Abstract
Imaging in rectal cancer has a vital role in staging disease, and in selecting and optimizing treatment planning. High-resolution MRI (HR-MRI) is the recommended method of first choice for local staging of rectal cancer for both primary staging and for restaging after preoperative chemoradiation (CT-RT). HR-MRI helps decide between upfront surgery and preoperative CT-RT. It provides high accuracy for prediction of circumferential resection margin at surgery, T category, and nodal status in that order. MRI also helps assess resectability after preoperative CT-RT and decide between sphincter saving or more radical surgery. Accurate technique is crucial for obtaining high-resolution images in the appropriate planes for correct staging. The phased array external coil has replaced the endorectal coil that is no longer recommended. Non-fat suppressed 2D T2-weighted (T2W) sequences in orthogonal planes to the tumor are sufficient for primary staging. Contrast-enhanced MRI is considered inappropriate for both primary staging and restaging. Diffusion-weighted sequence may be of value in restaging. Multidetector CT cannot replace MRI in local staging, but has an important role for evaluating distant metastases. Positron emission tomography-computed tomography (PET/CT) has a limited role in the initial staging of rectal cancer and is reserved for cases with resectable metastatic disease before contemplating surgery. This article briefly reviews the comprehensive role of imaging in rectal cancer, describes the role of MRI in local staging in detail, discusses the optimal MRI technique, and provides a synoptic report for both primary staging and restaging after CT-RT in routine practice.
Keywords: Imaging, local staging, MRI, staging rectal cancer
Background
Why imaging
The radiologist today plays a crucial part in the multidisciplinary team that manages rectal cancer. The reason for this is the paradigm shift in using imaging to select patients for optimal therapy. A high rate of local recurrence is a major concern in rectal cancers as it greatly influences the quality of life causing severe pain and immobility.[1] The primary reason for local recurrence in rectal cancers is incomplete removal,[2,3] adverse prognostic factors, and inadequate treatment. Advances in treatment such as surgery and chemo-radiotherapy (CT-RT) have, therefore, strived to minimize local recurrence. Advances in imaging have tried to identify the tumors with bad prognosis that can be given more intensive treatment.
The first advance in treatment of rectal cancers was the introduction of a standardized surgical technique called the total mesorectal excision (TME) in which the rectum along with the entire mesorectal fat containing perirectal lymph nodes limited by a thin fascial envelope called the mesorectal fascia (MRF) is removed.[4] This technique reduced the local recurrence rate to below 10%.[4] European trials have also experimented with preoperative radiotherapy (RT) in mobile rectal cancers[1] and the Swedish Rectal Cancer trial (in the pre-TME era) found that preoperative RT reduces local recurrence rates to 11%.[5] Although in the United States, postoperative CT-RT is practiced for select loco-regionally advanced cancers, the German Rectal Cancer Study Group Trial clearly showed preoperative long course CT-RT had lower 5-year local recurrence rates as compared to postoperative long course CT-RT in T3, T4, and node-positive cancers.[6] The Dutch TME trial compared TME alone with preoperative RT followed by TME; and found that the latter combined approach reduced the 2-year local recurrence rate to 2.4%,[7] although RT is not without side effects. The trial also identified different groups of tumors: the low-risk group that could be treated with surgery alone and the high-risk group that required preoperative long course CT-RT, also called neoadjuvant chemo-radiation (NACT-RT), followed by extensive surgery. Digital rectal examination (DRE) alone is clearly insufficient for identification of these varied groups[8] and imaging is therefore essential. So, if preoperative CT-RT is the standard of care, a sensitive imaging method is required to
Select patients for upfront surgery or for NACT-RT,
Plan surgery after NACT-RT and
Plan RT.
Learning Objectives
Discuss the role of various imaging methods in the comprehensive staging of rectal cancer
Describe the MRI technique and technical challenges in the local staging of rectal cancer
Describe MRI anatomy of the rectum
Discuss the treatment principles of rectal cancers with a brief overview of the various surgical procedures
Describe the issues in local staging of rectal cancer, the role of MRI and the pitfalls in staging
Provide a synoptic MRI report for pretreatment evaluation
Discuss imaging in restaging after NACT-RT
Provide a synoptic MRI report in the restaging setting.
Role of imaging methods in pretreatment staging of rectal cancer
Cancer staging usually requires information on the tumor stage (T), nodal stage (N), and metastases (M). The American Joint Committee on Cancer (AJCC) TNM staging of rectal cancers is provided in Table 1.[9] However, for complete local staging and deciding therapy in rectal cancers, additional information beyond the T and N staging is required.[10,11] These issues that are discussed in detail later in this article are enumerated below:
Table 1.
AJCC 7th edition TNM staging classification of rectal cancer

Circumferential resection margin (CRM) that provides information on the margin resection status for TME and influences local recurrence and therapy plan[1,10,11,12]
Extramural venous invasion (EMV), a feature that influences prognosis[11,13,14,15]
Sphincter complex status to decide sphincter-sparing surgery as well as the need for preoperative RT[11]
Extramesorectal nodes that can impact therapy planning, particularly RT.[1,11]
High-resolution MRI (HR-MRI) using a phased array external coil best addresses all these issues[1,11,16,17] and is the recommended method of first choice for overall primary staging of rectal cancer.[18]
MRI with endorectal coil (ER-MRI) is not recommended.[18] Although endorectal MRI can show five layers of the rectal wall and is highly accurate for T staging, the field of view (FOV) is limited and the MRF is not always visible (and hence, CRM status cannot be gleaned). Moreover, the endorectal coil cannot be inserted in stenosing/obstructing tumors and the extramesorectal nodes cannot be visualized.[1]
Endorectal ultrasound (ERUS) is highly accurate for T staging with a sensitivity of 94% and specificity of 86%[19] and is more accurate than MRI for T1 and T2 lesions[20] and similar in accuracy as MRI for T3 and T4 lesions.[21] However, like ER-MRI, the FOV is limited and information on the CRM or extramesorectal nodes is not always available. Hence, it is reserved for early tumors to differentiate between T1 and T2 tumors and is essential to identify T1 N0 tumors where endoanal excision is planned.[18] ERUS is operator dependent and more dependable results are obtained in high-volume centers.[22] ERUS is also not useful in stenosing lesions.[1] Majority of the cases presenting at our referral center are T2 and higher stage, and hence, ERUS is performed in <1% cases at our institute. Multidetector CT (MDCT) has been compared with MRI for local staging. It was found inadequate for prediction of CRM in low anterior tumors with inconsistency between readers, although the performance was adequate in mid-high rectal tumors.[23] The accuracy for T staging with MDCT was low[24] and sphincter status is difficult to predict. The peritoneal reflection that is visible with MRI (invasion of which upstages to T4a) is not visible with MDCT. Overall, MDCT cannot replace HR-MRI for local staging.[24]
However, MDCT has an essential role in the metastatic workup of rectal cancers. While MDCT chest, abdomen, and pelvis is the usual strategy, there is no uniform consensus in the chest strategy in colorectal cancers between CT chest and chest radiograph. CT chest alters the TNM stage in <1% and may show indeterminate pulmonary nodules in up to 25%.[25] Although CT chest is considered more useful when CT abdomen reveals a metastasis, a database review of 754 patients revealed isolated lung metastases in 12% patients with rectal cancer, particularly in advanced cases.[26]
Positron emission tomography-computed tomography (PET/CT) has no significant role in the initial staging in rectal cancers[27,28] in the absence of synchronous metastases. At our institute, we order PET/CT for cases with limited resectable liver or lung metastases, to rule out extensive metastases prior to planned resection. PET/CT is also ordered in locally advanced cancers where radical surgery like exenteration is planned.
