Abstract
Current standard of care for treatment of newly diagnosed high grade gliomas is surgery followed by concomitant radiotherapy (RT) and chemotherapy (CT) with temozolomide (TMZ). Recently, bevacizumab, an anti – angiogenic agent has also been approved for treatment of recurrent gliomas. Baseline imaging after excision is optimally obtained in the first 24 hours. When baseline postoperative imaging is delayed beyond 24 hours, subacute hemorrhage, subacute ischemia and inflammation at the resection margins render differentiation from residual tumor challenging. Radiation necrosis is a well recognized entity and is differentiated from recurrence based on morphology on structural imaging, presence of lipid – lactate complexes with lack of choline on spectroscopy and low normalized cerebral blood volume (CBV) ratios at perfusion imaging. Novel chemotherapies have lead to the occurrence of interesting but sometimes confusing post treatment imaging appearances including the phenomena of ‘pseudoprogression’ and ‘pseudoresponse’. Pseudoprogression refers to transient, self resolving focal enhancement mediated by TMZ-induced increased vascular permeability and local inflammatory response. Pathologically, these lesions do not have viable tumor. The lesions stabilize or regress without further treatment and are usually clinically asymptomatic. Pseudoresponse refers to rapid regression of enhancement, perfusion, mass effect and midline shift caused by the anti – angiogenic effect of bevacizumab. It is termed pseudoresponse since biological tumor persists as non-enhancing altered signal. It is important for radiologists to be aware of these entities seen on post treatment imaging of gliomas, as misinterpretation may lead to inappropriate management decisions and prognostication.
Keywords: Glioma, post treatment, pseudoprogression, pseudoresponse, radiation necrosis
Introduction
As novel therapies for high-grade gliomas (HGGs) emerge, “Macdonald criteria,”[1] representing the traditional guidelines for treatment response evaluation, are being challenged. Recently established standard of care for newly diagnosed HGG consists of surgery followed by concomitant radiotherapy (RT) and chemotherapy (CT) using temozolomide (TMZ).[2] Bevacizumab[3] is another chemotherapeutic agent with antiangiogenic properties that has been recently approved for treatment of recurrent gliomas. Changes in enhancement on follow-up imaging of gliomas treated with postoperative RT–TMZ and bevacizumab include the phenomena of pseudoprogression and pseudoresponse, respectively. Pseudoprogression is a transient, self-resolving increase in enhancement mediated by the chemotherapeutic agent, TMZ. Pseudoresponse is a regression in enhancement mediated by the antiangiogenic chemotherapeutic agent, bevacizumab. These changes in enhancement on post-treatment imaging reflect CT-related alteration in the stability of the blood–brain barrier (BBB) and not true tumor progression or regression, respectively.
The Macdonald criteria are based on measurement of enhancing lesion on magnetic resonance imaging (MRI), steroid dose, and clinical assessment to stratify treated gliomas into four categories: (1) Complete response: disappearance of enhancing lesion, neurologically stable/improved, and no steroids; (2) partial response: ≥50% reduction of enhancing lesion, neurologically stable/improved, and stable steroids; (3) progressive disease: ≥25% increase in enhancing lesion or new tumor, increased steroids, and neurologically worse; and (4) stable disease: all other situations.
A critical pitfall in these criteria is reliance on measurement of only the enhancing component of the tumor. In view of recently described non-tumoral changes in enhancement mediated by concomitant RT-CT, the Macdonald criteria are increasingly recognized as inaccurate[4] by the neuro-oncology and neuroradiology communities. Based on observation of this pitfall, updated guidelines for Response Assessment in Neuro-Oncology (RANO)[5] have been established. The aim of the RANO working group, a collective transnational, interdisciplinary committee, is to establish unanimous guidelines of response criteria for treated brain tumors.
Taking into consideration advances in chemoradiation and antiangiogenic therapy, this article reviews the role of conventional contrast MRI as well as explores the role of quantitative imaging biomarkers in the post-treatment imaging of HGGs.
