Abstract
Purpose
To evaluate the impact of dedicated reader education on accuracy/confidence of peripheral zone index cancer and anterior prostate cancer (PCa) diagnosis with mpMRI; secondary aim was to assess the ability of readers to differentiate low-grade cancer (Gleason 6 or below) from high-grade cancer (Gleason 7+).
Materials and methods
Five blinded radiology fellows evaluated 31 total prostate mpMRIs in this IRB-approved, HIPAA-compliant, retrospective study for index lesion detection, confidence in lesion diagnosis (1–5 scale), and Gleason grade (Gleason 6 or lower vs. Gleason 7+). Following a dedicated education program, readers reinterpreted cases after a memory extinction period, blinded to initial reads. Reference standard was established combining whole mount histopathology with mpMRI findings by a board-certified radiologist with 5 years of prostate mpMRI experience.
Results
Index cancer detection: pre-education accuracy 74.2%; post-education accuracy 87.7% (p = 0.003). Confidence in index lesion diagnosis: pre-education 4.22 ± 1.04; post-education 3.75 ± 1.41 (p = 0.0004). Anterior PCa detection: pre-education accuracy 54.3%; post-education accuracy 94.3% (p = 0.001). Confidence in anterior PCa diagnosis: pre-education 3.22 ± 1.54; post-education 4.29 ± 0.83 (p = 0.0003). Gleason score accuracy: pre-education 54.8%; post-education 73.5% (p = 0.0005).
Conclusions
A dedicated reader education program on PCa detection with mpMRI was associated with a statistically significant increase in diagnostic accuracy of index cancer and anterior cancer detection as well as Gleason grade identification as compared to pre-education values. This was also associated with a significant increase in reader diagnostic confidence. This suggests that substantial interobserver variability in mpMRI interpretation can potentially be reduced with a focus on education and that this can occur over a fellowship training year.
Keywords: Prostate cancer, Multiparametric prostate MRI, Reader education
Prostate cancer (PCa) is the most common non-cutaneous malignancy among men in the United States and second leading cause of cancer death [1]. Screening with prostate-specific antigen (PSA) has led to earlier diagnosis of smaller tumors and more localized disease. However, it is well known that the sensitivity and specificity of the test are not optimal. Due to its low specificity, PSA can lead to false positives and detection of small, low-grade lesions that may be clinically insignificant [2]. As such, the potential of over-diagnosis and over-treatment of low-grade disease is a true possibility. Pathologic diagnosis is made by random transrectal ultrasonography (TRUS)-guided biopsies, which are then used to provide the clinician with the disease Gleason score. Owing to the random nature of these biopsies, cancer located outside the routine sampling site is often missed or underdiagnosed. In a study by Mufarrij et al. [3], approximately 46% of patients who were candidates for active surveillance but underwent radical prostatectomy had a higher Gleason score on final histopathology than was originally diagnosed after TRUS biopsy. These inaccuracies have led to interest in targeted strategies in both diagnostic and therapeutic interventions.
Prostate MR is a non-invasive tool that is being used to assess disease burden and identify clinically significant PCa. Current prostate MR relies on a multiparametric approach, whereby typically 3 or more imaging sequences—including anatomic and functional data—are used in conjunction to arrive at a diagnosis [4]. Multiparametric prostate MRI (mpMRI)—including T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE-MRI) imaging—provides a wealth of functional information that has made possible vastly improved detection and characterization of PCa. Because of the inherent limitations of TRUS-guided biopsy in reaching the anterior aspect of the gland, mpMRI has particular value in the detection of anterior cancers.
Research has shown substantial interobserver variability in the interpretation of prostate MRI as a result of heterogeneous reader experience [5, 6]. To the best of the authors’ knowledge, there have been no studies to date that have investigated the role of dedicated reader education on accuracy of prostate cancer diagnosis using a comprehensive multiparametric approach. The purpose of our study was to evaluate the effect of dedicated reader education on diagnostic accuracy and confidence of peripheral zone (PZ) index cancer and anterior cancer diagnosis with mpMRI. A secondary aim of our study was to assess the ability of readers to differentiate low-grade cancer (Gleason 6 or below) from high-grade cancer (Gleason 7+).
