Abstract
18Fluorine-2-fluoro-2-Deoxy-d-glucose (18F-FDG) positron emission tomography/computerized tomography (PET/CT) is a well-established functional imaging method widely used in oncology. In this article, we have incorporated the various indications for 18FDG PET/CT in oncology based on available evidence and current guidelines. Growing body of evidence for use of 18FDG PET/CT in select tumors is also discussed. This article attempts to give the reader an overview of the appropriateness of using 18F-FDG PET/CT in various malignancies.
Keywords: Cancer, 18Fluorine-2-fluoro-2-deoxy-d-glucose, guidelines for PET/CT, oncology, positron emission tomography/computerized tomography, positron emission tomography
Introduction
Positron emission tomography/computerized tomography (PET/CT) is a technological advancement having a significant impact in oncology. 18Fluorine-2-fluoro-2-deoxy-D-glucose (18F-FDG) is an expensive, short-lived radiopharmaceutical used for PET/CT scanning and is synthesized in a cyclotron by a complex process. The net result is an expensive modality. However, with rising concerns of expenditure and radiation exposure, it becomes necessary to critically evaluate the utility of this modality. Health technology assessment boards of several countries have evaluated available evidence to decide on the appropriateness and cost-effectiveness of 18F-FDG PET/CT in cancer. In 2010, the International Atomic Energy Agency (IAEA) pooled expert evidence in a manual named “Appropriate use of PET/CT for the management of cancer patients.”[1] The National Comprehensive Cancer Network (NCCN), which represents a group of cancer institutions in the USA, frames guidelines for tumor groups. “Society of Nuclear Medicine and Molecular Imaging” (SNMMI), a professional body in the USA, produced a summary based on NCCN practice guidelines in March 2013.[2]
This article attempts to familiarize the reader with current appropriateness criteria for using 18F-FDG PET/CT in various malignancies based on evidence. The NCCN guidelines are based on availability of reimbursement for FDG PET/CT for various indications. The IAEA appropriateness criteria are recommendations based on the evidence published prior to 2009. This article amalgamates the recommendations of both guideline bodies. Attempt has been made to add evidence generated from 2010 till date and its possible impact on the appropriateness criteria.
Generally, to consider an investigation “appropriate,” there must be a clear evidence of better diagnostic performance with higher sensitivity, specificity, and accuracy compared to conventional imaging or existing standard of care. The information obtained from the investigation should change the clinical practice and impact patient outcome. The change could be in the form of adopting better therapeutic strategies or elimination of ineffective, morbid, and expensive practices. “Potentially appropriate” refers to evidence of clear radiological superiority compared to current imaging, but with inadequate evidence about impact on treatment and outcome. “Possibly appropriate” refers to a situation where there is inadequate evidence for use despite a large number of well-planned studies, but a strong rationale exists due to clinical benefit from PET shown by case reports or inadequately performed studies. “Inappropriate” refers to a situation where performance of PET is inferior to that of conventional imaging.[1]
The various utilities of PET/CT in oncology can be categorized and defined as follows:[1]
Diagnosis
To characterize a lesion to suggest whether it is benign or malignant
For the detection of a possible primary when the patient presents with metastases
To identify an appropriate site from which a biopsy would yield adequate representative tissue for diagnosis
Detection of malignancy when tumor markers are abnormal
Staging
After the histological diagnosis, to assess the extent of disease before the start of treatment
Response Evaluation
Assessment of response to treatment during or after therapy
Restaging
Assessment of the extent of the disease after treatment or after confirmed recurrence
Suspected Recurrence
Assessment of disease following clinical or biochemical suspicion of recurrence
Follow-up or Surveillance
Assessment of disease in the absence of critical evidence of recurrence
Radiotherapy Planning (RT)
When the study is used for contouring and planning the radiation fields.
The evidence discussed is based on the superior performance of FDG PET or FDG PET/CT compared to contrast-enhanced CT (CECT), which in most situations represents the conventional choice of imaging. CECT may be merged with FDG PET as FDG PET/CECT, as being practiced in many centers, which has shown improved radiological performance compared to FDG PET or FDG PET/CT in a few tumors.
The appropriate timing of the PET/CT study for maximum accuracy is important. To avoid false-positive, results, the best time to perform a PET/CT study is 8-12 weeks after completion of chemotherapy and radiotherapy. Postoperative inflammatory changes are seen till about 12 weeks or, at times, longer. The effect of colony stimulating factor (administered to stimulate production of blood cells after chemotherapy) is seen as intense metabolic activity in the marrow of the bones. This effect is less pronounced after 3 weeks. Ideal timing for performing a postoperative PET/CT study is also after 12 weeks.
Role of FDG PET/CT in Various Cancers
Lymphoma
There is an overwhelming body of evidence regarding the use of PET/CT in staging, restaging, and post-therapy evaluation in Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (NHL), except in a few low-grade lymphomas like small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL), marginal zone lymphoma (MZL), mucosa-associated lymphoid tissue (MALT), cutaneous T-cell lymphoma (CTCL), mycosis fungoides, and Sezary syndrome.[1,2,3]
FDG PET leads to upstaging of disease in about 30% of cases at initial staging[4] [Figure 1]. The sensitivity and specificity of PET/CT is higher as compared to CECT for detection of nodal as well as extranodal disease.[5,6] The International Harmonization Project recommends the use of a baseline FDG PET in HL and aggressive NHL.[7] There is no added advantage of adding CECT in staging or combining it with PET/CT.[8] This only increases the cost and radiation burden. It is also helpful in management of residual masses that are seen after completion of therapy by correctly predicting the presence or absence of viable disease [Figure 2]. CECT is the imaging modality of choice in staging all low-grade lymphomas,[9] except in MALT where FDG PET/CT may have a potential role.[10]
Figure 1 (A-F).
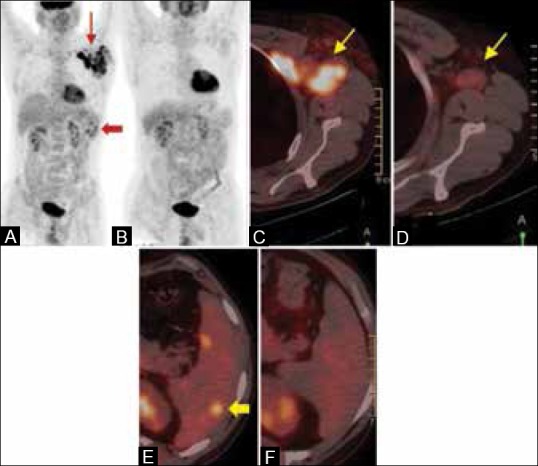
A case of Hodgkin's lymphoma. (A) The maximum intensity projection (MIP) PET pretreatment scan shows bulky left axillary adenopathy (arrows) and hypermetabolic splenic lesions - disease upstaged (block arrow). (B) MIP image of the same patient done after two cycles of chemotherapy (interim response assessment) shows complete metabolic resolution. The fused PET/CT image in the pretreatment scan (C) shows hypermetabolic left axillary nodes. The interim scan in (D) shows residual morphologic nodes with no significant FDG uptake. Hypermetabolic splenic lesions in (E) show complete metabolic and morphologic resolution in (F)
Figure 2 (A and B).
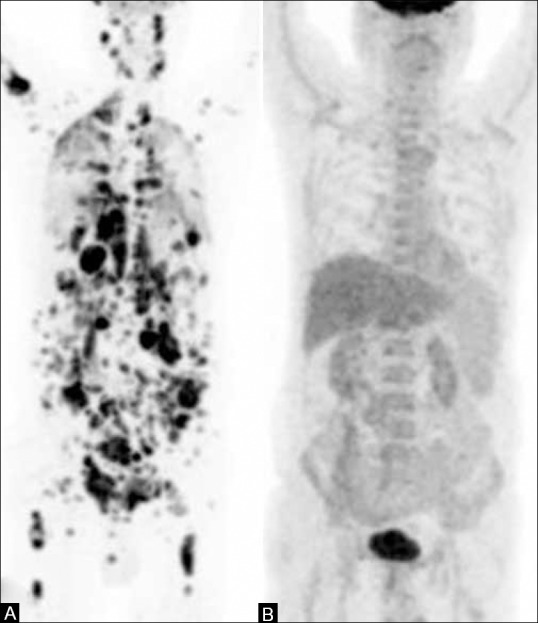
A 65-year-old male, a case of NHL. (A) The pretreatment PET MIP image shows extensive nodal and extranodal involvement (B) PET scan done after completion of six cycles of chemotherapy (post-treatment response assessment) shows complete metabolic resolution of disease
Bone marrow biopsy is an integral part of the work-up of lymphoma. FDG PET/CT has shown increased sensitivity in detection of marrow involvement, especially when the disease is focal in nature. Interim PET or early treatment response after two cycles of chemotherapy helps in prognostication and may modify treatment in some lymphomas[11] [Figure 1], if there is no response or there is progression of the disease seen on PET/CT.
