Abstract
To determine whether chemotherapy treatment at least 6 months prior to the detection of hepatic steatosis is associated with advanced hepatic fibrosis. Demographics, comorbid conditions, and laboratory data for cancer patients with hepatic steatosis were reviewed. The primary end point of this study was a low probability of fibrosis as calculated by the AST-to-platelet ratio index (APRI)—a surrogate for the absence of histologic bridging fibrosis and/or cirrhosis. Of 279 patients, 117 (41.9 %) were treated with chemotherapy and 197 (66.3 %) had a low probability of fibrosis by APRI. A smaller proportion of patients treated with chemotherapy had a low probability of hepatic fibrosis compared with untreated patients (64.1 vs. 75.3 %, p = 0.04). On multivariable analysis, chemotherapy treatment was a negative predictive factor for a low probability of fibrosis (OR 0.366 [95 % CI 0.184–0.708], p <0.01). Among chemotherapy-treated patients, 75 (64.1 %) had a low probability of fibrosis. There were no differences in chemotherapy duration (mean 7.8 vs. 7.5 cycles) and interval from last dose to steatosis diagnosis (24.3 vs. 21.4 months) between patients with and without a low probability of fibrosis. A smaller proportion of patients treated with irinotecan or 5-fluorouracil had a low probability of fibrosis (37.3 vs. 66.7 %, p = 0.04). On multivariable analysis, irinotecan or 5-fluorouracil treatment was a negative predictive factor for low probability of fibrosis (OR 0.277 [95 % CI 0.091–0.779], p = 0.02). Prior chemotherapy treatment, especially with 5-fluorouracil or irinotecan, is a negative predictor for the absence of advanced hepatic fibrosis among patients with steatosis.
Keywords: Nonalcoholic fatty liver disease, Chemotherapy, Hepatic fibrosis, Steatohepatitis
Introduction
In parallel with dramatic survival benefits derived from chemotherapy, evaluation and management of chemotherapy-related side effects are at the forefront of cancer care. Chemotherapy-associated hepatotoxicity is most often reversible and has been well studied in settings of neoadjuvant or conversion treatment before subsequent liver resection for colorectal cancer liver metastases [1–4]. However, long-term, irreversible, chemotherapy-associated hepatotoxicity has been reported after treatment with many agents [4–20]. Chemotherapy-associated liver toxicity (particularly that mediated by steatosis-related mechanisms) may be more common in the current era due to (1) improved patient survival for patients with malignancy, (2) increasing popularity of long-term “maintenance” therapy for the prevention of disease recurrence or progression, and (3) the rising prevalence of nonalcoholic fatty liver disease (NAFLD), the most common chronic liver disease in the USA with prevalence proportions of 20–46 % [21–23]. Deleterious effects of NAFLD include steatohepatitis and associated advanced hepatic fibrosis (e.g., bridging fibrosis and cirrhosis). Evolution of simple hepatic steatosis to these more adverse conditions is dependent on a multitude of factors including visceral adiposity, aberrations in intestinal microbiota and/or gut bacterial translocation, and hepatic mitochondrial dysfunction [24, 25]. In a “two-hit” model for progression [26, 27], hepatic insults (such as chemotherapy) [1, 28, 29] in the setting of metabolic derangements leading to steatosis may result in progression to steatohepatitis and associated advanced hepatic fibrosis.
The extent of liver injury among treated patients with persistent hepatic steatosis without cirrhosis detected on surveillance imaging is unclear. Routine imaging modalities, such as computed tomography, ultrasonography, and magnetic resonance imaging, will accurately diagnose advanced fibrosis only when features such as nodular hepatic parenchyma, caudate hypertrophy, widening of the umbilical fissure, and/or varices from portal hypertension are present [30]. Moreover, the long-term influence of chemotherapy on the evolution of fatty liver disease is not well understood. The purpose of this study was to determine whether chemotherapy treatment at least 6 months prior to the detection of hepatic steatosis is associated with advanced fibrosis. Among cancer patients with radiologic evidence of hepatic steatosis without cirrhosis, we hypothesize that the proportion of patients without histologic advanced hepatic fibrosis among those who have been off chemotherapy for at least 6 months prior to steatosis diagnosis is smaller compared with counterparts who have not been treated with chemotherapy.
Methods
Patient selection
After obtaining institutional review board approval, demographics, comorbid conditions, and laboratory data for patients with hepatic steatosis without evidence of cirrhosis on radiologic imaging as determined by board-certified abdominal radiologists at the University of Maryland Medical Center (UMMC) from January 2000 to April 2013 were reviewed. Patients were initially identified by searching all radiology reports for the words or phrases: “liver” or “hepatic” within ten words of “fat,” “fatty,” or “steatosis”; “fatty change”; “nonalcoholic”; and “steatohepatitis.” Each report was then reviewed to confirm the presence of hepatic steatosis, the absence of cirrhosis, and the presence of a solid tumor indication for radiologic imaging. Patients with prior chemotherapy treatment were only included in this study if the last dose of chemotherapy was at least 6 months prior to the date of the radiologic imaging which detected hepatic steatosis. Several exclusion criteria were then employed to arrive at the study cohort. These criteria included (1) the presence of other chronic diseases of the underlying liver, such as hepatitis B or C viral infection, primary sclerosing cholangitis, auto-immune hepatitis, and Wilson’s disease (as noted by documented diagnoses in the medical record, histopathology, and/or serology); (2) diagnosis of cirrhosis based on radiologic imaging; (3) alcohol consumption of greater than 21 or 14 drinks per week for men and women, respectively, (per the American Association for the Study of Liver Diseases consensus statement regarding the alcohol consumption threshold to distinguish alcoholic from nonalcoholic fatty liver disease) [31, 32], any detectable blood alcohol content at UMMC (either before or after hepatic steatosis diagnosis), or any documented history of alcohol abuse and/or dependence in the medical record; (4) extra-hepatic biliary obstruction; (5) hepatic trauma or shock from any etiology; (6) pregnancy as documented in the medical record or by serum or urine laboratory levels; (7) any prior history of treatments with medications with known hepatotoxicity such as glucocorticoids or methotrexate; (8) age less than 18 years; (9) thrombocytopenia in the presence of sepsis; and (10) missing laboratory data within 60 days of the hepatic steatosis diagnosis per radiologic imaging. The entire electronic medical record of each patient was reviewed to determine accurate study exclusion. Any patient whose medical record was missing information pertaining to alcohol use was excluded from this study.
