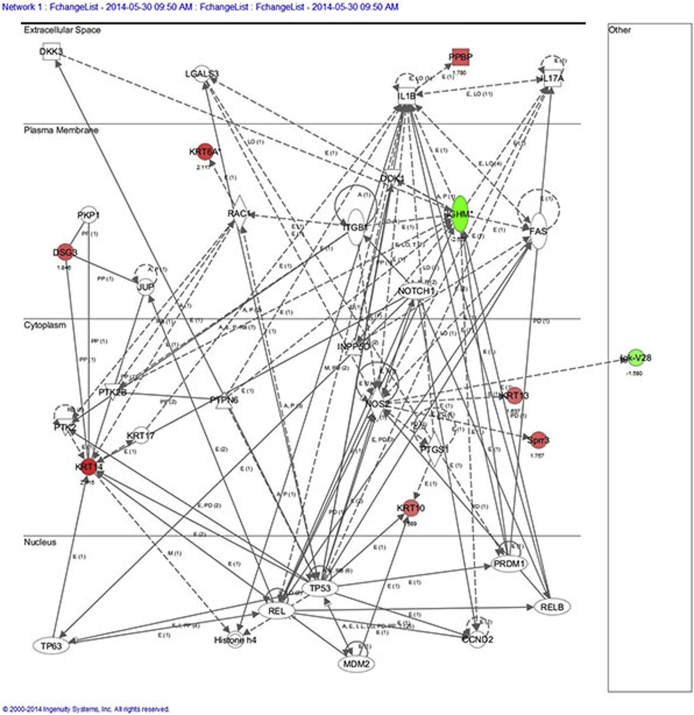
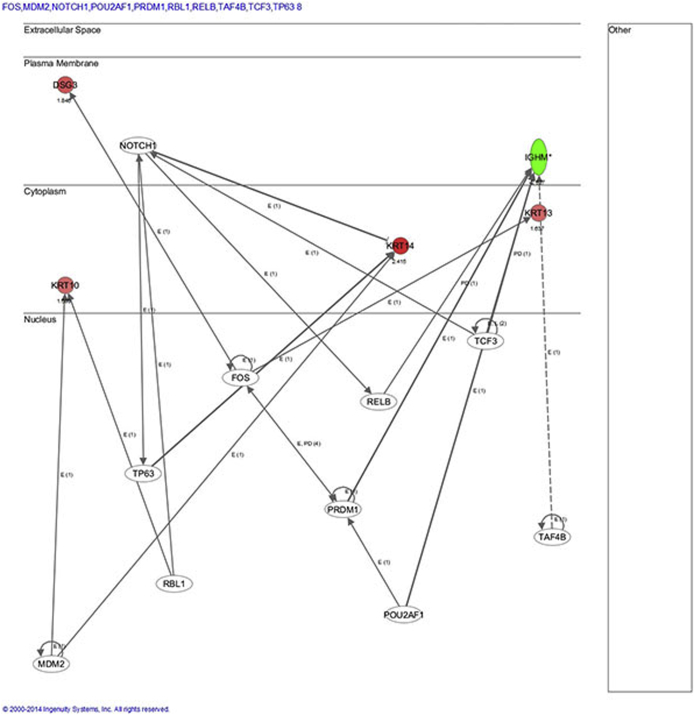
IPA analysis of female bladder microarray results identified cellular growth and proliferation, tissue development, and inflammatory response as the top disease network associated with Foxa1 knockout. Another disease network associated with Foxa1 knockout in female bladders included dermatological diseases (DSG, KRT10, KRT14, and KRT6A). This correlates well with the observed histological alterations after Foxa1 knockout in female mice (Supplemental Figure S3). We next performed Upstream Analysis to identify potential transcriptional regulators, which may additionally contribute to alterations in gene expression after Foxa1 knockout in female bladders, focusing on Krt14 and Krt10 because these keratins are implicated in human bladder cancer27–29 and associated with Foxa1/FOXA1 inactivation in murine and human tumors.30 Upstream analysis suggested Tp63 activation of Krt14, which has been previously implicated in bladder development and malignancy,31,32 as being potential factors associated with altered gene expression after Foxa1 knockout in female mice (Supplemental Figure S4). In addition, upstream pathway analysis suggested that the activation of MDM2 and RBL1 may be in part responsible for increased expression of Krt10. In summary, IPM analysis identified several interesting avenues for future studies.
