Abstract
Background
The overall incidence of myeloid malignancies is 8.6 per 100 000 persons. Allogeneic stem-cell transplantation (SCT) is a major therapeutic option despite its risks, which include graft-versus-host disease (GvHD) and infection. In Germany, about 1600 patients with myeloid malignancies undergo SCT each year. The indications for SCT have changed since the introduction of tyrosine kinase inhibitors (TKI) and improved methods of SCT.
Methods
This article is based on relevant guidelines from Germany and abroad and on a selective review of the literature from 2010 onward.
Results
The individual indication for SCT is based on the risk of disease progression, accompanying illnesses, the probability that SCT will result in cure, and the risk of complications. There is good evidence favoring allogeneic SCT in the following situations affecting 20% to 50% of patients with the respective disease: advanced chronic myeloid leukemia (CML) or CML that does not respond to TKI, Philadelphia chromosome–negative myeloproliferative neoplasm (Ph- MPN) or myelodysplastic syndrome (MDS) with a high risk of progression, and acute myeloid leukemia (AML) that has high-risk cytogenetic features or is recurrent. Good evidence is accumulating in favor of allogeneic SCT in older patients as well.
Conclusion
The prognosis of patients with myeloid neoplasm can now be assessed more accurately than before. This facilitates well-founded clinical decision-making about SCT, which is the only potentially curative treatment for most patients with myeloid neoplasm. Patients up to about age 75 should be referred to a transplantation center for consultation at an early stage of their disease so that the treatment options can be evaluated. A major goal of current research is to reduce toxicity with innovative forms of treatment.
The proliferation rate of hematopoietic precursor cells is associated with a high risk of malignant myeloid diseases. These include conditions whose presentation is initially chronic, such as chronic myeloid leukemia (CML), Philadelphia chromosome– negative myeloproliferative neoplasm (Ph–MPN), and myelodysplastic syndrome (MDS). They may switch to a more aggressive form and manifest as acute myeloid leukemia (AML) or a blast crisis. AML may also occur de novo. Myeloid diseases are found in every age group, with an overall incidence of 8.6/100 000 (1). However, the incidence increases with age.
In allogeneic stem cell transplantation (SCT) (Box) the patient first undergoes so-called conditioning and then receives an infusion of hematopoietic stem cells from another person. After maturation, the infused cells begin hematopoiesis. Thanks to the size and quality of today’s donor registries a suitable donor can be found for around 90% of all patients in Germany (e2).
Box. Typical course and essential characteristics of allogeneic stem cell transplantation (SCT)*.
-
HLA typing/search for donor (circa days –90 to –60)
In siblings (related donors); if unsuccessful, registry search (unrelated donor)
Typing of five genes: HLA-A, -B, -C, -DRB1, -DQB1; two alleles per gene, i.e., ten traits
Donor well matched if at least nine of ten traits identical (≥ 9/10)
If no matching donor found (<9/10), consider haploidentical donor (e.g. patient’s child)
-
Selection/preliminary investigation of donor (circa days –40 to –20)
Primarily on basis of HLA identity
Further factors (e.g., CMV constellation) if two or more HLA-identical donors available
-
Preliminary investigation of patient (circa days –40 to –15)
Risk of TRM on basis of risk scores (e.g., HCT-CI)
-
Conditioning (circa days –12 to –1)
TBI and/or chemotherapy to suppress hematopoiesis and patient’s immune system
-
Forms: myeloablative (cytopenia irreversible without SCT)
nonmyeloablative (cytopenia always reversible even without SCT)
reduced intensity (neither myeloablative nor nonmyeloablative; cytopenia may be reversible without SCT)
-
Stem cell harvesting from donor (day –1)
Apheresis of peripheral blood stem cells after G-CSF treatment or by bone marrow extraction
-
Transplantation (day 0)
Transfusion of stem cell preparation into patient
-
Engraftment (circa days +10 to +20)
“;Proliferation” of transplanted cells in patient’s body with regeneration of blood count
-
Medicinal immunosuppression (day –3 to circa day +100/+180)
Depending on GvHD risk, administration of anti-T-cell antibodies in context of conditioning
After transplantation, medication (e.g., cyclosporin A) until circa day +100/+180
-
GvHD
Reaction of donor’s immune system to recipient tissue
Risk depends among other factors on HLA identity and donor provenance (higher risk with unrelated donor)
-
Transplantation-associated morbidity and mortality
Cause: particularly GvHD, infections, and organ toxicity
Risk depends among other factors on patients’ conditioning, age, and comorbidities
*The stated times relate to the time of allogeneic SCT. The forms of conditioning are classified according to (e1).