Demographics, comorbid conditions, and serum laboratory values of patients with and without prior chemotherapy treatment were ascertained. Except in cases where the interval from last dose of chemotherapy to radiologic imaging was less than 6 months, the earliest imaging which detected hepatic steatosis and with no corresponding missing laboratory data was used for this study. Total number of chemotherapy cycles (not stratified by any particular component of the overall regimen) were reported as was concomitant anti-biologic therapy at some point during chemotherapy treatment. Because irinotecan and 5-fluorouracil are particularly associated with fatty liver disease, treatment with either of these agents at anytime was reported. Criteria for the metabolic syndrome were extrapolated from international guidelines [33] and included any three of the following: body mass index (BMI) greater than 28.8 kg/m2 (validated as a replacement for elevated waist circumference in men and women) [34] and documentation of, or medical treatment for, dyslipidemia, hypertension, and/or diabetes mellitus. To facilitate comparisons, malignant diagnoses were grouped per Supplementary Table 1.
Noninvasive determination of the absence of advanced hepatic fibrosis
The ideal end point for this study would be the presence of advanced hepatic fibrosis on histologic examination or as determined by a noninvasive test with a 100 % positive predictive value for the presence of histologic advanced hepatic fibrosis. However, histopathologic examination of the liver was not possible in this retrospective study and high probabilities of fibrosis based on noninvasive laboratory panels do not accurately predict the presence of histologic advanced hepatic fibrosis (i.e., low positive predictive value) [35–43]. Thus, the primary end point of this study was the absence of advanced histopathologic hepatic fibrosis. The surrogate used for the absence of advanced histopathologic hepatic fibrosis was a low probability of fibrosis as calculated by the AST-to-platelet ratio index (APRI). Work by our group and others revealed that low probability of hepatic fibrosis as calculated by APRI has a high negative predictive value (90–98 %) in excluding the histologic presence of advanced hepatic fibrosis among hospitalized and ambulatory patients with various chronic liver diseases including NAFLD [35–43]. The following formula was used to calculate the risk scores:
Interpretation : ≤ 0.5 = low probability
At our center, the highest normal value of AST is 36 U/L.
Statistical analysis
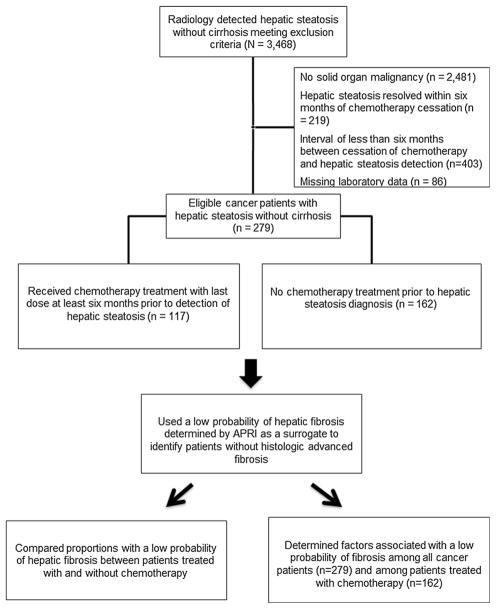
Statistical analyses were conducted using SAS® software, version 9.3 (SAS Institute, Cary, NC). Demographics, comorbid conditions, principal diagnoses, and serum laboratory values were compared between (1) cancer patients with and without chemotherapy treatment prior to the detection of hepatic steatosis, (2) cancer patients with and without a low probability of hepatic fibrosis as determined by APRI, and (3) cancer patients treated with prior chemotherapy with and without a low probability of hepatic fibrosis as determined by APRI (Fig. 1). Continuous variables were reported as means with standard errors (SE), and univariable comparisons were conducted with the two-tailed Student’s t test. Categorical variables were summarized as proportion percentages and compared with the χ2 test (or Fisher’s exact test in cases of low frequencies). A p <0.05 was considered significant for both tests. Multivariable logistic regression analyses with the end point of low probability of fibrosis determined by APRI were then conducted using variables in which the univariable comparisons had a p <0.10. Variables used to calculate probability of fibrosis (including serum platelet and AST levels) and composite variables (such as the metabolic syndrome) were not included in the multivariable analyses. A p <0.05 was considered significant for the multivariable analyses. Odds ratios (OR) and 95 % confidence interval (95 % CI) were reported.
Fig. 1.