Post-surgical Imaging
The first or baseline postoperative imaging is optimal within 24-48 hours of surgery. When baseline imaging is delayed beyond 72 hours, subacute hemorrhage/ischemia or reactive post-surgical enhancement may be confused with residual tumor. On baseline imaging, mild enhancement at the periphery of the resection cavity is likely postoperative granulation tissue.[6] However, larger areas of nodular and mass-like enhancement, similar in morphology to preoperative imaging, indicate residual tumor.[6]
When baseline imaging is obtained more than 72 h after surgery, postoperative blood with T1 shortening is difficult to separate from enhancing residual tumor; this concern may be addressed by comparison of pre-contrast and post-contrast T1 images in the same plane.
Diffusion imaging is the key to distinguish enhancing subacute ischemia from enhancing residual tumor follow-up imaging. Perioperative ischemia due to microvascular compromise is common at the resection margins. Acute ischemia displays restricted diffusion on early postoperative images and subacute ischemia displays enhancement on follow-up images; the latter is often misinterpreted as tumor. In these cases, comparison of areas of enhancement on follow-up imaging with diffusion images obtained at 24-48 h is recommended.[7,8] Enhancing subacute ischemia at follow-up shows restricted diffusion on review of 24-48 h scan; this is not seen in the case of enhancing tumor residue.
Fluid-attenuated inversion recovery (FLAIR) imaging of partially resected gliomas is sensitive to signal alteration of fluid in the postoperative cavity. Studies[9] have shown that increasing FLAIR signal in the resection cavity has high specificity for progression, even before the size of tumor increases.
Post-radiation Imaging
Side effects of RT are stratified into three types: acute (during RT), subacute/early-delayed (up to 3 months post-RT), and late (months to years post-RT). In the acute stage, MRI is usually normal, though sometimes diffuse parenchymal edema is observed. In subacute radiation injury, the MRI findings include non-enhancing white-matter signal alteration and occasionally focal new or increased enhancement. As opposed to acute–subacute post-radiation effects, late changes that occur months or even decades after radiation are usually progressive and irreversible. Late radiation effects in the brain include leukoencephalopathy, meningioma formation, radiation necrosis, and vascular changes such as telangiectasias, large vessel occlusion, Moya Moya syndrome, and lacunar infarcts. Stroke-like migraine attacks after radiation therapy (SMART) syndrome occurs 2-10 years following RT; patients present with headache and neurological deficit, similar to recurrence. The characteristic imaging in SMART syndrome consists of transient gyral thickening, restricted diffusion, and enhancement in the parieto-occipital parenchyma, reflecting a disturbance in the BBB of the more vulnerable posterior circulation.
Radiation necrosis appears as a ring-enhancing lesion with characteristic stellate margins and surrounding vasogenic edema. It usually occurs at the site of maximum RT dose in and around the tumor bed and within the margins of the irradiation field. The pattern of enhancement [Figures 1 and 2] has been described as “Swiss cheese” or “soap bubble” appearance.[10] Perfusion,[11,12] magnetic resonance spectroscopy (MRS),[13] diffusion, and diffusion tensor imaging are used to distinguish radiation necrosis from recurrence. Unlike tumor, the ring-enhancing lesions of radiation necrosis are hypoperfused with presence of lipid–lactate complexes and decrease in other metabolites including choline on MRS. Apparent diffusion co-efficient (ADC) and fractional anisotropy (FA) values in radiation necrosis are higher, as compared to tumor recurrence. Low ADC reflects higher cellularity of HGG. Table 1 demonstrates the role of advanced MRI techniques[14,15,16,17,18,19] in differentiating tumor from treatment necrosis. However, often, recurrent tumor and radiation necrosis co-exist [Figure 2A and B].
Figure 1 (A-I).
