Abstract
The effect of race on breast cancer outcome is confounded by tumor and treatment heterogeneity. We examined a cohort of women with stage II–III breast cancer treated uniformly with neoadjuvant chemotherapy to identify factors associated with racial differences in chemotherapeutic response and long-term survival. Using a prospective database, we identified women with stage II-III breast cancer treated with neoadjuvant chemotherapy from 1998 to 2011. Race was categorized as African-American (AA) or non-AA. Preplanned subtype analyses were stratified by hormone receptor (HR) and HER2. Pathologic response to chemotherapy (pCR), time to recurrence (TTR), and overall survival (OS) were assessed using logistic regression, Kaplan–Meier method, and Cox proportional hazards regression analyses. Of 349 women identified, 102 (29 %) were AA, who were younger (p = 0.03), more obese (p < 0.001), and less likely to have HR+/HER2–tumors (p = 0.01). No significant differences in pCR rate by race were found. At median follow-up of 6.5 years, AA had worse TTR (hazard ratio 1.51, 95 % CI 1.02–2.24), which was attenuated in multivariable modeling, and there was no significant difference in OS. When stratified by HR, worse outcomes were limited to HR+AA (TTR hazard ratio 1.85, 95 % CI 1.09–3.14; OS hazard ratio 2.42 95 % CI 1.37–4.28), which remained significant in multivariable analysis including pCR rate and BMI. With long-term follow-up, racial disparity in outcome was limited to HR+ breast cancer, with no apparent contribution of chemotherapy sensitivity. This suggests that disparity root causes may be driven by HR+ factors such as unmeasured molecular differences, endocrine therapy sensitivity, or adherence.
Keywords: Race, Breast cancer subtype, pCR, Obesity, Recurrence, Survival
Introduction
Despite improvements in treatment and overall gains in breast cancer survival, racial disparities in breast cancer outcome are well documented and persistent over time [1–3], with age-adjusted mortality among AA women 40 % higher than Caucasian women (30.8 vs. 22.1 deaths per 100,000) [4]. Disparities in outcome between AA and non-AA women are likely to be multifactorial with potential contributions from differences in breast cancer subtype frequencies, variability in comorbid conditions, access to and receipt of care, and other unmeasured biological factors. Advanced stage at diagnosis is more common for AA women but exhibit worse breast cancer-related outcomes even after stratification for stage compared to non-AA women [5, 6].Obesity is more prevalent in AA women compared to non-AA and is a prognostic variable for breast cancer survival, with poorly understood mechanisms to affect that endpoint [7–9]. Differences in access to care and treatment between race remain. Moreover some studies have found persistent racial disparities in outcome even among similarly treated women [10–12]. For example, a study from the U.S. Department of Defense, which entitles equal access to its health care system, demonstrated worse breast cancer survival rates among participating AA women than non-AA [13]. A case-control study of 317 AA and matched non-AA women participating in adjuvant cooperative group trials also found worse disease-free survival among AAs despite identical therapy [14].
Although AA women are more likely to have poor prognosis breast cancer subtypes [3, 15, 16], the core of race disparity in breast cancer outcomes may lie in HR + subtypes. An analysis of outcome by race in the population-based Carolina Breast Cancer Study (CBCS) found that the greatest racial difference in survival was seen in the good prognosis HR+/HER2 subset [17]. Similarly, in a randomized Phase III trial in which women were treated with adjuvant doxorubicin/cyclophosphamide (AC) chemotherapy and either paclitaxel (T) or docetaxel (Td), AA women with the HR+/HER2- subtype demonstrated worse disease-free and overall survival compared to non-AA women of the same subtype, whereas no difference in outcome was seen in triple-negative or HER2+ disease [18]. Those studies could not identify whether treatment responsiveness contributed to this outcome differential.
