Abstract
Persons over age 50 are not only aging with human immunodeficiency virus (HIV) infection but also represent a high proportion of new HIV infections. Neuropsychiatric symptoms, including depression, cognitive impairment, and substance abuse, are very common in individuals infected with HIV. However, there is little understanding of the relationship between these HIV-related comorbid conditions in newly infected elderly patients compared to uninfected elderly and those who have survived after 20 years of HIV/AIDS. We summarize the current theories and research that link aging and HIV with psychiatric illnesses and identify emerging areas for improved research, treatment, and patient care.
Keywords: Human immunodeficiency virus (HIV), Depression, Aging
Introduction
Once a fatal illness in many countries, the now chronic, manageable human immunodeficiency virus (HIV) infection and resulting acquired immunodeficiency syndrome (AIDS) have evolved into illnesses that are diagnosed and treated across the life span. While healthy, individuals are considered to be “elderly” over age 65; older persons living with HIV/AIDS are identified at age 50. The designation of “older” HIV patients as >50 years old is a statistical definition used by the Centers for Disease Control (CDC 2008), but appears to be an imprecise barometer of physical and mental health in seropositive individuals. An estimated 25 % of all HIV-seropositive individuals in the United States are >50 years old. (Martin et al. 2008; CDC 2008). As persons live longer, in part because of the widespread use of highly active antiretroviral therapy (HAART), the population of HIV patients 50 years and older will continue to increase. This population growth is attributed to both chronic HIV infections from earlier exposures and newly infected older adults. By the year 2015, 50 % of the AIDS cases in the USA and 15 % of newly diagnosed cases are predicted to fall into this older age group (CDC 2008).
Despite an estimated 15–25 % of potential new HIV+ cases diagnosed in the age >50 group (CDC 2008; Nguyen and Holodniy 2008), there is little data available regarding this unique population and limited information for treatment of medical and psychiatric comorbidities. Older HIV patients can be medically complicated and have been demonstrated to have increased risk and severity of depression and cognitive impairment (Martin et al. 2008; Valcour and Paul 2006; Valcour et al. 2004; McArthur et al. 2010), a shorter time period from infection to the development of AIDS (Nath et al. 2008; Zablotsky and Kennedy 2003; Butt et al. 2001), and decreased proliferation of T lymphocytes (Ellis et al. 2011; Goetz et al. 2001; Justice et al. 2004; Grabar et al. 2004). Older individuals appear to be more vulnerable to HIV infection because of lack of knowledge of personal risk, their low rate of condom use (Pappas and Halkitis 2011; Zablotsky and Kennedy 2003), and delayed or missed diagnosis (Nguyen and Holodniy 2008; Deeks 2010). Substance abuse is still common among older, seropositive gay and bisexual men, and club drug use appears to contribute to growing infection rates in older age groups (Pappas and Halkitis 2011). Although there is significant morbidity and mortality in elderly HIV patients, the trend, along with younger patients, has been to exclude medical comorbidities in research studies. This provides less than adequate information about optimal management for older populations with HIV that have substance abuse problems and mental illness.
While these studies represent an emerging body of literature that demonstrates that age exacerbates the HIV/AIDS disease course in older patients (McArthur et al. 2010), further advances are needed to understand the changes in mood, cognition, and behavioral symptoms in this age group. There is a paucity of newer studies in older adults with HIV, with much of the original data over a decade old. Ongoing discussions about strategic research from these earlier articles are needed to address the compounding physical and psychological factors of aging in a growing older group of HIV patients. In this article, we identify two hypothetical mechanisms related to aging, mental illness, and HIV. We review the clinical presentation of psychiatric disorders in older HIV populations and summarize the current knowledge and treatment regarding older HIV-seropositive individuals with comorbid mental health issues. We also identify emerging areas for improved research on treatment and patient care.
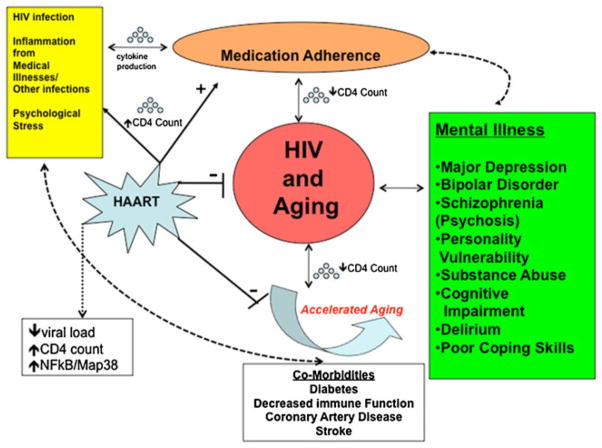
Mechanisms involved in aging, psychiatric comorbidities, and HIV
Medication adherence, increased survival, and reduced psychiatric symptoms
The ability to adhere to a prescribed medication regimen is one of the most important factors in long-term survival with HIV. In addition to poor prognosis for the patient, abandoning treatment leads to a potential threat of multi-drug resistant HIV in the greater community. The higher prevalence rates of age >50 may be directly linked to HAART medication compliance and viral replication (Fig. 1). A study showed that adherence to HAART must exceed 95 % to limit viral replication effectively. Patients with less than 80 % medication adherence had an 87 % virologic failure rate, whereas patients with medication adherence ranging from 80 to 90 % reported a virologic failure rate of 47 % (Ingersoll et al. 2011). Patients with an adherence rate more than 95 % had a 10 % virologic failure rate. Other studies have shown that older patients tended to achieve better virologic control when compared with younger patients, possibly due to better medication compliance (Grabar et al. 2004; Lodwick et al. 2010). Research conducted specifically among older HIV-infected adults (Wutoh et al. 2003) reported a significant inverse correlation between antiretroviral adherence and viral load among 100 HIV-infected adults older than 50 years of age.
Fig. 1.
Aging with HIV and its effects on related neuropsychiatric illnesses
Untreated neuropsychiatric manifestations in HIV+ individuals, independent of age, can promote risk-taking behavior leading to further spread of the disease (Pappas and Halkitis 2011; Lyketsos et al. 1993; Kopnisky et al. 2004). Along with depression, other conditions including dementia, anxiety, psychosis, delirium, substance abuse, and aversive life experiences are all psychiatric factors associated with nonadherence to HIV treatment (Mellins et al. 2009, Ettenhofer et al. 2009). Treatment of depression is associated with improved adherence to HAART (Koenig et al. 2008; Campos et al. 2008) and improved CD4 counts and disease outcomes.
While psychiatric comorbidities are common in HIV-related illness, there are limited data to determine if the neuropsychiatric symptoms are the result of the virus in the CNS, magnified by HAART treatment or comorbid with other chronic, inflammatory illness. During the earlier course of the epidemic, multiple reports documented that older age was associated with higher rates of seroconversion and worse prognosis once infected with HIV. With the advent of successful antiviral therapy, there has been a reduction in viral load and increase in CD4 counts in large numbers of HIV patients treated with HAART. Studies have shown that antiretroviral treatment can ameliorate depression symptoms and increase medication adherence that coincides with improvement in CD4 cell counts (Koenig et al. 2008). One study of homeless HIV-positive individuals with depression and substance abuse showed significant improvement in antiretroviral therapy adherence with antidepressant treatment (Tsai et al. 2010).
The antiretroviral therapies used to treat HIV illness decrease virus production, but may also precipitate or worsen cognition, mood, and daily functioning (Leserman 2008; van der Lee et al. 2007; van Servellen et al. 2002) (see below). There is overlap between neuropsychiatric complications of HIV and HAART itself (McArthur and Brew 2010; Ellis et al. 2010; Letendre et al. 2006) (Table 1). Peripheral neuropathy, hepatotoxicity, lactic acidosis, metabolic syndrome, dyslipidemia, and pancreatic and endocrine dysfunction have been associated with HAART. Older patients may be more likely to develop toxicities than younger patients because of normal age-related changes in these organ systems. More work is needed regarding the complex interactions between medication adherence, antiretroviral drug mechanisms, mood, cognition, and behavior. Future research that focuses on the relationship of long-term compliance with HAART to neuropsychiatric symptoms in older patients living with the HIV virus for several decades is essential for educating newly diagnosed patients about effective treatment of their viral illness and mental health (Table 2).