MRI technique
The optimal MRI technique is crucial for accurate locoregional staging. Many of the recommendations below are adapted from the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting 2012 on MRI in the clinical management of rectal cancer patients, with a few from the MERCURY Group.[11,18]
The optimal magnetic field strength is 1.5 T, minimal being 1.0 T. There is no consensus between field strengths of 1.5T and 3T. In our institute, we prefer a 1.5 T magnet
Endorectal coil is not recommended. A phased array external coil is used
Routine endorectal filling, enema, or spasmolytics are not recommended. Use of endorectal gel was favored by only 23% in the ESGAR 2012 consensus meeting[18] and is useful in those with small tumors <3 cm and in polypoid lesions (villus adenomas)[29]
Prior to MR imaging, the clinical notes should be studied for precise location of the tumor. The positioning of the 8-channel surface coil is dependent on tumor location such that the rectum with the entire tumor, mesorectum, and the anal complex are adequately included. Adequate coverage of nodal territory includes 5 cm above the level of tumor. It is recommended that patient should empty the bowel and bladder prior to MR imaging.
Only 2D T2-weighted (T2W) sequences are recommended for both primary and restaging. 3D T2W sequences and fat-suppressed sequences are not necessary for both primary and restaging. Diffusion-weighted imaging (DWI) sequences are not required for the primary staging (no added value), but maybe useful for restaging
Only T2W non-fat suppressed sequences are recommended. Gadolinium-enhanced sequences are not useful in routine practice and are considered inappropriate by some.[18] Studies have demonstrated no increase in the diagnostic yield for tumor staging and nodal staging[30,31]
The slice thickness is 3 mm (range 1-4 mm). We acquire a sagittal sequence first and plan the axial and coronal small FOV sequences orthogonal to the tumor. The coil may need repositioning depending on the location of tumor seen on sagittal sequences. The cranial border of the coil should not be higher than L5 and the caudal border of the coil should be 10 cm below the symphysis pubis in low rectal tumors. In upper and mid-rectal tumors, the axial and coronal sequences are obtained perpendicular and parallel to the tumor axis, respectively [Figure 1A]. In lower-third rectal tumors involving the region above the anorectal ring, the planning is similar to above; but for low rectal tumors below the anorectal ring, the scan plane should be perpendicular and parallel to the anal canal [Figure 1B]. At our institute, we also acquire two additional sequences, namely larger FOV axial T1 and T2W sequences, from the iliac crests to ischial tuberosities for complete coverage of nodes and any other co-existent pelvic pathology. This also helps us limit the metastatic workup to MDCT chest and abdomen.
The MRI parameters used at our institute are shown in Table 2
Technical challenges with MRI in staging rectal cancer include motion artifacts (from bowel, bladder, and anterior abdominal wall movements) and suboptimal signal-to-noise ratio (SNR).[29] Motion artifact due to movement of anterior abdominal wall can be offset by placement of a saturation band in the anterior abdominal region anterior to the bladder. Excessive bowel movements might need use of spasmolytics.
Figure 1 (A and B).
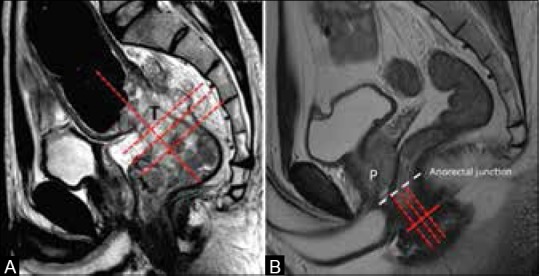
Planning MRI. (A) Sagittal T2W MRI. A bulky mid-rectal tumor (T); axial and coronal sequences are planned perpendicular and parallel to the tumor (lines). (B) Sagittal T2W MRI. Scan planes in the anal canal region (red lines). Anorectal junction in the male is at the level of apex of prostate (white line)
Table 2.
Imaging protocol for rectal cancer
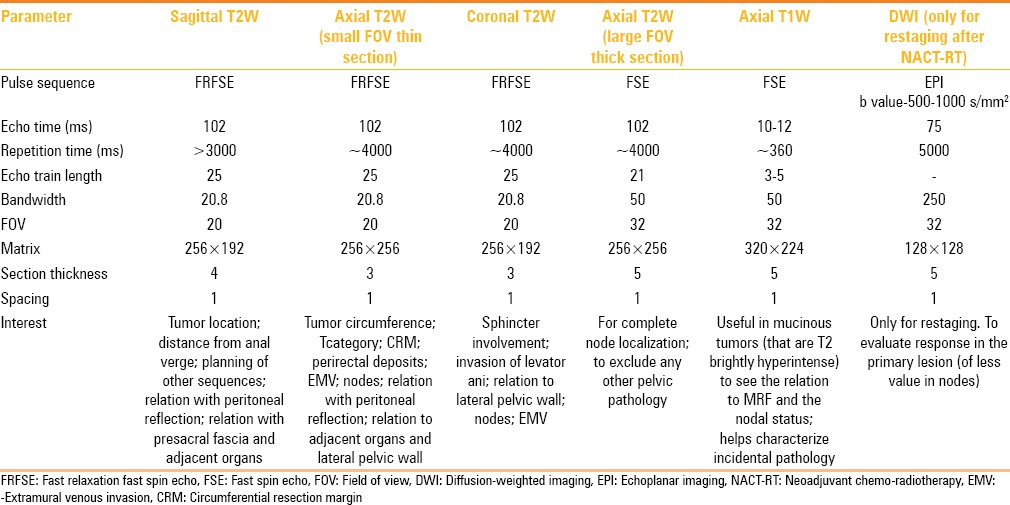
Improving SNR requires correct positioning of the external MRI coil as well as a compromise between appropriate matrix, FOV, and bandwidth. Our protocol is detailed in Table 2; however, the protocol needs standardizing on each MR scanner.
MRI anatomy of the rectum
The rectum is the terminal part of the alimentary tract, located from the anal verge (AV) to 15 cm above, nestling along the sacral curve. It is divided into three parts based on distance from AV into low rectum (0-5 cm), mid-rectum (5-10 cm), and upper rectum (10-15 cm) as shown in Figure 2A. It should be noted that this division implies that low rectum comprises the anal canal as well as the lowermost part of the rectum just above the anorectal junction. The rectosigmoid junction has a variable location from sacral promontory to S3 level [Figure 2A]. On axial sections, it can be identified at the point where the rectum leaves the sacral curve to extend anteriorly to the sigmoid colon [Figure 2B].
Figure 2 (A-C).
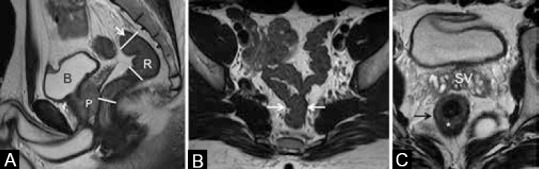
(A) Sagittal T2W MRI showing division into upper, mid, and lower rectum (R), bladder (b), prostate (P). (B and C) Axial T2W MRI. Arrows in (a and b) show rectosigmoid junction. (C) Section at the level of seminal vesicles (SV) shows normal rectum with hyperintense submucosa (*) and darkly hypointense muscularis propria (arrow)
Three layers of the rectum are visible on a phased array external MRI. The innermost mucosa is thin and hypointense, the middle submucosa is hyperintense, and the outer muscularis propria is darkly hypointense [Figure 2C]. The rectum has a serosal lining only above the peritoneal reflection, which is along the anterior and lateral surfaces in the upper-third and the anterior surface in the middle-third [Figure 3]. The peritoneal reflection is visible on high-resolution sagittal and axial sequences [Figure 4]. Below the peritoneal reflection, the rectum is surrounded by the mesorectal fat which is limited by a thin fascia called the MRF, which fuses with the retroprostatic or retrovaginal fascia anteriorly and the presacral fascia posteriorly [Figure 3A]. The MRF surrounds the rectum completely only in the lower third [Figure 3B]. It is best seen laterally as a thin hypointense line on T2W sequences [Figure 5]. Inferiorly, the MRF thins out as it reaches the levator ani, which forms the roof of the ischiorectal fossa [Figure 5B].
Figure 3 (A and B).
