Abstract
Endometrial carcinoma (EC) is the most common gynecologic malignancy in the United States. Prognosis depends on patient age, histological grade, depth of myometrial invasion and/or cervical invasion, and the presence of lymph node metastases. Although EC is staged surgically according to the International Federation of Gynecology and Obstetrics (FIGO) system, preoperative imaging can assist in optimal treatment planning. Several imaging techniques such as transvaginal ultrasonography (TVUS), computed tomography (CT), and magnetic resonance imaging (MRI) have been used as diagnostic tools for preoperative staging of EC. Recently, positron emission tomography (PET), PET/CT, and PET/MRI have also been used in staging these patients. In this article, we review the value of imaging in diagnosis, staging, treatment planning, and detection of recurrent disease in patients with EC.
Keywords: Endometrial cancer, magnetic resonance imaging, positron emission tomography
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy and the fourth most common cancer in women in the United States.[1] However, in developing countries, it is the second most common gynecologic malignancy with an incidence of 5.9 per 100,000 women. In India, the incidence is 4.3 per 100,000 women.[2] Around 52,630 new cases of cancer involving the uterine corpus, mostly endometrial, would be diagnosed and approximately 8590 deaths from this disease are estimated to occur in the United States in 2014.[1] Patients present with abnormal uterine bleeding (intermenstrual or postmenopausal) in more than 80% of cases. EC is more common during the 6th and 7th decades of life, with the mean age of patients being 65 years.[3]
Obesity, unopposed estrogen intake, nulliparity, diabetes mellitus, Stein–Leventhal syndrome, Lynch syndrome, and tamoxifen therapy are the known risk factors for the development of EC.[3] However, the etiology of EC is not completely clear. Definitive diagnosis of EC is generally made via endometrial biopsy or dilatation and curettage.
Histologically, ECs are divided into two subtypes. The most common is the endometrioid adenocarcinoma (type I) that accounts for 90% of the tumors. Type I ECs are associated with estrogen excess and obesity. These tumors often arise in a background of endometrial hyperplasia, occur in the early postmenopausal period, generally are low grade, and have a good prognosis. Based on the degree of differentiation, endometrioid adenocarcinomas are subdivided into three grades: Grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated tumors.[4]
Type II ECs include the clear-cell, serous papillary subtypes and carcinosarcomas. Type II cancers have no association with estrogen excess or atypical hyperplasia, generally occur in older women, carry a worse prognosis, and spread like ovarian cancer. All type II cancers and grade 3 endometrioid tumors are classified as high-grade tumors and are associated with a poor prognosis.[5] Recent studies suggest mutations may be present in individual genes such as p53, HER2/neu, and phosphatase and tensin homolog (PTEN) in patients with high-grade endometrial tumors. Mutant tumor suppressor p53 overexpression has been associated with poor histological grade, non-endometrioid histology, advanced stage, and poor survival rates.[6,7] Positive biomarker, p21, and microsatellite instability have a better prognosis[8] and PTEN mutations are typically associated with more favorable prognosis.[9] HER2/neu proto-oncogene overexpression has been associated with poor outcomes in breast, ovary, and endometrial cancer.[10]
Using histological type and local extension of the disease, EC can be classified as high-risk EC (high grade or stage ≥IB) and low-risk EC (low grade and stage IA). Parameters that impact prognosis and survival are: the stage of disease at diagnosis, histological grade, depth of myometrial invasion, lymphovascular invasion, and lymph node status.[11] Grade 3 histological type and the presence of greater than 50% depth of myometrial invasion are associated with poor survival and a high prevalence of pelvic and para-aortic lymph node metastases.[11] Owing to early symptoms, approximately 75% of women with EC are diagnosed with stage I disease. The mean 5-year survival rate for stage I is 85%, for stage II is 70%, for stage III is 50%, and for stage IV is 18%.[12]
EC spreads by direct infiltration or via lymphatic, transtubal peritoneal seeding or hematogenous routes. Locally, EC initially invades the myometrium and then the endocervix. After transserosal spread, direct invasion of the parametrium, bladder, or bowel may occur.[12]
The location of lymph nodes metastases reflects the portion of the uterus involved by the cancer. The parametrial, paracervical, and the obturator lymph nodes are involved when the cancer affects the middle and lower third of the uterus. The common iliac and obturator lymph nodes are involved when the tumor is located in the upper corpus or fundus of the uterus.[12]
Staging
Staging of EC is based on surgicopathologic International Federation of Gynecologic Oncology (FIGO) criteria.[13,14] The surgicopathologic staging system uses findings from exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, peritoneal lavage, and pelvic and para-aortic lymphadenectomy. Surgical staging is not performed in patients who are at increased risk for mortality or severe morbidity secondary to comorbidities. Para-aortic lymphadenectomy is usually performed in patients who have deep myometrial invasion or high histological tumor grade.
The FIGO revised the 1988 staging of gynecologic malignancies in 2009[13,14] [Table 1]. Stage I reflects ECs that are confined to the uterine corpus. It is further divided into stages IA and IB. Stage IA reflects tumors that are confined to the inner endometrium and invade less than 50% of the myometrial thickness. Stage IB represents tumors with more than 50% of myometrial thickness invasion. The difference in prognosis which is dependent on the depth of myometrial invasion makes its important to distinguish between stages IA and IB.
Table 1.
International federation of gynecology and obstetrics staging system for endometrial cancer, 2009[12]
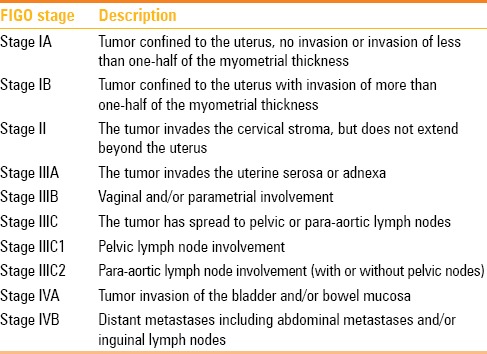
In relation to extension of tumors to the cervix, currently, tumors with endocervical glandular invasion only are considered stage I tumors and tumors with cervical stromal invasion are defined as stage II tumors.
Stage III represents tumor with local or regional spread beyond the uterus, but not outside the true pelvis. It is further divided into stage IIIA which includes tumors that invade the uterine serosa and/or adnexa, stage IIIB which includes tumors that extend into the parametrium and/or with vaginal involvement, and stage IIIC which includes tumors with spread to pelvic or para-aortic lymph nodes. Stage IIIC is further divided into stage IIIC1 when the tumor presents with pelvic lymph node involvement and stage IIIC2 when there is para-aortic lymph node involvement (with or without pelvic nodes).
Stage IV represents tumors that are locally advanced or have distant metastases. It is further divided into stage IVA that includes tumors with extension to the bladder or bowel mucosa and stage IVB consisting of tumors that have distant metastases.
