Abstract
Ischemic acute kidney injury is a serious untreatable condition. Activation of the G protein α12 (Gα12) subunit by reactive oxygen species is a major cause of tissue damage during renal ischemia-reperfusion injury. Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein that is highly up-regulated during acute kidney injury, but the physiologic significance of this up-regulation is unclear. Here, we report for the first time that Kim-1 inhibits Gα12 activation and protects mice against renal ischemia-reperfusion injury. We reveal that Kim-1 physically interacts with and inhibits cellular Gα12 activation after inflammatory stimuli, including reactive oxygen species, by blocking GTP binding to Gα12. Compared with Kim-1+/+ mice, Kim-1−/− mice exhibited greater Gα12 and downstream Src activation both in primary tubular epithelial cells after in vitro stimulation with H2O2 and in whole kidneys after unilateral renal artery clamping. Finally, we show that Kim-1–deficient mice had more severe kidney dysfunction and tissue damage after bilateral renal artery clamping, compared with wild-type mice. Our results suggest that KIM-1 is an endogenous protective mechanism against renal ischemia-reperfusion injury through inhibition of Gα12.
Acute kidney injury (AKI) is a serious medical condition that most often results from ischemia-reperfusion injury (IRI) and for which there is no effective treatment.1,2 Kidney injury molecule-1 (KIM-1) is a cell-surface glycoprotein receptor3 that is specifically up-regulated on the apical surface of proximal tubular epithelial cells (TECs) after AKI.4 It is a highly sensitive and specific biomarker of tubular injury that is virtually absent in healthy kidneys.5 Both mouse and human KIM-1 (also known as T-cell immunoglobulin, mucin domain-1 protein and hepatitis A virus cellular receptor-1) are small type I transmembrane glycoproteins that belong to the T-cell immunoglobulin mucin gene family.3 Structurally, KIM-1 is made up of an IgV-like domain, a mucin-like domain, a transmembrane domain, and an intracellular domain that is implicated in T-cell signaling.6,7 The pathophysiologic role of KIM-1 signaling in AKI remains unknown.
Here, we reveal a novel interaction between KIM-1 and G protein α-12 (Gα12). Gα12 is a ubiquitously expressed G protein that belongs to the G12 family of G proteins that has pleiotropic effects on cells, including inducing proliferation, focal adhesion assembly, cytoskeletal reorganization, apoptosis, and disruption of tight junctions.8 Recently, Yu et al9 demonstrated that activated Gα12 is a pivotal mediator of TEC injury caused by ischemia-reperfusion. Reactive oxygen species (ROSs) that are generated during ischemia-reperfusion stimulate Gα12 to activate Src-dependent injury pathways. Specifically, Gα12-deficient mice were protected from renal IRI, whereas mice with TEC-specific transgenic overexpression of constitutively active Gα12 exhibited worse tissue damage and delayed recovery after IRI.9 Given the relevance of KIM-1 and Gα12 in AKI, we investigated the biological interaction between these proteins by using in vitro and in vivo models of ischemic AKI.
Materials and Methods
Animal Preparation and Induction of IRI
All mouse experiments were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Western University. C57BL/6 wild-type (Kim-1+/+) mice were obtained from the Charles Rivers Laboratory (Wilmington, MA). Kim-1 (Havcr1) knockout (Kim-1−/−) mice were generously provided by Dr. Andrew N.J. McKenzie (MRC Laboratory of Molecular Biology, Cambridge, UK). The Kim-1−/− mice were generated using C57BL/6 embryos and backcrossed over six generations to the C57BL/6 strain, and homozygous Kim-1−/− were obtained by interbreeding the heterozygotes (Kim-1+/−). Genomic DNA was isolated from mice tail digests, and genotypes were screened with PCR by using primers ASEQ965 5′-ATATCTCAGGAATGGGATTGTGAC-3′ and ASEQ966 5′-CTACTGTATTTAACTGATTTGAAG-3′. Six- to 9-week-old male Kim-1+/+ or Kim-1−/− mice that weighed 20 to 25 g were subjected to unilateral or bilateral renal pedicle ligation for 25 to 35 minutes (as indicated) at 32°C, as described previously.10 To assess Gα12 activity in the ischemic and contralateral kidneys, after reperfusions, the kidneys were removed after being flushed with cold phosphate-buffered saline. Sham controls were treated with the same operative procedure as in the injury group, but kidneys were not clamped.
Renal Function and Histology
Serum creatinine was detected by a Jaffe reaction method with an automated CX5 clinic analyzer (Beckman, Pasadena, CA). Kidney sections (5 to 6 μm) from animals at 24 and 48 hours after reperfusion or sham surgery were stained with periodic acid-Schiff by a trained pathologist (A.H.), and the degree of tubular injury was graded by light microscopy by the same pathologist blinded to mouse strain by using an arbitrary scale that examined proximal tubule dilation, brush-border damage, proteinaceous casts, interstitial widening, and necrosis (0, none; 1, <11%; 2, 11% to 25%; 3, 26% to 45%; 4, 46% to 75%; 5, >75%).
Mass Spectrometry
KIM-1 immune complexes were derived from human embryonic kidney (HEK)-293 cells stably overexpressing hemagglutinin (HA)-tagged KIM-17 or vector alone.11 Plasmid-expressing KIM-1-HA was a kind gift from Dr. Joseph Bonventre (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), and it was generated in a similar manner as described before.7 Briefly, the full-length human KIM-1 with an N-terminal HA tag was generated with PCR by using phKIM1.2 as template and then subcloned into the EcoRV sites of eukaryotic expression vector pcDNA3 (Invitrogen, Carlsbad, CA). On transfection of KIM-1-HA, protein and surface expression were comparable with endogenous KIM-1 expressed in human renal adenocarcinoma cells (Supplemental Figure S1, A and B). Immunoprecipitation (IP) was done with anti-HA antibody (Santa Cruz Biotechnology, Santa Cruz, CA). SDS-PAGE was used to identify unique bands that co-immunoprecipitated with KIM-1, and a target protein band of approximately 38 kDa was excised and subjected to in-gel tryptic digestion, followed by mass spectrometry (microcapillary liquid chromatography/tandem mass spectrometry) and analyzed by the Taplin mass spectrometry facility (Harvard Medical School, Boston, MA).