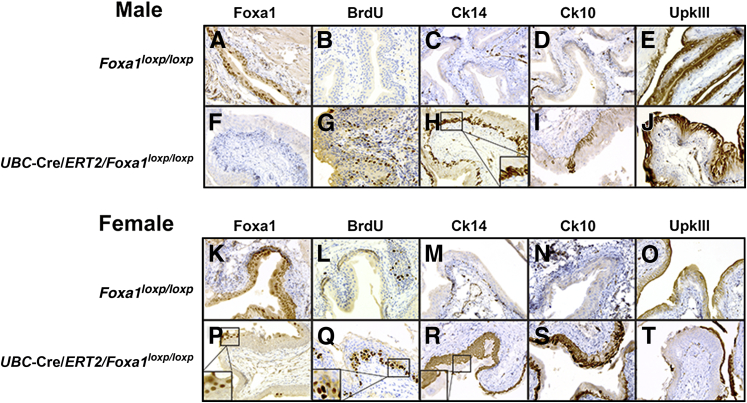
IHC performed on tissue dissected from Foxa1loxp/loxp (Figure 4, A and K) and UBC-Cre/ERT2/Foxa1loxp/loxp (Figure 4, F and P) confirmed that Foxa1 knockout was maintained 6 months after tamoxifen injection. In keeping with histological evidence of urothelial hyperplasia in male Foxa1 knockout mice and KSM in female Foxa1 knockout mice, urothelium in Foxa1 knockout bladders was proliferative by BrdU staining (Figure 4, B, G, L, and Q). Interestingly, BrdU staining was concentrated within the basal cell layer of the urothelium in female UBC-Cre/ERT2/Foxa1loxp/loxp tissue (Figure 4Q).
Figure 4.
Foxa1 knockout results in increased urothelial proliferation and alterations in cytokeratin expression. Immunostaining of Foxa1 (A), bromodeoxyuridine (BrdU) (B), cytokeratin 14 (Ck14) (C), cytokeratin 10 (Ck10) (D), and uroplakin III (UpkIII) (E) in male control Foxa1loxp/loxp mice 6 months after tamoxifen injection reveals normal expression patterns. Immunostaining of Foxa1 (F), BrdU (G), Ck14 (H), Ck10 (I), and UpkIII (J) in male UBC-Cre/ERT2/Foxa1loxp/loxp mice 6 months after tamoxifen injection shows that Foxa1 knockout activates urothelial proliferation and expansion of Ck14 and Ck10 expression. The inset in H shows the basal expression pattern of Ck14 after FOXA1 knockout. Immunostaining of Foxa1 (K), BrdU (L), Ck14 (M), Ck10 (N), and UpkIII (O) in female control Foxa1loxp/loxp mice 6 months after tamoxifen injection shows an expression pattern identical to male mice (A–E). Immunostaining of Foxa1 (P), BrdU (Q), Ck14 (R), Ck10 (S), and UpkIII (T) in female UBC-Cre/ERT2/Foxa1loxp/loxp mice 6 months after tamoxifen injection shows that Foxa1 knockout activates basal urothelial cell proliferation (inset and Q) and expansion of Ck14 and Ck10 expressing urothelial cells. In addition, areas of keratinizing squamous metaplasia were negative for UpkIII expression. P, inset: Retention of Foxa1 expression in small subset of superficial urothelium. R, inset: Foxa1+ superficial urothelium are Ck14.
Normally, Ck14 expression is extremely low in murine urothelium (Figure 4, C and M). However, Foxa1 knockout resulted in an expansion of the Ck14-positive cell population in the basal layer of male UBC-Cre/ERT2/Foxa1loxp/loxp mice (Figure 4H) and a remarkable expansion of Ck14-positive cells throughout the urothelium in female UBC-Cre/ERT2/Foxa1loxp/loxp bladder tissue (Figure 4R), confirming our microarray results.