Though surgical staging is accepted worldwide, cross-sectional imaging is frequently used to aid in pre-surgical evaluation and to help determine the type of therapy which is necessary. Potential advantages of preoperative imaging include: assessment of the depth of myometrial invasion, which in turn may predict the likelihood of lymph node involvement; determination of gross cervical invasion not detected by evaluation under anesthesia or by physical exam, which requires preoperative radiation therapy; and the identification of suspicious lymph nodes suggestive of metastatic disease, so that they can be sampled and patients may undergo neoadjuvant chemotherapy.
Imaging techniques
Ultrasound
TVUS is often used for the initial evaluation in women with history of postmenopausal bleeding because it is quick, inexpensive, and does not expose the patient to ionizing radiation. ECs typically present as thickening of the endometrium and TVUS diagnosis of endometrial cancer is based on endometrial thickness that is measured in the anteroposterior dimension [Figure 1]. The sensitivity and specificity of TVUS for detecting EC approach 96% and 61%, respectively, when an endometrial thickness threshold of 5 mm, in postmenopausal women, is used to define abnormal endometrial thickening.[15] A meta-analysis suggested a sensitivity of 68-100% and a specificity of 71-90% for subjective assessment of deep myometrial invasion.[16] Furthermore, the negative predictive value of a thin endometrium is very high. Also, cancer is more likely when the endometrium has a heterogeneous echotexture and irregular or poorly defined margins.
Figure 1 (A-D).
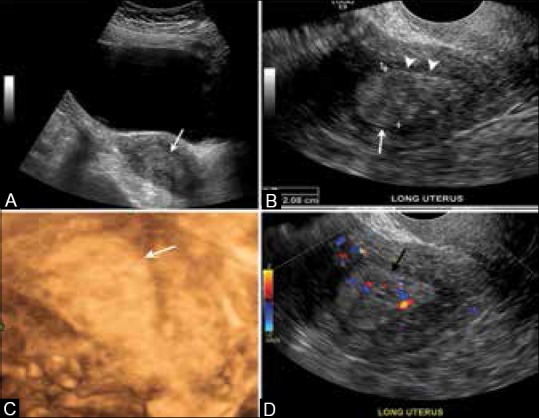
A 67-year-old female with endometrial cancer. (A) Longitudinal transabdominal scan. (B) Transvaginal scan and a 3-D reconstructed ultrasonography image (C) through the uterus demonstrate a thickened and heterogeneous endometrium measuring 2.0 cm (arrows). Note regular endometrial–myometrial border with no signs of invasion (arrowheads). (D) Note increased vascularity in the color Doppler US (black arrow)
It can be difficult to delineate the tumor margins on ultrasound, especially when it is diffusely infiltrating the myometrium [Figure 2]. The reported sensitivity and specificity for TVUS in determining the depth of myometrial invasion are 69% and 70%, respectively.[12] Myometrial invasion is suggested when there is irregularity of the endometrium - myometrium border and disruption of the subendometrial halo (inner layer of myometrium) or the tumor extends asymmetrically into the myometrium.[12] The use of saline infusion (i.e., sonohysterography) increases the accuracy of TVUS to 84-89% in evaluating deep myometrial invasion.[17] However, its use is controversial with several reports indicating that the procedure may disseminate malignant cells into the peritoneal cavity.[18]
Figure 2.
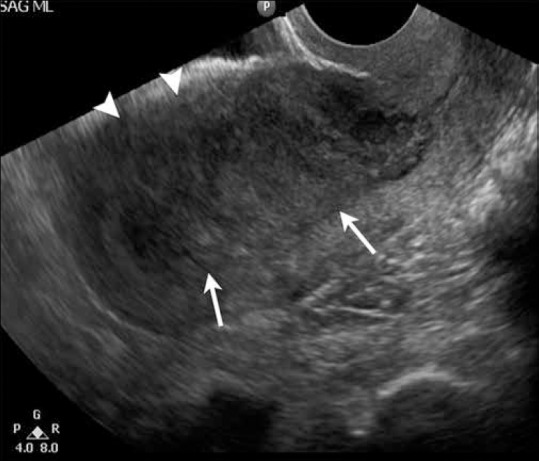
A 72-year-old female with endometrial cancer. Longitudinal transvaginal scan through the uterus demonstrates markedly thickened and heterogeneous endometrium (arrows) with ill-defined anterior border and no clear separation from the myometrium (arrowheads), suggestive of myometrial invasion
Limitations of TVUS include its operator dependence and limited field of view. TVUS may overestimate myometrial invasion in the presence of large tumors, adenomyosis, and lymphovascular space invasion. In addition, there is insufficient data about the value of TVUS in predicting cervical extension, parametrial invasion, or lymphadenopathy.[19]
Although color Doppler ultrasound often reveals increased vascularity with a multivessel pattern and spectral Doppler indices may have low impedance flow, it has a limited role in evaluating patients with EC since there is no significant difference in uterine blood flow between benign and malignant endometrial processes.[20]
Computed tomography
On contrast-enhanced CT, EC appears as a hypoattenuating and hypoenhancing mass in the endometrial cavity [Figure 3]. However, this appearance is nonspecific and the differential diagnosis of a hypoenhancing endometrial mass on CT includes submucosal leiomyomas, endometrial polyps, or cervical stenosis.
Figure 3 (A-C).
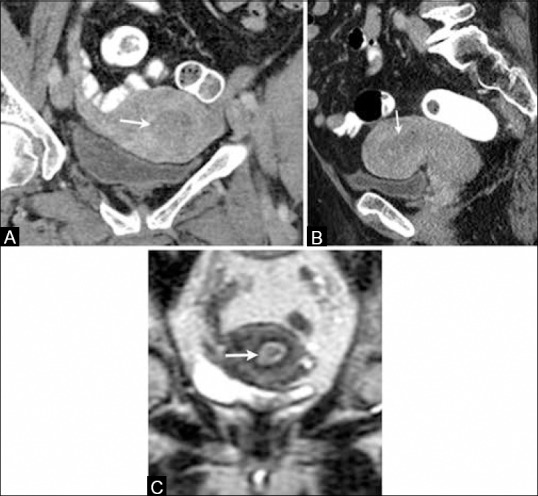
A 66-year-old female with endometrial cancer. (A) Coronal and (B) sagittal reformatted contrast-enhanced computed tomography images of the pelvis show thick hypodense and hypoenhancing endometrium (arrows). (C) Coronal T2W MR image showing a thick and heterogeneous endometrium (arrow) in this patient with biopsy-proven diagnosis of endometrial cancer
CT's poor soft tissue differentiation limits its use in the local staging of EC. CT is less sensitive and less specific in accurately visualizing myometrial invasion and cervical involvement than MRI. The sensitivity and specificity of CT in evaluating myometrial invasion range from 40% to 83% and from 42% to 75%, respectively.[21] A more recent study in preoperative evaluation of myometrial invasion and cervical extension of EC using multidetector CT showed improved diagnostic accuracies of 95% and 81%, respectively.[22] Currently, CT is used mainly in the assessment of advanced disease, i.e. for staging patients with EC by detecting nodal and distant metastases [Figure 4].
Figure 4 (A and B).
