Abstract
Collagen V mutations underlie classic Ehlers-Danlos syndrome, and joint hypermobility is an important clinical manifestation. We define the function of collagen V in tendons and ligaments, as well as the role of alterations in collagen V expression in the pathobiology in classic Ehlers-Danlos syndrome. A conditional Col5a1flox/flox mouse model was bred with Scleraxis-Cre mice to create a targeted tendon and ligament Col5a1-null mouse model, Col5a1Δten/Δten. Targeting was specific, resulting in collagen V–null tendons and ligaments. Col5a1Δten/Δten mice demonstrated decreased body size, grip weakness, abnormal gait, joint laxity, and early-onset osteoarthritis. These gross changes were associated with abnormal fiber organization, as well as altered collagen fibril structure with increased fibril diameters and decreased fibril number that was more severe in a major joint stabilizing ligament, the anterior cruciate ligament (ACL), than in the flexor digitorum longus tendon. The ACL also had a higher collagen V content than did the flexor digitorum longus tendon. The collagen V–null ACL and flexor digitorum longus tendon both had significant alterations in mechanical properties, with ACL exhibiting more severe changes. The data demonstrate critical differential regulatory roles for collagen V in tendon and ligament structure and function and suggest that collagen V regulatory dysfunction is associated with an abnormal joint phenotype, similar to the hypermobility phenotype in classic Ehlers-Danlos syndrome.
Ehlers-Danlos syndrome (EDS) is a hereditary connective tissue disorder characterized by joint hypermobility, skin extensibility, and connective tissue fragility.1–5 The combined prevalence of all types of EDS is approximately 1 in 5000. More than half of these cases are characterized by joint hypermobility,6 and hypermobility is one of the major diagnostic criteria for the classic subtype of EDS. Joint hypermobility can cause chronic joint and limb pain, recurring joint dislocation, and sports injuries with potential degenerative complications, including muscle weakness, precocious osteoarthritis, spondylosis, and lower bone mass.6–9
In more than 90% of patients with classic EDS, collagen V mutations have been identified,3,10 and approximately half are null-allele mutations resulting in COL5A1 haploinsufficiency.10–12 Besides the null-allele mutations, mutations scattered throughout COL5A1 and COL5A2 genes and some mRNA splicing mutations in COL5A1 also have been identified.10 This finding has led to the proposal that classic EDS is a collagen V disease resulting from altered collagen V expression.10
Collagen V is a quantitatively minor, regulatory component in collagen I–rich connective tissues, including dermis, tendons and ligaments, bones, blood vessels, and cornea.13 Collagen V content relative to collagen I varies from a high of 10% to 20% in cornea to 2% to 5% of the total fibril-forming collagens in most other tissues.14,15 Collagen V regulates collagen fibrillogenesis by nucleating fibril assembly in in vitro self-assembly assays, cell culture analyses, and mouse models.14,16–19 The data support a model whereby the collagen V:I ratio in different tissues determines the initial diameter and number of fibrils assembled. By varying the number of collagen V nucleation sites for a given collagen I concentration, the fibroblast can regulate fibril number and diameter in a tissue-specific manner, ie, more sites result in increased fibrils assembled with smaller diameters.13,17
Several mouse models have been established to elucidate the function of collagen V tissue-specific fibril assembly. A traditional homozygous deletion in the Col5a1 gene is embryonic lethal because of a virtual lack of fibril formation at the beginning of organogenesis in the early embryo, although the Col5a1−/− mice synthesize and secrete collagen I at a level comparable with that of wild-type controls.18 This model demonstrates that collagen V is essential for the assembly of collagen I–containing fibrils in the low-collagen-concentration environment of the embryo and is consistent with a critical role in nucleation of fibril assembly. The heterozygous Col5a1+/− mice demonstrate haploinsufficiency, with approximately 50% of wild-type collagen V expression. Col5a1+/− mice are excellent models of classic EDS.20 There were two subpopulations of fibrils in the mutant dermis. One had increased diameters and normal fibril structure, and these were immunoreactive for collagen V; the second group had very large diameters with aberrant fibril structure and were negative for collagen V reactivity. This suggests relatively normal assembly when nucleated with collagen, but the expression level of collagen V was insufficient to nucleate all available collagen I and, therefore, dysfunctional fibril assembly in the high-collagen-content environment. This abnormal fibril growth recapitulated that seen clinically in the dermis of EDS patients. In addition, the Col5a1+/− flexor digitorum longus tendon (FDL) also demonstrates decreased cross-sectional area and stiffness compared with that in the wild-type controls,21 consistent with the joint hypermobility and dislocations seen in EDS patients.
To overcome the embryonic lethal phenotype and permit analyses of the roles of collagen V in the development and maturation of a tissue-specific extracellular matrix, our group created a conditional collagen V–null mouse model by using a Cre/loxP approach.22 When targeted to the corneal stroma, a severe dysfunctional regulation of fibrillogenesis and corneal opacity was observed in the targeted collagen V–null mice. Unlike cornea, the tendon has a low collagen V content. The mature tendon contains uniaxial fibrils with a very heterogeneous population of fibril diameters, and the mechanical properties of the tendon depend on the increases in fibril diameter seen with development.23
Our aim was to explore specific regulatory roles for collagen V in tendons and ligaments, as well as its roles in the pathophysiology associated with joint hypermobility in classic EDS. Our conditional Col5a1flox/flox mouse model was targeted to tendons and ligaments using Cre driven by a Scleraxis (Scx) promoter (Scx-Cre) to produce the Col5a1Δten/Δten mouse model. Scx-Cre targets the deletion to tendons and ligaments. The data demonstrate that the absence of collagen V results in a disruption in tendon and ligament structure and function. In addition, there were consistent differences between the FDL tendon and the anterior cruciate ligament (ACL), indicating tissue-specific regulatory properties. The absence of collagen V also resulted in alterations in the joint consistent with the hypermobility seen in classic EDS.
Materials and Methods
Generation of Tendon- and Ligament-Specific Col5a1Δten/Δten Mice
Conditional Col5a1flox/flox mice were created by flanking exons 3 and 4 of the Col5a1 gene with loxP elements and have been described previously.22 Scx-Cre transgenic mice express Cre driven by the Scx promoter, thereby targeting expression to tendons and ligaments.24 Tendon- and ligament- specific expression was characterized by breeding Scx-Cre mice with Cre reporter mTmG (membrane-Tomato/membrane-Green) mice (The Jackson Laboratory, Bar Harbor, ME). To generate tendon- and ligament-specific collagen V–null mice, we crossbred Scx-Cre transgenic mice with conditional Col5a1flox/flox mice for two generations to create Scx-Cre+/Col5a1flox/flox (Col5a1Δten/Δten) mice. The primers for the genotyping and characterization of Col5a1Δten/Δten mice were described previously.22 All animal studies were performed in compliance with animal protocols approved by the Institutional Animal Care and Use Committee.
Immunoblotting
FDL tendons, ACL, skin, bone, and cornea were dissected from postnatal day (P)10 male mice. Protein extracts were prepared in 50 mmol/L Tris-HCl, pH 6.8, 1% SDS lysis buffer with proteinase inhibitors (Thermal Scientific, Waltham, MA). Protein lysates (20 μg) were separated on a 4% to 12% Bis-Tris gel (Life Technologies, Grand Island, NY) and transferred onto a Hybond-C membrane (GE Healthcare, Pittsburgh, PA). The membrane was hybridized with affinity purified anti-α1(V),18 and anti-β-actin antibodies (Millipore, Billerica, MA). To analyze collagen V expression in FDLs and ACLs from wild-type C57BL/6 mice, we dissected control Col5a1flox/flox mice and Col5a1Δten/Δten mice, and the same procedures were followed.
Real-Time PCR
FDLs and ACLs were dissected from the mice at P10 and were cut into small pieces. Total RNA from FDL was extracted using the RNeasy Micro Kit (Qiagen, Germantown, MD). Total RNA (3 ng per well) was subjected to reverse transcription by using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies), and real-time PCR was performed with SYBR Green PCR master mix (Life Technologies) on a StepOnePlus Real-Time PCR System (Life Technologies). The primer sequences were as follows: Col5a1: forward, 5′-AAGCGTGGGAAACTGCTCTCCTAT-3′, and reverse, 5′-AGCAGTTGTAGGTGACGTTCTGGT-3′; β-actin, forward, 5′-AGATGACCCAGATCATGTTTGAGA-3′, and reverse, 5′-CACAGCCTGGATGGCTACGT-3′. Each sample was run in triplicate, and data were analyzed using StepOne software version 2.0 (Life Technologies, Foster City, CA). β-Actin was used as an internal control to standardize the amount of sample total RNA.
Immunolocalization
FDL tendons were dissected from control Col5a1flox/flox mice and Col5a1Δten/Δten mice at P4 and P30 and were fixed in the fixative containing 4% paraformaldehyde, embedded in an OCT compound and frozen at −80°C. Cross sections (5 μm) were cut using an HM 505E cryostat (Thermo Fisher Scientific). For the ACLs, whole knee joints were removed from littermate control and knockout mice at P4, skinned, and trimmed of extra muscles. Sagittal sections (5 μm) containing longitudinal ACL were cut. Immunolocalization was performed using immunofluorescence microscopy to analyze expression of collagen V. Before the incubation with antibodies, the sections were pretreated with testicular hyaluronidase to enhance penetration. Anti-mouse collagen V antibody was used at 1:400. The secondary antibody was goat anti-rabbit IgG Alexa Fluor 568 (Life Technologies) at 1:400. Vectashield mounting solution with DAPI (Vector Laboratories, Burlingame, CA) was used as a nuclear marker. Images were captured using a CTR 5500 fluorescence microscope (Leica, Wetzlar, Germany) and DFC 340 FX digital camera (Leica). Antibody incubations and image acquisition were performed concurrently for sections of control Col5a1flox/flox and Col5a1Δten/Δten mice by using identical procedures and settings to facilitate comparison.