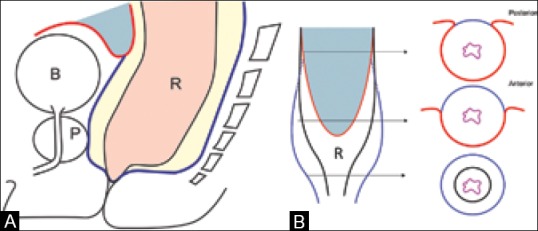
(A) Sagittal and (B) coronal diagrams show rectum (R), mesorectal fat (yellow), mesorectal fascia (MRF) in blue, anterior peritoneal reflection (red), bladder (B), prostate (P). (b) Cross section at upper, mid, and low rectum shows peritoneal reflection (red) and MRF (blue)
Figure 4 (A and B).
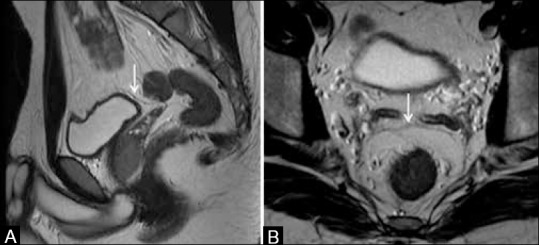
(A) Sagittal T2W MRI and (B) axial T2W MRI show the anterior peritoneal reflection (arrows) at the level of urinary bladder dome. Both sagittal and axial images have to be viewed
Figure 5 (A and B).
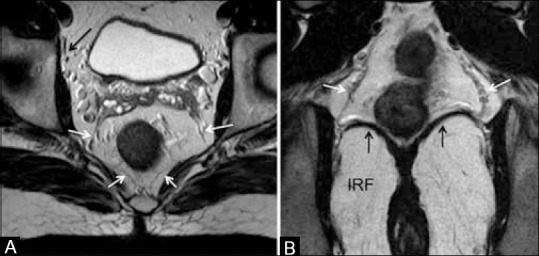
(A) Axial T2W MRI and (B) coronal T2W MRI. White arrows show mesorectal fascia (MRF). Black arrow in (A) shows obturator vessels. Vertical arrows in (B) show the levator ani, forming the roof of ischiorectal fossa (IRF). MRF thins out as it reaches the levator ani
The anal canal has the innermost mucosa and submucosa surrounded by the sphincter. The latter comprises the internal sphincter (continuation of the circular smooth muscle of the rectum), the longitudinal muscle outside this, and the outermost external sphincter made of striated muscles [Figure 6A]. The upper end of the anal canal (anorectal junction), best visualized on coronal MRI, is seen near the insertion of the levator ani into the puborectalis (continuous with the external sphincter) as in Figure 6B. Figure 1B shows the sagittal view of the anorectal junction.
Figure 6 (A and B).
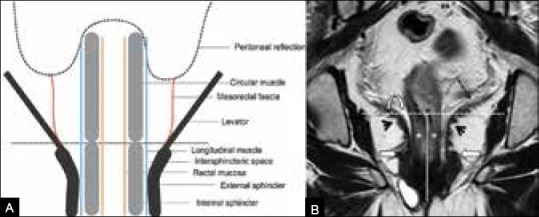
(A) Levator–sphincter complex. (B) Coronal T2W MRI. Levator ani (dashed arrow) inserting into puborectalis (arrowhead); anorectal junction (horizontal line); anal canal (vertical line); internal sphincter (*), intersphincteric space (curved arrow). Block arrows show the thickest part of external sphincter (continuous with puborectalis above)
The AV is the region where the lower end of the anal canal reaches the skin surface at the gluteal cleft and is seen in the last axial section where the circular lumen of the anal canal is visible [Figure 7A]. To precisely identify the AV on the sagittal scans, cross-referencing with the axial sequences can help [Figure 7A and B].
Figure 7 (A and B).
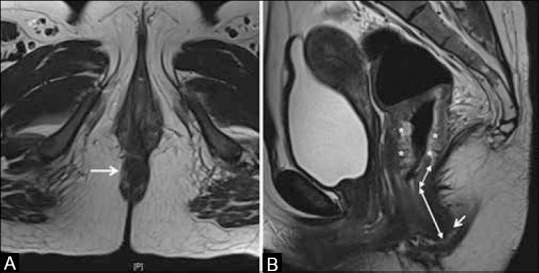
(A) Axial T2W MRI showing anal verge (arrow). (B) Sagittal T2W MRI shows circumferential mid-lower rectal tumor (*). Arrow shows anal verge, double-ended arrows show the measurement of distance of lower limit of tumor from anal verge
Treatment principles
Rectal cancers can be metastatic or non-metastatic. Non-metastatic rectal cancers can be early (T1 T2 N0) or locally advanced. Early rectal cancers can be treated by upfront surgery (TME), while T1N0 tumors can be treated with mucosal resection (endoanal excision).[32] On the other hand, the standard treatment practiced for locally advanced rectal cancers is NACT-RT or short course RT followed by surgery (either TME or more extensive surgery depending on the stage). The stratification into these varied groups for tailoring optimal treatment is based on imaging.
The various surgical procedures used for anorectal cancer merit brief description. While TME describes the optimal lateral clearance of disease, the caudal extent of resection can range from conservative to radical as described below:
Anterior resection (AR) is performed in upper rectal and mid-rectal cancers with anastomosis at 5 cm and 2 cm distal to tumor margin, respectively. Anal canal is intact.
Low AR is offered in low rectal cancers above the anorectal ring with anastomosis at 1 cm distal to the tumor margin. Sphincter is preserved.
Abdomino-perineal resection (APR) involves removal of the rectum and anal canal, requiring a permanent colostomy. It is performed in tumors reaching AV or less than 1 cm from AV, but sparing the intersphincteric space, levators, and adjacent pelvic organs.
Extralevator APR is performed in tumors that invade intersphincteric space, external sphincter/levators, but spare adjacent pelvic organs. Entire levator ani is removed along with APR.
Intersphincteric resection is performed in select tumors close to anorectal ring, which involve the internal sphincter but spare the intersphincteric space as well as adjacent pelvic organs. The external sphincter is preserved averting the need for permanent colostomy.
Exenteration is offered in rectal cancer invading adjacent organs, but not reaching lateral pelvic wall. It involves removal of the rectum with pelvic organs such as prostate, seminal vesicles, bladder, or vagina and/or uterus.
Spread up to or close to lateral pelvic wall is unresectable.
HR-MRI in pretreatment local staging
Distance from AV
The first information that is required while studying the MRI is the distance of the tumor from the AV. MRI should always be interpreted after studying the clinical notes of DRE or rigid sigmoidoscopy, which would specify the distance of lower limit of tumor from the AV. The DRE would also specify the precise circumferential location. MRI is needed primarily to evaluate the lateral spread of disease, but the distance from AV also needs mention in the MRI report and is measured as done with flexible sigmoidoscopy as shown in Figure 7B.[11] Pitfall in this interpretation may arise due to difficulty in precise identification of the position of the AV. Location of the AV has been illustrated in Figure 7 and described in the section on MR anatomy. The circumferential location of the tumor (seen on axial sections) needs mention at the o’clock position along with the tumor length (supero-inferior) measured in a similar fashion as the distance from AV.
T staging
T staging reflects the extent of the tumor within the rectal wall and extramural spread into the perirectal tissues and organs [Table 1 and Figure 8]. A recent meta-analysis reported an accuracy of 85%, sensitivity of 87%, and specificity of 75% for HR-MRI inT staging of rectal cancer.[33] On HR-MRI, T staging is decided by examining the T2W signal intensity of the normal rectum and of the tumor extending into the layers of the rectal walls and the mesorectal fat. The tumor usually has intermediate signal intensity on T2W MR images. However, mucinous tumors are brightly hyperintense on T2W MRI. In these tumors, where the contrast between the tumor and perirectal fat is inadequate, we find it useful to also examine the axial T1-weighted (T1W) sequence [Figure 9].
Figure 8.