Bone and soft tissue tumors
Both NCCN and IAEA recommend the use of PET in staging, restaging, and in surveillance with options of using either PET or bone scan.[1,2] A recent meta-analysis has shown that FDG PET is a valuable tool for staging and detecting recurrences in patients with Ewing's sarcoma (ES).[12] The pooled sensitivity and specificity of FDG PET/CT in detection of ES is 95%. It is more sensitive in detection of recurrent bone lesions, as compared with other imaging modalities.[13,14,15,16] It is also superior to bone scintigraphy and magnetic resonance imaging (MRI) in detection of bone metastases and may lead to change in management.[16] Earlier studies have reported low sensitivity of PET as compared to CT for detection of pulmonary metastases.[15,16,17] But now, with the availability of PET with diagnostic quality CT, lung nodules can be diagnosed using the CT component of PET/CT with similar accuracy, making it a one-stop-shop staging modality. A prospective multicenter trial has shown the usefulness of PET in detection of primary tumor and metastases with higher sensitivity and specificity as compared to conventional imaging modalities in ES, osteosarcoma, and rhabdomyosarcoma.[16]
There is growing evidence to suggest the role of FDG PET/CT in predicting the response to neoadjuvant chemotherapy (NACT) prior to surgery in osteosarcoma with a pooled sensitivity and specificity of 73% and 86%, respectively.[18] FDG PET/CT has shown promise in demonstrating sarcomatous change in osteochondromas by identifying the hypermetabolic sarcomatous focus.[19] It has also demonstrated clinical impact and outcome in chondrosarcomas.[20]
Breast cancer
FDG PET/CT is useful in providing more accurate metastatic (M) stage and metabolic information in locally advanced breast cancer and inflammatory breast cancer. It has limited/no role in initial detection of the primary and axillary nodes. It also predicts outcome after NACT.[21,22]
Gastrointestinal and hepatobiliary cancers
Though the NCCN and IAEA do not recommend the use of PET/CT in hepatocellular cancer (HCC), the role of functional imaging with PET/CT and PET/MR is gradually evolving. Triple-phase CECT and MRI are the gold standard for diagnosis and staging of HCC.[23] The uptake of FDG in HCC is dependent on the size and differentiation with well-differentiated tumors showing low FDG avidity and more undifferentiated tumors showing high FDG uptake.[24] It is useful in the detection of multifocal lesions and extra-hepatic disease with detection rates of 83-89.6%.[25,26,27] Preoperative PET helps in predicting tumor differentiation and post-surgical outcome. Higher standardized uptake values (SUVs) are associated with poor differentiation and worse prognosis.[28,29] PET is used in pre-transplant evaluation in HCC, with high recurrence rates seen in patients with positive PET.[30]
The work-up of cholangiocarcinoma is mainly by CECT and MRI with cholangio-pancreatography. Few studies have evaluated the role of PET/CT in diagnosis and staging of cholangiocarcinoma. PET has high sensitivity for the detection of intrahepatic cholangiocarcinoma, whereas the sensitivity is very low for extrahepatic cholangiocarcinoma (91-95% vs 60%).[31,32] Reported sensitivities for detection of distant metastases are in the range of 65-100%, with very low sensitivity for detection of lymph nodal metastases.[33,34] PET/CT is useful in detection of recurrences where anatomical imaging has limited use due to post-surgical distortion and fibrosis, with sensitivity and specificity of 94% and 100%, respectively, for PET/CT and 82% and 43%, respectively, for CT.[35]
There is scarcity of literature on the role of PET/CT in evaluation of gall bladder cancer, primarily because most of these are detected incidentally. FDG PET/CT seems to have a potential role in staging, as these cancers are intensely FDG avid. PET/CT has an overall diagnostic accuracy of 95.9% for the primary disease and 85.7% and 95.9% for the detection of lymph nodes and metastatic disease, respectively.[36] In our own experience, PET/CT scores over multi-detector CT (MDCT) in detection of occult metastatic disease.[37]
Pancreatic cancer
The primary imaging modality in staging pancreatic carcinoma is MDCT or MRI with/without MRCP (Magnetic Resonance cholangiopancreatography). FDG PET/CT may have a role in triaging patients for curative versus palliative treatment depending on the absence or presence of metastasis in locally advanced disease.[38] A recent meta-analysis has shown pooled sensitivity of 90% and specificity of 76% for FDG PET/CT. It has no additional benefit over the current conventional imaging modalities in diagnosing primary pancreatic cancer.[39] Current evidence regarding the role of FDG PET/CT in the evaluation of intraductal papillary mucinous neoplasms (IPMN) is limited.
Gastric cancer
CT is the modality of choice in defining resectability of gastric cancer due to exquisite anatomic detail, whereas FDG PET/CT has a role in predicting the biological behavior and in prognostication based on the metabolic activity.[40,41,42] PET/CT has low sensitivity for detection of the primary gastric tumor[43,44] because of normal physiological uptake of FDG in the stomach and due to certain histologies like signet cell and mucinous adenocarcinomas which are low FDG avid.[45] For nodal (N) staging, PET/CT has similar diagnostic performance as compared to CECT, with a sensitivity of 50% for detection of N2, N3 disease and a specificity of about 90%.[44,46,47] CECT has higher sensitivity than PET/CT (77% vs 35%) and lower specificity than PET/CT (92% vs 99%) for the detection of peritoneal metastases.[48] PET/CT has moderate sensitivity and specificity in the detection of recurrent gastric cancers, with pooled sensitivity of 76% and 86% and specificity of 82% and 88%, respectively.[49,50]
Colorectal cancer
The standard imaging work-up includes CT for colonic cancer, and MRI and CT with or without endorectal ultrasound for rectal cancer. NCCN guidelines recommend the use of FDG PET/CT as a problem-solving tool in the initial staging of colorectal cancer (CRC).[1,51] It is particularly useful in characterizing indeterminate liver lesions on CT. There is emerging evidence to suggest the role of PET/CT in evaluating extrahepatic disease before the surgical resection of liver metastases. PET/CT is more sensitive than CT for detection of extrahepatic disease with equal specificity.[52] There has been conflicting evidence regarding the use of PET/CT for response assessment post neoadjuvant chemo-radiotherapy (NACT-RT) in locally advanced rectal cancer.[53,54] But a recent systematic review has shown that PET predicts early response to NACT-RT with high accuracy.[55] However, PET/CT has an undisputed role in the evaluation of recurrent CRC with elevated CEA (carcinoembryonic antigen) and often with equivocal/negative CT [Figure 3]. A recent meta-analysis has quoted pooled sensitivity of 94.1% and specificity of 77.2% of PET/CT for detection of recurrence.[56]
Figure 3 (A-C).
