Abstract
Sensory hair cells are mechanoreceptors of the auditory and vestibular systems and are crucial for hearing and balance. In adult mammals, auditory hair cells are unable to regenerate, and damage to these cells results in permanent hearing loss. By contrast, hair cells in the chick cochlea and the zebrafish lateral line are able to regenerate, prompting studies into the signaling pathways, morphogen gradients and transcription factors that regulate hair cell development and regeneration in various species. Here, we review these findings and discuss how various signaling pathways and factors function to modulate sensory hair cell development and regeneration. By comparing and contrasting development and regeneration, we also highlight the utility and limitations of using defined developmental cues to drive mammalian hair cell regeneration.
Keywords: Wnt; FGF; Shh; Notch; Atoh1; p27Kip1, Cdkn1b
Summary: This Review compares the mechanisms at play during inner ear hair cell development and regeneration, highlighting gaps in our knowledge and proposing future directions in research and therapeutics.
Introduction
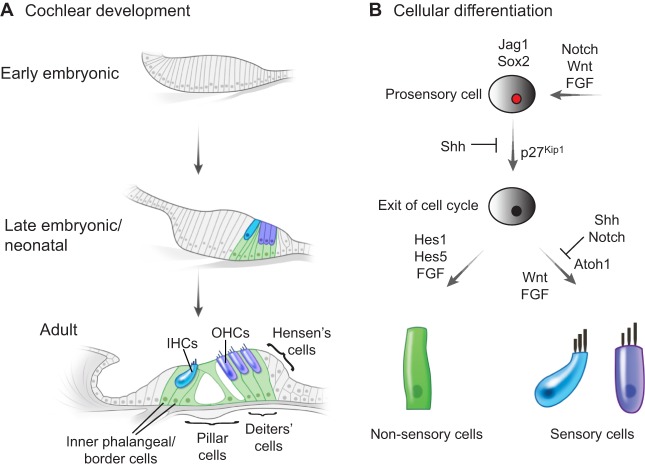
The inner ear sensory organs, which comprise the cochlea, utricle, saccule and the crista ampullaris of the three semicircular canals, serve to detect sound and motion. The hair cells that reside in these six sensory organs are mechanoreceptors, and their development and survival are crucial for these sensory functions. In the cochlea, hair cells are derived from prosensory cells in the floor of the cochlear duct (Fig. 1), which, in response to a variety of signals, enter terminal mitosis and begin to express Atoh1, a transcription factor that is necessary and sufficient for hair cell differentiation (Bermingham et al., 1999; Chen et al., 2002). Multiple events then occur within a relatively short time window as sensory hair cells appear in the prosensory region of the cochlear duct, eventually giving rise to one row of inner hair cells, three rows of outer hair cells, and supporting cell subtypes (Deiters' cells, pillar cells, inner phalangeal/border cells and Hensen's cells; Fig. 1). This orderly matrix of hair cells interdigitated by supporting cells forms the organ of Corti, which extends along the length of the cochlear duct and is the receptor organ for hearing.
Fig. 1.
Cell fate decisions and the development of the mammalian organ of Corti. (A) An overview of cochlear development, highlighting the location of inner hair cells (IHCs, blue), outer hair cells (OHCs, purple) and various supporting cell subtypes (green). (B) A simplified schematic highlighting some of the signaling pathways and factors responsible for the specification of prosensory, sensory and non-sensory cells. The FGF, Notch and Wnt signaling pathways, together with the transcription factor Sox2, are required for prosensory cell specification and/or maintenance. The timing of terminal mitosis and the upregulation of the cell cycle inhibitor p27Kip1 in Sox2-positive prosensory cells are governed by Shh signaling. Wnt and FGF signaling are required for Atoh1 expression and the induction of sensory cell fate, which is restricted by the Notch and Shh pathways. By contrast, the activation of Notch target genes (Hes1 and Hes5) promotes a supporting cell fate, with FGF signals regulating differentiation into supporting cell subtypes.
In the mature mammalian cochlea, hair cells are susceptible to damage from a variety of insults, ranging from aminoglycoside antibiotics to noise exposure. Once lost, hair cells are unable to spontaneously regenerate and the resulting hearing loss is thus permanent (Fig. 2). In stark contrast, the avian cochlea (the basilar papilla) is able to regenerate lost hair cells and auditory function can fully recover (Corwin and Cotanche, 1988; Cruz et al., 1987; Ryals and Rubel, 1988). During regeneration in the avian cochlea, the supporting cells that are interdigitated with hair cells serve as the source of regenerated hair cells through two mechanisms (Fig. 2): (1) via mitotic regeneration, whereby a supporting cell divides and then one or both of the daughter cells differentiates into a hair cell; and (2) via direct phenotypic conversion of a supporting cell into a hair cell without undergoing mitosis, a phenomenon termed direct transdifferentiation (Stone and Cotanche, 2007). Since the discovery of spontaneous hair cell regeneration in the avian cochlea, significant advances have been made towards elucidating the molecular mechanisms that underpin hair cell regeneration in this organ. In addition, studies of sensory organs with ongoing turnover and production of hair cells, including the chicken utricle, the zebrafish lateral line system and other non-mammalian vertebrate models (e.g. frogs, newts, lizards), have also shed light on potential key regulators of hair cell development and regeneration.
Fig. 2.
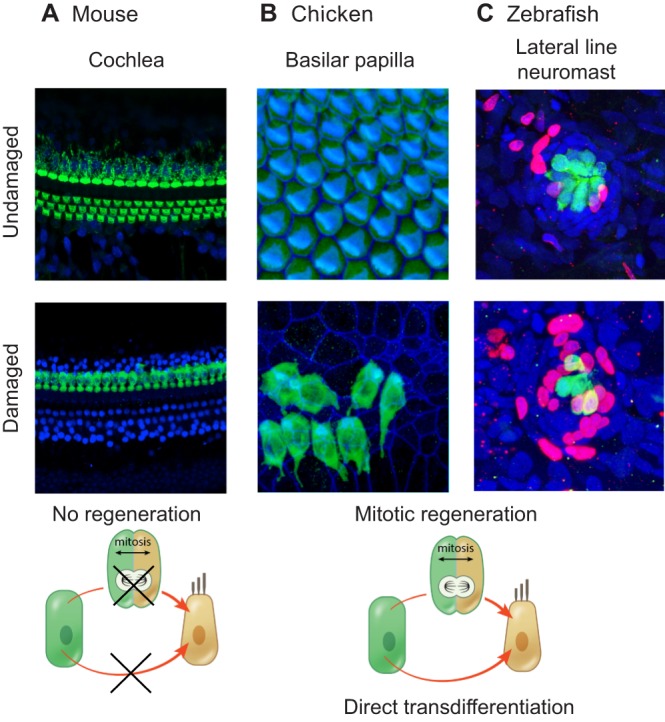
Regenerative capacities of the adult mouse cochlea, the chicken basilar papilla and the zebrafish lateral line system. (A) No sensory hair cell regeneration occurs in the adult mouse cochlea. Shown are the basal turns of postnatal day (P) 28-30 cochleae from control (undamaged) mice and from mice that were treated with sisomicin and furosemide at P21. Immunostaining was performed to detect myosin VIIa (green) and Sox2 (blue); the reduction in myosin VIIa-labeled hair cells indicates that the damaged hair cells are not able to regenerate. (B) Hair cell regeneration in the chicken basilar papilla. Organs were harvested from control chicks and from those that had received daily injections of streptomycin in the previous week. Myosin VIIa-labeled hair cells are in green and phalloidin staining (F-actin) is in blue. Image courtesy of M. Warchol. (C) Hair cell regeneration in the zebrafish lateral line system. Images show posterior lateral line neuromasts from control zebrafish and from those that were damaged with neomycin. Hair cells are labeled by GFP in green, while BrdU (red) marks proliferating cells and DAPI staining (blue) marks nuclei. Regeneration in the neuromast populations, as indicated by the number of BrdU-positive cells, occurs primarily through mitotic regeneration. Image courtesy of T. Piotrowski and A. Romero-Carvajal.
Here, we review the key factors (Atoh1 and p27Kip1) and cell signaling pathways [the Notch, fibroblast growth factor (FGF), Wnt and sonic hedgehog (Shh) pathways] that are involved in the development of hair cells, with a focus on the auditory organ, the cochlea. By highlighting similarities and differences in how these pathways and factors modulate hair cell development and regeneration, we then discuss the potential applicability and limitations of manipulating these signals individually or in combination to induce hair cell regeneration.
The transcription factor Atoh1 plays a key role in hair cell development
Atoh1 is the mammalian homolog of the Drosophila gene atonal, which was first described as a proneural gene involved in regulating the formation of mechanoreceptors and photoreceptors in Drosophila (Jarman et al., 1993, 1994). In the developing mouse cochlea, Atoh1 expression marks the prosensory region, and the Atoh1-positive lineage gives rise to sensory hair cells (Driver et al., 2013). In the absence of Atoh1 expression, prosensory cells fail to form hair cells and supporting cells and instead undergo cell death (Bermingham et al., 1999; Pan et al., 2011; Woods et al., 2004). Although Atoh1 is transiently expressed in developing hair cells until the early postnatal period, the deletion of Atoh1 at various developmental time points revealed that it is necessary for many of the processes needed to generate fully functional hair cells. Specifically, Atoh1 is crucial for hair cell survival soon after differentiation, which occurs from E15.5 to E17.5 (Cai et al., 2013; Chonko et al., 2013). Later on, Atoh1 expression is also required for hair cell maturation, including the development of stereociliary bundles (Cai et al., 2013). Collectively, these findings indicate that Atoh1 is necessary for hair cell differentiation, survival and maturation in the developing cochlea.