HLA, human leukocyte antigen; CMV, cytomegalovirus; TRM, transplantation-related morbidity; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; TBI, total-body irradiation; G-CSF, granulocyte colony-stimulating factor; GvHD, graft-versus-host disease
The graft-versus-malignancy (GvM) effect is decisive for the curative potential of the transplantation. This phenomenon involves an immune response of the donor’s immune system to the malignant cells of the transplant recipient.
The risks entailed in allogeneic SCT include infections during the phase of stem cell maturation and drug-induced immune suppression. Another danger is graft-versus-host disease (GvHD), i.e., an immune reaction of the transplant to the tissues of the recipient (2).
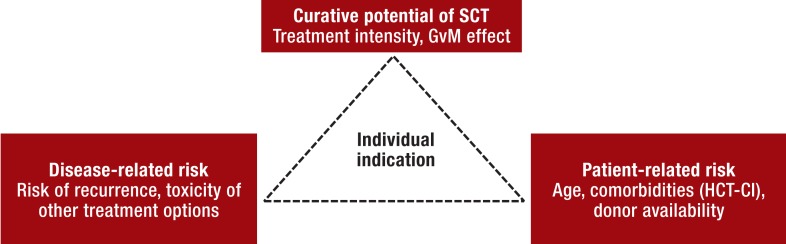
The risks of transplantation-associated morbidity and mortality is correlated both with patient age and with the comorbidities present. The indications and contraindications for transplantation therefore rest essentially on three factors that have to be weighed against each other (Figure). Partly because of the complexity of the treatment, only a limited number of randomized trials have been conducted. To a large extent, the data on allogeneic SCT for myeloid malignancies come from retrospective analyses. As far as possible, therefore, allogeneic SCT should be performed in the framework of a prospective study.
Figure.
Benefit–risk analysis to decide the indication for allogeneic SCT
The indication is determined on an individual basis for each patient and should be discussed with the patient and his/her family members. GvM, graft-versus-malignancy; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; SCT, stem cell transplantation
With the aim of reducing toxicity, reduced-intensity conditioning protocols have been developed. Furthermore, new drug treatments, e.g., tyrosine kinase inhibitors (TKI), have enhanced the treatment options in certain myeloid neoplasms. These two factors have combined to change the indications for allogeneic SCT: on one hand, patients of advanced age or with comorbidities can be treated with allogeneic SCT; on the other, effective TKI treatment means that SCT is less frequently indicated.
In general, the earlier the stage and the greater the success of conventional treatment in achieving remission, the better the chances that transplantation will result in cure.
Methods
A selective survey of the literature was carried out, using the search terms “;allogeneic,” “;transplantation,” and the respective disease.
The well-substantiated list of indications published by the European Society for Blood and Marrow Transplantation (EBMT) in 2010, which includes earlier studies, served as a starting point (3). Taking account of the new indication list of the German Study Group for Bone Marrow and Hematopoietic Stem Cell Transplantation (Deutsche Arbeitsgemeinschaft für Knochenmark- und Blutstammzelltransplantation, DAG-KBT) (4), the literature survey covered the period 2010–2014, so that changes and the current status could be included.
Chronic myeloid leukemia
Prior to the introduction of TKI, allogeneic SCT was a standard treatment for patients with CML. CML is considered a prime example of the action of the GvM effect (5).
Long-term remissions can be achieved with the help of TKI (6). However, allogeneic SCT remains the only treatment that regularly attains long-term molecular remission without the necessity for continued drug intake. Treatment-associated mortality is higher for allogeneic SCT than for TKI, so TKI treatment is standard for newly diagnosed CML (7).
