Abstract
This is a rare case report of a 30-year-old male, who was admitted to the Maxillofacial Surgery Department of the Dental College for a malunited fracture of the mandible and zygomatic bones. He was given oral medications namely, cefixime, metronidazole, ondansetron, and ranitidine for three days prior to the operation with complete normal preoperative workup. He had no significant past medical or family history. On the day of the operation, he was given injectable dexamethasone, cefotaxime, ondansetron, ranitidine, and metronidazole half-an-hour prior to the operation. In less than five minutes of giving a bolus ranitidine injection, the patient developed a cardiac arrest and was resuscitated by the anesthetist team on duty. He was transferred to the Intensive Care Unit (ICU) on a ventilator, which was soon removed and the patient was off vasopressors, with stable vitals for 24 hours after the event. He was then transferred to the general ward of Medicine Department and observed for a further two days during which the patient remained uneventful and was finally transferred back to the Dental Department.
Keywords: Cardiac arrest, intravenous bolus, intravenous infusion, ranitidine
CASE REPORT
A 30-year-old male, living in Baswada, Rajasthan, India, presented to the Maxillofacial Surgery Department of the Dental College with a complaint of reduced mouth opening since the last one month. He had suffered from traumatic facial injury following a road traffic accident in his village where he was hit by a bike. He was admitted to the hospital at the local place for 15 days, following which he was discharged. Since then he had reduced mouth opening.
A three-dimensional (3D) computed tomography (CT) scan reconstruction of the face revealed malunited fractures of the zygomatic and mandible bones on the right side, as shown in Figure 1. He was admitted on Tuesday, 23 July, 2013, and was planned for reduction surgery on Friday. He was investigated for operative fitness. The tests included human immunodeficiency virus (HIV) and hepatitis serology, with complete blood counts, renal and liver function tests with serum biochemistry, and electrocardiogram (EKG), all of which were normal. He had no significant past medical history or family history. He was a chronic tobacco chewer.
Figure 1.
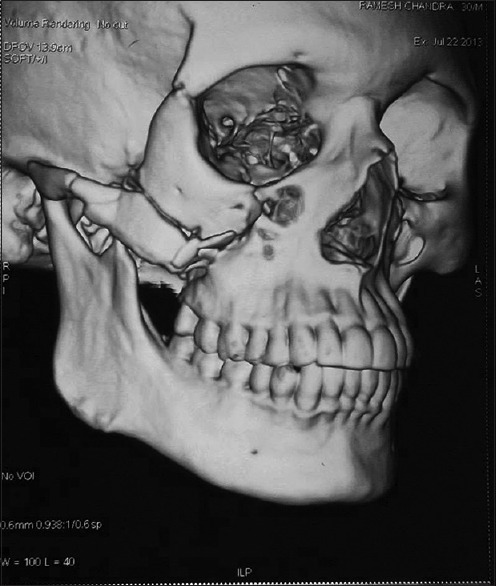
Fracture of the right zygomatic arch and right coronoid process of the mandible
During the preoperative days he was given oral antibiotics, which included cefixime and metronidazole, with supplement medications including ranitidine and ondansetron. On the day of the operation, as per protocol, the patient was given injectable drugs namely, dexamethasone (4 mg), cefotaxime (1 g), ondansetron (4 mg), pint of metronidazole (500 mg/100 ml), and when the bolus of intravenous ranitidine (50 mg/2 ml) was being pushed, the patient suddenly collapsed. The anesthetists on duty discovered asystole and started vigorous cardiopulmonary resuscitation with chest compression, adrenaline, dopamine, noradrenaline, and endotracheal intubation with an Artificial Manual Breathing Unit (AMBU) bag for delivery of adequate tidal volume and oxygen concentration, in view of the slowing and failing respiration.
The patient was immediately shifted to the ICU under the care of the Medicine Department. He was kept on ventilator support, with vasopressors and fluids according to central venous pressure monitoring and all serological investigations, as well as bedside 2D Echocardiography and venous Doppler of the upper limb, through which infusion was given were done. Gradually, the cardiac activity returned to normal and the patient was off the vasopressors. After two hours of ventilator support, the patient could be weaned and was kept on a T-piece with oxygen delivered at 6 L/minute. The next day, the patient was extubated successfully and remained uneventful during the 24 hours of continuous critical cardiac monitoring, including bedside echocardiography in the ICU, after which he was shifted to the general ward of the Medicine Department. He was observed further for two more days in the ward and finally transferred back to the Dental Department, after complete investigations, to ensure a normal cardiac and neurological status.
