Abstract
The marrow microenvironment and its components regulate hematopoietic stem and progenitor cell (HSC) fate. An abnormality in the marrow microenvironment and specific dysfunction of the HSC niche could play a critical role in initiation, disease progression and response to therapy of marrow failure syndromes. Therefore, the identification of changes in the HSC niche in marrow failure syndromes should lead to further knowledge of the signals that disrupt the normal microenvironment. In turn, niche disruption may contribute to disease morbidity resulting in pancytopenia and clonal evolution, and its understanding could point to new therapeutic targets for these conditions. We briefly (a) review evidence for the importance of the marrow microenvironment as a regulator of normal hematopoiesis, (b) summarize the current knowledge regarding the role of dysfunctions in the marrow microenvironment in marrow failure syndromes, and (c) propose a strategy through which niche stimulation can complement current treatment for MDS.
Introduction
The bone marrow (BM) is the exclusive site of production of all blood cells in humans. Aplastic anemia (AA) is a disorder of the BM resulting in the loss of its ability to produce mature blood cells. AA treatment is focused on intense immunosuppression and/or bone marrow transplantation. The study of therapeutic targets in AA has been limited by its rarity in the general population and the dearth of murine models of this disorder. The myelodysplastic syndromes (MDS) are also characterized by defects in the ability to form blood cells, resulting in pancytopenias. In contrast to AA, data suggest that the incidence of MDS is increasing. In fact, the SEER database underestimates the incidence of MDS by at least 3 fold.1 In MDS, the major morbidity and mortality results from the ineffective nature of the malignant clonal hematopoiesis and its suppression of residual normal hematopoiesis. All types of cytopenias are common among patients with both AA and MDS and are associated with symptomatic anemia, bleeding and infections. A large proportion of elderly patients with MDS are either hospitalized (62%) or use the emergency department (42%) within 3 months of diagnosis.2 While, as we will review, the role of the microenvironment in AA is well established, only recent studies suggest a role for the BM microenvironment (MME) in the pathogenesis and clinical features of MDS, and therapies targeting the MME in bone marrow failure are lacking. Moreover, to date the overwhelming majority of effort expended studying MDS has largely ignored the mechanisms by which the MDS clone alters its local microenvironment and suppresses residual normal marrow function. In this chapter, we will review the current understanding of the normal MME, examine evidence supporting MME disruption in marrow failure syndromes and highlight data supporting targeting the MME as a strategy for treatment. Disorders of hematopoiesis continue to have suboptimal clinical outcomes, highlighting the appeal of potential therapeutic manipulation of the MME in these situations.3
The Marrow Microenvironment in Normal Hematopoiesis
In mammalians, skeletal organs are essential for normal hematopoiesis.4 Within the marrow microenvironment, specific microenvironments, or niches, regulate HSC fate. Initial studies supported the central role of bone constituents in hematopoietic stem cell regulation.5–7 As our understanding of the system has progressed, and as a result of elegant genetic studiesand intravital microscopy, it became clear that the differentiation stage of mesenchymal cells is critical for their ability to support and regulate HSCs.8–10 In addition, heterogeneity of the marrow endothelium has been elucidated.11–13 Currently, niche cells with mesenchymal characteristics are thought to be found in close association with arterial structures located at endosteal sites.13 These cells are likely a subset of mesenchymal stem cells (MSCs), the multipotent stromal cells that give rise to osteolineage cells, adipocytes and chondrocytes. In the literature, this cell population is inconsistently defined, in part because the lack of consensus on its defining experimental characteristics (adherence to plastic vs functional characteristics vs cell surface markers) and the fact that in publications often the MSC abbreviation designates still heterogeneous preparations of human mesenchymal stromal precursor cells, which are now commercially available14,15. In addition to these immature cells, terminally differentiated hematopoietic cells, such as macrophages,16,17 osteoclasts,18 glia19 and T cells,20 have also been described as stimulatory components of the niche, while adipocytes are thought to inhibit HSCs.21 Niche composition and interactions with stem cells are coordinated by circadian rhythms,22 hormonal signals,23–25 oxygen tension26 and likely other physiologic stimuli.