Patients in whom TKI achieves no long-term benefit must be identified at an early stage and redirected to treatment by transplantation. According to the currently prevailing guidelines, the search for a donor and the evaluation of allogeneic SCT should ensue after initial diagnosis and also during the disease course, depending on individual risk profile (7, 8) (Table 1).
Table 1. Indication for donor search and allogeneic stem cell transplantation in chronic myeloid leukemia*1.
| Following diagnosis | During disease course | |
|---|---|---|
| HLA-typing of patient and search for family donor |
|
|
| Search for unrelated donor |
|
|
| Indication for allogeneic SCT |
|
|
*1A tiered approach is recommended, depending on initial risk constellation and disease course.
*2Clonal chromosomal aberrations in Philadelphia chromosome-positive cells, e.g., trisomy 8, trisomy Ph (+der(22)t(9;22)(q34;q11)), isochromosome 17 (i(17)(q10)), trisomy 19 and ider(22)(q10)t(9;22)(q34;q11)
SCT, stem cell transplantation; HLA, human leukocyte antigen; AP, accelerated phase; BC, blast crisis; TKI, tyrosine kinase inhibitor(s)
Both after initial diagnosis and during the later disease course, failure of TKI treatment or the presence of advanced disease or a T315I mutation, which induces resistance to the majority of licensed TKI, should prompt an urgent search for a donor and transplantation at the earliest possible time. Discovery of any other genetic aberrations associated with a poor prognosis (e3) should also trigger evaluation of the indication for allogeneic SCT (Table 1).
In an accelerated phase or if blast crisis is present, a second chronic phase should be induced whenever possible, either by means of the TKI that remain available or with chemotherapy. This distinctly increases the prospect of long-term survival after transplantation (9) (Table 2). If the blast crisis is uncontrolled, the prognosis is poor even after allogeneic SCT, with a 3-year survival rate of around 15% (12). Nevertheless, transplantation offers a chance of cure to a certain proportion of these patients (7).
Table 2. Selected results of allogeneic stem cell transplantation (SCT) in acute myeloid leukemia and myeloproliferative neoplasm including chronic myeloid leukemia*1.
| Inclusion criteria | Study design | Treatment (patients, N) | Median age (years)*2 | SCT regimen | OS (p-value)*2 | TRM (%)*2 | Reference |
|---|---|---|---|---|---|---|---|
| AML | |||||||
| <60 years, first cr |
Matched pair | Allo SCT (185) vs. conventional CTx (185) |
45 vs. 46 | MAC/RIC | 7 years: 58%vs. 46% (0.037) |
7 years: 24% vs. 6% (<0.001) |
(10) |
| <61 years, first cr, high risk |
Post-hoc analysis, prospective, multicenter |
Allo SCT (45) vs. conventional CTx (62) |
48 vs. 54 | MAC/RIC | 5 years: 19% vs. 9% (0.02) |
5 years: 15% vs. 2% (0.003) |
(e4) |
| <61 years, first cr |
Prospective, multicenter |
MAC (96) vs. RIC (99) |
45 vs. 44 | MAC/RIC | 3 years: 58% vs. 61% (NS) |
3 years: 18% vs. 13% (NS) |
(e5) |
| ≥ 50–70 years, first CR |
Retrospective, multicenter |
Allo SCT (152) vs. conventional CTx (884) |
55 vs. 61 | MAC/RIC | 3 years: 62% vs. 51% (0.012) |
3 years: 21% vs. 3% (<0.001) |
(e6) |
| ≥ 60 years, first CR |
Prospective, multicenter |
Allo SCT (123) | 65 | RIC | 2 years: 46% |
2 years: 14% |
(* 3) |
| CML and Ph– MPN | |||||||
| PMF/post-ET and post-PV MF | Prospective, multicenter |
Allo SCT (103) | 55 | RIC | 5 years: 67% |
1 year: 16% |
(e11) |
| PV or ET with progression (MF or AML) |
Retrospective, register |
Allo SCT (250) | 56 | RIC/MAC | 3 years: 55% |
3 years: 28% |
(e7) |
| CML with TKI failure | Prospective, multicenter |
Allo SCT in first CP (37) and allo SCT after BC/AP (28) |
38 and 38 | RIC/MAC | 3 years: 94% and 59% |
3 years: 5% and 18% |
(9) |
| CML, AP | Prospective, single-center |
Allo SCT (45) vs. imatinib (87) |
34 vs. 44 | MAC | 6 years: 83% vs. 51% (0.023) |
6 years: 11% (only SCT) |
(e8) |
*1Selected recent studies on the role of SCT in general and in older patients. With regard to myelodysplastic syndrome (MDS) we refer to a recent publication (e9).