Table 1.
Psychiatric complications in HIV patients >50
| Illness | Characteristic symptoms |
|---|---|
| Major depression | Persistent low mood, changes in sleep and appetite, poor concentration, lack of interests, guilt, suicidal ideations |
| Bipolar disorder (mania) | Decreased need for sleep, grandiosity, increased energy and libido, racing thoughts, pressured speech |
| Psychotic disorders (schizophrenia) | Auditory hallucinations, delusions, thought disorder |
| Anxiety disorders (generalized anxiety disorder) | Heightened arousal, nervousness, intrusive thoughts, anxiety |
| Cognitive impairment (HIV-associated Dementia) | Cognitive decline, language impairment, decreased attention, intact consciousness |
| Delirium | Waxing and waning consciousness, memory impairment |
| Substance abuse/dependence | Alcohol dependence, illicit drug use leading to impairment of social functioning |
| Adjustment disorders | Low mood and/or anxiety, less severe and related to an identified life stressor |
| Personality disorders | Maladaptive behaviors that interfere with interpersonal relationships |
Table 2.
Summary of guidelines for the management of psychiatric illness in HIV patients
|
Source: Ross Slotten, M.D., and http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/277/hiv-and-the-older-patient
Inflammation from HIV and “accelerated aging”
There has been widespread discussion of the concept that HIV “accelerates aging,” most likely through ongoing and increased inflammation (Fig. 1). Accelerated aging may lead to higher rates of major depression, bipolar disorder, anxiety disorders, psychosis, cognitive dysfunction, and substance abuse in older HIV+ individuals compared to healthy older adults (Havlik et al. 2011; Leserman 2008; Effros et al. 2008; Valcour et al. 2004). At the same time, the interrelationship between mental illness and further immunosuppression interferes with daily functioning, medication adherence, and ultimately with treatment of HIV/AIDS in the older HIV+ population. With aging, there are accelerated risks of developing, diabetes, hypertension, coronary artery disease, stroke, Alzheimer’s disease, and other immune system-related medical complications, all of which may increase the risks for neuropsychiatric conditions in HIV/AIDS. These comorbidities are typically not observed in the general population until the seventh decade of life. In contrast, HIV patients present with these multiple medical problems in their 40s and 50s.
Over age 50, HIV-positive adults appear to have higher rates of depression and poorer cognitive function (Nguyen and Holodniy 2008; Angelino and Treisman 2008) which may be in turn related to changes in immune profiles with age. The common link appears to be inflammation (Deeks 2010). In addition to the HIV virus, persistent inflammation from other viral infections, such as herpes, hepatitis, and CMV, may activate the immune system in older individuals with HIV and directly alter T cell production (Alciati et al. 2007).
The immune system has also been associated with HIV and age-related conditions at the cellular level. With normal T cells, one theory suggests that mitochondrial DNA functions less efficiently with age and produces fewer energy stores (Ozawa 1997). This may contribute to the physical exhaustion seen in HIV-related fatigue (Vance et al. 2010). HIV-related fatigue is an experience commonly reported in HIV patients over 50 (Siegel et al. 2004) and is often attributed to depression, HIV medications, and medical illness in older HIV-positive persons. Less energy at the cellular level also means lower metabolism for brain cells, which may contribute to a decline in mood and cognition. In patients aging with HIV, this is magnified by the direct viral depletion of the immune systems of cellular energy. Furthermore, since antiretroviral therapies, specifically the nucleoside reverse transcriptase inhibitors, are thought to decrease mitochondrial DNA, the combination results in higher rates of fatigue, depression, and cognitive changes in older HIV patients (Vance et al. 2010). There is strong evidence that fatigue and depression are interrelated. More research is needed to determine how cellular immune mechanisms contribute to fatigue, mood, and cognitive changes in older HIV patients.
The role of pro-inflammatory cytokines in the pathophysiology of mood is one of the best-supported hypotheses in the study of affective disorders (Schiepers et al. 2005). Inflammation is also strongly correlated with disease course and prognosis in aging and in HIV (Deeks 2010). Cytokines such as interferon-γ and IL-6, released in the periphery from macrophages and from activated microglia within the central nervous system, may provoke overstimulation of oxidative stress pathways and directly regulate serotonin production and trophic support from brain-derived neurotrophic factor and TNF-α. That deficit was independent of the presence of dementia in those patients.
Given the strength of the evidence for involvement of neuroinflammatory processes in HIV infection and neuropsychiatric symptoms, future studies are needed to expand the current data regarding the pathophysiology of HIV infection in older adults. More research is needed to understand the unique mechanism(s) by which neuropsychiatric illnesses manifest themselves in both patients with long-term HIV infection and those newly diagnosed as HIV positive later in life.
Psychiatric illnesses in older HIV patients
Major depressive disorder
Major depressive disorder (MDD) is the most common psychiatric diagnosis in HIV/AIDS. Approximately 25 to 45 % of HIV-infected patients have a lifetime prevalence of MDD, which is over twice the rates seen in the general population, which range from 5 to 7 % (CDC 2008; Rabkin et al. 2004a, b; Bing et al. 2001). In patients with CD4 cell counts below 200, MDD is even more severe (Alciati et al. 2001). Retrospective and prospective studies in late-stage HIV report a 2.5-fold increase in rates of MDD compared to the general population (Ickovics et al. 2001; Lyketsos et al. 1996b).
Newly diagnosed cases of depression and risk of depression generally decrease with normal aging. In contrast, older individuals with HIV have higher rates of lifetime depression compared to their HIV-negative cohort. A study looking at HIV-positive and HIV-negative veterans showed no difference in active depression symptoms in the young or old HIV-positive group. As HIV-negative individuals aged, depression symptoms declined. In every age group, lifetime prevalence of depression was increased in the HIV-positive group compared to HIV-negative controls (Justice et al. 2004). Similar high rates of depression were observed in a study of three groups, (1) male homosexual, non-IV drug users, without HIV; (2) young men with HIV; and (3) older men with HIV. Older, HIV-negative men had a significantly lower lifetime rate of depression, 20 %, compared to older men with HIV (36 %). Homosexual non-IV drug users had rates of 32 %, and younger HIV patients, 39 % (Rabkin et al. 2004a, b). Depression was associated with HIV infection in older, longitudinal studies (Lyketsos et al. 1996b; Ickovics et al. 2001; Leserman 2003) but not in short-term studies (Eich-Höchli et al. 1997; Rabkin et al. 1991; Perry et al. 2004), suggesting that the progression of HIVover time increases the risk for development of depression (Leserman 2003). The current research reflects the need for longitudinal studies of depression in older HIV patients with a distinction between older adults with new infections compared to long-term infections in adults who have aged with HIV/AIDS.
Depression in older HIV-positive individuals is also associated with higher rates of medical comorbidities. The Research on Older Adults with HIV study collected self-report data on depressive symptomology from over 1,000 HIV-positive men and women with HIV that were age 50 and older from 2005 to 2006. There appears to be significant correlations with depression scale scores and cardiovascular and pulmonary conditions (Havlik et al. 2011). They also found those with depression had a higher disease burden and an average of three other disease conditions (Havlik et al. 2011). Overall, more clinical studies are needed in HIV individuals with depression and multiple chronic illnesses, beyond monitoring CD4 count and viral load (Havlik et al. 2011).
An active major depressive episode may itself increase risk of contracting HIV. While cross-sectional studies and self-report measures have their own limitation in establishing a causal link between comorbid illness and neuropsychiatric symptoms, onset of depression has been reported to be present before HIV seroconversion and can be speculated to precede infection (Rabkin et al. 2004a, b). HIV-positive status may also exacerbate symptoms of depression (Lyketsos et al. 1996a, b). In addition, depression, has been hypothesized to worsen HIV progression (Nguyen and Holodniy 2008; Angelino and Treisman 2008; Dolder et al. 2004), leading to a cycle of HIV and depression exacerbating each other. To date, no studies have been specifically been done in the age >50 HIV-positive group. Through various biological and behavioral mechanisms, depression further promotes viral transmission and hinders effective treatment in HIV disease itself, thereby contributing to the disease progression and mortality (Leserman 2008; Riley et al. 2003). In a study of 36 HIV-infected patients with MDD treated with different potency antiretroviral therapies compared with 77 non-depressed HIV controls, depression was a significant predictor of natural killer (NK) cell number and percentage decline. However, treatment with antiretroviral therapy and depression–antiretroviral therapy interaction do not significantly influence depression-related NK cell changes (Alciati et al. 2007). Ultimately, MDD adversely affects HAART compliance (DiMatteo et al. 2000) which ultimately impacts quality of life (Lenz and Demal 2000; Meltzer-Brody and Davidson 2000), treatment outcome (Azar et al. 2010; DiMatteo et al. 2002; Holmes and House 2000), and function (Plummer et al. 2010; DiMatteo et al. 2002) in patients with HIV.