Materials and methods
Patient cohort
This was an IRB-approved, HIPAA-compliant study in which a total of 43 patients were prospectively recruited and enrolled in a research database between January 1st 2011 and February 28th, 2013. Inclusion criteria were patients who had undergone complete mpMRI with endorectal coil for detection of prostate cancer, including high-resolution, multiplanar T2-weighted anatomic imaging, diffusion-weighted imaging (DWI), and DCE-MRI at 3 Tesla (3 T) followed by radical prostatectomy and whole mount histology processing. Exclusion criteria included patients who had incomplete mpMRI, previous treatments for prostate cancer, or anatomic anomalies of the rectum.
Case selection for analysis by readers
Cases for analysis by readers were selected by combining information from mpMRI and whole mount histology after radical prostatectomy. The cases were selected by a board-certified, fellowship-trained abdominopelvic radiologist with 5 years of experience in interpreting mpMRI of the prostate in conjunction with a third-year medical student who assisted with case collection. The aim was to identify patients in which a discrete “index cancer” (defined as the largest lesion and the lesion thought to be responsible for the overall biological behavior of the cancer) could be identified on mpMRI and was confirmed on whole mount histology, as it has been shown that the volume of the “index cancer” has as much prognostic significance as total tumor volume [2, 7, 8].
MR-pathology correlation was performed in a similar manner as in previous published studies [9, 10]. In both studies, a positive correlation required that readers identify the exact location of the lesion. However, in order to account for possible changes in the pathologic specimen secondary to removal, fixation, and different slice thickness, some displacement in the cranio-caudal direction was allowed. The prostate was divided into 27 segments as previously described in the Dickinson et al. MRI reporting model [11, 12]. This scheme includes three axial levels (base, midgland, apex) that are each subdivided into four anterior glandular regions (right and left lateral and mediolobar), an anterior fibromuscular stroma (AFS) region and four posterior glandular regions, for a total of 12 anterior and posterior glandular regions, and three AFS regions. In this study, a finding was considered a true positive (TP) if it remained in the same lobe (right or left) and same zone (PZ) vs. central gland (CG) vs. AFS even if it was displaced up to one segment.
Our final cohort included 31 subjects, 24 with PZ prostate cancer and 7 with anterior prostate cancers (mean age 61.0, SD 7.7 years; range 47–74 years). The reason for choosing this split between PZ and anterior cases was to attempt to reflect the distribution of PCa in the general population [13]. The mean Gleason score for the 31 lesions was 7 (range 6–9) and the mean PSA was 6.7 ng/mL (range 2.2–30.1 ng/mL, SD 4.9 ng/mL). Mean lesion size across all index cancers, as measured on whole mount histology, was 2.8 cm (range 1.2–4.5 cm, SD 1.1 cm)
MR imaging
All MR imaging was performed on one of two 3.0 Tesla MR scanners (General Electric HDx, GE Healthcare, Waukesha, WI, USA; Siemens Skyra, Siemens Healthcare, Erlangan, Germany) using a single channel Medrad eCoil endorectal coil (Medrad, Indianola, PA, USA) as well as multichannel surface coils. Imaging sequences included high-resolution, thin-section (3-mm section thickness) fast spin echo T2-weighted images in the coronal, axial, and sagittal planes. Diffusion-weighted images were obtained using multiple b-values from which ADC maps were derived (b values 0 and 800). DCE MR sequences were obtained after administration of a weight-based dose of extracellular MR contrast agent with 5–6 s temporal resolution for 5–6 min. Analysis of the DCE-MRI was performed using colorized perfusion maps created on an independent workstation (VersaVue, ICad Inc., Nashua, NH, USA) and subsequently sent to the PACS system. Detailed MR acquisition parameters are provided in Appendix.
Prostate surgical removal
Radical prostatectomy was performed by one board-certified, fellowship-trained urologist with 15 years of experience. Prostates were radically removed using the da Vinci Surgical System. After excision, the prostates were formalin fixed for at least 24–48 h without being cut, and then processed for whole mount histology.
