Abstract
Bone morphogenetic protein (BMP) signaling is a critical regulator of cartilage differentiation and endochondral ossification. Gain-of-function mutations in ALK2, a type I BMP receptor, cause the debilitating disorder fibrodysplasia ossificans progressiva (FOP) and result in progressive heterotopic (extraskeletal) endochondral ossification within soft connective tissues. Here, we used murine mesenchymal progenitor cells to investigate the contribution of Alk2 during chondrogenic differentiation and heterotopic endochondral ossification (HEO). Alk2R206H/+ (gain-of-function), Alk2CKO (loss-of-function), and wild-type mouse embryonic fibroblasts were evaluated for chondrogenic potential. Chondrogenic differentiation was accelerated in Alk2R206H/+ cells, due in part to enhanced sensitivity to BMP ligand. In vivo, Alk2R206H/+ cells initiated robust HEO and recruited wild-type cell contribution. Despite expression of other type I BMP receptors (Alk3 and Alk6), chondrogenesis of Alk2CKO cells was severely impaired by absence of Alk2 during early differentiation. Alk2 is therefore a direct regulator of cartilage formation and mediates chondrogenic commitment of progenitor cells. These data establish that at least one effect of ALK2 gain-of-function mutations in FOP patients is enhanced chondrogenic differentiation which supports formation of heterotopic endochondral bone. This establishes ALK2 as a plausible therapeutic target during early chondrogenic stages of lesion formation for preventing heterotopic bone formation in FOP and other conditions.
Keywords: Alk2, Chondrogenesis, Bone morphogenetic protein signaling, Fibrodysplasia ossificans progressiva, Endochondral ossification
Introduction
Normal development of the skeletal long bones occurs through endochondral ossification, the process by which a cartilage template is replaced by bone. Condensing mesenchymal progenitor cells proliferate and differentiate into chondrocytes, then are subsequently replaced by osteoblasts and bone [1]. Such endochondral bone formation also occurs in acquired and inherited forms of heterotopic endochondral ossification (HEO), the formation of bone within nonskeletal tissues [2, 3]. HEO formation follows the same general cellular processes that form skeletal bone; however, the specific cell types and mechanisms that modulate the cascade of ectopic ossification remain undetermined.
Fibrodysplasia ossificans progressiva (FOP; MIM #135100), an inherited disease of HEO, is an autosomal dominant disorder characterized by progressive endochondral bone formation within soft connective tissues [2, 4–6]. Patients develop highly inflammatory and vascular swellings (lesion flare-up) foreshadowing the apoptosis of affected skeletal muscle and connective tissue and repopulation by mesenchymal progenitor cells [7–9]. These progenitor cells differentiate to cartilage that transitions to mature mineralized bone tissue [10, 11]. All confirmed cases of FOP are caused by mutations in the ACVR1 gene, which encodes ALK2, a type I bone morphogenetic protein (BMP) receptor [5, 6, 12]. Most FOP patients have the same specific R206H substitution in ALK2.
BMPs are extracellular ligands, part of the TGFβ superfamily, which exert their effects by binding to heteromeric complexes of type I and type II transmembrane serine/threonine kinase BMP receptors [13]. Signal transduction is mediated by four type I receptors (ALK2 [ACVR1], ALK3 [BMPR1A], ALK6 [BMPR1B], and ALK1 [ACVR1L]) and three type II receptors (ACTR2A, ACTR2B, and BMPR2). Upon ligand binding, the type II receptor phosphorylates the type I receptor GS domain. This facilitates activation of the neighboring protein kinase domain that subsequently induces downstream signal transduction by phosphorylating BMP-specific Smads (Smad1, Smad5, and Smad8) and/or components of the mitogen-activated protein kinase (MAPK) pathway to regulate gene transcription [14]. The ALK2R206H mutation in FOP appears to alter molecular interactions with the inhibitory protein FKBP12 and destabilize tertiary protein structure toward an activated conformation [15–18].
Signaling through BMPs and their receptors is a key regulator of chondrogenesis during development. BMP signaling is essential during mesenchymal cell condensation preceding initial chondrocyte formation [19] and further participates in the proliferation and maturation of chondrocytes during the development of cartilage and bone [20, 21]. Canonical BMP signal transduction through Smad protein phosphorylation is indispensable for proper chondrogenesis [22].
The Alk2R206H gain-of-function mutation enhances both canonical (phospho-Smad1/5/8) and noncanonical (phophop38) BMP signaling responses in the absence of ligand [17, 18, 23–25]. Furthermore, lesion biopsies from FOP patients and a R206H Acvr1 knockin mouse model revealed that cartilage differentiation occurs within regions of fibroproliferation [2, 10, 11, 26]. The induction of chondrogenesis is therefore an important early step in the pathology of FOP. Effects of the Alk2R206H mutation on in vitro chondrogenic differentiation were shown by over-expression of Alk2R206H in chick limb bud micromass cultures [17]. These experiments supported chondrogenic regulation by Alk2; however, did not reproduce the heterozygous mutant state that occurs in patients and, since limb bud cells are committed toward chondrogenesis, could not evaluate the early critical commitment stages of progenitor cells.
In this study, we examined heterozygous Alk2R206H expression in mesenchymal progenitor cells and determined that differentiation to cartilage in FOP patients is a direct consequence of heightened Alk2 signaling. We report that Alk2R206H/+ mouse embryonic fibroblasts (MEFs) have enhanced sensitivity toward chondrogenesis both in vitro and in vivo. In addition, chondrogenesis by Alk2-deficient cells demonstrated that Alk2 is a key regulator of chondrogenic commitment.
MATERIALS AND METHODS
Animal Care and Use
Knockin Alk2R206H/+ founder mice [26] generated germline Alk2R206H/+ and Alk2+/+ wild-type embryos for MEFs. Homozygous Alk2floxed/floxed mice [27] were bred to B6.Cg-Tg(CAG-cre/Esr1)5Amc/J mice [28] for Alk2fl/fl;Cre/Esr1 (Alk2CKO) embryos. C57BL/6-Tg(CAG-EGFP)10sb/J mice [29] were from Jackson Laboratory, Bar Harbor, ME, http://www.jax.org/. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at University of Pennsylvania.
Cell Culture
MEFs were isolated from 13.5 dpc mouse embryos [30]. With head and viscera removed, cells from each embryo were cultured individually in growth media (Dulbecco’s modified Eagle’s medium [DMEM, Gibco, Carlsbad, CA, http://www.lifetechnologies.com/] containing 10% fetal calf serum (FCS) [Invitrogen, Carlsbad, CA, http://www.lifetechnologies.com/]). MEFs at passages 3 and 4 were used for experiments. At least three individual embryo samples were used for experimental replicates.