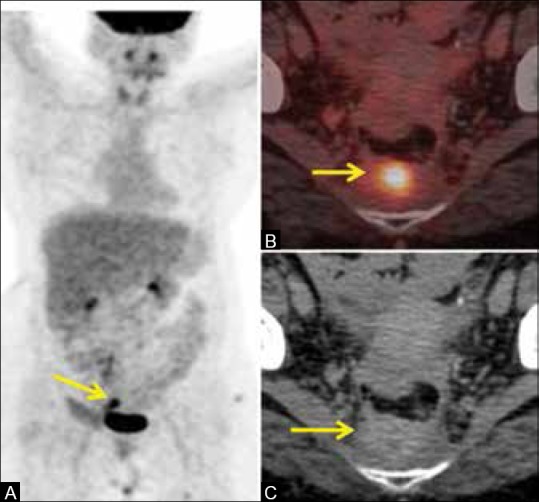
A case of carcinoma rectum post abdomino-perineal resection, 9 months post surgery with increasing CEA. (A) The MIP image of the PET scan shows a hypermetabolic focus in the pelvis (arrow) (B) Fused PET/CT image shows FDG-avid pre-sacral mass (arrow) suspicious for recurrent disease (C) CT image shows a pre-sacral mass. Indeterminate whether it is benign fibrosis or disease recurrence. Biopsy confirmed recurrence of adenocarcinoma
Gastrointestinal stromal tumors
FDG PET/CT has a definite role in staging and evaluation of the response to imatinib mesylate in gastrointestinal stromal tumors (GIST). CT-based Response Evaluation in Solid Tumors (RECIST) criteria are not very useful is assessing the treatment response as the size may not change in these tumors; whereas responses on PET/CT based on change in the metabolic activity are in tandem with the clinical response.[57,58,59]
Anal cancer
PET/CT can be considered for staging of anal cancer as it alters the stage as compared to conventional imaging modalities in 20-25 % cases.[60,61,62] The role of FDG PET/CT in RT planning in delineation of the target volume is evolving.[63]
Cervical cancer
Lymph nodal involvement is an important prognostic factor in cervical cancer management. FDG PET/CT has a potential role in detection of lymph nodal metastasis in locally advanced cervical carcinoma (≥IB2) in which extra-pelvic spread is common. PET can detect nodal metastasis when CT findings are normal.[64,65,66] In a retrospective study, Grigsby et al. compared CT and FDG PET in the evaluation of nodal metastasis and demonstrated that PET picked up more number of nodal metastasis as compared to CT and also that PET is a better predictor of survival.[66] PET/CT is valuable in treatment planning with external beam RT and brachytherapy. PET results in extension of the involved field radiation in about 18% of cases.[67] In brachytherapy, PET improves the tumor coverage without increasing the dose to the surrounding critical structures.[68] PET/CT has high accuracy of 92% in detection of recurrent disease with a sensitivity of 82% and specificity of 97%.[69] The role of predictive volumetric parameters derived from PET, such as metabolic tumor volume and total lesion glycolysis, is evolving.[70]
Ovarian cancer
CT is the primary imaging modality in the work-up of ovarian cancer. PET/CT has no additional benefit over CT in early ovarian cancer, but is useful in differentiating stage IIIC–IV from other stages with a sensitivity of 100% and specificity of 91%, whereas CT has a sensitivity of 97% and specificity of 64%.[71] PET has a higher sensitivity in the detection of peritoneal deposits and metastasis in normal-sized nodes. It is useful in the detection of recurrent ovarian cancer with increasing cancer antigen 125 (CA125) levels and negative CT or MRI, showing a high sensitivity and specificity of 94.5% and 100%, respectively.[72,73] A recent meta-analysis has shown that PET/CT has the highest sensitivity of 91% when compared with CA125, CT, and MRI.[74]
Head and neck cancers
FDG PET has a well-established role in the detection of distant metastasis and second primary in locally advanced head and neck cancers. In a recent meta-analysis, Xu et al. have shown PET and PET/CT to have a sensitivity of 83% and specificity of 96% for detection of distant malignancies, whereas for conventional imaging, the values are 44% and 96%, respectively.[75,76] Detection of distant metastasis early in the work-up algorithm leads to change in the treatment plan and in prognosis. A negative PET/CT study has nearly 100% accuracy in predicting the absence of distant metastatic disease and second primary.[77,78] Accurate response assessment after chemo-radiotherapy (CRT) is essential to plan salvage surgery in patients with advanced disease. PET/CT is not recommended before 8 weeks of completion of CRT because of high false-positive rates. The negative predictive value (NPV) of PET/CT done after 8–12 weeks is 95%.[79] In a meta-analysis of 51 studies, Gupta et al. have reported an NPV of 95% for both primary and nodal disease for response assessment.[80] In the post-treatment setting, the best timing for doing a PET/CT for response assessment is after 12 weeks of completion of treatment, but can be done sooner if there is clinical suspicion of recurrent disease,[81] though not before 8 weeks. PET/CT is the best modality for detection of recurrence in head and neck cancers [Figure 4].
Figure 4 (A-C).
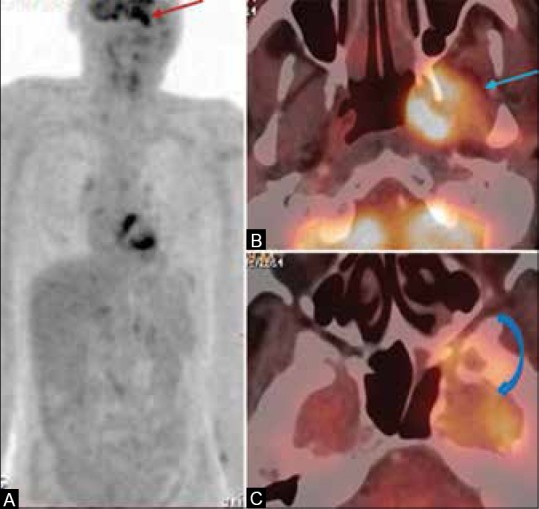
A 59-year-old male, a case of squamous carcinoma of left buccal mucosa, post surgery, chemotherapy, and external radiotherapy given a year back, presented with left facial palsy with no clinical evidence of disease. (A) The MIP image of the PET scan shows a hypermetabolic focus in the left infra-temporal fossa (ITF) (arrow) (B and C) Fused PET/CT image shows hypermetabolic mass in the left ITF involving the pterygoid muscles (arrow) with intracranial extension into the left cavernous sinus and left temporal lobe (curved arrow) suggestive of recurrent disease
Unknown primary
It is important to identify the primary in patients who present with metastatic cervical adenopathy. Inability to detect the primary leads to futile tonsillectomies and untargeted neck dissections. Varying detection rates of 29-54%, with sensitivity ranging from 62 to 97% and specificities of 33–93% have been reported[82,83,84,85] [Figure 5]. PET/CT leads to change in management in 38.9% of patients.[85] PET/CT done prior to pan-endoscopy and biopsy reduces futile endoscopies and false-positive PET studies[86] [Figure 5].
Figure 5 (A-D).
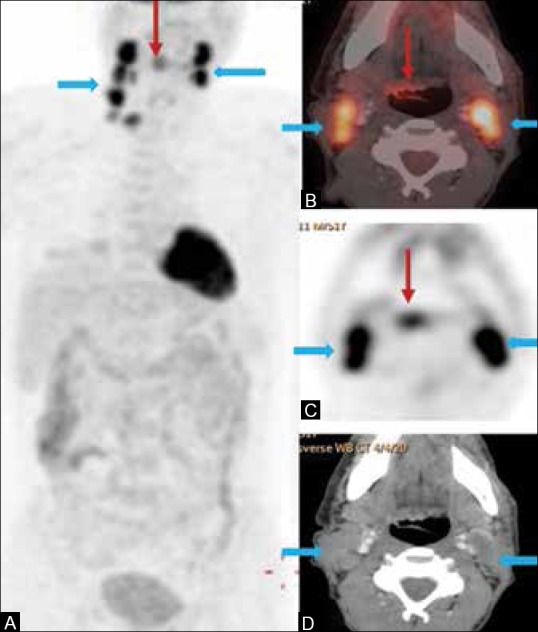
A 51-year-old old male presented with bilateral cervical adenopathy; biopsy revealed it as metastatic squamous cell cancer, unknown primary. (A) PET image shows hypermetabolic bilateral cervical adenopathy (block arrows) (B) Fused PET/CT image shows a subtle hypermetabolic lesion involving the right base tongue (BOT) and vallecula (arrow) (C) PET image shows the same (D) CECT image shows bilateral necrotic cervical nodes, but the primary lesion is not evident. Biopsy of the BOT and vallecula showed squamous cell carcinoma (primary detected)
Thyroid cancer
Poorly differentiated and undifferentiated thyroid cancers are FDG avid due to over-expression of glucose transporter (GLUT) receptors and negative on radioiodine scintigraphy due to loss of sodium iodide symporter responsible for iodine uptake in a thyroid follicular cell. This is in contrast to well-differential thyroid cancers which are radioiodine avid and negative on PET. This is known as flip-flop phenomenon and sets the basis of the appropriateness of FDG PET study in thyroid malignancies. It is considered appropriate to perform FDG PET in thyroid malignancies with high-risk pathology subtypes like tall cell, solid, insular variants, etc. In metastatic thyroid disease, current evidence suggests that FDG PET scan identifies the metastatic lesions not identified by radioiodine scan. This has important treatment implications. For undifferentiated and anaplastic thyroid tumors, FDG PET/CT is the imaging of choice.[1,2,87]
CNS lymphoma
FDG PET/CT has a potential role in the detection of primary CNS lymphoma (PCNSL). Though PET is not very useful in the detection of metastatic lesions due to high physiological uptake of FDG in the brain parenchyma, PCNSL usually demonstrates very intense FDG uptake, which is 2-2.5 times higher than normal physiological uptake. It also has high sensitivity in differentiating PCNSL from glioblastoma and metastatic disease.[88]
Lung cancer
FDG PET combined with CECT is an efficient modality for staging of lung cancers. PET/CT is the best modality to differentiate tumor from adjacent atelectasis, in identifying chest wall invasion, and in detection of pleural metastases.[89,90,91] PET has a high sensitivity of 95%, positive predictive value (PPV) of 95%, accuracy of 92%, and moderate specificity of 67% in the detection of pleural metastasis.[92] PET/CT has higher sensitivity and specificity in the mediastinal staging of lymph nodes with a sensitivity and specificity of 81% and 90%, respectively, as compared to CT which has a sensitivity and specificity of 59% and 79%, respectively.[93] PET has the added advantage of detection of metastatic nodes < 1 cm, but with a low PPV of 64%.[94,95,96] However, PET-positive nodes should still undergo mediastinoscopic sampling for confirmation of metastatic involvement.[97,98] On the contrary, PET has a high NPV of 95% and invasive mediastinoscopy can be omitted in PET-negative nodes,[98] except in centrally situated masses where perilesional mediastinal nodes can be masked due to high metabolic activity of the tumor.[99] PET detects occult metastatic disease in about 29% of cases.[100] It detects adrenal metastasis with a high sensitivity and specificity of >95% and >80%, respectively.[101,102] An invasive biopsy can usually be obviated in most cases. In cases of isolated adrenal metastasis with equivocal PET/CT, a biopsy or adrenalectomy can be contemplated. A meta-analysis comparing FDG PET/CT, MRI, and bone scintigraphy has shown that PET/CT is the best modality for detection of bone metastasis, with a pooled sensitivity and specificity of 92% and 98%, respectively, for PET/CT, 77% and 92%, respectively, for MRI, and 86% and 88%, respectively, for bone scintigraphy[103] [Figure 6]. For brain metastases, MRI is the imaging modality of choice. FDG PET/CT is not very suitable due to normal physiological FDG activity in the brain parenchyma. PET/CT-based RT planning reduces the inter-observer variability and leads to change in treatment plan in about 50% of patients, with enhanced tumor coverage.[89,104]
Figure 6 (A-D).