Conversely, the ectopic expression of Atoh1 has generated phenotypes that vary depending on the developmental stage of the cochlea. In the embryonic cochlea, Atoh1 overexpression results in the formation of supernumerary hair cells (Gubbels et al., 2008; Woods et al., 2004). In neonatal mice, supporting cells remain competent to form hair cells in response to Atoh1 overexpression, especially in the Kölliker's organ, which is directly medial to the organ of Corti (Kelly et al., 2012; Liu et al., 2012a, 2014; Shou et al., 2003; Yang et al., 2013; Zheng and Gao, 2000). However, this competence is significantly diminished in the undamaged, mature cochlea when assessed via either a transgenic approach or direct viral inoculation (Kawamoto et al., 2003; Liu et al., 2012a).
Building upon its crucial role in hair cell development, numerous studies have focused on Atoh1 in an attempt to regenerate hair cells in the damaged, mature cochlea. For example, Izumikawa and colleagues reported that ectopic expression of Atoh1 immediately after ototoxic injury results in the formation of immature hair cells and the rescue of hearing function in guinea pigs (Izumikawa et al., 2005). However, two subsequent studies showed that the efficacy of this approach might be variable and dependent on timing of expression after damage (Atkinson et al., 2014; Izumikawa et al., 2008). Although these studies illustrate the limited value of using Atoh1 as the sole agent to coerce a hair cell fate in the mature cochlea, they have served as foundations for other studies examining the signaling pathways that cooperatively govern Atoh1 expression and hair cell differentiation, several of which are discussed below.
Cell signaling pathways involved in hair cell development and regeneration
Notch signaling
The Notch pathway mediates short-range cell-cell communication and controls diverse cellular processes, including proliferation, differentiation and cell death in a context-dependent manner. Upon ligand activation, the Notch receptor is enzymatically cleaved, resulting in the release of the Notch intracellular domain (NICD). NICD then translocates into the nucleus, where it interacts with the DNA-binding protein and core effector of the canonical Notch pathway, RBPjk. Readers are referred to the following reviews for a more in-depth discussion of Notch signaling (Kiernan, 2013; Kopan, 2012; Louvi and Artavanis-Tsakonas, 2012).
The Notch pathway plays multiple roles during cochlear development. Several independent lines of research show that Notch signaling, acting via the process of lateral induction, is sufficient to specify prosensory cells. First, the Notch ligand jagged 1 (Jag1) is expressed prior to and during specification of the prosensory region, which is identified by Sox2 expression (Brooker et al., 2006; Kiernan et al., 2006). Second, conditional deletion of Jag1 leads to the downregulation of this prosensory marker, resulting in a malformed cochlear duct that contains few hair cells and supporting cells (Brooker et al., 2006; Kiernan et al., 2006). Third, cultured explants exposed to Notch inhibitors fail to form a prosensory domain (Munnamalai et al., 2012). Conversely, gain-of-function studies using virus-mediated overexpression of NICD in the chick cochlear duct (Daudet and Lewis, 2005) or transgenic approaches in the mouse inner ear (Hartman et al., 2010; Liu et al., 2012b; Pan et al., 2013) result in ectopic sensory patches composed of hair cells and supporting cells. Together, these studies suggest that the Notch pathway is both necessary and sufficient for specifying prosensory cells.
However, several other studies have challenged this interpretation. For example, an early in vitro study of the developing chick cochlea showed that Notch inhibition disrupts the maintenance but not the initial specification of prosensory cells (Daudet et al., 2007). Following up on this finding, two groups examined the in vivo effects of ablating RBPjk (Rbpj) prior to prosensory specification in mice (Basch et al., 2011; Yamamoto et al., 2011). They both observed the appearance of prosensory cells that subsequently underwent extensive cell death, further supporting the notion that the canonical Notch pathway is important for the maintenance rather than the specification of the prosensory region. Although canonical Notch signaling is classically mediated by RBPjk, one untested hypothesis for these conflicting results is that Notch signaling exerts its effects on prosensory specification in an RBPjk-independent manner. However, our understanding of the molecular machinery executing non-canonical Notch signaling is limited, and future studies to identify additional Notch effectors are required to clarify the role(s) of the Notch pathway during prosensory development.
After the initiation of hair cell differentiation, Notch-mediated lateral inhibition is involved in determining whether a prosensory cell becomes a hair cell or a supporting cell; differentiating hair cells express Notch ligands, and these activate Notch signaling in neighboring supporting cells, which, in turn, are prevented from adopting a hair cell fate. As a result, a mosaic pattern of organ of Corti cells is generated. Accordingly, mice deficient for the Notch ligand Jag2 exhibit an increase in hair cell number at the expense of surrounding non-sensory cells. This phenotype is more severe when another Notch ligand, delta-like 1 (Dll1), is also deficient, suggesting a graded regulation of this pathway by these ligands (Kiernan et al., 2005; Lanford et al., 1999). Unlike Jag1-deficient mice, Jag2 null and Jag2;Dll1 double mutants still form hair cells (Kiernan et al., 2005; Lanford et al., 1999). Similarly, conditional deletion of the Notch receptor Notch1 does not prevent formation of the prosensory domain, but instead leads to supernumerary hair cell formation and a concomitant reduction in the number of supporting cells (Kiernan et al., 2005). These studies suggest that different combinations of Notch ligands/receptors mediate prosensory specification and hair cell differentiation. This notion is supported by a recent study of the developing chick cochlea, which showed that different Notch ligands (Jag1 and Delta1) can activate the pathway at various levels (Petrovic et al., 2014).
Given the integral roles of the Notch signaling pathway during hair cell development, extensive work has been undertaken to characterize its involvement during hair cell regeneration. These studies have shown, for example, that hair cell damage in the avian cochlea and utricle, as well as in the zebrafish lateral line, results in the upregulation of Notch target genes (Daudet et al., 2009; Ku et al., 2014; Ma et al., 2008; Stone and Rubel, 1999). Furthermore, although the regenerative capacities of the mammalian cochlea and utricle are limited, the expression of Notch target genes in both of these organs also increases after damage (Batts et al., 2009; Korrapati et al., 2013; Lin et al., 2011; Mizutari et al., 2013; Wang et al., 2010).
Many studies have also characterized the effects of Notch inhibition on hair cell regeneration. Ma and colleagues, for example, demonstrated that mitotic hair cell regeneration after damage increases when γ-secretase inhibitors are used to block Notch signaling in the zebrafish lateral line system, whereas hair cell turnover during homeostasis is unaffected (Ma et al., 2008). Likewise, a study of the chick cochlea showed that treatment with γ-secretase inhibitors increases the number of regenerated hair cells, in this context via both mitotic and non-mitotic mechanisms (Daudet et al., 2009). Studies on the undamaged neonatal (immature) mammalian cochlea have also shown that supporting cells can acquire a hair cell fate when treated with Notch inhibitors (Doetzlhofer et al., 2009; Li et al., 2015; Yamamoto et al., 2006). Building on these findings, two recent studies examined the damaged neonatal mouse cochlea and demonstrated a similar ability of supporting cells to regenerate nascent hair cells after Notch inhibition (Bramhall et al., 2014; Korrapati et al., 2013). This pharmacological approach has also been applied to the damaged, mature mammalian cochlea, but yielded a significantly less robust regenerative response (Mizutari et al., 2013; Tona et al., 2014); unlike the effects observed in the zebrafish lateral line system and chick cochlea, the regenerated hair cells were attributed to direct transdifferentiation of supporting cells, as no cell proliferation was found. Although it is commonly postulated that the regeneration of a functional organ of Corti requires the coordination of supporting cell proliferation and hair cell differentiation such that a balanced ratio of sensory and non-sensory cell types is maintained, a modest functional improvement was observed using this pharmacological approach (Mizutari et al., 2013).
In contrast to the mature mammalian cochlea, the mammalian utricle, saccule and crista ampullaris of the three semicircular canals do exhibit spontaneous hair cell regeneration, albeit at a lower level than that observed in other organisms (Forge et al., 1993, 1998; Golub et al., 2012; Tanyeri et al., 1995; Warchol et al., 1993). In the absence of damage, pharmacological inhibition of Notch signaling in the adult utricle failed to promote a hair cell fate among supporting cells (Collado et al., 2011). However, after hair cell loss, the inhibition of Notch signaling via either inhibition of the target gene Hes5 or pharmacological inhibition of γ-secretase was able to significantly increase Atoh1 expression, with a subset of supporting cells maturing to myosin VIIa-expressing hair cells (Burns et al., 2012; Jung et al., 2013; Lin et al., 2011). Similarly, more hair cells were detected following Notch inhibition in the damaged crista ampullaris (Slowik and Bermingham-McDonogh, 2013).
Taken together, these studies draw similarities between the roles of Notch signaling in specifying hair cell fate during development and in regulating hair cell regeneration. Importantly, these findings also underscore the value of Notch inhibition as a promising approach for enhancing hair cell regeneration. In addition, they suggest that mitotic divisions among supporting cells are likely to be guided by Notch-independent signals. These advances also highlight the diminishing competence of mammalian cochlear supporting cells to respond to cues and regenerate as the organ matures. This notion is further supported by work on the postnatal cochlea, which, unlike the embryonic organ, does not generate ectopic hair cells following NICD overexpression (Liu et al., 2012b). If the process of hair cell regeneration were to simply recapitulate the process of hair cell development, one may schematize a strategy using sequential Notch activation and inhibition. However, the utility of this approach has not been tested and will require a better understanding of the factors that confer competence to respond to Notch manipulation in the developing cochlea – a topic to which we return later.
FGF signaling
The FGF pathway regulates the development of multiple sensory organs, including the inner ear where it plays an essential role in the induction of the otic placode, development of the otic vesicle and regulation of ensuing inner ear morphogenesis (Pirvola et al., 2000; Schimmang, 2007; Wright and Mansour, 2003). FGF also regulates the later stages of inner ear development and hair cell formation: tissue-specific deletion of FGF receptor 1 (Fgfr1) reduces prosensory cell proliferation, resulting in fewer prosensory cells and thus decreased numbers of hair cells and accompanying supporting cells (Ono et al., 2014; Pirvola et al., 2002). The treatment of cultured embryonic cochlear explants with chemical inhibitors of FGF signaling at a stage after terminal mitosis (E14) causes a decrease in Atoh1 expression, suggesting that FGF signaling is also required for hair cell differentiation (Hayashi et al., 2008). Two independent studies pinpointed FGF20 as the candidate ligand for FGFR1 in the cochlea, with both FGF20 and FGFR1 being required for the development of at least a subset of hair cells and supporting cells (Hayashi et al., 2008; Huh et al., 2012). The prosensory effect of FGF20 was further shown to act downstream of Notch signaling; FGF20 treatment rescues prosensory specification that is impeded by Notch inhibition (Munnamalai et al., 2012). Remarkably, deletion of Fgf20 did not lead to vestibular dysfunction, suggesting that the role of FGF20 in hair cell specification might be specific to the cochlea.