The neoadjuvant setting offers a rich opportunity to examine chemotherapeutic response as well as long-term outcomes among patients treated in a uniform manner. A large cohort study including HR+ disease treated with neoadjuvant therapy did not find a difference in pCR by race but did find a trend toward worse long-term survival outcomes among AA women. That particular study was limited by very low pCR rates and short follow-up of only 30 months making subtype-stratified survival analysis difficult [19]. In contrast, a study limited to triple-negative stage I–III breast cancers treated with neoadjuvant therapy found that race did not affect either response or survival in this subtype [20]. Taken together, these studies suggest that racial disparities in AA breast cancer survival may be more driven by factors related to HR+ disease than HR–.
The current study seeks to further define racial differences in subtype-specific outcomes using a prospectively maintained database of neoadjuvantly treated breast cancer patients at the University of North Carolina. Detailed clinical information in this database includes factors such as body mass index (BMI) as well as chemotherapy and endocrine therapy receipt and completion.
Patients and methods
Patient population
The study population is derived from the prospectively annotated, IRB-approved Neoadjuvant Database at the University of North Carolina, Lineberger Comprehensive Cancer Center. Race was according to patient self-report as documented in the medical record. The database is updated every 6 months and includes serial clinical, radiographic, and pathologic tumor measurement, treatment details, toxicity, and outcome. The cohort eligible for analysis consisted of women with adenocarcinoma of the breast with or without axillary lymph node metastases treated with neoadjuvant chemotherapy. Included patients had clinical stage II–III breast cancer at presentation including inflammatory breast cancers and had no evidence of distant metastases according to the American Joint Commission on Cancer, Fifth Edition [21]. Each tumor in bilateral or multi-centric disease was measured separately. Inflamma-tory breast cancer was clinically defined based on erythema with an erysipeloid edge or peau d'orange changes with or without dermal lymphatic invasion.
Receptor status was based on clinical assays including immunohistochemical (IHC) markers for hormone receptor (HR) status. HER2 status was determined by IHC with fluorescence in situ hybridization (FISH) confirmation. IHC subtypes were categorized as HR +/HER2-; HR+/ HER2+; HR-/HER2-; and HR-/HER2+. Pathologic complete response (pCR) was defined as the absence of residual invasive breast cancer in the breast or axillary lymph nodes; residual noninvasive disease was allowed [22]. Race was categorized as AA versus non-AA for all analyses.
Treatment
Neoadjuvant chemotherapy was categorized as (1) anthracycline-based (A) with no taxane, (2) taxane-based (T) with no anthracycline, or (3) both an anthracycline and a taxane (A+T). Completion of neoadjuvant A and/or T chemotherapy was defined as receipt of A ≥ 4 cycles and/ or T as ≥12 weekly cycles or T as ≥4 cycles every 2 or 3 weeks.
Medical records were reviewed to assess initiation and completion of endocrine or HER2-targeted adjuvant therapy for appropriate HR+ or HER2+ tumors and for body mass index (BMI). Completion of adjuvant endocrine therapy was defined by documentation of tamoxifen and/or aromatase inhibitor (AI) use for at least 4.5 years; anti-HER2 therapy completion was defined as 1 year of adjuvant trastuzumab. Daily medication adherence was unable to be adequately assessed in this cohort, and therefore not measured. Incomplete endocrine therapy was defined as treatment duration less than 4.5 years. The definition of incomplete endocrine therapy also included non-persistence of therapy longer than 6 months within 4.5 years of treatment. Women lost to follow-up prior to the defined period of endocrine therapy completion (4.5 years) were excluded from analysis (n = 18). Follow-up data abstraction was completed on August 31, 2012.
Statistical methods
Fisher's Exact and Wilcoxon rank sum tests were used to compare characteristics between AA and non-AA patients, in addition to evaluating associations with pCR. The Kaplan–Meier method was used to estimate and compare TTR and OS, and hazard ratios were estimated using Cox regression models. Multivariable logistic and Cox regression models evaluated the combined effect of covariates on outcomes of pCR, TTR, and OS. Variables included in the modeling of probability of pCR and survival included subtype, body mass index (BMI), age, stage at diagnosis, and race. Models for TTR and OS also included pCR as a time-varying covariate. Limited multivariable modeling, due to small sample size, was done separately within the HR ± cohorts. TTR was defined as time from diagnosis to local recurrence or distant recurrence, whichever occurred first, and deaths without recurrence were censored. Those with no events were censored at last contact. OS was defined as time from diagnosis to death, with patients censored at last contact. Unadjusted two-sided p values are reported, and all analyses were conducted using SAS statistical software, version 9.3 (Cary, NC).
