Abstract
Neutrophil migration to sites of inflammation and the subsequent execution of multiple functions are designed to contain and kill invading pathogens. These highly regulated and orchestrated processes are controlled by interactions between numerous receptors and their cognate ligands. Unraveling and identifying those that are central to inflammatory processes may represent novel therapeutic targets for the treatment of neutrophil-dominant inflammatory disorders in which dysregulated neutrophil recruitment, function, and elimination serve to potentiate rather than resolve an initial inflammatory insult. The first G protein–coupled receptor to be described on human neutrophils, formyl peptide receptor 1 (FPR1), is one such receptor that plays a significant role in the execution of these functions through multiple intracellular signaling pathways. Recent work has highlighted important observations with regard to both receptor function and the importance and functional relevance of FPR1 in the pathogenesis of a range of both sterile and infective inflammatory conditions. In this review, we explore the multiple components of neutrophil migration and function in both health and disease, with a focus on the role of FPR1 in these processes. The current understanding of FPR1 structure, function, and signaling is examined, alongside discussion of the potential importance of FPR1 in inflammatory diseases suggesting that FPR1 is a key regulator of the inflammatory environment.
More than 100 years ago, Metchnikoff described microphagocytes as important responders to sterile injury in starfish larvae.1 Now known to be neutrophils, they are key effector cells of the innate immune system and are pivotal in the containment and clearance of initial noxious stimuli of either infective or sterile origin.2 Accounting for 50% to 70% of circulating human leukocytes, these polymorphonuclear granulocytes patrol the vasculature and rapidly migrate into tissues in response to chemotactic signals.3 On arrival, neutrophils execute a variety of antimicrobial functions, including the generation of reactive oxygen species (ROS), phagocytosis of pathogens and dead and dying tissue, degranulation with the release of a variety of toxic products, expulsion of neutrophil extracellular traps, and paracrine signaling to recruit other cell types.4 Disruption of these processes or overwhelming neutrophil recruitment can cause significant dysregulation of the inflammatory cascade, resulting in failed resolution of inflammation, tissue injury, and acute or chronic disease.
The predominant mechanisms through which neutrophils sense inflammatory stimuli and surrounding environments are a plethora of cell surface receptors principally within the G protein–coupled receptor (GPCR) family. The first GPCR to be described on the human neutrophil was formyl peptide receptor 1 (FPR1) which, when activated, triggers a wide variety of functions, including chemotaxis, degranulation, ROS production, and phagocytosis.5,6 Although descriptions of FPR1 functions in vitro are well documented, increasing attention is being paid to its in vivo role, influence, and importance in the pathogenesis of both infective and sterile acute inflammatory disease.
The principal ligands for FPR1 are bacterial and mitochondrial formylated peptides, actively secreted by invading pathogens or passively released from dead and dying host cells after tissue injury. During infection, pathogens target and destroy host tissue with the simultaneous release of both bacteria-derived (when the pathogen is of bacterial origin) and host-derived formylated peptides (from host mitochondria), thereby linking FPR1 in both infective and sterile inflammatory processes. This review therefore provides an overview of the structure, signaling, and pathological functions of FPR1 as well as a focus on the recent developments in the understanding of formylated peptides and neutrophil FPR1 in the context of infective and sterile inflammation.
Formyl Peptide Receptors
The binding of the N-formyl methionine motifs of bacterial- and mitochondrial-derived peptides to FPR1 was initially described over 3 decades ago and was the starting point for the subsequent dissection of the many G-protein signaling cascades within neutrophils.7,8 The human FPR family constitutes FPR1, FPR2/ALX (lipoxin receptor), and FPR3, which are well-conserved GPCRs that have pluripotent and diverse roles in the initiation, propagation, and resolution of inflammation.9
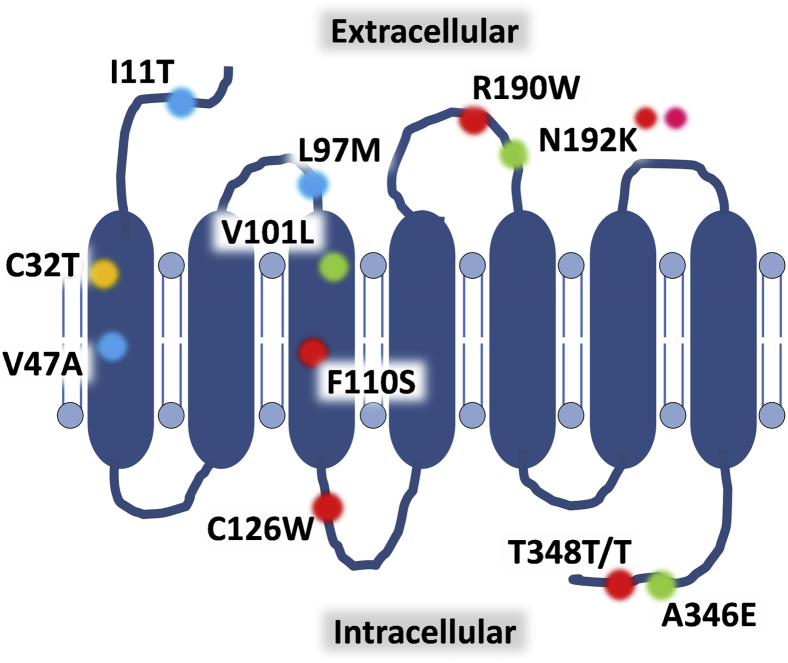
FPR1 was the first neutrophil GPCR to be cloned and sequenced, identifying it as a 350-residue protein with seven hydrophobic segments (Figure 1).5 A subsequent study characterized FPR1 as a seven-transmembrane receptor with the N-terminus and three loops exposed on the cell surface for ligand interactions and the C-terminus and the remaining loops found within the cytoplasm necessary for intracellular signaling.10 Using low-stringency hybridization with FPR cDNA as the probe, two separate but relatively conserved low-affinity receptors, initially termed FPR-like 1 (FPRL1) and FPR-like 2 (FPR2L), were cloned from an mRNA library of neutrophil-like promyelocytic HL-60 cells. These receptors have since been renamed FPR2/ALX and FPR3, respectively, as more has become known about their distinct biochemical and physiological roles.6 All three receptors are clustered together on chromosome 19q13.3 and share significant sequence homology. FPR1 has 69% amino acid identity with FPR2 and 56% with FPR3, whereas FPR2 and FPR3 share 83% identity.11
Figure 1.
Structure of the FPR1 receptor and key polymorphisms within the protein. Formyl peptide receptor 1 (FPR1) as a classic G-protein–coupled receptor has seven transmembrane-spanning regions with an extracellular N-terminus, whereas the C-terminus is found within the cytoplasm. Multiple amino acid substitutions have been found within the protein: recognized constitutive isoforms (green; FPR-26, FPR-98, and FPR-G6), polymorphisms associated with disease states including juvenile and aggressive periodontitis (red), hypertension in young adults (orange), gastric cancer (pink; denotes D192K), and those that have been observed but whose functional significance remains uncertain (blue).
Formyl Peptide Receptor 1
Regulation of FPR1 Expression
Constitutively expressed on the surface of quiescent neutrophils, FPR1 receptor expression is rapidly up-regulated in response to a wide number of inflammatory stimuli. In vitro, these stimuli include lipopolysaccharide (LPS), platelet-activating factor, unmethylated CpG oligodinucleotides, and tumor necrosis factor α12–15; FPR1 receptor up-regulation has also been described in the circulating neutrophils of patients with emphysema, Crohn disease, and sepsis.16–18 Such a rapid increase in FPR1 expression indicates its preformed presence within the cell. Subcellular fractionation and the study of neutrophil transcription within the bone marrow have determined that FPR1 synthesis occurs late in neutrophil maturation, with the receptor subsequently stored in azurophilic granules (also known as primary granules) and secretory vesicles.14,19 In response to agonists such as platelet-activating factor, secretory-vesicle FPR1 alone is mobilized; however, in response to powerful stimuli such as phorbol myristate acetate, azurophilic-granule FPR1 also localizes to the cell surface.14
Alongside FPR1 transport from intracellular compartments, neutrophil activation increases FPR1 protein synthesis. In nonstimulated cells, FPR1 mRNA is unstable, with a t1/2 of approximately 90 minutes.20 After incubation with LPS, increased FPR1 gene transcription occurs along with stabilization of the mRNA transcript (t1/2, approximately 20 hours), allowing for increased synthesis of the receptor. LPS, through myeloid differentiation marker MyD88–dependent signaling pathways, is a powerful inducer of FPR1 synthesis in mature neutrophils, whereas tumor necrosis factor α and other proinflammatory mediators also induce a similar response.21,22 The increases in receptor cycling and protein synthesis therefore increase FPR1 at the cell surface, facilitating the perpetuation of the inflammatory response as greater numbers of receptor are available on each cell to bind free ligand.