Gait Analysis
Footprints were recorded for gait analysis of individual mice. Hind limbs of the mouse were dipped in nontoxic paint, and the mouse was allowed to walk freely on a piece of white paper in a walkway consisting of two Plexiglas walls, spaced 8 cm apart. The footprint recording was repeated at least three times for each mouse. Parameters for stride length, intermediate toe spread, and total toe spread were calculated from walking tracks recorded on the paper. Stride length was the distance between adjacent prints made by the same hind limb.
Grip Strength
To assess musculoskeletal and motor function impairment of the conditional knockout mouse, we used a grip strength meter (San Diego Instruments, San Diego, CA) to record the peak force the animal exerted in grasping a grip placed at the forelimb. The grip strength meter was positioned horizontally, and the mouse was held by the tail and lowered toward the grip strength platform. The animal was allowed to grasp the forelimb grip with its forepaws. The mouse then was pulled steadily by the tail away from the rod until the mouse's grip was broken. The force applied to the grip just before the animal lost its grip was recorded as the peak tension. Ten measurements from each mouse were recorded, and the mean force was used to represent the grip strength for each mouse.
Biomechanics
Postnatal day 60 (P60) FDLs were analyzed as previously described25 by using eight wild-type control mice and eight Col5a1Δten/Δten mice. Briefly, cross-sectional areas of the P60 tendons were measured using a custom laser-based device.26 P60 tendons were clamped in custom test fixtures, and standard mechanical testing protocols were used as described. Student's t-tests were performed on cross-sectional area, stiffness, and elastic modulus comparing across genotype (significance at P < 0.05).
ACLs were analyzed using 19 control and 10 Col5a1Δten/Δten male mice at age P60. Briefly, hind limbs were obtained, and the ACL was dissected free of soft tissue, leaving only the tibia-ACL-femur complex intact, and Verhoeff stain was applied for optical strain tracking.27 The femur and tibia were affixed in custom-built testing fixtures such that the femur was vertical and the tibia was at approximately 60 degrees of flexion. The ACL was tested using a standard testing protocol as described for the FDL but adjusted for a 1-mm gauge length for the ACL. Cross-sectional area was calculated assuming an ellipsoidal shape, with length and width measured in the coronal and sagittal planes. Local strain was measured optically, and parameters, ie, modulus and stiffness, were calculated using custom Matlab software version 2012a (Mathworks, Natick, MA). Modulus is the slope of the linear portion of the stress-versus-strain curve, where stress is the force divided by cross-sectional area and strain is the change in length divided by the initial length. Stiffness is a structural parameter that can vary with tissue size, but modulus is a property of the material. Maximum stress was calculated as maximum load divided by initial area. Comparisons were made between groups by using Student's t-tests with significance set at P < 0.05.
Transmission Electron Microscopy
FDLs and ACLs from Col5a1Δten/Δten and control Col5a1flox/flox mice at ages P4 and P30 were used for ultrastructural analysis. The samples were prepared for transmission electron microscopy as previously described.25 Sections were examined and photographed at 80 kV by using a JEM-1400 transmission electron microscope (JEOL USA, Peabody, MA) and a Gatan Orius widefield side mount digital camera (Gatan, Warrendale, PA).
Fibril Diameter Measurement
Fibril diameter was analyzed as previously described.21,28 Collagen fibril diameters in FDLs and ACLs from three different P30 mice from each genotype were analyzed. Four nonoverlapping cross-sectioned digital images were obtained at ×60,000 from the central areas of each specimen. Diameters were measured along the minor axis of cross sections by using an RM Biometrics-Bioquant Image Analysis System (Nashville, TN). Data analysis and histograms were created using Microsoft Excel 2007 (Redmond, WA). Fibril density was obtained as the fibril number per unit area.
Histologic Analysis
Col5a1Δten/Δten mice and Col5a1flox/flox control mice at P4 and P30 were whole-body perfused with 4% paraformaldehyde. Intact knee joints were dissected, decalcified, and embedded in paraffin. Sections (5 μm) were cut in the frontal plane toward the back or sagittal plane from lateral toward medial. Sections were collected from the central weight-bearing region of the tibial plateau on the basis of the presence of the ACL and anatomy of the lateral meniscus. Sections were stained with either H&E or Safranin O. Histologic images were captured using an Olympus BX61 TRF microscope and a DP72 12.8-megapixel digital color camera (Olympus, Center Valley, PA).
Results
Generation of a Tendon- and Ligament-Specific Conditional Collagen V–Null Mouse Model
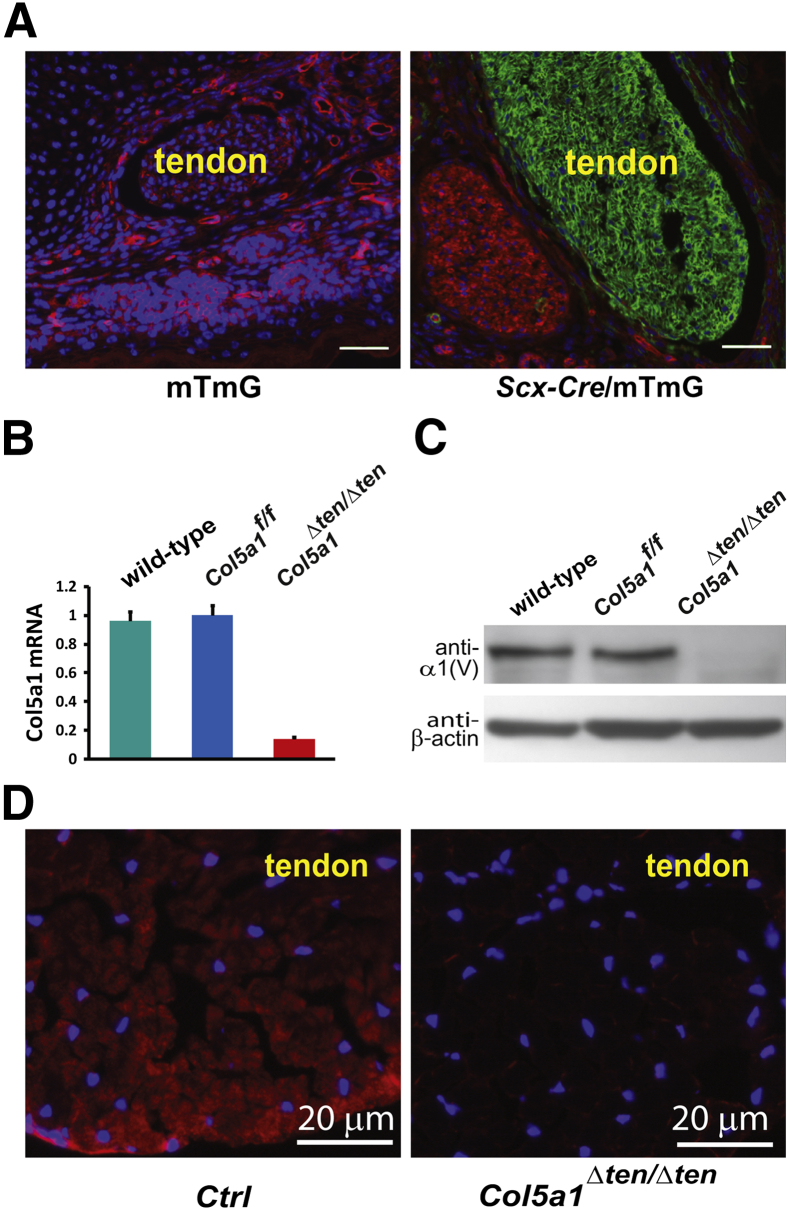
To overcome the embryonic lethal phenotype in the traditional Col5a1-null mice,18 a mouse line was established with Col5a1 exons 3 and 4 flanked by loxP elements.22 This conditional mouse line Col5a1flox/flox was crossed with a Scx-Cre mouse line. The Scx-Cre transgenic mouse line expresses Cre under the control of the regulatory sequence for the Scx gene that is expressed in tendon and ligament cells beginning with progenitor stages.29,30 To determine the tissue specificity and efficacy of recombination driven by Scx-Cre, we crossed Scx-Cre mice with a Cre reporter (mTmG) mouse line.31 The mTmG mouse is a dual-fluorescent Cre reporter mouse with loxP sites flanking a mT cassette. It expresses strong red fluorescence in all tissues including skin and tendon in the absence of Cre expression (Figure 1A). In contrast, when the mTmG mouse is crossed with an Scx-Cre mouse, the Cre recombinase in the tenocytes from the offspring excises the mT cassette and activates the downstream membrane-targeted enhanced green fluorescent protein (mG) cassette that expresses strong green fluorescence. The Scx-Cre recombinase activity is limited to tendons (Figure 1A) and ligaments (Figure 2A). The red fluorescence remained in other tissues, including skin, muscle, and bone tissues in the limb cross sections from P4 mice (Figure 1A). These data are consistent with a lack of Cre excision in these tissues and demonstrate specific tendon and ligament targeting by using the Scx-Cre mouse.
Figure 1.
Targeted deletion of collagen V in flexor digitorum longus (FDL) tendons. A: Cre excision was targeted to tendons by using a Scleraxis promoter (Scx-Cre) in a double reporter (mTmG) mouse. Analysis of cross sections from postnatal day 4 (P4) limbs shows ubiquitous expression of red fluorescence (mT) in control mTmG mice. In contrast, Scx-Cre/mTmG mice show green fluorescence (mG) in the tendon, indicating targeted Cre excision. Other tissues show no Cre recombinase activity. DAPI (blue), nuclear localization. B: Col5a1 mRNA expression in the postnatal day 10 Col5a1Δten/Δten FDL is at background levels at real-time PCR. In contrast, expression in Col5a1flox/flox (Col5a1f/f) control mice is comparable with that in the wild-type mice. C: Postnatal day 10 FDLs are collagen V null in Col5a1Δten/Δten mice. Immunoblot analysis using antibodies against the alpha 1 chain of collagen V [a1(V)] and against β-actin as a loading control. Control Col5a1flox/flox and wild-type mice express comparable collagen V. D: Collagen V immunoreactivity (red) is present in postnatal day 30 control (Ctrl) FDLs but absent in Col5a1Δten/Δten mice. DAPI (blue), nuclear localization. Scale bars = 20 μm (A).
