Abstract
Extracellular vesicles are signaling organelles that are released by many cell types and is highly conserved in both prokaryotes and eukaryotes. Based on the mechanism of biogenesis, these membranous vesicles can be classified as exosomes, shedding microvesicles and apoptotic blebs. It is becoming clearer that these extracellular vesicles mediate signal transduction in both autocrine and paracrine fashion by the transfer of proteins and RNA. Whilst the role of extracellular vesicles including exosomes in pathogenesis is well established, very little is known about their function in normal physiological conditions. Recent evidences allude that extracellular vesicles can mediate both protective and pathogenic effects depending on the precise state. In this review, we discuss the involvement of extracellular vesicle as mediators of signal transduction in neurodegenerative diseases and cancer. In addition, the role of extracellular vesicles in mediating Wnt and PI3K signaling pathways is also discussed. Additional findings on the involvement of extracellular vesicles in homeostasis and disease progression will promote a better biological understanding, advance future therapeutic and diagnostic applications.
Keywords: exosomes, extracellular vesicles, microvesicles, neurodegenerative diseases tumor microenvironment
Introduction
Ligand-receptor interactions and direct cell-to-cell contacts have long been considered as the predominant means of intercellular communication. In the last decade, a novel method of cell-to-cell communication mediated by membranous extracellular vesicles (EVs) has recently emerged [1, 2]. While the EV nomenclature is yet to be standardized [3], recent community efforts have urged researchers to name the vesicles based on the mode of biogenesis and not on the sample or cell type of origin [4]. In accordance with the consensus and current knowledge on biogenesis, the EVs could be classified into three main classes such as exosomes, shedding microvesicles (MVs) and apoptotic blebs. Exosomes are of endocytic origin and are the smallest among the EVs (40–150 nm in diameter), appear cup-shaped under the electron microscope and sediment at a buoyant density of 1.13–1.19 g/mL [5]. They are released by both normal and disease cells after the fusion of multivesicular bodies (MVB) with the plasma membrane (PM) [6]. Shedding microvesicles or ectosomes are large vesicles (50–1000 nm in diameter) that are directly formed from the PM while apoptotic blebs are released by dying cells [7]. While a large body of work supports the role of exosomes in signal transduction, shedding microvesicles and apoptotic blebs are relatively poorly characterized. For the purpose of this review, exosomes and MVs will be referred to as EVs from here on.
EVs are mediators of cellular communication and are involved in both normal physiological processes such as lactation, immune response and neuronal function [8], as well as implicated in pathological conditions such as liver diseases [9], neurodegenerative diseases [10] and cancer [2]. EVs contain a rich cargo of proteins, lipids, DNA and RNA that resemble the cell type of origin. Many studies have highlighted that EVs harbor specific proteins, mRNAs, miRNAs, and lipids rather than random cellular components [11, 12]. The tissue and cell type signature contained within EVs and its availability in bodily fluids such as blood plasma, urine and saliva, have created significant interests in exploiting EVs as potential source of disease biomarkers [2]. In addition, EVs are reported to be very stable in blood plasma and are considered as reservoirs of disease biomarkers [13]. These emerging interests have spurred a plethora of EV studies that employs high-throughput technologies including proteomics, transcriptomics and lipidomics. This review summarizes the role of EVs in various signaling pathways and also its involvement in pathological conditions including cancer and neurodegenerative disorders.
Role of EVs in cancer
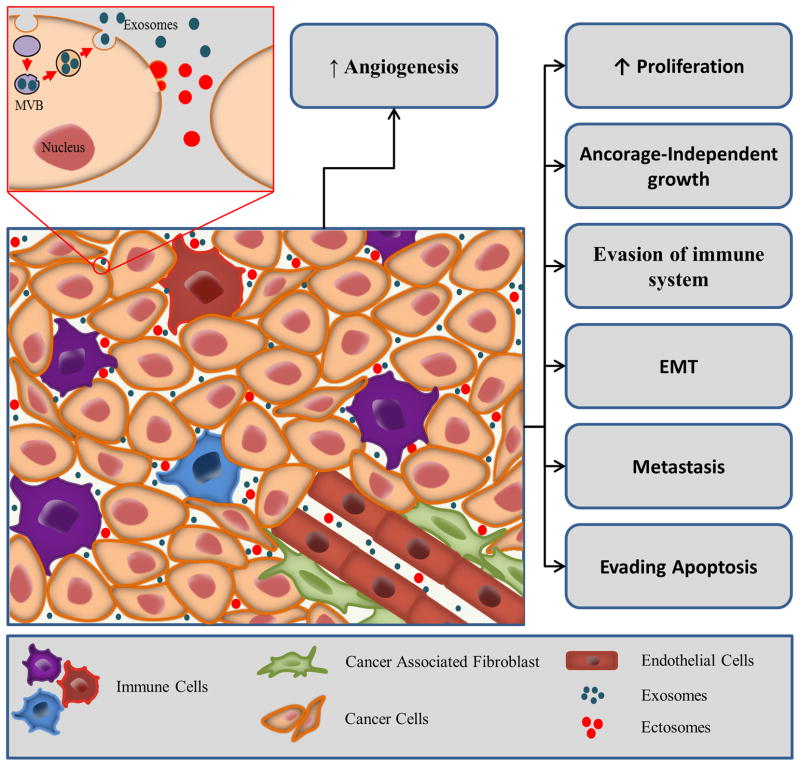
For tumors to progress, bidirectional crosstalk between different cells occurs within the tumor and its surrounding supporting tissue [14]. A tumor can be considered as a complex tissue or organ with abnormal cells harbouring genetic mutations, typically referred to as tumor or cancer cells, enmeshed within the surrounding and interwoven stroma, the epithelial parenchyma, which provides the connective tissue of the tumor. Stromal elements include the extracellular matrix as well as other cell types that are activated and/or recruited to the tumor microenvironment such as fibroblasts, immune and inflammatory cells, fat cells and endothelial cells of the blood and lymphatic circulation [15]. Recent literature indicated that all aspects of cellular tumorigenicity are profoundly influenced by reciprocal interactions between responding normal cells, their mediators, structural components of the extracellular matrix, and genetically altered neoplastic cells [16, 17]. EVs have recently been recognized as important mediators of the cross-talk in the tumor microenvironment (Fig. 1). EVs derived from tumor cells have been shown to have both pro- and anti-tumorigenic properties.