Polymorphisms within FPR1
With increasing attention being paid to the role of interindividual differences in response to infection and inflammation, variation in receptor number and function through polymorphisms within the FPR1 gene have been studied. Three isoforms of human FPR1 exist: FPR-26 (V101, N192, E346), FPR-98 (L101, N192, A346), and FPR-G6 (V101, K192, A346). These differ in amino acids 101 (the top of the third transmembrane loop), 192 (center of the second intracellular loop), and 346 (end of the C-terminus) (Figure 1).22,23 Functionally, FPR-98 and FPR-G6 have a partial defect in Gi-protein coupling relative to the more constitutively active FPR-26, with the amino acid glutamic acid at position 346 essential for the G-protein signaling of the latter isoform.24 Alongside these naturally occurring variants of FPR1, at least 30 other single-nucleotide polymorphisms (SNPs) have been described within the FPR1 gene.6
Neutrophils isolated from individuals with localized juvenile periodontitis demonstrate impaired responsiveness to formyl-methyl-leucine-phenylalanine (fMLF; alias fMLP), perhaps explaining increased rates of periodontal infection through reduced migration or impaired bacterial killing capacity.25 SNPs F110S and C126W in the second intracellular loop of the receptor may well account for this phenotype. Furthermore, a T348T/T mutation, although not altering FPR1 receptor number, was associated with impaired fMLF-induced neutrophil chemotaxis and aggressive periodontitis in an African-American population.26 Meanwhile, the association of the C32T SNP with the development of hypertension in younger adults, as well as the D192K polymorphism with the onset of gastric carcinoma in an elderly Japanese population, have also been described, although their mechanistic and functional relevance is yet to be determined.27,28 In contrast, the V101L SNP is associated with increased binding affinity for the FPR1 antagonist cyclosporin H.29 With increasing application of genome-wide association studies, it remains to be seen whether other SNPs are associated with altered susceptibility to inflammatory diseases or infection.
FPR1 Ligands
Although it was initially thought that FPR1 only bound N-formylated peptides,7 it is now widely recognized that the formyl group is not a prerequisite for receptor binding. The N-formylated version of any peptide containing a methionine residue at the 5′ terminus is at least 100-fold more potent than the identical nonformylated peptide. However, if the peptide contains five or more amino acids, the nonformylated moieties can also bind and activate FPR1.23 This finding has been further substantiated by the description of endogenous nonformylated ligands that bind FPR1, including cathepsin G, annexin A1 (AnnA1), and the recently described cytokine family with sequence similarity 19, member A4, although their functional effects differ from those of fMLF/FPR1-described functions (Table 1).34,42 For example, the neutrophil granule protein cathepsin G binding to FPR1 is unable to induce intracellular calcium flux but does, however, result in mitogen-activated protein kinase (MAPK) phosphorylation and neutrophil chemotaxis and dendritic cell recruitment.32 It is postulated that this discrepancy in receptor function is likely attributable to low-affinity binding, thereby inducing isolated components of the inflammatory cascade.10 Similarly, AnnA1 and its N-terminal peptide derivative Ac2-26 bind to FPR1 and induce anti-inflammatory and proresolution effects rather than a proinflammatory response through a variety of effects, including receptor heterodimerization with FPR2.35,42
Table 1.
The Principal Cell Types that Express Human Formyl Peptide Receptors and the Predominant Agonists and Antagonists of the Receptors
| Receptor/cell type | Agonists | Antagonists |
|---|---|---|
| FPR1 | ||
| Neutrophils8 | N-formylated peptides7 | Cyclosporin H30 |
| Macrophages20 | Synthetic fMLF5 | Cyclosporin A29 |
| Monocytes31 | Cathepsin G32 | Boc-MLF (Boc-1)10 |
| Dendritic cells33 | FAM19A434 | Boc-FLFLFL (Boc 2)10 |
| Epithelial cells35 | Annexin A1∗35 | CHIPS9 |
| Hepatocytes10 | Ac2-26∗35 | Spinorphin9 |
| Glial cells10 | ||
| Astrocytes10 | ||
| Keratinocytes36 | ||
| FPR2 | ||
| Neutrophils37 | N-formylated peptides38 | Boc-MLF (Boc-1)10 |
| Macrophages39 | fMLF40 | Boc-FLFLFL (Boc 2)10 |
| Monocytes41 | LL-3741 | WRW4 peptide10 |
| Dendritic cells9 | Serum amyloid A9 | |
| Microglia9 | β-Amyloid9 | |
| T lymphocytes9 | HIV gp41-derived peptide9 | |
| Epithelial cells35 | Annexin A1∗42 | |
| Ac2-26∗42 | ||
| Lipoxin A4∗41 | ||
| Humanin∗43 | ||
| FPR3 | ||
| Macrophages10 | F2L∗44 | WRW4 peptide10 |
| Monocytes10 | Humanin∗43 | |
| Dendritic cells10 | ||
| Eosinophils9 | ||
Ac2-26, N-terminal peptide of annexin 1; Boc-FLFLFL, Boc-Phe-Leu-Phe-Leu-Phe-OH; Boc-MLF, Boc-Met-Leu-Phe-OH; CHIPS, chemotaxis inhibitory protein of Staphylococcus aureus; FAM19A4, family with sequence similarity 19, member A4; fMLF, formyl-methyl-leucine-phenylalanine; FPR, formyl peptide receptor; gp, glycoprotein; LL-37, leucine, leucine 37; WRW4, Trp-Arg-Trp-Trp-Trp-Trp-CONH2 (WRWWWW).
Anti-inflammatory agonist; the ligands described elicit functional responses in a number of cell types.
FPR1 Binding Properties
Much is known about the structure and function of FPR1 at a molecular level and its interaction with the Escherichia coli–derived prototypic synthetic ligand fMLF. Firstly, the formyl group at the N-terminus allows hydrogen bond formation to a hydrogen acceptor within the binding pocket. Secondly, the methionine side chain may occupy a hydrophobic pocket within the receptor, with the sulfur atom in the ligand interacting with a positively charged portion of the receptor. Finally, further hydrophobic interactions may occur between the leucine and phenylalanine residues and FPR1.10 After binding to FPR1, fMLF is internalized within approximately 30 seconds and stimulates a variety of intracellular signaling cascades, resulting in a number of cellular responses. Within neutrophils, these include chemotaxis, ROS production, degranulation, cytokine expression, phagocytosis, and changes in cell surface marker expression.23,45,46
FPR1 Desensitization
As discussed, regulation of GPCR function is essential in vivo for controlling neutrophil functions, including chemotaxis and the exact localization of cells to the inflammatory site.9,23,47 After FPR1-ligand binding, the cell rapidly becomes unresponsive to subsequent stimulation by the same ligand (homologous desensitization). Multiple distinct processes account for this change in cell function, including receptor internalization and desensitization.10 The latter is mediated by phosphorylation of multiple serine and threonine residues within the carboxy-terminal, leading to G-protein dissociation and disruption of intracellular signaling pathways, a process that is governed in part by ERK1/2–GPCR kinase 2 (GPK2) and arrestin binding.48,49 Meanwhile, receptor internalization predominantly occurs after C-terminal phosphorylation through β-arrestin–independent and non–clathrin-mediated endocytosis.6,50
Heterologous desensitization of FPR1 can also occur after activation of CD88 [a complement component 5a (C5a) receptor] or chemokine (C-X-C motif) receptor 2 CXCR2; (an IL-8 receptor) due to shared components of intracellular signaling molecules and occurs principally through protein kinase C–mediated pathways.51 Due to the intracellular signaling hierarchy that exists, however, the capacity for FPR1's heterologous desensitization of other receptors far outstrips their reciprocal ability to inhibit FPR1 function,52 which was recently highlighted by the observation that the activation of FPR1 in monocytes induces cross-desensitization of CCR1 but not CCR2.52 This observation suggests the ability of FPR1 to act as a regulator of chemoreceptor hierarchy with regard to prioritizing not only its own signaling but also that of other potent and important agonists, such as those that bind CCR2.53 Importantly, these reductions in cell surface receptor number and function can be relatively short-lived, as receptor and ligand dissociate from each other after internalization and the receptor returns to the cell surface available for further ligand binding.