For signaling assays, MEFs were cultured in DMEM without serum for 2 hours prior to adding 15 ng/ml hrBMP4 (R&D Systems, Minneapolis, MN, http://www.rndsystems.com/) for 1 hour. For analysis of prechondrogenic markers, MEFs were in growth media. For growth curves, cells were plated at 1.5 × 104 cells per square centimeter and counted at time points by Trypan Blue (Gibco) exclusion.
Immunoblot Analysis
Total cell protein was recovered using M-PER containing Halt Protease and Halt Phosphatase Inhibitor Cocktails and quantified using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Wilmington, DE, http://www.fishersci.com/). Proteins were electro-phoresed through 10% SDS-polyacrylamide gels and transferred to nitrocellulose (Invitrogen). Membranes were blocked in 5% milk and incubated with primary antibodies against: phosphorylated Smad1/5/8 (1:750) and β-actin (1:3,000) (Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com/), at 4°C overnight. Bound antibodies were detected with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:6,000) (Cell Signaling Technology) at room temperature, 1 hour. Detected proteins were imaged with Immobilon Chemiluminescent HRP Substrate (Millipore, Billerica, MA, http://www.millipore.com) and quantified using ImageJ Software.
RNA Isolation and Real-Time RT-PCR
RNA was isolated from undifferentiated MEFs in monolayer or MEFs in alginate spheres using TRIzol (Invitrogen) and quantified. cDNA was synthesized using High Capacity RNA-to-cDNA reagents (Applied Biosystems, Foster City, CA, http://www.lifetechnologies.com/). Real-time quantitative PCR reactions contained forward/reverse primers (0.37 µM, Supporting Information Table S1), cDNA (1:10 dilution), and Fast SYBR Green PCR Master Mix (Applied Biosystems); each sample was analyzed in triplicate. Target gene mRNAs were quantified from standard curves and normalized to the indicated housekeeping gene.
Cell Differentiation
For adipogenesis and osteogenesis, cells were seeded at 2.5 × 104 cells per square centimeter and cultured to confluence.
Adipogenic media (10% FCS, 1 µM dexamethasone, 10 mg/ml insulin, 0.5 mM IBMX [Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com/], and 10 µm rosaglitazone [Cayman Chemical, Ann Arbor, MI, www.caymanchem.com] in high glucose DMEM) were replenished every 3 days. For assays, cells were homogenized in TRIzol reagent, extracted for total protein, or formalin-fixed and stained with 0.2% oil red O (Sigma-Aldrich). Isopropanol extracted oil red O for quantification at 550 nm absorbance; samples were normalized to total protein of replicate wells.
Osteogenic media (10% FCS, 50 µg/ml ascorbic acid, 10 mM β-glycerophosphate (Sigma-Aldrich), and 100 ng/ml hrBMP4, in high glucose DMEM) were replenished every 3 days. For assays, cells were homogenized in TRIzol reagent, extracted for total protein, or stained with Alizarin red (Ricca Chemical, Arlington, TX, http://www.riccachemical.com/). Solution of 0.5 N HCl, 5% SDS extracted the deposited Alizarin red for quantification at 405 nm absorbance; samples were normalized to total protein of replicate wells.
For chondrogenesis, cell suspensions at 6.7 × 106 cells per milliliter in 1.2% alginate (Sigma-Aldrich) solution were extruded through 16-guage needles into 102 mM CaCl2 (Thermo Fisher Scientific), forming alginate spheres of ~1.0 × 105 cells in 30 µl [31]. Chondrogenic media (0.1 µM dexamethasone, 50 mg/ml l-ascobate-2-phosphate, 40 mg/ml l-proline [Sigma-Aldrich], 100 µg/ml sodium pyruvate [Gibco], and 1:100 ITS+ culture supplement [BD Biosciences, San Jose, CA, http://www.bdbiosciences.com/]) in high glucose DMEM with or without indicated concentrations of hrBMP4 were replenished every 3 days. To recombine floxed Alk2CKO cells, 1.2 nM 4-hydroxytamoxifen (Sigma-Aldrich) was added to chondrogenic media containing alginate spheres for 48 hours; genomic DNA isolated from cell pellets was amplified to confirm efficient recombination equivalent to tamoxifen treatment of monolayer culture. To assay, alginate spheres were formalinfixed for histology or incubated with 55 mM sodium citrate (Sigma-Aldrich) to release cells.
Cell Implants
A modified Matrigel implant protocol for heterotopic ossification [7, 32] was used to insert wild-type and Alk2R206H/+ MEFs into the hind limbs of wild-type C57Bl/6-Tg(CAG-EGFP) 10sb/J mice (n = 4 per MEF genotype). Prior to implant, cells were labeled with Qtracker625 quantum dots (Qdots) (Invitrogen). Qdots localize to the cell cytoplasm, are unable to diffuse back out through the cell membrane, and maintain fluorescence for at least 8 weeks in vivo [33]. Labeled cells (2.67 × 106 cells per milliliter) in phenol red-free Matrigel (BD Biosciences) with 3.33 µg/ml hrBMP4 were injected (150 µl) into the right anterior tibialis muscles; contralateral left anterior tibialis muscles were injected with BMP/Matrigel (no cells). Upon injection, Matrigel solidifies into a porous scaffold that remains localized to the injection site and fully containing the cells. At 3 weeks postinjection, animals were analyzed.
MicroCT Analysis
High-resolution, cross-sectional images of injected hind limbs were obtained using a VivaCT 40 (Scanco, Nokomis, FL, http://www.scanco.com/) at a source voltage of 55 kV, a source current of 142 µA, and an isotropic voxel size of 38.0 µm. A three-dimensional (3D) image was reconstructed using Scanco microCT V6.1 software. The skeletal bone of the hind limbs and the sites of ectopic ossification were imaged separately, using two different thresholds to optimize visualization and quantification of HEO formation. The optimal threshold for the skeletal bone was a lower threshold of 212 Hounsfield and an upper threshold of 1,000 Hounsfield units. The optimal threshold for detecting ectopic ossification was a lower threshold of 150 Hounsfield and an upper threshold of 1,000 Hounsfield units. Detected ectopic mineralization was quantified using Scanco microCT V6.1 software.