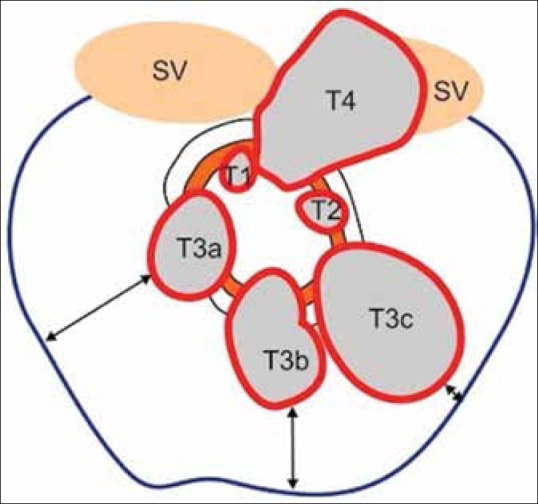
T staging and CRM. T2 tumors extend to muscularis propria. T3 tumors extend into mesorectal fat. T4 tumors invade adjacent organs. Blue line represents the mesorectal fascia (MRF). Double-ended arrows show the varying circumferential resection margin, which is the shortest distance between tumor edge and MRF
Figure 9 (A and B).
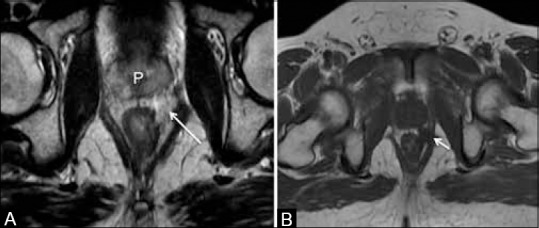
(A) Axial T2W MRI shows a hyperintense mucin containing node in the left periprostatic region (arrow), which could be overlooked due to inadequate contrast. (B) Axial T1W MRI shows the node (arrow) which is hypointense against the bright fat
T1 stage tumors extend upto the submucosa, while tumors extending into the muscularis propria without extension into perirectal tissues are T2 [Figure 10]. It is not possible to reliably distinguish between T1 and T2 tumors on MRI;[18] ERUS is used in such cases.[18,32] T3 tumors spread beyond the muscularis propria into the perirectal fat. This is well seen on HR-MRI as loss of continuity of the muscularis propria with extension of the tumor signal intensity into the perirectal fat or as perirectal fat stranding [Figure 11].
Figure 10 (A and B).
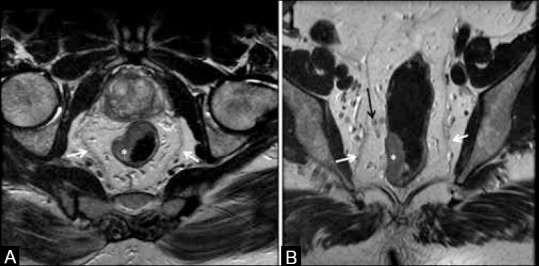
(A) Axial T2W MRI shows rectal tumor (*) from 7o’clock to 1o’clock position; T2 tumor (no spread into mesorectal fat). (B) Coronal T2W MRI. White arrows in (a and b)show mesorectal fascia; black arrow in (B) shows perirectal deposits (<3 mm). “Figure reproduced with permission from “Arya S, Rangarajan V, Purandare N. Principles of Cancer Diagnosis: Imaging. UICC Manual of Clinical Oncology. 9th edition. Wiley Publishers.
Figure 11 (A-C).
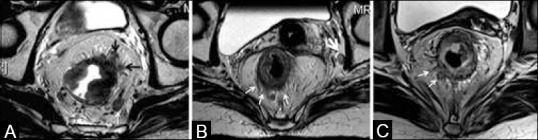
(A) Arrows at 1-2 o’clock show perirectal spread (T3 tumor). Left mesorectal node (white arrow). (B) Thin arrows show darkly hypointense spicules (T2 tumor with desmoplastic reaction). Left iliac node (thick arrow). (C) Arrows show perirectal spread of intermediate signal with broad pushing front (early T3 tumor)
The extent of spread into the perirectal fat has a prognostic significance. Hence, T3 tumors have been subclassified further by measuring the distance between the intact adjacent muscularis and the maximum extramural spread into T3 a-c in one system[29] [Figure 8 and Table 3] or T3 a-d in another subclassification.[11] The significance is that T3 tumors with ≤5 mm spread into perirectal tissues have 5 year survival of 85%, while T3 tumors > 5 mm extramural spread have a 5 year survival of 54% requiring more intensive treatment.[34] Hence, it is useful to specify the distance of extramural spread in millimeters. A discontinuous satellite lesion in the mesorectal fat is categorized as deposit if ≤3 mm in size and is stage N1C [Table 1]; >3-mm-sized structures in the mesorectal fat are classified as nodes.[11]
Table 3.
Subclassification of T3 based on prognostic patterns[29]

Invasion of adjacent organs indicates T4 stage. This is seen in low rectal cancers and it is important to examine the fat plane between the tumor and adjacent organ. There are three possibilities:[35] (a) when the intervening fat plane is intact, it is regarded as “no invasion” [Figure 12A]; (b) when there is loss of intervening fat plane and a corresponding T2 signal abnormality in the adjacent organ, it is “definite invasion” [Figure 12B]; and (C) when the fat plane is lost and the adjacent organ does not show any corresponding T2W signal intensity, it is “possible invasion” [Figure 12C]. In upper rectal cancers, invasion of the anterior peritoneal reflection by tumor is T4a[11] [Figure 13].
Figure 12 (A-C).
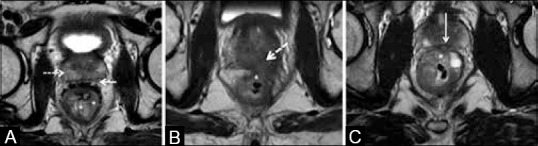
Axial T2W MRI. (A) No invasion: Solid arrow shows clear plane with prostate that shows normal signal intensity (dashed arrow). (B) Definite invasion: Tumor (*) invades prostate showing altered signal (arrow). (C) Possible invasion of prostate in midline (arrow) by tumor (*)
Figure 13 (A and B).
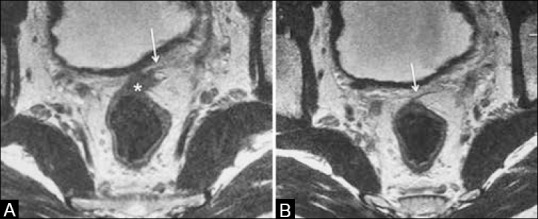
Axial T2W MRI. (a and b) The anterior peritoneal reflection (arrows) is invaded by a rectosigmoid tumor (*) –stage T4a; needs preoperative radiation to minimize local recurrence. Rectosigmoid tumors without peritoneal invasion are offered upfront surgery
Pitfalls in the T staging on MRI are as follows:
Difficulty in differentiating perirectal stranding due to desmoplastic reaction (T2) from borderline true tumor invasion (early T3). Nougaret et al.[11] recommend thinner sections and high-resolution imaging that can differentiate fine low signal intensity spicules suggestive of desmoplastic reaction from thicker intermediate signal intensity nodular bands of tumor [Figure 11B and C]. If it is still not possible to differentiate, any spiculation of the perirectal fat should be reported as “T2/early T3”[35]
Identification of the anterior peritoneal reflection invasion (T4a) in upper rectal tumors [Figures 4 and 13], which, if missed, can result in incorrect staging to T3. T4a tumors may require preoperative RT to minimize local recurrence.[11] High-resolution axial and sagittal images are required to visualize this.
Inability to conclusively establish adjacent organ invasion when there is “possible invasion” as described previously [Figure 12C]. Presence and extent of organ invasion influences the extent of resection requiring pelvic exenteration in advanced cases. Incorrect angulation while planning the sequences can cause blurring of the fat plane leading to misinterpretation of stage.