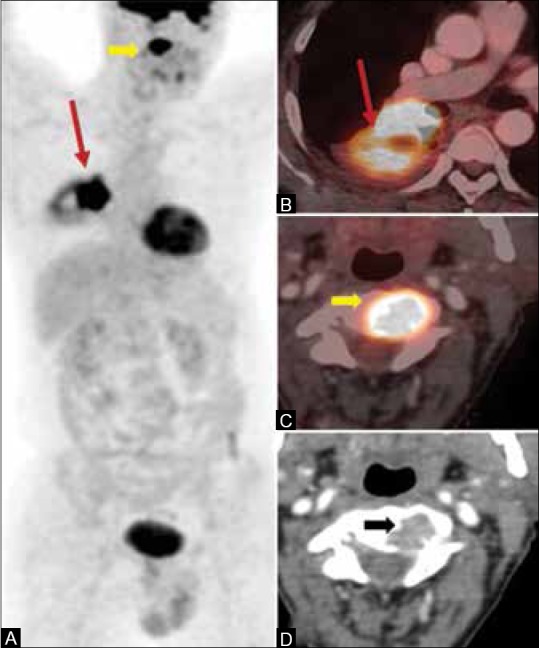
A 54-year-old male, diagnosed case of adenocarcinoma of the right lung. PET/CT scan done for initial staging shows hypermetabolic right lower lobe mass (arrows in A and B) and an unsuspected metastatic lesion in C2 vertebral body (block arrows in A, C, and D)
PET is a better predictor of response to treatment and the metabolic response correlates with outcome.[105] PET/CT detects recurrences with a high sensitivity and specificity of 93% and 89%, respectively, and an accuracy of 92%.[106]
Small cell lung cancer
PET/CT has higher sensitivity and specificity in the detection of distant metastases of small cell cancer as compared to CT, except for brain metastasis. PET leads to change in stage of the disease in 17% of cases, when compared to conventional imaging modalities.[107,108] PET-based RT planning also leads to change in radiation field in about 37% cases.[109]
Malignant pleural mesothelioma
PET/CT is recommended for staging and restaging of malignant mesothelioma. It correctly differentiates between benign and malignant pleural lesions with a sensitivity of 95-97% and specificity ranging from 78 to 92%.[110,111] FDG avidity with an SUVmax value >2-2.2 is helpful for this differentiation.[111] PET/CT identifies more sites of nodal involvement and distant metastases, as compared to CT.[112,113]
Thymic tumors
FDG PET/CT is recommended for the diagnosis and staging of thymic tumors. Studies have shown that PET can differentiate between low-risk thymomas (A, AB, B1 histological types), high-risk thymomas (B2, B3 histological types), and thymic carcinomas based on SUV values. The SUV values show an increasing trend from low to high-risk tumors.[114,115,116,117] PET/CT is better in identifying involved nodes of size <1 cm.[114]
Esophageal cancer
For initial tumor (T) staging of esophageal cancer, endoscopic ultrasound is the preferred modality of choice as it measures the depth of invasion most accurately.[118] PET/CT lacks the spatial resolution to demarcate the depth of invasion. The sensitivity increases with increasing depth of invasion, the value being 83% for T2 tumors, 97% for T3, and 100% for T4 tumors.[119] For detection of nodal disease, PET/CT has the highest sensitivity and specificity of 96% and 95%, respectively.[120] PET/CT is the best modality for detecting distant metastasis and prevents unnecessary surgical explorations by detection of metastatic disease.[121] In a study by Duong et al., PET/CT changed the clinical management from either curative to palliative or vice versa or changed the treatment modality in 40% of patients.[122] In our own experience, PET/CT detects unsuspected distant metastases in 16% of patients, changing the plan of treatment from curative to palliative.[123] PET/CT is useful in evaluating the response to NACT [Figure 7] and CRT by distinguishing residual viable tumor from fibrosis/necrosis based on the metabolic activity of the tumor.[124]
Figure 7 (A-F).
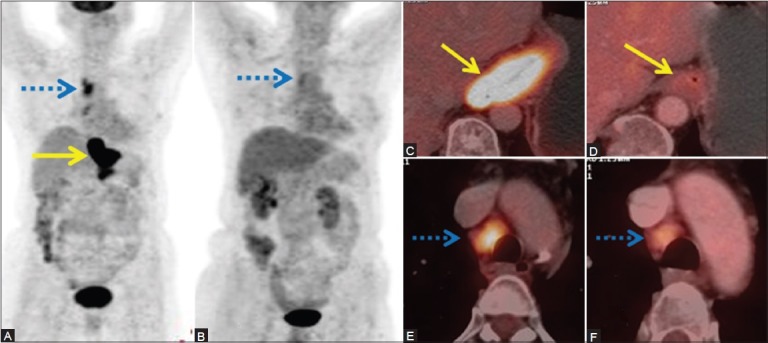
A case of adenocarcinoma of the distal esophagus. (A) Pretreatment MIP image shows hypermetabolic mass in the distal esophagus (arrow) and hypermetabolic metastatic mediastinal nodes (dashed arrow). (C and E) Fused PET/CT images showing the same. (B) Response assessment PET scan done after three cycles of neoadjuvant chemotherapy (NACT) shows complete metabolic and morphologic regression of the mass with partial metabolic and morphologic regression of the mediastinal nodes (dashed arrow) on MIP. (D), (F) The same is depicted in fused PET/CT images (arrow in d and dashed arrow in F)
Prostate cancer
FDG PET has a limited role in the staging and diagnosis of prostate cancer (PC) because of overlap of FDG uptake in normal, benign, and malignant lesions in the prostate.[125,126] Few recent studies have shown increased FDG uptake in aggressive lesions as compared to the indolent ones, with a sensitivity of 80%.[127,128]
Renal cell cancer
FDG PET/CT has limited a role in staging of renal cell cancer (RCC), but is useful in the detection of metastatic disease and in restaging. Wang et al. have shown in a meta-analysis that the pooled sensitivity and specificity for detection of metastases is 91% and 88%, respectively.[129]
Urinary bladder cancer
FDG PET/CT has a limited role in staging of bladder cancer due to excretion of FDG via the kidneys and subsequent accumulation in the bladder, masking the uptake in the primary. Many interventions like hydration, delayed imaging, and diuretic administration are performed to enhance the visualization of FDG uptake in the primary, showing good results.[130,131] A recent meta-analysis has reported good diagnostic accuracy for detection of metastatic lesions with a pooled sensitivity and specificity of 82% and 89%, respectively, for restaging patients.[132]
Testicular cancer
Presence of viable tumor in residual masses after chemotherapy is a major problem in germ cell tumors (GCT). Currently FDG PET/CT is the best imaging modality to solve this problem. De Santis et al., in a study of 56 seminomatous GCT patients, have shown that PET is the best predictor of viable disease in residual masses with a sensitivity of 80% and specificity of 100%, in comparison to CT which has a sensitivity of 70% and specificity of 74%.[133] The accuracy is 100% for masses >3 cm and 95% for masses <3 cm. Similar results were confirmed by a recent meta-analysis.[134,135] The use of PET in non-seminomatous GCT (NSGCT) is controversial. In NSGCT, PET has a PPV of 91% and NPV of 62% in differentiating viable from non-viable disease. This means that PET can predict the presence of viable disease with a reasonable accuracy, but a negative PET study cannot exclude the presence of disease.[136] PET has a definite role in the evaluation of seminoma, but cannot predict the presence of disease in NSGCT with negative PET study.