Cohort identification and study design flowchart
Results
Overall study cohort
From January 2000–April 2013, 3,468 patients had hepatic steatosis without evidence of cirrhosis observed on radiologic imaging by board-certified abdominal radiologists at UMMC. Of these patients, 279 met the inclusion and exclusion criteria and thus comprised the study cohort (Fig. 1). The majority of these patients were female, White, and had a body mass index (BMI) over 30 kg/m2 (Table 1). While 69.5 % of patients had hypertension, only 36.9 and 45.5 % of patients had dyslipidemia and diabetes mellitus, respectively. Consequently, only 37.6 % of patients had the metabolic syndrome. Gastrointestinal primary tumors (44.1 %) were the most common malignancies in this cohort. Computed tomography (CT) was by far the most common modality by which hepatic steatosis was detected. Most patients had serum blood count and chemistry levels within respective normal ranges. Nearly two-thirds of patients (66.3 %) in this cohort had a low probability of hepatic fibrosis as determined by APRI.
Table 1.
Demographics, comorbidities, serum laboratory values, and noninvasive fibrosis scores for all cancer patients and stratified by chemotherapy treatment
| Variable | Total (n = 279) | Chemotherapy (n = 117) | No chemotherapy (n = 162) | p |
|---|---|---|---|---|
| Demographics and comorbidities | ||||
| Age (years), mean ± SE | 59.1 ± 0.7 | 59.2 ± 1.1 | 59.0 ± 0.9 | 0.90 |
| Female, n (%) | 156 (55.9) | 64 (54.7) | 92 (56.8) | 0.73 |
| Ethnicity, n (%) | 0.02 | |||
| White | 186 (66.7) | 87 (74.4) | 99 (61.1) | |
| Black | 66 (23.7) | 17 (14.5) | 49 (30.3) | |
| Hispanic | 10 (3.6) | 4 (3.4) | 6 (3.7) | |
| Asian | 10 (3.6) | 4 (3.4) | 6 (3.7) | |
| Native American | 4 (1.4) | 2 (1.7) | 2 (1.2) | |
| Body mass index (kg/m2), mean ± SE | 31.0 ± 0.5 | 30.2 ± 0.7 | 31.6 ± 0.7 | 0.17 |
| Hypertension, n (%) | 194 (69.5) | 78 (66.7) | 116 (71.6) | 0.37 |
| Diabetes mellitus, n (%) | 103 (36.9) | 33 (28.2) | 70 (43.2) | 0.01 |
| Dyslipidemia, n (%) | 127 (45.5) | 51 (43.6) | 76 (46.9) | 0.58 |
| Metabolic syndrome, n (%) | 105 (37.6) | 35 (29.9) | 70 (43.2) | 0.02 |
| Cancer type | <0.01 | |||
| Gastrointestinal, n (%) | 123 (44.1) | 67 (57.3) | 56 (34.6) | |
| Soft tissue, n (%) | 77 (27.6) | 25 (21.4) | 52 (32.1) | |
| Genitourinary, n (%) | 45 (16.1) | 13 (11.1) | 32 (20.0) | |
| Other, n (%) | 34 (12.2) | 12 (10.3) | 22 (13.6) | |
| Serum laboratory values | ||||
| Alanine aminotransferase (U/L), mean ± SE | 38.8 ± 2.1 | 37.8 ± 3.0 | 39.5 ± 2.9 | 0.68 |
| Aspartate aminotransferase (U/L), mean ± SE | 36.9 ± 1.9 | 35.4 ± 2.3 | 38.0 ± 2.9 | 0.50 |
| Total bilirubin (mg/dL), mean ± SE | 0.76 ± 0.04 | 0.76 ± 0.08 | 0.77 ± 0.03 | 0.90 |
| Alkaline phosphatase (U/L), mean ± SE | 107.0 ± 5.6 | 108.9 ± 7.8 | 105.7 ± 8.0 | 0.77 |
| Albumin (mg/dL) | 3.53 ± 0.04 | 3.64 ± 0.05 | 3.44 ± 0.07 | 0.02 |
| Creatinine (mg/dL), mean ± SE | 0.91 ± 0.02 | 0.88 ± 0.02 | 0.93 ± 0.03 | 0.17 |
| Hemoglobin (g/dL), mean ± SE | 12.2 ± 0.2 | 12.2 ± 0.2 | 12.2 ± 0.2 | 0.95 |
| Hematocrit (%), mean ± SE | 36.5 ± 0.3 | 36.6 ± 0.5 | 36.5 ± 0.4 | 0.85 |
| Platelets (thousand/μL), mean ± SE | 252.0 ± 7.0 | 219.9 ± 8.0 | 275.7 ± 10.3 | <0.01 |
| Diagnostic radiologic imaging | 0.94 | |||
| Computed tomography, n (%) | 260 (93.2) | 109 (93.2) | 151 (93.2) | |
| Ultrasound, n (%) | 15 (5.4) | 5 (5.1) | 9 (5.6) | |
| Magnetic resonance imaging, n (%) | 4 (1.4) | 2 (1.7) | 2 (1.2) | |
| Noninvasive fibrosis scores | ||||
| APRI, mean ± SE | 0.498 ± 0.037 | 0.531 ± 0.043 | 0.475 ± 0.056 | 0.43 |
| Low probability of fibrosis, APRI | 197 (66.3) | 75 (64.1) | 122 (75.3) | 0.04 |
Specified p values are from comparisons between patients treated with and without chemotherapy
SE standard error, APRI AST-to-platelet ratio index
Comparisons between patients with and without prior chemotherapy treatment