Definition of the HSC niche in humans remains less understood, however both osteoblastic5 and mesenchymal stromal cells have been demonstrated to increase HSC support ex vivo.27 In addition to HSC niches, data support a role of the MME in both lymphopoiesis10,28 as well as myelopoiesis29. The therapeutic potential of the MME in HSC regulation has already found clinical relevance, as illustrated by de Lima and colleagues, who found that in patients receiving cord blood transplants for hematologic malignancies, engraftment is faster and more robust after co-culturing the cord blood cells with marrow mesenchymal stromal cells.27 While our understanding of the HSC niche is likely to continue to evolve, the concept of its heterogeneity has emerged and allows for multiple targets for potential therapeutic intervention in hematopoietic regeneration (Figure 1). Moreover, the concept of the niche allows for a unique frame of reference when considering initiation of hematopoietic pathologies and interactions of malignant cells with the MME.
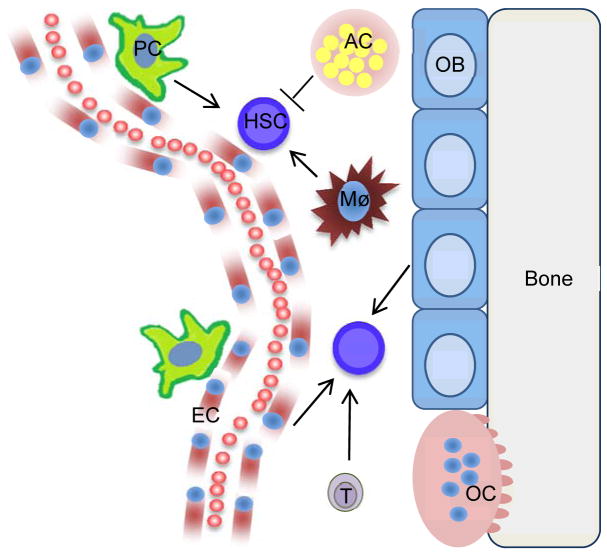
Figure 1. Schematic of HSCs within the niche.
Niche regulations of HSCs
For simplicity, only some MME cell types are depicted. Pericytes (PC), macrophages (Mø), Endothelial cells (EC), T-cells (T) and osteoblasts (OB) are supportive of HSCs under normal conditions while adipocytes (AC) have an inhibitory role.
The Marrow Microenvironment and Initiation of Marrow Failure Syndromes
Murine models have demonstrated that disruption of the MME can initiate myeloproliferative disease 30,31 and even leukemia.32 However, until recently, the role of MME in the pathogenesis of marrow failure syndromes was limited, with the exception of AA. AA was once thought to be the result of a quantitative HSC pool defect secondary to a toxin.33 In the early 1970s, however, Mathé and colleagues observed recovery of hematopoiesis in patients with AA who failed to engraft after allogeneic stem-cell transplantation with an immunosuppressive conditioning regimen, suggesting that the immune system in these patients was suppressing the growth and differentiation of HSCs.34 Also, attempts to treat patients with AA via transplantation of HSCs from an identical twin without conditioning the recipient with total body radiation or high-dose cytotoxic agents often failed to reconstitute hematopoiesis.35 Idiopathic AA is now largely considered to be an autoimmune disease in which activated T lymphocytes induce accelerated apoptosis of HSCs.33,36 Marrow lymphocytes from patients with AA can inhibit hematopoiesis when cultured with normal marrow.37 Oligoclonal or monoclonal expansions of CD8+ T cells have been found in patients with AA, a finding consistent with antigen-specific lymphocyte attack against hematopoietic tissue.38 Whereas the antigen(s) on HSCs that is targeted by T cells is unknown, cytokine expression by activated cytotoxic T cells may play an important role in the pathogenesis of AA. Those cytokines found to be prevalent in the bone marrow of patients with AA include the marrow suppressive cytokine interferon γ, as well as interleukins 17 and 27, both strong T-helper-1 cytokines.36
Bone marrow microenvironmental cells in AA have been characterized by a number of groups. Compared to controls, MSCs from patients with AA have aberrant morphology, decreased proliferation and clonogenic potential, and increased apoptosis. Relative to normal MSCs, those from AA patients were more difficult to induce to differentiate into osteoblasts and were more readily induced to differentiate into adipocytes. In addition to the abnormal biological features, the transcriptome of MSCs from aplastic anemia patients demonstrated the downregulation of numerous genes, including mediators of cell cycling, cell division, proliferation, chemotaxis,, and hematopoietic cell interactions and the upregulation of genes involved in apoptosis, adipogenesis, and the immune response were up-regulated in MSCs from aplastic anemia patients.39 These data suggested that MSCs represent another component of the deranged MME in aplastic anemia. Notably, T lymphocytes modulate some aspects of hormonal regulation of the HSC niche.40,41 Additionaly, T regulatory cells in mice are required for allogeneic HSC persistence.42 Whether altered T-cell signaling in AA contributes to abnormal MSC regulation is to our knowledge an unexplored, and as of yet an unanswered question.