Following the initiation of hair cell differentiation, interactions between FGF8, which is secreted by nascent inner hair cells, and FGFR3, which is expressed by supporting cells, are required for the proper development of pillar cells (Colvin et al., 1996; Jacques et al., 2007). At this stage, deficient FGF signaling (as apparent in Fgfr3 mutant mice) increases the number of outer hair cells at the expense of pillar cells and also distorts the relative proportion of supporting cell subtypes (Hayashi et al., 2007; Mansour et al., 2013; Puligilla et al., 2007). Unlike other supporting cell subtypes (e.g. Deiters’ cells), pillar cells do not normally convert to hair cells after inhibition of Notch signaling. However, FGF receptor blockage or Hey2 deficiency renders pillar cells responsive to Notch inhibition (Doetzlhofer et al., 2009), indicating that the two pathways – Notch and FGF – coordinate to maintain the fate of cochlear supporting cells.
The role of FGF signaling during hair cell regeneration has been more thoroughly investigated in the chicken utricle. When analyzing the transcriptome during regeneration of the chicken utricle in vitro, Ku and colleagues found a decrease in Fgf20 and Fgfr3 expression at the time when supporting cells robustly proliferate (Ku et al., 2014). Furthermore, although FGF20 treatment decreases the number of proliferative supporting cells, which is an effect previously observed with broad (basic) FGF treatment (Oesterle et al., 2000), no change was observed when a general FGF receptor inhibitor was applied (Ku et al., 2014). This suggests that FGF signaling inhibits proliferation during regeneration but that blocking FGF signaling alone is not sufficient to enhance proliferation. A decrease in Fgfr3 expression has also been observed after damage in the chicken cochlea and in the zebrafish lateral line (Bermingham-McDonogh et al., 2001; Jiang et al., 2014), whereas Fgfr3 upregulation occurs in the damaged, mammalian cochlea (Pirvola et al., 1995). Whether this difference in FGF receptor expression between mammalian and non-mammalian cochleae accounts for their contrasting proliferative capacities is currently unclear.
In summary, these studies indicate that FGF signaling helps to govern the specification of prosensory cells and their subsequent differentiation into hair cells and supporting cells during cochlear development. By contrast, FGF signaling appears to play a role in limiting proliferation during regeneration in the chick utricle. These studies also elucidated interactions between the Notch and FGF signaling pathways during multiple stages of cochlear development, raising the question of whether and how these pathways might also intersect during regeneration.
Wnt signaling
The Wnt signaling pathway is involved in a wide spectrum of processes during development, including cell proliferation, cell fate determination, maintenance of stem/progenitor cells, and several aspects of cellular and subcellular polarization. The binding of Wnt protein to a receptor complex on Wnt-responsive cells activates the pathway, which is generally separated into the canonical/β-catenin and non-canonical signaling pathways (Logan and Nusse, 2004). A large body of literature describes the key roles of Wnt signaling during inner ear development (reviewed by Groves and Fekete, 2012; Munnamalai and Fekete, 2013). Here, we focus on the roles of canonical Wnt signaling during prosensory cell specification and hair cell differentiation. It is important to point out that non-canonical Wnt signaling also plays a key role during inner ear development by mediating planar cell polarity of hair cells and convergent extension (Dabdoub et al., 2003; Qian et al., 2007). However, it is not clear how Wnt-secreting and Wnt-responsive cells transition from utilizing canonical Wnt signals for guiding cell fate decisions to using non-canonical signals for establishing cell polarity a few days later. This is an important issue to address if we are to understand whether Wnt activation can be applied to stimulate hair cell regeneration.
The examination of two reporter mouse strains (Lgr5-EGFP-IRES-CreERT2 and TCF/LEF:H2B-GFP) (Barker et al., 2007; Ferrer-Vaquer et al., 2010) indicates that Wnt signaling is active in the cochlear duct prior to hair cell differentiation (Chai et al., 2011; Jacques et al., 2012; Shi et al., 2012). Using cochlear explants, it was shown that the application of Wnt inhibitors prevents the proliferation of prosensory cells and their subsequent differentiation into hair cells in vitro (Jacques et al., 2012). The opposite effects were observed when chemical activators of Wnt signaling were applied: an increase in the proliferation of prosensory cells and in the number of hair cells (Jacques et al., 2012). Wnt activation stimulates hair cell proliferation via the upregulation of cyclin D1 in Sox2-positive prosensory cells, both before and after their terminal mitosis, giving rise to an expanded Sox2-expressing domain. The subsequent determination of cell fate in the expanded Sox2 domain occurs as in the normal Sox2-expressing domain, indicating that Notch-mediated lateral inhibition takes place in these tissues (Jacques et al., 2012). By contrast, when cochlear explants from late embryonic or neonatal mice were treated with Wnt activators, a mitogenic response was only seen among a subset of supporting cells, and no ectopic hair cell formation was observed (Jacques et al., 2012; Jan et al., 2013). These observations indicate that the canonical Wnt pathway can regulate both the proliferative state and the fate of prosensory cells during cochlear development. In agreement with this interpretation, a follow-up study used a transgenic approach to ablate β-catenin (Ctnnb1) during cochlear development in vivo, and this resulted in diminished proliferation of prosensory cells and reduced hair cell formation (Shi et al., 2014). Moreover, overexpression of β-catenin in the developing chicken otocyst results in ectopic sensory patches (Stevens et al., 2003), suggesting that the competence to respond to the prosensory effects of Wnt signaling, like those of Notch activation (Hartman et al., 2010; Liu et al., 2012b; Pan et al., 2013), is broader than the prosensory domain at this earlier stage of development.
The roles of canonical Wnt signaling during hair cell regeneration have also been closely examined in recent years, given its mitogenic and prosensory effects during development. In the neonatal cochlea, a subset of supporting cells expresses the Wnt target gene Lgr5 (Chai et al., 2011), which labels Wnt-responsive stem/progenitor cells in a variety of tissues (Barker and Clevers, 2006; Jaks et al., 2008). Although these Lgr5-positive supporting cells in the cochlea are normally quiescent both in vivo and in vitro, they become proliferative when isolated as single cells and can convert into hair cell-like cells in culture (Chai et al., 2012; Shi et al., 2012). Experiments using Wnt activators and inhibitors have shown that Wnt activity controls the proliferation of isolated Lgr5-positive supporting cells in vitro (Chai et al., 2012; Shi et al., 2012). In addition, the overexpression of β-catenin in colonies derived from isolated supporting cells modestly increases Atoh1 expression and hair cell formation in vitro (Shi et al., 2012). This finding is concordant with previous work demonstrating that the enhancer region of Atoh1 is a direct target of β-catenin in neural progenitors (Shi et al., 2010). In a similar vein, neonatal transgenic mice that conditionally express a stabilized form of β-catenin exhibit increased proliferation of supporting cells in the cochlea (Chai et al., 2012; Shi et al., 2013). Remarkably, among these Wnt-responsive supporting cells, the inner pillar cells also upregulate Atoh1, indicating that Wnt/β-catenin signaling can still exert a prosensory response in a limited fashion at this developmental stage. However, no mitogenic or prosensory effects were observed when this pathway was activated in supporting cells of the mature adult cochlea (Shi et al., 2013).
The role of Wnt signaling during hair cell regeneration has also been studied in the zebrafish lateral line. In this context, Wnt activators have been shown to increase the degree of regeneration after damage (Head et al., 2013; Jacques et al., 2014). Similarly, recent studies of the damaged neonatal mouse utricle revealed that Wnt/β-catenin activation increases both supporting cell proliferation and, subsequently, hair cell regeneration in vivo (Wang et al., 2015). It remains to be tested whether supporting cells in the damaged, adult mammalian cochlea can proliferate and regenerate in response to Wnt/β-catenin activation.
Collectively, these findings demonstrate that activating the canonical Wnt pathway can force neonatal cochlear supporting cells, which are otherwise mitotically quiescent, to proliferate and form new hair cells. It is thus feasible to formulate a regenerative strategy by first employing Wnt activation to stimulate progenitor/supporting cell proliferation before introducing a prosensory signal, such as Notch inhibition (via lateral inhibition) or Atoh1 overexpression, to direct hair cell differentiation. Although the interaction between the Notch and Wnt pathways is well characterized in other tissues, studies examining this interplay in the inner ear are rather limited (Jayasena et al., 2008; Li et al., 2015). Thus, more work is needed to further characterize this interaction and the mechanisms involved in reducing Wnt responsiveness as the cochlea matures, particularly in the context of damage paradigms.
Shh signaling
The Shh signaling pathway is essential for various aspects of organogenesis, including growth and patterning (Varjosalo and Taipale, 2008). In a simplified model, the secreted ligand Shh binds its receptor patched 1, resulting in the derepression of another membrane protein, smoothened (Smo), in target cells. This in turn activates the Gli transcription factors, which are the effectors of Shh signaling, leading to the expression or repression of Shh target genes.
Prior to hair cell differentiation in the developing mouse cochlea, Shh is expressed by spiral ganglia neurons (Liu et al., 2010). The subsequent expression of Shh is dynamic, and Shh levels decrease from basal to apical neurons, with the overall level of expression further declining to undetectable levels around birth. This spatiotemporal pattern closely matches that of hair cell differentiation in the cochlear duct, raising the hypothesis that Shh regulates the timing of hair cell differentiation. This is indirectly supported by the finding that loss of the spiral ganglia in Neurod1−/− and Neurog1−/− mice leads to premature cell cycle exit and hair cell differentiation in the cochlear duct (Jahan et al., 2010; Matei et al., 2005).