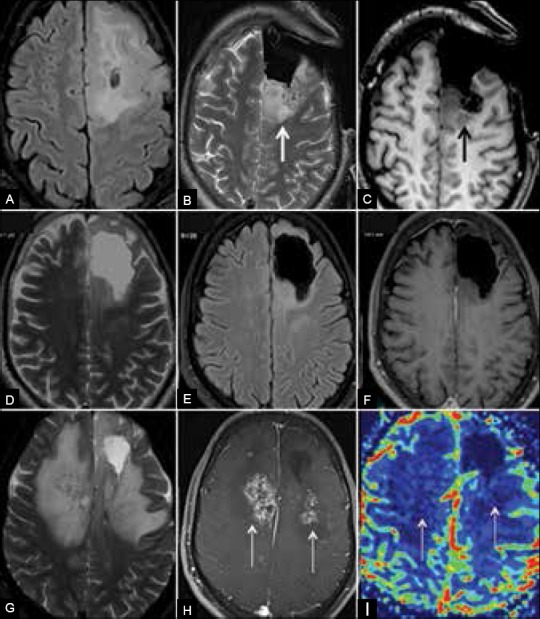
Radiation necrosis: (A) Preoperative MRI shows infiltrative glioma. (B and C) Intra-operative MRI shows residual lesion posterior to resection cavity. (D-F) Follow-up MRI 20 months after surgery and RT shows no significant residue. (G-I) Follow-up MRI 26 months after treatment shows new enhancing lesions with extensive edema in radiation fields. Hypoperfusion of the lesions validates diagnosis of radiation necrosis
Figure 2A (A-F).
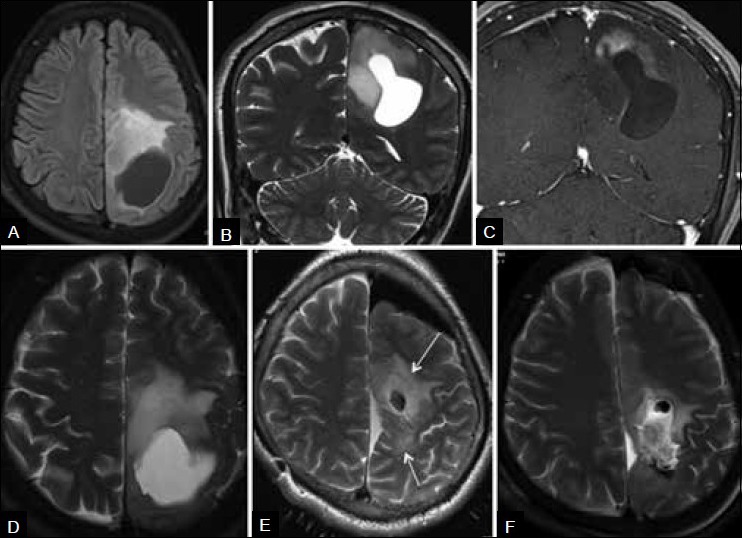
Mixed radiation necrosis and recurrence. (A-D) Preoperative MRI shows left cerebral glioma. (E) Intra-operative MRI shows residual infiltrative tumor (arrows) around the resection cavity, close to the eloquent paracentral lobule and precentral gyrus. Distortion of anatomy is due to intra-operative brain shift. Residue was further excised in the same surgery. (F) Postoperative MRI shows near-complete resection
Table 1.
Advanced MRI techniques for differentiation of tumor recurrence from treatment necrosis (modified from an analysis of literature by Alexiou et al.[14])
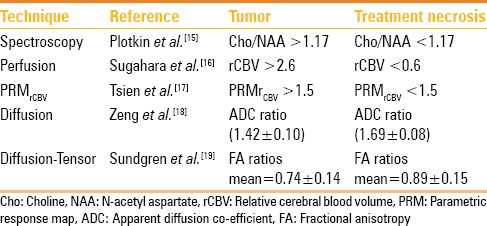
Figure 2B (A-I).