Frequently cited as some of the strongest evidence for MME dysfunction in MDS contributing to disease initiation and progression, Raaijmakers et al. showed that deletion of Dicer1 in murine osteoprogenitors results in development of dysplastic hematopoiesis, with an MDS-like phenotype. The Dicer1 deletion leads to decreased Sbds (the gene mutated in Shwachman-Diamond Syndrome) expression in osteoprogenitors; this murine model of MDS therefore provides data indicative of a role of the MME in SDS. Notably, the Dicer1 null murine model of MDS resulted in primary MME dysfunction leading to secondary development of hematologic malignancy, supporting the concept of niche-initiated oncogenesis.43
Therefore, data support the concept that defects in the MME can be initiating steps in the pathogenesis of marrow failure syndromes. AA is a paradigm for this concept since microenvironmental dysfunction, likely mediated at least in part by autoimmunity, is thought to lead to HSC pool depletion.Conceptually, therefore, the MME can now be considered a targetable component of the disease process initiating marrow failure syndromes.
Disruption of the Marrow Microenvironment in Inherited Marrow Failure Diseases
The rarity of the hereditary marrow failure syndromes likely accounts for a dearth of studies on the MME in these diseases. Dror and Freedman studied marrow stromal function in Shwachman-Diamond syndrome (SDS) in vitro using primary cells from patients. They found that SDS marrow stroma had decreased ability to support and maintain both normal and SDS-associated hematopoiesis.44 Over a decade later, Andre et al. studied a more select cell population, namely mesenchymal stem cells (MSCs), from patients with SDS. In comparison with normal MSCs, SDS MSCs did not show any differences with regard to morphology, growth kinetics, expression of surface markers, differentiation, HSC support or karyotype.45 Future studies are needed to identify the particular niche in the SDS-associated MME that contributes to dysfunctional hematopoiesis in this disease. Li et al. investigated MSCs in a murine model of Fanconi Anemia with deletion of the Fancg gene. They found Fancg −/− mice to have defective MSC proliferation and increased apoptosis. Introduction of human Fancg cDNA restored both normal proliferative capacity and normal cell survival to the Fancg-null MSCs. Fancg −/− MSCs had a reduced ability to support both HSC proliferation and differentiation in vitro, and engraftment of transplanted HSCs in vivo. These data supported the concept that Fanconi Anemia proteins were required in the MME to maintain normal hematopoiesis.46 Therefore, , while data on the role of the MME in inherited marrow failure syndromes is scarce, there is some that suggests its dysfunction in these diseases, aligning these disorders with acquired marrow failure syndromes, as we will review in the next section.
Disruption of the Marrow Microenvironment in Myelodysplastic Syndromes
In MDS the marrow is generally hyperproliferative but the disease is characterized by ineffective hematopoiesis and cytopenias. This paradox of myeloproliferation and cytopenias is ascribed to late precursor apoptosis in the marrow. Some groups have implicated the MME as the source of pro-apoptotic signals. Kitagawa et al. found the pro-apoptotic factor FAS ligand was produced by stromal cells, whereas its receptor was expressed in the hematopoietic cells of MDS marrow.47 Stromal populations from patients with MDS are defective in their ability to support HSCs. Tennant et al. demonstrated that adherent cell layers from MDS marrow were defective in supporting colony formation relative to adherent cell layers from normal marrow.48 Ferrer et al. co-cultured marrow mesenchymal stromal cells from MDS patients with hCD34+ cells from healthy donors and found decreased numbers of HSCs and colony-forming units, compared with co-cultures utilizing mesenchymal stromal cells from healthy donors.49 Geyh et al. also reported on the diminished capacity of MDS-derived mesenchymal stromal cells to support hCD34+ HSCs in long-term culture-initiating cell assays due to altered gene expression of known mediators of interactions with HSCs including osteopontin, Jagged-1, Kit-ligand, and angiopoietin.50
Some data support immune dysregulation in MDS. Sloand et al. found expansion of cytotoxic T-cell clones in all of 34 patients with MDS cytogenetically characterized by trisomy 8. Consistent with an underlying autoimmune pathophysiology in this subset of MDS, over 60% of patients with trisomy 8 as the sole cytogenetic abnormality (13 patients) who were treated with an antithymocyte globulin (ATG) based regimen had a response, with durable reversal of cytopenias that was coincident with a stable increase in trisomy 8 marrow mononuclear cells and normalization of the T-cell repertoire.51 Subsequently, Sloand et al. reported on a clinical trial in which patients with MDS classified as refractory anemia, refractory anemia with ringed sideroblasts, or refractory anemia with excess blasts, received immunosuppressive therapy with either equine ATG, ATG plus cyclosporine, or cyclosporine alone. Of the 129 patients treated, 30% achieved a hematologic response, with a median follow up of 3 years.52 