Figure 1. EV mediated signalling in tumor microenvironment.
Cancer cell secreted EVs are known to influence cancer progression. EVs can elicit various signalling pathways inducing proliferation, anchorage-independent growth and metastasis. EVs can trigger endothelial cells to promote angiogenesis, apoptosis of immune cells and increase the secretion of growth factor from stromal cells.
Pro-tumorigenic role of tumor-derived EVs
EVs can promote tumor growth and metastasis through several mechanisms as detailed by the cancer hallmarks [18]; evading apoptosis and resisting cell death, sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, enabling replicative immortality and inducing angiogenesis. The pro-tumorigenic role of EVs is highlighted in the following sections.
Evading apoptosis and sustaining proliferative signal
EVs can modulate immune-regulatory processes, induce T cell death and set up tumor escape mechanisms. Tumor cells may subvert recognition and killing by cytotoxic T lymphocytes and NK cells through EV membrane shedding of ligands such as MICA [19]. Similarly, EV membrane associated TGFβ aided tumors to overcome cytotoxic killing by lymphocytes. In addition, TGFβ containing tumor EVs mediated anti-proliferative effects on blood lymphocytes [20]. Qu et al. demonstrated that gastric cancer EVs induced degradation of PI3K subunit (p85) in Jurkat T cells. Simultaneously, the degradation of PI3K subunit leads to the down regulation of survival signals contributing to T cell apoptosis. Specific and reversible proteasome inhibitor PS341, was used to inhibit p85 degradation which substantially reduced T cell apoptosis induced by EVs [21]. In addition to evading apoptosis, the role of EVs in sustaining proliferative signals in the recipient cells is beginning to emerge. Over expression of EGFR has been associated with a number of cancers, including glioblastoma and lung cancer. Several studies have employed biochemical and proteomic techniques and identified both EGFR and EGFRvIII (constitutively active mutated form of receptor) in EVs originating from brain tumors [22, 23]. During the enforced expression of EGFRvIII in U373 glioma cells, an increase in the secretion of EVs was observed [23]. EGFRvIII can be transferred via EVs to distant cells that lack expression of EGFRvIII with concomitant activation of downstream signaling pathways in those cells. In addition, ligands such as EGF, TGFα and amphiregulin have also been found in EVs from breast and colorectal cancer cell lines [24]. These ligand containing EVs can stimulate the recipient cells and induce proliferation. However, it has to be established whether these ligands are functional in mediating an effect on target cells both in vitro and in vivo.
Activating invasion and metastasis
Intercellular contacts formed by dense populations of normal cells operate to suppress proliferation yielding confluent monolayers. Such contact inhibitions are abolished in cancer cells which show anchorage independent growth (AIG). EVs isolated from fetal bovine serum (FBS) has been shown to induce AIG in breast cancer cells [25]. The study identified that EV depleted FBS was not able to induce AIG confirming the functional role of EVs. To further profile the protein content of FBS EVs, in-gel digestion coupled with LC-MS/MS analysis was performed using a LTQ ion trap. EVs free and enriched fractions were analysed to identify the proteins and spectral counting was performed to obtain the relative abundance of the identified proteins. Even though the role of EVs in AIG was established, the EV proteins/RNA involved in AIG need to be identified with the use of new high-resolution MS instrumentation and transcriptomic technologies. In another proteomic study, it was established that aggressive cancer cells secrete Hsp90α containing EVs which contribute to their invasiveness. Hsp90α was shown to activate plasmin as well as increase plasmin dependent cell motility [26]. Similarly, TGFβ containing EVs released by mesothelioma cells triggered sustained changes in the actin cytoskeleton with increased expression of α-smooth muscle actin, a marker for fibroblast to myofibroblast transition. EV mediated activation of myofibroblasts may provide a favourable environment for tumor growth, angiogenesis and metastasis [27].
Tumor metastasis can be promoted by a (pre)metastatic niche formation. EVs which mediate long distance cross talk between a tumor and selected (pre)metastatic organs has been established to play an important role in niche preparation [28]. CD44v4 containing EVs have been noted to cause changes in several proteases, growth factors and adhesion molecules and create a long distance metastatic environment for tumor cells [29]. EV-based transfer of oncogenes from tumor to neighbouring cells can induce replicative immortality in these target cells. EVs isolated from mutant KRAS-expressing colon cancer cells enhanced the invasiveness of recipient cells [30]. A label-free proteomic analysis based on spectral counting revealed a change in protein cargo based on the KRAS mutational status. Specifically, EVs from mutant KRAS cells contained many tumor-promoting proteins including KRAS, EGFR, SRC family kinases and integrins. The study hypothesized that mutant KRAS-expressing cells promote metastatic progression by pre metastatic niche preparation.