Cell Lines and Culture
HEK-293 cells stably expressing control vector or human KIM-1 were cultured at 37°C in 5% (v/v) CO2 and maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) that contained 5% (v/v) fetal bovine serum and 800 μg/mL geneticin (G418) sulfate (Santa Cruz Biotechnology). Expression of KIM-1 was confirmed by Western blot analysis. HEK-293 cells were transfected with a truncated version of KIM-1 that contained its cytosolic domain fused to transmembrane domain (kind gift from Dr. Joseph Bonventre, Brigham and Women's Hospital, Harvard Medical School) by using lipofectamine 2000 (Invitrogen). Briefly, this plasmid was generated with PCR by using Not1 restriction sites of human KIM-1 plasmid.7 The resulting construct that contained the transmembrane and cytosolic domain of KIM-1 was subcloned into pFLAG-CMV-3 (Sigma-Aldrich, St. Louis, MO). The expression of the construct was confirmed with Western blot analysis (Supplemental Figure S1C). Kidneys isolated from 3- to 4-week-old C57BL/6 wild-type (Kim-1+/+) or Kim-1−/− mice were cultured to select for proximal TECs as described previously.12 Briefly, kidneys were homogenized, and the cells isolated were cultured for the first 6 days in serum-free Dulbecco’s modified Eagle’s medium/F-12 mixed media (1:1; Invitrogen) supplemented with 5% insulin-transferrin-selenium solution, 5% penicillin-streptomycin solution (Invitrogen), 0.5 μg/mL mouse epidermal growth factor (PeproTech, Rocky Hill, NJ), and 50 ng/mL hydrocortisone (Invitrogen). After 6 days, cells were cultured with the same media mixture supplemented with 5% fetal bovine serum.12
Western Blot Analysis
Confluent monolayer of cell lines (HEK-293 or primary TECs) or kidney cortices were homogenized and lyzed in ice-cold lysis buffer and were centrifuged at 15,000 × g for 10 minutes. The supernatant fluid was collected for Western blot analysis. Protein concentrations were determined by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL), and 50 μg of protein was used as input for the experiment. Proteins (1000 μg) were used for IP or glutathione S-transferase (GST)–fused tetratricopeptide repeat (TPR) domain of Ser/Thr protein phosphatase type 5 (GST-TPR) pull-down experiments. Input and immuneprecipitated samples were electrophoresed on 4% to 20% Mini-PROTEAN TGX gels (Bio-Rad Laboratories, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were blocked with blocking buffer [(tris-buffered saline, 0.1% Tween-20, and either 5% nonfat dried milk (Bioshop, Burlington, ON, Canada) or 5% bovine serum albumin solution (Sigma-Aldrich)] for 30 minutes and were then incubated overnight at 4°C with one of the following primary antibodies: cytosolic domain of human KIM-1 (custom antibody; Thermo Fisher Scientific),4 extracellular domain of human KIM-1 (Dr. Bonventre, Harvard Medical School),13 extracellular domain of mouse Kim-1 (AF1817; R&D Systems, Minneapolis, MN), Gα12/actin/glyceraldehyde-3-phosphate dehydrogenase or c-Src (Santa Cruz Biotechnology), or active Src (pY419-Src) antibody (Invitrogen). Membranes were washed and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (dilution 1:30,000; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature in blocking buffer. Proteins were visualized with SuperSignal West Femto chemiluminescent substrate (Thermo Fisher Scientific). The resulting protein bands were detected by autoradiography (Biomax; Denville Scientific, South Plainfield, NJ) and were scanned with a Brother scanner (Brother Electronics, Dollard-des-Ormeaux, QC, Canada), and the integrated density of each protein band was determined with ImageJ software version 1.49p (NIH, Bethesda, MD). Each densiometric graph represents at least three independent experimental results. To normalize for protein loading, the integrated density of each band was divided by the integrated density of the actin band in the same lane from the same membrane.14
IP and GST-Gα12 Pull-Down Assay
For IP, cells were lyzed with ice-cold lysis buffer [25 mmol/L HEPES (Sigma-Aldrich), 150 mmol/L NaCl, 15 mmol/L MgCl2, 1% Triton X-100, and Mini-protease inhibitors tablet (Roche Diagnostic, Basel, Switzerland)].15 After protein quantification, IP of 1000 μg of lysates was done with antibodies against Gα12, the cytosolic domain of KIM-1,7 or rabbit IgG control (Santa Cruz Biotechnology) and protein-A/G Sepharose beads (Santa Cruz Biotechnology). Lysates (representing 5% of total lysate) and IP samples were analyzed by SDS-PAGE and Western blot analysis as described in Western Blot Analysis. For GST-Gα12 pull-down, KIM-1-HA was in vitro translated and S35-labled in rabbit reticulocyte lysates as described previously.16 Protein lysates were incubated with GST-Gα12 or GST alone and eluted after 3 hours of incubation. Samples were analyzed by SDS-PAGE and autoradiography (Biomax; Denville Scientific, Metuchen, NJ).