Ck10 protein is not detected in normal urothelium (Figure 4, D and N) but was expanded in male UB-Cre/ERT2/Foxa1loxp/loxp bladder tissue (Figure 4I) and was detected at even greater levels in the urothelium of female UBC-Cre/ERT2/Foxa1loxp/loxp mice (Figure 4S), also consistent with our microarray results.
The terminal differentiation marker UpkIII is normally detected in murine urothelium (Figure 4, E and O) and was similarly present in the urothelium of male UBC-Cre/ERT2/Foxa1loxp/loxp bladders (Figure 4J). However, areas of squamous differentiation in the urothelium of female UBC-Cre/ERT2/Foxa1loxp/loxp bladders exhibited a loss of UpkIII expression (Figure 4T), consistent with the development of KSM.33 These results confirm that Foxa1 expression is required for the maintenance of a differentiated phenotype in the urothelium and that genetic ablation of Foxa1 results in sexually dimorphic phenotypes. Because FOXA1 loss is common in human UBC, increases in CK14 expression after FOXA1 knockout suggested that a similar relationship may exist in human UBC.
In Agreement with Knockout Mouse Studies, FOXA1 Loss Is Associated with High CK14 Expression in Human Bladder Cancer Specimens
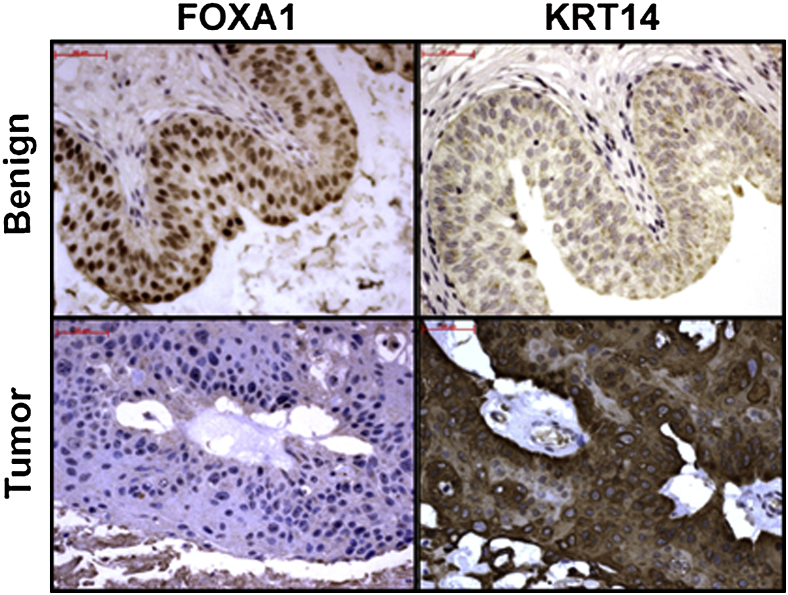
Increased CK14 expression has been reported in human UBC.11,34 In addition, basal UBC tumors express high CK14 levels, as well as diminished FOXA1 expression.12 Our studies indicate that ablation of Foxa1 expression is strongly associated with increased Ck14 expression throughout the bladder epithelium in female Foxa1 knockout mice and enriched in the basal urothelium of male Foxa1 knockout mice. In keeping with these observations, we used the human UBC cohort to determine whether the relationship between these markers extended to human disease. Interestingly, the prevalence of CK14 expression was more often seen in FOXA1-negative human bladder tumors (44%) compared with FOXA1-positive tumors (17%) (Spearman's test; Table 4 and Figure 5). On the other hand, CK10 expression in human FOXA1-negative and FOXA1-positive tumors was similar (24% versus 31%) (Spearman's test; data not shown). However, because FOXA1 expression is decreased and CK14 expression is increased with advancing tumor stage, we tested whether tumor stage was confounding the association between FOXA1 loss and CK14 gain. Implementation of the extended Mantel-Haenszel test-stratifying groups by tumor stage revealed that the association between decreased FOXA1 expression and increased CK14 expression is statistically significant (P = 0.004), independent of tumor stage. These results confirm a significant association between FOXA1 loss and CK14 gain in human UBC, first identified in our mouse model, and subsequently validated in our patient cohort, independent of tumor stage.