Melanoma
FDG PET/CT is the best modality for N and M staging of advanced cutaneous melanoma[137] [Figure 8] and for detection of metastasis in mucosal melanomas of head and neck and anorectum.
Figure 8 (A-E).
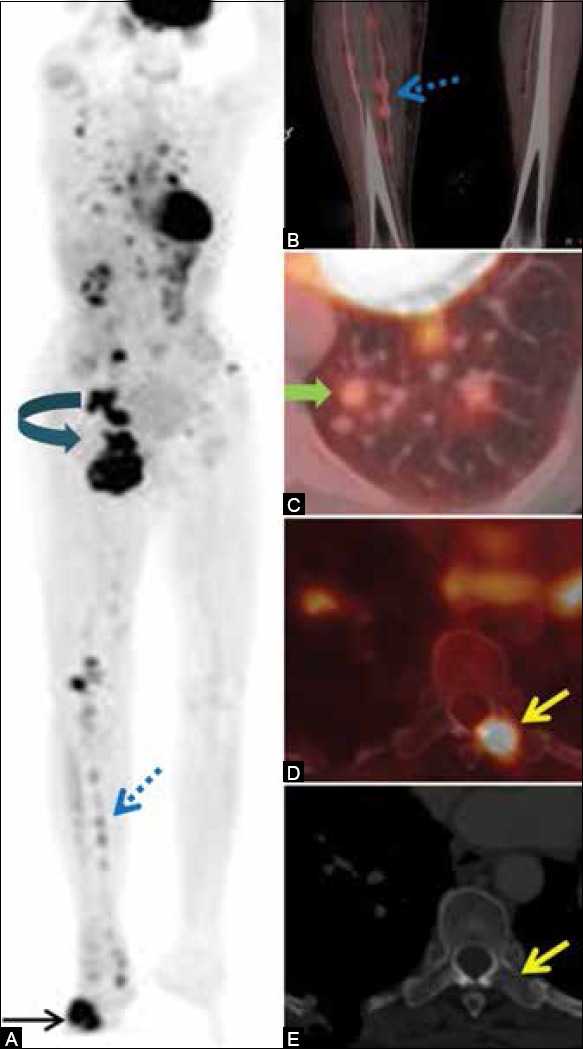
(A-E) A 65-year-old lady, a case of malignant melanoma of the right foot. PET/CT done for staging shows FDG-avid mass in the right foot [site of primary (arrow)], multiple interstitial nodules in the right lower leg (dashed arrows in A and B), right inguinal and pelvic nodal masses (curved arrow), multiple metastatic lung nodules (block arrow), and marrow metastasis in the dorsal vertebra (yellow arrow). PET/CT is a one-stop-shop imaging for detection of metastases in malignant melanoma
The indications for the use of PET/CT are summarized in Table 1.
Table 1.
Indications of PET/CT
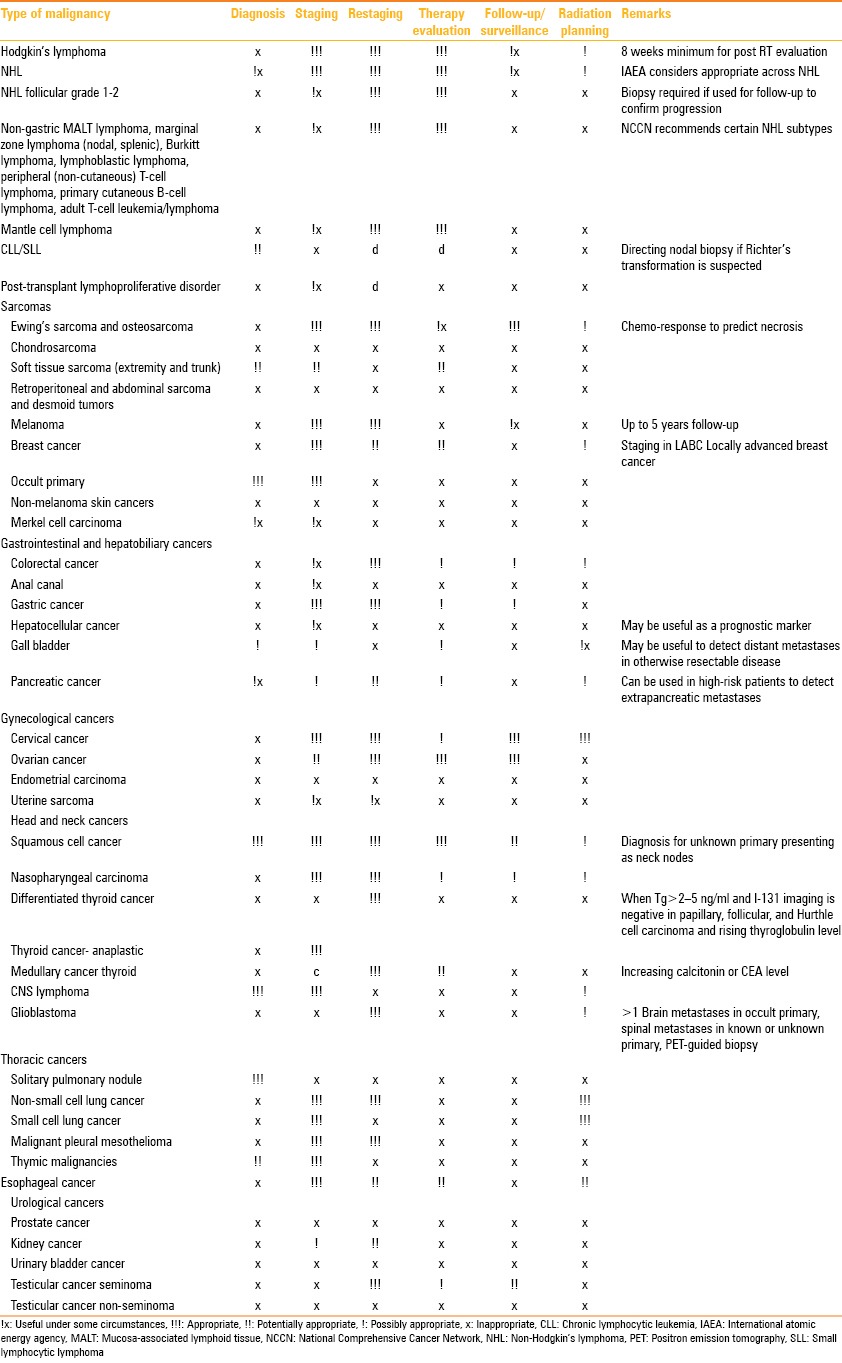
Conclusion
It is evident that the appropriateness criteria for FDG PET/CT in various malignancies are constantly evolving. The impact of FDG PET/CT on treatment management, as evident by the National Oncologic PET Registry (NOPR)[138,139] which is a collaboration of the American College of Radiology Imaging Network (ACRIN) to ensure access to Medicare reimbursement for various PET scan indications, has added a new dimension to the appropriateness criteria. The above recommendations provide an insight into the usefulness of PET/CT in oncology and can help the radiologist and clinician in choosing this hybrid technology appropriately to maximize information for optimal patient care.
Acknowledgement
We would like to thank Dr. Abhijith Singh and Mr. Ashish Jha for their help in preparing the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.A Guide to Clinical PET in Oncology. [Last accessed on 2014 Aug 16]. Available from: http://www.pub.iaea.org/MTCD/publications/PDF/te_1605_web.pdf .
- 2.PET/CT Practice Guidelines in Oncology-Society of Nuclear Medicine and Molecular Imaging. [Last accessed on 2014 Aug 16]. Available from: http://www.snm.org/docs/PET./OncologyPracticeGuidelineSummary.pdf .