Several elegant studies have been undertaken to characterize the role of Shh signaling in governing prosensory cell formation. Both Gli3-deficient cochlea and cochlear explants treated with chemical inhibitors of Shh signaling display an expanded prosensory domain, whereas Shh treatment prevents prosensory domain formation (Driver et al., 2008). Using a transgenic approach, it was shown that the ablation of Shh from the spiral ganglia at several developmental time points causes premature cell cycle exit and an abnormal wave of hair cell differentiation extending from the apex towards the base, in the opposite direction to normal development (Bok et al., 2013). In another study, Tateya and colleagues demonstrated that the conditional ablation of Smo in the developing cochlear epithelium results in accelerated hair cell differentiation in the apical region (Tateya et al., 2013). Conversely, the expression of a constitutively active allele of Smo in the cochlear epithelium prevents outer hair cell differentiation, with prosensory cells remaining largely undifferentiated (Tateya et al., 2013). This excessive Shh signaling inhibits hair cell formation by upregulating Fgf20; anti-FGF20 antibodies or FGF receptor blockage partially restored hair cell differentiation (Tateya et al., 2013). More recently, it was shown that the ablation of both Hey1 and Hey2, which are both well-characterized Notch target genes, results in premature hair cell differentiation without affecting the proliferation of prosensory cells (Benito-Gonzalez and Doetzlhofer, 2014). This study also demonstrated that Shh signals regulate Hey1 and Hey2 expression, suggesting that Shh signaling governs the timing of hair cell differentiation via Hey1 and Hey2, possibly by regulating FGF signaling as an intermediate step.
Although much work has been done to elucidate the roles of Shh signaling during cochlear development, its roles during hair cell regeneration remain largely unknown. One study has demonstrated that Shh treatment of cultured cochlea from neonatal rats inhibits retinoblastoma expression and induces the proliferation of supporting cells after hair cell damage (Lu et al., 2013). The crucial roles of Shh signaling in restricting both proliferation and hair cell differentiation during development, together with cumulative evidence of its interaction with other signaling pathways, should serve as an impetus for additional investigations to delineate the extent of these interactions during development and regeneration.
Regulation of the cell cycle during hair cell development
As highlighted above, many signaling molecules appear to affect the proliferation and differentiation of cells during cochlear development. Given this intimate relationship between the cell cycle and cell differentiation, the potential roles of cell cycle inhibitors during the development and regeneration of hair cells have been extensively explored (Schimmang and Pirvola, 2013). In the developing mammalian cochlea, prosensory cells enter terminal mitosis and begin expressing the cell cycle inhibitor p27Kip1 (Cdkn1b) in a wave extending from the apex towards the base (Chen and Segil, 1999; Ruben, 1967). Germline deletion of p27Kip1 leads to ongoing proliferation of organ of Corti cells extending into the postnatal period, when sporadic mitotic events are observed (Chen and Segil, 1999; Lowenheim et al., 1999). However, this overproduction of cells results in a dysfunctional organ, as p27Kip1 null mice exhibit hearing loss (Chen and Segil, 1999). In another mouse model, deletion of the cycle inhibitor p19Ink4d (Cdkn2d) similarly leads to prolonged proliferation of organ of Corti cells and to the eventual degeneration of hair cells, again causing hearing loss (Chen et al., 2003). These studies indicate that the expression of cell cycle inhibitors is required for the proper control of hair cell and supporting cell numbers, but not for the differentiation of hair cells.
A major difference between the regenerating avian cochlea and the non-regenerating mammalian counterpart is the ability of supporting cells in the former to restart proliferation upon damage (Warchol and Corwin, 1996) (Fig. 2). Furthermore, in the chicken utricle and the zebrafish lateral line system, hair cells are continuously replenished and new hair cells are generated via supporting cell division and differentiation. In these contexts, mitotic regeneration is increased when hair cells are damaged by treatment with ototoxins such as aminoglycosides (Harris et al., 2003; Hernández et al., 2007; Matsui et al., 2000). Conversely, in the mature mammalian organ of Corti, no spontaneous cell cycle re-entry of the supporting cells is observed (Fig. 3). This differential response to damage is postulated to be one of the reasons why the mature mammalian cochlea is unable to regenerate hair cells after damage.
Fig. 3.
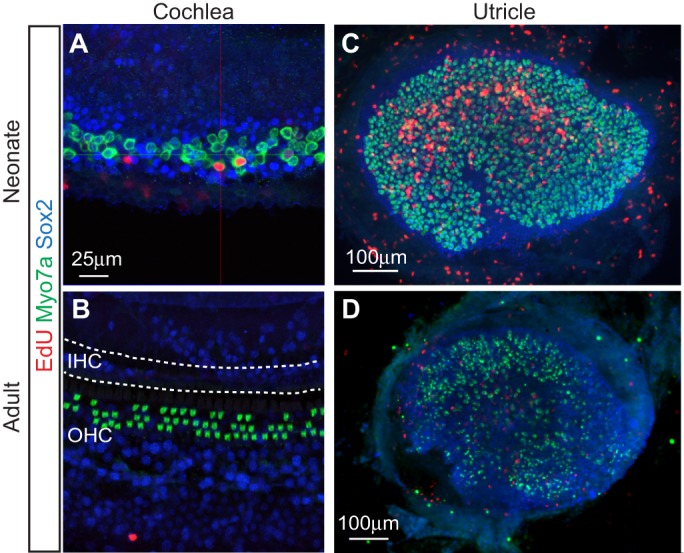
Comparison of the modes and capacity of hair cell regeneration in the neonatal and adult mouse cochlea and utricle. (A) Limited mitotic hair cell regeneration occurs in the neonatal cochlea following diphtheria toxin-induced damage. Shown is the apical turn of the cochlea from P7 Pou4f3DTR/+ mice, which received diphtheria toxin at P1 to ablate hair cells. Immunostaining for myosin VIIa (green) highlights hair cells, while Sox2-positive supporting cells are in blue. The presence of hair cells labeled with EdU (red) indicates mitotic hair cell regeneration. Image courtesy of R. Chai. (B) Conversely, no mitotic response (EdU labeling) or hair cell regeneration occurs in the damaged adult cochlea. The image shows a cochlea from Pou4f3DTR/+ mice that received diphtheria toxin one week previously. There is a complete loss of inner hair cells (IHC) and a partial loss of outer hair cells (OHC; green). (C) By contrast, robust mitotic hair cell regeneration (as indicated by the abundance of EdU-labeled hair cells) is observed in the neonatal utricle. The image is of a utricle from P30 Pou4f3DTR/+ mice that received diphtheria toxin at P1 to ablate hair cells. Image courtesy of R. Chai and T. Wang. (D) Scant proliferation and regeneration are observed in the damaged mature utricle. Shown is a utricle from an adult mouse previously treated with a vestibulotoxin.
Supporting cells in both the neonatal and mature mammalian cochlea are mitotically quiescent. When p27Kip1 is acutely ablated in vivo or suppressed in vitro, supporting cells in the neonatal cochlea proliferate but do not acquire a hair cell fate (Liu et al., 2012c; Maass et al., 2013; Oesterle et al., 2011). In the mature cochlea, the degree of mitotic response was significantly lower than in the neonatal organ (Liu et al., 2012c; Oesterle et al., 2011). These findings suggest that p27Kip1 also contributes to the mitotic quiescence of supporting cells in the postnatal period.
Like supporting cells, hair cells are mitotically quiescent. Deletion of p27Kip1 from hair cells in the neonatal mouse cochlea allowed cell cycle re-entry and the survival of newly added hair cells into the mature organ without compromising hearing function (Walters et al., 2014). This phenotype contrasts with that observed following deletion of retinoblastoma (Rb1) (a tumor suppressor gene downstream of p27Kip1) or co-deletion of p19Ink4d and p21Cip1 (Cdkn1a), in which proliferating hair cells apoptosed with ensuing hearing loss (Laine et al., 2007; Sage et al., 2005, 2006; Weber et al., 2008). Although the effect of blocking cell cycle inhibitors in the damaged cochlea is unclear, these studies posit p27Kip1 as another potential target to induce supporting cell divisions prior to the differentiation of regenerating hair cells. Alternatively, one tantalizing route to induce regeneration is to directly renew the proliferative capacity of surviving hair cells, a regenerative mechanism that is exhibited by differentiated cell types in other end-organs (Dor et al., 2004; Georgia and Bhushan, 2004; Porrello et al., 2011).
Age-dependent changes to regenerative capacity: implications for hair cell regeneration
A number of organs and tissues exhibit age-related changes to their regenerative capacity. For example, in the immature heart and lungs of neonatal mice, injury is met with a robust degree of regeneration/repair, but this phenomenon decreases with age (Jesty et al., 2012; Paxson et al., 2011; Porrello et al., 2011). Neural stem cells also decrease in number as the animal ages (Maslov et al., 2004), and this decrease in the stem cell reserve is implicated in the decline in tissue repair seen with aging (Jin et al., 2004). In the inner ear, a loss of competence to respond to multiple signaling pathways occurs as the cochlea matures (Walters and Zuo, 2013). As discussed above, this difference in responsiveness between the neonatal and adult cochlea is observed when overexpressing Atoh1, activating Wnt signaling, or manipulating Notch signaling and p27Kip1. When hair cells are damaged in the embryonic or neonatal immature cochlea, spontaneous hair cell replacement has been observed (Bramhall et al., 2014; Cox et al., 2014; Kelley et al., 1995). Remarkably, damage in the neonatal cochlea results in a limited degree of proliferation among supporting cells and in mitotic hair cell regeneration (Bramhall et al., 2014; Cox et al., 2014). This body of work illustrates that the mature cochlea is less capable than the neonatal organ of proliferating and forming new hair cells. In parallel, the mitotic response and degree of hair cell regeneration observed in the immature neonatal utricle are also more robust than in the mature adult organ (Burns et al., 2012; Golub et al., 2012) (Fig. 3).