Depressive illness in the elderly with HIV still remains underdiagnosed and undertreated in medical clinics (Patterson et al. 2006; Marwick and Kaaya 2010; Lyketsos et al. 1994). This is in part because older patients with HIV often present with somatic complaints, anger, and irritability instead of low mood (Krishnan et al. 2002). Because older patients with depression and HIV are more somatically focused, it may be difficult to delineate HIV symptoms from MDD. Poor sleep, changes in appetite, lack of motivation, decreased concentration, fatigue, and weight loss are overlapping symptoms in both HIV and MDD. This misinterpretation of symptoms in older HIV-infected older adults may contribute to the delay in the diagnosis and treatment of MDD, allowing the depression to have an insidious course until identified (Cherner et al. 2004; Malaspina et al. 2011). More comprehensive measures have been implemented in HIV-infected older adults to capture what the conventional Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) may have missed in this special patient population. One example is that by using more formal and descriptive methods of diagnosis, one group was able to accurately identify depression, cognitive symptoms, and alcohol abuse with increasing age in HIV populations (Justice et al. 2004).
Suicide, the most significant risk associated with depression, has been understudied in older HIV-infected patients. The limited data are surprising, given that older people have the highest rate of completed suicide and persons with HIV/AIDS and depression are more vulnerable to thoughts of suicide, given perceived lack of social support and higher ratings of emotional stress. One survey of a group of 113 individuals older than age 45 utilizing HIV-AIDS services reported thoughts of taking their own life in the previous week in 27 % of respondents. Greater levels of emotional distress and poorer health-related quality of life correlated with increased suicidal thoughts in this older, HIV-positive group. Increased suicidal thoughts and behaviors have also been associated with lower CD4 counts and higher viral loads (Cooperman and Simoni 2005; Haller and Miles 2003; Carrico et al. 2006; Alciati et al. 2001), and the cycle of reinforcement between depression and HIV illness is further exacerbated by self-defeating behaviors often seen in this patient population.
The pharmacological treatment of depression in older people with HIV/AIDS has not been extensively studied, and there are no specific treatment guidelines. We do know that antiretroviral therapy may have important interactions with selective serotonin reuptake inhibitors, tricyclic antidepressants, and benzodiazepines that are commonly used to manage depression in HIV patients. HIV patients should be counseled regarding the benefit of antidepressant treatment, but should also be aware of potential interactions with their HAART therapy and the need for close monitoring of medication dosages.
Mania and bipolar disorder
Although mania has been well documented in HIV-positive individuals in multiple regions in the world, only a handful of studies focus on older HIV patients. A chart review of an HIV/AIDS clinic showed manic syndromes affected 8 % of the clinic population. History of a mood disorder was also associated with mania presenting later in the course of HIV infections and correlated with higher rates of dementia (Lyketsos et al. 1993). Bipolar mania that predates HIV infection is distinguished from secondary mania or “AIDS mania” in the literature by age, HIV stage, and/or duration of illness. Secondary mania occurs in more advanced stages of immunosuppression and is associated with dementia and psychomotor retardation. Rates of secondary mania were at one time estimated as high as 4 % in some clinical populations (Kilbourne et al. 2001; Ellen et al. 1999), but there are no updated studies to determine if these rates have changes with widespread use of HAART. The general thinking is that mania in the early stages of HIV in individuals with a history of mood disorder and a CD4 count greater than 200 is indicative of bipolar disorder. In contrast, manic symptoms thought to be related to the HIV virus in the brain in the later stages of AIDS, with CD4 counts less than 200, are descriptive of secondary mania (Nakimuli-Mpungu et al. 2009). Bipolar mania and secondary mania should be characterized as different conditions, as they have distinct clinical presentations and management. AIDS mania, unlike bipolar disorder, tends to have a chronic, unremitting course, with the need for long-term pharmacotherapy treatment. To date, there are no studies that look at bipolar disorder compared to AIDS mania in HIV-positive individuals of age >50. One study in Uganda examines age differences in AIDS mania, and this was done in the absence of antiretroviral therapy. In examining 151 individuals admitted to a psychiatric hospital with acute mania, 18.5 % were HIV positive, with bipolar mania; 41.1 % were HIV positive and satisfied criteria for secondary mania; and 40.4 % were HIV negative, with bipolar mania. HIV-positive patients with bipolar disorder were older than the HIV-negative patients. This suggests that the HIV-positive patients had a longer duration of bipolar illness than the HIV-negative patients with bipolar mania (Nakimuli-Mpungu et al. 2009).
Like unipolar depression, bipolar disorder is associated with elevated risk of behaviors that increase the chance of contracting HIV. The risks appear to be more prominent in bipolar mania compared to the general population (Perretta et al. 1998) with prevalence of 2.3 % compared to 0.3 %, respectively. The manic behaviors including hypersexuality, impulsivity, high risk taking, and disinhibition lead to poor reality testing and may interfere with decision-making ability and use of safer-sex practices. Patients with HIV, including those age >50, tend to have higher rates of comorbid alcohol or substance abuse, which elevates risks (Sullivan et al. 2008). Irritability, more than an elevated mood, is seen in patients with long-term, advanced infections that tend to have AIDS mania (Lyketsos et al. 1997). Bipolar mania and AIDS mania may be associated with delusional beliefs. Most commonly, these involve inventing a cure for HIV or being cured of HIV (Lyketsos et al. 1997). HIV-related medication treatment, cocaine, amphetamines, and steroids can induce manic symptoms. Older HIV patients are more vulnerable to opportunistic infections and HIV neurotoxicity from medical complications, which may put them at higher risk for mania.
Bipolar disorder may be underrecognized in older HIV patients because of difficulty to distinguish symptoms from MDD or poor recall of illness episodes. In a sub-type of bipolar affective disorder, type II (BPAD II), which has milder manic episodes, there appears to be a higher lifetime prevalence in the AIDS mania, independent of HIV risk factors (Perretta et al. 1998). For example, although the patients with HIV had a chief complaint of depression, 78 % met criteria for BPAD II, 52 % had associated cyclothymic disorder, and 35 % had hyperthymic temperament. New onset of mood labiality and increased goal-directed activity in any older HIV patient may warrant referral to psychiatry for screening and management of acute mania.
There is little research on specific treatment recommendations for bipolar mania and AIDS mania in older HIV individuals. The treatment involves management of mania symptoms with antipsychotic medication and suppression of systemic viral load with antiretroviral medications. Older HIV patients may be sensitive to extrapyramidal symptoms (EPS) and anticholinergic agents, where medication toxicity and drug interactions are also a problem with increased age (Lyketsos et al. 1997). Very low doses of a single typical or atypical neuroleptic medication is preferable for late-stage AIDS mania as they can be dosed once daily, have a lower side effect profile, and are generally well tolerated and maintain compliance. The most common atypical neuroleptics, which are also approved for older HIV-positive patients, are quetiapine, risperidone, olanzapine, and aripiprazole. Mood stabilizers, such as lithium, the mainstay of treatment in bipolar disorder, can be problematic in HIV patients with mania, because serum drug levels need to be closely monitored and may be affected by HAART medications. There is also a risk of CNS toxicity if levels are supratherapeutic. Another mood stabilizer, valproic acid, has been associated with liver toxicity and bone marrow suppression in immunocompromised patients. The long-term use of antiretroviral therapy would be expected to decrease the incidence of AIDS mania in the population and potentially prevent relapse in individual patients. However, more research is needed to determine if AIDS mania rates are increasing as more long-term survivors of HIV age with the virus.