Inducing angiogenesis
EVs enriched in the tetraspanins CO-029 and TSPAN8 have been shown to activate endothelial cells and induce several genes required for angiogenesis [31, 32]. Furthermore, Mineo et al. showed that EVs from K562 chronic myeloid leukemia cells induced angiogenesis in human umbilical endothelial cells in a Src-dependent manner [33]. Kim et al. showed that sphingomyelin is an the active component for vesicle-induced endothelial cell migration, tube formation, and neovascularization [34]. Hegmans et al. analyzed several mesothelioma cell secreted EVs by MALDI-TOF MS and identified DEL-1 in EVs [35]. DEL-1 is structurally homologous to MFGE8 (also known as lactadherin) and is suggested to play a role in angiogenesis [36]. Though the functional role of mesothelioma EVs in angiogenesis was not tested, the study speculated the role of DEL-1 containing EVs as strong angiogenic factors that can increase the vascular development in the tumor microenvironment. Similarly, Dll4, a notch ligand that plays an important role in neo-vascularization and angiogenesis, is upregulated in endothelial and cancer cells and are secreted via EVs. Interestingly, the transfer of Dll4 from tumor cells to host endothelium via EVs enhanced vessel formation at distant locations [37]. MFGE8 is a major component of EVs from immature dendritic, as well as tumor cells. MFGE8 containing EVs promotes cell survival induced by an endothelial-specific growth factor, VEGF, and thus induces angiogenesis [38, 39].
Anti-tumorigenic role of tumor-derived EVs
Tumor-derived EVs are enriched in tumor-specific antigens that are expressed in the parental tumor cells such as carcinoembryonic antigen [40] and mesothelin [20]. This property could be used for tumor EV-based cancer vaccine development and T cell cross priming [41]. EVs isolated from patients with melanoma contained Mart1 tumor antigens that were delivered to dendritic cells for cross presentation to clones of cytotoxic T lymphocytes specific to Mart1, thereby mounting an antitumor response [42]. Similarly, tumor-derived EVs have been used as a source of tumor antigens to pulse dendritic cells resulting in induction of CD8+ T cell-dependent antitumor effects in mice [41]. In addition to tumor rejection antigens, EVs secreted by human pancreatic tumor cells induced (glyco) protein ligand-independent cell death and inhibited Notch-1 pathway which is particularly active during carcinogenesis and in cancer stem cells. Interestingly, these EVs were also reported to increase Bax and decrease Bcl-2 expression thereby triggering the tumor cells towards mitochondrial apoptotic pathway [43–45].
EVs as regulators of intracellular signals
Even though recent reports have implicated EVs in intercellular signaling, their influence in modulating signaling pathways in the target cells is far from being completely elucidated. Calzolari and colleagues demonstrated that activation of transferrin receptor 2 induces the activation of ERK1/2 and p38 MAPK pathways supporting the hypothesis that transferrin receptor may function as a signaling receptor. MAPK is a key signaling pathway activated in endothelial cells after the binding of angiogenic factors of EVs [46]. Through pathogen recognition receptors, such as Toll-like receptors, and their associated downstream signaling pathways, such as nuclear factor kappaB (NF-κB), EV components may also play a large role in the homeostasis and regulation of the innate immune response. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling through triggering NFκB and delaying STAT3 signaling pathway [47]. In addition to proteins, exosomal lipids are also shown to regulate Notch signaling [43]. Even though multiple pathways are studied, the role of EVs in PI3K/Akt and Wnt signaling has been extensively studied. The review will focus on the role of EVs in PI3K/Akt and Wnt signaling in the following sections.
Role of EVs in PI3K/Akt pathway
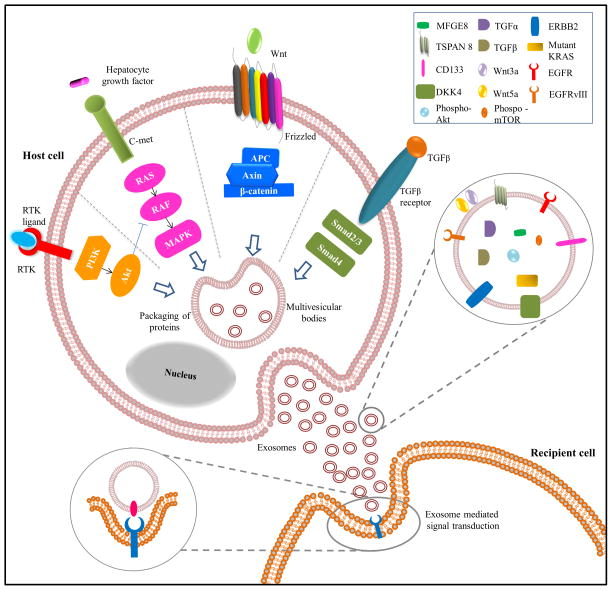
PI3K/Akt signaling pathway is a critical regulator of cell growth and survival and is implicated in cancer. Somatic mutations in genes regulating PI3K/Akt pathway may lead to dysregulated signaling resulting in cancer [48]. Due to its major role in cancer, multiple drugs targeting PI3K/Akt signaling pathway and their downstream effectors have been developed [48, 49]. However, the complexity of the pathway and various mutations in proteins implicated in the pathway hinder the effectiveness of the drugs. Activation of PI3K/Akt signaling pathway is controlled by several different protein receptor families including integrin, cytokine, GPCR and receptor tyrosine kinases [50]. Soluble secreted factors such as insulin, IGF, EGF, VEGF, HGF, IL-6, leptin and angiopetin-1 are known to activate PI3K/Akt signaling. Recent evidences suggest that EVs play a critical role in signal transduction by mediating the intercellular transfer of molecules regulating PI3K/Akt signaling (Fig. 2). Several possible ways as to how EVs mediate PI3K/Akt signaling pathway and its impact on cancer progression is discussed in the subsequent sections.
Figure 2. EV-mediated signaling in recipient cells.
Protein sorting during MVB formation results in packaging of key molecules in exosomes. Exosomes bound-signaling ligands once released from host cells interacts with the receptors on the recipient cells to induce downstream signaling cascades pivotal in the initiation and progression of cancer. This horizontal transfer of proteomic content of exosomes can mediate signaling pathways including Wnt, PI3K and TGFβ in recipient cells.