Whole mount histology processing
The excised radical prostatectomy specimens were fixed in formalin for 48 h and then sectioned and processed. Each prostate was hand sectioned into 2–3 mm thick axial slices, embedded in paraffin, and a microtome was used to cut a 5 λm thin single slice from each thick section. The thin slices were then attached to a glass slide that was processed with Hematoxylin and Eosin staining. A pathologist who was blinded to all imaging data, identified and marked the left–right and anterior–posterior aspect of each slide as well as anatomical structures including the urethra and verumontanum. He also identified BPH, atrophy, and PCa. Each prostate specimen yielded 8–21 whole mount slides. The histology slides were then digitized (0.04 × 0.04 mm) using a flat-bed scanner (Epson Perfection V750-M Pro Scanner with 640 dpi resolution).
Readers and image interpretation
Readers for this study included five board-certified radiologists near completion of their 1-year dedicated abdominal imaging fellowship and varying degrees of previous experience with prostate MR (all less than 50 cases in total). Readers were aware that each patient had undergone mpMRI for possible cancer diagnosis but were blinded to all other clinical and pathological data, including previous MRI reads, TRUS-guided biopsy results, and the fact that these patients had undergone subsequent radical prostatectomy for prostate cancer as not to bias their interpretation of the images.
Each reader was asked to independently interpret 31 mpMRI studies on a PACS workstation, closely mimicking our clinical practice. The cases were randomized and all patient identifiers were eliminated. Readers were asked to identify the presence of only one index lesion and assess its location and Gleason grade (Gleason 6 or lower or Gleason 7+). If more than one lesion was suspicious, readers were asked to identify the one thought most likely to represent the index cancer. If no focal lesion was observed, readers had the option of stating that no index cancer was detected.
The radiologists were also asked to rate their confidence in lesion detection on a 5-point scale (1, <25% confidence; 2, 25%–50% confidence; 3, 50%–75% confidence; 4, 75%–90% confidence; 5, near 100% confidence of diagnosis of prostate cancer). Following a dedicated education program on mpMRI (explained below) and blinded to their initial reads, readers were asked to interpret re-anonymized and re-randomized cases after a memory extinction period of at least 4 weeks.
Educational intervention/interactive didactic lectures
Following their initial interpretation of the first set of 31 mpMRIs, the readers were required to attend two didactic lectures given by a board-certified, fellowship-trained abdominopelvic radiologist with 5 years of experience in interpreting mpMRI of the prostate. The lectures covered current mpMRI techniques, the evolution of MR imaging in prostate cancer diagnosis, its current limitations, and future directions. In addition, multiple cases (different from those in the case cohort) were shown with radiologic–pathologic correlation. Specific aims were to show TP cases in the PZ and anterior prostate as well as representative false positive and false negative cases. The sessions were interactive and the readers were encouraged to participate and ask questions to clarify their understanding.
Statistical analysis
The pre- and post-education accuracies of index lesion detection were calculated across all readers (31 cases × 5 readers = 155 total cases). Accuracy was defined as the percentage of index lesions that were correctly identified, before and after training. A subanalysis of anterior cancer diagnostic accuracy (7 total anterior prostate index cancers × 5 readers = 35 total cases) and Gleason score identification (as Gleason 6 or below or Gleason 7 or above) were also performed.
To perform a combined analysis of the data across all readers, we fit appropriate generalized linear mixed models. We assumed a binomial distribution for detections, with probabilities of detection (sensitivity) given by a model of the form:
where pijk is the probability of detection for the i-th reader (i=1, 2, 3, 5, or 6), j-th case (j = 1, 2, …,31), and k-th read (k = pre or post). The model explains the log odds of this probability in terms of a baseline value μ, which corresponds to a pre-read, non-anterior nodule, λk, the effect of learning and ri, the effect of the i-th reader, assumed to be random, with a zero mean Gaussian distribution. We fit this model using restricted maximum likelihood (REML) via the lme4 package in the R computing platform (www.r-project.org). Similar models were also fit for false positives and the Gleason score. For subset analysis, we also fit a similar model for just the anterior cancers.