*2Results for treatments or patient groups in same order as in "Treatment" column.
*3Devine S, Owzar K, Blum W, et al.: A phase II study of allogeneic transplantation for older patients with AML in first complete remission using a reduced intensity conditioning regimen: results from CALGB 100103/BMT CTN 0502. Blood, ASH Annual Meeting Abstracts 2012; 120: abstract 230.
Allo SCT, allogeneic stem cell transplantation; AML, acute myeloid leukemia; AP, accelerated phase; BC, blast crisis; CML, chronic myeloid leukemia; CP, chronic phase;
CR, complete remission; CTx, chemotherapy; ET, essential thrombocythemia; MAC, myeloablative conditioning; MF, myelofibrosis; MPN, myeloproliferative neoplasm; NS, nonsignificant;
OS, overall survival; Ph – MPN, Philadelphia chromosome-negative myeloproliferative neoplasms; PMF, primary myelofibrosis; PV, polycythemia vera; RIC, reduced-intensity conditioning;
TKI, tyrosine kinase inhibitors; TRM, transplantation-associated mortality
Myeloablative conditioning is standard for allogeneic SCT in CML, but can be carried out only in younger patients in whom the risk is acceptable. It seems justified to set the age limit at 45–55 years. The age distribution of CML means there is a need for conditioning strategies with reduced toxicity. These enable high rates of long-term remission (13). There is thus no absolute upper age limit for allogeneic SCT in CML.
Philadelphia chromosome–negative neoplasms
In view of the good prognosis of polycythemia vera and essential thrombocythemia, allogeneic SCT is indicated only if the disease is progressing to myelofibrosis or AML (14). This is the case in around 2 to 15% of patients (15).
In contrast, allogeneic SCT is the only curative treatment option for primary myelofibrosis. Its use is limited, however, by high transplantation-associated mortality in the mostly elderly patients. Transplantation is recommended if median survival of less than 5 years can otherwise be expected (14).
This risk can be estimated with the aid of various models. The recently refined version of the Dynamic International Prognostic Scoring System Plus (DIPSS plus) takes account of transfusion requirement as well as cytogenetics and enables assessment of disease dynamics (16). According to this model, patients classed as intermediate and high risk can be expected to die of their disease within 5 years (Table 3). The greatest risk is the development of a blast crisis. Therefore, patients at risk should undergo allogeneic SCT at the earliest possible time. The same holds for patients with secondary myelofibrosis after polycythemia vera or essential thrombocythemia. The JAK2V617F mutation that is often found in Ph–MPN does not correlate with survival (17) and has no relevance, to date, with regard to the indications for transplantation.
Table 3. Indication in primary myelofibrosis and other Philadelphia chromosome-negative myeloproliferative neoplasms*.
| Primary myelofibrosis | Polycythemia vera/ essential thrombocythemia | ||
|---|---|---|---|
| On diagnosis | DIPSS plus score ≥ 2 | None | |
| During course | DIPSS plus score ≥ 2 Blast crisis |
Blast crisis Secondary myelofibrosis |
|
IPSS – 1 point for each of the following:
DIPSS plus – in addition, 1 point each for:
median survival by dipss plus score (16): | |||
| 0 points (low risk) | 15.4 years | 1 point (intermediate-1 risk) | 6.5 years |
| 2 or 3 points (intermediate-2 risk) | 2.9 years | ≥ 4 points (high risk) | 1.3 years |
*While in polycythemia vera and essential thrombocythemia the indication may arise during the course of the disease, in primary myelofibrosis the indication should be evaluated on initial diagnosis, depending on prognostic risk stratification.