EV-based transfer of key proteins involved in PI3K/Akt signaling pathway
The first key step of PI3K/Akt signaling pathway is the phosphorylation of phosphatidylinositol-3,4-diphosphate (PIP2) by PI3K. PIK3CA, a class I PI3K catalytic subunit, overexpression is linked with many types of cancer and is associated with poor prognosis [51]. PIK3CA has been shown to be secreted via EVs from nasopharyngeal carcinoma cells [52]. The presence of this PI3K catalytic subunit in EVs indicates the possibility of EV-based transfer of the PIK3CA from malignant cells to other distant cells thereby stimulating proliferation. EVs not only facilitate PI3K/Akt signaling pathway in cancer cells, it also mediates exchange of membrane (including proteins and lipids) between cells [1].
Recently, active phosphorylated proteins that can mediate PI3K/Akt signaling pathway were also identified in EVs. Biasutto et al. isolated EVs from retinal pigment epithelial cells and analysed them using reversed phase protein arrays to identify the proteins and phosphoproteins [53]. The study detected more than 41 phosphoproteins including PDK (S241), mTOR (S2481) and Akt (T308) in EVs from oxidative stress induced retinal pigment epithelial cells. Inhibition of tyrosine kinase using imatinib and dasatinib inhibits EV release from chronic myeloid leukaemia cells [33].Whilst further functional studies to confirm the activity of the phosphoproteins were limited; identification of these proteins in EVs in active state highlights the importance of EVs in autocrine/paracrine signaling. Importantly, it also underlines the potential of EVs to bypass ligand-receptor activation and null chemotherapeutics such as gefitinib and erlotinib which target malignant cells.
The chemotherapeutic drugs gefitinib and erlotinib interrupt signaling through EGFR which can activate several different pathways including PI3K/Akt. EGFR is reported to be secreted via EVs from bladder cancer, colorectal cancer, pancreatic adenocarcinoma and brain tumor cells [22, 40, 54, 55]. In addition, the secretion of EGFRvIII through EVs [23] highlights the importance of EVs in regulating PI3K/Akt signaling in the recipient cells.
Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) is known to activate several different pathways including PI3K and it also induces the overexpression of EGFR [56, 57]. Both LMP1 and EGFR were detected in EVs secreted by EBV infected cells. These secreted EVs were uptaken by fibroblasts, endothelial and epithelial cells simultaneously activating ERK and PI3K/Akt signaling pathways [52]. Similar observations have been found with EVs derived from primary effusion of lymphomas that were infected with Kaposi sarcoma-associated virus (KSHV). Quantitative proteomics analysis was performed on EVs isolated from 11 different B-cell lines either uninfected, EBV or KSHV infected. Analysis by 2D DIGE and spectral counting revealed 22 proteins that were preferentially enriched in KSHV+EVs. Using Ingenuity Pathway Analysis software, the authors predicted KHSV+EVs could potentially activate MAPK and PI3K signaling pathways in recipient cells [58]. While, uptake of LMP+ and KSHV+EVs could activate PI3K signaling pathway in recipient cells directly; uptake of EVs containing EGFR by endothelial cells increased VEGF secretion. Subsequently, secreted VEGF leads to activation of PI3K signaling pathway in the neighbouring cells [59]. Taken together, these studies emphasize the role of EVs in mediating PI3K signaling events in the recipient cells.
In addition to proteomic and transcriptomic studies intended to profile the molecular content of EVs, few functional studies have also shown that EVs can activate PI3K/Akt signaling pathway in the recipient cells. Qu et al. demonstrated that gastric cancer derived EVs can increase pAkt and proliferation of the recipient cells in a dose dependent manner. Inhibition of PI3K/Akt using LY294002 showed partial decrease in EVs induced-cell proliferation and pAkt levels [60]. This partial inhibition indicates that EVs could mediate PI3K/Akt signalling in two ways: through membrane binding ligands which are inhibited by the LY294002 and through the activated phosphorylated proteins (e.g., pAkt). EVs from mesenchymal stem cells can enhance myocardial viability after reperfusion injury. It has been shown that injection of purified EVs prior to reperfusion increased the level of pAkt which subsequently activates pro-survival signaling in injured cells [61].
Role of EVs in Wnt signaling pathway
Wnt signaling has gained enormous attention due to its role in embryonic development, cell migration and misregulation during cancer development [62]. Wnt pathway components are of main focus in cancer research owing to their association with human neoplasms. However, the process of Wnt trafficking between cells remained elusive. As Wnt proteins are insoluble due to their hydrophobic nature, carrier mechanisms that facilitate their export from endoplasmic reticulum to the cell surface are required. One of the proposed mechanisms of intercellular Wnt signaling involves EVs which act as couriers carrying Wnt ligands or oncogenes from malignant to non-malignant target cells. These EVs can ultimately lead to Wnt-driven cancers in the surrounding cells. However, the key mechanism of Wnt secretion by EVs remains unclear. EVs sequester Wnt ligands including Wnt activators and inhibitors. EVs carrying Wnt activators including Wnt3a and Wnt5a have been shown to stimulate Wnt signaling in the recipient cells, however the effect of Wnt inhibitors containing EVs needs to be explored upon interaction with target cells [63, 64]. A key question is how these two mechanisms are coordinated and balanced in the context of the recipient cells.
EV-based transfer of key proteins involved in Wnt signaling pathway
In a 2009, Korkut et al. demonstrated that both Evi and Wingless (Wnt1 homolog in Homo sapiens) were secreted together in EVs [65]. This Wingless secretion and transmission was investigated at the Drosophila larval neuromuscular junction and Evi was found to be essential for its secretion and trafficking from the cell body to presynaptic vesicles. This was the first demonstration of EV-based transfer of Wingless between cells establishing a novel mechanism potentially in the form of EVs for trans-synaptic communication. The study raised several questions whether Evi has a role in Wnt endocytosis or formation of EVs or both [65]. Follow up studies using RNA silencing assays demonstrated that the release of Evi containing EVs can be blocked by depleting Rab11, Syntaxin 1A and Myosin 5B in S2 cells. Rab11, Syntaxin 1A and Myosin 5B were also required for release of Evi containing EVs in vivo at neuromuscular junction in nervous system [66].