Intracellular Signaling
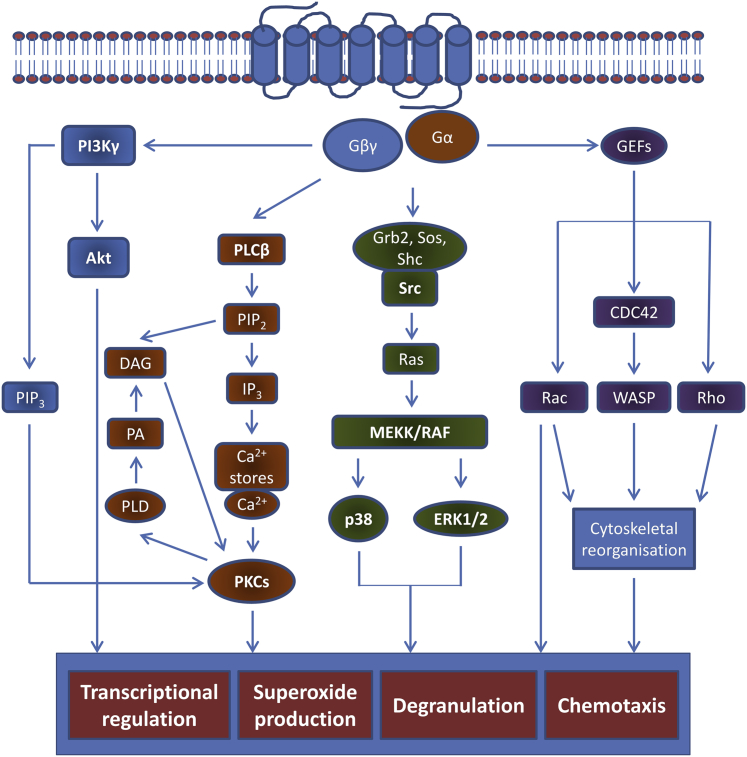
Characterization of the FPR1 signaling cascades has been conducted with fMLF as the stereotypical ligand and has revealed a highly complex and integrated chain of intracellular signaling events.10,23 Binding to FPR1 results in Gi-type G-protein activation, with the conversion of GDP to GTP inducing the dissociation of α from the βγ subunits (Figure 2). The latter liberated subunits activate both the phospholipase Cβ and phosphoinositide 3-kinase γ (PI3Kγ) signaling cascades. Phospholipase Cβ hydrolyses membrane-bound phosphoinositol-4,5-bisphosphate into diacylglycerol and inositol trisphosphate to mediate the release of intracellular calcium stores, principally from the endoplasmic reticulum. These events subsequently activate protein kinase C and are central to NADPH oxidase ROS production.54 Meanwhile, PI3Kγ-mediated conversion of phosphoinositol-4,5-bisphosphate to phosphoinositol-3,4,5-trisphosphate acts as the principal regulator of neutrophil cytoskeletal reorganization and respiratory burst after FPR1 activation as well as influences the chemotactic response.10,47
Figure 2.
Intracellular signaling events after FPR1 receptor activation. After binding of ligand to formyl peptide receptor 1 (FPR1), conversion of guanosine diphosphate to guanosine triphosphate induces dissociation of the α from the βγ subunits. These trigger a range of intracellular kinase pathways, resulting in the induction of a variety of cell functions, including neutrophil chemotaxis, degranulation, superoxide anion production, and transcriptional activity. The predominant signaling pathways are those of phosphoinositide-3 kinase (PI3K), mitogen-activated protein kinase (MAPK), and phospholipase C. The latter triggers release of intracellular calcium from the endoplasmic reticulum to activate protein kinase C (PKC) and subsequent reactive oxygen species production, whereas PI3K triggers protein kinase B (alias Akt)-mediated and phosphoinositol-3,4,5-trisphosphate (PIP3)-mediated signaling, with a variety of cellular effects. With PI3K pulled toward the plasma membrane by the β and γ G-protein subunits (Gβ and Gγ), activation of Ras family proteins and MAPK further contributes to oxidative burst and chemotaxis. CDC, cell division control protein; DAG, diacyl glycerol; Gα, G-protein α; GEF, guanine nucleotide exchange factor; Grb, growth factor receptor–bound protein; IP3, inositol trisphosphate; MEKK, mitogen-activated protein kinase kinase; PA, phosphatidic acid; PLCβ, phospholipase Cβ; PLD, phospholipase D; RAF, rapidly accelerated fibrosarcoma; Sos, son of sevenless; WASP, Wiskott-Aldrich syndrome protein.
As the βγ subunits activate PI3Kγ and pull it toward the plasma membrane, the activity of Src-like tyrosine kinases increases with the phosphorylation of the Src homology 2 domain–containing (Shc) adaptor protein, which, in turn, increases the association with growth factor receptor–bound protein 2 and son of sevenless and subsequently activates MAPK signaling pathways. ERK and p38 MAPK predominantly influence chemotaxis and FPR1-mediated transcriptional activity.10 Meanwhile, the activation of guanine–nucleotide exchange factors induces the activation of the Rho GTPases [Rho, Rac, and cell division control protein 42 (CDC42)]. These, in turn, regulate ROS production through control of the formation of NADPH oxidase complex6 as well as influence leukocyte adhesion, transmigration, actin polymerization, and phagocytosis.55
Although much of this work is well recognized, recent work using antibodies specific to C-terminal phosphorylation sites within the FPR1 protein has demonstrated that in some cases, in particular in the context of inflammatory bowel disease, G-protein–insensitive, formylated peptide–dependent FPR1 phosphorylation may also occur. This observation was noted to be predominantly at the surface of colonic crypt abscesses, leading Leoni et al56 to postulate that this possible reactivation of FPR1 at distinct sites of inflammation may contribute to perpetuation of the acute and often deleterious inflammatory response.
New facets of the conventional understanding of FPR1 signaling within neutrophils have begun to emerge in recent years, for example, the appreciation of its interconnection with autocrine release of ATP and subsequent purinergic signaling. Recent work by Bao et al57 demonstrated that FPR1 signaling induces a rapid, but reversible, increase in the mitochondrial membrane potential within neutrophils, with an associated oxidative burst and extracellular release of ATP through pannexin 1 channels. Despite the conventional understanding that neutrophil-mediated ATP production occurs through glycolysis, researchers demonstrate that the initial burst of ATP is mitochondria derived and important in initiating the FPR/purinergic response. The autocrine ATP appears to then bind purinergic P2Y2 and P2X receptors to augment neutrophil chemotaxis, intracellular calcium flux, and p38 and ERK p42/44 MAPK signaling but appears to have little effect on fMLF-mediated degranulation and phagocytosis. Interestingly, pannexin 1 channels and the purinergic receptors accumulate at the leading edge of the polarized neutrophil, providing opportunity for coordinated migratory activity.57–59
Also, localizing to the leading edge of the migrating neutrophil is the receptor of urokinase-type plasminogen activator serine protease to facilitate urokinase-type plasminogen activator–mediated degradation of the extracellular matrix, thus allowing for extravascular neutrophil transit. It is understood that urokinase-type plasminogen activator receptor expression is necessary for fMLF-induced chemotaxis through a variety of means, including the colocalization of FPR1 and β1-integrins at the cell surface, with urokinase-type plasminogen activator receptor acting as a bridging molecule to facilitate their respective functions.60 These novel observations, therefore, provide further insight into the highly regulated and integrated crosstalk between different ligands and their receptors in fine-tuning the functional response to inflammatory agents.
Formyl Peptide Receptor 2
In contrast to the specificity of FPR1, its relative FPR2/ALX is a highly promiscuous receptor that binds fMLF with low affinity.40 It was one of the first descriptions of a receptor capable of binding lipids, peptides, and proteins with ligands including serum amyloid A, lipoxin A4, and AnnA1 and has been reviewed in detail elsewhere.9,37,61 Importantly, these ligand-specific interactions are able to induce either proinflammatory or proresolution/anti-inflammatory effects. With regard to binding to formylated peptides, the binding affinity of FPR2 is determined principally by the charge of the C-terminus of peptide, contrary to FPR1 which, as discussed in FPR1 Ligands, is N-terminal dependent.
Binding of serum amyloid A or the cathelicidin-associated antimicrobial peptide leucine, leucine-37 (LL37) to FPR2/ALX results in proinflammatory responses with neutrophil NF-κB activation and cytokine release, increased neutrophil recruitment to sites of inflammation, and increased neutrophil lifespan.41,62 In contrast, binding of AnnA1 inhibits neutrophil migration, promotes neutrophil apoptosis, increases the rate of macrophage phagocytosis of apoptotic cells, and skews the macrophages toward a less proinflammatory phenotype.39 Lipoxin A4, again through FPR2/ALX, inhibits neutrophil migration while concomitantly augmenting monocyte recruitment. These distinct effects elicited from ligand binding to the same receptor have recently been attributed to different dimerization states after agonist binding that alter receptor conformation and subsequent intracellular signaling.42
Formyl Peptide Receptor 3
Distinct from the other members of the FPR family, the function of FPR3 remains relatively poorly understood. While not expressed on human neutrophils, it is found in eosinophils, monocytes, macrophages, and dendritic cells, leading to speculation that it may play a role in the pathogenesis of allergic disease.63 FPR3 is relatively insensitive to formylated peptides, and few specific endogenous ligands have been identified. F2L, an endogenous 21–amino acid acetylated amino-terminal peptide, is the most specific ligand described to date.64 Derived from cleavage of heme-binding protein 1 by cathepsin D, F2L activates FPR3 in low nanomolar concentrations.44 In doing so, it induces monocyte intracellular calcium flux, ERK1/2 phosphorylation, and chemotaxis while also augmenting LPS-mediated IL-12 production in dendritic cells, thereby inhibiting their maturation.33,64 Humanin, a neuroprotective peptide, also binds with high affinity to both FPR2 and FPR3.43 Despite the high sequence homology with FPR2/ALX, the behavior of FPR3 is surprising, with significantly higher basal levels of receptor phosphorylation and internalization and relative insensitivity to common FPR2/ALX ligands.65 This observation has led to the hypothesis that it may also act as a decoy receptor to bind extracellular ligands, thereby regulating the function of other formylated peptide receptors.65 Although there is likely to be some functional overlap with FPR2/ALX, the true functional role of FPR3 and its relevance in vivo remain to be determined.