Although our insights into the mechanisms responsible for such differences in responsiveness to gene manipulations in the neonatal and mature inner ear organs are limited, organs of these ages do display rather different gene expression profiles (reviewed by Walters and Zuo, 2013) and epigenetic differences, such as those at Sox2 enhancers (Waldhaus et al., 2012). In light of the important roles of epigenetic regulation in cellular senescence in other organs, one may postulate that epigenetic changes play a role in suppressing the expression of hair cell genes in supporting cells, possibly limiting the efficacy of reintroducing Atoh1. Thus, more studies are urgently needed to further elucidate epigenetic changes in neonatal and adult sensory organs, especially after damage.
Numerous studies have also closely probed for the existence of hair cell progenitors in the neonatal cochlea. Although cells from the neonatal mouse organ of Corti are mitotically quiescent, cochlear cells isolated from this stage can behave as progenitor cells by proliferating and forming new hair cells in vitro (Oshima et al., 2007; Savary et al., 2007; White et al., 2006). However, the quantity of colony-forming cells rapidly decreases from the neonatal to adult period (Oshima et al., 2007; White et al., 2006), implying a loss of stem cells and/or their accompanying niche. Several groups have used various defined markers to segregate distinct populations of cells in the neonatal cochlea (Chai et al., 2012; Jan et al., 2013; Oshima et al., 2007; Savary et al., 2007; Shi et al., 2012; Sinkkonen et al., 2011; White et al., 2006). Together, these studies suggest that Lgr5, which marks somatic stem cells in self-renewing organs (Barker et al., 2007; Jaks et al., 2008), is a promising enrichment marker for supporting cells that behave as hair cell progenitors in vitro. Specifically, isolated Lgr5-positive cells proliferate and form more hair cell-like cells than Lgr5-negative cells (Chai et al., 2012; Shi et al., 2012). Moreover, after damage in vitro and in vivo, Lgr5-marked supporting cells in the neonatal cochlea are capable of regenerating hair cells via a mitotic mechanism (Bramhall et al., 2014; Cox et al., 2014). Despite these promising findings that hair cells can indeed be regenerated via a mitotic mechanism, these cells undergo delayed cell death (Cox et al., 2014). Although informative as proof-of-principle experiments that putative hair cell progenitors exist in the neonatal cochlea, these studies have left several questions unanswered: which factors confer the competence to neonatal Lgr5-positive cells to mitotically regenerate hair cells; which survival factors are missing in these short-lived regenerated hair cells; and what is the function of Lgr5-positive cells in the adult, mature cochlea where regeneration does not occur? Below, we propose some possible explanations and approaches that could be used to tackle these open questions.
Future perspectives
Exploring the intersections between signaling pathways
Our understanding of the signaling mechanisms regulating cochlear development has leapt forward in recent years, aided by numerous studies characterizing the context-dependent roles of each signaling pathway. The availability of genetic tools has enabled us to further dissect components of these pathways. Moreover, these studies have begun to reveal the complex interplay that exists among different pathways at different developmental stages, ranging from otic placode specification (Jayasena et al., 2008) and sensory cell specification (Benito-Gonzalez and Doetzlhofer, 2014; Bok et al., 2013; Munnamalai et al., 2012; Tateya et al., 2013) to the maintenance of cell fate (Doetzlhofer et al., 2009; Li et al., 2015). As insights into the roles of additional pathways in these diverse contexts culminate (e.g. TGFβ, Hippo, microRNAs, retinoic acid, insulin growth factor), the extent to which they interact with one another is likely to prove extensive. Understanding these interactions is a challenging but important next step, as the concurrent or sequential manipulation of several pathways is more likely to be fruitful as we advance towards the goal of regenerating hair cells in the adult mammalian cochlea. The manipulation of individual pathways has already been shown to exert multiple effects (e.g. proliferative and prosensory roles of the canonical Wnt pathway), so titrating the activities of several signaling pathways will undoubtedly bring additional layers of complexity to future investigations.
Akin to the approaches used to guide the differentiation of embryonic stem cells and induced pluripotent stem cells, directing adult mammalian cochlear supporting cells to proliferate and regenerate hair cells is likely to involve the rekindling and orchestration of multiple developmental cues. The findings of studies that have probed the dynamics of gene expression during regeneration, such as those in the chicken inner ear organs and the zebrafish lateral line, have already illustrated such multiplex changes during the different phases of regeneration (Alvarado et al., 2011; Hawkins et al., 2007; Jiang et al., 2014; Ku et al., 2014; Steiner et al., 2014). As we gain knowledge about the transcriptome of the non-regenerating mammalian cochlea, we can begin to dissect its gene expression patterns and contrast them with those seen in regenerating sensory organs. We might also be able to decipher whether differences exist between the mammalian cochlea immediately and long-term after damage.
Cellular reprogramming
Another novel approach used in regenerative medicine is de novo cellular reprogramming, or ‘direct reprogramming’, using defined factors. The introduction of such putative reprogramming factor(s) has met with success in regenerating cardiomyocytes (Efe et al., 2011; Ieda et al., 2010; Jayawardena et al., 2012; Qian et al., 2012) and neurons (Guo et al., 2014; Kim et al., 2011; Niu et al., 2013; Vierbuchen et al., 2010). An analogous method to regenerate hair cells has also been developed using Atoh1 as the sole reprogramming factor, which, as mentioned above, yielded limited success in preclinical models of hearing loss. Nonetheless, an active clinical trial, in which an adenoviral vector carrying Atoh1 is directly inoculated into the inner ear, is still under way (ClinicalTrials.gov identifier: NCT02132130). Its results should reveal, first and foremost, whether such an approach is safe and, second, whether it is effective at promoting hair cell regeneration.
Other transcription factors that are crucial for hair cell development have also proven to be useful for generating new sensory cells (Ahmed et al., 2012; Masuda et al., 2012). For example, Ahmed and colleagues showed that the combined use of Eya1 and Six1 induces a hair cell fate in Sox2-expressing non-sensory cells in embryonic cochlear explants (Ahmed et al., 2012; Masuda et al., 2012). Interestingly, myosin VIIa-positive hair cells can be generated through Atoh1-dependent and -independent pathways, providing support for the existence of putative hair cell reprogramming factors aside from Atoh1. Additionally, transcription factors that are upregulated or downregulated during regeneration in non-mammalian sensory organs might prove to be valuable candidate reprogramming factors.
Possible differences between development and regeneration
In addition to the known and aforementioned similarities and differences in the functions of individual signaling pathways during development and regeneration, it is important to emphasize two major distinctions between the two processes.
The first concerns the timing of mitosis and hair cell formation. During development, prosensory cells enter terminal mitosis prior to differentiating into hair cells, yet the timing of these two events differs dramatically along the length of the cochlea, with the prosensory cells in the apical turn being the first to stop dividing and the last to change fate. By contrast, mitotic regeneration from supporting cells in avian inner ear tissues and the zebrafish lateral line system is much more synchronized. If a two-step strategy were employed (i.e. by first stimulating proliferation via Wnt activation or by inhibiting a cell cycle inhibitor, and then promoting hair cell induction through Notch inhibition or Atoh1 overexpression; Fig. 4), it will probably be necessary to tailor the timing of the two steps (and of other factors) to regeneration.
Fig. 4.
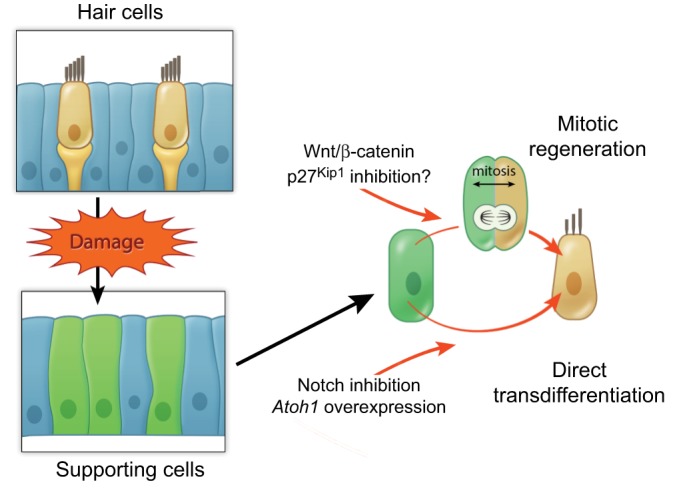
Proposed mechanisms of hair cell regeneration. Supporting cells can classically regenerate hair cells via two mechanisms: mitotic hair cell regeneration and direct transdifferentiation. Enhancing the Wnt/β-catenin signaling pathway or inhibiting cell cycle inhibitors such as p27Kip1 may constitute approaches to enhance mitotic regeneration. By contrast, Notch inhibition and Atoh1 overexpression may promote the direct conversion of supporting cells into hair cells.
The second distinction relates to hair cell differentiation, maturation and survival. After overcoming the challenge of inducing proliferation and a cell fate change among supporting cells in the damaged, mature mammalian cochlea, another obstacle may arise from the lack of hair cell maturation and survival cues that are native to the embryonic and neonatal cochlea. This difference might be attributed to a deficient progenitor cell response to damage. For example, regenerating hair cells in the neonatal cochlea lack the survival factor Pou4f3 and undergo delayed cell death (Cox et al., 2014). This difference might also stem from several other structural and/or physiological changes to the developing cochlea (e.g. the loss of the greater epithelial ridge, establishment of the tectorial membrane and endocochlear potential, a decrease in the number of tympanic border cells), which may also influence the maturation and survival of hair cells.