Of the overall study cohort, 117 (41.9 %) were treated with chemotherapy at least 6 months prior to the detection of hepatic steatosis. Types of chemotherapy regimens are included in Supplementary Table 2. The mean duration of chemotherapy was 7.7 cycles with a SE of 0.4 cycles. The mean interval from last dose of chemotherapy to date of detection of hepatic steatosis was 23.3 months with SE of 2.5 months. Of the patients treated with chemotherapy, 14 (12.0 %) were treated with anti-biologic therapy at some point during their chemotherapy treatment. Anti-biologic agents included bevacizumab (n = 6), cetuximab (n = 2), panitumumab (n = 2), sorafenib (n = 1), ipilimumab (n = 1), and trastuzumab (n = 2). In comparison with patients not treated with chemotherapy, higher proportions of patients treated with chemotherapy were White and had gastrointestinal primary tumors (Table 1). Lower proportions of patients treated with chemotherapy had diabetes mellitus and the metabolic syndrome. Patients treated with chemotherapy had higher serum albumin and lower platelet levels compared with patients not treated with chemotherapy. In contrast, there were no significant differences in age or BMI; proportions with female gender, hypertension, or dyslipidemia; and serum alanine aminotransferase (ALT), AST, creatinine, hemoglobin, or hematocrit levels between patients treated with and without chemotherapy. A smaller proportion of patients treated with chemotherapy had a low probability of hepatic fibrosis as calculated by APRI compared with patients not treated with chemotherapy (64.1 vs. 75.3 %, p = 0.04).
Comparisons between patients with and without a low probability of hepatic fibrosis
Demographics, comorbid conditions, and serum laboratory values were compared between 197 patients with and 82 patients without a low probability of hepatic fibrosis as determined by APRI (Table 2). On univariable analysis, patients with a low probability of hepatic fibrosis had significantly lower serum levels of ALT, AST, total bilirubin, alkaline phosphatase, hemoglobin, hematocrit, and platelet levels compared with other patients. Differences in AST and platelet levels are expected, given that these variables are used to determine APRI probability. In contrast, there were no significant differences in demographics, proportions of patients with comorbid conditions, or cancer type between patients with and without a low probability of hepatic fibrosis. A smaller proportion of patients with a low probability of hepatic fibrosis were treated with chemotherapy compared with that observed in other patients (38.1 vs. 51.2 %, p = 0.04). Multivariable analysis for the end point of low probability of hepatic fibrosis revealed ethnicity (group of Asian, Hispanic, and Native American compared with White); elevated serum ALT, total bilirubin, and alkaline phosphatase; and chemotherapy treatment to be independent negative predictive factors for a low probability of hepatic fibrosis (Table 2). Specifically, chemotherapy treatment had an OR of 0.366 (95 % CI 0.184–0.708), p <0.01, for a low probability of hepatic fibrosis.
Table 2.
Univariable and multivariable comparisons between demographics, comorbidities, treatments, and serum laboratory values for cancer patients stratified by probability of fibrosis as determined by APRI
| Variable | Low probability (n = 197) | Other (n = 82) | Univariable p | Multivariable p | OR [95 % CI] |
|---|---|---|---|---|---|
| Demographics and comorbidities | |||||
| Age (years), mean ± SE | 58.8 ± 0.8 | 59.9 ± 1.3 | 0.43 | – | – |
| Female, n (%) | 113 (57.4) | 43 (52.4) | 0.45 | – | – |
| Ethnicity, n (%) | 0.08 | ||||
| White | 136 (69.0) | 50 (61.0) | – | Reference | |
| Black | 47 (23.9) | 19 (23.2) | 0.17 | 0.568 [0.253–1.289] | |
| Other§ | 14 (7.1) | 13 (15.9) | <0.01 | 0.275 [0.106–0.709] | |
| Body mass index (kg/m2), mean ± SE | 31.2 ± 0.6 | 30.6 ± 1.0 | 0.58 | – | – |
| Hypertension, n (%) | 135 (68.5) | 59 (72.0) | 0.57 | – | – |
| Diabetes mellitus, n (%) | 72 (36.6) | 31 (37.8) | 0.84 | – | – |
| Dyslipidemia, n (%) | 93 (47.2) | 34 (41.5) | 0.38 | – | – |
| Metabolic syndrome, n (%) | 73 (37.1) | 32 (39.0) | 0.77 | – | – |
| Cancer type | 0.64 | – | – | ||
| Gastrointestinal, n (%) | 83 (42.1) | 40 (48.8) | – | – | |
| Soft tissue, n (%) | 57 (28.9) | 20 (24.4) | – | – | |
| Genitourinary, n (%) | 34 (17.3) | 11 (13.4) | – | – | |
| Other, n (%) | 23 (11.7) | 11 (13.4) | – | – | |
| Serum laboratory values | |||||
| Alanine aminotransferase (U/L), mean ± SE | 28.7 ± 1.6 | 63.1 ± 5.1 | <0.01 | <0.01 | 0.633 [0.523–0.735]* |
| Aspartate aminotransferase (U/L), mean ± SE | 24.6 ± 0.7 | 66.3 ± 5.1 | <0.01 | – | – |
| Total bilirubin (mg/dL), mean ± SE | 0.69 ± 0.03 | 0.94 ± 0.11 | 0.04 | 0.02 | 0.618 [0.414–0.889]† |
| Alkaline phosphatase (U/L), mean ± SE | 92.4 ± 4.3 | 142.4 ± 15.6 | <0.01 | 0.05 | 0.927 [0.849–0.997]‡ |
| Albumin (mg/dL) | 3.57 ± 0.05 | 3.43 ± 0.08 | 0.14 | – | – |