Figure 2.
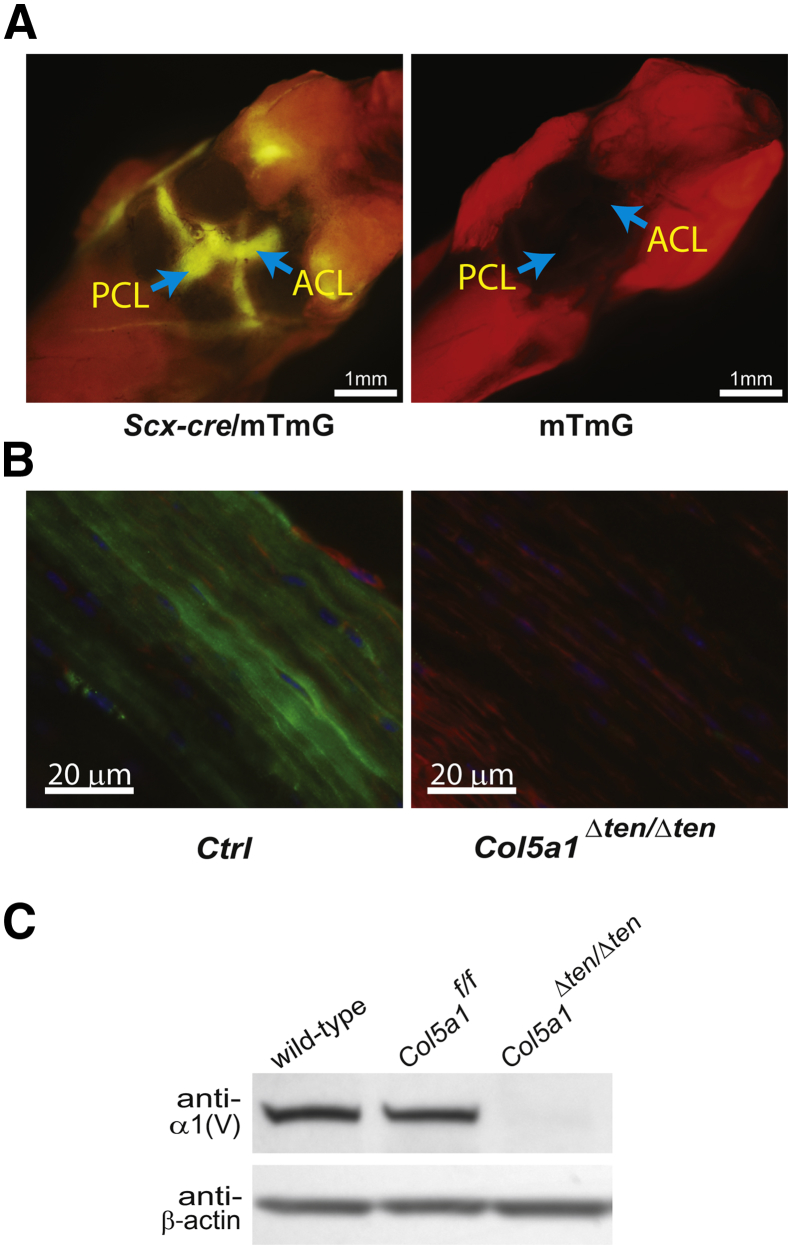
Targeted deletion of collagen V in anterior cruciate ligament (ACL) driven by Scleraxis-Cre (Scx-Cre). A: Posterior view of the knee joint of Scx-Cre/mTmG mouse at postnatal day 10 shows the ACL and posterior cruciate ligament (PCL) with green fluorescent protein expression (left panel). No green fluorescent protein is expressed in the mTmG control mouse knee joint (right panel). Arrows indicate the position of the ACL and PCL. The image was obtained with a fluorescence dissecting microscope. B: Immunofluorescence microscopy showed the depletion of collagen V expression in the Col5a1Δten/Δten ACL at postnatal day 10 (right panel) compared to the controls (left panel). Green, collagen V; red, phalloidin; blue, DAPI stained nucleus. C: Immunoblotting shows no expression of collagen V in Col5a1Δten/Δten ACLs. The Col5a1flox/flox (Col5a1f/f) mouse ACL expressed the same amount of collagen V as did that of wild-type C57BL/6 mice. Ctrl, control; double reporter, mTmG.
To produce a targeted tendon and ligament collagen V–null mouse model, we bred Scx-Cre mice with the conditional Col5a1flox/flox mouse line22 that produced Scx-Cre+/Col5a1flox/+ mice. Intercrossing Scx-Cre+/Col5a1flox/+ mice with Col5a1flox/flox mice generated mice homozygous for the floxed allele expressing Cre under the Scx promoter (Scx-Cre). The Cre excision of exons 3 and 4 resulted in mice with Col5a1-null tendons and ligaments (Col5a1Δten/Δten). The expression of Col5a1 mRNA and collagen V was analyzed in the Col5a1Δten/Δten mice. In the P10 Col5a1Δten/Δten FDL tendons, Col5a1 mRNA expression was decreased to background levels compared with that in the wild-type P10 FDL tendons. Col5a1 mRNA expression in the control Col5a1flox/flox mouse FDL was comparable with that in wild-type FDL (Figure 1B). Consistent with the lack of mRNA expression, collagen V was completely absent in tendons from P10 Col5a1Δten/Δten mice, whereas the expression in the control Col5a1flox/flox FDL was comparable with that in wild-type mice (Figure 1C). In the P30 control mouse FDL tendon, collagen V was homogeneously distributed throughout the fiber bundles. In the Col5a1Δten/Δten FDL, the expression of collagen V was absent (Figure 1D). In addition, collagen V expression also was not detected in the ACLs of Col5a1Δten/Δten mice at immunofluorescence microscopy (Figure 2B), and this finding was confirmed by using immunoblot analysis (Figure 2C). These data indicate that the Col5a1Δten/Δten mouse is a tendon- and ligament-specific collagen V–null mouse model line.
Conditional Inactivation of Tendon and Ligament Col5a1 Gene Expression Results in Abnormal Gait and Joint Phenotype
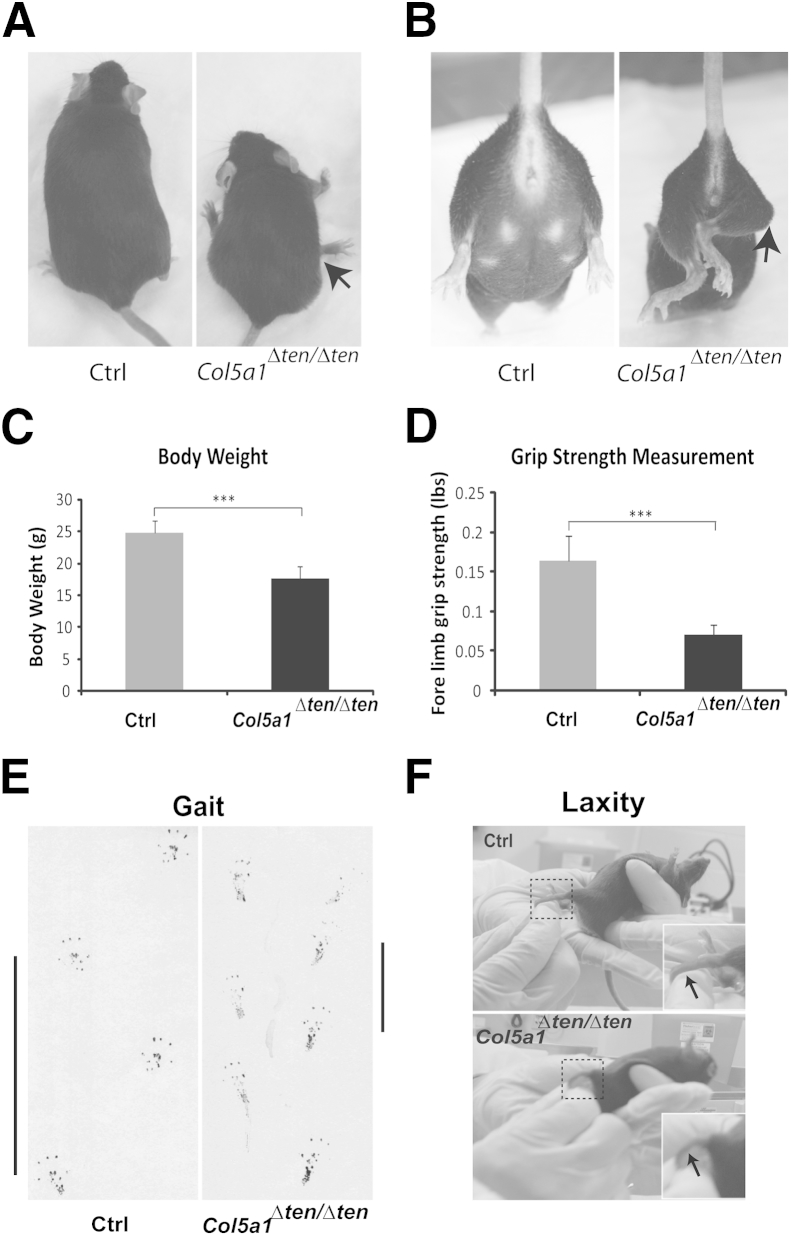
Col5a1Δten/Δten mice are viable; however, grossly they are easily distinguished from their Cre-negative littermates by the significantly smaller body size, slow movement, and abnormal gait in the young mice and joint dislocation in the older mice. We could speculate that the decreased body weight is the result of impaired mobility and function resulting in sedentary mice with difficulty feeding beginning at birth. In Col5a1Δten/Δten mice, the knee joints were positioned abnormally (Figure 3A). When the mouse was lifted gently by the tail, the two feet of the control mouse were positioned symmetrically, pointed to the front with the toes spread (Figure 3B). In contrast to the control mice, the feet of the Col5a1Δten/Δten mouse were not positioned symmetrically and usually were pointed outward or clutched together (Figure 3B). At age P60, the Col5a1Δten/Δten mouse was significantly smaller than the control mouse, and the body weight was 30% less than that of the control littermates (Figure 3C). Inside the cage, the Col5a1Δten/Δten mice tended to stay in one spot and were reluctant to move around. When walking freely on a piece of white paper in a walkway consisting of two Plexiglas walls, Col5a1Δten/Δten mice demonstrated major hindlimb differences in gait characteristics compared with that in Col5a1flox/flox mice. The Col5a1Δten/Δten mice dragged their hind limbs, with the body weight on the base of the foot. In contrast, the control mice placed their body weight on their toes and the middle part of the plantar surface. Compared with the mutant mice, the control mice had a consistently longer stride length and wider toe spread (Figure 3E). Col5a1Δten/Δten mice exhibited a less stable gait pattern than did Col5a1flox/flox mice.