Gα12 Activation Assay (GST-TPR Pull Down)
A construct of GST-fused TPR domain of Ser/Thr protein phosphatase type 5 was kindly provided by Dr. Danny N. Dhanasekaran (Temple University, Philadelphia, PA).16 GST-TPR was purified from Escherichia coli and was conjugated to glutathione-agarose beads (Thermo Fisher Scientific) as described.17 GST-TPR–conjugated beads were freshly made for each experiment. Kidney homogenates after IRI or cells unstimulated, stimulated with 5 mmol/L H2O2 (Bio Basic, Amherst, NY) or 2 U/mL α-thrombin (Enzyme Research Laboratories, South Bend, IN) were lyzed with ice-cold lysis buffer [50 mmol/L HEPES (at pH 7.5) (Sigma-Aldrich), 1 mmol/L EDTA, 3 mmol/L dithiothreitol, 2 mmol/L MgSO4, 1% polyoxyethylene (10) lauryl ether (C12E10), and mini-protease inhibitor tablet (Roche Diagnostic, Basel, Switzerland)].9 Supernatant fluids that contained 1 mg of the protein were incubated with GST-TPR coupled with glutathione-agarose beads at 4°C for 5 hours.16 Where indicated, cell lysates were loaded with 1 μmol/L nonhydrolyzable GTP analog (GTPγS; Cytoskeleton, Denver, CO) after protein quantification and were incubated for 15 minutes at room temperature before adding GST-TPR coupled with glutathione-agarose beads. Samples were centrifuged at 5000 × g for 5 minutes and washed with 1× phosphate-buffered saline twice before eluting the bound active Gα12. Both 50 μg of protein (input) and pull-down samples were analyzed by SDS-PAGE and Western blot analysis by probing for Gα12 to represent total (input) and active Gα12 (pull-down) in these samples.
Immunofluorescence and Confocal Microscopy
HEK-293 cells were cultured at subconfluent density on glass coverslips coated with poly-DL-lysine hydrobromide (Sigma-Aldrich). Cells were transfected with green fluorescent protein-tagged Gα12 construct18 by using lipofectamine 2000 (Invitrogen). Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and stained for surface KIM-1 by using antibody against the extracellular domain of KIM-1 overnight at 4°C. The slides were washed and incubated with Alexa 555–conjugated secondary antibody (Molecular Probes, Invitrogen). Coverslips were mounted with Shandon-Mount (Thermo Fisher Scientific) permanent mounting medium. Mice kidneys after IRI were extracted and quartered. The sections were fixed in 4% paraformaldehyde overnight for subsequent paraffin embedding. Kidney cortex sections (10 μm) were sliced at 50-μm intervals by using a Leica RM2125 microtome (Leica Microsystems, Buffalo Grove, IL) and were mounted on glass slides. Sections were blocked with 5% horse serum (Invitrogen) and hybridized overnight at 4°C with either extracellular domain of mouse Kim-1 (dilution 1:50; Sigma-Aldrich) or Gα12 (dilution 1:50; Santa Cruz Biotechnology). Sections were then washed and incubated with the appropriate fluorophore-conjugated secondary antibodies (dilution 1:100; Invitrogen). All of the sections were coverslipped with Shandon-Mount permanent mounting medium before imaging. Samples were viewed with FLUOVIEW X83I confocal microscopy (Olympus, Tokyo, Japan). Data were acquired and analyzed with FLUOVIEW FV10 ASW 4.0 viewer (Olympus), and ImageJ software version 1.49p (NIH) was used to determine Pearson's coefficient for colocalization of KIM-1 and Gα12. Quantification of the number of colocalization score was assessed in five random fields per sample and was done in four independent experiments.
Statistical Analysis
Statistical analyses were done with Prism version 5.01 (GraphPad Software Inc., La Jolla, CA). Mean or median differences between knockout and wild-type mice were compared with unpaired t-tests or U-tests as appropriate, proportions were compared with χ2 tests, and 95% CIs were calculated with the method of Wilson.19 P < 0.05 was considered significant. Error bars represent the SEM unless otherwise indicated.
Results
To identify putative signaling proteins downstream of KIM-1, we performed mass spectrometric analysis on KIM-1 (anti-HA) immunoprecipitates from HEK-293 cells stably overexpressing KIM-1 fused to a c-terminal HA tag7 or vector alone (pcDNA3) as described in Materials and Methods. Of the several target proteins identified (Table 1), we focused on Gα12 because of its previously described roles in the biology of TECs and AKI.9,20
Table 1.
Selected Homo sapiens KIM-1 Interacting Proteins
| Interacting proteins | Accession number |
|---|---|
| Guanine nucleotide-binding protein, α-12 subunit (Gα12) | Q03113 |
| Arginase 1 (liver-type arginase) | P05089 |
| Set protein (phosphatase 2a inhibitor i2pp2a) template activating factor i (Taf-i) | Q01105 |
| Fructose-bisphosphate aldolase c | P09972 |
| Nucleophosmin numatrin | P06748 |
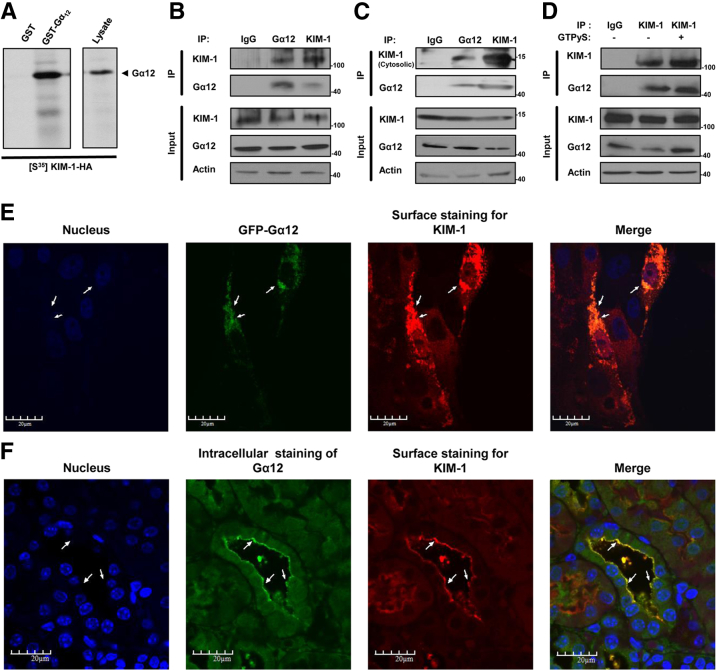
To confirm the mass spectrometric results, we performed pull-down experiments by using S35methionine-labeled KIM-1 from reticulocyte lysates with GST fused to Gα12 to determine whether Gα12 can interact directly with KIM-1.17 We found that KIM-1 with a c-terminal HA tag bound to GST-Gα12 but not unconjugated GST (Figure 1A). Co-IP of Gα12 and KIM-1 by using both anti–KIM-1 and anti-Gα12 but not the control antibody confirmed this interaction (Figure 1B). The cytosolic domain of KIM-1 was sufficient to mediate binding to Gα12 as indicated by co-IP of Gα12 with a truncated version of KIM-1 that contained its cytosolic domain fused to its transmembrane domain and an extracellular FLAG tag (Figure 1C).7 To determine whether activated Gα12 can also interact with KIM-1, we stimulated KIM-1–expressing HEK-293 cell lysates with GTPγS before performing co-IP21; GTPγS stimulation of Gα12 did not alter the interaction with KIM-1 (Figure 1D). To visualize the interaction of Gα12 with KIM-1, HEK-293 cells were cotransfected with KIM-1 and green fluorescent protein-Gα12 and colocalization of both proteins was assessed by confocal microscopy. A substantial proportion of KIM-1 was associated with Gα12 in cells expressing both proteins as indicated by Pearson's coefficient (0.409 ± 0.098; n = 7) (Figure 1E). In addition, we found colocalization of both murine Kim-1 and Gα12 in kidney tissue sections isolated from C57BL/6 wild-type (Kim-1+/+) mice that were subjected to renal IRI (bilateral renal pedicle clamping for 30 minutes followed by 24 hours of reperfusion) to allow for Kim-1 up-regulation (Figure 1F). Taken together, these data suggest that KIM-1 interacts constitutively with Gα12 via its cytosolic domain, independent of Gα12 activation.