For confidence ratings, we fit a model of the form:
where Cijk is the confidence of detection for the i-th reader (i=1, 2, 3, 5, or 6), j-th case (j = 1, 2, …, 31), and k-th read (k = pre or post). The model explain the expected confidence in terms of a baseline value μ, which corresponds to a pre-read, non-anterior nodule, λk, the effect of learning and ri, the effect of the i-th reader, assumed to be random, with a zero mean Gaussian distribution. Finally, εijk is measurement error, assumed to have a zero mean Gaussian distribution. We fit this model using REML via the nlme package in the R computing platform (www.r-project.org). Statistical significance was inferred at p < 0.05.
Results
Diagnostic accuracy and confidence: all index cancers
Data for 31 patients was analyzed in aggregate for the 5 readers, with 31 lesions per reader and n = 155 across all readers. Pre-education accuracy for index lesion detection was 74.2% (115/155) and post-education accuracy was 87.7% (136/155). There was a highly significant improvement in the odds of detection post learning (odds ratio (OR) 2.5, p = 0.003, Table 1). Reader confidence in index lesion detection was significantly higher post-training (4.22 ± 1.04) vs. pre-training (3.75 ± 1.41) (mean diff = 0.47, p = 0.0004, Table 2).
Table 1.
Estimated effects from generalized linear mixed model for detection for all index cancers
| Effect | Log odds | SE | z value | p value |
|---|---|---|---|---|
| Baseline | 1.06 | 0.18 | 5.75 | <0.0001 |
| Learning | 0.91 | 0.31 | 2.98 | 0.003 |
Table 2.
Estimated effects from linear mixed model for confidence for all index cancers
| Effect | Value | SE | DF | t value | p value |
|---|---|---|---|---|---|
| Baseline | 3.75 | 0.23 | 304.00 | 16.27 | <0.0001 |
| Learning | 0.47 | 0.13 | 304.00 | 3.57 | 0.0004 |
The value in the learning category refers to the increase over baseline
Diagnostic accuracy and confidence: anterior cancers
Subset analysis was then performed for anterior PCa with 7 total cases analyzed in aggregate for 5 readers for n = 35 lesions. Pre-education accuracy for anterior PCa detection was 54.3% (19/35) and post-education accuracy was 94.3% (33/35) (OR = 13.77, p = 0.001, Table 3). Confidence in anterior PCa diagnosis was significantly higher post-training (4.29 ± 0.83) vs. pre-training (3.22 ± 1.54) (mean diff = 1.06, p = 0.0003, Table 4) (Fig. 1).
Table 3.
Estimated effects from generalized linear mixed model for detection for anterior cancers
| Effect | Log odds | SE | z value | p value |
|---|---|---|---|---|
| Baseline | 0.17 | 0.34 | 0.51 | 0.61 |
| Learning | 2.63 | 0.80 | 3.28 | 0.001 |
Table 4.
Estimated effects from linear mixed model for confidence for anterior cancers
| Effect | Estimate | SE | DF | t value | p value |
|---|---|---|---|---|---|
| Baseline | 3.23 | 0.28 | 64.00 | 11.40 | <0.0001 |
| Learning | 1.06 | 0.28 | 64.00 | 3.81 | 0.0003 |
The value in the learning category refers to the increase over baseline
Fig. 1.