Epling-Bernette et al. found evidence of reduced natural killer (NK) cell function on the basis of in vitro assays with blood mononuclear cells from patients with MDS. Based on these results, the authors speculated that abnormal myeloid progenitors in MDS may expand partly due to evasion of NK immunosurveillance.53 Marcondes et al. similarly reported reduced NK cell function in in vitro assays utilizing blood samples from patients with MDS.54 It has been postulated that abnormal BM stroma in MDS may play an inhibitory role in NK cell development since contact between these two cell populations is required for full NK cell differentiation and accordingly, that loss of immunosurveillance may lead to accumulation of cells with DNA damage, contributing to MDS progression.55 Taken together, data are supportive of immune dysregulation as a contributing factor to MDS pathogenesis, at least in some subsets of the disease.
There are multiple signals and secreted factors from MDS marrow stroma that have been implicated as plausible mediators of dysfunctional interactions with hematopoietic cells. The precise roles of many of these factors including cytokines, angiogenic factors, metalloproteinases, and adhesion molecules are not well understood. TNFα produced by stromal cells plays a role in inducing apoptosis of maturing hematopoietic progeny.56–58 Vascular endothelial growth factor (VEGF) levels have been found to be increased in the marrow of patients with MDS. 59,60 Keith et al. found increased microvascular density in the marrow of patients with MDS, which correlated with increased VEGF expression.61 MDS stromal cells induce altered matrix metalloproteinase expression in clonally-derived monocytes but the significance of this finding is unclear.62 Expression of adhesion molecules including CD166 and CD29 was altered in MDS-derived mesenchymal stromal cells; however, it is not clear if these abnormalities influence the pathogenesis of MDS.49
Therefore, while the precise role of the MME in MDS pathogenesis is poorly characterized, the evidence is strong that it is altered in this disease and that its dysfunction contributes to disease progression.
MDS Cells Require their Niche
Murine xenotransplantation models of MDS provide an opportunity to study this disease in an in vivo setting. However, it has been difficult to attain engraftment of clonal CD34+ cells derived from the marrow of patients with MDS into non-obese diabetic/ severe combined immunodeficient (NOD/SCID) mice. These observations support the concept that MDS relies heavily on its MME. In fact, multiple studies demonstrate that marrow stroma is necessary for successful engraftment of clonal human MDS marrow-derived CD34+ cells into mice. Kerbauy et al. showed that irradiated NOD/SCID- β-2 −/− mice had engraftment of clonal MDS-derived hematopoietic precursors when normal stromal cells (from cell lines derived from a healthy marrow donor) were co-injected via the intramedullary route.63 Muguruma et al. performed intramedullary injections of marrow hCD34+ cells from MDS patients without or along with human MSCs into irradiated NOD/SCID mice with deletion of the T-cell receptor λ chain (NOG mice). Cells from three of six patients with MDS engrafted in NOG mice when co-injected with MSCs. Engraftment of MDS cells was not achieved with co-injection of stromal cells derived from sites other than marrow or non-stromal cells.64
HS27a is a normal human marrow stromal cell line that supports primitive hematopoietic cells, at least partially via cell-cell signaling through the Jagged-Notch pathway.65 This is in contrast to the HS5 human marrow stromal cell line, which supports more mature colony-forming cells. Li et al. found that clonal human MDS cell engraftment was achieved with intravenous co-injection of HS27a cells but not with HS5 cells in xenograft models.66 Highly expressed on HS27a cells but not on HS5 cells, the cell adhesion molecule glycoprotein CD146 may be important for engraftment of MDS clonal cells. CD146 is involved in multiple physiologic processes including signal transduction, cell migration and development, angiogenesis and mesenchymal cell differentiation.67 CD146+ cells are an important subset of stromal fibroblasts which express CXCL12 and contribute to the stem cell niche.68 Notably, levels of CXCL12 and its receptor CXCR4 are decreased in MDS cultures and this profile is associated with reduced induction of migration of CD34+ hematopoietic cells.69 Sorted CD146+ perivascular cells, but not CD146- cells, can support propagation of human HSCs with long-term reconstituting potential.70 Moreover, overexpression of CD146 in HS5 cells imparts capability to the HS5 cells to facilitate engraftment of hCD34+ clonal MDS cells in murine xenografts.66 CD146+ cells support long-term persistence of hematopoietic cells through cell-to-cell contact, with at least some reliance on Notch activation.71 Impaired Notch signaling via both anti-Notch1 antibodies and by inhibition of gamma-secretase decreased the CD146-mediated hematopoietic stem cell support by CD146+ cells.72 These data collectively suggest that a subset of human stromal cells which are CD146+ is most likely responsible for support of human MDS engraftment in xenograft models.