The mechanism of Wnt secretion through EVs was not only in Drosophila but also conserved in human cells. EVs were shown to carry Wnt ligands on their surfaces and were reported to induce Wnt signaling activity in the recipient cells [67]. The EV ride of Wnt molecules has also been demonstrated in yet another study that visualised Wnt3a trafficking to MVB in mouse L cells using fluorescence imaging. A pool of palmitoylated Wnt3a was detected in sections that were positive for EV markers CD63, CD81 and CD9. This specific targeting to the EVs implies that after Wnt3a is shuttled to the PM, they are subsequently secreted via EVs [68].
In addition to EV mediated-Wnt signaling, few studies also explored the role of Wnt ligands in EV secretion. Wnt3a after been internalised has been shown to stimulate the release of EVs from rat primary microglia by activating Wnt β-catenin signaling pathway. Primary rat microglia cells were treated with Wnt3a and EVs were collected from the culture medium. The proteomic profile of EVs derived from Wnt3a treated microglia was characterized using LC-MS/MS followed by database searching. The proteomic results confirmed the presence of Wnt3a in microglia derived EVs following Wnt3a treatment. The authors suggested that the Wnt3a induced EV secretion could possibly act as a clearance method to get rid of the undesirable proteins from the microglia [69].
Transitions of cell states either to side population cells (SP) or non-side population cells (nonSP) in large B-cell lymphoma through Wnt signaling are also facilitated by EVs. The SP cells showed increased expression of Wnt3a and considerably higher percentage of cellular β-catenin compared to nonSP cells from OCI Ly3 lymphoma cells. The culture supernatant from OCI Ly3 cells contained EVs that were enriched in Wnt3a and immunogold staining revealed the presence of this ligand at the limiting membrane. Furthermore, luciferase assay showed that EVs purified from OCI Ly3 cells stimulated Wnt activity in recipient cells. Overall, these results suggest that in the tumor microenvironment EVs may mediate the transfer of Wnt signaling molecules and switch the clonogenic states between cells by the regulation of Wnt signaling [64].
Ratajczak et al. showed that, EVs derived from murine embryonic cells D3 are highly enriched in Wnt3a surface expressed ligand as compared to parental cells which serves as an expansion model for murine hematopoietic progenitor cells [70]. Recently, the role of cancer-associated fibroblasts in influencing tumor progression has gained much attention. EVs secreted by cancer-associated fibroblasts are shown to carry Wnt components and induce Wnt signalling in distant sites. CD81 positive EVs released from L-fibroblast cells stimulate Wnt/Planar cell polarity signaling in breast cancer cells including MDA-MB-2331 and SUM-159PT to increase invasiveness [71–73]. These intriguing findings demonstrate the active role of EVs in facilitating stromal cells to promote metastasis. Non-canonical Wnt5a has also been noted to act as a signal to facilitate macrophage-stimulated invasion in vivo and activation of β-catenin-independent Wnt signaling in breast cancer cells [63]. EVs derived from breast cancer cells were shown to carry Wnt5a which was shuttled to the macrophages thus activating macrophage-induced invasiveness of the MCF-7 cells [74].
Taelman et al. reported the components of MVB that are important for the formation of EVs are also crucial for Wnt signaling pathway [62]. Fluorescence microscopy revealed that Wnt signaling relocated GSK3 from the cytoplasm into the lumen of MVB positive for Rab7 thereby removing GSK3 away from its cytoplasmic substrates. This might be a possible mechanism to inhibit GSK3 from phosphorylating β-catenin in the cytoplasm during Wnt activation. Additionally, depleting Vps27 and Vps4 (proteins involved in the formation of intraluminal vesicles) blocked Wnt activity suggesting that these two proteins are required for Wnt signal transduction pathway. This study shows that sequestration of GSK3 in MVBs links with the requirement for endocytosis during Wnt signaling. Together, these data clearly point the role of MVB in Wnt signaling events. Further research is needed to investigate whether blockage of MVB maturation reverses GSK3 release to the cytoplasm.
Role of neuronal EVs in the central nervous system
Functioning of the brain relies on intercellular communication between neural cells. Release of EVs from a variety of different neuronal cells has been described recently [75–78]. These EVs can be taken up by other neurons suggesting a novel way for inter-neuronal communication. In addition to its physiological function in inter-neuronal communication, EVs may also be involved in the pathogenesis of neurological diseases. They are implicated in the spread of toxic proteins within the nervous system in a number of neurodegenerative diseases, including Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Prion diseases, Alzheimer’s disease (AD) and tauopathies [79].
Role of EVs in the normal nervous system
It has been demonstrated, using primary cortical cultures, that neurons and astrocytes can secrete EVs [75]. These cortical neuron derived EVs were shown to contain AMPA receptors, GPI-anchored prion protein and the cell adhesion molecule L1CAM (expressed only by neurons in the central nervous system) [75, 80]. The long term depolarization of neurons with treatment (25 mM potassium) strongly increased the release of AMPA-R containing EVs, suggesting that EVs may have a regulatory function at synapses and could also mediate the intercellular exchange of membrane proteins within the brain [75]. Lachenal et al. speculated that calcium is a potent activator of MVB fusion to the PM thereby increasing the secretion of EVs at least in neurons. The study also hypothesised that the enhanced secretion of AMPA-R-containing EVs following glutamatergic synaptic activation is a way of local elimination of receptors at synapses undergoing plastic changes [81]. This loss of AMPA receptors upon extensive synaptic activation could be a mechanism of homeostatic synaptic down-scaling [82]. Taken together, these observations of EV secretion regulated by synaptic activity, demonstrates their function in the physiological conditions.