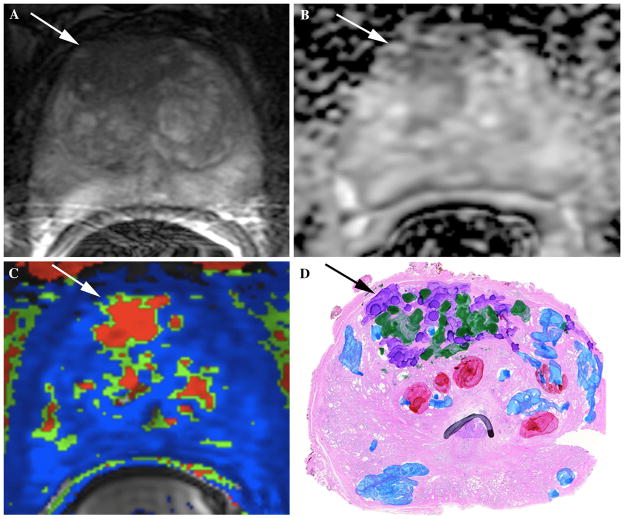
69-year-old male with increasing PSA value (7.8 ng/mL at time of mpMRI exam) and prior TRUS-guided biopsy showing Gleason 3+3 (6) tumor on the right apex in 1/12 cores. Patient was referred for mpMRI with endorectal coil for disease localization/characterization and formulation of a clinical management plan. A Axial T2W image at the level of the apex shows a large area of decreased T2 signal intensity in anterior right prostate gland with extension to the left (white arrow). B Axial ADC map shows corresponding area of markedly restricted diffusion and low ADC value (white arrow). This large lesion in the anterior right prostate apex was considered to be the index lesion and felt to represent a Gleason 7+ tumor in light of the markedly restricted diffusion. C Colorized perfusion map created using post-processing software from DCE-MRI acquisition shows evidence of suspicious perfusion kinetics (white arrow) in this region, corresponding with findings seen on T2W and ADC map. D Whole mount histology image at this location within the prostate confirms the presence of an anterior index lesion (black arrow) with components of Gleason 3 (green) and Gleason 4 (purple) disease. For this case, reader pre-education accuracy of index lesion detection was 60%, which improved to 100% after the educational intervention.
Gleason scores: all index cancers
Accuracy of Gleason score estimation as Gleason 6 or below vs. Gleason 7+ across all cases was 54.8% (85/155) pre-education and significantly improved to 73.5% (114/155) post-education (OR = 2.4, p = 0.0005, Table 5). Anterior cancer Gleason score estimation also significantly improved to 80.0% (28/35) from 45.7% (16/35) post-education (OR = 5.8, p = 0.002, Table 6).
Table 5.
Estimated effects from generalized linear mixed model for Gleason score characterization for all index cancers
| Effect | Log odds | SE | z value | p value |
|---|---|---|---|---|
| Baseline | 0.21 | 0.25 | 0.83 | 0.41 |
| Learning | 0.86 | 0.25 | 3.48 | 0.0005 |
Table 6.
Estimated effects from generalized linear mixed model for Gleason score characterization for anterior cancers
| Effect | Log odds | SE | z value | p value |
|---|---|---|---|---|
| Baseline | −0.17 | 0.34 | −0.51 | 0.61 |
| Learning | 1.75 | 0.56 | 3.11 | 0.002 |
False positives
The number of false positives across all cases was 30.6% (32/155) pre education and significantly decreased to 6.5% (10/155) post-education (OR = 0.26, p = 0.0005, Table 7).
Table 7.
Estimated effects from generalized linear mixed model for false positives
| Effect | Log odds | SE | z value | p value |
|---|---|---|---|---|
| Baseline | −1.35 | 0.20 | −6.79 | <0.0001 |
| Learning | −1.33 | 0.38 | −3.47 | 0.0005 |
Discussion
T2-weighted MR imaging has been used in the workup of patients with prostate cancer since the 1980s, yet the literature on its diagnostic accuracy was initially heterogeneous. The more recent addition of functional sequences to create an mpMRI has improved the ability to localize focal lesions [2]. Despite the improved technology and literature supporting its possible role in the detection of prostate cancer, the overall application of mpMRI in clinical practice remains relatively low, possibly related to inconsistent technique and interpretation across different centers.
Our study results show that a dedicated mpMRI prostate education program is associated with a statistically significant increase in diagnostic accuracy of index cancer lesion detection as compared to pre-education values (74.2% vs. 87.7%, p = 0.003) in a group of Abdominal Imaging fellows with similar levels of experience. In addition, diagnostic confidence increased post-education both overall and for anterior cancers. This suggests that, with continued focus on increasing reader education, mpMRI could begin to play a greater role in PCa detection and management.