Table 4.
Correlation of Immunohistochemical Scores for FOXA1 with Cytokeratin (CK)14 and CK10 in Human Cystectomy Samples
| CK | ρ | P value | n |
|---|---|---|---|
| CK14 | −0.35 | <0.001 | 446 |
| CK10 | 0.04 | 0.429 | 427 |
Figure 5.
In humans, absence of FOXA1 expression is significantly associated with increased cytokeratin 14 (CK14) expression, independent of tumor stage. Representative immunostaining of human benign and tumor tissue microarray bladder tissue samples for FOXA1 and CK14. Scale bars: 100 μm.
Discussion
Building on findings from our previous studies,10,13 as well as a recent study by the Cancer Genome Atlas11 and others,12 indicating that the loss of FOXA1 expression is a common event in human bladder cancers, we now report that decreased FOXA1 expression is an independent predictor of clinical outcome in UBC (Figure 1 and Table 2). Increasing evidence suggests that deregulation of FOX transcription factors is associated with neoplastic transformation and progression in a variety of malignant tumors.
Previously, we found that FOXA1 loss is associated with high-grade, late-stage UBC, as well as the aggressive phenotypic behavior typical of advanced UBC.10 To determine the prognostic significance of FOXA1 loss in clinical samples, we created a clinically annotated TMA of UBC samples from patients who underwent radical cystectomy. In addition to verifying our initial findings with a separate cohort from our institution, we determined that retention of FOXA1 expression is associated with improved overall survival on multivariate analysis after controlling for tumor stage, patient age, sex, and comorbidity status. This is the first report indicating that the decreased FOXA1 expression is independently associated with lower overall survival of UBC patients. The survival analysis performed here was limited in that recurrence-free and disease-specific survival could not be assessed because of the relatively low number of events (n = 39 for recurrence and n = 8 for UBC-specific deaths). In this study, we sought to examine tumor biology independent of the effects of systemic chemotherapy. Therefore, the TMA was specifically constructed to exclude those who underwent neoadjuvant chemotherapy. Because neoadjuvant therapy is now often used in the management of muscle-invasive bladder cancer to treat presumed micrometastases, future studies are needed to assess whether neoadjuvant chemotherapy alters the expression of FOXA1 or if its expression predicts response to chemotherapy.35
In our animal studies, Foxa1 knockout resulted in hyperplasia in male mice and KSM in female mice. These results offer further evidence that expression of Foxa1 is required to maintain differentiation of the urothelium, much as is the case for prostate epithelium.36 Men are at higher risk for developing UBC, and increased incidence of UBC among men has been historically attributed to higher rates of smoking and industrial exposure to carcinogens. As these epidemiological distinctions have subsided, there has been increased interest in determining the role of sex steroid receptors (and circulating sex steroids) in UBC incidence and progression. For instance, androgen receptor knockout prevented carcinogen-induced UBC in male and female mice, as did androgen deprivation.37 Moreover, knockout of estrogen receptor-α and estrogen receptor-β reportedly affects bladder tumorigenesis after carcinogen exposure.38,39 Both FOXA1 and FOXA2 physically interact with the androgen receptor,40,41 and FOXA1 interacts with estrogen receptor-α. Interestingly, genetic ablation of both Foxa1 and Foxa2 in the liver of mice reverses the sex differences in carcinogen-induced hepatocellular carcinoma.42 The ability of FOXA1 to interact with steroid receptors and its ability to modulate carcinogenesis in male versus female mice suggests in liver and now bladder a role for FOXA1 in sex-associated differences in UBC. However, forced overexpression of androgen receptor and estrogen receptors in the presence and absence of FOXA1 and steroid treatment in human bladder cancer cell lines did not result in detectable alterations in gene expression patterns similar to those observed in the in vivo experiments (data not shown). These findings may suggest that additional transcription factors and/or epigenetic alterations are active in sex-specific bladder tumorigenesis after inactivation of Foxa1 in mice and FOXA1 in humans. In addition to interacting with sex steroid receptors, we recently reported that FOXA1 interacts with the nuclear factor one family of transcription factors.43 Future identification of novel FOXA1-interacting proteins important for bladder cancer will reveal important insights into the molecular pathogenesis of this common malignant neoplasm.