Figure 1.
KIM-1 interacts with Gα12. A: Autoradiogram of pull-down with purified GST-Gα12 or GST of in vitro translated S35-methionine-labeled HA-tagged KIM-1. B: IP of KIM-1 and Gα12 in lysates from HEK-293 cells stably expressing human KIM-1 by using anti-Gα12, anti–KIM-1 (against cytosolic), or rabbit IgG control antibodies. C: Lysates of HEK-293 cells expressing truncated transmembrane and cytosolic domain of KIM-1 were subjected to IP with indicated antibodies. D: Lysates of HEK-293 cells stably expressing KIM-1 were pretreated with GTPγS to activate Gα12 and then subjected to IP. The input lane represents 5% of the lysate. Both lysates and IP samples were analyzed by SDS-PAGE and Western blot analysis for KIM-1 (against extracellular domain), Gα12, and actin. Results represent three independent experiments. E: Colocalization of both KIM-1 and Gα12 proteins in HEK-293 cells cotransfected with KIM-1 and GFP-tagged Gα12 constructs. KIM-1 was detected with an antibody against its extracellular domain. F: Kidney cortex cross-section from wild-type (Kim-1+/+) mice that were immunofluorescently stained with anti–Kim-1 (red) and anti-Gα12 antibody after renal artery clamping for 30 minutes, followed by reperfusion for 24 hours. Arrows indicate areas of colocalization between KIM-1/Kim-1 and Gα12. Original magnification: ×600 (E); ×400 (F). Gα12, G protein α-12; GFP, green fluorescent protein; GST, glutathione S-transferase; GTPγS, nonhydrolyzable GTP; HA, hemagglutinin; HEK, human embryonic kidney; IP, immunoprecipitation; KIM-1, Kidney injury molecule-1.
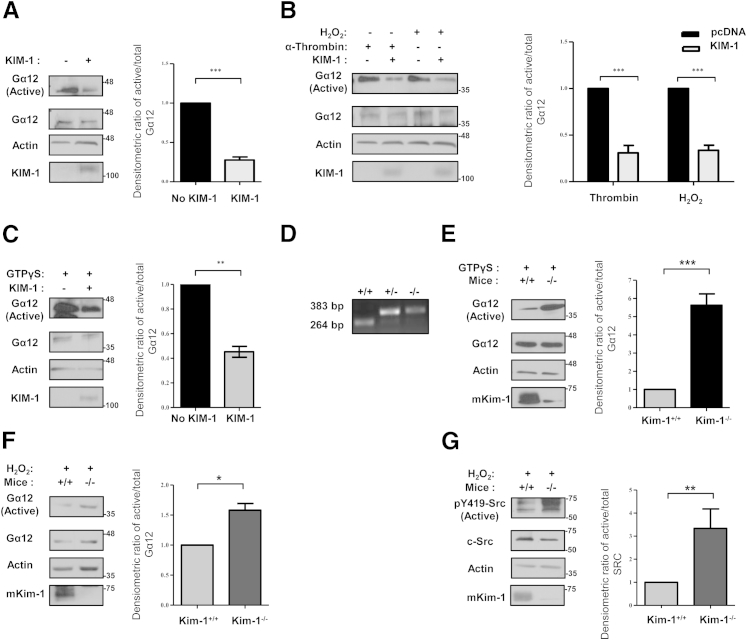
It has long been hypothesized that KIM-1 might play a protective role in AKI.4 Given that Gα12 activation instigates injury pathways in AKI, we hypothesized that KIM-1 might suppress endogenous Gα12 activation in TECs. Because Gα12 is activated by guanine nucleotide exchange, we studied its activation by using a GST-TPR pull-down assay.18 Figure 2A shows that endogenous Gα12 activity is inhibited in cells expressing KIM-1 but not the control vector. Next, to test if KIM-1 expression could inhibit Gα12 activation by physiologic ligands that stimulate its cognate G protein-coupled receptors, we measured the amount of active Gα12 after stimulating HEK-293 cells transiently expressing KIM-1 or control vector with α-thrombin (Figure 2B).9,20 Given that ROSs stimulate Gα12 activation in IRI, we tested whether KIM-1 expression affected Gα12 activation by H2O2; Gα12 activation was significantly blunted in KIM-1–expressing cells compared with control cells on stimulation with either α-thrombin or 5 mmol/L H2O2 (Figure 2B). In addition, we did not observe any effect of the amount of H2O2 used on cell viability (Supplemental Figure S2). Therefore, we suspected that KIM-1 might block nucleotide exchange to Gα12, thereby preventing its activation. To test this hypothesis, we exposed lysates from HEK-293 cells transiently transfected with either control vector or vector encoding KIM-1 to GTPγS to irreversibly lock Gα12 in the active conformation. We observed significantly less GTP-bound Gα12 in KIM-1–expressing cells than in cells not expressing KIM-1 (Figure 2C). Similar results were obtained when we used HEK-293 cells stably expressing KIM-1 or control vector (results not shown).