In 2010, Akin et al. [14] studied the role of interactive dedicated training in improving the accuracy of PCa diagnosis with T2-weighted MR imaging. While our data are in line with their findings that dedicated education improves accuracy, we feel that our study reflects a more current state of clinical practice as we have included functional MR imaging in addition to anatomic imaging, compared to their study in which only conventional anatomical MRI techniques were utilized [14]. The role and value of functional imaging sequences in the setting of prostate MRI has been studied by assessment of the performance of T2WI, DWI, DCE-MRI, and magnetic resonance spectroscopic imaging (MRSI) sequences as compared with histopathology after patients underwent radical prostatectomy. It has been shown that any two functional sequences [(DWI, DCE-MRI, and/or MR spectroscopic imaging (MRSI)] in addition to T2W1 yielded better results than one functional sequence alone and that addition of a third functional sequence did not add substantial value for diagnosis of PCa [15]. Our study included two functional imaging modalities (DWI and DCE-MRI), thus representing current mpMRI practice.
In 2012, Rosenkrantz et al. [9] evaluated the utility of mpMRI in PCa index lesion diagnosis. As stated above, our methods of determining interpretation accuracy were similar, however, in this study, we performed imaging with use of endorectal coils at 3 T and utilized more robust perfusion protocols with creation of colorized perfusion maps for analysis. In addition, our purpose was to assess the impact of dedicated education on accuracy of diagnosis, and therefore, we studied a fairly homogenous subset of readers (all board-certified radiologists, completing their Abdominal Imaging fellowship with an experience level of less than 50 cases). This is in contrast to the study from Rosenkrantz et al. in which four out of six readers had 2–5 years of experience with prostate imaging after completion of their fellowship and no dedicated educational program was implemented.
In addition to overall index lesion diagnosis, it is important to note that subset analysis of anterior prostate cancer detection also reflected significantly increased accuracy after dedicated reader education from 54.3% pre-education to 94.3% after education (p = 0.001). This finding is particularly important as anterior cancers can be missed on TRUS-guided biopsies as the needle may not be able to reach them with such an approach [13, 16]. MpMRI can facilitate more accurate lesion localization, especially in the anterior prostate, and could potentially fill an existing void in anterior prostate cancer diagnosis.
Reader confidence also statistically significantly improved between pre-education and post-education reading sessions. Confidence is a critical factor in assessing the likelihood that a patient harbors clinically significant prostate cancer and is important from a reporting standpoint. In some as yet unpublished data from our group, confidence scores of 4–5/5 were shown to have specificity of 86%–96% and negative predictive value (NPV) of 89%–92% in diagnosing clinically significant prostate cancer (size >0.2 mL and/or Gleason score of 7+). Of note, increasing the confidence threshold for definition of positive mpMRI from 3/5 to 4/5 resulted in only minor drops of sensitivity and almost unchanged NPVs but improved specificity and PPV by several percentage points; this suggests that equivocal mpMRI results (3/5 confidence score) could be considered negative with little or no deleterious impact on the diagnostic properties of the study [17]. Though the absolute value of change in confidence across readers was small between pre-education and post-education sessions for both all index lesions and anterior cancers, it was statistically significant. The fact that the confidence score after education was greater than 4/5 suggests that readers felt more certain that the index lesion identified was representative of clinically significant disease.
Finally, in addition to accurately identifying the location of the prostate cancer, the ability to determine which cancers may exhibit more aggressive behavior is important when making clinical management decisions. The Gleason score is one of the main tools used to stratify patients into risk-categories using the D’Amico criteria, to assess prognosis with nomograms such as the Partin tables, and to make treatment decisions [18, 19]. A number of studies have found that lower ADC values correlate with tumors that have higher Gleason scores [20–22]. Our results suggest that dedicated prostate cancer mpMRI training can lead to improved Gleason grade characterization (Fig. 2) and thus, potentially aid clinicians in management and treatment planning. These findings suggest an added value of training readers in mpMRI, especially in an era where there has been a shift from radical prostatectomy to more focal therapies and active surveillance for patients with perceived lower risk disease.
Fig. 2.