IPSS, International Prognostic Scoring System; DIPSS, Dynamic International Prognostic Scoring System
The 5-year survival rate for intermediate- and high-risk patients with myelofibrosis after allogeneic SCT is circa 30 to 40% (11, 18). The results of reduced-intensity conditioning are comparable to those attained with myeloablative protocols (11) (Table 2). There is no formal age limit for transplantation in patients with primary or secondary myelofibrosis.
JAK1/2 inhibitors represent a new treatment for some patients with Ph– MPN. Good control of symptoms can be achieved, and in some cases survival can be extended (19). The survival advantage appears much smaller, however, than that attained with TKI in CML. Therefore, the decision to carry out allogeneic SCT in suitable patients should not be postponed by treatment with JAK1/2 inhibitors. Since JAK inhibitors relieve constitutional symptoms, however, treatment can improve the patient’s general condition before transplantation.
If a blast crisis occurs in Ph– MPN the prognosis without SCT is extremely poor, with median survival of 3 months (20). Remission-inducing chemotherapy should be considered in such cases, with the aim of achieving a second chronic phase and performing allogeneic SCT (14). This strategy yields 2-year survival of >40% (21) (Table 2). The prognosis is poorer if remission cannot be realized, but even then allogeneic SCT attains long-term remission in around 20% of cases (e10).
Acute myeloid leukemia
The treatment goal in newly diagnosed AML is, in suitable patients, the induction of remission by means of intensive chemotherapy. Consolidation treatment is necessary, as without it the risk of recurrence is as high as 90% (22). Options for consolidation are conventional chemotherapy and allogeneic SCT.
The risk of recurrence of AML is determined mainly by the patient’s age and genetic factors (23). Several studies have shown that allogeneic SCT represents the only realistic chance of long-term remission in patients with a high cytogenetic risk (e11). Patients with acute promyelocytic leukemia, which can be cured with modern treatments in more than 95% of cases, do not undergo transplantation in first remission.
For the large number of patients with intermediate cytogenetic risk, it is unclear whether allogeneic SCT in first remission is beneficial or not. In Germany, this is currently being investigated by the ETAL-1 study. The data from previous studies support the hypothesis that allogeneic SCT can increase by 10% the proportion of patients in this group who survive for 5 years (10, e11).
Apart from cytogenetics, molecular markers such as the FLT3 and the NPM1 mutation are associated with the prognosis. Because of their uncertain predictive value, these markers so far play a minor role in the decision-making process for transplantation in AML. Further risk factors for recurrence are lack of complete remission after first induction treatment and a high initial leukocyte count.
Independent of other risk factors, the patient’s age is decisive for the prognosis of AML. Even with intensive chemotherapy, fewer than 20% of patients over the age of 65 survive for 5 years without allogeneic SCT (24). Transplantation with reduced-intensity conditioning should therefore be evaluated on an individual basis with no firm age limit.
Thus an increasingly sophisticated decision-making strategy has emerged for allogeneic SCT in patients with AML in first remission. Table 4 shows a risk stratification based on current recommendations (25). Transplantation, if indicated, should be carried out as early as possible in first complete remission.
Table 4. Indication for allogeneic stem cell transplantation in acute myeloid leukemia*.
| Risk of recurrence with conventional consolidation depending on selected disease characteristics | Acceptable risk of transplantation-associated mortality for SCT _(e.g.. according to HCT-CI) |
|---|---|
| Indication for allogeneic SCT in first complete remission | |
Low risk of recurrence (35–40%). e.g.:
|
Low (SCT justified only in absence of comorbidities) |
Moderate risk of recurrence (50–55%). e.g.:
|
Moderate (SCT justified only with low number of comorbidities) |
High risk of recurrence (70–80%). e.g.:
|
High (consider SCT even in presence of comorbidities) |
Very high risk of recurrence (>90%). e.g.:
|
Very high (consider SCT even in presence of comorbidities) |
| Indication for allogeneic SCT in recurrence | |
| All constellations | According to individual assessment |
*Allogeneic SCT in AML in first complete remission is always an individual decision. There is good evidence particularly for AML with high risk of recurrence. In cases with moderate or low risk of recurrence. assessment of the risks entailed in SCT is of special importance.