Figure 3.
Joint laxity and aberrant gait in Col5a1Δten/Δten mice. A and B:Col5a1Δten/Δten mice with a targeted deletion of collagen V in tendons and ligaments show smaller size and deformation of the limbs in representative 8-month-old female mice (arrows indicate joint dislocation). C: Decreased body weight in Col5a1Δten/Δten mice. Postnatal day 60 male wild-type (n = 8) and mutant mice (n = 8) mice were used. D: Forelimb grip strength measurement from the same group of mice as in C show weakness of Col5a1Δten/Δten mice relative to control mice. E: Abnormal gait in Col5a1Δten/Δten mice. Representative hindlimb prints of control and Col5a1Δten/Δten mice. The control mice walk in a straight line with regular, even steps and wide toe spread. The Col5a1Δten/Δten mice show short stride lengths (vertical bars), with small toe spread. F:Col5a1Δten/Δten mice exhibit excess joint laxity compared with that in the control mice when the joint is overextended passively (arrows, ankle joint). ∗∗∗P < 0.001. Ctrl, control.
To assess the musculoskeletal and motor function impairment of our mouse models, we measured the forelimb grip strength. The forelimb grip strength for the Col5a1Δten/Δten mice was significantly decreased compared with that of the control mice (P < 0.001) (Figure 3D). Because of the decreased grip strength, mutant mice could not hang on the cage wires only by their forelimbs. The Col5a1Δten/Δten mice also showed excessive joint laxity in the knee and ankle joints (Figure 3F). This increased joint laxity resembled the joint hypermobility seen in patients with classic EDS.2,5 This phenotype progressed as the mice aged. The joint laxity led to instability, easy joint dislocation, and hypermobility. The majority of P90 and older Col5a1Δten/Δten mice had at least one joint dislocation in their four limbs. Both sexes of the Col5a1Δten/Δten mice were fertile. However, pups were produced and raised to weaning at a low rate; this may have been due to the limited mobility in the adult Col5a1Δten/Δten mice (data not shown). These data indicate a severe impairment of tendon and ligament function in the absence of collagen V.
Early-Onset Osteoarthritis in Col5a1Δten/Δten Mice
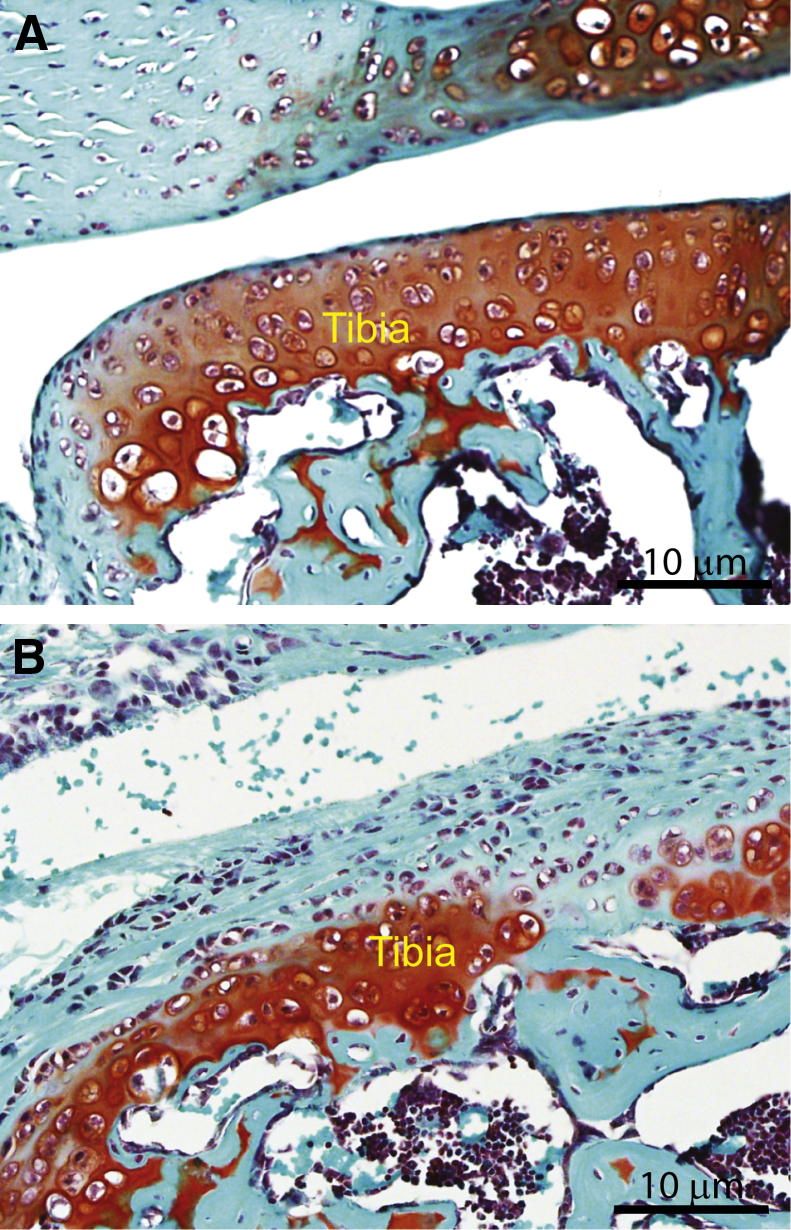
The development of osteoarthritis was examined in control and Col5a1Δten/Δten mouse knee joints. Safranin O stains glycosaminoglycans in cartilage and is used for grading the development of osteoarthritis.32 In the 1-month-old control mice, the articular cartilage of the medial and lateral tibial plateau or the medial and lateral femoral condyle had a smooth surface, evenly stained with Safranin O (Figure 4A). In the superficial zone of the articular cartilage, one or two layers of flat cells were arranged tangentially, and round cells were observed in the middle zone above the tidemark. In contrast, the Col5a1Δten/Δten mouse knees showed uneven staining with Safranin O in weight-bearing cartilage regions, with formation of fibrillations and further degeneration demonstrated by the presence of an osteophyte in severe cases (Figure 4B). These data demonstrate osteoarthritic progression as an indirect outcome of joint hypermobility as a result of collagen V deficiency in tendons and ligaments stabilizing the joint.
Figure 4.
Early-onset osteoarthritis in Col5a1Δten/Δten mice. Representative histologic sections of tibial condyles in control and Col5a1Δten/Δten mice at postnatal day 30. Decalcified knee joint embedded in OCT. Frontal sections of the knee joint were stained with Safranin O and Fast green. A: The cartilage surface in the control mouse is smooth. B: The lateral surface of the tibia condyles in Col5a1Δten/Δten knee joint is covered with fibrous tissue and chronic inflammation cells. The cartilage erosion, chondrocyte death, and proteoglycan depletion indicate the onset of osteoarthritis in Col5a1Δten/Δten knee joint.
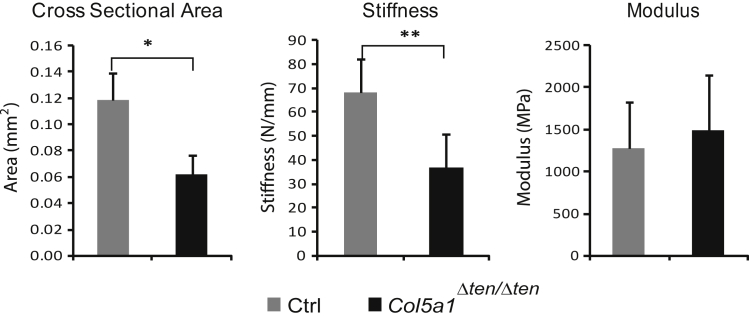
Altered Biomechanical Properties of FDLs in Col5a1Δten/Δten Mice
Cross-sectional area, stiffness, and modulus were analyzed (Figure 5). Cross-sectional area showed a significant difference between the control and mutant mice, with the latter being significantly smaller (P < 0.01). This is consistent with significant difference in body weight in these two groups of mice. Stiffness also showed a significant difference between the two genotypes, with the Col5a1Δten/Δten tendons being less stiff (P < 0.05). However, there were no significant differences detected in modulus between the groups (P = 0.24). These results indicate that the tendons of Col5a1Δten/Δten mice were smaller and, thus, were less stiff. The decreased stiffness is consistent with a role in joint laxity in EDS.
Figure 5.