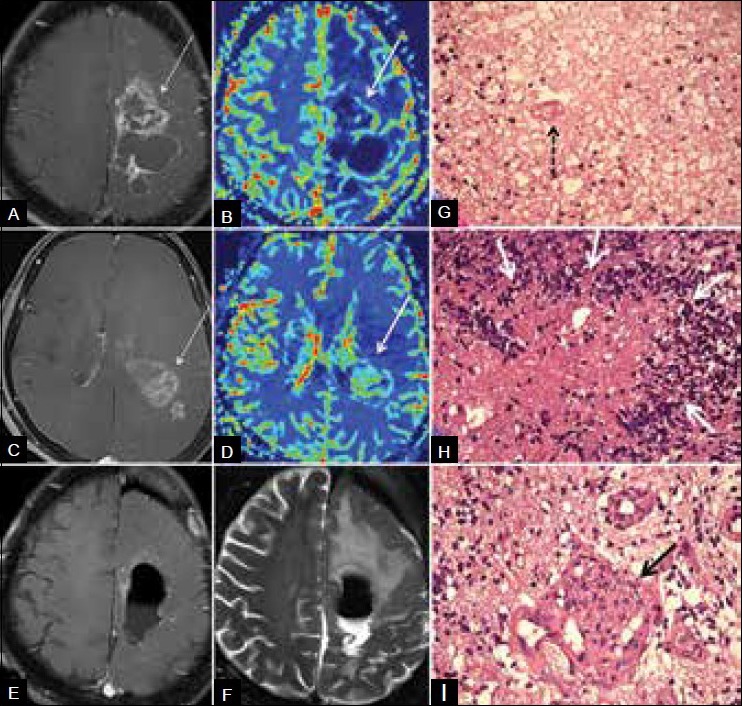
Mixed radiation necrosis and recurrence. Three years after surgery and RT–CT for grade 2 astrocytoma, patient presented with new multifocal mass. (A) Contrast (B) perfusion images show hypoperfused enhancing lesion (arrow) near the tumor bed, suggestive of radiation necrosis. (C and D) Caudal section in the same patient shows hyperperfused enhancing (arrows) tumor recurrence. (E and F) Postoperative MRI shows excision. (G) Histology from the tumor bed mass (H and E, ×40) shows RT necrosis comprising fibrinoid material with extravasated RBCs, inflammatory cells, and fibrinoid vasculopathy (arrow). No palisading of the necrosis is seen. (H) Histology from caudal lesion (H and E, ×40) shows recurrence with palisading of tumor necrosis by tumor cells forming wreath rosettes (arrows). (I) Histology from caudal lesion (H and E, ×40) shows recurrence with increased vascularity and endothelial proliferation giving glomeruloid appearance (arrow)
Pathophysiology of radiation toxicity in the brain includes vascular insult, white matter and glial damage, and activation of the fibrinolytic enzyme and immune systems.[11] However, it is vascular damage that is critical in radiation neurotoxicity. Radiation-mediated vascular insult includes disruption of the BBB. Histologic findings are characterized by endothelial injury with fibrinoid necrosis of small vessels and vessel wall hyalinization [Figure 2G] resulting in occlusive vasculopathy, thrombosis, and ischemia. At histology, areas of necrosis are frequently intermixed with tumor cells of doubtful viability. In contrast, the histological characteristic of tumor necrosis is palisading of necrosis by the tumor cells [Figure 2H] with microvascular proliferation [Figure 2I] and angiogenesis without vascular luminal obliteration.
Post-CT Imaging
Pseudoprogression refers to self-resolving, focal, new enhancement presenting most commonly in the first 3 months after RT–TMZ.[20,21] This imaging entity has been recognized after the 2005 randomized Phase 3 trial[2] that validated the addition of TMZ CT to RT in newly diagnosed malignant gliomas by demonstrating increase in mean survival from 12.1 to 14.6 months.