Figure 2.
KIM-1 inhibits cellular Gα12 activation by blocking GTP-binding. HEK-293 cells were transfected with either control vector (pcDNA) or KIM-1 plasmid. Cells were left untreated (A), stimulated with either 2 U/mL α-thrombin or 5 mmol/L H2O2 for 30 minutes (B), or treated with GTPγS for 15 minutes (C). D: Confirmation of genotype by using genomic DNA of wild-type (+/+), heterozygous (+/−), and homozygous Kim-1 knockout (−/−) C57BL/6 mice by PCR. TECs isolated from wild-type (Kim-1+/+) and Kim-1–deficient (Kim-1−/−) mice were stimulated with GTPγS (E) or 5 mmol/L H2O2 for 30 minutes (F). Samples in A–C, E, and F were subjected to GST-TPR pull-down assay to measure the amount of Gα12 activation. Lysates (total) and pull-down (active) samples were analyzed by SDS-PAGE and Western analysis with antibodies against Gα12, human KIM-1 or mKim-1, and actin where indicated. G: Activated and total Src were detected in total cell lysates from TECs stimulation with H2O2 by Western blot analysis by using anti–p-Src (Py419-Src) phosphospecific antibody and anti–c-Src antibody, respectively. Densitometric analysis of the ratio of active to total Gα12 (or pY419-Src to c-Src) relative to non–KIM-1-expressing cells or wild-type TECs (+/+) is shown as a representation the experiments. Data are expressed as means ± SEM. n = 3 independent experiments. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Gα12, G protein α-12; GST-TPR, glutathione S-transferase–fused tetratricopeptide repeat domain of Ser/Thr protein phosphatase type 5; GTPγS, nonhydrolyzable GTP analog; HEK, human embryonic kidney; KIM-1, kidney injury molecule-1; mKim-1, mouse kidney injury molecule-1; TEC, tubular epithelial cell.
To extend these findings to a physiologically relevant in vitro model, we compared Gα12 activation in primary TECs isolated from previously generated Kim-1–deficient mice (Kim-1−/−) (Figure 2D) to wild-type mice (Kim-1+/+). Concordant with previous reports,22 Kim-1−/− mice were phenotypically normal. Ex vivo culture of TECs from Kim-1+/+ mice resulted in KIM-1 up-regulation as previously reported,4 but it was expectedly absent in TECs isolated from Kim-1−/− mice. Consistent with data in HEK-293 cells, we observed significantly higher amounts of active Gα12 in TEC lysates from Kim-1−/− mice compared with TEC lysates from Kim-1+/+ mice loaded with GTPγS (Figure 2E). Similarly, the amount of Gα12 activation was significantly higher in TEC lysates from Kim-1−/− mice than that from Kim-1+/+ mice on stimulation with H2O2 (Figure 2F). Given that Src was found to intervene the ROS/Gα12-mediated epithelial injury,9 we examined Src activation by using pY419 antibodies (phosphorylated Src) in the total cell lysates from Kim-1−/− and Kim-1+/+ TECs after treatment with H2O2. Src activation was significantly enhanced in TECs from Kim-1−/− mice compared with TECs from Kim-1+/+ mice after H2O2 stimulation (Figure 2G). Taken together, these data suggested that KIM-1 constitutively inhibits Gα12/Src activation by blocking GDP-GTP exchange by Gα12.
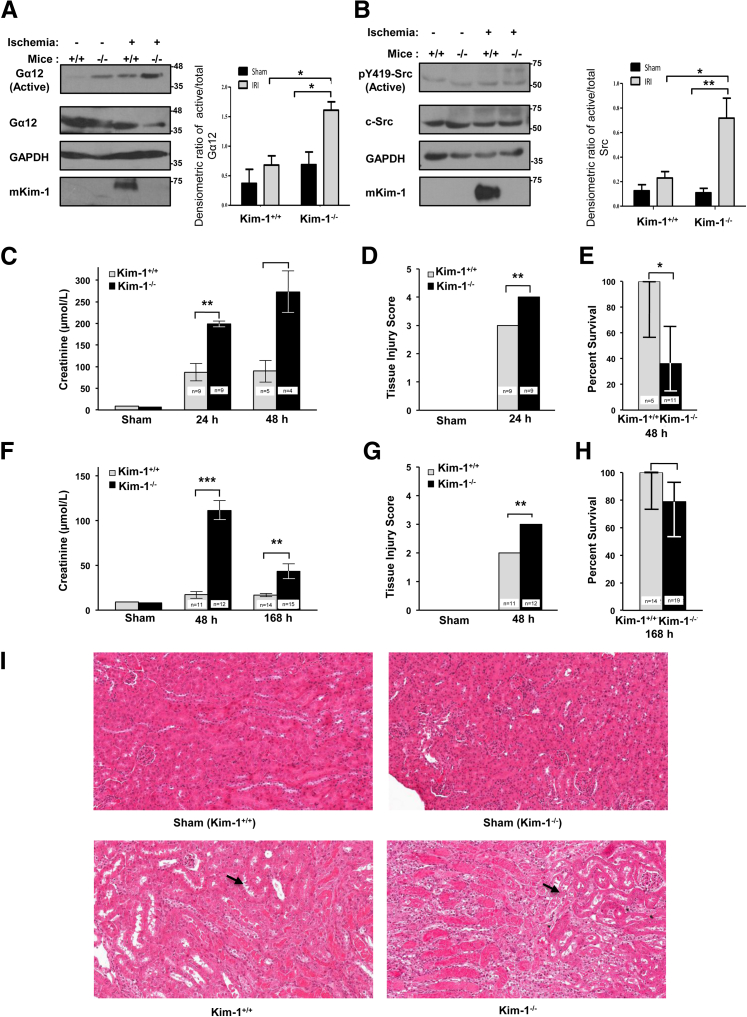
To extend these findings to a physiologically important model, we next determined whether up-regulation of KIM-1 by TECs during renal IRI would block pathogenic Gα12 activation in the kidneys by ROS. We subjected Kim-1+/+ and Kim-1−/− mice to 35 minutes of unilateral renal artery clamping, followed by 24 hours of reperfusion, and measured Gα12 activation in the affected and contralateral kidney tissues.9 As expected, we observed both an increase in Gα12 activation and an up-regulation in Kim-1 expression4,5 in the reperfused kidneys but not in the contralateral (nonischemic) kidneys as determined by Western blot analysis (Figure 3A). Predictably, the degree of Gα12 activation after IRI was significantly higher in the kidneys from Kim-1−/− mice than in kidneys from the Kim-1+/+ mice. Although there was a small difference in active Gα12 amounts in the contralateral kidneys between Kim-1−/− and Kim-1+/+ mice, it was not significant. In a parallel set of experiments, we also observed increased Src activation in the total cell lysates from Kim-1−/− kidneys compared with Kim-1+/+ kidneys subjected to ischemia-reperfusion consistent with previous findings (Figure 3B).9,15
Figure 3.