Circumferential resection margin
The MRF is the surgical plane limiting resection in TME surgery and is the potential CRM[11,35] [Figure 8]. It must be remembered that CRM is a term referring to the surgically dissected surface of the rectum corresponding to the non-peritonealized part of the rectum.[35] Hence, it is applicable to tumors below the peritoneal reflection of the rectum [Figure 3]. For upper rectal tumors, the CRM exists only posteriorly and in upper-mid rectal tumors, it is posterior and lateral [Figure 3B]. For example, an upper rectal tumor along the anterior wall invading the peritoneal reflection will be T4a, but CRM negative. In lower rectal cancers, CRM is circumferential [Figure 3B and 5]. In tumors close to the anorectal ring, where the MRF ends, the tumor relation to the levator ani is studied for the CRM status.[11]
The discussion on CRM is relevant only to T3 and higher stage tumors.[11] CRM is assessed by measuring the shortest distance between the outermost edge of the tumor and the MRF [Figure 8]. MRI with phased array coils is the most reliable technique to determine CRM invasion with high accuracy, high negative predictive value (NPV) of 94%, and a specificity of 94%.[33]
CRM can be negative, threatened, or positive, as described below:
A tumor–MRF distance >2 mm is CRM negative [Figure 14A].
A distance of <1 mm between the advancing tumor edge and MRF is indicative of a CRM-positive status [Figure 14B]. Also, CRM positivity could be due to tumor/perirectal nodes/deposits/tumor stranding[11] reaching <1 mm of the MRF [Figure 15A].
When the tumor/node/deposit–MRF distance is between 1 and 2 mm, the CRM is regarded as “threatened” [Figure 15B].
Figure 14 (A and B).
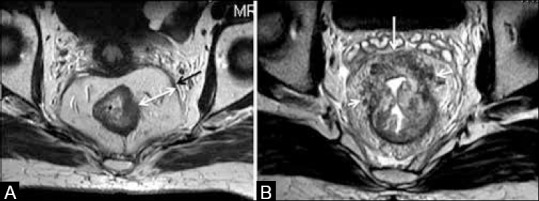
Axial T2W MRI. (A) Black arrow shows the mesorectal fascia (MRF). Distance between tumor and MRF is wide as shown by double-ended arrow (CRM −ve). (B) Perirectal tumoral stranding from 9 o’clock to 2o’clock position (arrows) reaching the MRF at 12o’clock position (CRM +ve)
Figure 15 (A and B).
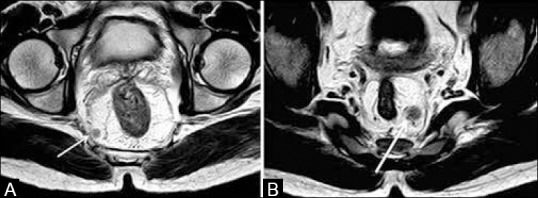
Axial T2W MRI. (a) Tumor deposit in the right mesorectal fat (arrow) located <1mm from the MRF (CRM +ve). (b) Left mesorectal node (arrow), 1-2 mm from the MRF indicating a threatened CRM. Node has heterogeneity and irregular margins
Importance of CRM
CRM is the best predictor of local recurrence and poor survival. The 5-year follow-up of the MERCURY study showed that when the CRM was involved on a preoperative MRI, the risk of local recurrence was significantly higher and the disease-free survival reduced.[17] Taylor et al. demonstrated that when MRI demonstrated a CRM >1 mm, the rate of local recurrence was as low as 3%.[12] CRM positivity/CRM threatened implies the need for treatment intensification with NACT-RT prior to restaging for assessing resectability.
Every MRI report needs to record the CRM status and the region where it is positive/threatened (at the precise o’clock position). The shortest distance between the tumor and MRF should be measured and recorded with the location (o’clock position). This information is needed for surgical planning. MRF is very close to the anterior rectal wall in lower third rectal cancers and tumors here are most likely to result in CRM positivity.
Extramural venous invasion
EMV refers to tumor invading veins within the mesorectal fat. It is applicable to tumors that are at least T3 and is not applicable to T1 or T2 tumors. It is a risk factor for local and distant recurrence as well as reduced survival,[13,14,15] requiring treatment intensification. It is well seen on MRI as intermediate signal intensity of tumor invading the vessel. This is seen as a rounded structure (in successive axial scans) or as an elongated structure (on coronal scans) in contiguity with the tumor [Figure 16]. The invaded vessel has similar T2W signal intensity and enhancement pattern as the tumor and may have a nodular outline. Expansion and disruption of the vessel also suggests EMV. Also, if the EMV is <1 mm from the MRF, CRM is regarded positive.[11]
Figure 16 (A and B).

(A) Axial T2W MRI and (B) coronal T2W MRI show rectal tumor (*) from 12o’clock to 7o’clock position with finger-like extramural venous invasion (EMV) into left perirectal veins (white arrows). Black arrow in (B) shows a metastatic left perirectal node
Nodal staging
MRI using the orthogonal T2W sequences has a sensitivity of 77% and a specificity of 71% for detecting nodal metastases, which is lower than that for T staging.[33] Metastatic mesorectal nodes [Figures 11, 15–17] are usually proximal to or at the level of the tumor.[36] Two MRI criteria in perirectal nodes favor metastases: (a) heterogeneity of signal intensity on T2W sequences and (b) irregular margins [Figure 15B]. Size criteria are unreliable as 30-50% metastatic nodes in rectal cancers are <5 mm in size.[37] However, a size ≥8 mm is unequivocally considered metastatic.[35]
Figure 17 (A and B).
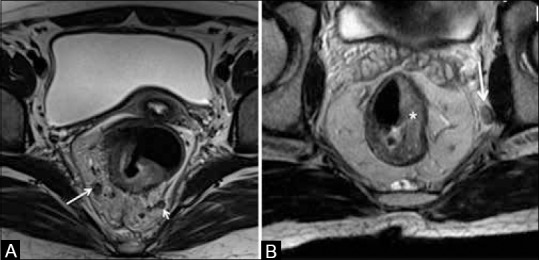
Axial T2 W MRI in (A) shows a 5-mm-sized right mesorectal node (long arrow) and a left mesorectal deposit <3mm (short arrow). (B) shows a T2 rectal tumor (*) with a left extramesorectal node (arrow)
Following endoanal resection of a malignant polyp (initially diagnosed as T1 rectal cancer), the final histopathology may reveal T1 tumor with poor prognostic factors, involved margins, or even a T2 tumor that would need a TME surgery. MRI is frequently ordered in such cases and may reveal reactive nodes following trans-anal excision of the polyp, which may be difficult to characterize. Perirectal inflammatory stranding may also be mistaken for a T3 tumor. Tailored treatment in such cases is given based on the multidisciplinary disease management group decision or patient's preference. It is not uncommon to give adjuvant (postoperative) RT, though evidence is limited.[38] Ultrasmall superparamagnetic iron oxide MRI (USPIO-MRI) would be useful as it is reported to improve specificity of detection of metastatic mesorectal nodes to 93% while the sensitivity remains the same as conventional MRI.[39,40] However, USPIO-MRI is not available for clinical use as it has not been approved by recommending agencies.
Extramesorectal nodes
The AJCC nodal staging depends on the number of abnormal nodes in the vicinity of tumor and not the nodal location [Table 1]. However, extramesorectal (pelvic sidewall) nodes along the iliac/obturator vessels need mention [Figures 11 and 17B]. Their presence influences the surgical and RT field planning. These are often treated only with preoperative RT that could sterilize nodes adequately and improve outcomes.[41] Larger nodes may require extension of the surgical field since they cannot be removed by performing a standard TME surgery.[11] Nodes with heterogeneous signal, irregular margins, and size ≥1 cm in short axis are suspicious.[11,18,35]
Sphincter complex
This issue is relevant to low rectal cancers or tumors extending into low rectum. MRI information on the involvement of the sphincter/anal complex helps predict sphincter-saving surgery that greatly impacts the quality of life. If sphincter invasion is seen, preoperative RT is indicated and can help achieve sphincter preservation by converting an APR into a low AR.[42]
MRI with small FOV coronal T2W sequences taken parallel to the anal canal axis helps excellent visualization of the sphincter complex [Figure 1B]. Pitfall in assessment arises from scan planes taken at an oblique axis to the anal canal. The distance of the lower limit of tumor from the AV helps decide sphincter involvement.