Medyouf and colleagues recently demonstrated that engraftment of NSG mice with HSCs from patients with MDS can be significantly enhanced by co-transplantation via intramedullary injection of mesenchymal stromal cells from the same patient. They further found a unique gene expression profile in mesenchymal stromal cells from MDS patients as compared to healthy control mesenchymal stromal cells, including upregulation of LIF. Using an in vitro coculture system, they demonstrated that healthy mesenchymal stromal cells could be induced to upregulate expression of LIF upon incubation with whole bone marrow from MDS patients, suggesting that MDS cells are capable of inducing changes within the MME. Overall their data supports the concept that MDS cells alter mesenchymal stromal cells so that they promote clonal expansion and that mesenchymal stromal cells play an important role in disease pathogenesis.73
Therefore, much data using murine xenotransplantation models which employ human stromal cells are demonstrative of a dependence of MDS HSCs on mesenchymal stromal cells.
Therapeutic Targeting of the MME in Marrow Failure Syndromes
In murine models, activation of the normal HSC niche improves recovery from radiation6,20,74 and chemotherapeutic injury75, and suppresses CML disease progression, impairing leukemic stem cell maintenance in a syngeneic model.76 In addition, a murine model of GVHD demonstrated targeting of the MME, which could be modulated to improve hematopoiesis.77.This body of work provides a rationale to consider MME modulation as a strategy to treat marrow failure syndromes.
The notion that the underlying pathophysiology in AA results from a MME that is suppressive to HSCs, largely due to activated T lymphocytes, was confirmed through the success of trials of immunosuppressive therapy (with ATG alone or in combination with glucocorticoids, cyclosporine or cyclophosphamide).33 AA is the only marrow failure syndrome in which successful therapeutic targeting of the MME (marrow T lymphocytes) has been realized.
Since MDS can be cured by allogeneic HSC transplantation and stromal cells remain of patient origin after allotransplant, it has been postulated that alterations in MDS stroma must be secondary to interactions with clonal MDS cells and reversible upon their eradication.69 Therefore, identification and targeting of MDS-initiated signals which modulate the MME represent an appealing therapeutic target for the treatment of MDS. The relatively recent availability of transgenic murine models of MDS78,79 will allow for the definition of the response of the normal MME to the development and progression of MDS in an immunocompetent system. These models could also be helpful in elucidating the degree of support for hematopoiesis by the MDS MME and whether it has a role in propagation of the disease. If so, these model systems could be suitable as preclinical models of MME manipulation in MDS (Figure 2). Since as reviewed above in the normal marrow and in certain myeloablative conditions (as well as CML) strategies have already been advanced identifying components of the MME that can be stimulated, defects in the MME of MDS become appealing therapeutic targets for improvement of hematopoiesis.