KIM-1–deficient mice exhibit increased renal Gα12 activation and tissue damage after ischemia-reperfusion injury. A: Wild-type (Kim-1+/+) and Kim-1–deficient (Kim-1−/−) mice underwent unilateral renal pedicle clamping for 35 minutes, followed by 24 hours of reperfusion. Active Gα12 was measured in renal cortical lysates obtained from clamped and contralateral kidneys by GST-TPR pull-down assay, followed by SDS-PAGE and Western blot analysis for active and total Gα12, mKim-1, and GAPDH. B: The amount of active pY419 compared with total Src was measured by Western blot analysis in renal cortical lysates of mice treated as in A. Samples were analyzed by SDS-PAGE and Western blot analysis for indicated antibodies. Densitometric analysis of the ratio of active Gα12 to total Gα12 (or pY419-Src to c-Src) is shown as a representation of experiments. C–I: Independent groups of Kim-1+/+ and Kim-1−/− mice underwent sham surgery or bilateral renal pedicle clamping for 30 minutes (C–E) or 25 minutes (F–I), followed by 24, 48, or 168 hours of reperfusion. C and F: Kidney function was determined by plasma creatinine. D and G: Quantification of tubular damage in whole kidneys after 48 hours of reperfusion. E and H: Percentage of survival after reperfusion. I: Representative periodic acid-Schiff–stained kidney sections after 48 hours of reperfusion (Sham represents Kim-1−/−). Arrows indicate areas with tubular injury. Data are expressed as means ± SEM. n = 3 per group in three independent experiments (A and B); n = 5 to 11 per group (C–E); n = 7 to 12 per group (F–I); n = 9 per group (D and G). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 0.10 mm. Original magnification, ×200. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Gα12, G protein α-12; GST-TPR, glutathione S-transferase–fused tetratricopeptide repeat domain of Ser/Thr protein phosphatase type 5; KIM-1, kidney injury molecule-1; mKim-1, mouse kidney injury molecule-1; pY419, phosphorylated Src.
In view of the pathogenic role of activated Gα12 in IRI9 and the above data indicating that KIM-1 inhibits Gα12 activation, we hypothesized that Kim-1–deficient mice would show more severe IRI than Kim-1+/+ mice. After 30 minutes of renal ischemia and 24 hours of reperfusion, renal function was significantly worse in Kim-1−/− mice than in Kim-1+/+ mice (mean creatinine, 198 versus 87 μmol/L, respectively; P < 0.01; n = 9 per group) (Figure 3C). Kim-1−/− mice also exhibited worse histologic renal damage than did Kim-1+/+ mice (Figure 3D). After 48 hours of reperfusion, 7 of 11 Kim-1−/− mice, whereas none of five Kim-1+/+ mice died (P = 0.05) (Figure 3E). When we reduced ischemia time to 25 minutes, Kim-1+/+ mice maintained near-normal renal function (mean creatinine, 17 μmol/L; n = 11), whereas Kim-1−/− mice developed severe renal impairment (mean creatinine, 111 μmol/L; n = 12) (P < 0.001) after 48 hours of reperfusion (Figure 3F). Tissue injury was also greater in Kim-1−/− mice than in Kim-1+/+ mice (Figure 3G). After 168 hours of reperfusion, a significant difference in renal function remained between Kim-1+/+ mice (mean creatinine, 17 μmol/L) and Kim-1−/− mice (mean creatinine, 43 μmol/L) (P < 0.01), although 4 of 19 Kim-1−/− mice and none of 14 Kim-1+/+ mice died (P = 0.067) (Figure 3, H and I).