Histology and Immunohistochemistry
Chondrogenic alginate spheres were formalin-fixed overnight then embedded in paraffin and sectioned serially at 7 µm. Deparaffinized sections were incubated with 55 mM sodium citrate (Sigma-Aldrich) at 37°C to remove alginate then stained with Alcian blue (pH 2.5) (Sigma-Aldrich) and counter-stained by nuclear fast red (American MasterTech, Lodi, CA, http://www.americanmastertech.com/). For type II collagen immunohistochemistry, deparaffinized sections were treated for antigen retrieval with Proteinase K (20 µg/ml) (Roche, Indianapolis, IN, http://www.roche.com/); endogenous peroxidase activity was quenched with hydrogen peroxide (3%) and incubation with Background Buster (Innovex Biosciences, Richmond, CA, http://innvx.com/). Type II collagen primary antibody (1:2,000) (Abcam, Cambridge, MA, http://www.abcam.com/), 4°C overnight, was followed by incubated with anti-rabbit HRP-linked secondary antibody and detection with DAB, 3,3′-Diaminobenzidine, Rabbit SuperPicTure Kit (Invitrogen). Counterstain was hematoxylin (Sigma-Aldrich).
For Tg(CAG-EGFP) tissues, muscle was dissected from skeletal bone, fixed in zinc-formaldehyde (4%), decalcified in EDTA (pH 6.5) (Gibco), then transferred to sucrose (30%) (Thermo Fisher Scientific), and embedded in optimal cutting temperature (OTC) (American MasterTech) before serial sectioning at 7 µm and staining with Harris-Modified hematoxylin and eosin Y, safranin-O (American MasterTech), and alcian blue-hematoxylin-orange G [34]. For green fluorescent protein (GFP) immunohistochemistry, sections were permeabilized with Triton X-100 (0.1%) (Thermo Fisher Scientific), quenched with hydrogen peroxide (3%), and incubated with Background Buster. GFP primary antibody (1:3,000) (Abcam) at 4°C overnight, followed by anti-rabbit HRP-linked secondary antibody and DAB, Rabbit SuperPicTure Kit were used for detection. Counterstain was hematoxylin.
Data Analysis
Values are expressed as the mean ± SEM in line and bar graphs. All data are from a minimum of three independent experiments. All data are normalized to wild-type levels in the absence of ligand where relevant. Student’s t test (two-sided, equal variance) was performed; significance was p < .05.
Results
BMP Signaling Is Dysregulated in Alk2R206H/+ Cells
Altered BMP signaling in response to the mutant ALK2R206H has been previously described in both transiently transfected mammalian cells and patient-derived cells [17, 18, 23–25]. However, such over-expression may lead to incorrect representation of the mutant receptor effects on biological processes. Furthermore, patient-derived cells (SHED and LCL) show variability in signaling levels due to varied genetic backgrounds of individual patients. To develop a stable and reproducible mesenchymal progenitor cell system, we isolated primary MEFs from Alk2R206H/+ knockin mouse embryos [26], in which the mutant receptor is expressed from the endogenous Acvr1 locus.
Both Alk2R206H/+ and wild-type cells express the full repertoire of known type I and type II BMP receptors (Alk1, Alk3, Alk6, Actr2a, Actr2b, and Bmpr2) at similar levels (Fig. 1A). Gain-of-function activity of Alk2R206H was confirmed by immunoblot assays for Smad1/5/8 phosphorylation (pSmad1/5/8). In the absence of exogenous BMP ligand, pSmad1/5/8 is negligible in wild-type cells, while signaling in Alk2R206H/+ cells is detectable due to leaky receptor activity (Fig. 1B). BMP ligand induces rapid pSmad1/5/8 but this is further enhanced in Alk2R206H/+ cells (Fig. 1B). The pSmad1/5/8 levels observed in MEFs are comparable to those of patient-derived cells [24]. We further quantified the BMP signaling response by qRT-PCR to detect expression of specific BMP responsive transcription factors: Id1, Id2, Id3, and Msx2 [35]. Without BMP ligand, increased expression of each factor was observed in Alk2R206H/+ cells compared to wild-type cells (Fig. 1C). In the presence of BMP4, Msx2 maintained increased expression relative to wild-type (Fig. 1C). Together, these results corroborate the dysregulated canonical BMP signaling in our MEF culture system that has been previously described in patient cells and over-expression systems [17, 18, 23–25, 36].
Figure 1.
Enhanced BMP signaling of Alk2R206H/+ mouse embryonic fibroblasts (MEFs). (A): Expression of type I and type II BMP receptor mRNAs in undifferentiated MEFs was quantified and normalized to Gapdh levels. (B): Immunoblot detection of pSmad1/5/8 in the absence and presence of BMP4. Quantified pSmad1/5/8 was normalized to β-actin. Lanes were run on the same gel but are noncontiguous. (C): BMP target genes expression without BMP (left) and in response to BMP4 (15 ng/ml) for 2 hours (right) detected by qRT-PCR and normalized to Gapdh levels. (D): Growth curves of wild-type and Alk2R206H/+ MEFs. All data represent mean ± SEM; **, p ≤ .01; ***, p ≤ .001; ns = not significant. Abbreviation: BMP, bone morphogenetic protein.
Dysregulated BMP Signaling Does Not Alter Cell Growth Characteristics
BMP signaling is reported to have both proliferative and antiproliferative effects depending on cell type and cell context [37–39]. In FOP, mesenchymal progenitor cells recruited during early phases of lesion formation, prior to endochondral ossification, undergo robust proliferation to form fibroproliferative regions that are positive for BMP2/4 [40]. We therefore investigated effects of the gain-of-function mutation on cell proliferation. MEFs display a typical fibroblast appearance, with indistinguishable morphologies between wild-type and Alk2R206H/+ cells (Supporting Information Fig. S1A). Doubling times for wild-type and Alk2R206H/+ MEFs, 25.4 ± 1.2 and 25.5 ± 1.3 hours, respectively, were not significantly different (Fig. 1D). Proliferation assayed by colony-forming unit-fibroblast (Supporting Information Fig. S1B) and BrdU incorporation in the absence and presence of BMP4 (Supporting Information Fig. S1C) and/or additional BMP ligands (data not shown) also showed no significant effect of the mutation on proliferation.