For tumors that are 5 cm or more above the AV, the sphincter is free
When the tumor is 0-5 cm from the AV, sphincter invasion needs mention. Tumor reaching upto internal sphincter is T2 disease[11,31] and can be offered an inter-sphincteric resection when not reaching the inter-sphincteric space and when at least 1 cm away from the AV [Figure 18A]. Tumors reaching upto or <1 cm from AV require an APR. Tumor invasion into inter-sphincteric space (T2 disease) or external sphincter (T3 disease) and into levator ani [Figure 18B–D] requires an extralevator APR after NACT-RT to ensure negative resection margins.[11] Figure 19 shows the incision lines of various surgical procedures.
Figure 18.
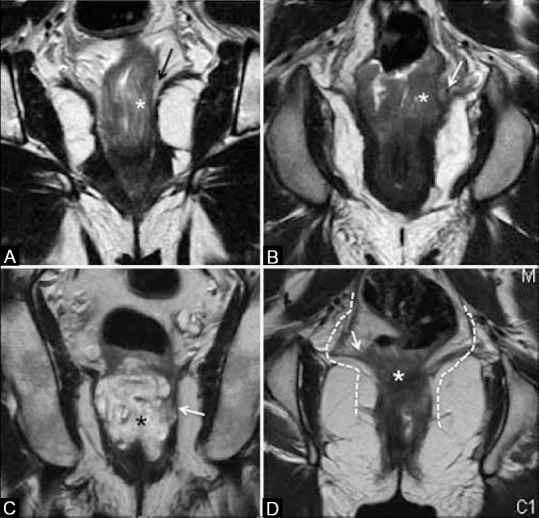
Coronal T2W MRI showing sphincter status. (A) Arrow shows intersphincteric space (ISS) spared by tumor (*). (B) Arrow shows ISS invasion. (C) Mucinous tumor (*) invades ISS andexternal sphincter (arrow). (D) Tumor (*) invades levator ani (arrow). Dashed lines show incision for extralevator abdomino-perineal resection
Figure 19.
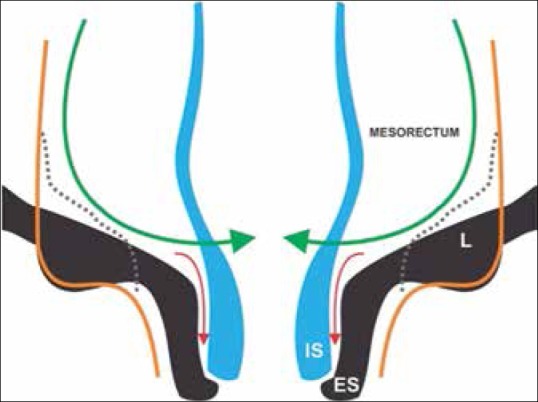
Incision lines for surgical procedures: Anterior resection (green); intersphincteric resection (red), APR (dotted), extralevator APR (orange). Sphincter is removed in the latter two surgeries. ES: External sphincter, IS: Internal sphincter
Distance from pelvic wall
In CRM-positive and advanced T4 disease, the distance from the lateral pelvic wall, adherence to presacral fascia, and contiguity to the penile bulb need to be specified to assess resectability status.
MRI report template for primary staging
MRI also influences RT planning. Table 4 gives a list of MRI findings that are treated with preoperative RT at our institute. An optimal MRI report should indicate all such features to assist adequate therapy. Pretreatment MRI is critical for adequately planning the RT portals, which are defined as per the T category and nodal location. For T3 tumors, the RT portal includes the internal iliac group of lymph nodes, whereas in T4 tumors, the external iliac group of lymph nodes is also included.
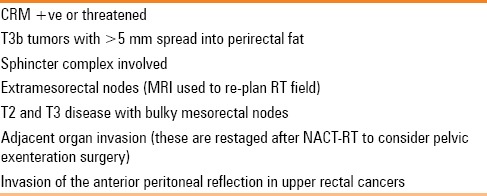
Table 4.
MRI findings that justify preoperative chemo-radiation
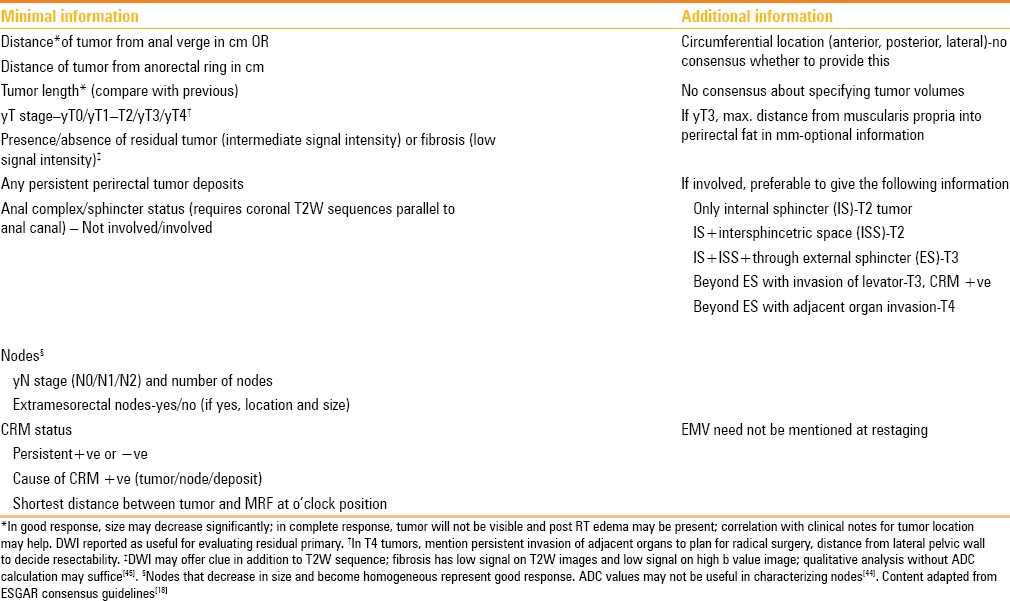
The minimal MRI report and additional information adapted from the ESGAR consensus guidelines[18] are given in Table 5. Nougaret et al. have designed an elegant mnemonic for a complete synoptic report,[11] called “DIS T A N C E” (Dis = distance from the AV, T = T stage, A = anal/sphincter complex, N = nodal status, C = CRM, E = EMV), which can be used to recall the guideline information in Table 5.
Table 5.
Synoptic MRI report (checklist) for primary staging

Imaging for restaging rectal cancer
Choice of imaging method
Although studies have reported MRI to accurately downstage tumor, predict the grade of tumor regression, and complete the pathologic response based on T2W signal intensity changes,[43,44] this could not be reproduced in the MERRION study.[45] PET/CT has been evaluated for response assessment after preoperative CT-RT in rectal cancer and promising results have been reported in a systematic review and meta-analysis.[46,47] PET/CT is also useful in poorly differentiated cancers with local progression to detect systemic metastasis. It is, however, not sensitive for lesions less than 1 cm and may not detect small peritoneal deposits.
Currently, MRI is the technique of choice for local restaging following NACT-RT and addition of a DWI sequence is useful.[11,18,48,49,50,51,52] The use of routine contrast-enhanced T1W images is currently not recommended even in the post treatment assessment protocol.[18] Post-treatment stage is indicated by the prefix “y.” Accuracy of MRI for predicting yT stage is 50% and for CRM at restaging is 66%, although the NPV is higher.[11] This may lead to overtreatment, but reduces the chances of positive resection margins. Accuracy for yN stage is 65%.[11] Despite this, response assessment with MRI helps assess resectability, plan for sphincter saving and radical surgery, and decide the need for further chemotherapy.