AML. acute myeloid leukemia; Evi-1. ectropic viral integration site 1; HCT-CI. hematopoietic cell transplantation-specific comorbidity index; FLT3. Fms-like tyrosine kinase receptor 3; _NPM1. nucleophosmin; SCT. stem cell transplantation
The role of allogeneic SCT in patients with low genetic risk is unclear, particularly in those who only have an NPM1 mutation. Recent data show that the prognosis of these patients is poorer if the NPM1 mutation can still be demonstrated in bone marrow after induction chemotherapy (26). In these cases transplantation may be advisable even on first remission.
On first diagnosis and in recurrences, one option for treatment of patients with refractory AML is cytoreductive chemotherapy followed immediately by conditioning in the aplasia phase. This achieves long-term survival in 30 to 50% of such cases (27).
Allogeneic SCT remains the standard treatment in patients with recurrent AML. It is unclear whether remission should first be induced by means of chemotherapy, as is practiced in many centers, or whether the goal should be prompt transplantation regardless of remission status. The latter option can avoid toxicity.
Myelodysplastic syndrome
The International Prognostic Scoring System (IPSS) can be used to estimate the prognosis of patients with MDS, particularly the risk of secondary AML. The newly developed IPSS-R score enables finer classification by taking account of cytogenetic aberrations (28). Most study data are based on the IPSS, which therefore continues to be used to determine the indication for allogeneic SCT (29). Recent data confirm the recommendation for transplantation in patients whose risk according to the IPSS is intermediate-2 or high (Table 5) (30). In patients with low-risk MDS, transplantation should generally not be carried out immediately; rather, one should wait until the disease progresses. Remission-inducing chemotherapy before scheduled allogeneic SCT should be carried out only if secondary AML has developed.
Table 5. Indication for allogeneic stem cell transplantation in myelodysplastic syndrome*.
| Risk (IPSS score) | Indication for allogeneic SCT | ||||
|---|---|---|---|---|---|
| Low (0) | None | ||||
| Intermediate-1 (0.5 or 1) | In special cases: high-risk cytogenetics or severe cytopenias | ||||
| Intermediate-2 (1.5 or 2) | Standard | ||||
| High (2.5 to 3.5) | Standard | ||||
| IPSS | |||||
| Variable | Score | ||||
| 0 | 0.5 | 1 | 1.5 | 2 | |
| BM blasts Cytogenetics Cytopenia |
<5 Good 0 or 1 |
5–10 Intermediate 2 or 3 |
Poor | 11–20 | 21–30 |
| Cytogenetics | Good Intermediate Poor |
Normal. only del(5q). only del(20q). only –Y All others Complex with >2 aberrations; anomalies of chromosome 7 |
|||
*The indication for allogeneic SCT is based on models for estimation of the risk of transformation into AML. To date the IPSS has been used. but the IPSS-R will offer an improved system in future.
IPSS. International Prognostic Scoring System; BM. bone marrow; SCT. stem cell transplantation
Because of their usually advanced age, only a small proportion of patients with MDS are suitable for myeloablative conditioning. Reduced-intensity conditioning entails a somewhat higher risk of recurrence but has a lower rate of transplant-associated mortality; overall, the long-term survival is similar (31). Randomized trials comparing reduced-intensity and myeloablative conditioning have taken place, but the final results have not yet been published. Transplantation, the only curative option, should therefore also be considered on an individual basis in older patients.
The data on chronic myelomonocytic leukemia (CMML)—classified in the MDS/MPN group of diseases by the World Health Organization (WHO)— are sparse. The indication for SCT should currently continue to be determined analogous to the risk stratification for MDS. New analyses show that apart from patient age and changes in blood composition, the prognosis is also affected by specific genetic aberrations (32). Accordingly, the indication for allogeneic SCT is decided on the merits of each individual case in patients with CMML. Around 30 to 40% of those treated survive for at least 3 years after transplantation (33).
Patients over 70 years old or with comorbidities
The use of allogeneic SCT in the elderly is restricted by its toxicity. However, optimization of conditioning, supportive treatment, immune suppression, and choice of donor have greatly improved the results (34, e12).