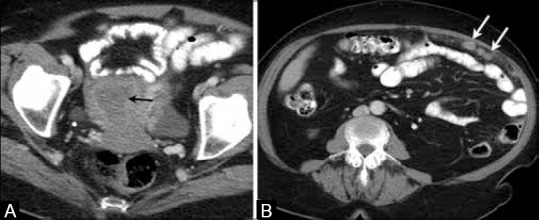
A 75-year-old female with endometrial cancer. (A) Axial contrast-enhanced computed tomography image of the pelvis shows the thick hypodense endometrium (black arrow). (B) Axial contrast-enhanced CT image shows peritoneal implants (white arrows) in this patient with clear cell endometrial carcinoma
Magnetic resonance imaging
MRI is considered the most accurate imaging modality for the pretreatment local staging of EC secondary to its excellent soft tissue delineation. On MRI, EC is usually seen as a hypo-to-isointense mass on T1-weighted images (T1WI) with an intermediate signal intensity lower than the normal endometrium on T2-weighted images (T2WI). On dynamic post-contrast images, EC enhances less than the myometrium[23] [Figure 5]. The overall staging accuracy of MR imaging is reported to be 83-92%.[24,25,26]
Figure 5 (A-E).
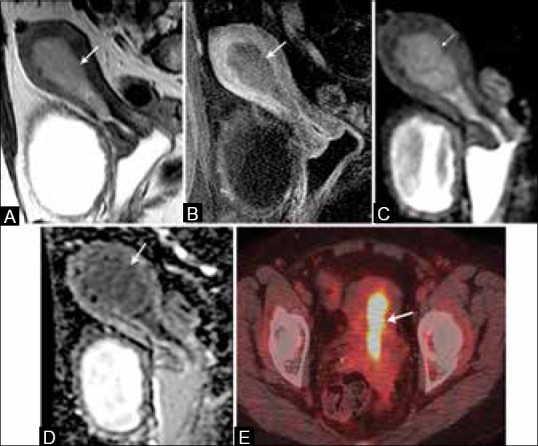
A 64-year-old female with endometrial cancer. (A) Sagittal T2W image show a hyperintense signal intensity tumor distending the endometrial cavity (arrow). (B) On T1W post-contrast image, the tumor (arrow) is low in signal compared to the enhancing adjacent myometrium. It presents restricted diffusion with high signal on DW images (C) and low signal on ADC map (D) (arrows). (E) It presents with high FDG uptake on FDG-PET/CT (arrow)
Depth of myometrial invasion is one of the most important prognostic factors[27] [Figures 6–11]. The depth of myometrial invasion is optimally depicted with T2-weighted sequences. However, there are some limitations such as thinning of the myometrium in postmenopausal women, tumor extension into the cornua, myometrial compression from a polypoid tumor, and presence of leiomyomas or adenomyosis.[28,29]
Figure 6 (A and B).
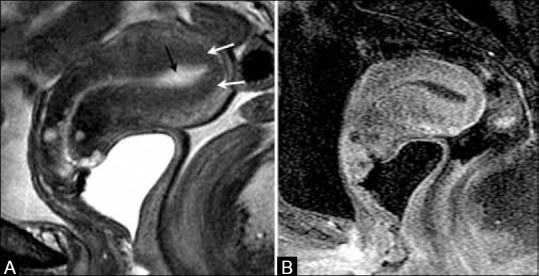
A 70-year-old female with endometrial cancer. (A) Sagittal T2W image showing high signal intensity fluid in endometrial cavity (black arrow) with intact low signal intensity junctional zone (white arrows). (B) T1W post-contrast image shows no evidence of myometrial invasion or cervical involvement indicating stage IA disease
Figure 11.
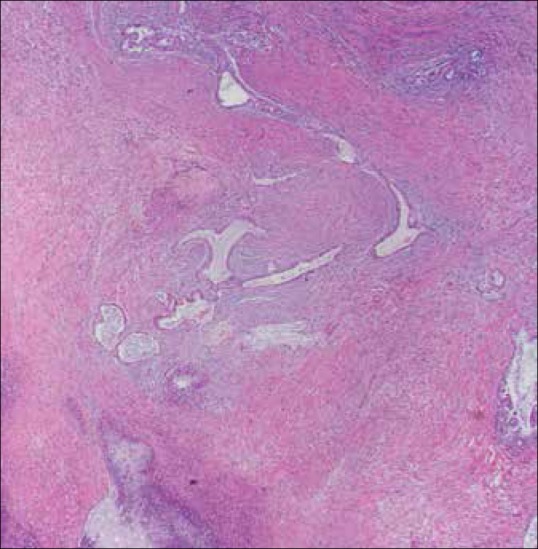
Endometrial adenocarcinoma (hematoxylin and eosin staining). Multiple foci of glandular-forming tumors intercalated within the smooth muscle bundles of the myometrium representing myometrial invasion
Figure 7 (A and B).
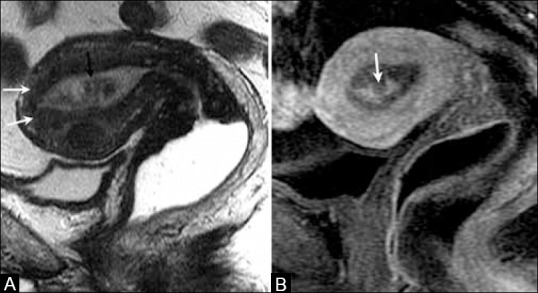
A 68-year-old female with endometrial cancer. (A) Sagittal T2W image showing a hypointense mass (black arrow) distending the endometrial cavity with integrity of the junctional zone (white arrows). (B) T1W post-contrast image shows heterogeneous enhancement of the endometrial cavity (arrow) with no evidence of myometrial invasion or cervical involvement indicating stage IA disease
Figure 8.
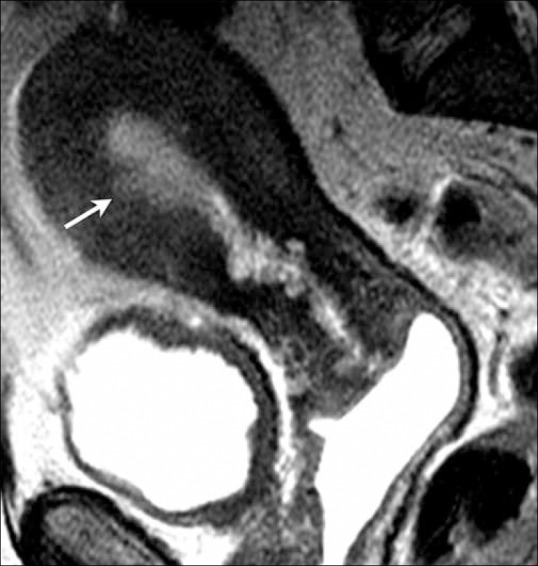
A 61-year-old female with endometrial cancer. Sagittal T2W image showing a focal area of endometrial thickening at the anterior wall (arrow) with irregular endometrium–myometrium interface with disruption of the junctional zone, but less than 50% invasion of the myometrium indicating stage IA disease
Figure 9 (A-C).