The case has been reported to the Central Drugs Standard Control Organization (CDSCO) as per the norms, by submitting the ‘suspected adverse drug reaction form’ via the Department of Pharmacology, B. J. Medical College, Ahmedabad, Gujarat, India. According to the Naranjo Adverse Drug Reaction (ADR) probability scale and the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) scale, ranitidine is the probable cause of the event, as no re-challenge was carried out.
DISCUSSION
Histamine means ‘tissue amine’ and is almost ubiquitously present in animal tissues. Asch and Schild classified the receptors for histamine, for the first time in 1966, into H1 and H2. Sir James Black in 1972, developed the first H2 blocker, burimamide and confirmed this classification.[1]
The H2 receptors involve the adenylyl cyclase activation pathway, leading to a rise in cyclic adenosine monophosphate (cAMP) and phosphorylation of the specific proteins.[2] The H2 receptors are distributed in various tissues, such as, gastric glands, blood vessels, heart, uterus, brain, and so on. Blockade of the H2 receptors decreases gastric acid secretion, which is the main clinical utility of this class of drugs. Cimetidine, the prototype drug of this class has fallen out of use due to its significant drug interactions via inhibition of the cytochrome P-450 enzyme. Newer available H2 blockers lacking this side effect include ranitidine, famotidine, roxatidine and loxatidine.
Ranitidine causes minor side effects, such as, headache, nausea, and constipation, which usually resolve with continued therapy. Effects on the Central Nervous System (CNS), such as, a confusional state, restlessness, hallucinations, delirium, convulsions, and coma, have occurred in elderly patients and in those with renal impairment, especially with large doses infused intravenously.
A bolus intravenous injection can cause bradycardia, arrhythmias and cardiac arrest.[3] The adverse cardiac effects of ranitidine may be due to H2 receptor antagonism in the coronary smooth muscle via vasoconstriction, a rise in the plasma histamine levels[4] or by cholinesterase inhibition.[5]
To the best of our knowledge, there are only two case reports on cardiac arrest due to ranitidine in the PUBMED database, namely: (1) BMJ 1989[6] and (2) Rev Esp Anestesiol Reanim. 1998.[7] This case, according to the author, is probably the third in the world and the first in India.
According to the Food and Drug Administration (FDA) label information for ranitidine, dilute ranitidine injection, 50 mg in 0.9% sodium chloride injection or other compatible IV solution to a concentration no greater than 2.5 mg/mL (20 mL) must be injected at a rate no greater than 4 mL/minute (five minutes).[8] The FDA label information does not provide any issue of interaction between ondansetron and ranitidine. Moreover there was a sufficient lag time between the injections of ondansetron and ranitidine, as the injection of metronidazole infusion was given in between the two, and there was no cardiac adverse event during that period also. Hence, injectable ranitidine appears to be the only probable cause for the event.
This case report, therefore, clearly provides a message that injection ranitidine must always be given in a slow diluted bolus dose, as mentioned in the FDA label information.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tripathi KD. Histamine and Antihistaminics. In: Tripathi M, editor. Essentials of Medical Pharmacology. 5th ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2003. pp. 135–44. [Google Scholar]
- 2.Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Professional; 2006. pp. 623–34. [Google Scholar]
- 3.Camarri E, Chirone E, Fanteria G, Zocchi M. Ranitidine-induced bradycardia. Lancet. 1982;2:160. doi: 10.1016/s0140-6736(82)91129-1. [DOI] [PubMed] [Google Scholar]
- 4.Baumann G, Loher U, Felix SB, Heidecke CD, Riess G, Ludwig L, et al. Deleterious effects of cimetidine in the presence of histamineon coronary circulation. Possible clinical implications in anaphylactic states in individuals with coronary heart disease. Res Exp Med (Berl) 1982;180:209–13. doi: 10.1007/BF01852292. [DOI] [PubMed] [Google Scholar]
- 5.Hansen WE, Bertl S. Inhibition of cholinesterases by ranitidine. Lancet. 1983;1:235. doi: 10.1016/s0140-6736(83)92605-3. [DOI] [PubMed] [Google Scholar]
- 6.Hart AM. Cardiac arrest associated with ranitidine. BMJ. 1989;299:519. doi: 10.1136/bmj.299.6697.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira TA, Pensado A, Rama P, Molins N, Valdés C, Pose P. Asystole after intravenous administration of ranitidine. Rev Esp Anestesiol Reanim. 1998;45:30. [PubMed] [Google Scholar]
- 8.Internet. [Last accessed on 2014 July 06]. Available from: www.fda.gov.http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019090s053,019593s042lbl.pdf .