Discussion
AKI is a serious medical condition with no known treatment and unresolved pathogenic mechanisms. It was recently discovered that Gα12 has a crucial pathogenic role in renal IRI.9,15 Specifically, ROS stimulates Gα12 to activate tubular injury pathways, including disruption of tight junctions via Src activation. To our knowledge, regulation of this pathway has never been described. Here, we demonstrate that KIM-1 blocks GTP binding onto Gα12 and thus is an inhibitor of Gα12 activation. In support of this finding, we found that Kim-1−/− mice exhibited exaggerated Gα12 and Src activation in vivo during renal IRI, compared with wild-type mice. In addition, we observed worse renal dysfunction and histology after bilateral pedicle clamping in Kim-1−/− mice than in wild-type mice. Taken together, our findings suggest that KIM-1 protects against Gα12-mediated tissue damage during ischemic AKI. Whether KIM-1 has additional, Gα12-independent effects on TECs that protect from IRI is an area of further study.23
Our results provide particular insight into the regulation of G-protein signaling. Gα12, like all G proteins, is a molecular switch that is activated by GTP binding (on GDP dissociation) and is inactivated when bound GTP is hydrolyzed to GDP.8 We found that KIM-1 reduces binding of GTPγS to Gα12. To our knowledge, KIM-1 is the first guanine nucleotide dissociation inhibitor against Gα12 to be identified.24 Interestingly, the cytosolic domain of KIM-1 does not contain the 19 amino acid sequence GoLoco motif that is typical of guanine nucleotide dissociation inhibitor.25 The residues responsible for KIM-1 inhibition of Gα12 are unknown. It is conceivable that targeting Gα12 by using an exogenous chemical inhibitor might ameliorate renal IRI.9 However, such an inhibitor does not exist and may in fact be toxic, given that Gα12 is expressed ubiquitously and also regulates many crucial cellular functions.8
Our results suggest that KIM-1 represents a natural, endogenous mechanism to protect against tissue damage during renal IRI. Given that KIM-1 is expressed by TECs only during the injury and returns to baseline (undetectable) amounts on renal recovery (after day 7 after IRI),26 Gα12 inhibition by KIM-1 is likely transient. Interestingly, the up-regulation of KIM-1 seems to overlap with Gα12 activation by ROS during ischemia-reperfusion. The down-regulation of KIM-1 after AKI is equally important because conditional overexpression of KIM-1 in TECs in the absence of an injury stimulus results in kidney fibrosis.27 It is proposed that antagonizing KIM-1 signaling may represent a novel therapeutic target to ameliorate renal fibrosis in chronic kidney disease. However, our identification of KIM-1 in regulating Gα12 and in protection against renal dysfunction during IRI suggests that caution is warranted in targeting KIM-1 in chronic kidney disease, as such a therapeutic agent may exacerbate AKI episodes that are linked to progression of chronic kidney disease.28 It would also be interesting to know if transiently overexpressing KIM-1 during IRI would further protect against tissue damage. We speculate that strategies to enhance KIM-1 expression or function may be particularly important in patients with polymorphic variants of KIM-1 that might confer reduced ability to inhibit Gα12.
Acknowledgments
We thank Drs. Andrew McKenzie (MRC Laboratory of Molecular Biology) for providing the Kim-1−/− and Kim-1+/− frozen embryos, Terry Strom (Harvard Medical School) for reviving the frozen embryos, Joseph Bonventre (Harvard Medical School) for providing the vital reagents, and Danny N. Dhanasekaran (Temple University) for GST-fused TPR domain of Ser/Thr PP5.
O.I., X.Z., and J.W. performed research and analyzed data; A.H. graded histology; B.M.D. provided vital reagents, performed part of the research, and edited the manuscript; R.S.S. and A.S. analyzed data and edited the manuscript; L.G. designed the experiments and analyzed data; O.I. and L.G. wrote the manuscript.
Footnotes
Supported by Canadian Institutes of Health Research grants HDK 232429 and 244945 (L.G.), NIH grant GM55223 (B.M.D.), Lawson Studentship (O.Z.I.), KRESCENT and AMOSO salary awards (L.G.), and Junior 1 scholarship award from the Fonds de Recherche du Québec – Santé (R.A.S.).
Disclosures: None declared.
Supplemental Data
Protein expression of KIM-1-HA and KIM-1 with truncated transmembrane/cytosolic domain in HEK-293 cells. A: HEK-293 cells were transfected with either control vector (pcDNA) or KIM-1-HA–expressing vector. Cell lysates were compared with an endogenous amount of KIM-1 expression in human renal adenocarcinoma cell line (769P) by Western blot analysis against full-length KIM-1 (extracellular domain) and actin. B: Surface staining for KIM-1 in HEK-293 cells expressing pcDNA or KIM-1-HA as determined by flow cytometry. C: HEK-293 cells were transfected with either full-length KIM-1 or truncated flag-tagged KIM-1 construct that contained only the TM and the cytosolic domain of KIM-1. Lysate of these cells were run on Western blot analysis and probed for full-length KIM-1 (antibody against extracellular domain of KIM-1), the cytosolic domain of KIM-1 (antibody against cytosolic domain of KIM-1), and actin. Lysate of human renal adenocarcinoma cells (769P) was used as a positive control. Data represent three independent experiments. HA, hemagglutinin; HEK, human embryonic kidney; KIM-1, kidney injury molecule-1; TM, transmembrane.
Treatment with H2O2 does not affect the viability of proximal tubule cells. Proximal tubule cells isolated from either wild-type (A) or Kim-1–deficient (B) mice were treated with increasing concentrations of H2O2 [mM (mmol/L)] or H2O (control) as indicated. Percentage of cell death was determined by the percentage of PI+ cells as determined by flow cytometry in comparison with control treatment (water). No significant difference was found between H2O2 treated and the respective control. n = 3. Kim-1, kidney injury molecule-1; PI, propidium iodide.