Psychotic disorders
Psychosis is a cluster of symptoms, not a specific illness itself, which involves alteration of perception and thinking. Hallucinations, delusions, disorganized and paranoid thoughts, apathy, and blunted emotion responses are some of the characteristic symptoms. Patients with psychotic disorders, the most severe symptoms of mental illness, have higher rates of HIV infection compared to the general population (Hinkin et al. 2001; Kalichman et al. 1994). Psychosis may contribute to greater morbidity and mortality in patients with HIV by interfering with reality testing, creating barriers to medication compliance, difficulty with communicating symptoms to the clinician, and minimization of HIV symptom severity on the part of clinical staff (Dolder et al. 2004; Sewell 1996). To date, aging, HIV, and psychosis have only been circumstantially studied with mainly indirect evidence regarding prevalence, clinical course, and treatment in HIV-seropositive populations with these three confounding factors. Older HIV patients have been studied with regard to subclassification of psychotic disorder; primary—associated with ongoing schizophrenia, mood disorders, and substance abuse and dependence and secondary—associated with new-onset symptoms during HIV infection.
Schizophrenia, schizoaffective disorder, and bipolar disorder are pre-existing mental disorders that are part of a spectrum of psychosis and seen in HIV seropositivity. This group of patients appears to be more vulnerable to higher rates of HIV infection compared to the general population. Psychotic patients may be paranoid about medication side effects or may be too confused to follow a medication regimen. Subjective evidence has suggested an adverse impact of HIV disease because individuals with schizophrenia may be more vulnerable to the stresses related to HIV infection and have fewer resources to manage these issues (Sewell 1996). Interestingly, in an examination of claims histories of Medicaid beneficiaries with both HIV infection and schizophrenia (conducted before the widespread use of protease inhibitors), no difference was found in the likelihood of receiving antiretroviral medications between HIV-infected individuals with or without schizophrenia (Walkup et al. 2010). A recent study published in 2011, also based on Medicaid claims, showed that the HIV risks among people living with schizophrenia varied across the eight states reviewed in the study but were closely linked to local epidemiologic patterns of HIV among IV drug users (Walkup et al. 2011).
Aging may have a direct effect on HIV in patients with ongoing psychotic disorders. A recent retrospective cohort study using the national Veterans Health Administration HIV Clinical Case Registry examined the prevalence of severe mental disorders, including schizophrenia and bipolar disorder in older veterans with access to HIV treatment and medical care. Age, race, CD4 count, and antiretroviral compliance were associated with shorter time to an AIDS defining illness and death. Psychosis in schizophrenia and bipolar disorder was associated with lower survival rates in older veterans (Nurutdinova et al. 2012). Few studies have examined the rates of HIV in older compared to younger adults with pre-existing psychotic disorders. Additional studies are needed in HIV-seropositive individuals with pre-existing psychotic illness to better understand the impact of psychotic symptoms on HIV disease progression.
Secondary psychosis, often termed “new-onset psychosis,” should be distinguished from an episode of psychosis in individuals with pre-existing psychiatric disorders. New-onset psychosis has been described as a symptom in late-stage HIV/AIDS, with CD4 counts often <200. Common reasons for new-onset psychosis include central nervous system infection, tumors, HIV invasion into the brain, and cognitive impairment. Estimates of new-onset psychosis, depending on pre-HAART vs. post-HAART treatment error, range from 0.23 to 15.2 % (Dolder et al. 2004). There are no definitive estimates of new-onset psychosis in patients >50 with HIV. Persecutory and grandiose delusions are more prominent that hallucinations. Compared to primary psychosis, suicidal thoughts, first-rank symptoms, and bizarre delusions are much less common (Harris et al. 1991). Although the clinical presentation is variable, there appears to be a higher mortality rate in new-onset psychosis when compared to non-psychotic patients (Sewell 1996). However, with treatment, new-onset psychosis is self-limited and eventually remits in over half of the cases observed. Psychotic symptoms, along with depression and mania, have been reported as secondary side effects associated with HIV and HAART treatments. Medications, including efavirenz, interferon, metoclopramide, clonidine, anabolic steroids, corticosteroids, and muscle relaxants have been associated with neuropsychiatric symptoms in HIV patients (Angelino and Treisman 2008; Dolder et al. 2004). Despite stopping the offending agent, these symptoms may persist and require treatment.
Patients with new-onset psychosis, like AIDS mania, tend to be older and respond to treatment with neuroleptic medications. Because of age, these patients are often more vulnerable to medication side effects and extrapyramidal motor symptoms (EPS). The most common extrapyramidal symptoms observed in older patients treated with neuroleptics are akinesia, the inability to initiate movement, and akathisia or difficulty remaining still. One study comparing HIV-seropositive individuals to HIV-seronegative individuals, even after controlling for the dose administered, demonstrated EPS rates of 50–78 % (Hriso et al. 1991). In HIV-negative individuals with schizophrenia, randomized, controlled trials have demonstrated that atypical neuroleptics were associated with lower rates of EPS and tardive dyskinesia compared with traditional agents like haloperidol and thorazine (Dolder et al. 2004). The safety and efficacy of antipsychotics in the treatment of new-onset psychosis have been addressed in a case series (Singh et al. 1997) and small pilot studies (Dolder et al. 2004), but older individuals with HIV have not been well studied. Much of our information comes from the use of antipsychotic agents in older adult populations, but it is unclear if these are generalizable to HIV patients >age 50.
Anxiety disorders
While anxiety disorders are the most common of all psychiatric disorders in the general population, there is far less research in HIV-positive patients compared to mood and psychotic disorders. One review estimates anxiety rates up to 38 % in the general HIV population (Elliott 1998), but no specific studies have looked at rates of anxiety in older HIV patients or in those with advanced stages of HIV. Anxiety was a strong independent predictor of sexual risk and substance use in one study of 302 substance-using, HIV-negative, and unknown-status gay/bisexual men at risk for HIV infection. Age appeared to be a moderating factor for anxiety and sex-risk outcomes, where older and more anxious participants had more frequent instances of sexual risk (Lelutiu-Weinberger et al. 2011). Anxiety that develops from stressors that develop during HIV treatment and pre-existing generalized anxiety disorder, panic disorder, and/or posttraumatic stress disorder has been reported in individuals with HIV. Those diagnoses with HIV had higher rates of generalized anxiety disorder (GAD) than simple phobias, with social anxiety common in both groups (Bing et al. 2001). Rates of GAD, panic disorder (PD), and combined GAD and PD were estimated to be 15.8, 10.5, and 5 %, respectively, in an older review of a nationwide analysis of HIV-positive individuals (Elliott 1998).
Women with HIV tend to report higher rates of anxiety. Subsequently, the HIV literature on anxiety disorders tends to be gender specific. One study used the Hamilton Depression Rating Scale and the Hamilton Anxiety Rating Scale to compare anxiety symptoms in 93 HIV-seropositive women and 62 seronegative women in a clinic setting. There was no significant between-group difference in the rate of anxiety disorders. However, HIV-seropositive women had significantly higher anxiety symptom scores, over two times higher, compared to seronegative women (Morrison et al. 2002). HIV-positive women reported higher rates of GAD, bereavement, and suicidal thoughts compared to HIV-positive men after the death of a loved one (Summers et al. 2004).
HIV-positive women appear to have more exposure to trauma-related disorders and posttraumatic stress disorder (PTSD), with PTSD rates as high as 42 % (Martinez et al. 2002; Klinkenberg and Sacks 2004). In one study of 61 HIV-positive homosexual/bisexual men assessed for posttraumatic stress disorder in response to HIV infection, 30 % met criteria for PTSD in response to HIV diagnosis. PTSD has an onset greater than 6 months after initial HIV infection diagnosis in over one third of the cases, but was also significantly associated with a pre-HIV history of PTSD from non-HIV causes (Kelly et al. 1998).