DWI-MRI added to T2W sequences helps evaluate the response of the primary lesion, but was less useful in predicting response in the nodes.[18,48] DWI-MRI also had high NPV in ruling out complete responders, but the positive predictive value was lower.[51] Residual primary tumor is inferred when the signal intensity of the lesion visible on T2W images is high on the b 1000 s/mm2 image and low on the ADC (apparent diffusion coefficient) map.[49] Using these qualitative criteria, Park et al. reported that it improved prediction of tumor clearance from the MRF after NACT-RT and significantly improved consistency among readers.[49] Quantitative measurements using ADC have not been standardized and are difficult to interpret.[50] Heijnen et al. also found DWI-MRI to be unreliable in differentiating between benign and metastatic nodes as their ADC values overlapped.[48]
There is also research in progress evaluating the accuracy of dynamic contrast-enhanced MRI (DCE-MRI) in detecting response in rectal cancer. Alberda et al. found it a useful tool for nodal staging, but not for tumor stage or CRM involvement or detecting complete response.[53] Lymph node specific MRI contrast agent (gadofosveset-enhanced MRI) has also been evaluated for nodal restaging in rectal cancer with reported high performance.[54]
Evaluation of response on MRI
Ideally, response is evaluated by comparing with pre NACT-RT MRI. However, if the pretherapy MRI is not available, the disease stage and information for surgical planning could be described in the post-treatment MRI (that is ordered 6-8 weeks after completion of NACT-RT). Tumor response (yT stage and CRM) and nodal response (yN stage) are assessed by studying the T2 signal intensity. The appearances can be challenging to interpret due to post-therapy changes that include the following:
Edematous submucosa that appears uniformly T2 hyperintense masking the residual tumor [Figure 20A]. Precise DRE notes are invaluable in assessing the MR images
Intense perirectal stranding may be difficult to evaluate. Dark hypointensity in the stranding could represent fibrosis, particularly if the previous stranding showed intermediate signal intensity
Thickening of the MRF circumferentially could be due to post RT fibrosis [Figure 20B]
Darkly hypointense tissue extending into the mesorectal fat may represent fibrosis, but could harbor small residual tumor [Figure 21A and B]. Intermediate signal intensity usually represents residual tumor, but cannot rule out fibro-inflammatory response. Only complete disappearance of the tumor with a normal two-layered rectal wall is a sign of yT0 [Figure 20A], i.e. complete pathologic response.[18]
Figure 20 (A and B).
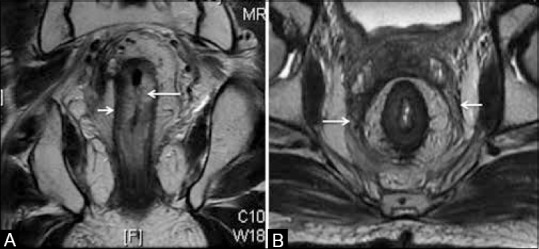
Post RT appearances. (A) Coronal T2W MRI shows thickened hyperintense submucosa due to edema (long arrow) with the intact muscularis (short arrow). (B) Axial T2W MRI with diffusely thickened mesorectal fascia (arrows)
Figure 21.
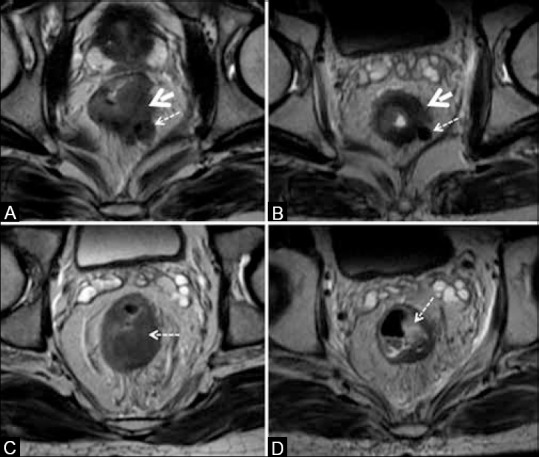
Tumor response. (A and B) The tumor (solid arrows) and node (dashed arrows) with intermediate signal in (A) show darkly hypointense signal in (B); both regressed in size. (C and D) Nonmucinous tumor (arrow) in (c) shows mucinous degeneration seen as hyperintense focus (arrow) in (D)
Response is assessed by the following:
Studying tumor signal intensity in the submucosa, muscularis propria, and extramural component. If the outer surface of the muscularis is intact with a complete dark hypointense ring with no mesorectal extension, the tumor is yT2 stage; but persistent mesorectal extension represents a tumor of yT3 stage [Figure 21]. Non-mucinous tumors may show response in the form of mucinous degeneration.[11] This is seen as pools of mucin that are homogeneously brightly hyperintense areas on T2W-MRI [Figure 21C and D]. Mucinous tumors may respond by disappearance of the previous intermediate signal intensity areas (persistence of which signifies non-response).
Regression in tumor size: (a) by measuring the craniocaudal extent in cm; (b) there is no consensus on using tumor volumetry measured with dedicated software.[18]
Regression in CRM status: if a well-defined fat plane (that was previously absent) appears between the MRF and the stranding, it represents response.[11,18]
Regression in nodal size with homogeneity replacing previous heterogeneity is an indicator of sterilized node[18] [Figure 21B]. Increase in size and number of nodes indicates progression.
Synoptic MRI report for restaging
The synoptic MR report for restaging after NACT-RT treatment is provided in Table 6.
Table 6.
Synoptic MR report (checklist) for restaging after preoperative chemo-radiation
Conclusion
High-resolution phased array external MRI is the investigation of choice for local issues in the primary staging of rectal cancer as well as for restaging after NACT-RT. It provides the highest accuracy for issues in pretreatment local staging, while MDCT chest, abdomen, and pelvis is used for metastatic workup. Non-fat suppressed T2W MRI sequences in sagittal, axial, and coronal planes are mandatory. Contrast-enhanced MRI is not recommended.
Acknowledgements
Mr Wagh, Scientific Officer, Tata Memorial Hospital, Mumbai
Mr Nilesh Ganthade, Medical Artist, Tata Memorial Hospital, Mumbai.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Beets-Tan RG, Beets GL. Rectal cancer: Review with emphasis on MR imaging. Radiology. 2004;232:335–46. doi: 10.1148/radiol.2322021326. [DOI] [PubMed] [Google Scholar]
- 2.Sagar PM, Pemberton JH. Surgical management of locally recurrent rectal cancer. Br J Surg. 1996;83:293–304. doi: 10.1002/bjs.1800830305. [DOI] [PubMed] [Google Scholar]
- 3.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;2:996–9. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 4.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 5.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 7.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 8.Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, et al. Effectiveness of preoperative staging in rectal cancer: Digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23–9. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Joint Committee on Cancer Staging. Colon and Rectum Cancer Staging. 7th ed. [Last accessed on 2015 Mar 14]. Available from: https://cancerstaging.org/referencestools/quickreferences/Documents/ColonMedium.pdf .