Results
Patient characteristics
A total of 349 women with clinical stage II-III breast cancer were included, of whom 102 (29 %) were AA (Table 1). Patients included in the non-AA demographic self-identified as Caucasian (224, 64 %), Hispanic (17, 5 %), Asian (1, <1 %), American Indian (1, <1 %), and “other” (4, 1 %). Consistent with previous studies, AA women were significantly younger, and obesity, defined as BMI ≤ 30 kg/m2, was more prevalent in AA women (62 vs. 28 %, p < 0.001). In this high-risk cohort, 3 (<1 %) women had bilateral breast cancer, 85 (24 %) had multi-centric or multi-focal disease, and the remaining were unifocal (76 %). Eighteen percent of patients had inflammatory disease, which was equally represented between AA (19 %) and non-AA (18 %) women. The representation of poorly differentiated tumors was similar between cohorts (grade 3 tumors at diagnosis: AA 69 % vs non-AA 65 %, p = 0.5) (Table 1). The largest IHC subtype group was HR+/HER2- (42 %), followed by HR–/HER2–(30 %), HR+/HER2+ (15 %), and HR-/HER2+ (13 %). As expected, the distribution of subtypes differed significantly by race (p = 0.01), with a disproportionate percentage of AA women having HR-/HER2–, or triple-negative disease. Since progesterone receptor (PR) expression is seen as a prognostic differentiator in recurrence-free survival, this cohort was assessed and no difference was observed between AA and non-AA PR-tumors (p = 0.8) [23, 24].
Table 1.
Patient characteristics and receipt of therapy
| Overall | Non-AA | AA | p value | |
|---|---|---|---|---|
| Sample size | 349 | 247 (70.7 %) | 102 (29.2 %) | |
| Age (median, range) | 48 (24–78) | 49 (24–78) | 45 (27–69) | 0.03 |
| Tumor grade at diagnosis | ||||
| Grade III | (65 %) | (69 %) | 0.50 | |
| Stage at diagnosis | ||||
| II | 159 (45.6 %) | 117 (47.3 %) | 42 (41.2 %) | 0.34 |
| III | 190 (54.4 %) | 130 (52.7 %) | 60 (58.8 %) | |
| Body mass index | ||||
| BMI (median, range) | 27.6 (15.3, 70.7) | 26.6 (15.4, 70.7) | 31.6 (15.3, 60.9) | <0.0001 |
| <30 | 216 (61.9 %) | 177 (71.7 %) | 39 (38.2 %) | <0.0001 |
| ≥30 | 133 (38.1 %) | 70 (28.3 %) | 63 (61.8 %) | |
| IHC subtype | ||||
| HR-/HER2– | 105 (30.0 %) | 61 (24.7 %) | 44 (43.1 %) | 0.01 |
| HR-/HER2+ | 46 (13.2 %) | 34 (13.8 %) | 12 (11.7 %) | |
| HR+/HER2– | 147 (42.1 %) | 115 (46.5 %) | 32 (31.4 %) | |
| HR+/HER2+ | 51 (14.6 %) | 37 (15.0 %) | 14 (14.7 %) | |
| Neoadjuvant regimen | ||||
| A + T | 291 (83.5 %) | 205 (83.0 %) | 86 (84.3 %) | 0.94 |
| A, no T | 30 (8.5 %) | 21 (8.5 %) | 9 (8.8 %) | |
| T, no A | 28 (8.0 %) | 21 (8.5 %) | 7 (6.9 %) | |
| Adjuvant therapy | ||||
| Endocrine therapy receipt (HR+ only) | 192/198 (97.0 %) | 149/152 (98.0 %) | 43/46 (93.4 %) | 0.14 |
| Post-mastectomy radiation | 184 (52.7 %) | (94 %) | (88 %) | 0.20 |
| Pathologic complete response (pCR) | ||||
| pCR event rate | 85/349 (24.3 %) | 64/247 (25.9 %) | 21/102 (20.5 %) | 0.34 |
Treatment received