Altered biomechanical properties in Col5a1Δten/Δten flexor digitorum longus. Cross-sectional area, stiffness, and modulus were measured in postnatal day 60 (P60) male flexor digitorum longus tendons from Col5a1Δten/Δten and Scx-Cre control mice. The significant decrease in cross-sectional area and stiffness in Col5a1Δten/Δten FDLs is consistent with the joint laxity phenotype. The modulus is comparable in both genotypes. ∗P < 0.05, ∗∗P < 0.01.
Aberrant Fiber Structure and Organization in Collagen V–Null FDL Tendons
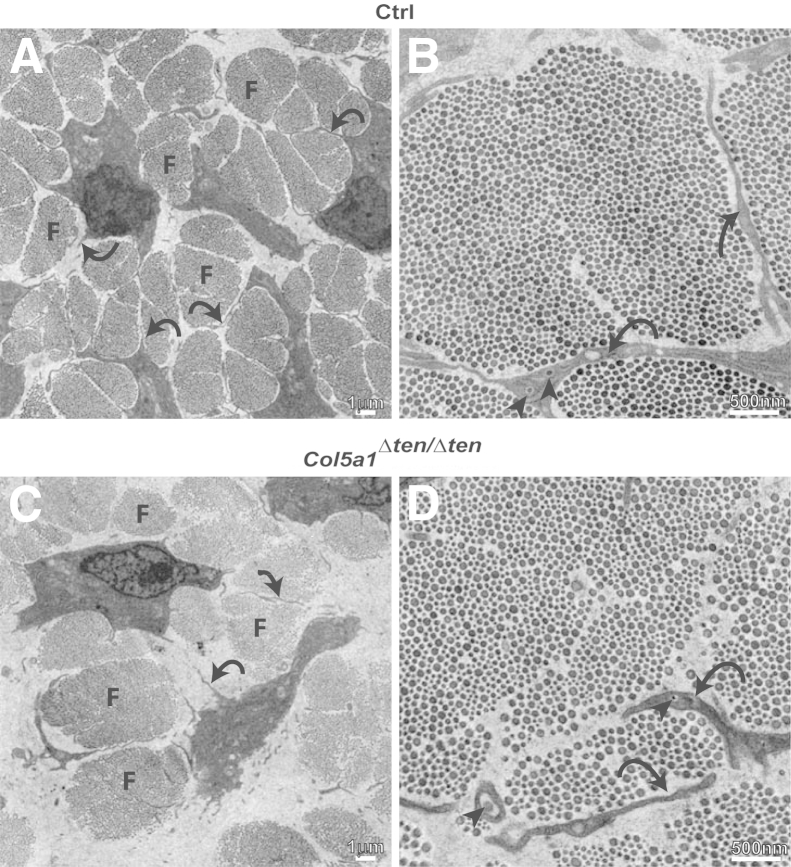
The regulatory roles of collagen V in the assembly of fiber structure, ie, organization of fibrils into fibers associated with tendon fibroblasts, was analyzed at P4 during an early stage of tendon development. In the wild-type mouse FDL, fibers were organized uniaxially throughout the tendon. The fibers were relatively uniform in size and were defined by tenocyte cytoplasmic processes that defined microdomains where fibrils were organized into fibers. Collagen protofibrils can be seen in the cytoplasmic processes during deposition (Figure 6, A and B). In contrast, the Col5a1Δten/Δten tendon demonstrated less-organized fibers. Fibril organization into fibers also was disrupted, with fewer, larger fibers that were less organized than in the wild-type controls. The fibers were packed less regularly, with more and irregular spaces separating them. In addition, the tenocyte processes defining the microdomains were less organized than in the controls and were associated with the disruption in fiber organization (Figure 6, C and D).
Figure 6.
Aberrant fiber structure and organization in Col5a1Δten/Δten tendons during early tendon development. A and B: Postnatal day 4 control (Ctrl) mouse flexor digitorum longus (FDL) contained organized fibers (F) that are relatively uniform in size. The fibers were present in microdomains that were defined by tenocyte cytoplasmic processes (curved arrows). B and D: Collagen protofibrils can be seen during deposition (arrowhead). C and D:Col5a1Δten/Δten mouse FDLs have less-organized fibers (F). In Col5a1Δten/Δten FDLs, there are fewer fibers than in the wild-type controls. The collagen V–null FDLs have larger fibers with more heterogeneity in size than in control tendons. In addition, in the absence of collagen V, the fibers are less organized, ie, less regularly packed, with more space separating them than in the controls. In addition, the tenocyte processes (curved arrows) defining the microdomains are less organized than in the controls.
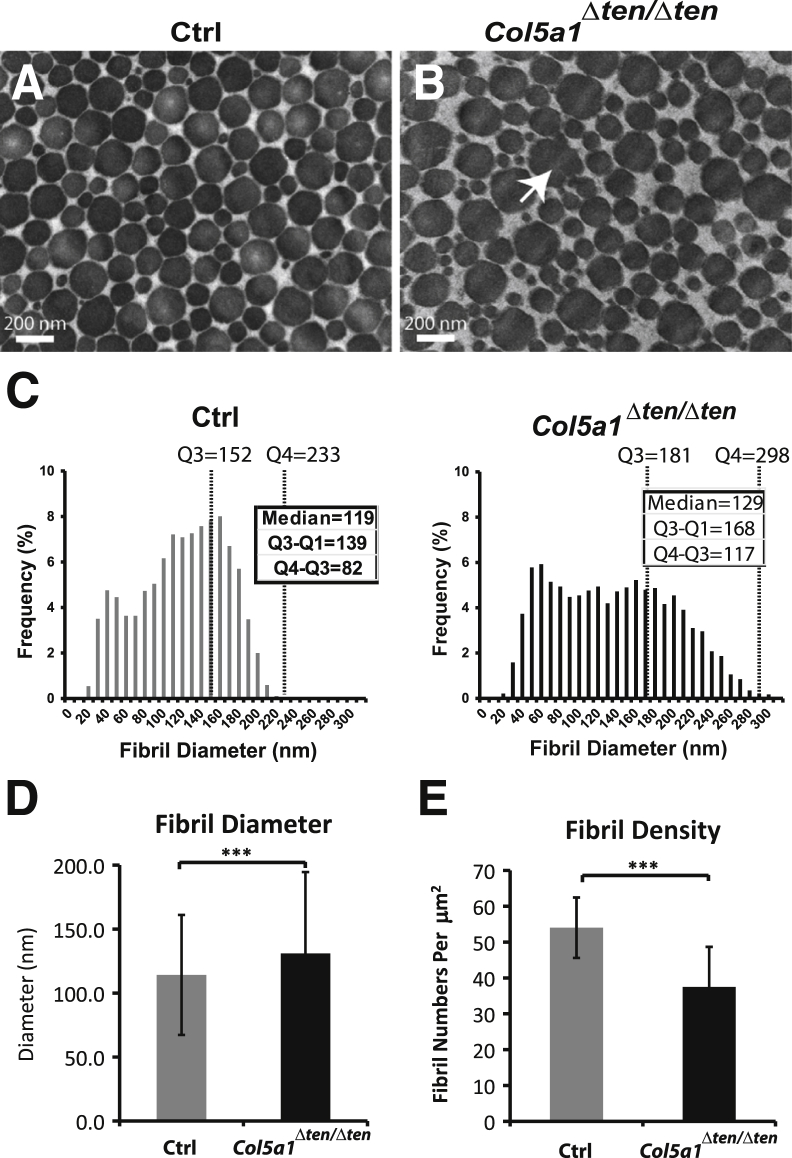
Fibril assembly also was altered in the Col5a1Δten/Δten compared with in the wild-type FDL. Fibril diameters were more heterogeneous in P30 Col5a1Δten/Δten FDLs than in the wild-type tendons (Figure 7, A and B). In addition, the mutant fibrils often demonstrated irregular cross-sectional profiles compared with the circular profiles seen in control mice. Analyses of the diameter distributions demonstrated a broader distribution with a right-hand shoulder representing larger-diameter fibrils in the Col5a1Δten/Δten than in the control FDLs (Figure 7C). In addition, a broadening of the diameter distribution was observed as an increase in the median from 119 nm in the control mouse FDL to 129 nm in the Col5a1Δten/Δten FDL; the interquartile range Q4 to Q3 increased from 82 to 117 nm, and the interquartile range Q3 to Q1 increased from 139 to 168 nm. The fibril diameter (means ± SD) was significantly increased from 114 ± 47 nm in the control mouse FDL to 131 ± 64 nm in the Col5a1Δten/Δten FDL (Figure 7D). The number of fibrils decreased, with fibril density approximately 30% less than in control FDLs (Figure 7E). The assembly of fewer and larger-diameter fibrils is consistent with dysregulated fibril nucleation and assembly in the absence of collagen V.21
Figure 7.
Abnormal fibril structure in mature Col5a1Δten/Δten tendons. A and B: The Col5a1Δten/Δten flexor digitorum longus (FDL) has larger and more heterogeneous fibrils than do the wild-type controls. In addition, fibrils assembled in collagen V–null tendons show aberrant structures (arrow). C: Histograms represent the distribution of fibril diameters in the FDL tendon of control and Col5a1Δten/Δten mice. The Col5a1Δten/Δten mice have broader distribution of the fibril diameters, with increased fibril numbers of both small-diameter and large-diameter fibrils. The diameter was measured in three different mice of each group, with seven images from each mouse. D: The mean fibril diameter is increased significantly in the FDL of Col5a1Δten/Δten mice. E: The fibril density is decreased significantly in the FDL of Col5a1Δten/Δten mice. The means ± SD, n = 21, and Student's t-test (D and E). All FDLs were from male mice at postnatal day 30. ∗∗∗P < 0.001. Ctrl, control; Q, quartile.
Joint laxity and dislocation are the major gross phenotypes in the Col5a1Δten/Δten mice, consistent with a classic EDS joint phenotype. The structural and functional data from the FDL are consistent with a role in joint stability. Data derived from one tendon or ligament often is generalized to tendons and ligaments, but this flexor tendon does not function directly to stabilize joints in vivo. Therefore, a ligament intrinsic to the joint, the ACL, which is a critical ligament in maintaining knee stability, was analyzed.