Conclusions
In recent years, tremendous progress has been made in revealing the complex mechanisms involved in the development of the mammalian cochlea. These studies have helped to define the functions of individual signaling pathways at different stages of cochlear development, and also the complex interplay among them. In addition, gain-of-function studies have identified that prosensory signals (e.g. Notch and Atoh1) can drive the formation of sensory cells in regions outside the prosensory domain, possibly facilitating the characterization of novel factors that confer competence to generate sensory cells. Lastly, knowledge gained from analyses of hair cell progenitors in other species and the identification of mammalian progenitors in the immature cochlea and vestibular organs should accelerate additional discoveries on the path towards regenerating the mammalian cochlea.
Acknowledgements
We thank B. Cox, O. Bermingham-McDonogh, S. Heller, S. Billings and L. Jansson for insightful comments on the manuscript; R. Chai, T. Wang, M. Warchol, T. Piotrowski, A. Romero-Carvajal for generously sharing figures; E. Rubel for sharing the Pou4f3-DTR mice; and C. Gralapp for figure illustration. We apologize to those whose work we have omitted to cite owing to limitations of space.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the Lucile Packard Foundation for Children's Health, Stanford [NIH-NCATS-CTSA UL1 TR001085]; Child Health Research Institute of Stanford University (E.H.N.); Stanford University Medical Scholars Research Program; Howard Hughes Medical Institute Medical Fellows Program (Z.N.S.); NIH/NIDCD [K08DC011043, RO1DC01910]; Department of Defense [W81XWH-14-1-0517]; Akiko Yamazaki and Jerry Yang Faculty Scholar Fund; and California Initiative in Regenerative Medicine [RN3-06529] (A.G.C.). Deposited in PMC for release after 12 months.
References
- Ahmed M., Wong E. Y. M., Sun J., Xu J., Wang F. and Xu P.-X. (2012). Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 22, 377-390 10.1016/j.devcel.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado D. M., Hawkins R. D., Bashiardes S., Veile R. A., Ku Y.-C., Powder K. E., Spriggs M. K., Speck J. D., Warchol M. E. and Lovett M. (2011). An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J. Neurosci. 31, 4535-4543 10.1523/JNEUROSCI.5456-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson P. J., Wise A. K., Flynn B. O., Nayagam B. A. and Richardson R. T. (2014). Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS ONE 9, e102077 10.1371/journal.pone.0102077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. and Clevers H. (2006). Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5, 997-1014 10.1038/nrd2154 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J. et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003-1007 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Ohyama T., Segil N. and Groves A. K. (2011). Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J. Neurosci. 31, 8046-8058 10.1523/JNEUROSCI.6671-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts S. A., Shoemaker C. R. and Raphael Y. (2009). Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear. Res. 249, 15-22 10.1016/j.heares.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Gonzalez A. and Doetzlhofer A. (2014). Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J. Neurosci. 34, 12865-12876 10.1523/JNEUROSCI.1494-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N. A., Hassan B. A., Price S. D., Vollrath M. A., Ben-Arie N., Eatock R. A., Bellen H. J., Lysakowski A. and Zoghbi H. Y. (1999). Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837-1841 10.1126/science.284.5421.1837 [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Stone J. S., Reh T. A. and Rubel E. W. (2001). FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev. Biol. 238, 247-259 10.1006/dbio.2001.0412 [DOI] [PubMed] [Google Scholar]
- Bok J., Zenczak C., Hwang C. H. and Wu D. K. (2013). Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. USA 110, 13869-13874 10.1073/pnas.1222341110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N. F., Shi F., Arnold K., Hochedlinger K. and Edge A. S. B. (2014). Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep. 2, 311-322 10.1016/j.stemcr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R., Hozumi K. and Lewis J. (2006). Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133, 1277-1286 10.1242/dev.02284 [DOI] [PubMed] [Google Scholar]
- Burns J. C., Cox B. C., Thiede B. R., Zuo J. and Corwin J. T. (2012). In vivo proliferative regeneration of balance hair cells in newborn mice. J. Neurosci. 32, 6570-6577 10.1523/JNEUROSCI.6274-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T., Seymour M. L., Zhang H., Pereira F. A. and Groves A. K. (2013). Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neurosci. 33, 10110-10122 10.1523/JNEUROSCI.5606-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R., Xia A., Wang T., Jan T. A., Hayashi T., Bermingham-McDonogh O. and Cheng A. G.-L. (2011). Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455-469 10.1007/s10162-011-0267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R., Kuo B., Wang T., Liaw E. J., Xia A., Jan T. A., Liu Z., Taketo M. M., Oghalai J. S., Nusse R. et al. (2012). Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA 109, 8167-8172 10.1073/pnas.1202774109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. and Segil N. (1999). p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581-1590. [DOI] [PubMed] [Google Scholar]
- Chen P., Johnson J. E., Zoghbi H. Y. and Segil N. (2002). The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495-2505. [DOI] [PubMed] [Google Scholar]
- Chen P., Zindy F., Abdala C., Liu F., Li X., Roussel M. F. and Segil N. (2003). Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat. Cell Biol. 5, 422-426 10.1038/ncb976 [DOI] [PubMed] [Google Scholar]
- Chonko K. T., Jahan I., Stone J., Wright M. C., Fujiyama T., Hoshino M., Fritzsch B. and Maricich S. M. (2013). Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev. Biol. 381, 401-410 10.1016/j.ydbio.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M. S., Thiede B. R., Baker W., Askew C., Igbani L. M. and Corwin J. T. (2011). The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs. J. Neurosci. 31, 11855-11866 10.1523/JNEUROSCI.2525-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G. and Ornitz D. M. (1996). Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390-397 10.1038/ng0496-390 [DOI] [PubMed] [Google Scholar]
- Corwin J. T. and Cotanche D. A. (1988). Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772-1774 10.1126/science.3381100 [DOI] [PubMed] [Google Scholar]
- Cox B. C., Chai R., Lenoir A., Liu Z., Zhang L., Nguyen D.-H., Chalasani K., Steigelman K. A., Fang J., Rubel E. W. et al. (2014). Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141, 816-829 10.1242/dev.103036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz R. M., Lambert P. R. and Rubel E. W. (1987). Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch. Otolaryngol. Head Neck Surg. 113, 1058-1062 10.1001/archotol.1987.01860100036017 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Donohue M. J., Brennan A., Wolf V., Montcouquiol M., Sassoon D. A., Hseih J.-C., Rubin J. S., Salinas P. C. and Kelley M. W. (2003). Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130, 2375-2384 10.1242/dev.00448 [DOI] [PubMed] [Google Scholar]
- Daudet N. and Lewis J. (2005). Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132, 541-551 10.1242/dev.01589 [DOI] [PubMed] [Google Scholar]
- Daudet N., Ariza-McNaughton L. and Lewis J. (2007). Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development 134, 2369-2378 10.1242/dev.001842 [DOI] [PubMed] [Google Scholar]
- Daudet N., Gibson R., Shang J., Bernard A., Lewis J. and Stone J. (2009). Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev. Biol. 326, 86-100 10.1016/j.ydbio.2008.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A., Basch M. L., Ohyama T., Gessler M., Groves A. K. and Segil N. (2009). Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 16, 58-69 10.1016/j.devcel.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I. and Melton D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41-46 10.1038/nature02520 [DOI] [PubMed] [Google Scholar]
- Driver E. C., Pryor S. P., Hill P., Turner J., Ruther U., Biesecker L. G., Griffith A. J. and Kelley M. W. (2008). Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J. Neurosci. 28, 7350-7358 10.1523/JNEUROSCI.0312-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver E. C., Sillers L., Coate T. M., Rose M. F. and Kelley M. W. (2013). The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol. 376, 86-98 10.1016/j.ydbio.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe J. A., Hilcove S., Kim J., Zhou H., Ouyang K., Wang G., Chen J. and Ding S. (2011). Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 13, 215-222 10.1038/ncb2164 [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A., Piliszek A., Tian G., Aho R. J., Dufort D. and Hadjantonakis A.-K. (2010). A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev. Biol. 10, 121 10.1186/1471-213X-10-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Li L., Corwin J. T. and Nevill G. (1993). Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259, 1616-1619 10.1126/science.8456284 [DOI] [PubMed] [Google Scholar]
- Forge A., Li L. and Nevill G. (1998). Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J. Comp. Neurol. 397, 69-88 [DOI] [PubMed] [Google Scholar]
- Georgia S. and Bhushan A. (2004). Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 114, 963-968 10.1172/JCI200422098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub J. S., Tong L., Ngyuen T. B., Hume C. R., Palmiter R. D., Rubel E. W. and Stone J. S. (2012). Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci. 32, 15093-15105 10.1523/JNEUROSCI.1709-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. K. and Fekete D. M. (2012). Shaping sound in space: the regulation of inner ear patterning. Development 139, 245-257 10.1242/dev.067074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels S. P., Woessner D. W., Mitchell J. C., Ricci A. J. and Brigande J. V. (2008). Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537-541 10.1038/nature07265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Zhang L., Wu Z., Chen Y., Wang F. and Chen G. (2014). In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell 14, 188-202 10.1016/j.stem.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. A., Cheng A. G., Cunningham L. L., MacDonald G., Raible D. W. and Rubel E. W. (2003). Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 4, 219-234 10.1007/s10162-002-3022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. H., Reh T. A. and Bermingham-McDonogh O. (2010). Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc. Natl. Acad. Sci. USA 107, 15792-15797 10.1073/pnas.1002827107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Bashiardes S., Powder K. E., Sajan S. A., Bhonagiri V., Alvarado D. M., Speck J., Warchol M. E. and Lovett M. (2007). Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS ONE 2, e525 10.1371/journal.pone.0000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Cunningham D. and Bermingham-McDonogh O. (2007). Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev. Dyn. 236, 525-533 10.1002/dvdy.21026 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ray C. A. and Bermingham-McDonogh O. (2008). Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28, 5991-5999 10.1523/JNEUROSCI.1690-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. R., Gacioch L., Pennisi M. and Meyers J. R. (2013). Activation of canonical Wnt/beta-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Dev. Dyn. 242, 832-846 10.1002/dvdy.23973 [DOI] [PubMed] [Google Scholar]