Mouse FPR Receptors
FPR1 has been described across several species, including horse, rabbits, and rodents, with marked differences in functional responses to formylated peptides observed.6 In comparison to the three FPR receptors described in humans, the mouse genome encodes multiple FPR-related receptors from chromosome 17A3.2.66 Fpr1 is the murine orthologue of human FPR1, sharing 77% homology, expression on similar cell types, and induction of the same effects of neutrophil chemotaxis, degranulation, cytokine production, and phagocytosis.67 Genes Fpr2 and Fpr3 together encode receptors that mimic human FPR2/ALX. Fpr2 encodes the ALX receptor specific for lipoxin A4, whereas Fpr3 encodes Fpr2, which binds formylated peptides, serum amyloid A, and other similar ligands.6 Similarities between murine Fpr2 and human FPR3 with regard to responsiveness to F2L have also been observed.68 At present, the function and cognate ligands of the remaining murine Fpr receptors are poorly characterized and understood. Fpr-related sequences 3, 4, 6, and 7 may function as chemoreceptors in vomeronasal olfactory sensory neurons,69 whereas Fpr-related sequence 8 appears to affect the lifespan of mice, although the reason for this remains unknown at present.66
Although there is relatively high sequence homology of FPR1 between humans and mice, and the intracellular domain structure is highly conserved, there are distinct differences in the affinity of murine Fpr1 for fMLF, which is approximately 100-fold less than that of its human counterpart.68 This difference in affinity is attributed to alterations in the folding of the transmembrane and extracellular domains, as determined by the apposition of multiple noncontiguous residues.70 Although differences in the affinity of a receptor to prototypic E. coli–derived fMLF are described, it should be noted that, with regard to other bacterial formylated peptides, murine Fpr1 remains a high-affinity receptor, in particular to Staphylococcus aureus, Listeria monocytogenes, and mitochondria-derived formylated peptides.68 Although it is important to be aware of such differences, inferences from mouse models of disease remain relevant and have, to date, significantly advanced our understanding of FPR biology and neutrophil function in both the physiological and pathophysiological states.
FPR1 in Health and Disease
Mitochondrial Formylated Peptides
With regard to neutrophil FPR1 function, the principal proinflammatory effects are mediated through bacterial and mitochondrial formylated peptides. Given the increasing interest in the role of FPR1 in sterile inflammation, we focus on the synthesis, release, and function of the latter in greater detail. It has been proposed that mitochondria arose over 2 billion years ago after the inclusion of α-proteobacteria within the ancient precursors of eukaryotic cells. Much of the initial genetic material contained within these bacteria has either been lost or transferred to the nuclear genome, which now encodes the vast majority of the approximately 1500 mitochondrial proteins.71 What has been left, however, is approximately 16 Kb of CpG-rich, unmethylated mitochondrial DNA (mtDNA), which encodes for 13 mitochondrial peptides that make up the key components of the respiratory chain within the inner mitochondrial membrane. In addition, mtDNA also contains coding information of 22 tRNAs and two mitochondrial ribosome (mitoribosome)-coding RNAs, vital for the processes of translation and peptide synthesis.72
Distinct from eukaryotic cytoplasmic ribosomes, prokaryotic and mitochondrial protein synthesis require an N-terminal fMet residue to initiate translation. Mitoribosomes are located in the mitochondrial matrix, with approximately 100 per organelle, and contain 39S and 28S ribosomal subunits.72 Unlike cytoplasmic mRNA, mitochondrial mRNA has no upstream leader sequence to coordinate mitoribosome binding but instead starts at the 5′ end, with the N-terminal fMet binding to the mRNA start codon. N-terminal fMet binding to the small (28S) ribosome is facilitated by mitochondrial translational initiation factor 2 in a GTP-dependent process. Importantly, it is only N-terminal fMet and not Met-tRNA that is recognized by mitochondrial translational initiation factor 2 as the initiator tRNA, hence the crucial and irreplaceable nature of fMet in mitochondrial peptide synthesis.73
Although extracellular mitochondrial formylated peptides are recognized as potent damage-associated molecular patterns, within mitochondria they are naturally subjected to post-translational modification. Some peptides, however, undergo removal of the formyl group which principally occurs through the action of peptide deformylase, a zinc- and iron-binding metalloproteinase that hydrolytically cleaves the N-terminal formyl moiety.74 Inhibition of peptide deformylase causes a global reduction in the accumulation of mtDNA-encoded proteins, with impaired subsequent assembly of components of the respiratory chain and therefore mitochondrial energy production.75 Despite these observations, the exact role and contribution of peptide deformylase and the balance between mature formylated and deformylated peptides within the mitochondria remain poorly understood. Importantly, it appears that peptide deformylase is not capable of deformylating all mitochondria-synthesized peptides.76 Indeed, deformylation does not completely remove the presence of formylated peptides within eukaryotic cells, as both formylated and deformylated versions of the same peptides coexist.75 It is also interesting to note that extracellular deformylase enzymes within rat intestinal mucosa that sequentially cleave bacterial formylated peptides to reduce the abundance of proinflammatory mediators have been described.77
At present, there is no direct evidence of active release of mitochondrial formylated peptides from cells; rather, it is considered a passive process after necrotic cell death. This method of release is obviously distinct from bacteria, which can actively secrete formylated peptides into the extracellular milieu.78 Given the description of autophagy-mediated high-mobility group box protein 1 (HMGB1), IL-1β, mtDNA, and ATP release, it is not inconceivable that formylated peptides are also actively released by similar mechanisms.79
FPR1 in Sterile Inflammation
The importance of mitochondrial formylated peptides in influencing neutrophil function was originally described by Carp,7 who demonstrated their ability in vitro, distinct from nonformylated mitochondrial proteins, to induce neutrophil chemotaxis through binding to FPR1. This finding was substantiated by the subsequent demonstration of their ability to induce neutrophil-like promyelocytic HL-60 cell intracellular calcium flux and superoxide anion production through binding to both FPR1 and FPR2/ALX.38 Further appreciation of their in vivo significance as important damage-associated molecular patterns came with the description of elevated mtDNA levels, as a general marker of liberated mitochondrial products, in the circulation of patients with aseptic, trauma-induced systemic inflammatory response syndrome.30 Alongside this observation, Zhang et al30 demonstrated the FPR1-dependent peritoneal neutrophil recruitment after i.p. injection of isolated mitochondrial damage-associated molecular patterns. Furthermore, they were able to induce alveolar neutrophil accumulation and pulmonary extravascular leak after i.v. administration of mitochondrial damage-associated molecular patterns in rats. Together with further in vitro demonstration of mitochondrial damage-associated molecular patterns–induced FPR1-dependent chemotaxis, intracellular calcium flux, and degranulation, they provided the first robust evidence of the in vivo importance of mitochondrial formylated peptide–mediated neutrophil activation and migration in driving local and distant inflammation.30,80,81 These findings have subsequently been supported by the discovery that, in a mouse model of paracetamol-induced liver injury in which increased levels of circulating mtDNA were detected, dual blockade of FPR1 and chemokine (C-X-C motif) receptor 2 attenuated both local hepatotoxicity and distant neutrophil migration into the lung.82
Alongside these important observations, the role of mitochondrial formylated peptides was further emphasized in eloquent work using a mouse model of focal heat–induced sterile liver injury and studied with spinning-disk intravital microscopy.83 After hepatic necrosis, intravascular gradients of chemokines such as macrophage inhibitory protein 2, interacting with chemokine (C-X-C motif) receptor 2, were shown to guide neutrophils crawling along the vascular endothelium to regions of sterile inflammation. Having arrived within the vascular system, neutrophils then sensed formylated peptides, exiting the capillary bed and moving through the tissue toward the area of necrosis in an FPR1-dependent manner. This observation was confirmed by the nondirectional migration of FPR1−/− neutrophils within the tissue and their failure to enter the necrotic zone, distinct from wild-type neutrophils, which rapidly accumulated within the necrotic area generated by the thermal injury.83 Importantly, this appears not to be an organ-specific phenomenon, as the same hierarchy of chemotactic signals was observed within areas of sterile skin inflammation even though the route of neutrophil migration was predominantly interstitial rather than intravascular.84