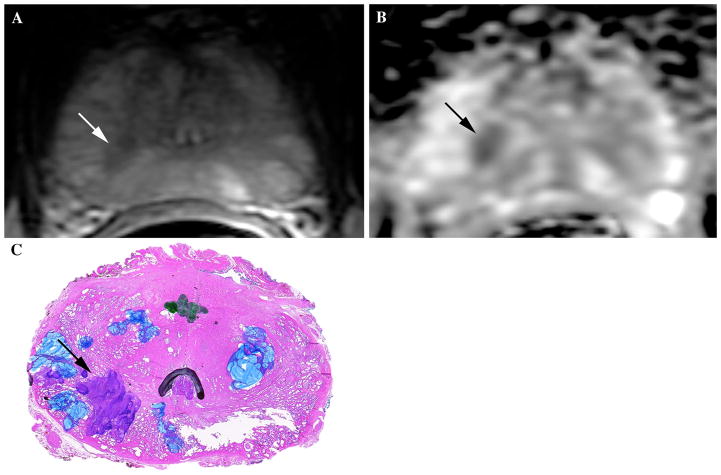
61-year-old male with elevated PSA value (6.1 ng/mL at time of mpMRI exam) and prior TRUS-guided biopsy showing moderate-high volume Gleason 5+4 (9) tumor on the right in 4/6 cores and low volume Gleason 3+3 (6) tumor on the left in 2/6 cores. Patient was referred for mpMRI with endorectal coil for pre-operative planning. A Axial T2W image at the level of the midgland shows an area of decreased T2 signal intensity in posterior medial right PZ (white arrow). B Axial ADC map shows corresponding area of markedly restricted diffusion and low ADC value (black arrow). This lesion was considered to be the index lesion and felt to represent a Gleason 7+ tumor in light of the markedly restricted diffusion. C Whole mount histology image at this location within the prostate confirms the presence of an index lesion (black arrow) with components of Gleason 4 (purple) disease, corresponding with findings on initial biopsy and mpMRI. Of note, the blue marking on the image reflects prostatic atrophy and the green marking in the center refers to a small low-grade focus of prostate cancer that was not deemed to be the index cancer. This case is an example of one used in the dedicated reader education program.
We do acknowledge that our study had some limitations. First, our stringent inclusion criteria included only men with PCa that underwent mpMRI at 3 T with an endorectal coil and radical prostatectomy with processed whole mount histology. These conditions excluded a large number of PCa patients from our institution, leading to a relatively small sample size of cases for analysis. In addition, we had to ensure that index cancers seen on histology were in fact visible on mpMRI. As such, we were able to identify 31 total cases. A larger study sample with individual analysis by reader would be an excellent avenue for future research. Second, our readers were blinded to all clinical data, which is not routine in the clinical setting. The readers did not have access to biopsy results, which tend to include the location of the positive biopsy cores. Thus, overall accuracy levels might have been initially underestimated as the diagnosis was based on mpMRI findings alone. However, blinding was necessary to appropriately assess the effect of dedicated reader education on improving accuracy, and it was critical that the readers not know that a diagnosis of prostate cancer existed in order to minimize bias during image interpretation.
In conclusion, a dedicated reader education program on PCa detection with mpMRI was associated with a statistically significant increase in diagnostic accuracy of index cancer and anterior cancer detection as well as Gleason grade identification as compared to pre-education values. This also was associated with a significant increase in reader diagnostic confidence, which is critical for accurate mpMRI reporting. Of note, our study was completed within a fellowship training year, indicating that a prostate-specific mpMRI training program could be achieved within this timeframe. This suggests that with continued focus on increasing reader education, mpMRI could begin to play a greater role in targeted prostate cancer detection and management.
Acknowledgments
The authors gratefully acknowledge the contributions of Steven Breault, M.D., Andrew Buck, M.D., Lauren Burke, M.D., Ghaneh Fananapazir, M.D., Alex Kim, M.D., Evan Kulbacki, M.D., Samantha Lipman, B.S., John Madden, M.D, Ph.D., Kathryn Nightingale, Ph.D., Arthur Parsee, M.D., and Jose Pratts, M.D., without whom this project would not have been possible.
Appendix: Multiparametric MRI acquisition parameters
Array spatial sensitivity-encoding technique (parallel imaging) factor of 2–3 was used in all sequences.