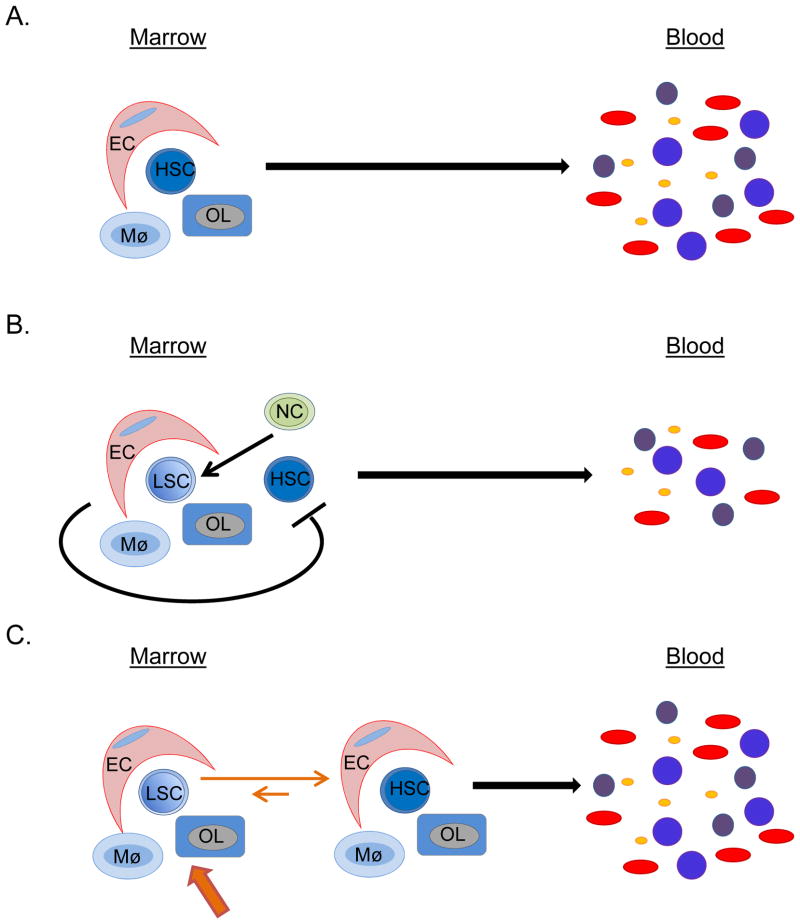
Figure 2. Conceptual models of MME-HSC interactions are illustrated.
Altered MME-HSC interactions in hematologic malignancies
A) Normally, the HSC is supported by various niche cells, including endothelial cells (EC), macrophages (Mø) and osteolineage cells (OL). B) In hematologic malignancies, clonal neoplastic cells (NC) alter the MME so that it becomes supportive of leukemic stem cells (LSC) and becomes less supportive of normal HSCs, ultimately leading to decreased normal hematopoiesis. C) There is a strong rationale for therapeutic targeting of the MME in hematologic malignancies, so as to push the MME into becoming less supportive of LSCs and more supportive of HSCs, in an effort to restore normal hematopoiesis.
Conclusion
The regulatory role of the MME in determining normal HSC fate and supporting hematopoiesis, while still being described, provides a rationale for studying the role of the MME in hematologic disorders. Disruption of the MME in inherited and acquired marrow failure syndromes has long been reported. AA, given that T-cells are considered a component of the MME, is the first marrow failure syndrome in which therapeutic targeting of the MME, in the form of immunosuppressive therapy, has met with success. More and larger studies are needed to further delineate whether an altered MME is involved in the pathogenesis and progression of inherited marrow failure syndromes. Recent progress has been made in our understanding of the role of the MME in disease pathogenesis and support of MDS. Further definition of the role of the MME could facilitate its therapeutic targeting in marrow failure syndromes, to exploit the biology of the niche by interfering with its disruption and by stimulating its supportive components as an additional tool for improvement of hematopoiesis and mitigation of its associated morbidities.
Learner objectives.
To understand the definition and current constituents of the hematopoietic stem cell niche in the bone marrow.
To review the role for the bone marrow microenvironment in marrow failure syndromes ontology and progression.
Acknowledgments
The authors would like to thank Drs. Michael Becker and Marshall Lichtman for helpful discussion. This work is supported by the National Institutes of Health (NIDDK: DK081843; NIAID: AI091036 and AI107276; NCI: CA166280; NIA: AG046293) and the Department of Defense (BM110106) to LMC. SRB is supported by the Wilmot Cancer Research Fellowship.
Contributor Information
Sophia R. Balderman, Instructor of Medicine, Division of Hematology/Oncology, Wilmot Cancer Center, University of Rochester School of Medicine.