- 3.Kostakoglu L, Cheson BD. Current role of FDG PET/CT in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1004–27. doi: 10.1007/s00259-013-2686-2. [DOI] [PubMed] [Google Scholar]
- 4.Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, et al. Positron emission tomography with or without computed tomography in the primary staging of Hodgkin's lymphoma. Haematologica. 2006;91:482–9. [PubMed] [Google Scholar]
- 5.Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: A systematic review. Blood. 2008;111:504–16. doi: 10.1182/blood-2007-07-101899. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer NG, Hany TF, Taverna C, Seifert B, Stumpe KD, von Schulthess GK, et al. Non-Hodgkin lymphoma and Hodgkin disease: Coregistered FDG PET and CT at staging and restaging-do we need contrast-enhanced CT? Radiology. 2004;232:823–9. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 8.Raanani P, Shasha Y, Perry C, Metser U, Naparstek E, Apter S, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006;17:117–22. doi: 10.1093/annonc/mdj024. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M, Wöhrer S, Becherer A, Chott A, Streubel B, Kletter K, et al. 18F-fluoro-deoxy-glucose positron emission tomography in lymphoma of mucosa-associated lymphoid tissue: Histology makes the difference. Ann Oncol. 2006;17:1761–5. doi: 10.1093/annonc/mdl295. [DOI] [PubMed] [Google Scholar]
- 10.Treglia G, Zucca E, Sadeghi R, Cavalli F, Giovanella L, Ceriani L. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: A meta-analysis. Hematol Oncol. 2014 doi: 10.1002/hon.2152. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Lister TA. Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–68. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treglia G, Salsano M, Stefanelli A, Mattoli MV, Giordano A, Bonomo L. Diagnostic accuracy of 18F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: A systematic review and a meta-analysis. Skeletal Radiol. 2012;41:249–56. doi: 10.1007/s00256-011-1298-9. [DOI] [PubMed] [Google Scholar]
- 13.Franzius C, Sciuk J, Daldrup-Link HE, Jürgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: Comparison with bone scintigraphy. Eur J Nucl Med. 2000;27:1305–11. doi: 10.1007/s002590000301. [DOI] [PubMed] [Google Scholar]
- 14.Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jürgens H, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: Comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:2209–36. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 15.Györke T, Zajic T, Lange A, Schäfer O, Moser E, Makó E, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun. 2006;27:17–24. doi: 10.1097/01.mnm.0000186608.12895.69. [DOI] [PubMed] [Google Scholar]
- 16.Völker T, Denecke T, Steffen I, Misch D, Schönberger S, Plotkin M, et al. Positron emission tomography for staging of pediatric sarcoma patients: Results of a prospective multicentre trial. J Clin Oncol. 2007;25:5435–41. doi: 10.1200/JCO.2007.12.2473. [DOI] [PubMed] [Google Scholar]
- 17.Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jürgens H, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: Comparison with spiral CT. Ann Oncol. 2001;12:479–86. doi: 10.1023/a:1011111322376. [DOI] [PubMed] [Google Scholar]
- 18.Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: A meta-analysis. Surg Oncol. 2012;21:e165–70. doi: 10.1016/j.suronc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Purandare NC, Rangarajan V, Agarwal M, Sharma AR, Shah S, Arora A, et al. Integrated PET/CT in evaluating sarcomatous transformation in osteochondromas. Clin Nucl Med. 2009;34:350–4. doi: 10.1097/RLU.0b013e3181a34525. [DOI] [PubMed] [Google Scholar]
- 20.Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31:189–95. doi: 10.1007/s00259-003-1353-4. [DOI] [PubMed] [Google Scholar]
- 21.Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 22.Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: Comparison to conventional staging. J Nucl Med. 2013;54:5–11. doi: 10.2967/jnumed.112.106864. [DOI] [PubMed] [Google Scholar]
- 23.Sharma B, Martin A, Zerizer I. Positron emission tomography-computed tomography in liver imaging. Semin Ultrasound CT MR. 2013;34:66–80. doi: 10.1053/j.sult.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–21. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961–8. doi: 10.1007/s00535-004-1427-5. [DOI] [PubMed] [Google Scholar]
- 26.Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: Preliminary findings. Liver Int. 2006;26:781–8. doi: 10.1111/j.1478-3231.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 27.Kawaoka T, Aikata H, Takaki S, Uka K, Azakami T, Saneto H, et al. FDG positron emission tomography/computed tomography for the detection of extrahepatic metastases from hepatocellular carcinoma. Hepatol Res. 2009;39:134–42. doi: 10.1111/j.1872-034X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 28.Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736–41. doi: 10.1007/s00268-005-0791-5. [DOI] [PubMed] [Google Scholar]
- 29.Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumour differentiation, P- glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 30.Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 31.Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, et al. Positron emission tomography with [(18) F] fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029–35. doi: 10.1053/jhep.2001.23912. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Yun M, Lee WJ, Kim KS, Lee JD. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging. 2003;30:1467–72. doi: 10.1007/s00259-003-1297-8. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Tsukamoto E, Kuge Y, Katoh C, Nambu T, Nobuta A, et al. Clinical role of (18) F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur J Nucl Med Mol Imaging. 2002;29:1047–54. doi: 10.1007/s00259-002-0852-z. [DOI] [PubMed] [Google Scholar]
- 34.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Jadvar H, Henderson RW, Conti PS. [F-18] fluorodeoxyglucose positron emission tomography and positron emission tomography: Computed tomography in recurrent and metastatic cholangiocarcinoma. J Comput Assist Tomogr. 2007;31:223–8. doi: 10.1097/01.rct.0000237811.88251.d7. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Font C, Gómez-Rio M, Rodríguez-Fernández A, Jiménez-Heffernan A, Sánchez Sánchez R, Llamas-Elvira JM. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol. 2014;109:218–24. doi: 10.1002/jso.23476. [DOI] [PubMed] [Google Scholar]
- 37.Shukla PJ, Barreto SG, Arya S, Shrikhande SV, Hawaldar R, Purandare N, et al. Does PET-CT scan have a role prior to radical re-resection for incidental gallbladder cancer? HPB (Oxford) 2008;10:439–45. doi: 10.1080/13651820802286910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrikhande SV, Barreto SG, Goel M, Arya S. Multimodality imaging of pancreatic ductal adenocarcinoma: A review of the literature. HPB (Oxford) 2012;14:658–68. doi: 10.1111/j.1477-2574.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: A meta-analysis. Eur J Surg Oncol. 2014;40:794–804. doi: 10.1016/j.ejso.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer. 2014;14:1–6. doi: 10.5230/jgc.2014.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929–35. doi: 10.1007/s00432-010-0852-5. [DOI] [PubMed] [Google Scholar]
- 42.Park JC, Lee JH, Cheoi K, Chung H, Yun MJ, Lee H, et al. Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: The maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging. 2012;39:1107–16. doi: 10.1007/s00259-012-2116-x. [DOI] [PubMed] [Google Scholar]
- 43.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–6. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: Comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183–8. doi: 10.1016/j.ejrad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003;44:690–9. [PubMed] [Google Scholar]
- 46.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18) F-FDG PET: A comparison study with CT. J Nucl Med. 2005;46:1582–8. [PubMed] [Google Scholar]
- 47.Yang QM, Kawamura T, Itoh H, Bando E, Nemoto M, Akamoto S, et al. Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology. 2008;55:782–5. [PubMed] [Google Scholar]
- 48.Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249–56. doi: 10.3348/kjr.2006.7.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LM, Hu JN, Hua J, Gu HY, Zhu J, Xu JR. 18 F-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2012;27:472–80. doi: 10.1111/j.1440-1746.2011.06919.x. [DOI] [PubMed] [Google Scholar]
- 50.Zou H, Zhao Y. 18FDG PET-CT for detecting gastric cancer recurrence after surgical resection: A meta-analysis. Surg Oncol. 2013;22:162–6. doi: 10.1016/j.suronc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Bipat S, Niekel MC, Comans EF, Nio CY, Bemelman WA, Verhoef C, et al. Imaging modalities for the staging of patients with colorectal cancer. Neth J Med. 2012;70:26–34. [PubMed] [Google Scholar]
- 52.Patel S, McCall M, Ohinmaa A, Bigam D, Dryden DM. Positron emission tomography/computed tomographic scans compared to computed tomographic scans for detecting colorectal liver metastases: A systematic review. Ann Surg. 2011;253:666–71. doi: 10.1097/SLA.0b013e31821110c9. [DOI] [PubMed] [Google Scholar]
- 53.Guillem JG, Ruby JA, Leibold T, Akhurst TJ, Yeung HW, Gollub MJ, et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: A prospective study. Ann Surg. 2013;258:289–95. doi: 10.1097/SLA.0b013e318277b625. [DOI] [PubMed] [Google Scholar]