Of the 349 women prescribed neoadjuvant chemotherapy, 291 (84 %) patients received combination of anthracycline/taxane-based treatment (Table 1). Of these women nearly half (141, 48 %) received dose-dense every-2-weeks doxorubicin/cyclophosphamide for 4 cycles followed by paclitaxel either every 2 weeks for 4 cycles or 12 weekly cycles (ddAC-T). 30 patients (9 %) received anthracycline-based therapy without a taxane, and 28 (8 %) received taxane-based therapy without an anthracycline. There was no significant difference in type of prescribed chemotherapy (anthracycline-based, taxane-based, or both) by race. No significant racial differences in completion of chemotherapy were observed, regardless of chemotherapy type (AA: 84/101, 83 %, vs. non-AA: 214/245, 87 %, p = 0.31). Forty-two patients (12 %) received additional chemotherapy postoperatively, without a difference in the receipt of adjuvant chemotherapy between AA and non-AA women (p = 1.0). No difference was seen in the receipt of radiation therapy, as treatment following either breast conservation surgery or mastectomy. Of those who received breast conservation treatment (n = 107), all but 1 received adjuvant radiation. Following mastectomy, almost all women (92 %) received radiation and no significant difference was observed between AA (88 %) vs non-AA (94 %), p = 0.2 (Table 1).
Adjuvant endocrine therapy was received by most HR+ women (192/198, 97 %) with no significant difference seen in the prescription of endocrine therapy (tamoxifen and aromatase inhibitors) by race (p = 0.14) (Table 1). There were no significant differences in rates of endocrine therapy completion at 4.5 years, also known as treatment persistence, for women with HR + breast cancer (AA: 36/43, 84 %, vs. non-AA 118/144, 82 %, p = 0.49).
Trastuzumab was approved for adjuvant therapy of HER2+ breast cancer in 2005. Prior to that time, both neoadjuvant and adjuvant clinical trials of the agent were available. Receipt of trastuzumab in the neoadjuvant or adjuvant setting was less common for AA (15/26, 58 % AA, vs. 56/71, 79 % non-AA, p = 0.07).
Pathologic response to chemotherapy
Overall, 85 of 349 (24 %) patients achieved pCR to neoadjuvant chemotherapy (Table 1), with expected differences by clinical subtype (Supplemental Table 1). Although AA had uniformly lower rates of response (Supplemental Table 1), pCR rates did not significantly differ by race overall (21/102, 21 % AA vs 64/247, 26 % non-AA, p = 0.34) or when compared within any clinical subtype. A multivariable model including race, subtype, age, stage, and BMI did not find a significant difference in pCR rates for AAs compared to non-AAs (OR 0.57, 95 % CI 0.31–1.06).
Recurrence and survival
There were 107 (31 %) breast cancer recurrences and 98 (28 %) deaths in this cohort. Most deaths during the study period were preceded by recurrence (81, 83 %), and of the women who recurred, 26 (24 %) were alive at time of last follow-up. Median follow-up of survivors was 6.5 years, and median OS for the entire cohort was 13.4 years. At 5 years, 73 % (95 % CI 68–77 %) were recurrence free and 79 % (95 % CI 74–83 %) were alive.