Alk2R206H/+ Does Not Promote Spontaneous Chondrogenic Differentiation in the Absence of BMP Stimulation
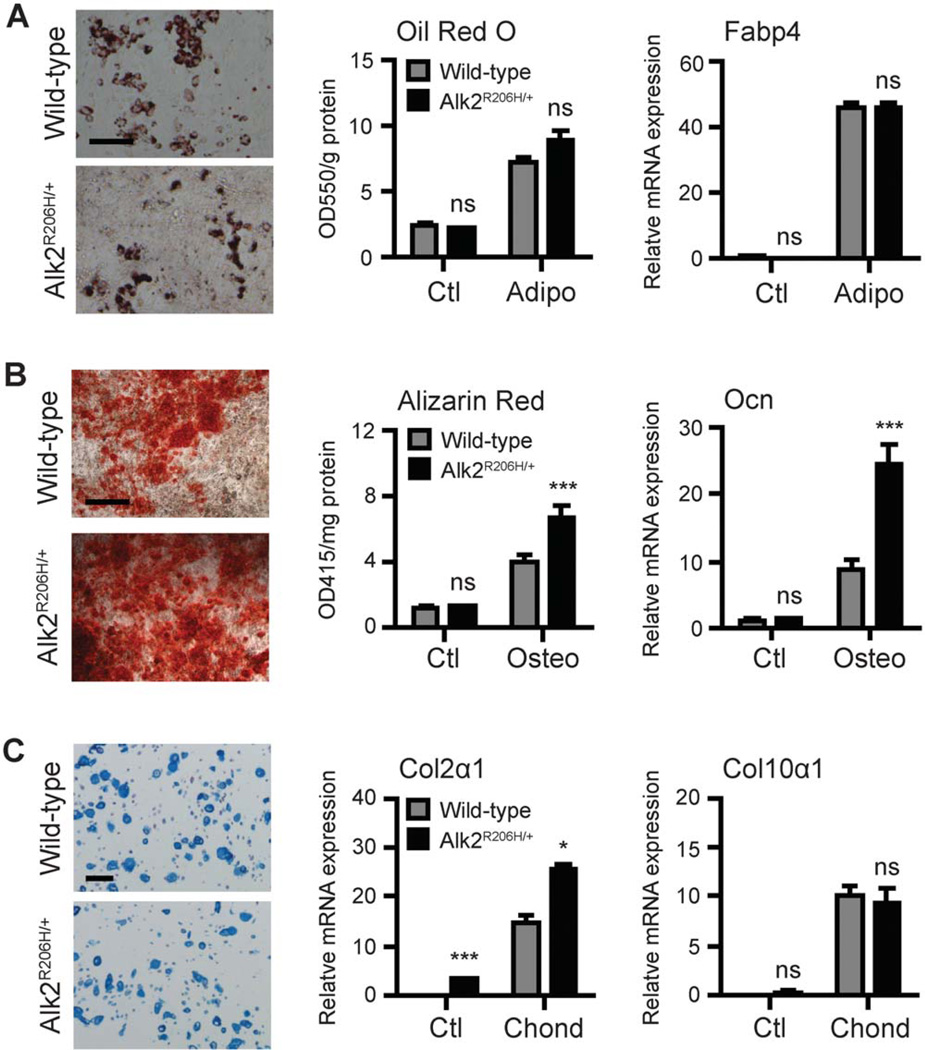
Several reports have used MEFs as a tool to study cellular differentiation, commonly in the context of embryonic lethal genotypes. MEFs behave similarly to bone marrow-derived mesenchymal stromal cells in being plastic adherent with fibroblast-like morphology and having multipotent mesenchymal lineage potential both in vitro and in vivo [41–44].We confirmed that both wild-type and Alk2R206H/+ MEFs are functionally multipotent progenitor cells through in vitro differentiation toward the adipocyte, osteoblast, and chondrocyte lineages. Differentiation in adipogenic media showed accumulation of lipid-containing vacuoles and increased adipocytespecific Fatty acid binding protein 4 (Fabp4) mRNA for both wild-type and Alk2R206H/+ cultures (Fig. 2A). Of note, differentiation to adipocytes was less efficient compared to other lineages. Osteoblast differentiation was confirmed by staining for calcium deposition and mRNA quantification of osteoblast-specific osteocalcin (Ocn) (Fig. 2B). Enhanced osteogenesis of Alk2R206H/+ cells agrees with results previously reported for FOP cells and the R206H Alk2 mutation [17, 18, 24, 25]. Chondrogenic differentiation in 3D alginate culture showed chondrocyte morphology with sulfated-glycosaminoglycans in the extracellular matrix and increased mRNAs for type II (Col2α1) and X collagen (Col10α1), with higher Col2α1 levels in mutant cells (Fig. 2C).
Figure 2.
Mesenchymal multipotency of mouse embryonic fibroblasts (MEFs). (A): MEFs in adipogenic media (14 days) were stained with oil red O to visualize lipid droplets and quantified. Fabp4 expression was normalized to Gapdh. (B): MEFs in osteogenic media (14 days) were stained with Alizarin Red to visualize calcium deposition and quantified. Ocn expression was normalized to 18S rRNA. (C): MEFs in chondrogenic media containing BMP4 (100 ng/ml) (14 days) were stained with Alcian blue to visualize chondrocyte morphology and matrix. Col2α1 and Col10α1 expressions were normalized to 18s rRNA. All data represent mean ± SEM; n = 3; *, p ≤ .05; ***, p ≤ .001; ns = not significant. Scale bars = 100 µm.
To determine whether undifferentiated Alk2R206H/+ cells are primed toward chondrogenesis, we examined early chondrogenic marker expression in the absence of chondrogenic inducers. During early stages of commitment toward chondrocytes, transcription factors including Nkx3.2/Bapx1 and Sox5, 6, and 9 (the sox trio) increase in expression [45, 46]. Sox9, considered the master regulator of chondrogenesis, must be expressed in order for differentiation to occur [47]. Decreased expression of fibroblast markers (Fsp1 and Prrx1) and increased expression of early chondrogenic markers (Nkx3.2 and Sox5/6/9) would suggest that Alk2R206H/+ cells are poised toward chondrogenesis, however, quantification of these markers in undifferentiated wild-type and Alk2R206H/+ cells showed no significant differences (Fig. 3A). Protein levels of Fsp1 and Sox9 were also examined and were consistent with mRNA data (data not shown).
Figure 3.
Alk2R206H/+ does not predispose cells toward chondrogenesis. (A): Expression of Prrx1 and Fsp1 (fibroblast) and Nxk3.2 and Sox5/6/9 (prechondrogenic) mRNAs were quantified in undifferentiated mouse embryonic fibroblasts (MEFs) and normalized to 18S rRNA. (B): MEFs cultured in three-dimensional alginate spheres with chondrogenic media in the absence (control) or presence of BMP4 (100 ng/ml) for 21 days were sectioned and stained with Alcian blue to visualize chondrocyte morphology and matrix. All data represent mean ± SEM; ns = not significant.
Previous studies demonstrated that over-expression of human R206H ACVR1 in chick limb bud micromass culture induces BMP-independent chondrogenesis [17]. Using 3D chondrogenic alginate sphere cultures [31], we examined the effect of endogenous heterozygous expression of R206H Alk2 on spontaneous chondrogenesis in the absence of growth factors. We observed no spontaneous differentiation in wild-type or Alk2R206H/+ cells, even after 3 weeks in chondrogenic media, and determined that addition of BMP ligand was necessary for chondrogenesis (Fig. 3B), as previously reported [43].We found variable induction of chondrogenesis by TGFβ superfamily ligands (BMP2, BMP4, BMP6, BMP7, and TGFβ3) at static dose and time (Supporting Information Fig. S2), with the most robust chondrogenesis in our culture system induced by BMP4.