Role of EVs in the pathological nervous system
Parkinson’s disease (PD)
The protein α-synuclein is a mediator of neurodegeneration in PD and its aggregation plays a central role in the pathology [83]. MS-based proteomic studies and immunoblotting has revealed that α-synuclein expressing cells secrete α-synuclein both extracellularly and in association with EVs. Interestingly, cell culture medium from α-synuclein expressing cells, induced apoptosis and were toxic to neuroblastoma SH-5YSY cells and primary cortical neurons. Therefore the EV pathway has been identified to play a potential role in delivering α-synuclein to healthy cells thereby transferring the pathogenic effects associated with this protein [83]. Following on from these findings, Alvarez-Erviti et al. were able to confirm EVs released by α–synuclein overexpressing SH-5YSY cells were able to transfer α-synuclein to wild type SH-5YSY cells [84, 85]. Sonication of EVs prior to incubation with target cells prevented the transfer of α-synuclein highlighting the importance of intact EVs for the mechanism of transfer [85]. Moreover, the assay also confirmed that the EVs are the primary regulators of this transfer.
Recent evidence suggests that increased expression of P-type ATPase ion pump, PARK9/ATP13A2 suppresses α–synuclein toxicity in primary neurons [86]. Increased ATP13A2 levels positively correlated with decreased levels of intracellular α–synuclein and increased α–synuclein containing EVs secretion. Further to validate the relevance of ATP13A2, the investigators performed ATP13A2 knockdown which reduced EV α–synuclein release in HEK293 cells. Interestingly, the overexpression of ATP13A2 also correlated with increased Hsp70 levels in EVs. Hsp70 has previously been shown to be associated with externalised α–synuclein and protect against in vitro toxicity and in vivo α–synuclein aggregates [87]. While several studies suggested that EVs play a role in the transfer of pathogenic proteins between neighbouring cells involved in neurodegeneration [83, 85], Kong et al. suggested a contradictory role wherein EVs relieves the neurodegenerative disease burden in surviving DA neurons with elevated ATP13A2 levels [86].
Amyotrophic lateral sclerosis (ALS)
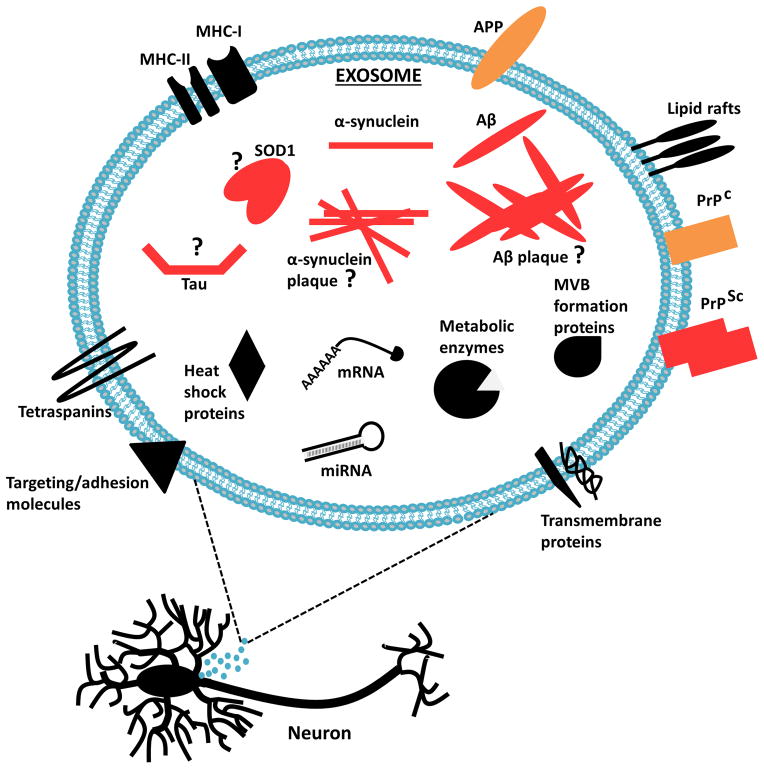
A familial form of the neurodegenerative disease ALS is caused by dominant mutations in the cytosolic SOD1 [88]. Extracellular mutant SOD1 has been shown to trigger microgliosis and death of motor neurons in culture. Yet, the mechanism of SOD1 secretion and transfer between cells during ALS remains elusive. One of the potential mechanisms of the endogenous mouse SOD1 secretion is via EVs. Conditioned media from WT or mutant SOD1 expressing NSC-34 cells revealed the presence of both forms of SOD1 in the EV fractions [88]. Grad and colleagues further characterised the transmission of SOD1 from NSC-34 cells transfected with WT or mutant SOD1 [89]. Interestingly, immunoelectron microscopy revealed that approximately 80–90% of misfolded SOD1 was associated with exosomes derived from NSC-34 cells stably expressing the WT or mutant SOD1 variants. In addition to the misfolded SOD1, both WT and mutant SOD1 were exported from the cells via EVs [89]. While the knowledge on the transport of other proteins implicated in ALS via EVs is limited, the transfer of misfolded SOD1 emphasizes the critical involvement of EVs in ALS (Fig. 3).
Figure 3. Neuronal EV-mediated release of proteins implicated in neurodegenerative diseases.
EVs released from infected neurons carry disease associated proteins; PrPSc, α-synuclein, Aβ, tau, SOD1 and possibly including α-synuclein and Aβ aggregates. Exosomal release and transfer to surrounding neuronal cells may facilitate in neurodegenerative disease progression.
Prion diseases
EV pathway is exploited for the intercellular transfer of prion proteins (PrP), the infectious particle responsible for the transmissible neurodegenerative diseases such as Creutzfeldt–Jakob disease (CJD) and Gerstmann–Straüssler–Scheinker Syndrome (GSS) of humans or bovine spongiform encephalopathy (BSE) of cattle. The normal prion protein PrPC is expressed in all tissues of the human body, with the highest levels of expression observed in tissues of the central nervous system and brain. Both PrPC and its abnormal form PrP scrapie (PrPSc) have been isolated in association with EVs, and PrPSc containing EVs were infectious in both animal and cell bioassays [90]. Following on from these findings, Vella et al. were also able to demonstrate the EV-based transfer of pathogenic PrPSc to cells from the same or different cellular origin both in vitro and in vivo. Tga20 mice (overexpressing mouse PrPC in CNS) treated with cell lysates or EVs derived from PrPSc infected cells showed symptoms associated with prion infectivity [10]. In a similar study, neuroblastoma N2a cells were noted to release prion proteins predominantly via EVs [91]. Taken together, there are strong evidences suggesting that the dissemination of prion proteins is mediated by EV transfer to neighbouring cells.