- Hernández P. P., Olivari F. A., Sarrazin A. F., Sandoval P. C. and Allende M. L. (2007). Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev. Neurobiol. 67, 637-654 10.1002/dneu.20386 [DOI] [PubMed] [Google Scholar]
- Huh S.-H., Jones J., Warchol M. E. and Ornitz D. M. (2012). Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol. 10, e1001231 10.1371/journal.pbio.1001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M., Fu J.-D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B. G. and Srivastava D. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375-386 10.1016/j.cell.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M., Minoda R., Kawamoto K., Abrashkin K. A., Swiderski D. L., Dolan D. F., Brough D. E. and Raphael Y. (2005). Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11, 271-276 10.1038/nm1193 [DOI] [PubMed] [Google Scholar]
- Izumikawa M., Batts S. A., Miyazawa T., Swiderski D. L. and Raphael Y. (2008). Response of the flat cochlear epithelium to forced expression of Atoh1. Hear. Res. 240, 52-56 10.1016/j.heares.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques B. E., Montcouquiol M. E., Layman E. M., Lewandoski M. and Kelley M. W. (2007). Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134, 3021-3029 10.1242/dev.02874 [DOI] [PubMed] [Google Scholar]
- Jacques B. E., Puligilla C., Weichert R. M., Ferrer-Vaquer A., Hadjantonakis A.-K., Kelley M. W. and Dabdoub A. (2012). A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development 139, 4395-4404 10.1242/dev.080358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques B. E., Montgomery W. H., Uribe P. M., Yatteau A., Asuncion J. D., Resendiz G., Matsui J. I. and Dabdoub A. (2014). The role of Wnt/beta-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev. Neurobiol. 74, 438-456 10.1002/dneu.22134 [DOI] [PubMed] [Google Scholar]
- Jahan I., Pan N., Kersigo J. and Fritzsch B. (2010). Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS ONE 5, e11661 10.1371/journal.pone.0011661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V., Barker N., Kasper M., van Es J. H., Snippert H. J., Clevers H. and Toftgård R. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291-1299 10.1038/ng.239 [DOI] [PubMed] [Google Scholar]
- Jan T. A., Chai R., Sayyid Z. N., van Amerongen R., Xia A., Wang T., Sinkkonen S. T., Zeng Y. A., Levin J. R., Heller S. et al. (2013). Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development 140, 1196-1206 10.1242/dev.087528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A. P., Grau Y., Jan L. Y. and Jan Y. N. (1993). atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell 73, 1307-1321 10.1016/0092-8674(93)90358-W [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y. and Jan Y. N. (1994). Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398-400 10.1038/369398a0 [DOI] [PubMed] [Google Scholar]
- Jayasena C. S., Ohyama T., Segil N. and Groves A. K. (2008). Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135, 2251-2261 10.1242/dev.017905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena T. M., Egemnazarov B., Finch E. A., Zhang L., Payne J. A., Pandya K., Zhang Z., Rosenberg P., Mirotsou M. and Dzau V. J. (2012). MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465-1473 10.1161/CIRCRESAHA.112.269035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty S. A., Steffey M. A., Lee F. K., Breitbach M., Hesse M., Reining S., Lee J. C., Doran R. M., Nikitin A. Y., Fleischmann B. K. et al. (2012). c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 109, 13380-13385 10.1073/pnas.1208114109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Romero-Carvajal A., Haug J. S., Seidel C. W. and Piotrowski T. (2014). Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 111, E1383-E1392 10.1073/pnas.1402898111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Minami M., Xie L., Sun Y., Mao X. O., Wang Y., Simon R. P. and Greenberg D. A. (2004). Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell 3, 373-377 10.1111/j.1474-9728.2004.00131.x [DOI] [PubMed] [Google Scholar]
- Jung J. Y., Avenarius M. R., Adamsky S., Alpert E., Feinstein E. and Raphael Y. (2013). siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol. Ther. 21, 834-841 10.1038/mt.2013.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K., Ishimoto S., Minoda R., Brough D. E. and Raphael Y. (2003). Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 23, 4395-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M. W., Talreja D. R. and Corwin J. T. (1995). Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J. Neurosci. 15, 3013-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. C., Chang Q., Pan A., Lin X. and Chen P. (2012). Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci. 32, 6699-6710 10.1523/JNEUROSCI.5420-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E. (2013). Notch signaling during cell fate determination in the inner ear. Semin. Cell Dev. Biol. 24, 470-479 10.1016/j.semcdb.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Cordes R., Kopan R., Gossler A. and Gridley T. (2005). The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132, 4353-4362 10.1242/dev.02002 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J. and Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 10.1371/journal.pgen.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Efe J. A., Zhu S., Talantova M., Yuan X., Wang S., Lipton S. A., Zhang K. and Ding S. (2011). Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 108, 7838-7843 10.1073/pnas.1103113108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R. (2012). Notch signaling. Cold Spring Harb. Perspect. Biol. 4, a011213 10.1101/cshperspect.a011213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrapati S., Roux I., Glowatzki E. and Doetzlhofer A. (2013). Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS ONE 8, e73276 10.1371/journal.pone.0073276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku Y.-C., Renaud N. A., Veile R. A., Helms C., Voelker C. C. J., Warchol M. E. and Lovett M. (2014). The transcriptome of utricle hair cell regeneration in the avian inner ear. J. Neurosci. 34, 3523-3535 10.1523/JNEUROSCI.2606-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine H., Doetzlhofer A., Mantela J., Ylikoski J., Laiho M., Roussel M. F., Segil N. and Pirvola U. (2007). p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J. Neurosci. 27, 1434-1444 10.1523/JNEUROSCI.4956-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford P. J., Lan Y., Jiang R., Lindsell C., Weinmaster G., Gridley T. and Kelley M. W. (1999). Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21, 289-292 10.1038/6804 [DOI] [PubMed] [Google Scholar]
- Li W., Wu J., Yang J., Sun S., Chai R., Chen Z. and Li H. (2015). Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. USA 112, 166-171 10.1073/pnas.1415901112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V., Golub J. S., Nguyen T. B., Hume C. R., Oesterle E. C. and Stone J. S. (2011). Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J. Neurosci. 31, 15329-15339 10.1523/JNEUROSCI.2057-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Owen T., Zhang L. and Zuo J. (2010). Dynamic expression pattern of Sonic hedgehog in developing cochlear spiral ganglion neurons. Dev. Dyn. 239, 1674-1683 10.1002/dvdy.22302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Dearman J. A., Cox B. C., Walters B. J., Zhang L., Ayrault O., Zindy F., Gan L., Roussel M. F. and Zuo J. (2012a). Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J. Neurosci. 32, 6600-6610 10.1523/JNEUROSCI.0818-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Owen T., Fang J. and Zuo J. (2012b). Overactivation of Notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PLoS ONE 7, e34123 10.1371/journal.pone.0034123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Walters B. J., Owen T., Brimble M. A., Steigelman K. A., Zhang L., Mellado Lagarde M. M., Valentine M. B., Yu Y., Cox B. C. et al. (2012c). Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J. Neurosci. 32, 10530-10540 10.1523/JNEUROSCI.0686-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Fang J., Dearman J., Zhang L. and Zuo J. (2014). In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS ONE 9, e89377 10.1371/journal.pone.0089377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y. and Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781-810 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Louvi A. and Artavanis-Tsakonas S. (2012). Notch and disease: a growing field. Semin. Cell Dev. Biol. 23, 473-480 10.1016/j.semcdb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H., Furness D. N., Kil J., Zinn C., Gultig K., Fero M. L., Frost D., Gummer A. W., Roberts J. M., Rubel E. W. et al. (1999). Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl. Acad. Sci. USA 96, 4084-4088 10.1073/pnas.96.7.4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N., Chen Y., Wang Z., Chen G., Lin Q., Chen Z.-Y. and Li H. (2013). Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein. Biochem. Biophys. Res. Commun. 430, 700-705 10.1016/j.bbrc.2012.11.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E. Y., Rubel E. W. and Raible D. W. (2008). Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 28, 2261-2273 10.1523/JNEUROSCI.4372-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass J. C., Berndt F. A., Cánovas J. and Kukuljan M. (2013). p27Kip1 knockdown induces proliferation in the organ of Corti in culture after efficient shRNA lentiviral transduction. J. Assoc. Res. Otolaryngol. 14, 495-508 10.1007/s10162-013-0383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Li C. and Urness L. D. (2013). Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 27, 2320-2331 10.1101/gad.228957.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov A. Y., Barone T. A., Plunkett R. J. and Pruitt S. C. (2004). Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24, 1726-1733 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Pak K., Chavez E. and Ryan A. F. (2012). TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Dev. Biol. 372, 68-80 10.1016/j.ydbio.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V., Pauley S., Kaing S., Rowitch D., Beisel K. W., Morris K., Feng F., Jones K., Lee J. and Fritzsch B. (2005). Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 234, 633-650 10.1002/dvdy.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J. I., Oesterle E. C., Stone J. S. and Rubel E. W. (2000). Characterization of damage and regeneration in cultured avian utricles. J. Assoc. Res. Otolaryngol. 1, 46-63 10.1007/s101620010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K., Fujioka M., Hosoya M., Bramhall N., Okano H. J., Okano H. and Edge A. S. B. (2013). Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58-69 10.1016/j.neuron.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V. and Fekete D. M. (2013). Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 24, 480-489 10.1016/j.semcdb.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V., Hayashi T. and Bermingham-McDonogh O. (2012). Notch prosensory effects in the Mammalian cochlea are partially mediated by Fgf20. J. Neurosci. 