- 10.Beets-Tan RG, Beets GL. Local staging of rectal cancer: A review of imaging. J Magn Reson Imaging. 2011;33:1012–9. doi: 10.1002/jmri.22475. [DOI] [PubMed] [Google Scholar]
- 11.Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: Have you checked the “DISTANCE”? Radiology. 2013;268:330–44. doi: 10.1148/radiol.13121361. [DOI] [PubMed] [Google Scholar]
- 12.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. MERCURY Study Group. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98:872–9. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- 13.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517–22. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 14.Koh DM, Smith NJ, Swift RI, Brown G. The relationship between MR demonstration of extramural venous invasion and nodal disease in rectal cancer. Clin Med Oncol. 2008;2:267–73. doi: 10.4137/cmo.s370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619–23. doi: 10.1016/j.crad.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Engelen SM, Maas M, Lahaye MJ, Leijtens JW, van Berlo CL, Jansen RL, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49:2311–20. doi: 10.1016/j.ejca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 18.Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: Recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522–31. doi: 10.1007/s00330-013-2864-4. [DOI] [PubMed] [Google Scholar]
- 19.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–65. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 20.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: Local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–83. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 21.Beaumont C, Pandey T, Gaines Fricke R, Laryea J, Jambhekar K. MR evaluation of rectal cancer: Current concepts. Curr Probl Diagn Radiol. 2013;42:99–112. doi: 10.1067/j.cpradiol.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Harewood GC. Assessment of publication bias in the reporting of EUS performance in staging rectal cancer. Am J Gastroenterol. 2005;100:808–16. doi: 10.1111/j.1572-0241.2005.41035.x. [DOI] [PubMed] [Google Scholar]
- 23.Vliegen R, Dresen R, Beets G, Daniels-Gooszen A, Kessels A, van Engelshoven J, et al. The accuracy of Multi-detector row CT for the assessment of tumor invasion of the mesorectal fascia in primary rectal cancer. Abdom Imaging. 2008;33:604–10. doi: 10.1007/s00261-007-9341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizlin ZV, Brown JA, So G, Brown C, Phang TP, Walker ML, et al. Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer? Dis Colon Rectum. 2010;53:308–14. doi: 10.1007/DCR.0b013e3181c5321e. [DOI] [PubMed] [Google Scholar]
- 25.McQueen AS, Scott J. CT staging of colorectal cancer: What do you find in the chest? Clin Radiol. 2012;67:352–8. doi: 10.1016/j.crad.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Tan KK, Lopes Gde L, Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13:642–8. doi: 10.1007/s11605-008-0757-7. [DOI] [PubMed] [Google Scholar]
- 27.Cipe G, Ergul N, Hasbahceci M, Firat D, Bozkurt S, Memmi N, et al. Routine use of positron-emission tomography/computed tomography for staging of primary colorectal cancer: Does it affect clinical management? World J Surg Oncol. 2013;11:49. doi: 10.1186/1477-7819-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NCCN Guidelines. Rectal Cancers Version 3.2014.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). NCCN.org [Google Scholar]
- 29.Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, et al. MR imaging for preoperative evaluation of primary rectal cancer: Practical considerations. Radiographics. 2012;32:389–409. doi: 10.1148/rg.322115122. [DOI] [PubMed] [Google Scholar]
- 30.Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, et al. Rectal cancer: MR imaging in local staging--is gadolinium-based contrast material helpful? Radiology. 2005;234:179–88. doi: 10.1148/radiol.2341031403. [DOI] [PubMed] [Google Scholar]
- 31.Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW. Evaluation of gadolinium-enhanced T1-weighted magnetic resonance imaging in the preoperative assessment of local staging in rectal cancer. Colorectal Dis. 2010;12:1139–48. doi: 10.1111/j.1463-1318.2009.01959.x. [DOI] [PubMed] [Google Scholar]
- 32.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR. Can endoscopic ultrasound predict early rectal cancers that can be resected endoscopically? A meta-analysis and systematic review. Dig Dis Sci. 2010;55:1221–9. doi: 10.1007/s10620-009-0862-9. [DOI] [PubMed] [Google Scholar]
- 33.Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–23. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 34.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. MERCURY Study Group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann Surg. 2011;253:711–9. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 35.Al Sukhni E, Milot M, Friutman M, Brown G, Schmocker S, Kennedy E. User's guide for the Synoptic MRI report for Rectal Cancer. Cancer Care Ontario. Canadian Cancer Society. [Last accessed on 2014 Jun 13]. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=133269 .
- 36.Engelen SM, Beets-Tan RG, Lahaye MJ, Kessels AG, Beets GL. Location of involved mesorectal and extramesorectal lymph nodes in patients with primary rectal cancer: Preoperative assessment with MR imaging. Eur J Surg Oncol. 2008;34:776–81. doi: 10.1016/j.ejso.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Kotanagi H, Fukuoka T, Shibata Y, Yoshioka T, Aizawa O, Saito Y, et al. The size of regional lymph nodes does not correlate with the presence or absence of metastasis in lymph nodes in rectal cancer. J Surg Oncol. 1993;54:252–4. doi: 10.1002/jso.2930540414. [DOI] [PubMed] [Google Scholar]
- 38.Wagman R, Minsky BD, Cohen AM, Saltz L, Paty PB, Guillem JG. Conservative management of rectal cancer with local excision and postoperative adjuvant therapy. Int J Radiat Oncol Biol Phys. 1999;44:841–6. [PubMed] [Google Scholar]
- 39.Koh DM, George C, Temple L, Collins DJ, Toomey P, Raja A, et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: Findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol. 2010;194:W505–13. doi: 10.2214/AJR.08.1819. [DOI] [PubMed] [Google Scholar]
- 40.Lahaye MJ, Engelen SM, Kessels AG, de Bruïne AP, von Meyenfeldt MF, van Engelshoven JM, et al. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: Predictive criteria. Radiology. 2008;246:804–11. doi: 10.1148/radiol.2463070221. [DOI] [PubMed] [Google Scholar]
- 41.Shihab OC, Taylor F, Bees N, Blake H, Jeyadevan N, Bleehen R, et al. MERCURY Study Group. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. 2011;98:1798–804. doi: 10.1002/bjs.7662. [DOI] [PubMed] [Google Scholar]
- 42.Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236–42. doi: 10.1097/SLA.0b013e318195e17c. [DOI] [PubMed] [Google Scholar]
- 43.Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–60. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 44.Kluza E, Rozeboom ED, Maas M, Martens M, Lambregts DM, Slenter J, et al. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur Radiol. 2013;23:253–61. doi: 10.1007/s00330-012-2578-z. [DOI] [PubMed] [Google Scholar]
- 45.Hanly AM, Ryan EM, Rogers AC, McNamara DA, Madoff RD, Winter DC MERRION Study Group. Multicenter evaluation of rectal cancer reimaging pOst neoadjuvant (MERRION) therapy. Ann Surg. 2014;259:723–7. doi: 10.1097/SLA.0b013e31828f6c91. [DOI] [PubMed] [Google Scholar]
- 46.Memon S, Lynch AC, Akhurst T, Ngan SY, Warrier SK, Michael M, et al. Systematic review of FDG-PET prediction of complete pathological response and survival in rectal cancer. Ann Surg Oncol. 2014;21:3598–607. doi: 10.1245/s10434-014-3753-z. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Lan X, Yuan H, Feng H, Xia X, Zhang Y. 18F-FDG PET predicts pathological response to preoperative chemoradiotherapy in patients with primary rectal cancer: A meta-analysis. Ann Nucl Med. 2014;28:436–46. doi: 10.1007/s12149-014-0837-6. [DOI] [PubMed] [Google Scholar]
- 48.Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, et al. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23:3354–60. doi: 10.1007/s00330-013-2952-5. [DOI] [PubMed] [Google Scholar]
- 49.Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: Added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology. 2011;260:771–80. doi: 10.1148/radiol.11102135. [DOI] [PubMed] [Google Scholar]
- 50.Boone D, Taylor SA, Halligan S. Diffusion weighted MRI: Overview and implications for rectal cancer management. Colorectal Dis. 2013;15:655–61. doi: 10.1111/codi.12241. [DOI] [PubMed] [Google Scholar]
- 51.Sassen S, de Booij M, Sosef M, Berendsen R, Lammering G, Clarijs R, et al. Locally advanced rectal cancer: Is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol. 2013;23:3440–9. doi: 10.1007/s00330-013-2956-1. [DOI] [PubMed] [Google Scholar]
- 52.Hötker AM, Garcia-Aguilar J, Gollub MJ. Multiparametric MRI of rectal cancer in the assessment of response to therapy: A systematic review. Dis Colon Rectum. 2014;57:790–9. doi: 10.1097/DCR.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 53.Alberda WJ, Dassen HP, Dwarkasing RS, Willemssen FE, van der Pool AE, de Wilt JH, et al. Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis. 2013;28:573–80. doi: 10.1007/s00384-012-1576-6. [DOI] [PubMed] [Google Scholar]
- 54.Heijnen LA, Lambregts DM, Martens MH, Maas M, Bakers FC, Cappendijk VC, et al. Performance of gadofosveset-enhanced MRI for staging rectal cancer nodes: Can the initial promising results be reproduced? Eur Radiol. 2014;24:371–9. doi: 10.1007/s00330-013-3016-6. [DOI] [PubMed] [Google Scholar]