Laura M. Calvi, Email: Laura_Calvi@URMC.Rochester.edu, Professor of Medicine, Pharmacology and Physiology, Neurologic Surgery, Wilmot Cancer Center, University of Rochester School of Medicine, 601 Elmwood Avenue Box 693, Rochester, NY 14642, (585) 275-5011.
Literature Cited
- 1.Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood. 2011;117(26):7121–7125. doi: 10.1182/blood-2011-02-337964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindquist KJ, Danese MD, Mikhael J, Knopf KB, Griffiths RI. Health care utilization and mortality among elderly patients with myelodysplastic syndromes. Ann Oncol. 2011;22(5):1181–1188. doi: 10.1093/annonc/mdq552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scadden DT. Nice Neighborhood: Emerging Concepts of the Stem Cell Niche. Cell. 2014;157(1):41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts [see comments] Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 5.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87(2):518–524. [PubMed] [Google Scholar]
- 6.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 8.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62. [PubMed] [Google Scholar]
- 15.Simmons PJ, Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78(11):2848–2853. [PubMed] [Google Scholar]
- 16.Ludin A, Itkin T, Gur-Cohen S, et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13(11):1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 17.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209(3):537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Adams J, Calvi LM, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012 doi: 10.1182/blood-2012-06-438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 23.Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120(2):303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollet O, Vagima Y, D'Uva G, et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia. 2013;27(10):2006–2015. doi: 10.1038/leu.2013.154. [DOI] [PubMed] [Google Scholar]
- 25.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulzele K, Krause DS, Panaroni C, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121(6):930–939. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YW, Koo BK, Jeong HW, et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. 2008;112(12):4628–4638. doi: 10.1182/blood-2008-03-148999. [DOI] [PubMed] [Google Scholar]
- 31.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2(5702):131–136. doi: 10.1136/bmj.2.5702.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinterberger W, Rowlings PA, Hinterberger-Fischer M, et al. Results of transplanting bone marrow from genetically identical twins into patients with aplastic anemia. Ann Intern Med. 1997;126(2):116–122. doi: 10.7326/0003-4819-126-2-199701150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Dolberg OJ, Levy Y. Idiopathic aplastic anemia: Diagnosis and classification. Autoimmun Rev. 2014;13(4–5):569–573. doi: 10.1016/j.autrev.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman R, Zanjani ED, Lutton JD, Zalusky R, Wasserman LR. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. 1977;296(1):10–13. doi: 10.1056/NEJM197701062960103. [DOI] [PubMed] [Google Scholar]
- 38.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004;364(9431):355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Yang S, Lu S, et al. Differential gene expression profile associated with the abnormality of bone marrow mesenchymal stem cells in aplastic anemia. PLoS One. 2012;7(11):e47764. doi: 10.1371/journal.pone.0047764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JY, Adams J, Calvi LM, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012;120(22):4352–4362. doi: 10.1182/blood-2012-06-438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li JY, Adams J, Calvi LM, Lane TF, Weitzmann MN, Pacifici R. Ovariectomy expands murine short-term hemopoietic stem cell function through T cell expressed CD40L and Wnt10B. Blood. 2013;122(14):2346–2357. doi: 10.1182/blood-2013-03-487801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dror Y, Freedman M. Shwachman-Diamond Syndrome: An Inherited Preleukemic Bone Marrow Failure Disorder With Aberrant Hematopoietic Progenitors and Faulty Marrow Microenvironment. Blood. 1999;94:3048–3054. [PubMed] [Google Scholar]
- 45.Andre V, Longoni D, Bresolin S, et al. Mesenchymal stem cells from Shwachman-Diamond syndrome patients display normal functions and do not contribute to hematological defects. Blood Cancer J. 2012;2:e94. doi: 10.1038/bcj.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Chen S, Yuan J, et al. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg−/− mice in vivo. Blood. 2009;113(10):2342–2351. doi: 10.1182/blood-2008-07-168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitagawa M, Yamaguchi S, Takahashi M, Tanizawa T, Hirokawa K, Kamiyama R. Localization of Fas and Fas ligand in bone marrow cells demonstrating myelodysplasia. Leukemia. 1998;12(4):486–492. doi: 10.1038/sj.leu.2400980. [DOI] [PubMed] [Google Scholar]