Abnormal enhancement in these patients is due to TMZ-induced cell hypoxia that leads to expression of hypoxia-regulated molecules from the tumor. These molecules mediate local inflammatory response including breakdown of the BBB and increased vascular permeability seen on imaging as new areas of enhancement. Pathologically, pseudoprogression corresponds to gliosis and reactive treatment-related changes with no evidence of viable tumor.[4,20,21,22] Radiation sensitizes the neuroparenchyma to the effects of TMZ.
Pseudoprogression is seen in about 20% of patients[22] on concomitant RT–TMZ and accounts for nearly half of the cases of new enhancement seen at the end of therapy. Cases with methylation of O6-methyl guanine-DNA methyl transferase (MGMT) gene have a higher incidence of pseudoprogression. Patients with pseudoprogression are often asymptomatic despite the presence of enhancing lesions. Interestingly, presence of pseudoprogression is correlated with better response to CT and even better survival. Recognition of this entity is important since misdiagnosis as true tumor progression may lead to inappropriate discontinuation of effective CT and unnecessary change to second-line therapy for recurrence.
When new enhancing lesions appear at 3-6 months after TMZ, differentiation between true progression and pseudoprogression is not possible on the initial MRI. The definite diagnosis of pseudoprogression is made with resolution of the lesion on follow-up imaging [Figure 3]. The only useful sign for true progression on initial MRI is subependymal enhancement; although the specificity of this finding for the diagnosis of true progression is high (93.3%), it has low sensitivity (38.1%) and modest negative predictive value (41.8%).[23] According to the modified RANO recommendations, true progression on initial MRI must be reported only if there is new enhancement outside the radiation field (beyond the high-dose region or 80% isodose line) or if tumor is seen on histopathological sampling.[5]
Figure 3 (A-C).
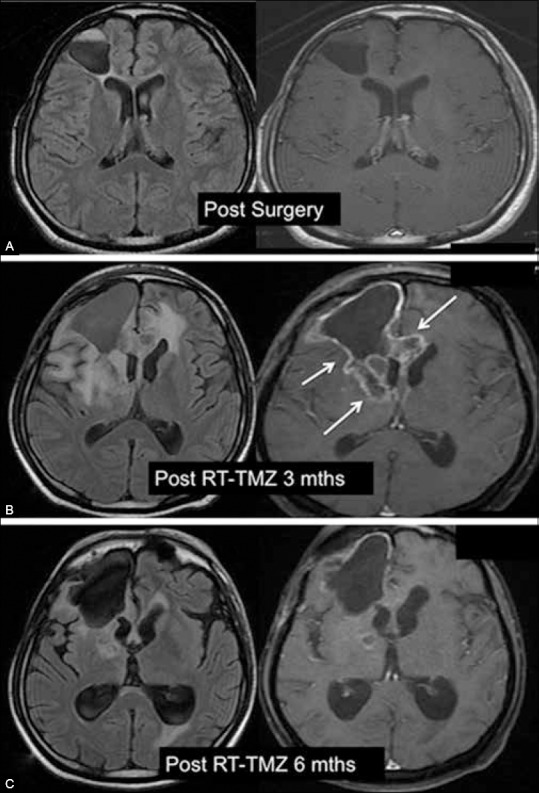
Pseudoprogression. (A) Postoperative MRI demonstrates resection cavity and complete excision of a malignant glioma. (B) 3 months post RT–TMZ MRI shows new enhancing lesions (arrows) at the margins of the resection cavity. (C) 6 months post RT–TMZ MRI shows spontaneous resolution of enhancing lesions
Recent studies have shown that perfusion, capillary permeability imaging, diffusion and diffusion tensor imaging and sodium imaging may have a future role in differentiation of progression from pseudoprogression on early scans. Some studies have shown that pseudoprogression has lower rCBV (relative cerebral blood volume) ratios,[24] as compared to true progression. However, subsequent studies have shown that change in rCBV ratios on serial imaging is more sensitive than normalized rCBV ratios on a single study. These studies use parametric analysis map (PRM), a voxel-wise analytical method applied to perfusion maps to quantify hemodynamic changes following treatment. PRMCBV is defined as the measure of the difference between serial CBV maps for each voxel in the target volume. These studies have shown that PRMCBV can be considered a potential biomarker to distinguish tumor recurrence from pseudoprogression.