References
- 1.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Freeman G.J., Casasnovas J.M., Umetsu D.T., DeKruyff R.H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L., Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya V.S., Ozer J.S., Dieterle F., Collings F.B., Ramirez V., Troth S., Muniappa N., Thudium D., Gerhold D., Holder D.J., Bobadilla N.A., Marrer E., Perentes E., Cordier A., Vonderscher J., Maurer G., Goering P.L., Sistare F.D., Bonventre J.V. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza A.J., Oriss T.B., O'Malley K.J., Ray A., Kane L.P. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Humphreys B.D., Bonventre J.V. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol. 2007;18:2704–2714. doi: 10.1681/ASN.2007030325. [DOI] [PubMed] [Google Scholar]
- 8.Dhanasekaran N., Dermott J.M. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 9.Yu W., Beaudry S., Negoro H., Boucher I., Tran M., Kong T., Denker B.M. H2O2 activates G protein, alpha 12 to disrupt the junctional complex and enhance ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2012;109:6680–6685. doi: 10.1073/pnas.1116800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z.X., Shek K., Wang S., Huang X., Lau A., Yin Z., Sun H., Liu W., Garcia B., Rittling S., Jevnikar A.M. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. J Immunol. 2010;185:967–973. doi: 10.4049/jimmunol.0903245. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka M., Goto K., Tsuchiya S., Aramaki Y. Phosphatidylserine-specific receptor contributes to TGF-beta production in macrophages through a MAP kinase, ERK. Biol Pharm Bull. 2005;28:1707–1710. doi: 10.1248/bpb.28.1707. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe C.C., Dockrell M.E. Primary culture of human renal proximal tubule epithelial cells and interstitial fibroblasts. Methods Mol Biol. 2012;806:175–185. doi: 10.1007/978-1-61779-367-7_12. [DOI] [PubMed] [Google Scholar]
- 13.Bailly V., Zhang Z., Meier W., Cate R., Sanicola M., Bonventre J.V. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi R., Yi J., Ha J., Shi H., Ismail O., Nathoo S., Bonventre J.V., Zhang X., Gunaratnam L. Accelerated receptor shedding inhibits kidney injury molecule-1 (KIM-1)-mediated efferocytosis. Am J Physiol Renal Physiol. 2014;307:F205–F221. doi: 10.1152/ajprenal.00638.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer T.N., Hunt J., Schwesinger C., Denker B.M. Galpha12 regulates epithelial cell junctions through Src tyrosine kinases. Am J Physiol Cell Physiol. 2003;285:C1281–C1293. doi: 10.1152/ajpcell.00548.2002. [DOI] [PubMed] [Google Scholar]
- 16.Zhu D., Kosik K.S., Meigs T.E., Yanamadala V., Denker B.M. Galpha12 directly interacts with PP2A: evidence FOR Galpha12-stimulated PP2A phosphatase activity and dephosphorylation of microtubule-associated protein, tau. J Biol Chem. 2004;279:54983–54986. doi: 10.1074/jbc.C400508200. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y., Katoh H., Mori K., Negishi M. Galpha(12) and Galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12:1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- 18.Andreeva A.V., Kutuzov M.A., Voyno-Yasenetskaya T.A. G alpha12 is targeted to the mitochondria and affects mitochondrial morphology and motility. FASEB J. 2008;22:2821–2831. doi: 10.1096/fj.07-104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson E.B. On confidence intervals. Proc Natl Acad Sci U S A. 1942;28:88–93. doi: 10.1073/pnas.28.3.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanamadala V., Negoro H., Gunaratnam L., Kong T., Denker B.M. Galpha12 stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of IkappaBalpha. J Biol Chem. 2007;282:24352–24363. doi: 10.1074/jbc.M702804200. [DOI] [PubMed] [Google Scholar]
- 21.Ohga N., Kikuchi A., Ueda T., Yamamoto J., Takai Y. Rabbit intestine contains a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. Biochem Biophys Res Commun. 1989;163:1523–1533. doi: 10.1016/0006-291x(89)91153-4. [DOI] [PubMed] [Google Scholar]
- 22.Wong S.H., Barlow J.L., Nabarro S., Fallon P.G., McKenzie A.N. Tim-1 is induced on germinal centre B cells through B-cell receptor signalling but is not essential for the germinal centre response. Immunology. 2010;131:77–88. doi: 10.1111/j.1365-2567.2010.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimura T., Brooks C.R., Bonventre J.V. Kim-1/Tim-1 and immune cells: shifting sands. Kidney Int. 2012;81:809–811. doi: 10.1038/ki.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siderovski D.P., Willard F.S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimple R.J., Kimple M.E., Betts L., Sondek J., Siderovski D.P. Structural determinants for GoLoco-induced inhibition of nucleotide release by Galpha subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 26.Ko G.J., Grigoryev D.N., Linfert D., Jang H.R., Watkins T., Cheadle C., Racusen L., Rabb H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010;298:F1472–F1483. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys B.D., Xu F., Sabbisetti V., Grgic I., Naini S.M., Wang N., Chen G., Xiao S., Patel D., Henderson J.M., Ichimura T., Mou S., Soeung S., McMahon A.P., Kuchroo V.K., Bonventre J.V. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein expression of KIM-1-HA and KIM-1 with truncated transmembrane/cytosolic domain in HEK-293 cells. A: HEK-293 cells were transfected with either control vector (pcDNA) or KIM-1-HA–expressing vector. Cell lysates were compared with an endogenous amount of KIM-1 expression in human renal adenocarcinoma cell line (769P) by Western blot analysis against full-length KIM-1 (extracellular domain) and actin. B: Surface staining for KIM-1 in HEK-293 cells expressing pcDNA or KIM-1-HA as determined by flow cytometry. C: HEK-293 cells were transfected with either full-length KIM-1 or truncated flag-tagged KIM-1 construct that contained only the TM and the cytosolic domain of KIM-1. Lysate of these cells were run on Western blot analysis and probed for full-length KIM-1 (antibody against extracellular domain of KIM-1), the cytosolic domain of KIM-1 (antibody against cytosolic domain of KIM-1), and actin. Lysate of human renal adenocarcinoma cells (769P) was used as a positive control. Data represent three independent experiments. HA, hemagglutinin; HEK, human embryonic kidney; KIM-1, kidney injury molecule-1; TM, transmembrane.
Treatment with H2O2 does not affect the viability of proximal tubule cells. Proximal tubule cells isolated from either wild-type (A) or Kim-1–deficient (B) mice were treated with increasing concentrations of H2O2 [mM (mmol/L)] or H2O (control) as indicated. Percentage of cell death was determined by the percentage of PI+ cells as determined by flow cytometry in comparison with control treatment (water). No significant difference was found between H2O2 treated and the respective control. n = 3. Kim-1, kidney injury molecule-1; PI, propidium iodide.