Alzheimer’s disease (AD)
EV proteins have been noted to be enriched in amyloid plaques from the post-mortem brains of AD patients suggesting a potential role for EVs in the pathogenesis of AD [92]. In AD, cleavage of amyloid precursor protein (APP) by the enzyme β-secretase occurs in a subset of endosomes and a fraction of the Aβ protein is released out of the cells through EVs [92, 93]. CHO-APP695 cells released full length APP and several distinct proteolytically cleaved products of APP, including Aβ through EVs. These fragments could be modulated using inhibitors of the proteases (e.g. γ-secretase inhibition using L685,458) involved in APP cleavage. Therefore, it has been suggested that EVs may potentially be a site of APP processing [93]. Extracellular accumulation of tau protein is another pathological hallmark of AD. In a recent MS-based proteomics study, M1C cells have been found to selectively secrete phosphorylated tau via EVs [94]. The study utilized MS to identify prominent peaks in EVs that were specific for tau phosphoepitopes associated with AD. Interestingly, quantitative comparisons between peaks identified in EV fractions and cell lysate samples showed enrichment for the phosphorylated sites in the proline-rich domain of tau in EV fractions.
Are EVs protective or pathogenic?
Whilst the role of EVs in pathological conditions is well established in many instances, studies aimed at underpinning the physiological role of EVs are limited and warrants further research. EVs are constitutively secreted in physiological conditions and may have a central role in tissue/organ homeostasis. For instance, EVs have been implicated indirectly to have a role in cellular senescence. Hayflick and colleague’s identified cellular senescence about 5 decades ago which lead to the hypotheses that cellular senescence may be either beneficial or detrimental to the cells and/or surrounding cells or both [95]. It has been previously shown that cells in senescence secrete a wide variety of soluble factors and also the release of EVs known as senescence-associated secretory phenotype [96, 97]. Lehman and colleague’s also identified approximately a 3-fold increase in EV release in irradiation induced senescence in the prostate cancer cells 22Rv1 and a >15-fold increase from NHDF cells once it reached a natural state of replicative senescence [96]. In agreement, Arscott and colleagues also identified an increased release of EVs from irradiated glioma and nonglioma cell lines compared to nonirradiated controls [98]. The study also showed that radiation derived EVs from U87MG cells significantly enhanced cell migration in vitro compared to nonirradiated derived EVs. Taken together, EVs may have a significant undiscovered role in the normal physiological conditions. The release of EVs as previously discussed in cancer, neurodegenerative or other diseases alludes to a biological function. Whether it is a protective or pathogenic signal remains elusive. In a protective role, the cells may get rid of pathogenic or oncogenic proteins/RNA through EVs so as to minimize the damage within [99]. Alternatively, the EVs could be used as amplifiers to spread pathogenic molecules between cells. Even though EV mediated signaling remains a grey area, it can be concluded that the exact role of EVs will depend on the precise conditions and functional state. May be the role of senescence secreted EVs may be a mixed blessing in disease progression.
Conclusions
There is an explosion of interest in the functional studies as well as applications of EVs especially in the last decade. Even though the importance of EVs in distant cell communication is recognised, its complex role in physiological and pathological conditions is yet to unwind. Additionally, there are many issues in the EV field which need to be dealt with in order to harness its true potential. The different terminologies used to name EVs need to be standardized and a general consensus agreed upon. In addition, very little is known about the role of EVs in homeostasis. In addition, functional studies conducted so far have demonstrated the role of EVs in vitro using varying amount of exosomes and the physiological concentration remains elusive. Hence, in the physiological context, the stoichiometry of EVs needs to be understood in order to completely understand the mechanism of EV-based functional response.