Understanding of the neutrophil response to infection explains the potentially significant role of mitochondrial formylated peptides in the inflammatory process. As neutrophils are essential for bacterial clearance, they need to rapidly migrate through tissues and localize invading pathogens by responding to bacteria-derived chemoattractants, including formylated peptides, and not be distracted by endogenous factors contained within the surrounding inflammatory milieu.85 The dominant role of bacterial formylated peptides, in the presence of other competing mediators, in driving neutrophil chemotaxis has been recently described.47 Interstitial neutrophil migration is mediated by PI3K and p38 MAPK signaling pathways, with the latter the more dominant process. In an environment of multiple signaling mediators, neutrophil PI3K is inactivated by phosphatase and tensin homologue, allowing p38 and phospholipase A2 to drive neutrophil migration toward bacterial products.47 In Pten−/− mice, this effect is lost and neutrophils fail to prioritize and become distracted by other chemokines, leading to impaired neutrophil migration with a subsequent reduction in bacterial clearance in vivo.47 As mitochondrial formylated peptides appear to exert a similarly dominant hierarchical effect on neutrophil migration and function, their putative importance in the pathogenesis of sterile inflammation and tissue injury is clear.86
FPR1 and Infection
The classic description of the role of FPR1 is in the migration of neutrophils into sites of infection for the subsequent containment and killing of the microorganism. Although this role had been suggested from in vitro observations,83 it was the generation of the Fpr1−/− mouse that allowed for the demonstration of this role in vivo.87 Replacement of a 150-bp sequence with a neomycin-resistance cassette of the Fpr1 gene, within the part encoding for the first extracellular loop to the fourth transmembrane domain, allowed for the complete absence of Fpr1 expression at both the protein and mRNA levels. The neutrophils were shown to be completely unresponsive to formylated peptides with regard to intracellular calcium flux as well as chemotaxis both in vitro and in vivo.87 Injection of i.v. L. monocytogenes resulted in increased bacterial load in both the liver and spleen of Fpr1−/− mice relative to wild-type mice, attributable to reductions in neutrophil migration and diminished ROS production.46,87 Furthermore, Streptococcus pneumoniae meningeal infection is associated with poorer outcome in Fpr1−/− animals but, interestingly, a paradoxical increase in neutrophil number within the brain.88 Consistent with FPR1 having a central role in pathogen containment, S. aureus secretes an FPR1 inhibitory protein as a potential immune-evasion strategy.89
In contrast, within the lung, susceptibility to pulmonary S. pneumoniae infection is not increased in Fpr1−/− mice or in wild-type animals treated with pharmacological inhibitors of FPR1, such as cyclosporin H.90,91 Indeed, a reduction in neutrophil number after pharmacological FPR1 inhibition has instead been demonstrated to have no effect on bacterial burden.91 In a model of bacterial endotoxin-mediated lung injury, Fpr1−/− mice had a reduction in alveolar, interstitial, and circulating neutrophil numbers, as well as extravascular leak, 4 hours after injury with nebulized LPS.92 Whether these seemingly paradoxical observations are due to organ, pathogen, or model-specific differences remains to be seen.
Cigarette smoke contains a wide variety of bacterial products, including LPS and formylated peptides.93 Demonstrating the importance of FPR1 in both the initiation and propagation of pulmonary inflammation, Cardini et al94 found that wild-type mice chronically exposed to cigarette smoke over a 7-month period developed characteristic emphysematous changes. In contrast, Fpr1−/− animals were protected from emphysema, suggesting that the formylated peptides within cigarette smoke are central to disease pathogenesis. A similarly beneficial reduction in inflammatory response, with fewer migrating neutrophils and macrophages and lower proinflammatory cytokine levels, was observed after acute or subacute exposure (1 to 3 days) to cigarette smoke.94 Whether the chronic changes observed are attributable to an alteration in initial inflammatory cell phenotype or are a result of a modulation of the chronic inflammatory response through both innate and adaptive immune response remains to be elucidated.
Further novel perspectives on the role of FPR1 in infection have come through the description of the essential role of neutrophil-derived IL-1β in abscess formation and bacterial clearance after cutaneous S. aureus infection.95 Alongside animals deficient in Toll-like receptor (Tlr) 2 and nucleotide-binding oligomerization domain (Nod) 2, Fpr1−/− mice were noted to have a reduced neutrophil number at the site of infection, with an associated reduction in IL-1β release after direct bacterial infection in vitro, suggesting another mechanism through which this GPCR exerts its antibacterial functions.95
FPR1 in Other Cell Types
The most frequently described roles for FPR1 are with regard to neutrophil chemotaxis, degranulation, and ROS production, but FPR1 activation also exerts effects in other cell types. In isolated human monocytes, FPR1 activation augments IL-8 release in a manner cooperative with other mitochondrial damage-associated molecular patterns31 while also influencing dendritic cell maturation, migration, and phenotype.33,96 Importantly, the number of FPR1 receptors on the macrophage surface appears linked to their phenotype, at least in vitro, as treatment with cytokines associated with inducing an alternatively activated phenotype (IL-4 and IL-13) reduced FPR1 through Stat6-dependent mechanisms, whereas interferon γ (associated with a classically activated phenotype) increased receptor expression.97,98 Importantly, in the former group, fMLF-induced migration was completely abrogated, suggesting regulation of cell migration into the inflammatory environment by autocrine and paracrine manipulation of GPCR expression.
In addition to the direct regulation of immune cells, the role of bacteria-derived formylated peptides in mediating nociceptive responses to infection has recently been delineated. S. aureus skin infection causes hyperalgesic responses that were found to be independent of infiltrating immune cells but instead dependent on formylated peptides and the bacterial pore-forming toxin α-hemolysin, with protection in Fpr1−/− mice noted.99 Interestingly, inhibition of nociceptor responses led to enhanced local inflammation, demonstrating that formylated peptides can have indirect immune-modulatory properties acting via neuronal cell types.99
Recent evidence has also emerged of a protective role for FPR1 in the maintenance of epithelial integrity and mucosal repair after colonic injury in vivo. Indeed, formylated peptides released by commensal bacteria within the colon promote enterocyte migration and proliferation through FPR1 and NADPH oxidase 1.100 Furthermore, binding of AnnA1 and Ac2-26, its N-terminal derivative, to epithelial FPR1 results in Src kinase and NADPH oxidase 1 activation and increased ROS production. Subsequent oxidative inactivation of regulatory phosphatases, phosphatase and tensin homologue, and protein tyrosine phosphatase containing proline, glutamic acid, serine, and threonine residues (PTP-PEST) leads to paxillin and focal adhesion kinase–mediated epithelial migration and wound closure.35 In vivo, delivery of exogenous AnnA1 resulted in accelerated recovery after chemical-induced colitis.35
Despite this latter observation, a similarly important role for FPR1 has been seen in vitro in human bronchial epithelial cell migration, with an FPR1-dependent response to mitochondrial formylated peptides observed after linear scratch injury to cells in culture.101 The in vivo corollary and functional relevance of FPR1-mediated pulmonary epithelial homeostasis is, however, yet to be established.101 Additional evidence for the importance of Fpr1 in epithelial homeostasis lies in the observation that Fpr1−/− mice develop spontaneous lens degeneration in the absence of any preceding inflammatory events,102 whereas the presence of formylated peptides accelerates human retinal pigment epithelial cell migration after injury.103 Conversely, up-regulation of expression in malignant cells may confer a more aggressive and invasive phenotype due to an increased propensity of cells to migrate.104
In keratinocytes, FPR1 has been shown, within specific clinical contexts, to play an important role in the control of necroptosis (caspase-independent programed cell death). In patients with severe blistering skin conditions, such as Stevens-Johnson syndrome, keratinocytes, when cultured in vitro, were sensitive to the FPR1 agonists fMLF and AnnA1, resulting in necroptosis through ROS production and receptor-interacting protein.36 The limited nature of this observation is attributed to differences in inducible expression of FPR1, which is greater in Stevens-Johnson syndrome than in other similar dermatological conditions despite no differences in promoter sequence.36 This role in keratinocyte death is in contrast to the proposed beneficial effects of FPR1,35 in particular on binding AnnA1, in the maintenance of gastrointestinal epithelial integrity and warrants further investigation.
Inhibition of FPR1
Accompanying the increased understanding of the pathophysiological role of FPR1 is the appreciation of its potential as a therapeutic target in chronic inflammatory disorders or acute sterile inflammation either through the use of agonists or antagonists, depending on the context. With regard to pharmacological antagonists, there are, at present, limited FPR1-specific compounds available. Initially, Boc-Met-Leu-Phe-OH (Boc-MLF; alias Boc-1) and subsequent Boc-Phe-Leu-Phe-Leu-Phe-OH (Boc-FLFLF; alias Boc-2) were described,105 but their relative low potency and lack of specificity given associated FPR2 inhibition have led to cyclosporin H, an inverse agonist to FPR1, being the more readily used agent in preclinical studies.80,83,91,94 Its specificity is limited by the off-target effects on calcium/calmodulin–dependent phosphorylation of elongation factor 2.106 Studies that are based on observations made entirely using these compounds should therefore be interpreted with caution and need to be accompanied by adjunctive demonstrations of FPR1 inhibition with monoclonal antibodies or, in the murine context, the use of Fpr1 knockout animals.