| Creatinine (mg/dL), mean ± SE | 0.89 ± 0.02 | 0.96 ± 0.05 | 0.22 | – | – |
| Hemoglobin (g/dL), mean ± SE | 12.4 ± 0.1 | 11.8 ± 0.3 | 0.04 | 0.19 | 1.056 [0.973–1.147]¥ |
| Hematocrit (%), mean ± SE | 37.1 ± 0.4 | 35.1 ± 0.7 | <0.01 | – | – |
| Platelets (thousand/μL), mean ± SE | 277.6 ± 8.5 | 191.6 ± 9.7 | <0.01 | – | – |
| Diagnostic radiologic imaging | 0.20 | – | – | ||
| Computed tomography, n (%) | 187 (94.9) | 73 (89.0) | – | ||
| Ultrasound, n (%) | 8 (4.1) | 7 (8.5) | – | ||
| Magnetic resonance imaging, n (%) | 2 (1.0) | 2 (2.4) | – | ||
| Chemotherapy treatment | 75 (38.1) | 42 (51.2) | 0.04 | <0.01 | 0.366 [0.184–0.708] |
Multivariable analysis for the end point of low probability of fibrosis is included
SE standard error, APRI AST-to-platelet ratio index
Includes Hispanic, Native American, and Asian populations
Units:
10 U/L;
0.5 mg/dL;
20 U/L;
0.5 g/dL
Subgroup analysis of patients treated with chemotherapy
To determine which aspects of chemotherapy are associated with a low probability of hepatic fibrosis, we performed a subgroup analysis consisting of the 117 chemotherapy-treated patients (Table 3). As in the previous analysis, similar significant differences in univariable comparisons between serum laboratory values were observed between patients with and without a low probability of hepatic fibrosis. Also, there were no significant differences in demographics, proportions of patients with comorbid conditions, or cancer type between patients with and without a low probability of hepatic fibrosis in this subgroup analysis. A smaller proportion of patients with a low probability of hepatic fibrosis were treated with irinotecan or 5-fluorouracil (37.3 vs. 66.7 %, p <0.01). A greater proportion of patients with a low probability of hepatic fibrosis were treated with anti-biologic agents, although this difference was not statistically significant. Neither duration of chemotherapy treatment nor interval from last chemotherapy treatment to diagnosis of hepatic steatosis was associated with a low probability of hepatic fibrosis. Multivariable analysis for the end point of low probability of hepatic fibrosis revealed elevated serum ALT and total bilirubin levels and irinotecan or 5-fluorouracil treatment to be independent negative predictive factors for a low probability of hepatic fibrosis. Specifically, irinotecan or 5-fluorouracil treatment had an OR of 0.277 (95 % CI 0.091–0.779), p = 0.02, for a low probability of hepatic fibrosis.
Table 3.
Univariable comparisons between demographics, comorbidities, treatments, and serum laboratory values for cancer patients treated with chemotherapy stratified by probability of fibrosis as determined by APRI
| Variable | Low probability (n = 75) | Other (n = 42) | Univariable p | Multivariable p | OR (95 % CI) |
|---|---|---|---|---|---|
| Demographics and comorbidities | |||||
| Age (years), mean ± SE | 58.5 ± 1.4 | 60.4 ± 1.8 | 0.41 | – | – |
| Female, n (%) | 40 (53.3) | 24 (57.1) | 0.69 | – | – |
| Ethnicity, n (%) | 0.28 | – | – | ||
| White | 59 (78.7) | 28 (66.7) | – | – | |
| Black | 10 (13.3) | 7 (16.7) | – | – | |
| Other | 6 (8.0) | 7 (16.7) | – | – | |
| Body mass index (kg/m2), mean ± SE | 29.9 ± 0.8 | 30.7 ± 1.6 | 0.67 | – | – |
| Hypertension, n (%) | 51 (68.0) | 27 (64.3) | 0.68 | – | – |
| Diabetes mellitus, n (%) | 21 (28.0) | 12 (28.6) | 0.95 | – | – |
| Dyslipidemia, n (%) | 35 (46.7) | 16 (38.1) | 0.37 | – | – |
| Metabolic syndrome, n (%) | 24 (32.0) | 11 (26.2) | – | – | |
| Cancer type | 0.16 | – | – | ||
| Gastrointestinal, n (%) | 39 (52.0) | 28 (66.7) | – | – | |
| Soft tissue, n (%) | 17 (22.7) | 8 (19.1) | – | – | |
| Genitourinary, n (%) | 8 (10.7) | 5 (11.9) | – | – | |
| Other, n (%) | 11 (14.7) | 1 (2.4) | – | – | |
| Serum laboratory values | |||||
| Alanine aminotransferase (U/L), mean ± SE | 26.8 ± 1.5 | 57.4 ± 6.9 | <0.01 | <0.01 | 0.449 [0.291–0.638] |
| Aspartate aminotransferase (U/L), mean ± SE | 24.1 ± 1.0 | 55.7 ± 4.9 | <0.01 | – | – |
| Total bilirubin (mg/dL), mean ± SE | 0.61 ± 0.03 | 1.02 ± 0.21 | 0.08 | 0.03 | 0.419 [0.185–0.877]† |
| Alkaline phosphatase (U/L), mean ± SE | 98.4 ± 8.3 | 127.7 ± 15.6 | 0.10 | 0.45 | 1.066 [0.890–1.241]‡ |
| Albumin (mg/dL) | 3.69 ± 0.05 | 3.56 ± 0.08 | 0.20 | – | – |
| Creatinine (mg/dL), mean ± SE | 0.88 ± 0.03 | 0.88 ± 0.05 | 0.87 | – | – |