Expression of Collagen V Is Higher in ACL than in FDL
Collagen V was analyzed in ACL, FDL, and other connective tissues in wild-type mice at P10. Consistent with other results from reports, collagen V expression in FDL was very low and in cornea was very abundant, accounting for 10% to 20% of total collagen.14 Collagen V expression in ACL was substantially higher than that in the FDL; ACL expression was comparable with or higher than that in skin and bone but lower than that in cornea (Figure 8). Col5a1 mRNA expression in P10 mice also indicated higher Col5a1 mRNA expression in the ACL relative to that in the FDL (data not shown). These data suggested that the different collagen V expression levels in ACL versus FDL may contribute to tissue-specific structure and function in tendons and ligaments.
Figure 8.
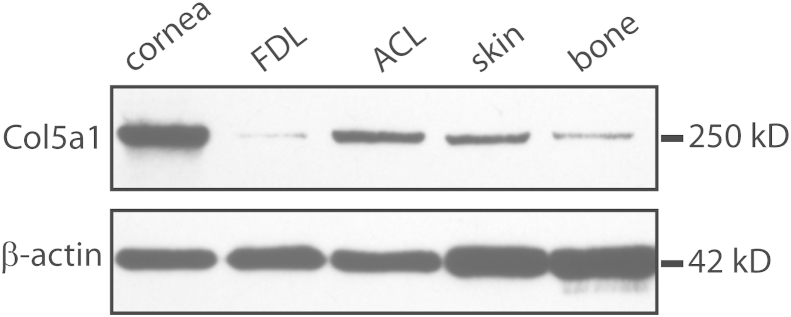
High collagen V content in anterior cruciate ligament (ACL) versus flexor digitorum longus (FDL). Immunoblot analysis shows different collagen V expression levels in different tissues of postnatal day 30 wild-type C57BL/6 mice. Cornea expresses the most collagen V, followed by ACL, which is comparable with that in the skin but much higher than that in FDL and bone. β-Actin was used as the protein loading control.
Altered ACL Fibrillogenesis in Col5a1Δten/Δten Mice
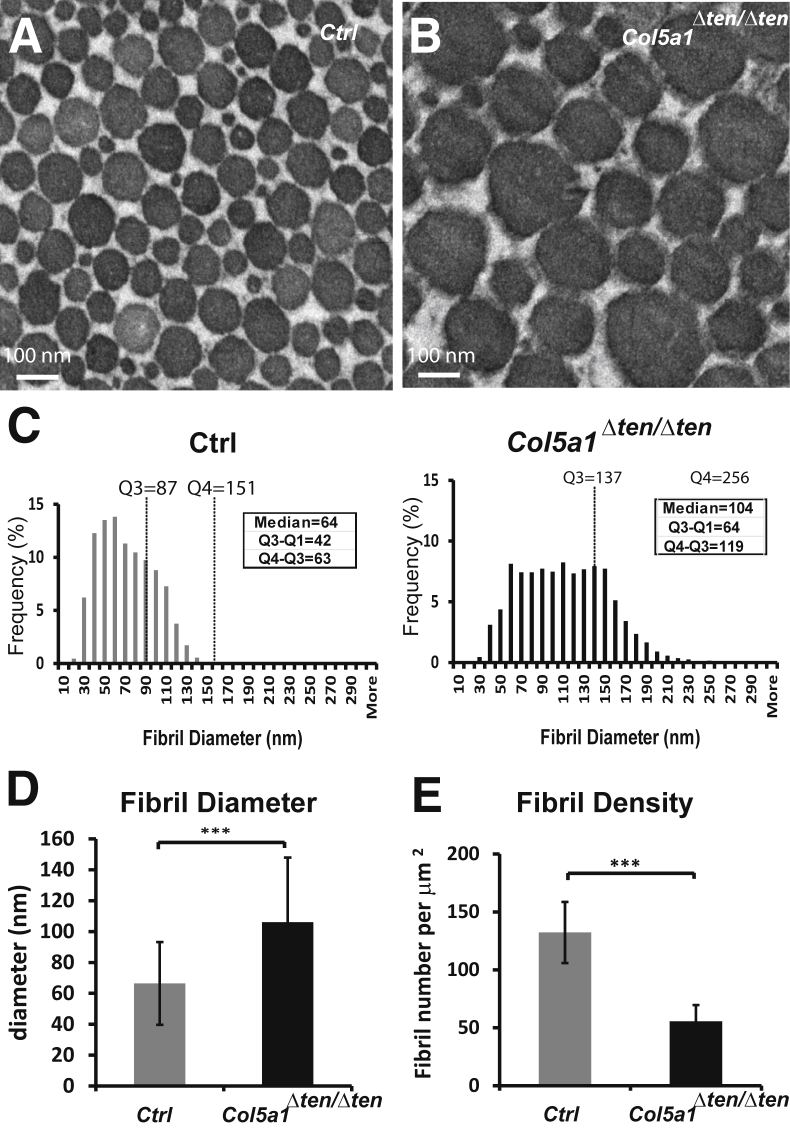
The ACL showed a severe disruption of fibrillogenesis in Col5a1Δten/Δten mice relative to the control mice at age P30 (Figure 9, A and B). Unlike in FDL tendon, the fibril bundles in ACL are not aligned in parallel, especially at the distal and proximal ends. However, the fibril arrangement is in parallel to the axis, and fibril cross sections were compared only in the ACL midsubstance. At P30, the fibril diameter distribution patterns were similar in the control ACLs and FDLs. The fibril diameters were heterogeneous, with larger and smaller diameter fibrils interspersed (Figure 9A). However, the fibril diameters in ACLs were smaller than in FDLs of comparable age. Diameters (means ± SD) were 67 ± 27 nm in the ACL (Figure 9D) compared with 118 ± 47 nm in the FDL (Figure 7D) at age P30. Compared with those in control mice, Col5a1Δten/Δten ACLs had a broader fibril diameter distribution (Figure 9C). The fibril diameter distribution showed a significant shift to the larger fibrils in Col5a1Δten/Δten mice, with a reduction in small-diameter fibrils in ACLs from Col5a1Δten/Δten mice. The median diameter of the fibrils was 104 nm, ranging from 23 to 256 nm, and the interquartile range Q4 to Q3 was 119 nm. In contrast, the median fibril diameter for the control mice was 64 nm, ranging from 13 to 151 nm, and the interquartile range Q4 to Q3 was 63 nm (Figure 9C). A statistical analysis demonstrated a significant increase in the mean fibril diameter (P < 0.001) in the ACL of Col5a1Δten/Δten mice compared with that in control mice (Figure 9D). In addition, the number of fibrils assembled in the Col5a1Δten/Δten ACLs was reduced compared with that in controls, with a significant decrease in fibril density (P < 0.001) (Figure 9E). The decrease in density was associated with an increase in fibril diameter, as well as an increase in interfibril spacing. These data indicated that collagen V is required for normal fibrillogenesis in the ACL ligament.
Figure 9.
Abnormal anterior cruciate ligament (ACL) structure in the Col5a1Δten/Δten mouse. A and B: Transmission electron microscopy shows a significant increase in fibril diameter and aberrant fibril structure in the Col5a1Δten/Δten ACL. The alterations in the collagen V–null ACL were more severe. C: Histograms show the broader distribution of fibril diameters in the ACL of Col5a1Δten/Δten mice compared with that in the control mice. D: The mean fibril diameter is increased significantly in the ACL of Col5a1Δten/Δten mice ACL. E: The fibril density was decreased significantly in Col5a1Δten/Δten mice. Fibril diameter and density were measured in three different mice of each group, with three to seven images from each mouse; the data are presented as means ± SD, and Student's t-test was used (C–E). All mice were postnatal day 30 males. ∗∗∗P < 0.001. Ctrl, control; Q, quartile.
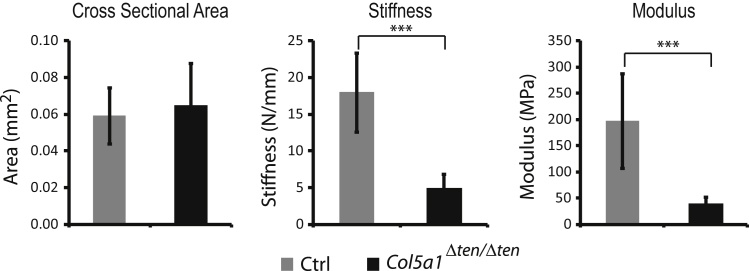
Severe Reduction in the Biomechanical Properties of Collagen V–Null ACL
During tissue preparation and biomechanical testing, the ACLs from Col5a1Δten/Δten mice were very fragile. ACLs frequently were broken before dissecting, and even very small loads during careful preparation of the ACLs for testing caused some ligaments to fail. This occurred only in the Col5a1Δten/Δten mice and not in the control mice. The Col5a1Δten/Δten ACLs demonstrated a significantly lower maximum load and maximum stress compared with that in wild-type controls (data not shown). In contrast to the FDL, there was no significant difference in cross-sectional area between Col5a1Δten/Δten and wild-type ACLs. However, the Col5a1Δten/Δten ACLs had a significantly lower stiffness and modulus compared with those in the controls (P < 0.001) (Figure 10). In the absence of a change in cross-sectional area, results support a change in the material properties of the ACL in the absence of collagen V. The mechanical changes in the ACL were much more dramatic than the changes in the FDL, suggesting that collagen V has a greater role in the ACL.
Figure 10.
Altered biomechanical properties in anterior cruciate ligament (ACL) of Col5a1Δten/Δten mice. The biomechanical properties are altered significantly in the absence of collagen V, with a significant decrease in stiffness and modulus in the Col5a1Δten/Δten ACL compared with that in the controls (Ctrl). ∗∗∗P < 0.001.