- 48.Tennant GB, Walsh V, Truran LN, Edwards P, Mills KI, Burnett AK. Abnormalities of adherent layers grown from bone marrow of patients with myelodysplasia. Br J Haematol. 2000;111(3):853–862. [PubMed] [Google Scholar]
- 49.Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013;98(11):1677–1685. doi: 10.3324/haematol.2013.083972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geyh S, Oz S, Cadeddu RP, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013;27(9):1841–1851. doi: 10.1038/leu.2013.193. [DOI] [PubMed] [Google Scholar]
- 51.Sloand EM, Mainwaring L, Fuhrer M, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106(3):841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epling-Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105(8):2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Epling-Burnette PK, List AF. Advancements in the molecular pathogenesis of myelodysplastic syndrome. Curr Opin Hematol. 2009;16(2):70–76. doi: 10.1097/MOH.0b013e3283257ac7. [DOI] [PubMed] [Google Scholar]
- 56.Campioni D, Secchiero P, Corallini F, et al. Evidence for a role of TNF-related apoptosis-inducing ligand (TRAIL) in the anemia of myelodysplastic syndromes. Am J Pathol. 2005;166(2):557–563. doi: 10.1016/S0002-9440(10)62277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawanobori M, Yamaguchi S, Hasegawa M, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res. 2003;27(7):583–591. doi: 10.1016/s0145-2126(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 58.Flores-Figueroa E, Gutierrez-Espindola G, Montesinos JJ, Arana-Trejo RM, Mayani H. In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndrome. Leuk Res. 2002;26(7):677–686. doi: 10.1016/s0145-2126(01)00193-x. [DOI] [PubMed] [Google Scholar]
- 59.Bellamy WT. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97(5):1427–1434. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- 60.Verstovsek S, Estey E, Manshouri T, et al. Clinical relevance of vascular endothelial growth factor receptors 1 and 2 in acute myeloid leukaemia and myelodysplastic syndrome. Br J Haematol. 2002;118(1):151–156. doi: 10.1046/j.1365-2141.2002.03551.x. [DOI] [PubMed] [Google Scholar]
- 61.Keith T, Araki Y, Ohyagi M, et al. Regulation of angiogenesis in the bone marrow of myelodysplastic syndromes transforming to overt leukaemia. Br J Haematol. 2007;137(3):206–215. doi: 10.1111/j.1365-2141.2007.06539.x. [DOI] [PubMed] [Google Scholar]
- 62.Iwata M, Pillai M, Ramakrishnan A, et al. Reduced expression of inducible gelatinase B/matrix metalloproteinase-9 in monocytes from patients with myelodysplastic syndrome: Correlation of inducible levels with the percentage of cytogenetically marked cells and with marrow cellularity. Blood. 2007;109(1):85–92. doi: 10.1182/blood-2006-05-020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerbauy DM, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood. 2004;104(7):2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 64.Muguruma Y, Matsushita H, Yahata T, et al. Establishment of a xenograft model of human myelodysplastic syndromes. Haematologica. 2011;96(4):543–551. doi: 10.3324/haematol.2010.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Milner LA, Deng Y, et al. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8(1):43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Marcondes AM, Ragoczy T, Telling A, Deeg HJ. Effect of intravenous coadministration of human stroma cell lines on engraftment of long-term repopulating clonal myelodysplastic syndrome cells in immunodeficient mice. Blood Cancer J. 2013;3:e113. doi: 10.1038/bcj.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330(2):150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 68.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Deeg HJ. Murine xenogeneic models of myelodysplastic syndrome: an essential role for stroma cells. Exp Hematol. 2014;42(1):4–10. doi: 10.1016/j.exphem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121(15):2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Levesque JP. A niche in a dish: pericytes support HSC. Blood. 2013;121(15):2816–2818. doi: 10.1182/blood-2013-02-485144. [DOI] [PubMed] [Google Scholar]
- 73.Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic Cells in Patients Reprogram Mesenchymal Stromal Cells to Establish a Transplantable Stem Cell Niche Disease Unit. Cell Stem Cell. 2014 doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Porter RL, Georger MA, Bromberg O, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31(2):372–383. doi: 10.1002/stem.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche (vol 25, pg 238, 2007) Nature Biotechnology. 2007;25(8):944–944. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 76.Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19(11):1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115(26):5401–5411. doi: 10.1182/blood-2009-11-253559. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Li Z, He Y, et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123(4):541–553. doi: 10.1182/blood-2013-05-500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106(1):287–295. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]