[17,25] Capillary permeability imaging[4] with MRI measures contrast leakage from intravascular to extravascular compartment. The rate of contrast leakage from the intravascular to the extravascular compartment, defined as contrast transfer co-efficient (Ktrans), is another biomarker whose role in post-treatment imaging is being investigated. Sodium (23Na) MRI shows promise in confirming true progression by demonstrating increased total tissue sodium concentration (TSC) as a result of depolarization of the cell membrane prior to cell proliferation in malignant glioma.[26] Finally, amino acid-positron emission tomography (AA-PET) using 18Fluoro-O-(2) fluoroethyl-l-tyrosine ([18F] FET) and [11C] methionine (MET) has shown to be superior to MRI in studies[27] to differentiate tumor from treatment necrosis.
In spite of the developments in physiologic and metabolic MRI techniques described above, at present, there is not enough evidence to incorporate the same into the RANO guidelines. Hence, follow-up imaging with conventional contrast MRI continues as the standard of practice.
The terms “pseudoprogression” and “radiation necrosis” overlap as both display tumor bed enhancement without true tumor. Pseudoprogression likely represents a self-limiting and milder variant.[28] Radiation necrosis is classically seen between 18 and 24 months after treatment. Pseudoprogression is seen much earlier than radiation necrosis, usually in the first 3 months after treatment.
Pseudoresponse refers to unique and sometimes confusing post-treatment imaging findings[29] in recurrent gliomas treated with the new antiangiogenic agent, bevacizumab (Avastin; Genentech). In 2009, on the basis of two Phase 2 trials,[3,30] bevacizumab monotherapy was approved for patients with recurrent glioblastoma.
Bevacizumab attaches to vascular endothelial growth factor (VEGF) and stabilizes the BBB resulting in decreased neovascularity. This is manifested on imaging as reduction of enhancement, perfusion, edema, and mass effect, soon after initiation of therapy [Figures 4 and 5]. This radiological response corresponds to reduced symptoms (decrease in mass effect), reduced steroid dependence (decrease in edema), and better quality of life (QOL). Although there is modest effect on overall survival, the 6-month progression-free survival increases.[31]
Figure 4 (A-C).
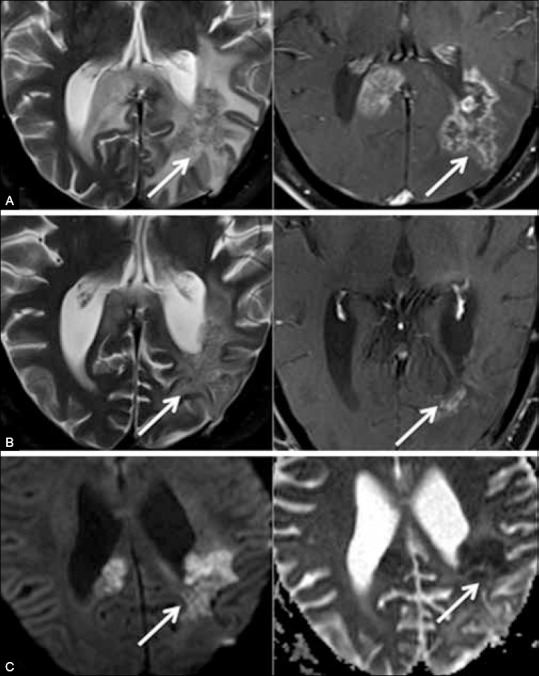
Pseudoresponse. Recurrent glioma in a 63-year-old man. (A) Follow-up T2 (left) and contrast-enhanced (right) images show enhancing recurrence (arrows) in the splenium. (B) Imaging after six cycles of bevacizumab continues to show abnormal T2 signal in the splenium (biological tumor), despite marked regression of surrounding edema and of enhancement (pseudoresponse). (C) DW (diffusion weighted) (left) and ADC (right) images show restricted diffusion with reduced ADC values representing persistent biological tumor. The paradox between progressive non-enhancing tumor and decreasing enhancement is a recognized effect of antiangiogenic agents and is referred to as pseudoresponse
Figure 5 (A and B).