Alk2R206H/+ Accelerates BMP-Induced Chondrogenesis
To examine the sensitivity of Alk2R206H/+ cells toward BMP-induced chondrogenesis, we examined responses to increasing concentrations of BMP4. Both wild-type and Alk2R206H/+ cells showed a dose-dependent response, with increasing BMP4 producing greater numbers of chondrocytes detected by histological staining of sulfated-glycosaminoglycans (Fig. 4A, 4B). However, Alk2R206H/+ cells showed enhanced sensitivity with a twofold increase in the number of cells differentiated to chondrocytes at low BMP4 doses; these differences between wild-type and Alk2R206H/+ cultures diminished as the cultures reached maximal differentiation (Fig. 4B).
Figure 4.
Increased sensitivity and accelerated chondrogenic differentiation by Alk2R206H/+. (A): Mouse embryonic fibroblasts (MEFs) in three-dimensional chondrogenic culture with increasing BMP4 concentrations were stained with Alcian blue (7 days). (B): Alcian bluepositive cells were quantified as the percentage of chondrocytes relative to total cells in field. (C): MEFs in chondrogenic media with 15 ng/ml BMP4 were detected for collagen type II over 14 days. Negative control is without primary antibody. (D): Expression of chondrocyte-specific genes over time was quantified by qRT-PCR and normalized to 18S rRNA. All data represent mean ± SEM; *, p ≤ .05; **, p ≤ .01; ***, p ≤ .001; ns = not significant. Scale bars = 100 µm.
To further investigate the heightened BMP-induced chondrogenic differentiation of Alk2R206H/+ cells, we quantified the progression of wild-type and Alk2R206H/+ cells toward chondrogenesis over time in the presence of low-dose BMP4 (15 ng/ml). Type II collagen detection (Fig. 4C) demonstrated that Alk2R206H/+ cells more rapidly achieved chondrocyte properties. Quantification of type II collagen-positive cells showed an increase in the number of chondrocytes present in Alk2R206H/+ cultures compared to wild-type at days 7 and 10 (data not shown), and also indicated that wild-type differentiation levels reach those of Alk2R206H/+ cells with time.
Quantified expression of early chondrocyte-specific mRNAs Sox9, Col2α1, and aggrecan (Acan) [48] showed a significant increase in Sox9 and Col2α1 mRNA in differentiating Alk2R206H/+ cells compared to wild-type beginning at 7 days, while Acan expression increased at 10 days (Fig. 4D). These data support that the mutation affects chondrogenesis at earlier stages of differentiation and suggest that early chondrogenic stage transcript expression is prolonged by the mutation. Together, these results suggest that Alk2R206H/+ MEFs differentiate to chondrocytes more rapidly and with increased production of chondrogenic transcripts and matrix proteins.
Alk2R206H/+ Cells Contribute to and Promote HEO In Vivo
We investigated whether Alk2R206H/+ cells could specifically induce HEO in vivo by implanting cells into skeletal muscle. Wild-type or Alk2R206H/+ donor cells labeled with red Qdots were implanted with BMP4 (nonosteoinductive amounts; 500 ng) into wild-type host mice ubiquitously expressing GFP. After 21 days, histological sections through the implants were evaluated for tissue morphology and to determine the fate of implanted donor cells (Fig. 5A). Donor wild-type cell implants are detected as undifferentiated or fibroblast-like cells within the implant region. By contrast, Alk2R206H/+ donor implants differentiated to both immature (low proteoglycans) and mature hypertrophic (high proteoglycans and anuclear) chondrocytes within the implant region. A fraction of chondrocytes retained Qdots, with which MEFs were initially labeled, indicating that implanted Alk2R206H/+ donor cells directly differentiated to chondrocytes (Fig. 5A). Within wild-type cell implants, Qdots are in undifferentiated fibroblast-like cells. To determine host cell contributions to HEO, cells within the implants were probed with GFP antibody to detect GFP-tagged host cells. Regardless of wild-type or mutant donor cells, GFP-positive host cells migrated into implants. Within areas of HEO induced by Alk2R206H/+ cells, both GFP-positive and GFP-negative chondrocytes were present indicating that Alk2R206H/+ cells support a permissive environment for HEO and that wild-type cells are recruited to contribute to ectopic cartilage.
Figure 5.
Alk2R206H/+ cells induce heterotopic endochondral ossification (HEO) in vivo. (A): Serial sections through implants were examined for endochondral ossification. Hematoxylin and eosin staining shows implant (im) adjacent to normal skeletal muscle (sm) tissue. Safranin-O (red) detects chondrocytes. Red fluorescent Qdots, from labeled donor cells, are present within chondrocytes of Alk2R206H/+ cell implants (arrows). GFP antibody detection identifies infiltrated GFP-tagged host cells within the implants (all conditions) and a mixture of GFP− (donor) and GFP+ (host) chondrocytes participating in HEO. Alcian blue/orange G staining differentiates cartilage (blue) from surrounding fragments of mature bone (bright pink; arrows). Scale bars = 100 µm. (B): Wild-type or Alk2R206H/+ mouse embryonic fibroblasts with 500ng BMP4 were injected into mouse hind limb muscles (right); the contralateral limb (left) was injected with BMP alone and microCT evaluated ossification after 21 days. Insets highlight detected mineralization in regions of cell implants. Where ectopic mineralization was detected the total mineralized volume (mm3) was quantified using Scanco software and is shown numerically above the sample. Data represent mean ± SEM; ***, p ≤ .001. Scale bars = 1 µm. Abbreviation: GFP, green fluorescent protein.
MicroCT demonstrated that control limbs receiving BMP4 without cells did not develop detectable mineralization (Fig. 5B). (BMP implant models for heterotopic ossification require a minimal dose of 2.5 µg BMP for consistent bone formation [7].) Limbs implanted with wild-type cells developed no measureable mineralization, with the exception of one mouse with very low levels of mineralization (animal 189), while all limbs with Alk2R206H/+ cells developed robust mineralization (Fig. 5B). Quantification confirmed that significantly more mineralization occurred in the presence of implanted Alk2R206H/+ cells compared to wild-type cells (Fig. 5B); this appears due to the presence of mature mineralized cartilage although bone is also present as shown by detection of type 1 collagen (Supporting Information Fig. S3). Small fragments of bone are observed neighboring regions containing hypertrophic chondrocytes (Fig. 5A).