Disruption of Higher-Order Structure in ACLs from Col5a1Δten/Δten Mice
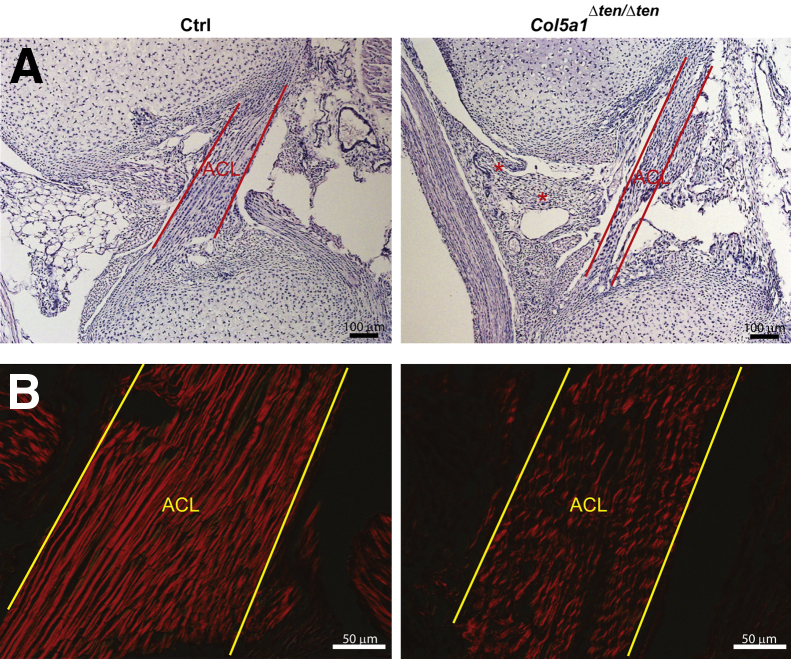
In wild-type ACLs, running from the anterior intercondylar region of the proximal tibia to the medial aspect of the lateral femoral condyle within the intercondylar groove, well-organized fibril bundles and fibroblasts were aligned parallel to the stress axis in the ACL midsubstance (Figure 11A). In contrast, in the knees from Col5a1Δten/Δten mice, the orientation of ACL fibril bundles was disorganized and wavy. Patchy infiltration of lymphocytes and increased vasculature also were present around the knee joint, consistent with soft-tissue inflammation, suggesting a secondary defect resulting from ligament injury in the unstable joint (Figure 11A). Collagen fiber organization in the ACL was analyzed using picrosirius red staining with polarized light microscopy (Figure 11B). Large bright, orange-red collagen fibers were arranged in parallel and were well organized in control mouse ACL. In contrast, small and wavy collagen fibers were sparse and disorganized in Col5a1Δten/Δten mouse ACL, indicating that collagen fibers were smaller and less organized. In the Col5a1Δten/Δten ACL, it is noteworthy that there was a consistent difference in crimping when compared with that in the controls (Figure 11B), which would be consistent with increased elasticity and joint hypermobility.
Figure 11.
Targeted depletion of collagen V in tendon and ligament induces joint instability. A: Joint laxity and chronic fibrous inflammation presented in Col5a1Δten/Δten mice knee joints at postnatal day 4. Sagittal sections of knee joints from postnatal day 4 mice show that the fibroblasts and fibers of the anterior cruciate ligament (ACL) align parallel in the ACL midsubstance in the control (Ctrl) mice. In the Col5a1Δten/Δten ACL, the fibers were disorganized and wavy. The asterisks show synovial inflammation that contained fibrous connective tissue, blood vessels, and chronic inflammatory cells in the Col5a1Δten/Δten ACL. Stain: H&E. B: Polarized light microscopy in control mice shows large, well-organized ACL fibers. In contrast, the fibers in Col5a1Δten/Δten ACL are wavy and smaller. The specimens were the same as in A. Stain: picrosirius red.
Discussion
The targeted deletion of collagen V in tendons and ligaments created a mouse model (Col5a1Δten/Δten) that faithfully recapitulates the joint laxity seen clinically in patients with classic EDS. The joint phenotype is the result of structural and functional changes in tendons and ligaments, especially the ACL, that is essential for the stabilization of the lower limb joint. Tendons and ligaments are multiunit hierarchical structures that contain collagen molecules, collagen fibrils, collagen fibers, and fascicles organized parallel to the geometric axis.33–35 Collagen I accounts for the majority of the collagen mass, whereas collagen V forms heterotypic fibrils with collagen I in the matrix of different tissues to regulate the fibril nucleation and initiation of fibrillogenesis.18,21,36 The Col5a1−/− mouse model was embryonic lethal because of a virtual lack of fibril formation. However, the Col5a1Δten/Δten mice are viable and fertile. The severe disruption in tendon and ligament structure and function results in sedentary mice with impaired mobility. However, unlike in the Col5a1−/− mouse model, the tendons and ligaments of the Col5a1Δten/Δten mice assembled fibrils. The embryo 10.5 days post coitum has a low-collagen-concentration environment, and the presence of collagen V as a nucleator to lower the critical concentration required for initiation of fibril assembly is essential. In contrast, developing tendons have a high-collagen-concentration environment in which fibril assembly can occur in the absence of collagen V, as is observed with in vitro self-assembly assays.17 However, the regulation conferred by heterotypic collagen I/V interactions is absent, resulting in the dysfunctional fibril and matrix assembly observed.
Altered Fibrillogenesis in Collagen V Mouse Models
Previous work in a Col5a1+/− haploinsufficient mouse model demonstrated increased fibril diameters and a subpopulation of fibrils that were large and structurally aberrant in the dermis, consistent with the classic EDS skin phenotype.20 However, tendon structure in the haploinsufficient mouse model did not show major structural changes.21 In contrast, the Col5a1Δten/Δten mouse model demonstrates major changes in tendon and ligament fibril structure, including an increase in fibril diameter and decrease in fibril density. This structural change is consistent with the fibril changes in EDS patients.37 This suggests that collagen V must be present above a specific concentration for assembly of normal fibrils, but higher concentrations are required for structural and functional integrity of the tendon.
Altered Fiber Structure and Organization in Collagen V–Null Mice
In addition to the changes in the fibril formation, collagen fiber organization also is altered in the Col5a1Δten/Δten tendon. Col5a1Δten/Δten FDLs have less-organized tenocytes, resulting in fewer and larger fibers that are disorganized compared with those in the wild-type controls. This finding suggests that the loss of collagen V may alter cell–extracellular matrix interactions. Tendon fibroblasts establish a hierarchy of extracellular compartments associated with assembly of fibrils, fibril bundles, and fibers during development.23,38,39 This hierarchy of microdomains allows control over the extracellular events in matrix assembly. Results from a recent study indicate that collagen V is localized preferentially on the tenocyte surface as distinct foci in tendons and in cell culture. This indicates that collagen V associates with the tenocyte surface, where it is proposed to function in regulation of collagen assembly and cell-directed fibril deposition.40 Collagen V also is present in a pericellular–cell-associated matrix extract from developing tenocytes.41 Collagen V binds to extracellular matrix proteins such as collagens, enzymes, and growth factors through the α1-N-propeptide and is involved in extracellular matrix homeostasis and bridging function in the cell-matrix environment.42 Depletion of collagen V in tendon may destroy the binding of extracellular matrix proteins to the cells and further affect the tenocyte-directed fibril deposition, fiber formation, and higher-level hierarchical organization. However, the detailed mechanism is still unclear and requires further investigation.
Altered Biomechanical Properties in Col5a1Δten/Δten Mice
Biomechanical studies of the Col5a1+/− haploinsufficient20 and Col5a1Δten/Δten FDLs demonstrate decreased cross-sectional area and stiffness. However, the modulus does not change significantly. From a mechanical perspective, this finding suggests that the material is functionally the same between groups, with differences primarily due to the size of the tissue. The changes in FDLs are relatively mild when compared with the severe biomechanical phenotype of the Col5a1Δten/Δten ACL, with a change in stiffness and modulus but no change in cross-sectional area compared with those in the controls. Unlike the FDL data, these data suggest a change in the material properties of the ACL because of the alterations in the structural organization. The FDL tendon is neither a weight-bearing tendon nor a direct joint-stabilizing tendon. Conversely, the ACL is intrinsic to the joint and essential for its stabilization, as well as one of the most vulnerable tendons or ligaments in the body.43 The differences in mechanical and structural properties in the FDL and ACL suggest that collagen V is a critical regulator of tendon- and ligament-specific properties. To our surprise, compared with collagen V expression in the FDL, collagen V expression is substantially higher in the ACL. The collagen V level in ACL was comparable with or slightly higher than that in skin and substantially higher than that in FDL and bone, although it is still lower than that in cornea. This is the first time that the collagen V expression level in ACL has been reported. It is possible that the higher collagen V expression in the ACL confers tissue-specific regulatory properties. The resulting ACL-specific fibrillogenesis would yield a matrix structure with a primary function in joint stabilization.
Mouse Model for Classic EDS
When collagen V is knocked out in Col5a1Δten/Δten tendons and ligaments, there is a significant increase in fibril diameter and decrease in fibril density. The alterations in collagen organization and structure influence the biomechanical properties. A similar disorganization and dysfunction is found in the tendons of classic EDS patients bearing a COL5A1 mutation.37 The smaller tendon, reduced stiffness, and modulus are consistent with a hypermobile joint phenotype supported by the loose joint capsule, changes in gait, and changes in walking patterns. Col5a1Δten/Δten ligaments are easy to rupture. This finding, coupled with joint laxity and dislocation, could lead to early-onset osteoarthritis as a secondary effect to joint hypermobility, as was observed in the Col5a1Δten/Δten mouse model. The joint hypermobility phenotype progressed dramatically with age. The phenotype in the Col5a1Δten/Δten is more severe than in EDS patients because Col5a1Δten/Δten tendons and ligaments are null for collagen V, whereas most EDS patients harbor COL5A1 mutations in only one allele. However, the mouse provides a valid model that faithfully recapitulates the clinical joint phenotype. Male mice were used in this initial study to ensure that the studies were controlled appropriately so that phenotypic differences would be explained only by gene dosage effects. In future work, it would be of interest to analyze potential sex differences in the mouse model to address the fact that female patients present more with classic EDS than do male patients.