This study also confirmed a strong association between the absence of FOXA1 expression and increased CK14 levels in human UBC. An association between FOXA1 and CK14 in basal and squamous UBC was recently found by molecular subtyping studies of the Cancer Genome Atlas and by others.11,12 In addition, markers for these proteins have been suggested to be useful for the identification of specific molecular subtypes of UBC.30 This pattern was also mirrored after Foxa1 knockout in the bladders of mice and suggests that this model is appropriate for future studies regarding the relationship between FOXA1 and CK14 expression in human UBC. Volkmer et al44 recently found that a cell population enriched for high CK14 expression is capable of causing various differentiation states present in UBC tumors, suggesting that the tumor cell of origin model may apply to UBC. Moreover, Volkmer et al44 also found that increased CK14 is associated with worse overall survival in UBC, as well as increased tumor recurrence and progression of pTa tumors. Although we did not find significant associations between CK14 expression and overall survival (data not shown), we observed that CK14 expression was associated with increasing tumor stage. Because increased CK14 is potentially associated with a tumor-initiating cell phenotype in UBC, and we found that FOXA1 loss is associated with the aggressive phenotype attributed to UBC,10 diminished FOXA1 expression may also be associated with this molecular phenotype. Future mechanistic studies will focus on the relationship between FOXA1 loss and increased CK14 in UBC.
In summary, our results indicate that loss of FOXA1 expression is an independent adverse predictor of overall survival in UBC. In addition, we found that abrogation of Foxa1 in mice results in sexually dimorphic histological alterations and an increase in the expression of Ck14, which is also associated with decreased FOXA1 expression in humans. These results provide further evidence of an important role of FOXA1 in human UBC progression and clinical outcome and provide important insight into the potential role of forkhead family members in the sexual dimorphic nature of UBC in regard to incidence and disease progression.
Acknowledgments
We thank Tom Case and Manik Paul for their technical help in conducting these experiments.
Footnotes
Supported by National Cancer Institute grants 1K99CA172122 and R00CA172122 (D.J.D.) and 1P01CA165980-01 (X.-R.W.); a Young Investigator Award from the Bladder Cancer Advocacy Network (D.J.D.); National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK055748-15 (R.J.M.); the Vanderbilt Medical Scholars Program and NIH CTSA grant TL1 TR000447 (O.L.T.); the National Center for Advancing Translational Sciences, NIH, Vanderbilt-Ingram Cancer Center support grant 5P30 CA068485; and the Vanderbilt Institute for Clinical and Translational Research grant support UL1 TR000445.
Disclosures: D.J.D., P.E.C., and R.J.M. have filed a patent application (PCT/US2011/059762) for the use of FOXA1 as a diagnostic and/or prognostic marker for bladder cancer. There are no further products in development or marketed products to declare.
Supplemental Data
Supplemental Figure S1.
Most enriched pathways after Foxa1 knockout in male bladders. Ingenuity pathway analysis (IPA) identified inflammatory disease, inflammatory response, and cell cycle as the top network after Foxa1 knockout in male bladders. Genes associated with this pathway included Clec4d, Clec4e, Nos2, S100a8, Saa3, and Reg3g. In addition, IPA identified a potential role for Dmbt1, Kap, Retnla/b, and Serpine1, all which are implicated in tumorigenesis.
Supplemental Figure S2.
Upstream analysis focused on transcription factors after Foxa1 knockout in male bladders. Transcriptional regulators implicated in induction of urothelial hyperplasia included CTNNB1/β-catenin, suggesting a potential mechanism whereby loss of Foxa1 drives activation of β-catenin during male bladder tumorigenesis.
Supplemental Figure S3.