Alk2 Expression Is Required During Initial Stages of Chondrogenesis
The accelerated chondrogenesis of Alk2R206H/+ cells in vitro coupled with their induction of robust HEO in vivo suggested that enhanced BMP signaling through Alk2 contributes significantly in these cellular events; however, it remained undetermined whether these effects are downstream of general BMP signaling or dependent on signaling specifically through Alk2. Quantification of type I BMP receptor mRNA expression during chondrogenesis revealed unique transcriptional regulation patterns of each receptor during progenitor cell commitment to chondrocytes (Fig. 6A). Alk2 mRNA was most abundant in undifferentiated MEFs and decreased rapidly upon differentiation, while Alk3 mRNA remained relatively stable throughout and Alk6 mRNA was most abundant in differentiated chondrocytes. The rapid and early decrease of Alk2 mRNA suggested that Alk2 has a specific contribution to early stage chondrogenesis and the accelerated phenotype observed in Alk2R206H/+ cells.
Figure 6.
Alk2 expression is required during early chondrogenesis. (A): Expression of Alk2, Alk3, and Alk6 type I BMP receptors during early chondrogenic differentiation of wild-type mouse embryonic fibroblasts (MEFs) was determined by qRT-PCR and normalized to 18S rRNA. (B): BMP signaling in wild-type and Alk2CKO MEFs in the presence of BMP4 was detected by immunoblot analysis of pSmad1/5/8 and normalized to α-tubulin. (C): Schematic shows timing of Alk2 knockout (arrow) before or during chondrogenic differentiation. Chondrocyte-specific markers were assessed by qRT-PCR after 14 days and normalized to β-2-microglobulin mRNA. All data represent mean ± SEM; *, p ≤ .05; **, p ≤ .01; ***, p ≤ .001; ns = not significant.
To investigate this, primary Alk2fl/fl;Esrl/Cre MEFs, which knockout Alk2 (Alk2CKO) upon tamoxifen-induced Cre recombination, were assayed in vitro. Alk2CKO cells show a twofold decrease of pSmad1/5/8 compared to wild-type cells, indicating that Alk2 contributes significantly to BMP signaling (Fig. 6B). Loss of Alk2 prior to chondrogenic induction (−48 hours) severely inhibited differentiation, with only an occasional chondrocyte observed and mRNA expression of chondrocyte markers Sox9, Col2α1, and Acan all significantly decreased at 14 days of culture (Fig. 6C). To identify the critical time window during which Alk2 is required, Alk2CKO cells were deleted for Alk2 at various times prior to and during chondrogenic differentiation (Fig. 6C). Knockout of Alk2 concurrently with chondrogenic induction (0 hours) maintained a significant decrease in chondrocyte markers. However, knockout of Alk2 at >24 hours postchondrogenic induction (24 and 48 hours) showed differentiation comparable to wild-type cells (Fig. 6D). Together, these data indicate that Alk2 signaling directly modulates chondrocyte differentiation potential and support that the enhanced signaling by of Alk2R206H during initial stages of chondrogenesis is sufficient to accelerate the chondrogenic program.
Discussion
FOP is a unique disorder in which one tissue (skeletal muscle, tendon, or ligament) is replaced with another—endochondral bone. Although gain-of-function ALK2 mutations are identified as the sole genetic cause of heterotopic (extraskeletal) ossification in FOP [6], current understanding of disease progression at the cellular and molecular levels is limited. It is well established that ALK2R206H/+ progenitor cells have enhanced BMP signaling and osteogenic differentiation [17, 18, 24, 25]; however, a direct effect of the endogenous patient mutation on chondrogenic differentiation, a key process that precedes osteoblastogenesis during HEO, remained to be established. In this study, we recapitulated the heterozygous FOP patient mutation in MEFs to determine the contribution of Alk2R206H in chondrogenesis which is known to precede and provide the proper environmental context for ectopic endochondral bone formation in FOP. We report that Alk2R206H/+ cells have enhanced sensitivity toward chondrogenesis both in vitro and in vivo in the presence of BMP ligand, indicating a direct consequence of heightened Alk2 signaling. In vivo, Alk2R206H/+ progenitor cells appear to play a role in establishing a HEO permissive environment, evidenced by recruitment of wild-type cells. Furthermore, we determined that signaling through Alk2 regulates early chondrogenic commitment that is not compensated by other type I BMP receptors.
Several reports have used MEFs as a tool to study cellular differentiation, commonly in the context of embryonic lethal genotypes for which bone marrow mesenchymal stem cells (MSCs) or other adult tissue-derived stem cells are not obtainable. MEFs behave similarly to bone marrow MSCs in that they are plastic adherent, express specific surface antigens, and have multipotent potential toward mesenchymal lineages in vitro and in vivo [41, 43, 44, 49–51], demonstrating that MEFs fulfill the minimal criteria for MSCs [52]. Germline transmission of knockin Alk2R206H/+ is perinatal lethal [26] and harvesting MEFs as mesenchymal progenitor cells enabled us to investigate the effects of endogenous heterozygous expression of the mutant receptor. This approach is advantageous compared to over-expression systems which may introduce artificial or exaggerated interpretations of receptor function in biological processes. We confirmed that our MEFs, as a progenitor cell model, possessed multipotent potential in vitro, and both wild-type and Alk2R206H/+ MEFs differentiate to adipocytes, osteoblasts, and chondrocytes.
In the absence of ligand, Alk2R206H/+ MEF progenitor cells showed mild leaky BMP pathway activation that was increased ~20% over wild-type. This finding contrasts with over-expression systems in which signaling appears at near maximum detectable capacity in the absence of ligand [17, 18, 25], but is similar to levels observed for patient-derived cells [24]. While Alk2R206H/+ MEFs have increased BMP signaling in the absence of ligand, this enhancement was not sufficient to promote spontaneous, BMP-independent, chondrogenic differentiation as was reported in an ALK2R206H over-expression system [17]. BMP signaling promotes expression of the Sox9 transcription factor in the context of chondrogenic induction [53], but we found no significant differences in Sox9 mRNA levels between undifferentiated wild-type and Alk2R206H/+ cells or for other early chondrogenic markers. Fibroblast-specific gene expression was also consistent between undifferentiated wild-type and Alk2R206H/+ cells, not decreased for Alk2R206H/+, further supporting that mutant cells are not precommitted. Wild-type and Alk2R206H/+ cells were indistinguishable by several other analyses including cell morphology, growth rates, and BMP receptor repertoire.