| Hemoglobin (g/dL), mean ± SE | 12.5 ± 0.2 | 11.6 ± 0.3 | 0.04 | 0.05 | 1.153 [0.996–1.345]¥ |
| Hematocrit (%), mean ± SE | 37.7 ± 0.6 | 34.7 ± 0.9 | <0.01 | – | – |
| Platelets (thousand/μL), mean ± SE | 246.0 ± 9.4 | 173.5 ± 11.6 | <0.01 | – | – |
| Diagnostic radiologic imaging | 0.91 | – | – | ||
| Computed tomography, n (%) | 70 (93.3) | 39 (92.9) | – | – | |
| Ultrasound, n (%) | 4 (5.3) | 2 (4.8) | – | – | |
| Magnetic resonance imaging, n (%) | 1 (1.3) | 1 (2.3) | – | – | |
| Duration of chemotherapy (cycles) | 7.8 ± 0.5 | 7.5 ± 0.7 | 0.67 | – | – |
| Irinotecan or 5-fluorouracil | 28 (37.3) | 28 (66.7) | <0.01 | 0.02 | 0.277 [0.091–0.779] |
| Interval from last chemotherapy (months) | 24.3 ± 2.9 | 21.4 ± 4.6 | 0.547 | – | – |
| Anti-biologic therapy | 12 (16.0) | 2 (4.8) | 0.08 | 0.46 | 1.901 [0.395–14.241] |
Multivariable analysis for the end point of low probability of fibrosis is included
SE standard error, APRI AST-to-platelet ratio index
Units:
10 U/L;
0.5 mg/dL;
20 U/L;
0.5 g/dL
Discussion
The purpose of this study was to determine whether chemotherapy treatment at least 6 months prior to the detection of hepatic steatosis is associated with advanced fibrosis. As most patients with hepatic steatosis do not have associated advanced fibrosis [21, 22, 44] (and are thus at relatively low risk for hepatic-related morbidity), it is important to know whether chemotherapy treatment alters the pathologic spectrum of fatty liver disease. To achieve this aim, we examined cancer patients with hepatic steatosis and without any other chronic liver diseases besides NAFLD. A 6-month interval from last dose of chemotherapy to hepatic steatosis diagnosis was chosen arbitrarily as we postulated that perturbations in serum laboratory levels due to chemotherapy treatment and reversible hepatic steatosis would have resolved by this time. Importantly, the mean interval from last chemotherapy dose to the detection of hepatic steatosis was nearly 2 years in this study. While the gold standard for determining the presence of advanced hepatic fibrosis is liver biopsy, associated complications in up to 5 % of patients and the lack of sufficient resources preclude performing biopsies on every patient with fatty liver disease [45–48]. Thus, noninvasive scoring panels, such as APRI, have been developed, which accurately predict the absence (but not presence) of histologic advanced hepatic fibrosis. Since previous studies have shown that a low probability of fibrosis as determined by APRI has a negative predictive value of 90–98 % in excluding the histologic presence of advanced fibrosis [8, 9, 35–43], we used a low calculated probability of fibrosis as a surrogate for the absence of advanced hepatic fibrosis.
Results of this study have important implications regarding the management of patients with hepatic steatosis after prior chemotherapy treatment. Persistence of steatosis for 6 months or longer after cessation of chemotherapy is not a benign finding as the absence of advanced hepatic fibrosis is less likely in these situations when compared to NAFLD without prior chemotherapy treatment. This relationship is not influenced by the duration of chemotherapy treatment, the interval from last dose of chemotherapy to diagnosis of hepatic steatosis, or elements of the metabolic syndrome. As expected given prior studies in colorectal cancer liver metastases [1–3, 5–7], 5-fluorouracil and irinotecan treatment in particular was associated with advanced hepatic fibrosis. Hence, cancer patients with hepatic steatosis after prior chemotherapy treatment should be further evaluated for the presence of associated advanced hepatic fibrosis by methods other than noninvasive laboratory risk scores, such as new magnetic resonance imaging techniques or liver biopsy. Advanced hepatic fibrosis has several potential harmful consequences for patients with malignancy. Numerous studies show that advanced hepatic fibrosis from any cause is associated with increased morbidity and mortality among hospitalized patients, including those with conditions common to cancer patients such as critical illness, infection, and sepsis [49–53]. Impairment of liver function due to advanced fibrosis results in heightened toxicity for most chemotherapy agents [54]; thus, identification of advanced fibrosis before initiating additional lines of chemotherapy upon disease progression is essential to reduce potentially lethal side effects.