One challenge is individual determination of the risks in a given patient. Transplantation-associated mortality can be estimated with the aid of various scoring systems, e.g., the HCT-CI (Box) (35, 36). However, analyses in specific categories of patients have shown the limitations of this system (37). The rates of transplantation-associated mortality between 2 and 5 years after SCT have been reported as 10 to 30% in recent studies (34, e12) (Table 2). In view of the enhanced treatment options, the numerical age is merely a guide. This is particularly relevant for myeloid neoplasms, the incidence of which rises with increasing age. Patients over age 40–55 years treated with allogeneic SCT should receive reduced-intensity conditioning. Depending on the prognosis, allogeneic SCT may be justified in patients over 70.
Dependence of indication on donor availability
High agreement of HLA characteristics between patient and donor is associated with low transplant-related mortality (38). Data in AML patients show that in the event of HLA identity the result of allogeneic SCT is almost the same with sibling donors and unrelated donors (39). The indication for transplantation should therefore not depend primarily on differentiation between related and unrelated donors.
If no HLA-identical donor is available, transplantation from a haploidentical family member can be considered. The transplant-associated mortality of haploidentical SCT is higher than that of HLA-identical transplantation but has been reduced by refinement of the SCT procedure (40, e13). Umbilical cord blood is a further option but is rarely used in adults in Germany.
Conclusions
The results of randomized trials and large retrospective analyses of registry data yield high evidence in favor of the use of allogeneic SCT in the following situations:
CML in an advanced stage or after failure of TKI
High-risk Ph– MPN and MDS
High-risk and recurrent AML.
On the basis of single-center and registry-based studies, together with early randomized trials, there is increasingly better evidence in favor of allogeneic SCT in patients >60 years.
The decision with regard to transplantation is taken after consideration of the danger of recurrence and the risks entailed in SCT. To facilitate the decision-making process, all patients with myeloid neoplasms up to an age of circa 75 years should be referred to a transplantation center immediately after diagnosis, without recourse to any further preliminary investigations. A decision for or against allogeneic SCT can then be taken with the involvement of the patient, his/her relatives, the treating hematologist, and the primary care physician. The transplantation center staff then determine what measures are necessary before transplantation, and the appropriate investigations are carried out in cooperation with the referring physician. In this way the best approach for each patient is determined on an individual basis.
Key Messages.
Allogeneic stem cell transplantation (SCT) should be considered above all in patients with any of the following: acute myeloid leukemia, myelodysplastic syndrome or myelofibrosis with high risk of progression, and advanced chronic myeloid leukemia.
For many patients with these diseases, allogeneic SCT is the only curative treatment.
The indication is determined by weighing the risks of the disease and the patient’s comorbidities against the curative potential and the risks inherent in SCT.
Allogeneic SCT should be evaluated in patients up to the age of 75 years.
Patients should be referred to a transplantation center at an early stage so that the advisability of treatment by means of SCT can be assessed together with the patient, his/her relatives, the treating hematologist, and the primary care physician.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Dr. Müller has received congress registration fees and reimbursement of travel costs from Astellas, Janssen, Sanofi, Gentium, and Gilead. He has received honoraria for lectures from Novartis, Celgene, and Pfizer. He has acted as a consultant for Celgene and CTI Life Sciences.
Prof. Müller-Tidow has received honoraria for consultancy (advisory board) from Janssen and for authorship or co-authorship of a publication on the topic covered in this article from Celgene. He has received reimbursement of congress registration costs from Novartis, and Novartis and Celgene have assumed travel costs on his behalf. He has received honoraria for lectures from Celgene and Janssen. His research has been supported by Novartis and Celgene (third-party funding of commissioned clinical trials and materials).