By contrast, wild-type and Alk2R206H/+ cells showed significant divergence when treated with BMP ligand. A clear dose effect for BMP4-induced chondrogenesis was observed for wild-type and Alk2R206H/+ cells, but with increased sensitivity toward differentiation at lower concentrations for Alk2R206H/+ cells. This effect is likely due to the already active BMP signaling in mutant MEFs and FOP patient-dtatic BMP4 concentration, Alk2R206H/+ cells additionally show accelerated differentiation with earlier appearance of chondrocyte morphology, extracellular matrix, and increased levels of chondrocyte-specific transcripts. In a previous study designed to demonstrate ligand-independent signaling of Alk2R206H, cells over-expressing the mutation in the presence of the BMP antagonist Noggin showed increased Sox9 and Col2α1 expression compared to wild-type Alk2 over-expression [17]. Our results show that while endogenous Alk2R206H/+ expression levels are insufficient to initiate chondrogenesis, the mutant cells are primed and show a sensitized response to ligand with enhanced expression of the early chondrogenic markers Sox9 and Col2α1.
We further demonstrated that Alk2R206H/+ progenitor cells alone, that is in the context of a wild-type cell/tissue environment, can form endochondral extraskeletal bone tissue in vivo. As in our in vitro chondrogenesis experiments, low concentration of BMP4 was required to activate the cells. However, the concentration used is well below osteo-inductive levels (approximately fivefold less) [32] and was not sufficient to promote HO in the absence of implanted Alk2R206H/+ cells; wild-type cell implants appear as dense undifferentiated fibroblast-like cells. Of note, BMP2/4 ligand is detected in patient lesions prior to the appearance of chondrocytes [40], suggesting that the mutation, together with endogenous BMPs, may direct lineage decisions toward cartilage.
We also observed that ectopic chondrocytes consist of not only implanted Alk2R206H/+ cells but also recruited wild-type host cells. These data are consistent with the spontaneous HEO that forms in chimeric knockin Alk2R206H/+ mice in which ectopic cartilage included both Alk2R206H/+ mutant cells and wild-type cells [26]. These data support that Alk2R206H/+ progenitor cells at the site of lesions not only participate in the formation of ectopic cartilage but also alter the tissue environment to support the differentiation of wild-type cells.
Alk2 mRNA levels were highest in undifferentiated cells and Alk2 expression rapidly decreased during chondrogenic differentiation of wild-type MEFs. Previous studies on chick limbs indicated that Alk2 mRNA expression is higher in resting and proliferating chondrocytes compared to hypertrophic chondrocytes [54]. Expression patterns in undifferentiated MEFs therefore appear to correlate with immature chondrocytes of the growth plate. Other type I BMP receptors, Alk3 and Alk6, were regulated differently than Alk2 in our cultures and align with known patterns of these receptors in the mouse growth plate [21, 55]. Alk3 and Alk6 have essential and relatively overlapping contributions to BMP signaling in the mouse growth plate with Alk3 protein most highly expressed in hypertrophic chondrocytes and Alk6 in proliferating and prehypertrophic chondrocytes [21, 55]. Little information is available for Alk1 expression patterns [21] and Alk1 was not abundant in MEFs.
We determined that Alk2 deletion prior to or during the first 24 hours of chondrogenic induction caused substantial inhibition of BMP-induced chondrogenesis. By contrast, delaying Alk2 knockout until 24 hours after chondrogenic induction resulted in a wild-type phenotype. Interestingly, in MEFs, the remaining type I receptors Alk3 and Alk6 were not able to compensate for early loss of Alk2, indicating that signaling through Alk2 is not equivalent to signaling through Alk3 and/ or Alk6, at least in the context of chondrogenesis, and/or that Alk2 is an obligate partner in type I receptor heterodimers during early chondrogenesis [56]. Loss of Alk2 has also been demonstrated to reduce proliferation, extension, and fusion of mandibular Meckel’s cartilage of Alk2/Wnt1-Cre knockout mice, where proper development of the mandible requires tight regulation of BMP signaling [57]. In agreement with this study that examined embryonic skeletal development specifically from the neural crest lineage, our data provide support for an important role for Alk2 in postnatal bone formation as well. Together these data support that Alk2 signaling is important for commitment toward chondrogenesis and that Alk2 modulates the progression of differentiation. Whether Alk2 is essential for terminal chondrogenic differentiation remains to be elucidated. In comparing the inhibited differentiation of Alk2CKO cells with accelerated differentiation of Alk2R206H/+ cells, we conclude that stimulation of Alk2R206H with BMP4 within the first 24 hours results in an enhanced and potentially unique signaling mechanism to promote chondrogenesis.
Conclusions
Many outstanding questions remain including the origins of progenitor cells in lesions, how inflammation preceding chondrogenesis may influence differentiation, and understanding how Alk2 signaling during early chondrogenic induction is distinct from contributions by other type I receptors. We demonstrate for the first time that heterozygous R206H Alk2 directly impacts progenitor cell differentiation toward chondrogenesis and that this process may be mechanistically regulated by unique receptor signaling during early chondrogenic commitment, thereby clarifying a direct role for Alk2R206H in promoting FOP HEO and indentifying Alk2-specific BMP signaling at the onset of chondrogenesis as a therapeutic target to prevent heterotopic ossification.
Supplementary Material
Acknowledgments
This study was supported in part by the International Fibrodysplasia Ossificans Progressiva Association, the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (AR050950), the Institute on Aging at the University of Pennsylvania Pilot Grant Award Program, the National Institutes of Health (R01AR41916), the Isaac and Rose Nassau Professorship (to F.S.K.), and the Cali/Weldon Professorship (to E.M.S.). We thank Dr. Brad Johnson (University of Pennsylvania School of Medicine) for EGFP mice and Dr. Vesa Kaartinen (University of Michigan School of Dentistry) for the gift of Alk2fl/fl null mice. Sincere thanks to Robert Caron, Deyu Zhang, Vitali Lounev, Julia Haupt, Meiqi Xu, Linda Wang, and Kate Mentzinger for their technical support and/or helpful discussions for this work.