The presence of PTSD, GAD, and panic disorder appears to affect the treatment and management of HIV. PTSD and anxiety have been proposed to interfere with adherence to antiretroviral treatment, but other studies suggest the association is due to depression more so than anxiety (Sledjeski et al. 2005), particularly in those aging with HIV. Middle-age and older individuals with HIV and suicidal thoughts also reported greater symptoms of anxiety, somatization, hostility, and interpersonal sensitivity (Kalichman et al. 2005). Higher stress ratings in these older HIV/AIDS patients tended to correspond with fewer social support, more feelings of stigma related to diagnosis, and higher rates of social isolation. Anxiety symptoms are common among patients dealing with a new diagnosis of HIV, and clinicians should be aware that individuals are vulnerable to acute stress disorder and changes in mood after receiving the diagnosis.
Personality vulnerabilities and adjusting to aging with HIV
HIV-positive individuals tend to have higher prevalence rates of personality disorders (Perkins et al. 1993; Jacobsberg et al. 1995). By definition, personality vulnerabilities can still persist in older HIV patients and lead to a negative perception of the illness and affect adherence. However, there is scant literature on personality and temperament in individuals living long term with HIV or in older individuals newly diagnosed with HIV. Much of the data we present are related to HIV risk in all age groups. Some personality characteristics are associated with higher risk-taking behaviors, including low conscientiousness and high neuroticism (Trobst et al. 2000). In part, individuals with these traits often do not perceive their behaviors as risky. Borderline personality disorder negatively affected medication adherence in HIV-positive individuals in a methadone treatment clinic (Palmer et al. 2003). Evidence of the relationship between personality and disease progression in HIV comes from a longitudinal study of HIV-seropositive patients that completed the NEO-PI-R personality assessment and underwent comprehensive psychological assessment, CD4 count, and viral load blood sampling every 6 months for 4 years. “Go Getters” and “Directed” personality traits had slower disease progression, whereas the “homebody” profile (low extraversion–low openness) was significantly associated with faster disease progression (Ironson et al. 2008). Personality disorders are thus considered an important mediator of risk behaviors and treatment success and therefore warrant further investigation in older populations.
Personality vulnerabilities also relate to coping with medical and mental illness in addition to dealing with the diagnosis of HIV. Older populations appear to be less accepting of an HIV diagnosis and have fewer potential resources and support. Older patients report feeling lonely because of the profound stigma of HIV perceived by this age group (Grov et al. 2010). They may conceal their infection from family and friends, resulting in further social isolation. Nondisclosure to their support systems is also related to further lack of practical (27 %) and emotional (42 %) support in older HIV-positive individuals (Montoya and Whitsett 2003). A recent study in HIV patients over 50 suggests that focusing efforts to reduce HIV-related stigma and loneliness may have lasting effects in reducing major depressive symptoms and improving perceived health (Grov et al. 2010). Offering additional support groups to older adults may provide the additional social support these patients need to manage their illness.
There appears to be a lack of knowledge among older adults about education and prevention regarding HIV transmission. This may also be related to the minimal number of services available to older adults compared to those that target young individuals at risk for HIV. While the rates of HIV infection are growing in older adults, 90 % of adults surveyed with risk factors reported they had not been tested for HIV, because they did not believe they were at risk (Zelnetz and Epstein 1998). In a nursing study of 166 persons with a mean age of 71 years, although they were knowledgeable about the human immunodeficiency virus and 10 % had unprotected sex outside of a long-term relationship, the majority did not believe that they were able to contract HIV because of their age (Maes and Louis 2003).
As all patients age, they begin to think about economic stability with regard to access to healthcare and managing medications. In older HIV patients, economic stressors can become a point of emotional distress as the cost of medications continue to increase. Even with Medicare and Medicaid, there may be a financial strain on the patient’s already fixed income. African–Americans, Latinos, and Native Americans have higher rates of newly diagnosed HIV in the 50-and-older age range and thus may be disproportionately affected by the economic burden of medical illness (Montoya and Whitsett 2003). Overall, adult patients are more compliant than younger individuals, but the ability to afford medications can be one of the major barriers to medication adherence (Klinkenberg and Sacks 2004). One study found increased risk of nonadherence in elderly patients who live alone or have few family support systems in place (Mehta et al. 1997). Additional services that provide illness education and specific medication adherence support strategies may be beneficial in older adults with HIV and financial stressors.
Minor cognitive disorder and HIV dementia
Clinicians and researchers have proposed that older age may potentiate or act as a risk factor for neurocognitive impairment in HIV disease. HIV patients over age 50, regardless of duration of infection appear to be at greater risk for cognitive decline. Age and HIV seropositivity in general are predisposing factors for this altered mental status, which may be precipitated by normal aging, central nervous system infection, opportunistic infections, medications, fever, narcotics, benzodiazepines, and/or steroids (Uldall et al. 2000a, b; Angelino and Treisman 2008). Older adults with HIV tend to generally have more progressive cognitive decline than younger HIV patients. Incidence of cognitive disorders was 7.3 % and associated with larger viral load but not age, in a 1-year prospective longitudinal study of older and younger adults with HIV (Becker et al. 2004).
Currently, there is a spectrum of cognitive impairment related to HIV infection. Minor cognitive motor disorder (MCMD) is associated with minor impairment while HIV-associated dementia (HAD) has more severe impairment in memory and executive functioning (Valcour et al. 2004). Through a comprehensive evaluation, HIV-related neurocognitive disorders are usually diagnoses of exclusion. Although the Mini-Mental State examination is used as a screen for general changes in mental status, the International HIV Dementia Scale and other specific HIV dementia scales appear to be more sensitive in identifying individuals at risk for HIV dementia in both the industrialized world and the developing world (Sacktor et al. 2005). The diagnosis of MCMD or HAD is then confirmed through a series of neuropsychological tests of memory, attention, language, and executive function. The criteria for MCM include at least two of the following features: poor memory, irritability, mood labiality, disrupted attention or concentration, mental slowing, slowed movements, impaired coordination, and/or personality change. MCMD does not necessarily develop into HAD.
HIV dementia is probably the most important neuropsychiatric condition in aging patients, but is reviewed extensively elsewhere in this volume. HAD is a subcortical dementia and characterized by two or more cognitive domains causing functional impairment, progression and decline in motor dysfunction, and/or lack in motivation or emotional control. Unlike delirium, there is no alteration in consciousness and often no confounding etiology. Despite the declining incidence of HAD, the overall cases of HAD are increasing as the number of older individuals with HIV increases. HAD almost defines the advanced stages of HIV, although the precise pathophysiology is unclear. HAD can be diagnosed with CD4 counts above 200 but is more often seen in patients with higher depression rating scales and lower CD4 counts. HAD is twice as common in older adults (25.2 %) as compared to adults living with HIV under the age of 40 (13.7 %) in a study of the Hawaii Aging with HIV-1 Cohort (Valcour et al. 2004).
HIV patients with cognitive impairment should be ruled out for opportunistic infection as well as medication side effects. Treatment with HAART should be implemented in those with a suppressed immune system. Interventions such as assistance with medication administration, home care, and activities of daily living, through occupational therapy, are important as these individuals may forget doses and appointments if they are caring for themselves.
Delirium
Although classified as a medical disorder, delirium is the most frequent reason for psychiatric consultation in the hospital and in nursing home settings. Fluctuations in level of consciousness, confusion, poor concentration, and disorganized thinking are all part of the clinical presentation of delirium. Delirium is traditionally associated with frail elderly patients and occurs frequently in patients with HIV/AIDS (Angelino and Treisman 2008). Hospitalized patients with HIV/AIDS often have high rates of delirium (Uldall et al. 2000a, b; Uldall et al. 2000a, b). One study found that 46 % of the AIDS patients at a skilled nursing facility had at least one episode of delirium (Uldall and Berghuis 1997). More subtle delirium is common in immunocompromised patients, although assessment can be difficult. In addition, delirium has been shown to be a marker for decreased survival in patients with AIDS. In comparison, strong, pre-morbid functioning and low viral load appear to protect older HIV patients from delirium (Cole et al. 2007)
In delirium, psychotic symptoms consisting of visual or auditory hallucinations and delusions are common and may cause distress in the patient. Behavioral and mood symptoms are common and often unpredictable. The syndrome has an acute or a sub-acute onset and remits fairly rapidly once the underlying etiology is treated. If untreated, patients have a marked increased risk of mortality, with estimates of about 20 % in hospitalized patients.