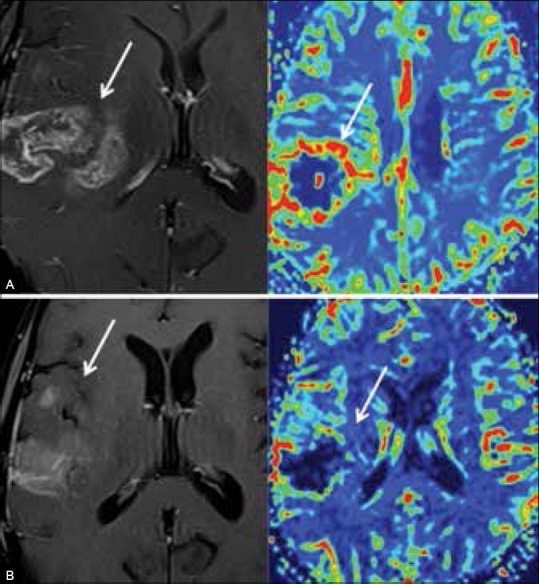
Pseudoresponse. Recurrent anaplastic astrocytoma treated with bevacizumab. (A) T1-enhanced image (left) and perfusion map (right) demonstrate enhancing, hyperperfused recurrence. (B) T1-enhanced image (left) and perfusion map (right) after four infusions of bevacizumab show rapid regression of enhancement and perfusion
Since biological tumor persists, this phenomenon is referred to as pseudoresponse. Biological tumor is seen as non-enhancing hyperintense signal on FLAIR images, often with restricted diffusion [Figure 4]. New areas of non-enhancing hyperintense FLAIR signal, remote from the primary lesion, representing multifocal tumor progression in patients on antiangiogenic medication may be due to vascular co-option[32] leading to development of another invasive non-enhancing phenotype.
Conclusion
HGG is the most common malignant tumor of the brain and remains a challenge for both the oncology and radiology communities. It portends a bleak prognosis related to innate cellular, genetic, and histological complexity. Most patients have recurrence within 12 months. Five-year survival is dismal.
The recent approval and subsequent clinical use of novel therapies for malignant gliomas has marginally improved survival, but has led to the recognition of unusual treatment-related phenomena on follow-up imaging. Radiation necrosis, pseudoprogression, and pseudoresponse have been described in this article. Interestingly, both pseudoresponse and pseudoprogression are associated with favorable patient outcome. Both are conclusively diagnosed on follow-up imaging as no radiological technique is currently capable of providing a categorical diagnosis of true tumor versus enhancement change due to treatment-mediated BBB alteration.
Perfusion, diffusion, and diffusion tensor imaging show promise, but at present are work-in-progress and require further validation, technical consistency in image acquisition and post processing, and further experience in interpretation. Meanwhile, it is important for the clinical radiologist to recognize these entities. In addition to influencing individual patient care, appropriate interpretation of these imaging changes also has an effect on clinical trials of newer therapies.