- 54.Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: Is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658–66. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- 55.Maffione AM, Chondrogiannis S, Capirci C, Galeotti F, Fornasiero A, Crepaldi G, et al. Early prediction of response by 18 F-FDG PET/CT during preoperative therapy in locally advanced rectal cancer: A systematic review. Eur J Surg Oncol. 2014;40:1186–94. doi: 10.1016/j.ejso.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Lu YY, Chen JH, Chien CR, Chen WT, Tsai SC, Lin WY, et al. Use of FDG-PET or PET/CT to detect recurrent colorectal cancer in patients with elevated CEA: A systematic review and meta-analysis. Int J Colorectal Dis. 2013;28:1039–47. doi: 10.1007/s00384-013-1659-z. [DOI] [PubMed] [Google Scholar]
- 57.Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist. 2008;13(Suppl 2):4–7. doi: 10.1634/theoncologist.13-S2-4. [DOI] [PubMed] [Google Scholar]
- 58.Van den Abbeele AD. GIST Collaborative PET Study Group. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) Proc Am Soc Clin Oncol. 2001;20:362a. [Google Scholar]
- 59.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 60.Winton Ed, Heriot AG, Ng M, Hicks RJ, Hogg A, Milner A, et al. The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer. 2009;100:693–700. doi: 10.1038/sj.bjc.6604897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mistrangelo M, Pelosi E, Bellò M, Ricardi U, Milanesi E, Cassoni P, et al. Role of positron emission tomography-computed tomography in the management of anal cancer. Int J Radiat Oncol Biol Phys. 2012;84:66–72. doi: 10.1016/j.ijrobp.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen BT, Joon DL, Khoo V, Quong G, Chao M, Wada M, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol. 2008;87:376–82. doi: 10.1016/j.radonc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Krengli M, Milia ME, Turri L, Mones E, Bassi MC, Cannillo B, et al. FDG-PET/CT imaging for staging and target volume delineation in conformal radiotherapy of anal carcinoma. Radiat Oncol. 2010;5:10. doi: 10.1186/1748-717X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Magné N, Chargari C, Vicenzi L, Gillion N, Messai T, Magné J, et al. New trends in the evaluation and treatment of cervix cancer: The role of FDG-PET. Cancer Treat Rev. 2008;34:671–81. doi: 10.1016/j.ctrv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 67.Belhocine T, Thille A, Fridman V, Albert A, Seidel L, Nickers P, et al. Contribution of whole-body 18FDG PET imaging in the management of cervical cancer. Gynecol Oncol. 2002;87:90–7. doi: 10.1006/gyno.2002.6769. [DOI] [PubMed] [Google Scholar]
- 68.Lin LL, Yang Z, Mutic S, Miller TR, Grigsby PW. FDG-PET imaging for the assessment of physiologic volume response during radiotherapy in cervix cancer. Int J Radiat Oncol Biol Phys. 2006;65:177–81. doi: 10.1016/j.ijrobp.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 69.Wong TZ, Jones EL, Coleman RE. Positron emission tomography with 2-deoxy-2- [(18) F] fluoro- D-glucose for evaluating local and distant disease in patients with cervical cancer. Mol Imaging Biol. 2004;6:55–62. doi: 10.1016/j.mibio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Sharma DN, Rath GK, Kumar R, Malhotra A, Kumar S, Pandjatcharam J, et al. Positron emission tomography scan for predicting clinical outcome of patients with recurrent cervical carcinoma following radiation therapy. J Cancer Res Ther. 2012;8:23–7. doi: 10.4103/0973-1482.95169. [DOI] [PubMed] [Google Scholar]
- 71.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Fukasawa I, Inaba N, et al. Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: Comparison with enhanced CT. Eur J Nucl Med Mol Imaging. 2008;35:1912–20. doi: 10.1007/s00259-008-0890-2. [DOI] [PubMed] [Google Scholar]
- 72.Thrall MM, DeLoia JA, Gallion H, Avril N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol Oncol. 2007;105:17–22. doi: 10.1016/j.ygyno.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 73.Takekuma M, Maeda M, Ozawa T, Yasumi K, Torizuka T. Positron emission tomography with 18F-fluoro-2-deoxyglucose for the detection of recurrent ovarian cancer. Int J Clin Oncol. 2005;10:177–81. doi: 10.1007/s10147-005-0489-6. [DOI] [PubMed] [Google Scholar]
- 74.Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: A systematic review and meta-analysis. Eur J Radiol. 2009;71:164–74. doi: 10.1016/j.ejrad.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 75.Xu G, Li J, Zuo X, Li C. Comparison of whole body positron emission tomography (PET)/PET-computed tomography and conventional anatomic imaging for detecting distant malignancies in patients with head and neck cancer: A meta-analysis. Laryngoscope. 2012;122:1974–8. doi: 10.1002/lary.23409. [DOI] [PubMed] [Google Scholar]
- 76.Xu GZ, Guan DJ, He ZY. (18) FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol. 2011;47:560–5. doi: 10.1016/j.oraloncology.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 77.O’Neill JP, Moynagh M, Kavanagh E, O’Dwyer T. Prospective, blinded trial of whole-body magnetic resonance imaging versus computed tomography positron emission tomography in staging primary and recurrent cancer of the head and neck. J Laryngol Otol. 2010;124:1274–7. doi: 10.1017/S0022215110001398. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JT, Branstetter BF., 4th PET/CT in head and neck oncology: State-of-the-art 2013. Laryngoscope. 2014;124:913–5. doi: 10.1002/lary.23942. [DOI] [PubMed] [Google Scholar]
- 79.McDermott M, Hughes M, Rath T, Johnson JT, Heron DE, Kubicek GJ, et al. Negative predictive value of surveillance PET/CT in head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2013;34:1632–6. doi: 10.3174/ajnr.A3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta T, Master Z, Kannan S, Agarwal JP, Ghosh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: A systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–95. doi: 10.1007/s00259-011-1893-y. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura S, Toriihara A, Okochi K, Watanabe H, Shibuya H, Kurabayashi T. Optimal timing of post-treatment [18F] fluorodeoxyglucose-PET/CT for patients with head and neck malignancy. Nucl Med Commun. 2013;34:162–7. doi: 10.1097/MNM.0b013e32835bdfe3. [DOI] [PubMed] [Google Scholar]
- 82.Tantiwongkosi B, Yu F, Kanard A, Miller FR. Role of (18) F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J Radiol. 2014;6:177–91. doi: 10.4329/wjr.v6.i5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudmik L, Lau HY, Matthews TW, Bosch JD, Kloiber R, Molnar CP, et al. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: A prospective clinical trial. Head Neck. 2011;33:935–40. doi: 10.1002/hed.21566. [DOI] [PubMed] [Google Scholar]
- 84.Zhu L, Wang N. 18F-fluorodeoxyglucose positron emission tomography-computed tomography as a diagnostic tool in patients with cervical nodal metastases of unknown primary site: A meta-analysis. Surg Oncol. 2013;22:190–4. doi: 10.1016/j.suronc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Dandekar MR, Kannan S, Rangarajan V, Purandare NC, Chaukar DA, Deshmukh A, et al. Utility of PET in unknown primary with cervical metastasis: A retrospective study. Indian J Cancer. 2011;48:181–6. doi: 10.4103/0019-509X.82882. [DOI] [PubMed] [Google Scholar]
- 86.Johansen J, Buus S, Loft A, Keiding S, Overgaard M, Hansen HS, et al. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck. 2008;30:471–8. doi: 10.1002/hed.20734. [DOI] [PubMed] [Google Scholar]
- 87.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 88.Kawai N, Miyake K, Yamamoto Y, Nishiyama Y, Tamiya T. 18F-FDG PET in the diagnosis and treatment of primary central nervous system lymphoma. Biomed Res Int 2013. 2013 doi: 10.1155/2013/247152. 247152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradley J, Bae K, Choi N, Forster K, Siegel BA, Brunetti J, et al. A phase II comparative study of gross tumor volume definition with or without PET/CT fusion in dosimetric planning for non-small-cell lung cancer (NSCLC): Primary analysis of Radiation Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 2012;82:435–41.e1. doi: 10.1016/j.ijrobp.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nestle U, Walter K, Schmidt S, Licht N, Nieder C, Motaref B, et al. 18F-deoxyglucose positron emission tomography (FDG-PET) for the planning of radiotherapy in lung cancer: High impact in patients with atelectasis. Int J Radiat Oncol Biol Phys. 1999;44:593–7. doi: 10.1016/s0360-3016(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 91.Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol. 2009;193:1203–11. doi: 10.2214/AJR.09.3193. [DOI] [PubMed] [Google Scholar]
- 92.Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF. FDG PET of pleural effusions in patients with non-small cell lung cancer. AJR Am J Roentgenol. 2000;175:245–9. doi: 10.2214/ajr.175.1.1750245. [DOI] [PubMed] [Google Scholar]
- 93.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: A meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 94.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123(Suppl):137S–46S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 95.Fischer BM, Mortensen J, Højgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: A systematic, quantitative review. Lancet Oncol. 2001;2:659–66. doi: 10.1016/S1470-2045(01)00555-1. [DOI] [PubMed] [Google Scholar]