Most enriched pathways after Foxa1 knockout in female bladders. Ingenuity pathway analysis of female bladder microarray results identified cellular growth and proliferation, tissue development, and inflammatory response as the top disease network associated with Foxa1 knockout. In addition, this approach identified dermatological diseases (Dsg, Krt10, Krt14, and Krt6A) as a top enriched disease network in female Foxa1 knockout mice.
Supplemental Figure S4.
Upstream analysis focused on transcription factors after Foxa1 knockout in female bladders. For this analysis, focus was placed on identifying the transcriptional regulators of Krt14 and Krt10 because these keratins are implicated in human bladder cancer and associated with Foxa1/FOXA1 inactivation in murine and human tumors. Upstream analysis suggested Tp63 activation of Krt14. In addition, upstream pathway analysis suggested that the activation of MDM2 and RBL1 may be in part responsible for increased expression of Krt10.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Fajkovic H., Halpern J.A., Cha E.K., Bahadori A., Chromecki T.F., Karakiewicz P.I., Breinl E., Merseburger A.S., Shariat S.F. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29:457–463. doi: 10.1007/s00345-011-0709-9. [DOI] [PubMed] [Google Scholar]
- 3.Hall M.C., Chang S.S., Dalbagni G., Pruthi R.S., Seigne J.D., Skinner E.C., Wolf J.S., Jr., Schellhammer P.F. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Grossman H.B., Natale R.B., Tangen C.M., Speights V.O., Vogelzang N.J., Trump D.L., deVere White R.W., Sarosdy M.F., Wood D.P., Jr., Raghavan D., Crawford E.D. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Lynch C.F., Cohen M.B. Urinary system. Cancer. 1995;75:316–329. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Ehdaie B., Maschino A., Shariat S.F., Rioja J., Hamilton R.J., Lowrance W.T., Poon S.A., Al-Ahmadie H.A., Herr H.W. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol. 2012;187:74–79. doi: 10.1016/j.juro.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oottamasathien S., Wang Y., Williams K., Franco O.E., Wills M.L., Thomas J.C., Saba K., Sharif-Afshar A.R., Makari J.H., Bhowmick N.A., DeMarco R.T., Hipkens S., Magnuson M., Brock J.W., 3rd, Hayward S.W., Pope JCt, Matusik R.J. Directed differentiation of embryonic stem cells into bladder tissue. Dev Biol. 2007;304:556–566. doi: 10.1016/j.ydbio.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi D., Molotkov A., Batourina E., Schneider K., Dan H., Reiley M., Laufer E., Metzger D., Liang F., Liao Y., Sun T.T., Aronow B., Rosen R., Mauney J., Adam R., Rosselot C., Van Batavia J., McMahon A., McMahon J., Guo J.J., Mendelsohn C. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell. 2013;26:469–482. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeGraff D.J., Clark P.E., Cates J.M., Yamashita H., Robinson V.L., Yu X., Smolkin M.E., Chang S.S., Cookson M.S., Herrick M.K., Shariat S.F., Steinberg G.D., Frierson H.F., Wu X.R., Theodorescu D., Matusik R.J. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PLoS One. 2012;7:e36669. doi: 10.1371/journal.pone.0036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi W., Porten S., Kim S., Willis D., Plimack E.R., Hoffman-Censits J., Roth B., Cheng T., Tran M., Lee I.L., Melquist J., Bondaruk J., Majewski T., Zhang S., Pretzsch S., Baggerly K., Siefker-Radtke A., Czerniak B., Dinney C.P., McConkey D.J. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strand D.W., DeGraff D.J., Jiang M., Sameni M., Franco O.E., Love H.D., Hayward W., Lin-Tsai O., Wang A., Cates J.M., Sloane B., Schoenmakers E., Chatterjee K., Matusik R.J., Hayward S. Deficiency in Metabolic Regulators PPARγ and PTEN cooperates to drive keratinizing squamous metaplasia in novel models of human tissue regeneration. Am J Pathol. 2012;182:1950–1961. doi: 10.1016/j.ajpath.