Several limitations to this study should be considered. Occult alcohol use and potential inaccuracies in degrees of alcohol consumption obtained from retrospective chart reviews may have clouded the etiology of hepatic steatosis. We minimized this limitation by only including those patients in which the medical record specified the amount of alcohol intake. Because serum triglyceride, high-density lipoprotein, fasting glucose levels, waist circumference, and blood pressure measurements were not available for most patients, we used surrogates for each parameter, including notation of each disorder in the medical record, medication treatment, and BMI. While there were thus likely some patients with unrecognized elements of the metabolic syndrome in this study, we believe that these methods of comorbidity identification are consistent with clinical practice in most patients. As the application of noninvasive risk scores is only useful for patients without apparent cirrhosis, our results most accurately apply to those patients without clear evidence of cirrhosis on clinical exam or radiologic imaging. Since we used radiologic imaging for cohort identification, patients with less than 33 % of hepatic steatosis (below the threshold for detection) [55, 56] were likely not included in this study. While the determination of hepatic steatosis was made by dedicated abdominal radiologists, it is unknown if established criteria were used in ascertaining each diagnosis. Again, we believe that this method of steatosis detection is consistent with clinical practice in most patients. We also did not differentiate between severities of steatosis noted on radiologic imaging. Extensive variations in durations, combinations, and dosages of chemotherapeutic regimens precluded further determination of the influence of specific agents on advanced hepatic fibrosis.
The proportion of cancer patients with radiologic evidence of hepatic steatosis who have been off chemotherapy for 6 months or longer without advanced hepatic fibrosis is smaller compared with cancer patients with hepatic steatosis who have not been treated with chemotherapy. Chemotherapy treatment is an independent negative predictor factor for the absence of advanced hepatic fibrosis. Given the deleterious consequences of advanced hepatic fibrosis, patients with persistent hepatic steatosis 6 months after chemotherapy treatment should be further evaluated for the presence of advanced hepatic fibrosis.
Supplementary Material
Acknowledgments
Dr. Reddy is supported by NIH 2K12HD043489-11. Dr. Mindikoglu is supported by NIH K23DK089008. The project described was supported in part by Grant Number 5 K23 DK089008-04 from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (to Ayse L. Mindikoglu, M.D., M.P.H.) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the NIH.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12032-014-0971-y) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Srinevas K. Reddy, Email: sreddy@smail.umaryland.edu, Departments of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Rm S3AX30, Baltimore, MD 21201, USA. Department of Veterans Affairs Maryland Healthcare System, Baltimore, MD 21201, USA
Colleen Reilly, Departments of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Rm S3AX30, Baltimore, MD 21201, USA.
Min Zhan, Departments of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Ayse L. Mindikoglu, Departments of Internal Medicine, University of Maryland School of Medicine, Baltimore, MD 21201, USA
Yixing Jiang, Departments of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Barton F. Lane, Departments of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD 21201, USA
H. Richard Alexander, Departments of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Rm S3AX30, Baltimore, MD 21201, USA.
William J. Culpepper, Department of Veterans Affairs Maryland Healthcare System, Baltimore, MD 21201, USA. Departments of Neurology, University of Maryland School of Medicine, Baltimore, MD 21201, USA
Samer S. El-Kamary, Departments of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD 21201, USA. Departments of Pediatrics, University of Maryland School of Medicine, Baltimore, MD 21201, USA
References
- 1.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal liver metastases. J Clin Oncol. 2006;24:2065–72. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 2.Pawlik TM, Olino K, Gleisner AL, et al. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–8. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PS, Park JO, Bao F, et al. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: a single institution experience. J Am Coll Surg. 2013;216:41–9. doi: 10.1016/j.jamcollsurg.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–6. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 5.Peppercorn PD, Reznek RH, Wilson P, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008–11. doi: 10.1038/bjc.1998.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorensen P, Edal AL, Madsen EL, et al. Reversible hepatic steatosis in patients treated with interferon alfa-2a and 5-fluorouracil. Cancer. 1995;15:2592–6. doi: 10.1002/1097-0142(19950515)75:10<2592::aid-cncr2820751029>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG, Fleming TR, Macdonald JS, et al. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J Clin Oncol. 1993;11:2386–90. doi: 10.1200/JCO.1993.11.12.2386. [DOI] [PubMed] [Google Scholar]
- 8.Morling JR, Fallowfield JA, Guha IN, et al. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. J Hepatol. 2014;60:384–91. doi: 10.1016/j.jhep.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg JW, Lidsky MD. Cyclophosphamide associated hepatotoxicity. South Med J. 1985;78:222–3. doi: 10.1097/00007611-198502000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Robinson K, Lambiase L, Li J, et al. Fatal cholestatic liver failure associated with gemcitabine therapy. Dig Dis Sci. 2003;48:1804–8. doi: 10.1023/a:1025415616592. [DOI] [PubMed] [Google Scholar]
- 12.Aviles A, Herrera J, Ramos E, et al. Hepatic injury during doxorubicin therapy. Arch Pathol Lab Med. 1084;108:912–3. [PubMed] [Google Scholar]
- 13.Field KM, Dow C, Michael M. Part I: Liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Loeb E, MacLellan A, et al. Clinical studies of platinum coordination compounds in the treatment of various malignant diseases. Cancer Chemother Rep. 1975;59:647–59. [PubMed] [Google Scholar]