Conclusions
Our results indicate that collagen V plays a critical regulatory role in the fibrillogenesis of tendons and ligaments. The deficiency of collagen V targeted to tendons and ligaments impairs normal FDL and ACL function, with differential tissue-specific effects. The absence of collagen V results in altered structure and functional properties, resulting in a severe joint hypermobility phenotype analogous to that observed in EDS patients. This mouse model provides, for the first time, the ability to target collagen V specifically in the musculoskeletal system, resulting in a severe joint phenotype that faithfully recapitulates the clinical phenotype observed in classic EDS patients. Therefore, an animal model of joint laxity has been created, providing a critical tool for the development of therapeutic interventions for EDS patients, as well as a larger group of non-EDS patients with joint laxity. Model availability provides a unique opportunity to evaluate a host of potential therapeutic agents, such as matrix metalloproteinase inhibitors like doxycycline,44,45 or agents that have been tried in attempts to control metastatic cancer but failed because of patients developing the complication of dose-dependent, reversible joint stiffness.46,47 In summary, the Col5a1Δten/Δten mouse model is a model for the EDS and hypermobile joint phenotype and can be used in future preclinical studies of injury, as well as clinical interventions for treatment of EDS and patients with hypermobile joints.
Acknowledgments
We thank Drs. Michael Mienaltowski (University of California Davis) and Patricia Teran-Yengle (University of South Florida) for helpful discussions and Qingmei Yao (University of South Florida) for the expert technical assistance with the maintenance of the mouse lines.
Footnotes
Supported by NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants AR044745 (D.E.B.) and AR065995 (L.J.S. and D.E.B.).
Disclosures: None declared.
References
- 1.Beighton P., De Paepe A., Steinmann B., Tsipouras P., Wenstrup R.J. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers- Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.De Paepe A., Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet. 2012;82:1–11. doi: 10.1111/j.1399-0004.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- 3.Malfait F., De Paepe A. The Ehlers-Danlos syndrome. Adv Exp Med Biol. 2014;802:129–143. doi: 10.1007/978-94-007-7893-1_9. [DOI] [PubMed] [Google Scholar]
- 4.Malfait F., Wenstrup R.J., De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12:597–605. doi: 10.1097/GIM.0b013e3181eed412. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann B., Royce P.M., Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce P.M., Steinmann B., editors. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. ed 2. Wiley-Liss; New York: 2002. pp. 431–523. [Google Scholar]
- 6.Castori M. Ehlers-danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012;2012:751–768. doi: 10.5402/2012/751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beighton P., Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51:444–453. [PubMed] [Google Scholar]
- 8.Stanitski D.F., Nadjarian R., Stanitski C.L., Bawle E., Tsipouras P. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Orthop Relat Res. 2000:213–221. doi: 10.1097/00003086-200007000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Ainsworth S.R., Aulicino P.L. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. 1993:250–256. [PubMed] [Google Scholar]
- 10.Symoens S., Syx D., Malfait F., Callewaert B., De Backer J., Vanakker O., Coucke P., De Paepe A. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012;33:1485–1493. doi: 10.1002/humu.22137. [DOI] [PubMed] [Google Scholar]
- 11.Malfait F., Coucke P., Symoens S., Loeys B., Nuytinck L., De Paepe A. The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 2005;25:28–37. doi: 10.1002/humu.20107. [DOI] [PubMed] [Google Scholar]
- 12.Malfait F., De Paepe A. Molecular genetics in classic Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2005;139C:17–23. doi: 10.1002/ajmg.c.30070. [DOI] [PubMed] [Google Scholar]
- 13.Birk D.E., Bruckner P. Collagens, suprastructures and collagen fibril assembly. In: Mecham R.P., editor. Vol 1. Springer; New York: 2011. pp. 77–115. (In The Extracellular Matrix: an Overview). [Google Scholar]
- 14.Birk D.E. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 15.Segev F., Héon E., Cole W.G., Wenstrup R.J., Young F., Slomovic A.R., Rootman D.S., Whitaker-Menezes D., Chervoneva I., Birk D.E. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 16.Marchant J.K., Hahn R.A., Linsenmayer T.F., Birk D.E. Reduction of type V collagen using a dominant-negative strategy alters the regulation of fibrillogenesis and results in the loss of corneal-specific fibril morphology. J Cell Biol. 1996;135:1415–1426. doi: 10.1083/jcb.135.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birk D.E., Fitch J.M., Babiarz J.P., Doane K.J., Linsenmayer T.F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 18.Wenstrup R.J., Florer J.B., Brunskill E.W., Bell S.M., Chervoneva I., Birk D.E. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 19.Wenstrup R.J., Florer J.B., Cole W.G., Willing M.C., Birk D.E. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004;92:113–124. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- 20.Wenstrup R.J., Florer J.B., Davidson J.M., Phillips C.L., Pfeiffer B.J., Menezes D.W., Chervoneva I., Birk D.E. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 21.Wenstrup R.J., Smith S.M., Florer J.B., Zhang G., Beason D.P., Seegmiller R.E., Soslowsky L.J., Birk D.E. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun M., Chen S., Adams S.M., Florer J.B., Liu H., Kao W.W., Wenstrup R.J., Birk D.E. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124:4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G., Young B.B., Ezura Y., Favata M., Soslowsky L.J., Chakravarti S., Birk D.E. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- 24.Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J.L., Pryce B.A., Johnson R.L., Tabin C.J., Schweitzer R., Zelzer E. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17:861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansorge H.L., Meng X., Zhang G., Veit G., Sun M., Klement J.F., Beason D.P., Soslowsky L.J., Koch M., Birk D.E. Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J Biol Chem. 2009;284:8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltz C.D., Perry S.M., Getz C.L., Soslowsky L.J. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connizzo BK, Freedman BR, Fried JH, Sun M, Birk DE, Soslowsky LJ. Regulatory role for collagen V in establishing mechanical properties of tendons and ligaments is tissue dependent. J Orthop Res, In press. [DOI] [PMC free article] [PubMed]
- 28.Chen S., Oldberg A., Chakravarti S., Birk D.E. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dyn. 2010;239:844–854. doi: 10.1002/dvdy.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brent A.E., Schweitzer R., Tabin C.J. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 30.Schweitzer R., Chyung J.H., Murtaugh L.C., Brent A.E., Rosen V., Olson E.N., Lassar A., Tabin C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 31.Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 32.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A., Salter D., van den Berg W.B. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Elliott D.H. Structure and function of mammalian tendon. Biol Rev Camb Philos Soc. 1965;40:392–421. doi: 10.1111/j.1469-185x.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 34.Silver F.H., Freeman J.W., Seehra G.P. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin M., Ralphs J.R. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- 36.Birk D.E., Fitch J.M., Babiarz J.P., Linsenmayer T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen R.H., Couppé C., Jensen J.K., Olsen M.R., Heinemeier K.M., Malfait F., Symoens S., De Paepe A., Schjerling P., Magnusson S.P., Remvig L., Kjaer M. Low tendon stiffness and abnormal ultrastructure distinguish classic Ehlers-Danlos syndrome from benign joint hypermobility syndrome in patients. FASEB J. 2014;28:4668–4676. doi: 10.1096/fj.14-249656. [DOI] [PubMed] [Google Scholar]
- 38.Birk D.E., Zycband E. Assembly of the tendon extracellular matrix during development. J Anat. 1994;184:457–463. [PMC free article] [PubMed] [Google Scholar]
- 39.Birk D.E., Trelstad R.L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith S.M., Zhang G., Birk D.E. Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis. Matrix Biol. 2014;33:47–53. doi: 10.1016/j.matbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith S.M., Thomas C.E., Birk D.E. Pericellular proteins of the developing mouse tendon: a proteomic analysis. Connect Tissue Res. 2012;53:2–13. doi: 10.3109/03008207.2011.602766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Symoens S., Renard M., Bonod-Bidaud C., Syx D., Vaganay E., Malfait F., Ricard-Blum S., Kessler E., Van Laer L., Coucke P., Ruggiero F., De Paepe A. Identification of binding partners interacting with the alpha1-N-propeptide of type V collagen. Biochem J. 2011;433:371–381. doi: 10.1042/BJ20101061. [DOI] [PubMed] [Google Scholar]
- 43.Cimino F., Volk B.S., Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. Am Fam Physician. 2010;82:917–922. [PubMed] [Google Scholar]
- 44.Kessler M.W., Barr J., Greenwald R., Lane L.B., Dines J.S., Dines D.M., Drakos M.C., Grande D.A., Chahine N.O. Enhancement of Achilles tendon repair mediated by matrix metalloproteinase inhibition via systemic administration of doxycycline. J Orthop Res. 2014;32:500–506. doi: 10.1002/jor.22564. [DOI] [PubMed] [Google Scholar]
- 45.Arnoczky S.P., Lavagnino M., Egerbacher M., Caballero O., Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763–769. doi: 10.1177/0363546506296043. [DOI] [PubMed] [Google Scholar]
- 46.Heath E.I., Grochow L.B. Clinical potential of matrix metalloprotease inhibitors in cancer therapy. Drugs. 2000;59:1043–1055. doi: 10.2165/00003495-200059050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Fenlon D., Addington-Hall J.M., O'Callaghan A.C., Clough J., Nicholls P., Simmonds P. A survey of joint and muscle aches, pain, and stiffness comparing women with and without breast cancer. J Pain Symptom Manage. 2013;46:523–535. doi: 10.1016/j.jpainsymman.2012.10.282. [DOI] [PubMed] [Google Scholar]