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Allred D.C., Clark G.M., Elledge R., Fuqua S.A., Brown R.W., Chamness G.C., Osborne C.K., McGuire W.L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 17.Morgan T.M., Barocas D.A., Chang S.S., Phillips S.E., Salem S., Clark P.E., Penson D.F., Smith J.A., Jr., Cookson M.S. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol. 2013;31:871–877. doi: 10.1016/j.urolonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y., Huo L., Liu P., Sneige N., Sun X., Ueno N.T., Lucci A., Buchholz T.A., Valero V., Cristofanilli M. Polycomb group protein EZH2 is frequently expressed in inflammatory breast cancer and is predictive of worse clinical outcome. Cancer. 2011;117:5476–5484. doi: 10.1002/cncr.26179. [DOI] [PubMed] [Google Scholar]
- 19.Gao N., LeLay J., Vatamaniuk M.Z., Rieck S., Friedman J.R., Kaestner K.H. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam H., Sehgal L., Kundu S.T., Dalal S.N., Vaidya M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell. 2011;22:4068–4078. doi: 10.1091/mbc.E10-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao H., Sayers J.M., Wilson N.J., Irvine A.D., Mellerio J.E., Baselga E., Bayliss S.J., Uliana V., Fimiani M., Lane E.B., McLean W.H., Leachman S.A., Smith F.J. A spectrum of mutations in keratins K6a, K16 and K17 causing pachyonychia congenita. J Dermatol Sci. 2007;48:199–205. doi: 10.1016/j.jdermsci.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Toulza E., Mattiuzzo N.R., Galliano M.F., Jonca N., Dossat C., Jacob D., de Daruvar A., Wincker P., Serre G., Guerrin M. Large-scale identification of human genes implicated in epidermal barrier function. Genome Biol. 2007;8:R107. doi: 10.1186/gb-2007-8-6-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs S., Fijneman R., Wiegant J., van Kessel A.G., van De Putte P., Backendorf C. Molecular characterization and evolution of the SPRR family of keratinocyte differentiation markers encoding small proline-rich proteins. Genomics. 1993;16:630–637. doi: 10.1006/geno.1993.1240. [DOI] [PubMed] [Google Scholar]
- 24.Rickman L., Simrak D., Stevens H.P., Hunt D.M., King I.A., Bryant S.P., Eady R.A., Leigh I.M., Arnemann J., Magee A.I., Kelsell D.P., Buxton R.S. N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma. Hum Mol Genet. 1999;8:971–976. doi: 10.1093/hmg/8.6.971. [DOI] [PubMed] [Google Scholar]
- 25.Muller F.B., Huber M., Kinaciyan T., Hausser I., Schaffrath C., Krieg T., Hohl D., Korge B.P., Arin M.J. A human keratin 10 knockout causes recessive epidermolytic hyperkeratosis. Hum Mol Genet. 2006;15:1133–1141. doi: 10.1093/hmg/ddl028. [DOI] [PubMed] [Google Scholar]
- 26.Lin C., Yin Y., Stemler K., Humphrey P., Kibel A.S., Mysorekar I.U., Ma L. Constitutive beta-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 2013;73:5914–5925. doi: 10.1158/0008-5472.CAN-12-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harnden P., Southgate J. Cytokeratin 14 as a marker of squamous differentiation in transitional cell carcinomas. J Clin Pathol. 1997;50:1032–1033. doi: 10.1136/jcp.50.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjodahl G., Lauss M., Lovgren K., Chebil G., Gudjonsson S., Veerla S., Patschan O., Aine M., Ferno M., Ringner M., Mansson W., Liedberg F., Lindgren D., Hoglund M. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 29.Sjodahl G., Lovgren K., Lauss M., Patschan O., Gudjonsson S., Chebil G., Aine M., Eriksson P., Mansson W., Lindgren D., Ferno M., Liedberg F., Hoglund M. Toward a molecular pathologic classification of urothelial carcinoma. Am J Pathol. 2013;183:681–691. doi: 10.1016/j.ajpath.