References
- 1.Visser O, Trama A, Maynadie M, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48:3257–3266. doi: 10.1016/j.ejca.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Wolff D, Bertz H, Greinix H, et al. The treatment of chronic graft-versus-host disease: consensus recommendations of experts from Germany, Austria, and Switzerland. Dtsch Arztebl Int. 2011;108:732–740. doi: 10.3238/arztebl.2011.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, Bregni M, Brune M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45:219–234. doi: 10.1038/bmt.2009.141. [DOI] [PubMed] [Google Scholar]
- 4.Deutsche Arbeitsgemeinschaft für Knochenmark- und Blutstammzelltransplantation e. V. (DAG-KBT) Indikationsliste DAG-KBT. www.dag-kbt.de/content/public/Indikationsliste-DAG.pdf. (last accessed on 1 March 2015)
- 5.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 6.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 7.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochhaus A, Baerlocher G, Brümmendorf TH, et al. Chronische myeloische Leukämie (CML) Leitlinie. www.dgho-onkopedia.de/de/onkopedia/leitlinien/cml. (last accessed on 1 March 2015) [Google Scholar]
- 9.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 10.Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32:288–296. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 11.Kroger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–5270. doi: 10.1182/blood-2009-07-234880. [DOI] [PubMed] [Google Scholar]
- 12.Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47:810–816. doi: 10.1038/bmt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 14.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–770. doi: 10.1200/JCO.2010.31.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 17.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 18.Scott BL, Gooley TA, Sorror ML, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119:2657–2664. doi: 10.1182/blood-2011-08-372904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guglielmelli P, Biamonte F, Rotunno G, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123:2157–2160. doi: 10.1182/blood-2013-11-536557. [DOI] [PubMed] [Google Scholar]
- 20.Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol. 2011;29:2410–2415. doi: 10.1200/JCO.2011.34.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121:2725–2733. doi: 10.1182/blood-2012-10-464248. [DOI] [PubMed] [Google Scholar]
- 22.Buchner T, Urbanitz D, Hiddemann W, et al. Intensified induction and consolidation with or without maintenance chemotherapy for acute myeloid leukemia (AML): two multicenter studies of the German AML Cooperative Group. J Clin Oncol. 1985;3:1583–1589. doi: 10.1200/JCO.1985.3.12.1583. [DOI] [PubMed] [Google Scholar]
- 23.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 24.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32:2541–2552. doi: 10.1200/JCO.2014.55.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 26.Kronke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 27.Schmid C, Schleuning M, Ledderose G, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–2964. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31:2662–2670. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–411. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 32.Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31:2428–2436. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 33.Bacher U, Haferlach T, Schnittger S, et al. Recent advances in diagnosis, molecular pathology and therapy of chronic myelomonocytic leukaemia. Br J Haematol. 2011;153:149–167. doi: 10.1111/j.1365-2141.2011.08631.x. [DOI] [PubMed] [Google Scholar]
- 34.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gratwohl A, Stern M, Brand R, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–4726. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 36.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birninger N, Bornhauser M, Schaich M, et al. The hematopoietic cell transplantation-specific comorbidity index fails to predict outcomes in high-risk AML patients undergoing allogeneic transplantation—investigation of potential limitations of the index. Biol Blood Marrow Transplant. 2011;17:1822–1832. doi: 10.1016/j.bbmt.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124:2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlenk RF, Dohner K, Mack S, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28:4642–4648. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 40.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Zentrales Knochenmarkspende-Register Deutschland. www.zkrd.de (last accessed on 3 December 2014)
- e3.Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–6768. doi: 10.1182/blood-2011-08-373902. [DOI] [PubMed] [Google Scholar]
- e4.Cornelissen JJ, Breems D, van Putten WL, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30:2140–2146. doi: 10.1200/JCO.2011.39.6499. [DOI] [PubMed] [Google Scholar]
- e5.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- e6.Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant. 2011;17:401–411. doi: 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- e7.Lussana F, Rambaldi A, Finazzi MC, et al. Allogeneic hematopoietic stem cell transplantation in patients with polycythemia vera or essential thrombocythemia transformed to myelofibrosis or acute myeloid leukemia: a report from the MPN Subcommittee of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:916–921. doi: 10.3324/haematol.2013.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Jiang Q, Xu LP, Liu DH, et al. Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood. 2011;117:3032–3040. doi: 10.1182/blood-2010-09-308510. [DOI] [PubMed] [Google Scholar]
- e9.Germing U, Kobbe G, Haas R, et al. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int. 2013;110:783–790. doi: 10.3238/arztebl.2013.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Alchalby H, Zabelina T, Stubig T, et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation: a study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:279–281. doi: 10.1016/j.bbmt.2013.10.027. [DOI] [PubMed] [Google Scholar]
- e11.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–221. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- e13.Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematology Am Soc Hematol Educ Program. 2012;2012:230–236. doi: 10.1182/asheducation-2012.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]