Acknowledgements
The author thanks Dr. Meenal Hastak for pathology images and Mr. Bobby Matthew for MR images.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Prados MD, Wen PY, Mikkelsen T, Abrey LE, Schiff D, et al. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) J Clin Otol. 2008;26(Suppl 15):2010b. [Google Scholar]
- 4.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–85. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 6.Oser AB, Moran CJ, Kaufman BA, Park TS. Intracranial tumor in children: MR imaging findings within 24 hours of craniotomy. Radiology. 1997;205:807–12. doi: 10.1148/radiology.205.3.9393539. [DOI] [PubMed] [Google Scholar]
- 7.Wang LL, Leach JL, Breneman JC, McPherson CM, Gaskill-Shipley MF. Critical role of imaging in the neurosurgical and radiotherapeutic management of brain tumors. Radiographics. 2014;34:702–21. doi: 10.1148/rg.343130156. [DOI] [PubMed] [Google Scholar]
- 8.Farace P, Amelio D, Ricciardi GK, Zoccatelli G, Magon S, Pizzini F, et al. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J Neurooncol. 2013;111:177–85. doi: 10.1007/s11060-012-0997-y. [DOI] [PubMed] [Google Scholar]
- 9.Winterstein M, Münter MW, Burkholder I, Essig M, Kauczor HU, Weber MA. Partially resected gliomas: Diagnostic performance of fluid-attenuated inversion recovery MR imaging for detection of progression. Radiology. 2010;254:907–16. doi: 10.1148/radiol09090893. [DOI] [PubMed] [Google Scholar]
- 10.Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–84. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 11.Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatment-related change versus tumor recurrence in high-grade gliomas: A diagnostic conundrum-use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol. 2012;198:19–26. doi: 10.2214/AJR.11.7417. [DOI] [PubMed] [Google Scholar]
- 12.Barajas RF, Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253:486–96. doi: 10.1148/radiol.2532090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock JP, Hearshen D, Scarpace L, Croteau D, Gutierrez J, Fisher JL, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51:912–20. doi: 10.1097/00006123-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD. Glioma recurrence versus radiation necrosis: Accuracy of current imaging modalities. J Neurooncol. 2009;95:1–11. doi: 10.1007/s11060-009-9897-1. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin M, Eisenacher J, Bruhn H, Wurm R, Michel R, Stockhammer F, et al. 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: A comparative study. J Neurooncol. 2004;70:49–58. doi: 10.1023/b:neon.0000040810.77270.68. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: The value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21:901–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsien C, Galbán CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-gradeglioma. J Clin Oncol. 2010;28:2293–9. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng QS, Li CF, Liu H, Zhen JH, Feng DC. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68:151–8. doi: 10.1016/j.ijrobp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24:1131–42. doi: 10.1016/j.mri.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–3. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 21.Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: Pitfalls in neurooncology. Neuro Oncol. 2008;10:361–7. doi: 10.1215/15228517-2008-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–10. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 23.Young RJ, Gupta A, Shah AD, Graber JJ, Zhang Z, Shi W, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76:1918–24. doi: 10.1212/WNL.0b013e31821d74e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangla R, Singh G, Ziegelitz D, Milano MT, Korones DN, Zhong J, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256:575–84. doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 25.Cha J, Kim ST, Kim HJ, Kim BJ, Kim YK, Lee JY, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35:1309–17. doi: 10.3174/ajnr.A3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laymon CM, Oborski MJ, Lee VK, Davis DK, Wiener EC, Lieberman FS, et al. Combined imaging biomarkers for therapy evaluation in glioblastoma multiforme: Correlating sodium MRI and F-18 FLT PET on a voxel-wise basis. Magn Reson Imaging. 2012;30:1268–78. doi: 10.1016/j.mri.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Götz I, Grosu AL. [(18) F] FET-PET imaging for treatment and response monitoring of radiation therapy in malignant glioma patients-A review. Front Oncol. 2013;3:104. doi: 10.3389/fonc.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–8. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 29.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9:241–6. doi: 10.1007/s11910-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 30.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 32.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