32, 12876-12884 10.1523/JNEUROSCI.2250-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W., Zang T., Zou Y., Fang S., Smith D. K., Bachoo R. and Zhang C.-L. (2013). In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 15, 1164-1175 10.1038/ncb2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle E. C., Bhave S. A. and Coltrera M. D. (2000). Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J. Comp. Neurol. 424, 307-326 [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Chien W.-M., Campbell S., Nellimarla P. and Fero M. L. (2011). p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle 10, 1237-1248 10.4161/cc.10.8.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Kita T., Sato S., O'Neill P., Mak S.-S., Paschaki M., Ito M., Gotoh N., Kawakami K., Sasai Y. et al. (2014). FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 10, e1004118 10.1371/journal.pgen.1004118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Grimm C. M., Corrales C. E., Senn P., Martinez Monedero R., Géléoc G. S. G., Edge A., Holt J. R. and Heller S. (2007). Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 8, 18-31 10.1007/s10162-006-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N., Jahan I., Kersigo J., Kopecky B., Santi P., Johnson S., Schmitz H. and Fritzsch B. (2011). Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear. Res. 275, 66-80 10.1016/j.heares.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Jin Y., Chen J., Rottier R. J., Steel K. P. and Kiernan A. E. (2013). Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J. Neurosci. 33, 16146-16157 10.1523/JNEUROSCI.3150-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson J. A., Gruntman A., Parkin C. D., Mazan M. R., Davis A., Ingenito E. P. and Hoffman A. M. (2011). Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS ONE 6, e23232 10.1371/journal.pone.0023232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic J., Formosa-Jordan P., Luna-Escalante J. C., Abello G., Ibanes M., Neves J. and Giraldez F. (2014). Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development 141, 2313-2324 10.1242/dev.108100 [DOI] [PubMed] [Google Scholar]
- Pirvola U., Cao Y., Oellig C., Suoqiang Z., Pettersson R. F. and Ylikoski J. (1995). The site of action of neuronal acidic fibroblast growth factor is the organ of Corti of the rat cochlea. Proc. Natl. Acad. Sci. USA 92, 9269-9273 10.1073/pnas.92.20.9269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Spencer-Dene B., Xing-Qun L., Kettunen P., Thesleff I., Fritzsch B., Dickson C. and Ylikoski J. (2000). FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 20, 6125-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Ylikoski J., Trokovic R., Hébert J. M., McConnell S. K. and Partanen J. (2002). FGFR1 is required for the development of the auditory sensory epithelium. Neuron 35, 671-680 10.1016/S0896-6273(02)00824-3 [DOI] [PubMed] [Google Scholar]
- Porrello E. R., Mahmoud A. I., Simpson E., Hill J. A., Richardson J. A., Olson E. N. and Sadek H. A. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331, 1078-1080 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C., Feng F., Ishikawa K., Bertuzzi S., Dabdoub A., Griffith A. J., Fritzsch B. and Kelley M. W. (2007). Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev. Dyn. 236, 1905-1917 10.1002/dvdy.21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X. and Chen P. (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121-133 10.1016/j.ydbio.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Huang Y., Spencer C. I., Foley A., Vedantham V., Liu L., Conway S. J., Fu J.-d. and Srivastava D. (2012). In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593-598 10.1038/nature11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben R. J. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. Suppl 220, 221-244. [PubMed] [Google Scholar]
- Ryals B. M. and Rubel E. W. (1988). Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240, 1774-1776 10.1126/science.3381101 [DOI] [PubMed] [Google Scholar]
- Sage C., Huang M., Karimi K., Gutierrez G., Vollrath M. A., Zhang D.-S., García-Añoveros J., Hinds P. W., Corwin J. T., Corey D. P. et al. (2005). Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 307, 1114-1118 10.1126/science.1106642 [DOI] [PubMed] [Google Scholar]
- Sage C., Huang M., Vollrath M. A., Brown M. C., Hinds P. W., Corey D. P., Vetter D. E. and Chen Z.-Y. (2006). Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc. Natl. Acad. Sci. USA 103, 7345-7350 10.1073/pnas.0510631103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary E., Hugnot J. P., Chassigneux Y., Travo C., Duperray C., Van De Water T. and Zine A. (2007). Distinct population of hair cell progenitors can be isolated from the postnatal mouse cochlea using side population analysis. Stem Cells 25, 332-339 10.1634/stemcells.2006-0303 [DOI] [PubMed] [Google Scholar]
- Schimmang T. (2007). Expression and functions of FGF ligands during early otic development. Int. J. Dev. Biol. 51, 473-481 10.1387/ijdb.072334ts [DOI] [PubMed] [Google Scholar]
- Schimmang T. and Pirvola U. (2013). Coupling the cell cycle to development and regeneration of the inner ear. Semin. Cell Dev. Biol. 24, 507-513 10.1016/j.semcdb.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Shi F., Hu L. and Edge A. S. B. (2013). Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proc. Natl. Acad. Sci. USA 110, 13851-13856 10.1073/pnas.1219952110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Cheng Y.-F., Wang X. L. and Edge A. S. B. (2010). Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J. Biol. Chem. 285, 392-400 10.1074/jbc.M109.059055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Kempfle J. S. and Edge A. S. B. (2012). Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 32, 9639-9648 10.1523/JNEUROSCI.1064-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Hu L., Jacques B. E., Mulvaney J. F., Dabdoub A. and Edge A. S. B. (2014). beta-Catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 34, 6470-6479 10.1523/JNEUROSCI.4305-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J., Zheng J. L. and Gao W.-Q. (2003). Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci. 23, 169-179 10.1016/S1044-7431(03)00066-6 [DOI] [PubMed] [Google Scholar]
- Sinkkonen S. T., Chai R., Jan T. A., Hartman B. H., Laske R. D., Gahlen F., Sinkkonen W., Cheng A. G., Oshima K. and Heller S. (2011). Intrinsic regenerative potential of murine cochlear supporting cells. Sci. Rep. 1, 26 10.1038/srep00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowik A. D. and Bermingham-McDonogh O. (2013). Hair cell generation by notch inhibition in the adult mammalian cristae. J. Assoc. Res. Otolaryngol. 14, 813-828 10.1007/s10162-013-0414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. B., Kim T., Cabot V. and Hudspeth A. J. (2014). Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 111, E1393-E1401 10.1073/pnas.1318692111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. B., Davies A. L., Battista S., Lewis J. H. and Fekete D. M. (2003). Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 261, 149-164 10.1016/S0012-1606(03)00297-5 [DOI] [PubMed] [Google Scholar]
- Stone J. S. and Cotanche D. A. (2007). Hair cell regeneration in the avian auditory epithelium. Int. J. Dev. Biol. 51, 633-647 10.1387/ijdb.072408js [DOI] [PubMed] [Google Scholar]
- Stone J. S. and Rubel E. W. (1999). Delta1 expression during avian hair cell regeneration. Development 126, 961-973. [DOI] [PubMed] [Google Scholar]
- Tanyeri H., Lopez I. and Honrubia V. (1995). Histological evidence for hair cell regeneration after ototoxic cell destruction with local application of gentamicin in the chinchilla crista ampullaris. Hear. Res. 89, 194-202 10.1016/0378-5955(95)00137-7 [DOI] [PubMed] [Google Scholar]
- Tateya T., Imayoshi I., Tateya I., Hamaguchi K., Torii H., Ito J. and Kageyama R. (2013). Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development 140, 3848-3857 10.1242/dev.095398 [DOI] [PubMed] [Google Scholar]
- Tona Y., Hamaguchi K., Ishikawa M., Miyoshi T., Yamamoto N., Yamahara K., Ito J. and Nakagawa T. (2014). Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC Neurosci. 15, 66 10.1186/1471-2202-15-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M. and Taipale J. (2008). Hedgehog: functions and mechanisms. Genes Dev. 22, 2454-2472 10.1101/gad.1693608 [DOI] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z. P., Kokubu Y., Südhof T. C. and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035-1041 10.1038/nature08797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldaus J., Cimerman J., Gohlke H., Ehrich N., Müller M. and Löwenheim H. (2012). Stemness of the organ of Corti relates to the epigenetic status of sox2 enhancers. PLoS ONE 7, e36066 10.1371/journal.pone.0036066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B. J. and Zuo J. (2013). Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration. Hear. Res. 297, 68-83 10.1016/j.heares.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B. J., Liu Z., Crabtree M., Coak E., Cox B. C. and Zuo J. (2014). Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing. J. Neurosci. 34, 15751-15763 10.1523/JNEUROSCI.3200-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-P., Chatterjee I., Batts S. A., Wong H. T., Gong T.-W., Gong S.-S. and Raphael Y. (2010). Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear. Res. 267, 61-70 10.1016/j.heares.2010.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chai R., Kim G. S., Pham N., Jansson L., Nguyen D.-H., Kuo B., May L. A., Zuo J., Cunningham L. L. et al. (2015). Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat. Commun. (in press) 10.1038/ncomms7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M. E. and Corwin J. T. (1996). Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J. Neurosci. 16, 5466-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M. E., Lambert P. R., Goldstein B. J., Forge A. and Corwin J. T. (1993). Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259, 1619-1622 10.1126/science.8456285 [DOI] [PubMed] [Google Scholar]
- Weber T., Corbett M. K., Chow L. M. L., Valentine M. B., Baker S. J. and Zuo J. (2008). Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proc. Natl. Acad. Sci. USA 105, 781-785 10.1073/pnas.0708061105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. M., Doetzlhofer A., Lee Y. S., Groves A. K. and Segil N. (2006). Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984-987 10.1038/nature04849 [DOI] [PubMed] [Google Scholar]
- Woods C., Montcouquiol M. and Kelley M. W. (2004). Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci. 7, 1310-1318 10.1038/nn1349 [DOI] [PubMed] [Google Scholar]
- Wright T. J. and Mansour S. L. (2003). Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130, 3379-3390 10.1242/dev.00555 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Chang W. and Kelley M. W. (2011). Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev. Biol. 353, 367-379 10.1016/j.ydbio.2011.03.016 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Tanigaki K., Tsuji M., Yabe D., Ito J. and Honjo T. (2006). Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J. Mol. Med. 84, 37-45 10.1007/s00109-005-0706-9 [DOI] [PubMed] [Google Scholar]
- Yang J., Cong N., Han Z., Huang Y. and Chi F. (2013). Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci. Lett. 549, 7-11 10.1016/j.neulet.2013.04.053 [DOI] [PubMed] [Google Scholar]
- Zheng J. L. and Gao W.-Q. (2000). Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580-586 10.1038/75753 [DOI] [PubMed] [Google Scholar]