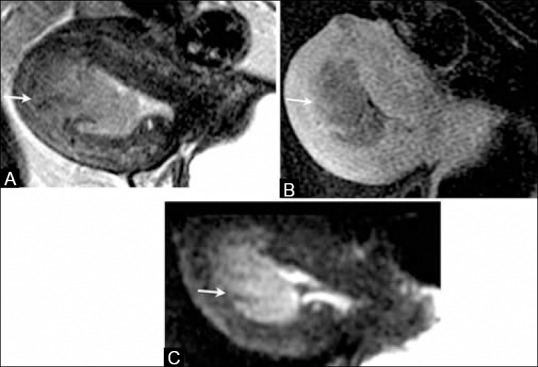
A 68-year-old female with endometrial cancer. (A) Sagittal T2W MR image, (B) T1W post-contrast image, and (C) DW image show endometrial tumor with more than 50% of myometrial invasion in the anterior wall (arrows) indicating stage IB disease
Figure 10 (A and B).
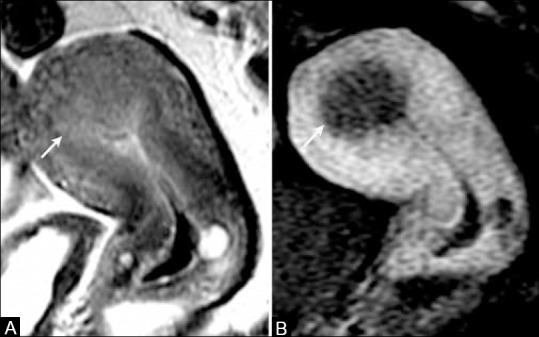
A 74-year-old female with endometrial cancer. (A) Sagittal T2W MR image shows a high signal intensity mass in the endometrial cavity (arrow). (B) T1W post-contrast MR image show a mass in the uterine fundus with greater than 50% of myometrial invasion in anterior wall (arrows) indicating stage IB disease
Dynamic contrast-enhanced MR imaging improves the accuracy of the assessment of the depth of myometrial invasion. EC enhances less than normal myometrium after administration of intravenous gadolinium.[30] Dynamic contrast-enhanced MRI has a pooled sensitivity and specificity of 81% and 72%, respectively,[31] and T2-weighted imaging has a pooled sensitivity and specificity of 87% and 58%, respectively, in the assessment of myometrial invasion.[31] Dynamic contrast-enhanced images and T2WI together have an accuracy of 98% for assessing myometrial invasion.[32] The negative predictive value of dynamic contrast-enhanced MRI is better than the positive predictive value for myometrial invasion and can help guide in optimal treatment planning.[31]
The normal cervical stroma has low signal intensity on T2WI, in contrast to the high-intermediate signal intensity from tumor infiltration.[33]
The sensitivity, specificity, and diagnostic accuracy of MRI imaging assessment of cervical invasion have been reported to be 100%, 87%, and 90%, respectively, on T2WI; 100%, 95%, and 96%, respectively, on post-contrast T1WI; and 100%, 100%, and 100%, respectively,[34] on dynamic MRI [Figure 12]. In one study that correlated imaging findings with fractional curettage and hysteroscopy results, MRI had an accuracy of 86% and CT had an accuracy of 83% for diagnosing cervical involvement in EC.[35]
Figure 12 (A-C).
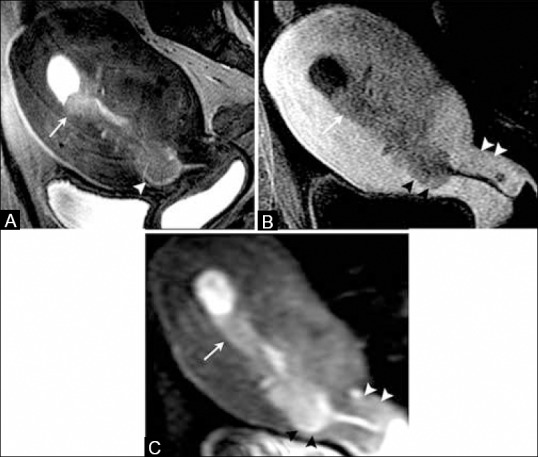
A 65-year-old female with endometrial cancer. (A) Sagittal T2W MR image, (B) T1W post-contrast image, (C) DW image, and (D) ADC map image show a large and irregular endometrial mass (white arrows) which disrupts the cervical stroma (black arrowheads), but does not extend beyond the uterus indicating stage II disease. Note normal posterior cervical lip (white arrowheads)
MRI's superior soft tissue resolution makes it better than other cross-sectional imaging modalities in assessing adnexal metastases [Figure 13], vaginal involvement [Figure 14], and invasion into the bladder and rectum [Figure 15].
Figure 13.
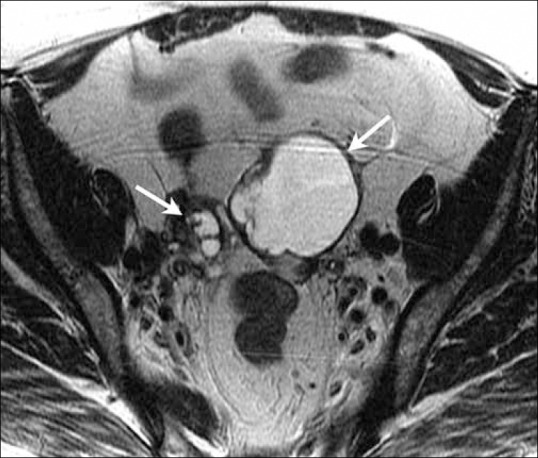
A 60-year-old female with endometrial cancer. Axial T2W MR image demonstrates bilateral large ovarian masses (arrows) suggestive of ovarian involvement indicating stage IIIA disease
Figure 14 (A-C).
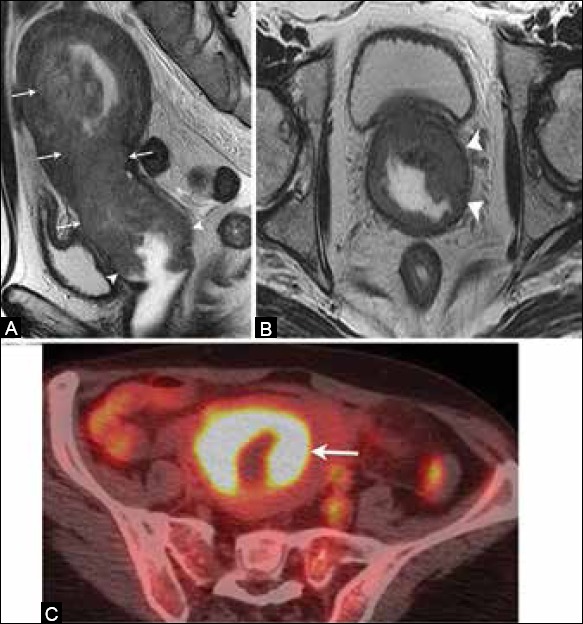
A 62-year-old female with endometrial cancer. (A) Sagittal and (B) axial T2W MR images show an endometrial mass (arrow) extending inferiorly and involving the vagina (arrowheads) indicating stage IIIB disease. (C) FDG-PET/CT demonstrates increased FDG uptake by the tumor (arrow)
Figure 15.