- 96.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: Mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 97.Darling GE, Maziak DE, Inculet RI, Gulenchyn KY, Driedger AA, Ung YC, et al. Positron emission tomography-computed tomography compared with invasive mediastinal staging in non-small cell lung cancer: Results of mediastinal staging in the early lung positron emission tomography trial. J Thorac Oncol. 2011;6:1367–72. doi: 10.1097/JTO.0b013e318220c912. [DOI] [PubMed] [Google Scholar]
- 98.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl):e211–50S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 99.Sahiner I, Vural GU. Positron emission tomography/computerized tomography in lung cancer. Quant Imaging Med Surg. 2014;4:195–206. doi: 10.3978/j.issn.2223-4292.2014.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schrevens L, Lorent N, Dooms C, Vansteenkiste J. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist. 2004;9:633–43. doi: 10.1634/theoncologist.9-6-633. [DOI] [PubMed] [Google Scholar]
- 101.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: A proposal for an efficient diagnostic algorithm. Radiology. 2009;250:523–30. doi: 10.1148/radiol.2502080219. [DOI] [PubMed] [Google Scholar]
- 102.Cho AR, Lim I, Na II, Choe du H, Park JY, Kim BI, et al. Evaluation of adrenal masses in lung cancer patients using F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011;45:52–8. doi: 10.1007/s13139-010-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qu X, Huang X, Yan W, Wu L, Dai K. A meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur J Radiol. 2012;81:1007–15. doi: 10.1016/j.ejrad.2011.01.126. [DOI] [PubMed] [Google Scholar]
- 104.Pommier P, Touboul E, Chabaud S, Dussart S, Le Pechoux C, Giammarile F, et al. Impact of (18) F-FDG PET on treatment strategy and 3D radiotherapy planning in non-small cell lung cancer: A prospective multicenter study. AJR Am J Roentgenol. 2010;195:350–5. doi: 10.2214/AJR.09.3981. [DOI] [PubMed] [Google Scholar]
- 105.Mac Manus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 106.Hellwig D, Gröschel A, Graeter TP, Hellwig AP, Nestle U, Schäfers HJ, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- 107.Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, et al. Impact of [18F] FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614–20. doi: 10.1007/s00259-004-1606-x. [DOI] [PubMed] [Google Scholar]
- 108.Fischer BM, Mortensen J, Langer SW, Loft A, Berthelsen AK, Petersen BI, et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: Comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol. 2007;18:338–45. doi: 10.1093/annonc/mdl374. [DOI] [PubMed] [Google Scholar]
- 109.Kamel EM, Zwahlen D, Wyss MT, Stumpe KD, von Schulthess GK, Steinert HC. Whole-body (18) F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med. 2003;44:1911–7. [PubMed] [Google Scholar]
- 110.Duysinx B, Nguyen D, Louis R, Cataldo D, Belhocine T, Bartsch P, et al. Evaluation of pleural disease with 18-fluorodeoxyglucose positron emission tomography imaging. Chest. 2004;125:489–93. doi: 10.1378/chest.125.2.489. [DOI] [PubMed] [Google Scholar]
- 111.Yildirim H, Metintas M, Entok E, Ak G, Ak I, Dundar E, et al. Clinical value of fluoro deoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: An observational pilot study. J Thorac Oncol. 2009;4:1480–4. doi: 10.1097/JTO.0b013e3181c0a7ff. [DOI] [PubMed] [Google Scholar]
- 112.Plathow C, Staab A, Schmaehl A, Aschoff P, Zuna I, Pfannenberg C, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: Initial results. Invest Radiol. 2008;43:737–44. doi: 10.1097/RLI.0b013e3181817b3d. [DOI] [PubMed] [Google Scholar]
- 113.Ambrosini V, Rubello D, Nanni C, Farsad M, Castellucci P, Franchi R, et al. Additional value of hybrid PET/CT fusion imaging vs. conventional CT scan alone in the staging and management of patients with malignant pleural mesothelioma. Nucl Med Rev Cent East Eur. 2005;8:111–5. [PubMed] [Google Scholar]
- 114.Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: Usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–34. [PubMed] [Google Scholar]
- 115.Endo M, Nakagawa K, Ohde Y, Okumura T, Kondo H, Igawa S, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;61:350–5. doi: 10.1016/j.lungcan.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Kaira K, Sunaga N, Ishizuka T, Shimizu K, Yamamoto N. The role of [18 F] fluorodeoxyglucose positron emission tomography in thymic epithelial tumors. Cancer Imaging. 2011;11:195–201. doi: 10.1102/1470-7330.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Otsuka H. The utility of FDG-PET in the diagnosis of thymic epithelial tumors. J Med Invest. 2012;59:225–34. doi: 10.2152/jmi.59.225. [DOI] [PubMed] [Google Scholar]
- 118.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–90. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–56. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- 120.Roedl JB, Blake MA, Holalkere NS, Mueller PR, Colen RR, Harisinghani MG. Lymph node staging in esophageal adenocarcinomas with PET-CT based on a visual analysis and based on metabolic parameters. Abdom Imaging. 2009;34:610–7. doi: 10.1007/s00261-008-9447-x. [DOI] [PubMed] [Google Scholar]
- 121.vanWestreenen HL, Heeren PA, van Dullemen HM, van der Jagt EJ, Jager PL, Groen H, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54–61. doi: 10.1016/j.gassur.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 122.Duong CP, Demitriou H, Weih L, Thompson A, Williams D, Thomas RJ, et al. Significant clinical impact and prognostic stratification provided by FDG-PET in the staging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:759–69. doi: 10.1007/s00259-005-0028-8. [DOI] [PubMed] [Google Scholar]
- 123.Purandare NC, Pramesh CS, Karimundackal G, Jiwnani S, Agrawal A, Shah S, et al. Incremental value of 18F-FDG PET/CT in therapeutic decision-making of potentially curable esophageal adenocarcinoma. Nucl Med Commun. 2014;35:864–9. doi: 10.1097/MNM.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 124.Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Masuda N, et al. Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg. 2002;184:279–83. doi: 10.1016/s0002-9610(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 125.Salminen E, Hogg A, Binns D, Frydenberg M, Hicks R. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–9. doi: 10.1080/028418602320405005. [DOI] [PubMed] [Google Scholar]
- 126.Jadvar H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–23. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, et al. The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–7. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 128.Shiiba M, Ishihara K, Kimura G, Kuwako T, Yoshihara H, Sato H, et al. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Ann Nucl Med. 2012;26:138–45. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 129.Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY, Lin WY, et al. Meta-analysis of the diagnostic performance of [18F] FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging. 2012;12:464–74. doi: 10.1102/1470-7330.2012.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48:764–70. doi: 10.2967/jnumed.106.036350. [DOI] [PubMed] [Google Scholar]
- 131.Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase F-fluorodeoxyglucose- PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–9. doi: 10.4103/0971-3026.59746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–6. doi: 10.1016/j.ejrad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 133.De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: An update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004;22:1034–9. doi: 10.1200/JCO.2004.07.188. [DOI] [PubMed] [Google Scholar]
- 134.Zhao JY, Ma XL, Li YY, Zhang BL, Li MM, Ma XL, et al. Diagnostic accuracy of 18F-FDG-PET in patients with testicular cancer: A meta-analysis. Asian Pac J Cancer Prev. 2014;15:3525–31. doi: 10.7314/apjcp.2014.15.8.3525. [DOI] [PubMed] [Google Scholar]
- 135.Müller J, Schrader AJ, Jentzmik F, Schrader M. Assessment of residual tumours after systemic treatment of metastatic seminoma: 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography-meta-analysis of diagnostic value. Urologe A. 2011;50:322–7. doi: 10.1007/s00120-010-2469-3. [DOI] [PubMed] [Google Scholar]
- 136.Kollmannsberger C, Oechsle K, Dohmen BM, Pfannenberg A, Bares R, Claussen CD, et al. Prospective comparison of [18F] fluorodeoxyglucose positron emission tomography with conventional assessment by computed tomography scans and serum tumor markers for the evaluation of residual masses in patients with nonseminomatous germ cell carcinoma. Cancer. 2002;94:2353–62. doi: 10.1002/cncr.10494. [DOI] [PubMed] [Google Scholar]
- 137.Gu H, Xu W, Song X, Dai D, Zhu L, Wang J. Diagnostic value of (18) F-fluoro deoxyglucose positron emission tomography/computed tomography for N-and M-staging of malignant melanoma. Zhonghua Yi Xue Za Zhi. 2014;94:1309–12. [PubMed] [Google Scholar]
- 138.Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF, Hanna L, et al. The impact of positron emission tomography (PET) on expected management during cancer treatment: Findings of the National Oncologic PET Registry. Cancer. 2009;115:410–8. doi: 10.1002/cncr.24000. [DOI] [PubMed] [Google Scholar]
- 139.Tunis S, Whicher D. The National Oncologic PET Registry: Lessons learned for coverage with evidence development. J Am Coll Radiol. 2009;6:360–5. doi: 10.1016/j.jacr.2009.01.016. [DOI] [PubMed] [Google Scholar]