Emphasis has therefore shifted to the development of alternative synthetic peptide ligands and small molecules, agonists and antagonists that were recently reviewed elsewhere.106 Several synthetic ligands inhibiting fMLF-induced neutrophil chemotaxis have been described107,108; however, their inhibitory effects may be through the inhibition of downstream signaling pathways rather than direct FPR1 antagonism. Given difficulties in the synthesis and delivery of peptides, a complementary focus has been on the development of small-molecule antagonists and the use of high-throughput screening and structure–activity relationship–directed design and synthesis.109,110 Notably, this approach has recently identified particular chromones and isoflavones that are selective for FPR1 and do not alter FPR2-mediated ERK phosphorylation and intracellular calcium flux.111
Conclusion
FPR1, as a stereotypical GPCR, has facilitated significant advances in our understanding of receptor signaling and control of multiple cell functions at a single-cell level. It has, however, been the identification of surrogate biomarkers of the release of mitochondrial products (elevated plasma mtDNA in trauma, sepsis, and systemic inflammatory response syndrome), the development and application of transgenic mouse models into a variety of disease models, and the awareness of FPR1 functions in nonmyeloid cells that has now firmly established the functional importance of FPR1 in the pathophysiology of a plethora of inflammatory diseases. Whether FPR1 antagonism, which interferes with the bactericidal capacity of neutrophils, risks precipitating or exacerbating intercurrent infection remains to be shown in appropriate animal models. However, in the context of the many neutrophil-dominant sterile inflammatory disease processes that affect humans, FPR1 antagonism may prove beneficial. Therefore, the development of small-molecule antagonists and the appreciation of its ligand-dependent and potential proresolution effects have placed FPR1 in the arena of attractive novel therapeutic targets.
Footnotes
Supported by Wellcome Trust grants WT096497 and WT094415 (D.A.D. and C.D.L.) and by Medical Research Council grant MR/K013386/1 (C.H. and A.G.R.).
Disclosures: None declared.
References
- 1.Cavaillon J.M. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol. 2011;90:413–424. doi: 10.1189/jlb.0211094. [DOI] [PubMed] [Google Scholar]
- 2.Jaillon S., Galdiero M.R., Del Prete D., Cassatella M.A., Garlanda C., Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol. 2013;35:377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 3.Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 4.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 5.Boulay F., Tardif M., Brouchon L., Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990;168:1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- 6.Ye R.D., Boulay F., Wang J.M., Dahlgren C., Gerard C., Parmentier M., Serhan C.N., Murphy P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffmann E., Corcoran B.A., Wahl S.M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufton N., Perretti M. Therapeutic anti-inflammatory potential of formyl-peptide receptor agonists. Pharmacol Ther. 2010;127:175–188. doi: 10.1016/j.pharmthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Rabiet M.J., Huet E., Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao L., Gerard N.P., Eddy R.L., Jr., Shows T.B., Gerard C. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi F., Means T.K., Luster A.D. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 13.Kitchen E., Rossi A.G., Condliffe A.M., Haslett C., Chilvers E.R. Demonstration of reversible priming of human neutrophils using platelet-activating factor. Blood. 1996;88:4330–4337. [PubMed] [Google Scholar]
- 14.Sengeløv H., Boulay F., Kjeldsen L., Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J. 1994;299:473–479. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Flaherty J.T., Rossi A.G., Redman J.F., Jacobson D.P. Tumor necrosis factor-alpha regulates expression of receptors for formyl-methionyl-leucyl-phenylalanine, leukotriene B4, and platelet-activating factor. Dissociation from priming in human polymorphonuclear neutrophils. J Immunol. 1991;147:3842–3847. [PubMed] [Google Scholar]
- 16.Anton P.A., Targan S.R., Shanahan F. Increased neutrophil receptors for and response to the proinflammatory bacterial peptide formyl-methionyl-leucyl-phenylalanine in Crohn’s disease. Gastroenterology. 1989;97:20–28. doi: 10.1016/0016-5085(89)91410-8. [DOI] [PubMed] [Google Scholar]
- 17.Stockley R.A., Grant R.A., Llewellyn-Jones C.G., Hill S.L., Burnett D. Neutrophil formyl-peptide receptors. Relationship to peptide-induced responses and emphysema. Am J Respir Crit Care Med. 1994;149:464–468. doi: 10.1164/ajrccm.149.2.8306047. [DOI] [PubMed] [Google Scholar]
- 18.Tennenberg S.D., Solomkin J.S. Neutrophil activation in sepsis: The relationship between fmet-leu-phe receptor mobilization and oxidative activity. Arch Surg. 1988;123:171–175. doi: 10.1001/archsurg.1988.01400260051005. [DOI] [PubMed] [Google Scholar]
- 19.Cowland J.B., Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999;66:989–995. doi: 10.1002/jlb.66.6.989. [DOI] [PubMed] [Google Scholar]
- 20.Mandal P., Novotny M., Hamilton T.A. Lipopolysaccharide induces formyl peptide receptor 1 gene expression in macrophages and neutrophils via transcriptional and posttranscriptional mechanisms. J Immunol. 2005;175:6085–6091. doi: 10.4049/jimmunol.175.9.6085. [DOI] [PubMed] [Google Scholar]
- 21.Hirotani T., Yamamoto M., Kumagai Y., Uematsu S., Kawase I., Takeuchi O., Akira S. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem Biophys Res Commun. 2005;328:383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 22.Boulay F., Tardif M., Brouchon L., Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 23.Prossnitz E.R., Ye R.D. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel-Seifert K., Seifert R. Functional differences between human formyl peptide receptor isoforms 26, 98, and G6. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:509–515. doi: 10.1007/s00210-003-0714-7. [DOI] [PubMed] [Google Scholar]
- 25.Gwinn M.R., Sharma A., De Nardin E. Single nucleotide polymorphisms of the N-formyl peptide receptor in localized juvenile periodontitis. J Periodontol. 1999;70:1194–1201. doi: 10.1902/jop.1999.70.10.1194. [DOI] [PubMed] [Google Scholar]
- 26.Maney P., Walters J.D. Formylpeptide receptor single nucleotide polymorphism 348T>C and its relationship to polymorphonuclear leukocyte chemotaxis in aggressive periodontitis. J Periodontol. 2009;80:1498–1505. doi: 10.1902/jop.2009.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Shamieh S., Herbeth B., Azimi-Nezhad M., Benachour H., Masson C., Visvikis-Siest S. Human formyl peptide receptor 1 C32T SNP interacts with age and is associated with blood pressure levels. Clin Chim Acta. 2012;413:34–38. doi: 10.1016/j.cca.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Otani T., Ikeda S., Lwin H., Arai T., Muramatsu M., Sawabe M. Polymorphisms of the formylpeptide receptor gene (FPR1) and susceptibility to stomach cancer in 1531 consecutive autopsy cases. Biochem Biophys Res Commun. 2011;405:356–361. doi: 10.1016/j.bbrc.2010.12.136. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C., Zhou Y., Wang J., Feng Y., Wang H., Xue J., Chen Y., Ye R.D., Wang M.W. V101L of human formyl peptide receptor 1 (FPR1) increases receptor affinity and augments the antagonism mediated by cyclosporins. Biochem J. 2013;451:245–255. doi: 10.1042/BJ20121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crouser E.D., Shao G., Julian M.W., Macre J.E., Shadel G.S., Tridandapani S., Huang Q., Wewers M.D. Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit Care Med. 2009;37:2000–2009. doi: 10.1097/CCM.0b013e3181a001ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun R., Iribarren P., Zhang N., Zhou Y., Gong W., Cho E.H., Lockett S., Chertov O., Bednar F., Rogers T.J., Oppenheim J.J., Wang J.M. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 33.Kang H.K., Lee H.Y., Kim M.K., Park K.S., Park Y.M., Kwak J.Y., Bae Y.S. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. J Immunol. 2005;175:685–692. doi: 10.4049/jimmunol.175.2.685. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Li T., Wang X., Yuan W., Cheng Y., Zhang H., Xu E., Zhang Y., Shi S., Ma D., Han W. FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cell Mol Immunol. 2014 Aug 11 doi: 10.1038/cmi.2014.61. 10.1038/cmi.2014.61 doi: [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leoni G., Alam A., Neumann P.A., Lambeth J.D., Cheng G., McCoy J., Hilgarth R.S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Neish A.S., Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito N., Qiao H., Yanagi T., Shinkuma S., Nishimura K., Suto A., Fujita Y., Suzuki S., Nomura T., Nakamura H., Nagao K., Obuse C., Shimizu H., Abe R. An annexin A1–FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014;6:245ra95. doi: 10.1126/scitranslmed.3008227. [DOI] [PubMed] [Google Scholar]