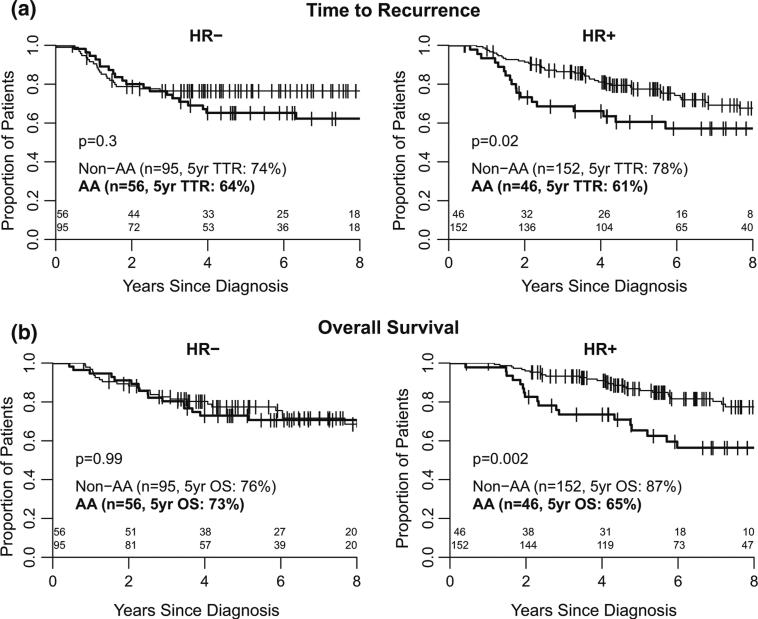
In univariable analyses, AAs had significantly higher risk of recurrence compared to non-AA women (hazard ratio TTR: 1.51, 95 % CI 1.02–2.24, p = 0.04), although overall survival was not statistically significantly worse (hazard ratio OS: 1.47 95 % CI 0.98–4.28, p = 0.06). In multivariable models including subtype, stage, BMI, age, and pCR, race was not significantly associated with recurrence or death (Table 2); in the overall cohort, very strong and independent predictors of outcome were pCR and stage at diagnosis. No significant pairwise interactions of race, subtype, and BMI were identified. However, in stratified univariable analyses by HR status, significantly inferior outcomes for AA were identified, and were limited to the HR+ subset (Table 3). Five-year estimates for the percentage of patients who were recurrence free were 61 % (95 % CI 44–73 %) in AA and 78 % (95 % CI 69–84 %) in non-AA women (p = 0.02) (Fig. 1a); similar discrepancies were seen for OS with five-year estimates of 65 % (95 % CI 49–78 %) in AA and 87 % (95 % CI 80–92 %) in non-AA (p = 0.002) (Fig. 1b). In multivariable models controlling for pCR and BMI, AA women in the HR+ subset had a significantly higher risk of recurrence and death when compared to non-AAs (hazard ratio TTR 1.91, 95 % CI 1.07–3.40 and OS 2.21, CI 95 % 1.16–4.21) (Table 3). Sample size did not permit the analysis of fully adjusted models within the HR+ subset. Within the HR-cohort, differences in TTR and OS were significantly associated with pCR, but not with race (Table 3).
Table 2.
Cox proportional hazard models of recurrence and overall survival (n = 349)
| Time to recurrence hazard ratio (95 % CI) |
Overall survival hazard ratio (95 % CI) |
|||
|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |
| Race | ||||
| AA | 1.51 (1.02–2.24)* | 1.29 (0.83–2.00) | 1.47 (0.98–2.21) | 1.17 (0.74–1.85) |
| non-AA | 1.00 | 1.00 | 1.00 | 1.00 |
| Subtype | ||||
| HR–/HER2+ | 0.83 (0.44–1.57) | 0.99 (0.51–1.93) | 0.93 (0.47–1.83) | 1.16 (0.57–2.35) |
| HR–/HER2– | 0.99 (0.63–1.56) | 1.18 (0.73–1.89) | 1.48 (0.92–2.37) | 1.91 (1.16–3.14)* |
| HR+/HER2+ | 1.06 (0.60–1.87) | 1.21 (0.68–2.14) | 1.56 (0.88–2.75) | 1.89 (1.07–3.37)* |
| HR+/HER2– | 1.00 | 1.00 | 1.00 | 1.00 |
| Stage | ||||
| III | 1.76 (1.18–2.63)** | 1.66* (1.10–2.50) | 2.10 (1.36–3.25)** | 2.13 (1.37-3.32)** |
| II | 1.00 | 1.00 | 1.00 | 1.00 |
| Body mass index (BMI) continuous | 1.02 (1.00–1.04) | 1.01 (0.98–1.01) | 1.03 (1.01–1.05)** | 1.02 (0.99–1.04) |
| Age continuous | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) |
| pCR | ||||
| Yes | 0.33** (0.17–0.61) | 0.33** (0.17–0.63) | 0.33** (0.17–0.63) | 0.28** (0.14–0.55) |
| No | 1.00 | 1.00 | 1.00 | 1.00 |
p ≤ 0.05
p ≤ 0.01
Table 3.