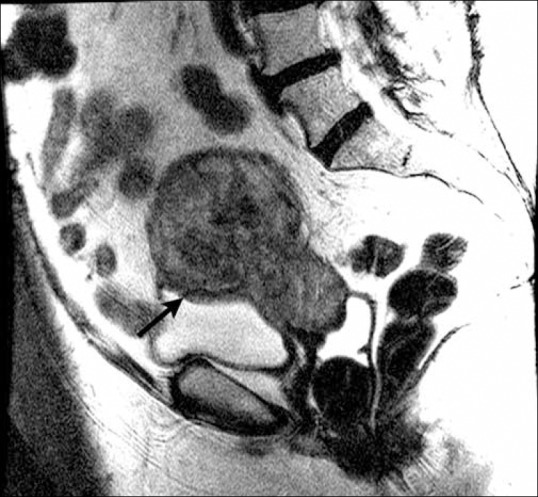
A 71-year-old female with endometrial cancer. Sagittal T2W MR image shows focal loss of low signal intensity wall of the bladder (arrow) suggestive of bladder involvement indicating stage IVA disease
Patient preparation is necessary to obtain diagnostic images. Fasting for about 4-6 h is usually recommended to reduce the artifact from bowel motion. Emptying the urinary bladder prior to the scan will also help delineate the plane between the uterus and bladder. Glucagon, an antiperistaltic agent, may be used as it reduces the bowel motion which may produce artifacts that obscure the endometrium and cervix. When administered intravenously, glucagon lasts for about 10 min; however, if administered intramuscularly, it can last for approximately 30 min.
A multichannel pelvic phased array coil is the preferred option for pelvic imaging. However, if unavailable, a multi-channel coil may be used. A body coil is the best option in extremely obese patients. The coil should be appropriately positioned to cover the pelvic floor and the lower para-aortic nodal chains and to prevent signal loss.
The imaging protocol should include small field of view images (28 mm), sagittal T2, axial T2, sagittal 3D dynamic fat-suppressed, pre- and post-contrast axial T1 sequences with fat suppression, along with diffusion and apparent diffusion coefficient (ADC) maps. One coronal T2 sequence is needed to assess retroperitoneal adenopathy and hydronephrosis.
The 2009 FIGO revision includes lymph nodes in staging.[36] Recent literature suggests that routine lymphadenectomy does not provide any survival benefit in early-stage EC.[37] Lymphadenectomy is associated with increasing morbidity, especially in obese patients, and should be performed only in patients who harbor metastasis in their lymph nodes.[38]
Thus, there is a need for an optimal imaging modality to assess for lymph node metastases, so that only high-risk patients undergo surgical resection. Most cross-sectional modalities, including MRI, use a short-axis diameter of 10 mm or greater to identify suspicious lymph nodes [Figure 16]. However, there is significant overlap between the sizes of metastatic and reactive lymph nodes. In one study, using this size criterion, MRI had a sensitivity of 44% and specificity of 98% in the detection of lymph node metastases.[39] More recently, various functional imaging modalities have been used to assess lymph node involvement in malignant disease. These modalities include MRI with ultrasmall superparamagnetic iron oxide (USPIO), diffusion-weighted (DW) imaging, and PET/CT.[40]
Figure 16.
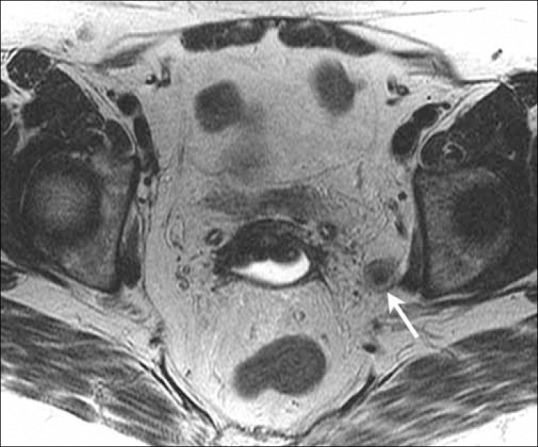
A 66-year-old female with endometrial cancer. Axial T2W MR images show an enlarged left pelvic lymph node (arrow) suggestive of regional pelvic lymph node involvement indicating stage IIIC1 disease
Advances in MRI have allowed the use of DW images in evaluation of uterine tumors. EC demonstrates high signal intensity on DW images and low signal intensity on ADC maps[41] [Figure 5].
DW imaging has an added value in EC as it helps in better depiction of the primary tumor, increasing the detection of local extension in the cervix and skipping metastases to the vagina, and in diagnosing extrauterine spread to the peritoneum and lymph nodes.[41]
Recent literature demonstrated that DW imaging has superior diagnostic accuracy in the assessment of myometrial invasion and significant higher staging accuracy compared to dynamic contrast enhanced (DCE) MR imaging. In a prospective study, Rechini et al. found that DWI was very accurate in assessing myometrial invasion, and perhaps could replace dynamic imaging for preoperative evaluation of EC.[42] Their study reported sensitivity, specificity, positive predictive value, and negative predictive value of 84.6%, 70.6%, 52.4%, and 92.3%, respectively, for DW images for assessing myometrial invasion, compared to 69.2%, 61.8%, 40.9%, and 84%, respectively for dynamic images.[42]
In patients with nephrogenic systemic fibrosis or patients who have contraindication to contrast, DW imaging will be helpful in assessing myometrial invasion, as it is considered to be as accurate or better than dynamic imaging.[43,44]
PET/CT
PET/CT allows for simultaneous acquisition of anatomic and metabolic information and has become an essential diagnostic tool in the oncologic staging and surveillance of patients with different types of cancer.
Fluorodeoxyglucose (FDG) generally accumulates in malignant lesions secondary to their high glucose metabolism. Kitajima et al. reported that the mean standardized uptake value (SUV) of EC is 11.2 ± 5.9 (SD).[45] Although EC shows intense FDG uptake, the added value of PET/CT in initial staging of early stage EC is restricted due to limited spatial resolution and physiologic uptake in pre-menopausal women.[46] However, recent studies have shown that pre-operative SUVmax of endometrial tumors seems to be an independent prognostic marker of recurrence and death.[47]
PET/CT is highly sensitive and specific for detecting positive pelvic and/or para-aortic lymphadenopathy as well as distant metastases in selected high-risk patients with EC.