T2-weighted Imaging Parameters
TR range/TE range = 3700–4610/84–102 (milliseconds, ms)
Matrix size = 448 × 360 (Axial); 384 × 230–260 (Coronal, Sagittal)
Echo-train length = 25
Number of signals acquired = 3
Section thickness = 3 mm
Intersection gap = 0 mm
FOV = 16 cm
Diffusion-weighted imaging parameters
TR range/TE range = 8000/80
Matrix size = 160 × 160
b values = 0 and 800 s/mm2
Number of signals acquired = 6
Slice thickness = 3 mm
Gap = 0 mm
FOV = 16 cm
T1-weighted, 3D, gradient-echo, and free-breathing axial DCE MR images covering the entire prostate were acquired starting 11 s before the IV administration of gadopentetate dimeglumine (Magnevist, Bayer Pharma AG) at a dose of 0.1 mmol/kg, followed by a 20-mL saline flush at a rate of 2.0 mL/s.
DCE MRI Parameters
TR range/TE range = 4.09/1.44
Matrix size = 192 × 160
Flip angle = 15°
Interpolated slice thickness = 3 mm with temporal resolution of 5–6 s for approximately 5–6 min (approximately 70 sets of images were acquired to monitor the time course of contrast agent uptake and clearance within the prostate).
Footnotes
This project was performed at the Departments of Radiology, Surgery and Biomedical Engineering at Duke University Medical Center.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2011. [Accessed 8 June 2013]. based on November 2012 SEER data submission, posted to the SEER web site. http://www.seercancergov/csr/1975_2010. [Google Scholar]
- 2.Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB. The state of prostate MRI in 2013. Oncology. 2013;27(4):262–270. [PubMed] [Google Scholar]
- 3.Mufarrij P, Sankin A, Godoy G, Lepor H. Pathologic outcomes of candidates for active surveillance undergoing radical prostatectomy. Urology. 2010;76(3):689–692. doi: 10.1016/j.urology.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 4.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruprecht O, Weisser P, Bodelle B, Ackermann H, Vogl TJ. MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol. 2012;81(3):456–460. doi: 10.1016/j.ejrad.2010.12.076. [DOI] [PubMed] [Google Scholar]
- 6.Mullerad M, Hricak H, Wang L, et al. Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology. 2004;232(1):140–146. doi: 10.1148/radiol.2321031254. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi M, Stamey TA, McNeal J, Nolley R. Prognostic factors formultifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol. 2003;170(2):459–463. doi: 10.1097/01.ju.0000070928.49986.04. [DOI] [PubMed] [Google Scholar]
- 8.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60(2):264–269. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkrantz AB, Deng F-M, Kim S, et al. Prostate cancer: multiparametric MRI for index lesion localization: a multiple-reader study. AJR Am J Roentgenol. 2012;199(4):830–837. doi: 10.2214/AJR.11.8446. [DOI] [PubMed] [Google Scholar]
- 10.Turkbey B, Mani H, Shah V, et al. Multiparametric 3 T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson L, Ahmed HU, Allen C, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imag. 2013;37(1):48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59(4):477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Bott S, Young M, Kellett M, Parkinson M. Anterior prostate cancer: is it more difficult to diagnose? BJU Int. 2002;89(9):886–889. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 14.Akin O, Riedl CC, Ishill NM, et al. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol. 2010;20(4):995–1002. doi: 10.1007/s00330-009-1625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riches SF, Payne GS, Morgan VA, et al. MRI in the detection of prostate cancer: combined apparent diffusion coefficient, metabolite ratio, and vascular parameters. AJR Am J Roentgenol. 2009;193(6):1583–1591. doi: 10.2214/AJR.09.2540. [DOI] [PubMed] [Google Scholar]
- 16.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188(6):2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsivian M, Gupta RT, et al. Multiparametric magnetic resonance imaging for detection and localization of prostate cancer: diagnostic properties. in press. [Google Scholar]
- 18.Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111(1):22–29. doi: 10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 20.Kobus T, Vos PC, Hambrock T, et al. Prostate cancer aggressiveness: in vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology. 2012;265(2):457–467. doi: 10.1148/radiol.12111744. [DOI] [PubMed] [Google Scholar]
- 21.Woodfield CA, Tung GA, Grand DJ, et al. Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194(4):W316–W322. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 22.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–461. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]