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Biton A., Bernard-Pierrot I., Lou Y., Krucker C., Chapeaublanc E., Rubio-Perez C., Lopez-Bigas N., Kamoun A., Neuzillet Y., Gestraud P., Grieco L., Rebouissou S., de Reynies A., Benhamou S., Lebret T., Southgate J., Barillot E., Allory Y., Zinovyev A., Radvanyi F. Independent component analysis uncovers the landscape of the bladder tumor transcriptome and reveals insights into luminal and basal subtypes. Cell Rep. 2014;9:1235–1245. doi: 10.1016/j.celrep.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Castillo-Martin M., Domingo-Domenech J., Karni-Schmidt O., Matos T., Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol. 2010;28:401–408. doi: 10.1016/j.urolonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Karni-Schmidt O., Castillo-Martin M., Shen T.H., Gladoun N., Domingo-Domenech J., Sanchez-Carbayo M., Li Y., Lowe S., Prives C., Cordon-Cardo C. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol. 2011;178:1350–1360. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang F.X., Bosland M.C., Huang H., Romih R., Baptiste S., Deng F.M., Wu X.R., Shapiro E., Sun T.T. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho P.L., Lay E.J., Jian W., Parra D., Chan K.S. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeks J.J., Bellmunt J., Bochner B.H., Clarke N.W., Daneshmand S., Galsky M.D., Hahn N.M., Lerner S.P., Mason M., Powles T., Sternberg C.N., Sonpavde G. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 36.DeGraff D.D., Grabowska M.M., Case T.C., Yu X., Herrick M.K., Hayward W., Strand D.W., Cates J.M., Hayward S., Gao N., Walter M., Buttyan R., Yi Y., Kaestner K.H., Matusik R.J. Foxa1 deletion in luminal epithelium causes prostatic hyperplasia and alteration of differentiated phenotype. Lab Invest. 2014;94:726–739. doi: 10.1038/labinvest.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto H., Yang Z., Chen Y.T., Ishiguro H., Uemura H., Kubota Y., Nagashima Y., Chang Y.J., Hu Y.C., Tsai M.Y., Yeh S., Messing E.M., Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 38.Hsu I., Chuang K.L., Slavin S., Da J., Lim W.X., Pang S.T., O'Brien J.H., Yeh S. Suppression of ERbeta signaling via ERbeta knockout or antagonist protects against bladder cancer development. Carcinogenesis. 2014;35:651–661. doi: 10.1093/carcin/bgt348. [DOI] [PubMed] [Google Scholar]
- 39.Hsu I., Yeh C.R., Slavin S., Miyamoto H., Netto G.J., Tsai Y.C., Muyan M., Wu X.R., Messing E.M., Guancial E.A., Yeh S. Estrogen receptor alpha prevents bladder cancer via INPP4B inhibited akt pathway in vitro and in vivo. Oncotarget. 2014;5:7917–7935. doi: 10.18632/oncotarget.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao N., Zhang J., Rao M.A., Case T.C., Mirosevich J., Wang Y., Jin R., Gupta A., Rennie P.S., Matusik R.J. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17:1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 41.Yu X., Gupta A., Wang Y., Suzuki K., Mirosevich J., Orgebin-Crist M.C., Matusik R.J. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann N Y Acad Sci. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- 42.Li Z., Tuteja G., Schug J., Kaestner K.H. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabowska M.M., Elliott A.D., DeGraff D.J., Anderson P.D., Anumanthan G., Yamashita H., Sun Q., Friedman D.B., Hachey D.L., Yu X., Sheehan J.H., Ahn J., Raj G., Piston D.W., Gronostajski R.M., Matusik R.J. NFI transcription factors interact with FOXA1 to regulate prostate specific gene expression. Mol Endocrinol. 2014;28:949–964. doi: 10.1210/me.2013-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkmer J.P., Sahoo D., Chin R.K., Ho P.L., Tang C., Kurtova A.V., Willingham S.B., Pazhanisamy S.K., Contreras-Trujillo H., Storm T.A., Lotan Y., Beck A.H., Chung B.I., Alizadeh A.A., Godoy G., Lerner S.P., van de Rijn M., Shortliffe L.D., Weissman I.L., Chan K.S. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A. 2012;109:2078–2083. doi: 10.1073/pnas.1120605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.