The sensitivity, specificity, and accuracy of PET/CT in detection of lymph node metastases based on increased tracer uptake by the lymph node and independent of size were reported as 53%, 99%, and 98%, respectively.[48] Park et al. compared PET/CT and MRI for detectability of lymph node metastases in 53 patients with EC, and found that PET/CT had better sensitivity and specificity than MRI for both pelvic and para-aortic lymph node metastases.[49] However, many authors showed that detectability using PET/CT is low for metastatic lymph node with a short-axis diameter of 5 mm or less.[48]
In a study comparing DWI and PET/CT for preoperative evaluation of pelvic lymph node metastases in uterine cancer, DWI showed higher sensitivity (83% vs. 38%) and lower specificity (51% vs. 96%) than PET/CT and the accuracy was 57% and 86% for DWI and PET/CT, respectively. Based on these findings, the authors concluded that neither DWI nor PET/CT was sufficiently accurate to replace lymphadenectomy.[50]
PET/MRI
PET/MR imaging systems have recently been developed to take advantage of MRI's high soft tissue resolution and improve the anatomic assessment of cancers.[51] Adding the strengths of PET in staging nodal and distant metastatic disease to the strengths of MRI in local staging allows for a more accurate staging in oncologic patients. Kuhn et al. compared PET/MR with PET/CT and showed that the conspicuity of primary tumors was significantly better for T1, T2, and contrast-enhanced PET/MR imaging in head and neck cancer patients.[52] Queiroz et al. reported almost identical accuracy between PET/CT and PET/MR imaging in primary tumor and lymph node detection, but superior lesion conspicuity for PET/MR in a study with 87 head and neck cancer patients.[53] At the moment, there are no published results for EC using PET/MR.
Diagnostic value of PET/MR for recurrent EC and treatment response assessment with PET/MRI have yet to be reported as this new technology has only been recently introduced into the clinical arena. However, it is predicted that PET/MRI is likely to have a higher diagnostic accuracy over each modality when performed alone.
Also, further improvement in PET/MRI technology, with investigation of new positron tracers, and increased availability are likely to make PET/MRI a very important tool in the preoperative staging and surveillance of patients with EC. Recently, a study reported that fused PET/MRI complements the individual advantages of MRI and PET and helps in assessment of the primary EC and nodal staging.[54]
Treatment
Surgery is the therapy of choice for patients with noninvasive disease. Patients with stage I disease are treated with hysterectomy and bilateral salpingo-oophorectomies [Table 2]. The addition of lymphadenectomy is controversial. Some authors argue that patients with stage 1A and grade 1 or 2 are unlikely to have lymph node involvement, and systematic lymphadenectomy is not indicated in these patients.[55] Other gynecologic oncologic surgeons believe that lymphadenectomy is indicated for all patients regardless of stage and some others do not recommend routine lymphadenectomy in any patient.[16] If lymph node metastases are confirmed by histology, adjuvant radiation or chemotherapy could be considered.
Table 2.
Treatment of endometrial cancer according to stage

Conversely, patients with incurable advanced disease can benefit from surgical intervention such as surgical debulking of large tumoral masses of hysterectomy to stop the bleeding, in addition to various palliative measures. In experienced hands, laparoscopic hysterectomy with adnexal removal and lymphadenectomy seems to be just as safe and effective as an open abdominal procedure.
Recurrence
The rate of recurrent disease in patients with type I EC is low, usually around 10%.[56] The risk of recurrence in type II EC is much higher than in type I. Approximately 25-30% of patients with advanced disease and poor prognostic factors may develop recurrent disease.[57] The serum marker cancer antigen 125 (CA-125) has been shown to be useful in the surveillance of patients with epithelial ovarian cancer and endometrial cancer.[58] Our institution follows high-risk patients with CA-125 every 3-6 months for the first 2 years and yearly thereafter, and elevation of this tumor marker is highly suspicious for recurrence.[58]
The modalities used for assessing recurrence are CT, MRI, and PET/CT. Recurrence usually occurs at an overall median time of 13 months after primary surgery.[59] The most frequently observed sites of relapse are the vagina, lymph nodes, peritoneum, and lungs [Figures 17–19]. Port site metastases may develop in patients who undergo laparoscopic surgery. The most common site for recurrent EC within the vagina is the vaginal apex. Recurrent tumor appears as a mass with high signal intensity on T2WI and intensely enhances following contrast administration. On FDG-PET, recurrent tumor appears as a focal area of increased uptake. Also, FDG PET/CT plays a role in the detection of recurrent lesions in EC [Figure 17].[60] Kitajima et al. reported a sensitivity, specificity, and accuracy of 90.9%, 93.5%, and 92.2%, respectively, of FDG-PET/CT in the diagnosis of recurrent disease in patients with EC.[51]
Figure 17.
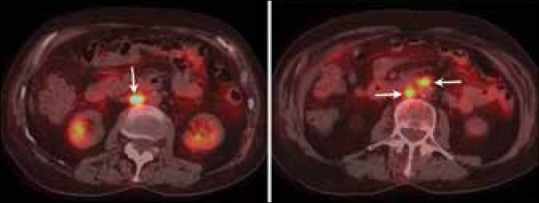
A 73-year-old female with history of endometrial cancer surgically treated with hysterectomy. FDG-PET/CT images show FDG uptake in retroperitoneal lymph nodes (arrows) that were biopsied and gave diagnosis of recurrent disease
Figure 19 (A and B).
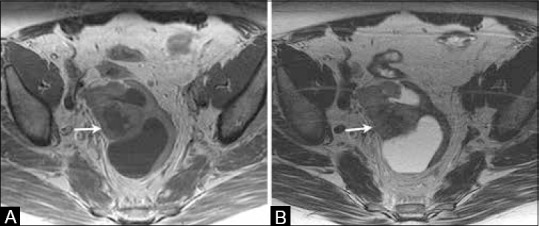
A 69-year-old female with history of treated endometrial cancer surgically treated with hysterectomy. (A) Axial T1W and (B) T2W MR images show a complex cystic solid lesion in the pelvic region suggestive of recurrent disease
Figure 18 (A and B).
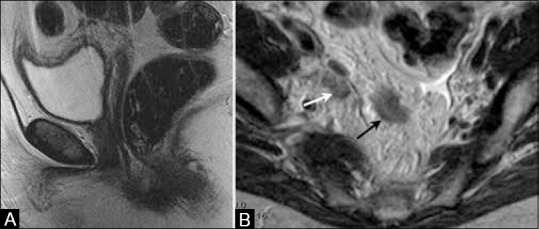
A 65-year-old female with history of treated endometrial cancer surgically treated with hysterectomy. (a) Sagittal T2W MR image show status post-hysterectomy. (b) Axial T2W MR image shows right internal iliac adenopathy and presacral implant suggestive of regional lymph node recurrence (white arrow) and peritoneal recurrence (black arrow)
The treatment for recurrent EC depends on the anatomic location of the recurrence. If the recurrence is confined to the pelvis and the patient has not received whole pelvic radiation therapy, radiotherapy may still be the treatment of choice. In some cases, isolated pelvic recurrences can be treated with exenteration. Patients with systemic disease can be treated with chemotherapy or hormonal therapy.