Aside from general risk factors such as older age, multiple medical problems, multiple medications, impaired visual acuity, and previous episodes of delirium, patients with HIV-associated dementia are at increased risk to develop delirium. The differential diagnosis of delirium includes HIV-associated dementia (especially with AIDS mania), minor cognitive–motor disorder, major depression, bipolar disorder, panic disorder, and schizophrenia. Delirium usually can be differentiated on the basis of its rapid onset, fluctuating level of consciousness, and link to medical etiology.
The cause of delirium should be aggressively sought. Particular considerations in HIV patients include hypoxia with Pneumocystis pneumonia, malnutrition, CNS infections and neoplasms, systemic infections (e.g., mycobacteria, CMV, bacterial sepsis), HIV nephropathy, substance intoxication and withdrawal, medication toxicity, and poly-pharmacy. Variations in hydration or electrolyte status also may profoundly affect patients with HIV who already have cerebral compromise. HIV infection itself also may produce an acute encephalopathy similar to that reported with CMV (Deeks 2010).
Substance abuse and addiction
Issues of substance abuse and addiction are common in older patients and put them at higher risk for HIV infection. Drug use accounts for more than 16 % of new HIV infections in people over age 50 (CDC 2008). As opposed to the general population where substance abuse rates decline in people age over 50, older HIV-positive patients seem to maintain steady rates of substance abuse and dependence (Rabkin et al. 2004a, b; Justice et al. 2004). Screening for substance abuse, including illicit drug use, is important for all older patients, especially because of estimated 2- to 10-fold risks of HIV infection (Martinez et al. 2002).
Alcohol abuse and illicit drug use over the lifespan have been also associated with a greater number of comorbid conditions in these older HIV patients. There are several studies that address age-related incidence and prevalence of alcohol abuse and depression in HIV patients. The HIV-Aging Study, a cross-sectional study designed to investigate neuropsychiatric complications in adults aging with HIV (Zanjani et al. 2007), hopes to provide longitudinal data related to substance abuse and older HIV patients. One study examined the impact of alcohol use on depressive symptoms in 400 individuals with HIV and a documented history of alcohol-related diagnoses (Sullivan et al. 2011). Alcohol dependence and heavy alcohol use were significantly associated with higher depression scores in HIV in unadjusted models. However, in adjusted analyses, the association of alcohol dependence persisted, but the effect of heavy alcohol abuse was no longer noted. This was in contrast to a prior study by the same group with a smaller number of subjects that concluded only alcohol dependence in older HIV-infected patients worsened depression (Sullivan et al. 2008).
Substance use, particularly alcohol in older HIV patients, has a profound impact on adherence (Zanjani et al. 2007; Bing et al. 2001). One study looking at the predictors of antiretroviral adherence among 148 HIV-infected adults, with a particular focus on advancing age, neuropsychological dysfunction, and substance abuse, showed older patients (50 years) demonstrating significantly better medication adherence than younger patients (87.5 vs. 78.3 %) (Hinkin et al. 2004). Among the older patients, those who were classified as poor adherers performed significantly worse on neuropsychological testing, particularly on measures of executive function and psychomotor speed. Current drug abuse/dependence, but not current alcohol abuse/dependence, was also associated with sub-optimal medication adherence. Some speculate that this is related to the social isolation that develops from broken relationships after long-term drug use as well as the stigma from the illness itself.
Addiction treatment and rehabilitation can improve adherence and disease outcomes (Azar et al. 2010), but the relationship between adherence and substance abuse can be complicated to manage. Health care providers should be aware of the rates of substance abuse in older HIV-positive individuals, conduct appropriate screening, and offer substance abuse treatment in an effort to decrease the long-term transmission rates of HIV in the older population.
Conclusion
Infection with HIV in older adults is emerging as a major public health problem in the USA and the developing world. Given that HIV is a preventable and treatable illness, research and patient education about the morbidity and mortality in older individuals newly diagnosed with the disease and adults who have aged with the disease are of critical importance. The prevalence of depression, mania, psychosis, cognitive impairment, and substance abuse in the older HIV population underscores the importance of addressing these interdependent psychiatric issues in patients aging with HIV.
Acknowledgments
The authors report no relevant financial relationships. We would like to acknowledge Julia Skapik for providing a critical review of the literature.
Contributor Information
Crystal C. Watkins, Email: cwatkins@jhmi.edu, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Hospital, The Johns Hopkins University School of Medicine, Meyer 119, Baltimore, MD 21287, USA
Glenn J. Treisman, Email: glenn@jhmi.edu, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Hospital, The Johns Hopkins University School of Medicine, Meyer 119, Baltimore, MD 21287, USA. Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
References
- Alciati A, Starace F, Scaramelli B, Campaniello M, Adriani B, Mellado C, Cargnel A. Has there been a decrease in the prevalence of mood disorders in HIV-seropositive individuals since the introduction of combination therapy? Eur Psychiatry. 2001;16:491–496. doi: 10.1016/s0924-9338(01)00611-3. [DOI] [PubMed] [Google Scholar]
- Alciati A, Gallo L, Monforte AD, Brambilla F, Mellado C. Major depression-related immunological changes and combination antiretroviral therapy in HIV-seropositive patients. Hum Psychopharmacol. 2007;22:33–40. doi: 10.1002/hup.813. [DOI] [PubMed] [Google Scholar]
- Angelino AF, Treisman GJ. Issues in co-morbid severe mental illnesses in HIV infected individuals. Int Rev Psychiatry. 2008;20:95–101. doi: 10.1080/09540260701861989. [DOI] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl 1):S11–S18. [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Butt AA, Dascomb KK, DeSalvo KB, Bazzano L, Kissinger PJ, Szerlip HM. Human immunodeficiency virus infection in elderly patients. South Med J. 2001;94:397–400. [PubMed] [Google Scholar]
- Campos LN, Guimaraes MD, Remien RH. Anxiety and depression symptoms as risk factors for non-adherence to antiretroviral therapy in Brazil. AIDS Behav. 2008;14:289–299. doi: 10.1007/s10461-008-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, Duran RE, Ironson G, Penedo F, Fletcher MA, Klimas N, Schneiderman N. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-Positive gay men treated with HAART. Ann Behav Med. 2006;31:155–164. doi: 10.1207/s15324796abm3102_7. [DOI] [PubMed] [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18 (Suppl 1):S27–S34. [PubMed] [Google Scholar]
- Cole MA, Margolick JB, Cox C, Li X, Selnes OA, Martin EM, Becker JT, Aronow HA, Cohen B, Sacktor N, Miller EN. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology. 2007;69:2213–2220. doi: 10.1212/01.WNL.0000277520.94788.82. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS among persons age 50 and older. 2008 At: http://www.cdc.gov/hiv/az.htm.