- 37.Bozinovski S., Anthony D., Anderson G.P., Irving L.B., Levy B.D., Vlahos R. Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways. Pharmacol Ther. 2013;140:280–289. doi: 10.1016/j.pharmthera.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Rabiet M.J., Huet E., Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Cai L., Wang H., Wu P., Gu W., Chen Y., Hao H., Tang K., Yi P., Liu M., Miao S., Ye D. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;30:3887–3899. doi: 10.1038/onc.2011.112. [erratum in: Oncogene. 2011;30:4373–4374] [DOI] [PubMed] [Google Scholar]
- 40.He H.Q., Troksa E.L., Caltabiano G., Pardo L., Ye R.D. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J Biol Chem. 2014;289:2295–2306. doi: 10.1074/jbc.M113.509216. [erratum in: J Biol Chem. 2014;289:4814] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan M., Godson C., Guiry P.J., Agerberth B., Haeggström J.Z. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 42.Cooray S.N., Gobbetti T., Montero-Melendez T., McArthur S., Thompson D., Clark A.J., Flower R.J., Perretti M. Ligand-specific conformational change of the G-protein–coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada M., Habata Y., Hosoya M., Nishi K., Fujii R., Kobayashi M., Hinuma S. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 44.Devosse T., Dutoit R., Migeotte I., De Nadai P., Imbault V., Communi D., Salmon I., Parmentier M. Processing of HEBP1 by cathepsin D gives rise to F2L, the agonist of formyl peptide receptor 3. J Immunol. 2011;187:1475–1485. doi: 10.4049/jimmunol.1003545. [DOI] [PubMed] [Google Scholar]
- 45.Dorward D.A., Lucas C.D., Alessandri A.L., Marwick J.A., Rossi F., Dransfield I., Haslett C., Dhaliwal K., Rossi A.G. Technical advance: autofluorescence-based sorting: rapid and nonperturbing isolation of ultrapure neutrophils to determine cytokine production. J Leukoc Biol. 2013;94:193–202. doi: 10.1189/jlb.0113040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J.L., Murphy P.M., Wang J.M. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep. 2012;2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heit B., Robbins S.M., Downey C.M., Guan Z., Colarusso P., Miller B.J., Jirik F.R., Kubes P. PTEN functions to ‘prioritize' chemotactic cues and prevent ‘distraction' in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Ma B., Malik A.B., Tang H., Yang T., Sun B., Wang G., Minshall R.D., Li Y., Zhao Y., Ye R.D., Xu J. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13:457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maaty W.S., Lord C.I., Gripentrog J.M., Riesselman M., Keren-Aviram G., Liu T., Dratz E.A., Bothner B., Jesaitis A.J. Identification of C-terminal phosphorylation sites of N-formyl peptide receptor-1 (FPR1) in human blood neutrophils. J Biol Chem. 2013;288:27042–27058. doi: 10.1074/jbc.M113.484113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue M., Hsieh G., Raymond-Stintz M.A., Pfeiffer J., Roberts D., Steinberg S.L., Oliver J.M., Prossnitz E.R., Lidke D.S., Wilson B.S. Activated N-formyl peptide receptor and high-affinity IgE receptor occupy common domains for signaling and internalization. Mol Biol Cell. 2007;18:1410–1420. doi: 10.1091/mbc.E05-11-1073. [erratum in: Mol Biol Cell. 2007;18:2778] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali H., Richardson R.M., Haribabu B., Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 52.Heit B., Tavener S., Raharjo E., Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bednar F., Song C., Bardi G., Cornwell W., Rogers T.J. Cross-desensitization of CCR1, but not CCR2, following activation of the formyl peptide receptor FPR1. J Immunol. 2014;192:5305–5313. doi: 10.4049/jimmunol.1302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Flaherty J.T., Jacobson D.P., Redman J.F., Rossi A.G. Translocation of protein kinase C in human polymorphonuclear neutrophils. Regulation by cytosolic Ca2(+)-independent and Ca2(+)-dependent mechanisms. J Biol Chem. 1990;265:9146–9152. [PubMed] [Google Scholar]
- 55.Morris A.C., Brittan M., Wilkinson T.S., McAuley D.F., Antonelli J., McCulloch C., Barr L.C., McDonald N.A., Dhaliwal K., Jones R.O., Mackellar A., Haslett C., Hay A.W., Swann D.G., Anderson N., Laurenson I.F., Davidson D.J., Rossi A.G., Walsh T.S., Simpson A.J. C5a-mediated neutrophil dysfunction is RhoA-dependent and predicts infection in critically ill patients. Blood. 2011;117:5178–5188. doi: 10.1182/blood-2010-08-304667. [DOI] [PubMed] [Google Scholar]
- 56.Leoni G., Gripentrog J., Lord C., Riesselman M., Sumagin R., Parkos C.A., Nusrat A., Jesaitis A.J. Human neutrophil formyl peptide receptor phosphorylation and the mucosal inflammatory response. J Leukoc Biol. 2015;97:87–101. doi: 10.1189/jlb.4A0314-153R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bao Y., Ledderose C., Seier T., Graf A.F., Brix B., Chong E., Junger W.G. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P.A., Junger W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 59.Bao Y., Chen Y., Ledderose C., Li L., Junger W.G. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013;288:22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorrasi A., Li Santi A., Amodio G., Alfano D., Remondelli P., Montuori N., Ragno P. The urokinase receptor takes control of cell migration by recruiting integrins and FPR1 on the cell surface. PLoS One. 2014;9:e86352. doi: 10.1371/journal.pone.0086352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y.H., Morand E., Leech M. Annexin A1: potential for glucocorticoid sparing in RA. Nat Rev Rheumatol. 2013;9:595–603. doi: 10.1038/nrrheum.2013.126. [DOI] [PubMed] [Google Scholar]
- 62.El Kebir D., József L., Khreiss T., Pan W., Petasis N.A., Serhan C.N., Filep J.G. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 63.Devosse T., Guillabert A., D'Haene N., Berton A., De Nadai P., Noel S., Brait M., Franssen J.D., Sozzani S., Salmon I., Parmentier M. Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J Immunol. 2009;182:4974–4984. doi: 10.4049/jimmunol.0803128. [DOI] [PubMed] [Google Scholar]
- 64.Migeotte I., Riboldi E., Franssen J.D., Grégoire F., Loison C., Wittamer V., Detheux M., Robberecht P., Costagliola S., Vassart G., Sozzani S., Parmentier M., Communi D. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabiet M.J., Macari L., Dahlgren C., Boulay F. N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation. J Biol Chem. 2011;286:26718–26731. doi: 10.1074/jbc.M111.244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tiffany H.L., Gao J.L., Roffe E., Sechler J.M., Murphy P.M. Characterization of Fpr-rs8, an atypical member of the mouse formyl peptide receptor gene family. J Innate Immun. 2011;3:519–529. doi: 10.1159/000327718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao J.L., Chen H., Filie J.D., Kozak C.A., Murphy P.M. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51:270–276. doi: 10.1006/geno.1998.5376. [DOI] [PubMed] [Google Scholar]
- 68.He H.Q., Liao D., Wang Z.G., Wang Z.L., Zhou H.C., Wang M.W., Ye R.D. Functional characterization of three mouse formyl peptide receptors. Mol Pharmacol. 2013;83:389–398. doi: 10.1124/mol.112.081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rivière S., Challet L., Fluegge D., Spehr M., Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 70.Gao J.L., Murphy P.M. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 71.Taylor S.W., Fahy E., Ghosh S.S. Global organellar proteomics. Trends Biotechnol. 2003;21:82–88. doi: 10.1016/S0167-7799(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 72.Taanman J.W. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 73.Spencer A.C., Spremulli L.L. Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 2004;32:5464–5670. doi: 10.1093/nar/gkh886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Becker A., Schlichting I., Kabsch W., Groche D., Schultz S., Wagner A.F. Iron center, substrate recognition and mechanism of peptide deformylase. Nat Struct Biol. 1998;5:1053–1058. doi: 10.1038/4162. [DOI] [PubMed] [Google Scholar]
- 75.Escobar-Alvarez S., Gardner J., Sheth A., Manfredi G., Yang G., Ouerfelli O., Heaney M.L., Scheinberg D.A. Inhibition of human peptide deformylase disrupts mitochondrial function. Mol Cell Biol. 2010;30:5099–5109. doi: 10.1128/MCB.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Escobar-Alvarez S., Goldgur Y., Yang G., Ouerfelli O., Li Y., Scheinberg D.A. Structure and activity of human mitochondrial peptide deformylase, a novel cancer target. J Mol Biol. 2009;387:1211–1228. doi: 10.1016/j.jmb.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen K.T., Pei D. Purification and characterization of enzymes involved in the degradation of chemotactic N-formyl peptides. Biochemistry. 2005;44:8514–8522. doi: 10.1021/bi050191o. [DOI] [PubMed] [Google Scholar]