Footnotes
Author Contributions
A.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; S.C.: data analysis and interpretation and final approval of manuscript; E.T. and T.B.: collection and/or assembly of data, data analysis and interpretation, and final approval of manuscript; F.K.: financial support, data analysis and interpretation, and final approval of manuscript; E.S.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Mackie EJ, Tatarczuch L, Mirams M. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211:109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 2.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J Surg Orthop Adv. 2010;19:54–61. [PubMed] [Google Scholar]
- 4.Cremin B, Connor JM, Beighton P. The radiological spectrum of fibrodysplasia ossificans progressiva. Clin Radiol. 1982;33:499–508. doi: 10.1016/s0009-9260(82)80159-1. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodysplasia ossificans progressiva (Fop) phenotypes are caused by mutations in the bone morphogenetic protein (Bmp) type I receptor Acvr1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the Bmp type I receptor Acvr1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 7.Lounev VY, Ramachandran R, Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medici D, Shore EM, Lounev VY, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wosczyna MN, Biswas AA, Cogswell CA, et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust Bmp-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon FH, Valentine BA, Shore EM, et al. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998:19–25. [PubMed] [Google Scholar]
- 11.Kaplan FS, Tabas JA, Gannon FH, et al. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Culbert AL, Chakkalakal SA, Convente MR, et al. Fibrodysplasia (myositis) ossificans progressiva. In: Thakker RV, Whyte MP, Eisman JA, Igarashi T, editors. Genetics of Bone Biology and Skeletal Disease. New York, NY: Elsevier; 2013. pp. 375–396. [Google Scholar]
- 13.Sieber C, Kopf J, Hiepen C, et al. Recent advances in bmp receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in Tgf-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 15.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the Acvr1 (R206h) mutation in Fop. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 16.Chaikuad A, Alfano I, Kerr G, et al. Structure of the bone morphogenetic protein receptor Alk2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287:36990–36998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Q, Little SC, Xu M, et al. The fibrodysplasia ossificans progressiva R206h Acvr1 mutation activates Bmp-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dinther M, Visser N, de Gorter DJ, et al. Alk2 R206h mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the bmp type i receptor and sensitizes mesenchymal cells to Bmp-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- 19.Pizette S, Niswander L. Bmps are required at two steps of limb chondrogenesis: Formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol. 2000;219:237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 20.Valcourt U, Gouttenoire J, Moustakas A, et al. Functions of transforming growth factor-beta family type i receptors and smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J Biol Chem. 2002;277:33545–33558. doi: 10.1074/jbc.M202086200. [DOI] [PubMed] [Google Scholar]
- 21.Zou H, Wieser R, Massague J, et al. Distinct roles of type i bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retting KN, Song B, Yoon BS, et al. Bmp canonical smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiori JL, Billings PC, de la Pena LS, et al. Dysregulation of the Bmp-P38 Mapk signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (Fop) J Bone Miner Res. 2006;21:902–909. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 24.Billings PC, Fiori JL, Bentwood JL, et al. Dysregulated Bmp signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (Fop) J Bone Miner Res. 2008;23:305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda T, Kohda M, Kanomata K, et al. Constitutively activated Alk2 and increased Smad1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakkalakal SA, Zhang D, Culbert AL, et al. An Acvr1 R206h knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27:1746–1756. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaartinen V, Nagy A. Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis. 2001;31:126–129. doi: 10.1002/gene.10015. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 29.Okabe M, Ikawa M, Kominami K, et al. ‘Green Mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 30.Xu J. Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr Protoc Mol Biol. 2005;28(28):21. doi: 10.1002/0471142727.mb2801s70. [DOI] [PubMed] [Google Scholar]
- 31.De Ceuninck F, Lesur C, Pastoureau P, et al. Culture of chondrocytes in alginate beads. Methods Mol Med. 2004;100:15–22. doi: 10.1385/1-59259-810-2:015. [DOI] [PubMed] [Google Scholar]
- 32.Glaser DL, Economides AN, Wang L, et al. In vivo somatic cell gene transfer of an engineered noggin mutein prevents Bmp4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Rosen AB, Kelly DJ, Schuldt AJ, et al. Finding fluorescent needles in the cardiac haystack: Tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 34.Nowalk JR, Flick LM. Visualization of different tissues involved in endochondral ossification with alcian blue hematoxylin and orange G/eosin counterstain. J Histotechnol. 2008;31:19–21. [Google Scholar]
- 35.Hollnagel A, Oehlmann V, Heymer J, et al. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 36.de la Pena LS, Billings PC, Fiori JL, et al. Fibrodysplasia ossificans progressiva (Fop), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of Bmpria. J Bone Miner Res. 2005;20:1168–1176. doi: 10.1359/JBMR.050305. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vortkamp A. Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis Cartilage. 2001;9(Suppl A):S109–S117. [PubMed] [Google Scholar]
- 39.Xu RH, Peck RM, Li DS, et al. Basic Fgf and suppression of Bmp signaling sustain undifferentiated proliferation of human ES Cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 40.Gannon FH, Kaplan FS, Olmsted E, et al. Bone morphogenetic protein 2/4 in early fibromatous lesions of fibrodysplasia ossificans progressiva. Hum Pathol. 1997;28:339–343. doi: 10.1016/s0046-8177(97)90133-7. [DOI] [PubMed] [Google Scholar]
- 41.Garreta E, Genove E, Borros S, et al. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 42.Kim KA, Kim JH, Wang Y, et al. Pref-1 (preadipocyte factor 1) activates the Mek/ extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lengner CJ, Lepper C, van Wijnen AJ, et al. Primary mouse embryonic fibroblasts: A model of mesenchymal cartilage formation. J Cell Physiol. 2004;200:327–333. doi: 10.1002/jcp.20118. [DOI] [PubMed] [Google Scholar]
- 44.Saeed H, Taipaleenmaki H, Aldahmash AM, et al. Mouse embryonic fibroblasts (Mef) exhibit a similar but not identical phenotype to bone marrow stromal stem cells (BMSC) Stem Cell Rev. 2012;8:318–328. doi: 10.1007/s12015-011-9315-x. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl A):S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 46.Murtaugh LC, Zeng L, Chyung JH, et al. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote bmp-dependent axial chondrogenesis. Dev Cell. 2001;1:411–422. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 47.Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 49.Qi C, Surapureddi S, Zhu YJ, et al. Transcriptional coactivator Prip, the peroxisome proliferator-activated receptor gamma (Ppargamma)-interacting protein, is required for Ppargamma-mediated adipogenesis. J Biol Chem. 2003;278:25281–25284. doi: 10.1074/jbc.C300175200. [DOI] [PubMed] [Google Scholar]
- 50.Quintana L, Muinos TF, Genove E, et al. Early tissue patterning recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng Part A. 2009;15:45–54. doi: 10.1089/ten.tea.2007.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang E, Bi Y, Jiang W, et al. Conditionally immortalized mouse embryonic fibroblasts retain proliferative activity without compromising multipotent differentiation potential. PLoS One. 2012;7:e32428. doi: 10.1371/journal.pone.0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 53.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 54.Zhang D, Schwarz EM, Rosier RN, et al. Alk2 functions as a Bmp Type I receptor and induces indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18:1593–1604. doi: 10.1359/jbmr.2003.18.9.1593. [DOI] [PubMed] [Google Scholar]
- 55.Yoon BS, Ovchinnikov DA, Yoshii I, et al. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type i receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudas M, Sridurongrit S, Nagy A, et al. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.