- Cooperman NA, Simoni JM. Suicidal ideation and attempted suicide among women living with HIV/AIDS. J Behav Med. 2005;28:149–156. doi: 10.1007/s10865-005-3664-3. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2010;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dolder CR, Patterson TL, Jeste DV. HIV, psychosis and aging: past, present and future. AIDS. 2004;18(Suppl 1):S35–S42. [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF, High KP. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich-Höchli E, Niklowitz MW, Luthy R, Opravil M. Are immunological markers, social and personal resources, or a complaint-free state predictors of progression among HIV-infected patients? Acta Psychiatr Scand. 1997;95:476–484. doi: 10.1111/j.1600-0447.1997.tb10135.x. [DOI] [PubMed] [Google Scholar]
- Ellen SR, Judd FK, Mijch AM, Cockram A. Secondary mania in patients with HIV infection. Aust N Z J Psychiatry. 1999;33:353–368. doi: 10.1046/j.1440-1614.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Elliott A. Anxiety and HIV Infection. STEP Perspect. 1998;98:11–14. [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA. Grant I CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–51. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, Myers H, Wright MJ, Foley J. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry. 2009;17:281–290. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15:1576–1579. doi: 10.1097/00002030-200108170-00017. [DOI] [PubMed] [Google Scholar]
- Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, Enel P, Jung C, Mahamat A, Lang JM, Costagliola D. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029–2038. doi: 10.1097/00002030-200410210-00007. [DOI] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22:630–9. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller DL, Miles DR. Suicidal ideation among psychiatric patients with HIV: psychiatric morbidity and quality of life. AIDS Behav. 2003;7:101–108. doi: 10.1023/a:1023985906166. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Jeste DV, Gleghorn A, Sewell DD. New-onset psychosis in HIV-infected patients. J Clin Psychiatry. 1991;52:369–376. [PubMed] [Google Scholar]
- Havlik RJ, Brennan M, Karpiak SE. Comorbidities and depression in older adults with HIV. Sex Health. 2011;8:551–559. doi: 10.1071/SH11017. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54(Suppl):S44–S52. doi: 10.1016/s0895-4356(01)00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J, House A. Psychiatric illness predicts poor outcome after surgery for hip fracture: a prospective cohort study. Psychol Med. 2000;30:921–929. doi: 10.1017/s0033291799002548. [DOI] [PubMed] [Google Scholar]
- Hriso E, Kuhn T, Masdeu JC, Grundman M. Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. Am J Psychiatry. 1991;148:1558–1561. doi: 10.1176/ajp.148.11.1558. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, Marzani-Nissen G. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011;116:177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson GH, O’Cleirigh C, Schneiderman N, Weiss A, Costa PT., Jr Personality and HIV disease progression: role of NEO-PI-R openness, extraversion, and profiles of engagement. Psychosom Med. 2008;70:245–253. doi: 10.1097/PSY.0b013e31816422fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsberg L, Frances A, Perry S. Axis II diagnoses among volunteers for HIV testing and counseling. Am J Psychiatry. 1995;152:1222–1224. doi: 10.1176/ajp.152.8.1222. [DOI] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Atkinson JH, Heaton RK, Young C, Sadek J, Madenwald T, Becker JT, Conigliaro J, Brown ST, Rimland D, Crystal S, Simberkoff M. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS. 2004;18(Suppl 1):S49–S59. [PubMed] [Google Scholar]
- Kalichman SC, Kelly JA, Johnson JR, Bulto M. Factors associated with risk for HIV infection among chronic mentally ill adults. Am J Psychiatry. 1994;151:221–227. doi: 10.1176/ajp.151.2.221. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Heckman T, Kochman A, Sikkema K, Bergholte J. Depression and thoughts of suicide among middle-aged and older persons living with HIV-AIDS. Psychiatr Serv. 2005;51:903–907. doi: 10.1176/appi.ps.51.7.903. [DOI] [PubMed] [Google Scholar]
- Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, Dunne M, Burrows G. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry. 1998;20(6):345–52. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Justice AC, Rabeneck L, Rodriguez-Barradas M, Weissman S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol. 2001;54(Suppl 1):S22–S28. doi: 10.1016/s0895-4356(01)00443-7. [DOI] [PubMed] [Google Scholar]
- Klinkenberg WD, Sacks S. HIV/AIDS Treatment Adherence, Health Outcomes and Cost Study Group. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16 (Suppl 1):S22–S42. doi: 10.1080/09540120412331315303. [DOI] [PubMed] [Google Scholar]
- Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27:159–169. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Stoff DM, Rausch DM. Workshop report: the effects of psychological variables on the progression of HIV-1 disease. Brain Behav Immun. 2004;18:246–261. doi: 10.1016/j.bbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Delong M, Kraemer H, Carney R, Spiegel D, Gordon C, McDonald W, Dew M, Alexopoulos G, Buckwalter K, Cohen PD, Evans D, Kaufmann PG, Olin J, Otey E, Wainscott C. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52(6):559–88. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- Lenz G, Demal U. Quality of life in depression and anxiety disorders: an exploratory follow-up study after intensive inpatient cognitive behaviour therapy. Psychopathology. 2000;33:297–302. doi: 10.1159/000029161. [DOI] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, Durelle J, Grant I, Everall I. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Lodwick R, Costagliola D, Reiss P, Torti C, Teira R, Dorrucci M, Ledergerber B, Mocroft A, Podzamczer D, Cozzi-Lepri A, Obel N, Masquelier B, Staszewski S, Garcia F, De Wit S, Castagna A, Antinori A, Judd A, Ghosn J, Touloumi G, Mussini C, Duval X, Ramos J, Meyer L, Warsawski J, Thorne C, Masip J, Perez-Hoyos S, Pillay D, van Sighem A, Lo Caputo S, Gunthard H, Paredes R, De Luca A, Paraskevis D, Fabre-Colin C, Kjaer J, Chene G, Lundgren JD, Phillips AN. Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med. 2010;170:410–419. doi: 10.1001/archinternmed.2009.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Hanson A, Fishman M, McHugh PR, Treisman GJ. Screening for psychiatric morbidity in a medical outpatient clinic for HIV infection: the need for a psychiatric presence. Int J Psychiatry Med. 1994;24:103–113. doi: 10.2190/URTC-AQVJ-N9KG-0RL4. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M. Depression and survival among HIV-infected persons. JAMA. 1996a;275:35–36. doi: 10.1001/jama.1996.03530250039021. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch JE, Bing EG, Treisman GJ. Changes in depressive symptoms as AIDS develops. The Multicenter AIDS Cohort Study. Am J Psychiatry. 1996b;153:1430–1437. doi: 10.1176/ajp.153.11.1430. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, VanRaden MJ, Treisman GJ, Morgenstern H. Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. JAMA. 1993;270:2563–2567. [PubMed] [Google Scholar]
- Lyketsos CG, Schwartz J, Fishman M, Treisman G. AIDS mania. J Neuropsychiatry Clin Neurosci. 1997;9:277–279. doi: 10.1176/jnp.9.2.277. [DOI] [PubMed] [Google Scholar]
- Maes CA, Louis M. Knowledge of AIDS, perceived risk of AIDS, and at-risk sexual behaviors among older adults. J Am Acad Nurse Pract. 2003;15(11):509–16. doi: 10.1111/j.1745-7599.2003.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D, Grant I. Successful cognitive aging in persons living with HIV infection. J Neurovirol. 2011;17:110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CP, Fain MJ, Klotz SA. The older HIV-positive adult: a critical review of the medical literature. Am J Med. 2008;121:1032–1037. doi: 10.1016/j.amjmed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Martinez A, Israelski D, Walker C, Koopman C. Posttraumatic stress disorder in women attending human immunodeficiency virus outpatient clinics. AIDS Patient Care STDS. 2002;16:283–291. doi: 10.1089/10872910260066714. [DOI] [PubMed] [Google Scholar]
- Marwick KF, Kaaya SF. Prevalence of depression and anxiety disorders in HIV-positive outpatients in rural Tanzania. AIDS Care. 2010;22:415–419. doi: 10.1080/09540120903253981. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24:1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665–1670. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, Santamaria EK, Bell J. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009;21:168–177. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Davidson JR. Completeness of response and quality of life in mood and anxiety disorders. Depress Anxiety. 2000;12 (Suppl 1):95–101. doi: 10.1002/1520-6394(2000)12:1+<95::AID-DA14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Have TT, Gettes DR, Chiappini MS, Weber AL, Brinker-Spence P, Bauer MR, Douglas SD, Evans DL. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159:789–796. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E, Musisi S, Mpungu SK, Katabira E. Clinical presentation of bipolar mania in HIV-positive patients in Uganda. Psychosomatics. 2009;50:325–330. doi: 10.1176/appi.psy.50.4.325. [DOI] [PubMed] [Google Scholar]