Conclusion
Imaging plays an important role in the diagnosis, staging, and surveillance of EC patients. Currently, MRI imaging is the most widely used modality for preoperative local staging, with CT or PET/CT used to evaluate distant metastases. Recent technologic advances have introduced new modalities such as FDG PET/CT and FDG PET/MR. These new imaging techniques will aid in the process to provide a comprehensive assessment of distant metastases, contributing to a better management of patients with EC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam G, Sushama S, Rasika B, Mahantshetty U. Hospital-based study of endometrial cancer survival in Mumbai, India. Asian Pac J Cancer Prev. 2013;14:977–80. doi: 10.7314/apjcp.2013.14.2.977. [DOI] [PubMed] [Google Scholar]
- 3.Arora V, Quinn MA. Endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2012;26:311–24. doi: 10.1016/j.bpobgyn.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 5.Tirumani SH, Shanbhogue AK, Prasad SR. Current concepts in the diagnosis and management of endometrial and cervical carcinomas. Radiol Clin North Am. 2013;51:1087–110. doi: 10.1016/j.rcl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Fadare O, Gwin K, Desouki MM, Crispens MA, Jones HW, 3rd, Khabele D, et al. The clinicopathologic significance of p53 and BAF-250a (ARID1A) expression in clear cell carcinoma of the endometrium. Mod Pathol. 2013;26:1101–10. doi: 10.1038/modpathol.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attias-Geva Z, Bentov I, Kidron D, Amichay K, Sarfstein R, Fishman A, et al. p53 Regulates insulin-like growth factor-I receptor gene expression in uterine serous carcinoma and predicts responsiveness to an insulin-like growth factor-I receptor-directed targeted therapy. Eur J Cancer. 2012;48:1570–80. doi: 10.1016/j.ejca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Steinbakk A, Malpica A, Slewa A, Skaland I, Gudlaugsson E, Janssen EA, et al. Biomarkers and microsatellite instability analysis of curettings can predict the behavior of FIGO stage I endometrial endometrioid adenocarcinoma. Mod Pathol. 2011;24:1262–71. doi: 10.1038/modpathol.2011.75. [DOI] [PubMed] [Google Scholar]
- 9.Minaguchi T, Yoshikawa H, Oda K, Ishino T, Yasugi T, Onda T, et al. PTEN mutation located only outside exons 5, 6, and 7 is an independent predictor of favorable survival in endometrial carcinomas. Clin Cancer Res. 2001;7:2636–42. [PubMed] [Google Scholar]
- 10.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, et al. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104:1391–7. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 11.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120:383–97. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 12.Ascher SM, Reinhold C. Imaging of cancer of the endometrium. Radiol Clin North Am. 2002;40:563–76. doi: 10.1016/s0033-8389(01)00013-6. [DOI] [PubMed] [Google Scholar]
- 13.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. 7th ed. New York: Springer; 2010. American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual; pp. 403–18. [Google Scholar]
- 15.Smith-Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, Segal M, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510–7. doi: 10.1001/jama.280.17.1510. [DOI] [PubMed] [Google Scholar]
- 16.Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28:721–39. doi: 10.1016/j.bpobgyn.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Takac I. Transvaginal ultrasonography with and without saline infusion in assessment of myometrial invasion of endometrial cancer. J Ultrasound Med. 2007;26:949–57. doi: 10.7863/jum.2007.26.7.949. [DOI] [PubMed] [Google Scholar]
- 18.Dessole S, Rubattu G, Farina M, Capobianco G, Cherchi PL, Tanda F, et al. Risks and usefulness of sonohysterography in patients with endometrial carcinoma. Am J Obstet Gynecol. 2006;194:362–8. doi: 10.1016/j.ajog.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Barwick TD, Rockall AG, Barton DP, Sohaib SA. Imaging of endometrial adenocarcinoma. Clin Radiol. 2006;61:545–55. doi: 10.1016/j.crad.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Sladkevicius P, Valentin L, Marsál K. Endometrial thickness and Doppler velocimetry of the uterine arteries as discriminators of endometrial status in women with postmenopausal bleeding: A comparative study. Am J Obstet Gynecol. 1994;171:722–8. doi: 10.1016/0002-9378(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 21.Hardesty LA, Sumkin JH, Hakim C, Johns C, Nath M. The ability of helical CT to preoperatively stage endometrial carcinoma. AJR Am J Roentgenol. 2001;176:603–6. doi: 10.2214/ajr.176.3.1760603. [DOI] [PubMed] [Google Scholar]
- 22.Tsili AC, Tsampoulas C, Dalkalitsis N, Stefanou D, Paraskevaidis E, Efremidis SC. Local staging of endometrial carcinoma: Role of multidetector CT. Eur Radiol. 2008;18:1043–8. doi: 10.1007/s00330-007-0839-z. [DOI] [PubMed] [Google Scholar]
- 23.Akin O, Mironov S, Pandit-Taskar N, Hann LE. Imaging of uterine cancer. Radiol Clin North Am. 2007;45:167–82. doi: 10.1016/j.rcl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Hirano Y, Kubo K, Hirai Y, Okada S, Yamada K, Sawano S, et al. Preliminary experience with gadolinium-enhanced dynamic MR imaging for uterine neoplasms. Radiographics. 1992;12:243–56. doi: 10.1148/radiographics.12.2.1561414. [DOI] [PubMed] [Google Scholar]
- 25.Hricak H, Rubinstein LV, Gherman GM, Karstaedt N. MR imaging evaluation of endometrial carcinoma: Results of an NCI cooperative study. Radiology. 1991;179:829–32. doi: 10.1148/radiology.179.3.2028000. [DOI] [PubMed] [Google Scholar]
- 26.Lien HH, Blomlie V, Tropé C, Kaern J, Abeler VM. Cancer of the endometrium: Value of MR imaging in determining depth of invasion into the myometrium. AJR Am J Roentgenol. 1991;157:1221–3. doi: 10.2214/ajr.157.6.1950869. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi R, Mirk P, Maresca G, Margariti PA, Testa A, Zannoni GF, et al. Local-regional staging of endometrial carcinoma: Role of MR imaging in surgical planning. Radiology. 2004;231:372–8. doi: 10.1148/radiol.2312021184. [DOI] [PubMed] [Google Scholar]
- 28.Beddy P, O’Neill AC, Yamamoto AK, Addley HC, Reinhold C, Sala E. FIGO staging system for endometrial cancer: Added benefits of MR imaging. Radiographics. 2012;32:241–54. doi: 10.1148/rg.321115045. [DOI] [PubMed] [Google Scholar]
- 29.Foti PV, Farina R, Coronella M, Ruggeri C, Palmucci S, Montana A, et al. Endometrial carcinoma: MR staging and causes of error. Radiol Med. 2013;118:487–503. doi: 10.1007/s11547-012-0861-3. [DOI] [PubMed] [Google Scholar]
- 30.Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: Implications for MR imaging. Radiographics. 2012;32:1805–27. doi: 10.1148/rg.326125519. [DOI] [PubMed] [Google Scholar]