- 15.Tran A, Housset C, Boboc B, et al. Etoposide (VP 16-213) induced hepatitis. Report of three cases following standard-dose treatments. J Hepatol. 1991;12:36–9. doi: 10.1016/0168-8278(91)90905-q. [DOI] [PubMed] [Google Scholar]
- 16.Saif MW, Shahrokni A, Cornfield D. Gemcitabine-induced liver fibrosis in a patient with pancreatic cancer. JOP. 2007;8:460–7. [PubMed] [Google Scholar]
- 17.Huizing MT, Misser VH, Pieters RC, et al. Taxanes: a new class of antitumor agents. Cancer Invest. 1995;13:381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 18.Creemers GJ, Lund B, Verweij J. Topoisomerase I inhibitors: topotecan and irinotecan. Cancer Treat Rev. 1994;20:73–96. doi: 10.1016/0305-7372(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 19.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–76. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DH, Greco FA, Wolff SN. Etoposide induced hepatic injury: a potential complication of high-dose therapy. Cancer Treat Rep. 1983;67:1023–4. [PubMed] [Google Scholar]
- 21.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–40. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1998 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Krawczyk M, Bonfrate L, Portincasa P. Nonalcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2010;24:695–708. doi: 10.1016/j.bpg.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Levene AP, Goldin RD. The epidemiology, pathogenesis, and histopathology of fatty liver disease. Histopathology. 2012;61:141–52. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- 26.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 27.Bohinc BN, Diehl AM. Mechanisms of disease progression in NASH: new paradigms. Clin Liver Dis. 2012;16:549–65. doi: 10.1016/j.cld.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–86. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 29.Reddy SK, Marsh JW, Varley PR, et al. Underlying steatohepatitis, but not simple steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 2012;56:2221–30. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 30.Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol. doi: 10.1136/jclinpath-2013-201620. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel RH, Alberti KGMM, Grundy SM, et al. The metabolic syndrome. Lancet. 2010;375:181–3. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 34.Ascha MS, Hanounch IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–8. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 35.Reddy SK, Reilly C, Twaddell WS, et al. Low probability of hepatic fibrosis from noninvasive risk panels accurately excludes advanced fibrosis due to nonalcoholic fatty liver disease among hospitalized patients. World J Gastroenterol. 2014 manuscript submitted. [Google Scholar]
- 36.McPherson S, Steward SF, Hendeson E, et al. Simple non-invasive fibrosis scoring systems can reliably excluded advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 37.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 38.Petersen JR, Stevenson HL, Kasturi KS, et al. Evaluation of the aspartate aminotransferase/platelet ratio index and enhanced liver fibrosis tests to detect significant fibrosis due to chronic hepatitis C. J Clin Gastroenterol. 2013 Sep 16; doi: 10.1097/MCG.0b013e3182a87e78. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celikbilek M, Dogan S, Gursoy S, et al. Noninvasive assessment of liver damage in chronic hepatitis B. World J Hepatol. 2013;5:439–45. doi: 10.4254/wjh.v5.i8.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loaeza-del-Castillo A, Paz-Pineda F, et al. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350–7. [PubMed] [Google Scholar]
- 41.Palmeri ML, Wang MH, Rouze NC, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666–72. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruger JC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101:477–80. [PubMed] [Google Scholar]
- 43.Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol. 2013;66:1033–45. doi: 10.1136/jclinpath-2013-201620. [DOI] [PubMed] [Google Scholar]
- 44.Nakahara T, Hyogo H, Yoneda M, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol. 2013 Nov 26; doi: 10.1007/s00535-013-0911-1. epub. [DOI] [PubMed] [Google Scholar]
- 45.Gunneson TJ, Menon KV, Wiesner RH, et al. Ultrasound-assisted percutaneous liver biopsy performed by a physician assistant. Am J Gastroenterol. 2002;97:1472–5. doi: 10.1111/j.1572-0241.2002.05789.x. [DOI] [PubMed] [Google Scholar]
- 46.Szymczak A, Simon K, Inglot M, et al. Safety and effectiveness of blind percutaneous liver biopsy: analysis of 1412 procedures. Hepat Mon. 2012;12:32–7. doi: 10.5812/kowsar.1735143x.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behrens G, Ferral H. Transjugular liver biopsy. Semin Intervent Radiol. 2012;29:111–7. doi: 10.1055/s-0032-1312572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller M, Kratzer W, Oeztuerk S, et al. Percutaneous ultrasonographically guided liver punctures: an analysis of 1961 patients over a period of ten years. BMC Gastroenterol. 2012;12:173. doi: 10.1186/1471-230X-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–20. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 50.Pavon A, Binquet C, Kara F, et al. Profile of the risk of death after septic shock in the present era: an epidemiological study. Crit Care Med. 2013 Aug 10; doi: 10.1097/CCM.0b013e31829a6e89. epub. [DOI] [PubMed] [Google Scholar]
- 51.Akiyama T, Chikuda H, Yasunaga H, et al. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013;25:3. doi: 10.1136/bmjopen-2012-002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Hasan MN, Lahr BD, Eckel-Passow JE, et al. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect. 2012 Nov 8; doi: 10.1111/1469-0691.12085. epub. [DOI] [PubMed] [Google Scholar]
- 53.Kang CI, Song JH, Chung DR, et al. Liver cirrhosis as a risk factor for mortality in a national cohort of patients with bacteremia. J Infect. 2011;63:336–43. doi: 10.1016/j.jinf.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Field KM, Micheal M. Part II: Liver function in oncology: towards safer chemotherapy use. Lancet Oncol. 2008;9:1181–90. doi: 10.1016/S1470-2045(08)70307-3. [DOI] [PubMed] [Google Scholar]
- 55.Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–45. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 56.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.