Acknowledgments
SM is supported by the Australian NH&MRC fellowship (1016599), Australian Research Council Discovery project grant (DP130100535), Ramaciotti Establishment Grant and Award U54-DA036134 supported by the NIH Common Fund through the Office of Strategic Coordination/Office of the NIH Director. HK is supported by a Victoria India Doctoral Scholarship from the Department of State Development, Business and Innovation (DSDBI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AIG
Anchorage independent growth
- EBV
Epstein-Barr virus
- EV
Extracellular vesicle
- MV
Microvesicles
- MVB
Multivesicular bodies
- PM
Plasma membrane
Footnotes
Conflict of interest
The authors have declared no conflict of interest
References
- 1.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Simpson RJ, Mathivanan S. Extracellular Microvesicles: The Need for Internationally Recognised Nomenclature and Stringent Purification Criteria. J Proteomics Bioinform. 2012;5:ii–ii. [Google Scholar]
- 4.Kalra H, Simpson RJ, Ji H, Aikawa E, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 6.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 8.Admyre C, Johansson SM, Qazi KR, Filen JJ, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 9.Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. 2013;59:621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vella LJ, Sharples RA, Lawson VA, Masters CL, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 11.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 12.Mathivanan S, Lim JW, Tauro BJ, Ji H, et al. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9:197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra H, Adda CG, Liem M, Ang CS, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 14.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 16.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34:206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 21.Qu JL, Qu XJ, Zhao MF, Teng YE, et al. The role of cbl family of ubiquitin ligases in gastric cancer exosome-induced apoptosis of Jurkat T cells. Acta Oncologica. 2009;48:1173–1180. doi: 10.3109/02841860903032817. [DOI] [PubMed] [Google Scholar]
- 22.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, et al. Proteomic and immunologic analyses of brain tumor exosomes. The FASEB Journal. 2009;23:1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 24.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochieng J, Pratap S, Khatua AK, Sakwe AM. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp Cell Res. 2009;315:1875–1888. doi: 10.1016/j.yexcr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 28.Peinado H, Aleckovic M, Lavotshkin S, Matei I, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gesierich S, Berezovskiy I, Ryschich E, Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6. 1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 32.Nazarenko I, Rana S, Baumann A, McAlear J, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 33.Mineo M, Garfield SH, Taverna S, Flugy A, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CW, Lee HM, Lee TH, Kang C, et al. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer research. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 35.Hegmans JP, Bard MP, Hemmes A, Luider TM, et al. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho HK, Jang JJ, Kaji S, Spektor G, et al. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation. 2004;109:1314–1319. doi: 10.1161/01.CIR.0000118465.36018.2D. [DOI] [PubMed] [Google Scholar]
- 37.Sheldon H, Heikamp E, Turley H, Dragovic R, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 38.Thery C, Regnault A, Garin J, Wolfers J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvestre JS, Thery C, Hamard G, Boddaert J, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 40.Mathivanan S, Lim JW, Tauro BJ, Ji H, et al. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Molecular & Cellular Proteomics. 2010;9:197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfers J, Lozier A, Raposo G, Regnault A, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 42.Andre F, Schartz NE, Movassagh M, Flament C, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 43.Beloribi S, Ristorcelli E, Breuzard G, Silvy F, et al. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PloS one. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 45.Ristorcelli E, Beraud E, Verrando P, Villard C, et al. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. Faseb J. 2008;22:3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 46.Taverna S, Flugy A, Saieva L, Kohn EC, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. International journal of cancer Journal international du cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bretz NP, Ridinger J, Rupp AK, Rimbach K, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. The Journal of biological chemistry. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 49.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 50.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi J, Yao D, Liu W, Wang N, et al. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC cancer. 2012;12:50. doi: 10.1186/1471-2407-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, et al. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welton JL, Khanna S, Giles PJ, Brennan P, et al. Proteomics analysis of bladder cancer exosomes. Molecular & Cellular Proteomics. 2010;9:1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adamczyk KA, Klein-Scory S, Tehrani MM, Warnken U, et al. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life sciences. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Miller WE, Earp HS, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. Journal of virology. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris MA, Dawson CW, Young LS. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncology. 2009;5:811–825. doi: 10.2217/fon.09.53. [DOI] [PubMed] [Google Scholar]
- 58.Meckes DG, Jr, Gunawardena HP, Dekroon RM, Heaton PR, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A. 2013;110:E2925–2933. doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proceedings of the National Academy of Sciences. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu JL, Qu XJ, Zhao MF, Teng YE, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Digestive and liver disease. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Arslan F, Lai RC, Smeets MB, Akeroyd L, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pukrop T, Klemm F, Hagemann T, Gradl D, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch R, Demant M, Aung T, Diering N, et al. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood. 2014 doi: 10.1182/blood-2013-08-523886. [DOI] [PubMed] [Google Scholar]
- 65.Korkut C, Ataman B, Ramachandran P, Ashley J, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koles K, Nunnari J, Korkut C, Barria R, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 68.Gao X, Hannoush RN. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat Chem Biol. 2014;10:61–68. doi: 10.1038/nchembio.1392. [DOI] [PubMed] [Google Scholar]
- 69.Hooper C, Sainz-Fuertes R, Lynham S, Hye A, et al. Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci. 2012;13:144. doi: 10.1186/1471-2202-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ratajczak J, Miekus K, Kucia M, Zhang J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 71.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 72.Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843–6847. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 73.Suetsugu A, Honma K, Saji S, Moriwaki H, et al. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Menck K, Klemm F, Gross JC, Pukrop T, et al. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4:2057–2066. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faure J, Lachenal G, Court M, Hirrlinger J, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Vingtdeux V, Hamdane M, Loyens A, Gele P, et al. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem. 2007;282:18197–18205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- 77.Putz U, Howitt J, Lackovic J, Foot N, et al. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J Biol Chem. 2008;283:32621–32627. doi: 10.1074/jbc.M804120200. [DOI] [PubMed] [Google Scholar]
- 78.Ghidoni R, Paterlini A, Albertini V, Glionna M, et al. Cystatin C is released in association with exosomes: a new tool of neuronal communication which is unbalanced in Alzheimer’s disease. Neurobiol Aging. 2011;32:1435–1442. doi: 10.1016/j.neurobiolaging.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 80.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 81.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69:337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 85.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kong SM, Chan BK, Park JS, Hill KJ, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 87.Danzer KM, Ruf WP, Putcha P, Joyner D, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. Faseb J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu,Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. 2007;428:43–46. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 89.Grad LI, Yerbury JJ, Turner BJ, Guest WC, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2014;111:3620–3625. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fevrier B, Vilette D, Archer F, Loew D, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alais S, Simoes S, Baas D, Lehmann S, et al. Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol Cell. 2008;100:603–615. doi: 10.1042/BC20080025. [DOI] [PubMed] [Google Scholar]
- 92.Rajendran L, Honsho M, Zahn TR, Keller P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharples RA, Vella LJ, Nisbet RM, Naylor R, et al. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. Faseb J. 2008;22:1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- 94.Saman S, Kim W, Raya M, Visnick Y, et al. Exosome-associated Tau Is Secreted in Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in Early Alzheimer Disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 96.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Re RN, Cook JL. Senescence, apoptosis, and stem cell biology: the rationale for an expanded view of intracrine action. Am J Physiol Heart Circ Physiol. 2009;297:H893–901. doi: 10.1152/ajpheart.00414.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arscott WT, Tandle AT, Zhao S, Shabason JE, et al. Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol. 2013;6:638–648. doi: 10.1593/tlo.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]