- 31.Wu WJ, Yu MS, Su HY, Lin KS, Lu KL, Hwang KS. The accuracy of magnetic resonance imaging for preoperative deep myometrium assessment in endometrial cancer. Taiwan J Obstet Gynecol. 2013;52:210–4. doi: 10.1016/j.tjog.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Peungjesada S, Bhosale PR, Balachandran A, Iyer RB. Magnetic resonance imaging of endometrial carcinoma. J Comput Assist Tomogr. 2009;33:601–8. doi: 10.1097/RCT.0b013e31818d4279. [DOI] [PubMed] [Google Scholar]
- 33.Haldorsen IS, Husby JA, Werner HM, Magnussen IJ, Rørvik J, Helland H, et al. Standard 1.5-T MRI of endometrial carcinomas: Modest agreement between radiologists. Eur Radiol. 2012;22:1601–11. doi: 10.1007/s00330-012-2400-y. [DOI] [PubMed] [Google Scholar]
- 34.Murakami T, Kurachi H, Nakamura H, Tsuda K, Miyake A, Tomoda K, et al. Cervical invasion of endometrial carcinoma-evaluation by parasagittal MR imaging. Acta Radiol. 1995;36:248–53. [PubMed] [Google Scholar]
- 35.Takahashi K, Yoshioka M, Kosuge H, Iizuka Y, Musha T, Yamauchi I, et al. The accuracy of computed tomography and magnetic resonance imaging in evaluating the extent of endometrial carcinoma. Nihon Sanka Fujinka Gakkai Zasshi. 1995;47:647–54. [PubMed] [Google Scholar]
- 36.Petru E, Lück HJ, Stuart G, Gaffney D, Millan D, Vergote I. Gynecologic Cancer Intergroup (GCIG). Gynecologic Cancer Intergroup (GCIG) proposals for changes of the current FIGO staging system. Eur J Obstet Gynecol Reprod Biol. 2009;143:69–74. doi: 10.1016/j.ejogrb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Kang S, Todo Y, Watari H. Risk assessment of lymph node metastasis before surgery in endometrial cancer: Do we need a clinical trial for low-risk patients? J Obstet Gynaecol Res. 2014;40:322–6. doi: 10.1111/jog.12281. [DOI] [PubMed] [Google Scholar]
- 38.Todo Y, Watari H, Kang S, Sakuragi N. Tailoring lymphadenectomy according to the risk of lymph node metastasis in endometrial cancer. J Obstet Gynaecol Res. 2014;40:317–21. doi: 10.1111/jog.12309. [DOI] [PubMed] [Google Scholar]
- 39.Rockall AG, Meroni R, Sohaib SA, Reynolds K, Alexander-Sefre F, Shepherd JH, et al. Evaluation of endometrial carcinoma on magnetic resonance imaging. Int J Gynecol Cancer. 2007;17:188–96. doi: 10.1111/j.1525-1438.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 40.Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–21. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 41.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: What the radiologist needs to know. Radiology. 2013;266:717–40. doi: 10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 42.Rechichi G, Galimberti S, Signorelli M, Perego P, Valsecchi MG, Sironi S. Myometrial invasion in endometrial cancer: Diagnostic performance of diffusion-weighted MR imaging at 1.5-T. Eur Radiol. 2010;20:754–62. doi: 10.1007/s00330-009-1597-x. [DOI] [PubMed] [Google Scholar]
- 43.Gallego JC, Porta A, Pardo MC, Fernández C. Evaluation of myometrial invasion in endometrial cancer: Comparison of diffusion-weighted magnetic resonance and intraoperative frozen sections. Abdom Imaging. 2014;39:1021–6. doi: 10.1007/s00261-014-0134-9. [DOI] [PubMed] [Google Scholar]
- 44.Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: A systematic review and meta-analysis. Eur Radiol. 2014;24:1327–38. doi: 10.1007/s00330-014-3139-4. [DOI] [PubMed] [Google Scholar]
- 45.Kitajima K, Murakami K, Kaji Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR Am J Roentgenol. 2010;195:737–43. doi: 10.2214/AJR.09.4074. [DOI] [PubMed] [Google Scholar]
- 46.Brunetti J. PET/CT in gynecologic malignancies. Radiol Clin North Am. 2013;51:895–911. doi: 10.1016/j.rcl.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Ghooshkhanei H, Treglia G, Sabouri G, Davoodi R, Sadeghi R. Risk stratification and prognosis determination using (18) F-FDG PET imaging in endometrial cancer patients: A systematic review and meta-analysis. Gynecol Oncol. 2014;132:669–76. doi: 10.1016/j.ygyno.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 48.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol. 2009;19:1529–36. doi: 10.1007/s00330-008-1271-8. [DOI] [PubMed] [Google Scholar]
- 49.Park JY, Kim EN, Kim DY, Suh DS, Kim JH, Kim YM, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008;108:486–92. doi: 10.1016/j.ygyno.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 50.Kitajima K, Yamasaki E, Kaji Y, Murakami K, Sugimura K. Comparison of DWI and PET/CT in evaluation of lymph node metastasis in uterine cancer. World J Radiol. 2012;4:207–14. doi: 10.4329/wjr.v4.i5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Morita S, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent uterine cancer: Comparison with PET and enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36:362–72. doi: 10.1007/s00259-008-0956-1. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn FP, Hüllner M, Mader CE, Kastrinidis N, Huber GF, von Schulthess GK, et al. Contrast-enhanced PET/MR imaging versus contrast-enhanced PET/CT in head and neck cancer: How much MR information is needed? J Nucl Med. 2014;55:551–8. doi: 10.2967/jnumed.113.125443. [DOI] [PubMed] [Google Scholar]
- 53.Queiroz MA, Hüllner M, Kuhn F, Huber G, Meerwein C, Kollias S, et al. PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur J Nucl Med Mol Imaging. 2014;41:1066–75. doi: 10.1007/s00259-014-2707-9. [DOI] [PubMed] [Google Scholar]
- 54.Kitajima K, Suenaga Y, Ueno Y, Kanda T, Maeda T, Takahashi S, et al. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with 18F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur J Radiol. 2013;82:1672–6. doi: 10.1016/j.ejrad.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Kehoe SM, Miller DS. The role of lymphadenectomy in endometrial cancer. Clin Obstet Gynecol. 2011;54:235–44. doi: 10.1097/GRF.0b013e318218577b. [DOI] [PubMed] [Google Scholar]
- 56.Carlson MJ, Thiel KW, Leslie KK. Past, present, and future of hormonal therapy in recurrent endometrial cancer. Int J Womens Health. 2014;6:429–35. doi: 10.2147/IJWH.S40942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugimura K, Okizuka H. Postsurgical pelvis: Treatment follow-up. Radiol Clin North Am. 2002;40:659–80. doi: 10.1016/s0033-8389(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 58.Ozcan Kara P, Kara T, Kaya B, Kara Gedik G, Sari O. The value of FDG-PET/CT in the post-treatment evaluation of endometrial carcinoma: A comparison of PET/CT findings with conventional imaging and CA 125 as a tumour marker. Rev Esp Med Nucl Imagen Mol. 2012;31:257–60. doi: 10.1016/j.remn.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: Patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62:28–36. doi: 10.1016/j.crad.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Kadkhodayan S, Shahriari S, Treglia G, Yousefi Z, Sadeghi R. Accuracy of 18-F-FDG PET imaging in the follow up of endometrial cancer patients: Systematic review and meta-analysis of the literature. Gynecol Oncol. 2013;128:397–404. doi: 10.1016/j.ygyno.2012.10.022. [DOI] [PubMed] [Google Scholar]