Adjusted cox proportional hazard models of recurrence and survival stratified by HR subset (n = 349)
| Time to recurrence hazard ratio (95 % CI) |
Overall survival hazard ratio (95 % CI) |
|||
|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |
| HR– | ||||
| AA vs non-AA | 1.40 (0.77–2.55) | 1.07 (0.58–1.98) | 1.00 (0.55–1.82) | 0.76 (0.41–1.41) |
| pCR | ||||
| Yes | 0.31 (0.14–0.66)** | 0.34 (0.14–0.68)** | 0.29 (0.14–0.63)** | 0.29 (0.13–0.62)** |
| No | ||||
| BMI | 1.03 (1.00–1.07) | 1.03 (0.99–1.07) | 1.02 (0.99–1.06) | 1.02 (0.99–1.05) |
| HR+ | ||||
| AA vs non-AA | 1.85 (1.09–3.14)* | 1.91 (1.07–3.40)* | 2.42 (1.37–4.28)** | 2.21 (1.16–4.21)* |
| pCR | ||||
| Yes | 0.32 (0.10–1.03) | 0.31 (0.10–1.00)* | 0.27 (0.07–1.10) | 0.26 (0.06–1.08) |
| No | ||||
| BMI | 1.02 (0.98–1.05) | 1.00 (0.97–1.03) | 1.04 (1.01–1.07)* | 1.02 (0.98–1.05) |
p ≤ 0.05
p ≤ 0.01
Fig. 1.
a Time to recurrence, b overall survival
Discussion
This study found that AA breast cancer patients were younger, had higher BMI, and were more likely to have triple-negative breast cancer than their non-AA counterparts, all of which can contribute to racial disparities in outcome. However, the factors most strongly associated with survival were pathologic complete response to chemotherapy and stage at diagnosis, neither of which significantly differed between AA and non-AA (although it should be noted that this study was deliberately limited to stages II–III). A significant difference in outcome by race was seen in HR+ tumors, with a 20 % lower rate of survival at 5 years for AA women, with no apparent difference among HR breast cancers. The AA and non-AA women in this cohort shared similar treatment patterns and rates of pathologic response with little evidence to suggest a difference in chemotherapeutic sensitivity.
This finding of racial disparity in outcomes driven by differences in HR+ subtypes mirrors and extends the findings of the population-based CBCS study, which found that the greatest racial disparity in outcome was noted in HR+ disease within a large cohort of patients with heterogeneous stages and treatments [17]. In an earlier study, Chavez-MacGregor and colleagues observed only a trend toward inferior outcomes in AA HR + patients treated with neoadjuvant therapy [19], but the median follow-up in that study was short and may have been immature for this clinical subset at risk for late relapses. Our findings contrast those presented from an Atlanta population-based study which demonstrated significantly worse survival for AA women with HR-tumors [25], but this study was limited to patients younger than 55 years of age, thus excluding a substantial subset of post-menopausal women.
The mainstay of systemic therapy for HR + breast cancer consists of at least 5 years of oral endocrine therapy. Our results as well as those of other investigators raise the question of whether racial differences in initiation, efficacy, or adherence to endocrine therapies may contribute to racial outcome disparities in this group. Both persistence (continuing to take the medication) and adherence (taking the medication at the prescribed dose and schedule) are known to be suboptimal among breast cancer patients. While literature on endocrine therapy has generally indicated equivalent rates of persistence among AAs and whites [26–30], several studies have found lower medication adherence among AA women [26, 27, 29-32]; inadequate adherence is associated with worse outcomes [31]. We observed an equivalent rate of endocrine therapy persistence between AA and non-AA; however, this dataset was not able to address the specific question of endocrine therapy adherence.