- 78.Karlsson A., Markfjall M., Stromberg N., Dahlgren C. Escherichia coli-induced activation of neutrophil NADPH-oxidase: lipopolysaccharide and formylated peptides act synergistically to induce release of reactive oxygen metabolites. Infect Immun. 1995;63:4606–4612. doi: 10.1128/iai.63.12.4606-4612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Q., Kang R., Zeh H.J., 3rd, Lotze M.T., Tang D. DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy. 2013;9:451–458. doi: 10.4161/auto.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hauser C.J., Sursal T., Rodriguez E.K., Appleton P.T., Zhang Q., Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010;24:534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raoof M., Zhang Q., Itagaki K., Hauser C.J. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1334. doi: 10.1097/TA.0b013e3181dcd28d. discussion 1332-4. [DOI] [PubMed] [Google Scholar]
- 82.Marques P.E., Amaral S.S., Pires D.A., Nogueira L.L., Soriani F.M., Lima B.H., Lopes G.A., Russo R.C., Avila T.V., Melgaco J.G., Oliveira A.G., Pinto M.A., Lima C.X., De Paula A.M., Cara D.C., Leite M.F., Teixeira M.M., Menezes G.B. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 83.McDonald B., Pittman K., Menezes G.B., Hirota S.A., Slaba I., Waterhouse C.C., Beck P.L., Muruve D.A., Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [erratum in: Science. 2011;331:1517] [DOI] [PubMed] [Google Scholar]
- 84.Ng L.G., Qin J.S., Roediger B., Wang Y., Jain R., Cavanagh L.L., Smith A.L., Jones C.A., de Veer M., Grimbaldeston M.A., Meeusen E.N., Weninger W. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011;131:2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 85.Kim D., Haynes C.L. Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal Chem. 2012;84:6070–6078. doi: 10.1021/ac3009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pittman K., Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–323. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao J.L., Lee E.J., Murphy P.M. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oldekamp S., Pscheidl S., Kress E., Soehnlein O., Jansen S., Pufe T., Wang J.M., Tauber S.C., Brandenburg L.O. Lack of formyl peptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology. 2014;143:447–461. doi: 10.1111/imm.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prat C., Haas P.J., Bestebroer J., de Haas C.J., van Strijp J.A., van Kessel K.P. A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J Immunol. 2009;183:6569–6578. doi: 10.4049/jimmunol.0801523. [DOI] [PubMed] [Google Scholar]
- 90.Fillion I., Ouellet N., Simard M., Bergeron Y., Sato S., Bergeron M.G. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J Immunol. 2001;166:7353–7361. doi: 10.4049/jimmunol.166.12.7353. [DOI] [PubMed] [Google Scholar]
- 91.Gauthier J.F., Fortin A., Bergeron Y., Dumas M.C., Champagne M.E., Bergeron M.G. Differential contribution of bacterial N-formyl-methionyl-leucyl- phenylalanine and host-derived CXC chemokines to neutrophil infiltration into pulmonary alveoli during murine pneumococcal pneumonia. Infect Immun. 2007;75:5361–5367. doi: 10.1128/IAI.02008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grommes J., Drechsler M., Soehnlein O. CCR5 and FPR1 mediate neutrophil recruitment in endotoxin-induced lung injury. J Innate Immun. 2014;6:111–116. doi: 10.1159/000353229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasday J.D., Bascom R., Costa J.J., Fitzgerald T., Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–835. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- 94.Cardini S., Dalli J., Fineschi S., Perretti M., Lungarella G., Lucattelli M. Genetic ablation of the fpr1 gene confers protection from smoking-induced lung emphysema in mice. Am J Respir Cell Mol Biol. 2012;47:332–339. doi: 10.1165/rcmb.2012-0036OC. [DOI] [PubMed] [Google Scholar]
- 95.Cho J.S., Guo Y., Ramos R.I., Hebroni F., Plaisier S.B., Xuan C., Granick J.L., Matsushima H., Takashima A., Iwakura Y., Cheung A.L., Cheng G., Lee D.J., Simon S.I., Miller L.S. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tae Y.M., Park H.T., Moon H.G., Kim Y.S., Jeon S.G., Roh T.Y., Bae Y.S., Gho Y.S., Ryu S.H., Kwon H.S., Kim Y.K. Airway activation of formyl peptide receptors inhibits Th1 and Th17 cell responses via inhibition of mediator release from immune and inflammatory cells and maturation of dendritic cells. J Immunol. 2012;188:1799–1808. doi: 10.4049/jimmunol.1102481. [DOI] [PubMed] [Google Scholar]
- 97.Gemperle C., Schmid M., Herova M., Marti-Jaun J., Wuest S.J., Loretz C., Hersberger M. Regulation of the formyl peptide receptor 1 (FPR1) gene in primary human macrophages. PLoS One. 2012;7:e50195. doi: 10.1371/journal.pone.0050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dai Y., Major J., Novotny M., Hamilton T.A. IL-4 inhibits expression of the formyl peptide receptor gene in mouse peritoneal macrophages. J Interferon Cytokine Res. 2005;25:11–19. doi: 10.1089/jir.2005.25.11. [DOI] [PubMed] [Google Scholar]
- 99.Chiu I.M., Heesters B.A., Ghasemlou N., Von Hehn C.A., Zhao F., Tran J., Wainger B., Strominger A., Muralidharan S., Horswill A.R., Bubeck Wardenburg J., Hwang S.W., Carroll M.C., Woolf C.J. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alam A., Leoni G., Wentworth C.C., Kwal J.M., Wu H., Ardita C.S., Swanson P.A., Lambeth J.D., Jones R.M., Nusrat A., Neish A.S. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao G., Julian M.W., Bao S., McCullers M.K., Lai J.P., Knoell D.L., Crouser E.D. Formyl peptide receptor ligands promote wound closure in lung epithelial cells. Am J Respir Cell Mol Biol. 2011;44:264–269. doi: 10.1165/rcmb.2010-0246RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider E.H., Weaver J.D., Gaur S.S., Tripathi B.K., Jesaitis A.J., Zelenka P.S., Gao J.L., Murphy P.M. The leukocyte chemotactic receptor FPR1 is functionally expressed on human lens epithelial cells. J Biol Chem. 2012;287:40779–40792. doi: 10.1074/jbc.M112.411181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X.G., Hui Y.N., Huang X.F., Du H.J., Zhou J., Ma J.X. Activation of formyl peptide receptor-1 enhances restitution of human retinal pigment epithelial cell monolayer under electric fields. Invest Ophthalmol Vis Sci. 2011;52:3160–3165. doi: 10.1167/iovs.10-5156. [DOI] [PubMed] [Google Scholar]
- 104.Huang J., Chen K., Chen J., Gong W., Dunlop N.M., Howard O.M., Gao Y., Bian X.W., Wang J.M. The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells. Br J Cancer. 2010;102:1052–1060. doi: 10.1038/sj.bjc.6605591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stenfeldt A.L., Karlsson J., Wennerås C., Bylund J., Fu H., Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–229. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 106.Schepetkin I.A., Khlebnikov A.I., Giovannoni M.P., Kirpotina L.N., Cilibrizzi A., Quinn M.T. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr Med Chem. 2014;21:1478–1504. doi: 10.2174/0929867321666131218095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brullo C., Spisani S., Selvatici R., Bruno O. N-Aryl-2-phenyl-2,3-dihydro-imidazo[1,2-b]pyrazole-1-carboxamides 7-substituted strongly inhibiting both fMLP-OMe- and IL-8-induced human neutrophil chemotaxis. Eur J Med Chem. 2012;47:573–579. doi: 10.1016/j.ejmech.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 108.Selvatici R., Brullo C., Bruno O., Spisani S. Differential inhibition of signaling pathways by two new imidazo-pyrazoles molecules in fMLF-OMe- and IL8-stimulated human neutrophil. Eur J Pharmacol. 2013;718:428–434. doi: 10.1016/j.ejphar.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 109.Unitt J., Fagura M., Phillips T., King S., Perry M., Morley A., MacDonald C., Weaver R., Christie J., Barber S., Mohammed R., Paul M., Cook A., Baxter A. Discovery of small molecule human FPR1 receptor antagonists. Bioorg Med Chem Lett. 2011;21:2991–2997. doi: 10.1016/j.bmcl.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 110.Pinilla C., Edwards B.S., Appel J.R., Yates-Gibbins T., Giulianotti M.A., Medina-Franco J.L., Young S.M., Santos R.G., Sklar L.A., Houghten R.A. Selective agonists and antagonists of formylpeptide receptors: duplex flow cytometry and mixture-based positional scanning libraries. Mol Pharmacol. 2013;84:314–324. doi: 10.1124/mol.113.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kirpotina L.N., Khlebnikov A.I., Schepetkin I.A., Ye R.D., Rabiet M.J., Jutila M.A., Quinn M.T. Identification of novel small-molecule agonists for human formyl peptide receptors and pharmacophore models of their recognition. Mol Pharmacol. 2010;77:159–170. doi: 10.1124/mol.109.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]