- Montoya ID, Whitsett DD. New frontiers and challenges in HIV research among older minority populations. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S218–S221. doi: 10.1097/00126334-200306012-00019. [DOI] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Nurutdinova D, Chrusciel T, Zeringue A, Scherrer JF, Al-Aly Z, McDonald JR, Overton ET. Mental health disorders and the risk of AIDS-defining illness and death in HIV-infected veterans. AIDS. 2012;26:229–234. doi: 10.1097/QAD.0b013e32834e1404. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- Palmer NB, Salcedo J, Miller AL, et al. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care STDS. 2003;17:635–644. doi: 10.1089/108729103771928690. [DOI] [PubMed] [Google Scholar]
- Pappas MK, Halkitis PN. Sexual risk taking and club drug use across three age cohorts of HIV-positive gay and bisexual men in New York City. AIDS Care. 2011;23:1410–6. doi: 10.1080/09540121.2011.565027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Young C, Woods SP, Vigil O, Grant I, Atkinson JH. Screening for major depression in persons with HIV infection: the concurrent predictive validity of the Profile of Mood States Depression-Dejection Scale. Int J Methods Psychiatr Res. 2006;15:75–82. doi: 10.1002/mpr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Davidson EJ, Leserman J, et al. Personality disorder in patients infected with HIV: a controlled study with implications for clinical care. Am J Psychiatry. 1993;150:309–315. doi: 10.1176/ajp.150.2.309. [DOI] [PubMed] [Google Scholar]
- Perretta P, Akiskal HS, Nisita C, et al. The high prevalence of bipolar II and associated cyclothymic and hyperthymic temperaments in HIV-patients. J Affect Disord. 1998;50:215–224. doi: 10.1016/s0165-0327(98)00111-6. [DOI] [PubMed] [Google Scholar]
- Perry EB, Berman RM, Sanacora G, Anand A, Lynch-Colonese K, Charney DS. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65:238–243. doi: 10.4088/jcp.v65n0215. [DOI] [PubMed] [Google Scholar]
- Plummer ML, Watson-Jones D, Lees S, Baisley K, Matari S, Changalucha J, Clayton T, Mugeye K, Tanton C, Weiss HA, Ross DA, Hayes RJ. A qualitative study of participant adherence in a randomized controlled trial of herpes suppressive therapy for HIV prevention in Tanzania. AIDS Care. 2010;22:499–508. doi: 10.1080/09540120903202889. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Wagner GJ, McElhiney MC, Rabkin R, Lin SH. Testosterone versus fluoxetine for depression and fatigue in HIV/AIDS: a placebo-controlled trial. J Clin Psychopharmacol. 2004a;24:379–385. doi: 10.1097/01.jcp.0000132442.35478.3c. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Ferrando SJ. Mood and substance use disorders in older adults with HIV/AIDS: methodological issues and preliminary evidence. AIDS. 2004b;18(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- Rabkin JG, Williams JB, Remien RH, Goetz R, Kertzner R, Gorman JM. Depression, distress, lymphocyte subsets, and human immunodeficiency virus symptoms on two occasions in HIV-positive homosexual men. Arch Gen Psychiatry. 1991;48:111–119. doi: 10.1001/archpsyc.1991.01810260019002. [DOI] [PubMed] [Google Scholar]
- Riley ED, Wu AW, Perry S, Clark RA, Moss AR, Crane J, Bangsberg DR. Depression and drug use impact health status among marginally housed HIV-infected individuals. AIDS Patient Care STDS. 2003;17:401–406. doi: 10.1089/108729103322277411. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. Epub 2005 Jan 25. [DOI] [PubMed] [Google Scholar]
- Sewell DD. Schizophrenia and HIV. Schizophr Bull. 1996;22(3):465–73. doi: 10.1093/schbul/22.3.465. [DOI] [PubMed] [Google Scholar]
- Siegel K, Brown-Bradley CJ, Lekas H-M. Strategies for coping with fatigue among HIV-positive individuals fifty years and older. AIDS Patient Care STDS. 2004;18:275–288. doi: 10.1089/108729104323076016. [DOI] [PubMed] [Google Scholar]
- Singh AN, Golledge H, Catalan J. Treatment of HIV-related psychotic disorders with risperidone: a series of 21 cases. J Psychosom Res. 1997;42:489–493. doi: 10.1016/s0022-3999(96)00373-x. [DOI] [PubMed] [Google Scholar]
- Sledjeski EM, Delahanty DL, Bogart LM. Incidence and impact of posttraumatic stress disorder and comorbid depression on adherence to HAART and CD4+ counts in people living with HIV. AIDS Patient Care STDS. 2005;19:728–736. doi: 10.1089/apc.2005.19.728. [DOI] [PubMed] [Google Scholar]
- Sullivan PS, Zapata A, Benbow N. New U.S. HIV incidence numbers: heeding their message. Focus. 2008;23(4):5–7. [PubMed] [Google Scholar]
- Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend. 2011;117:158–163. doi: 10.1016/j.drugalcdep.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J, Zisook S, Sciolla AD San Diego HIV Neurobehavioral Research Center (HNRC) Group et al. Gender, AIDS, and bereavement: a comparison of women and men living with HIV. Death Stud. 2004;28:225–241. doi: 10.1080/07481180490276562. [DOI] [PubMed] [Google Scholar]
- Trobst KK, Wiggins JS, Costa PT, Jr, et al. Personality psychology and problem behaviors: HIV risk and the five-factor model. J Pers. 2000;68:1233–1252. doi: 10.1111/1467-6494.00133. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67:1282–1290. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldall KK, Berghuis JP. Delirium in AIDS patients: recognition and medication factors. AIDS Patient Care STDS. 1997;11:435–441. doi: 10.1089/apc.1997.11.435. [DOI] [PubMed] [Google Scholar]
- Uldall KK, Harris VL, Lalonde B. Outcomes associated with delirium in acutely hospitalized acquired immune deficiency syndrome patients. Compr Psychiatry. 2000a;41:88–91. doi: 10.1016/s0010-440x(00)90138-x. [DOI] [PubMed] [Google Scholar]
- Uldall KK, Ryan R, Berghuis JP, Harris VL. Association between delirium and death in AIDS patients. AIDS Patient Care STDS. 2000b;14:95–100. doi: 10.1089/108729100318037. [DOI] [PubMed] [Google Scholar]
- Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis. 2006;42:1449–1454. doi: 10.1086/503565. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee MJ, Blenke AA, Rongen GA, Verwey-van Wissen CP, Koopmans PP, Pharo C, Burger DM. Interaction study of the combined use of paroxetine and fosamprenavir-ritonavir in healthy subjects. Antimicrob Agents Chemother. 2007;51:4098–4104. doi: 10.1128/AAC.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Servellen G, Chang B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care STDS. 2002;16:269–281. doi: 10.1089/10872910260066705. [DOI] [PubMed] [Google Scholar]
- Vance DE, Ross JA, Moneyham L, Farr KF, Fordham P. A model of cognitive decline and suicidal ideation in adults aging with HIV. J Neurosci Nurs. 2010;42:150–156. doi: 10.1097/jnn.0b013e3181d4a35a. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Akincigil A, Amin S, Hoover D, Siegel M, Crystal S. Prevalence of diagnosed HIV disease among medicaid beneficiaries with schizophrenia in U.S. metropolitan areas. J Nerv Ment Dis. 2010;198:682–686. doi: 10.1097/NMD.0b013e3181ef21a2. [DOI] [PubMed] [Google Scholar]
- Walkup J, Akincigil A, Hoover DR, Siegel MJ, Amin S, Crystal S. Use of Medicaid data to explore community characteristics associated with HIV prevalence among beneficiaries with schizophrenia. Public Health Rep. 2011;126(Suppl 3):89–101. doi: 10.1177/00333549111260S314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelutiu-Weinberger C, Pachankis JE, Golub SA, Walker JJ, Bamonte AJ, Parsons JT. Age cohort differences in the effects of gay-related stigma, anxiety and identification with the gay community on sexual risk and substance use. AIDS Behav. 2012 doi: 10.1007/s10461-011-0070-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutoh AK, Elekwachi O, Clarke-Tasker V, Daftary M, Powell NJ, Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S106–S114. doi: 10.1097/00126334-200306012-00007. [DOI] [PubMed] [Google Scholar]
- Zablotsky D, Kennedy M. Risk factors and HIV transmission to midlife and older women: knowledge, options, and the initiation of safer sexual practices. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S122–S130. doi: 10.1097/00126334-200306012-00009. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Saboe K, Oslin D. Age difference in rates of mental health/substance abuse and behavioral care in HIV-positive adults. AIDS Patient Care STDS. 2007;21:347–355. doi: 10.1089/apc.2006.0043. [DOI] [PubMed] [Google Scholar]
- Zelnetz PD, Epstein ME. HIV in elderly. AIDS Patient Care STDS. 1998;17:255–262. doi: 10.1089/apc.1998.12.255. [DOI] [PubMed] [Google Scholar]