With these findings, we must consider differences in estrogen metabolism and bioavailability as a possible explanation for the racial disparity in outcome. As the Suppression of Ovarian Function Trial (SOFT) suggested, improved breast cancer outcomes were seen in young women who received ovarian suppression and remained amenorrheic following chemotherapy [33]. It is plausible that a lower rate of chemotherapy-related amenorrhea in AA women, who are more likely to present pre-menopausal at diagnosis, may translate to worse long-term survival. Information related to menopausal status before and after breast cancer treatment was not obtained for this cohort, but warrants further consideration for future study. Lower sensitivity to endocrine therapy among AAs is also hypothesized. Lund and colleagues reported that AA women with HR+ tumors are more likely to present with higher 21-gene recurrence scores rendering endocrine therapy less effective for this group [34]. Obesity and its role in estrogen metabolism of breast tumors is complex and inconsistently associated with worse breast cancer outcome. The results of the ATAC trial suggested the benefit of aromatase inhibition was abrogated by higher body mass index [35]. AA women in our study were more likely to be obese (BMI ≥ 30 kg/m2), which may contribute to the worse outcome in this subset.
This study has several limitations. Although our sample contained a high proportion of AA women, the size of the cohort limits the detection of recurrence and survival differences by stratification, particularly among HER2+. While the cohort was assembled and outcome data collected prospectively, our analysis is retrospective. This limits our control over data elements not prospectively collected such as detailed information regarding adherence to endocrine therapy. We also do not have specific details regarding chemotherapy doses, timing of initiation, or treatment delays. However, institutional chemotherapy guidelines throughout the timeframe of this cohort recommended dosing according to actual weight rather than ideal weight, and therefore weight-based dose limits are unlikely to have affected the results. It should also be noted that clinical IHC-based subtypes were used and may not recapitulate tumor biology [36]; molecular subtype data for this cohort are not available.
In summary, among a cohort of women with stage II–III tumors treated similarly, we observed markedly worse long-term outcomes among AA patients with HR+ breast cancer. Within this subset, there appears to be an independent effect of race on breast cancer recurrence that is not explained by factors such as increased BMI or variations in persistence with prescribed endocrine therapy. The root causes of this racial disparity in outcome for AA women within those tumors considered least aggressive, and the most common, needs to be determined, including whether there are differences in endocrine therapy sensitivity, adherence, or other unmeasured biologic variables.
Supplementary Material
Acknowledgments
This work was supported by Breast Cancer Research Foundation (13295 to LAC); National Research Service Award T32, Academic Training in Oncology at the University of North Carolina at Chapel Hill (5T32CA128590-05 to JRT).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-015-3350-2) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
References
- 1.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–7841. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 2.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. 1975–2010 [Google Scholar]
- 5.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande AD, Jeffe DB, Gnerlich J, Iqbal AZ, Thummalakunta A, Margenthaler JA. Racial disparities in breast cancer survival: an analysis by age and stage. J Surg Res. 2009;153:105–113. doi: 10.1016/j.jss.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Drygalski A, Tran TB, Messer K, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. doi: 10.4061/2011/523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29:3358–3365. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauscher GH, Allgood KL, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. J Womens Health (Larchmt) 2012;21:154–160. doi: 10.1089/jwh.2010.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 12.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 13.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98:894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 14.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 16.Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116:4926–4932. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez-Macgregor M, Litton J, Chen H, et al. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116(17):4168–4177. doi: 10.1002/cncr.25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawood S, Broglio K, Kau SW, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming I. AJCC Cancer Staging Manual. American Joint Committee on Cancer, American Cancer Society, American College of Surgeons; Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 22.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 23.Zong Y, Zhu L, Wu J, et al. Progesterone receptor status and Ki-67 index may predict early relapse in luminal B/HER2 negative breast cancer patients: a retrospective study. PLoS One. 2014;9(8):e95629. doi: 10.1371/journal.pone.0095629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancers. J Clin Oncol. 2013;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 26.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 28.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 31.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hershman DL, Tsui J, Wright JD, et al. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.3062. pii: JCO.2014.58.3062. [Epub ahead of print] PMID: 25691670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund MJ, Mosunjac M, Davis KM, et al. 21-gene recurrence scores: racial differences in testing, scores, treatment and outcome. Cancer. 2012;118(3):788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 35.Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences of tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 36.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.