Abstract
The Paleozoic–Mesozoic transition is characterized by the most massive extinction of the Phanerozoic. Nevertheless, an impressive adaptive radiation of herbivorous insects occurred on gymnosperm-dominated floras not earlier than during the Middle to Late Triassic, penecontemporaneous with similar events worldwide, all which exhibit parallel expansions of generalized and mostly specialized insect herbivory on plants, expressed as insect damage on a various plant organs and tissues. The flora from Monte Agnello is distinctive, due to its preservation in subaerially deposited pyroclastic layers with exceptionally preserved details. Thus, the para-autochthonous assemblage provides insights into environmental disturbances, caused by volcanic activity, and how they profoundly affected the structure and composition of herbivory patterns. These diverse Middle Triassic biota supply extensive evidence for insect herbivore colonization, resulting in specific and complex herbivory patterns involving the frequency and diversity of 20 distinctive damage types (DTs). These DT patterns show that external foliage feeders, piercer-and-suckers, leaf miners, gallers, and oviposition culprits were intricately using almost all tissue types from the dominant host plants of voltzialean conifers (e.g., Voltzia), horsetails, ferns (e.g., Neuropteridium, Phlebopteris, Cladophlebis and Thaumatopteris), seed ferns (e.g., Scytophyllum), and cycadophytes (e.g., Bjuvia and Nilssonia).
Keywords: Plant–animal interactions, Plant fossils, Italy, Longobardian, Southern Alps, Volcanic activity
Introduction
Continental arthropods and vascular plants have been major elements of terrestrial ecosystems worldwide for nearly 400 million years, and their varied ectophytic and endophytic associations can provide a unique and direct record of the plant–insect interactions in the past (e.g., Labandeira & Currano, 2013). In 2006, Labandeira proposed four pulses of herbivore expansion, where the observed Palaeozoic arthropod herbivory patterns—covering the first two phases—are mainly expressed by damage patterns caused by mites and apterygote and/or basal pterygote herbivores on pteridophyte and basal gymnospermous plant hosts and are profoundly different from those that originated after the end-Permian mass extinction (Labandeira, 2006a; Labandeira, 2006b). Preliminary work on plant–insect interactions from early to late Permian floras of the Southern Alps indicates a moderately diverse pattern of damage occurring in a variety of habitats prior to the P-Tr crisis (T Wappler, pers. obs., 2013), penecontemporaneous with similar events in US Southwest (e.g., Schachat et al., 2014), Gondwana (Adami-Rodrigues et al., 2004; Adami-Rodrigues, Iannuzzi & Pinto, 2004; Cariglino & Gutiérrez, 2011; Gallego, Cúneo & Escapa, 2014; Iannuzzi & Labandeira, 2008; Prevec et al., 2009; Slater, McLoughlin & Hilton, 2012), or Cathaysia (e.g., Glasspool et al., 2003). Nevertheless, herbivory expansion 2 was profoundly disrupted by environmental perturbations at the P-Tr boundary. The Early Triassic has been traditionally viewed as an unusual time marked by suppressed origination rates and low diversity (e.g., Benton & Emerson, 2007) generally attributed to the effects of extreme environmental conditions inflicted on Early Triassic ecosystems (e.g., Looy et al., 1999; Grauvogel-Stamm & Ash, 2005; Roopnarine et al., 2007; Tong et al., 2007) but taphonomical biases cannot be excluded at least for European floras (Kustatscher et al., 2014). In general, records of Early Triassic insects or of insect damage on plants are scant worldwide (comp. Table 1), so little is known about the mechanics and timing of diversification of this ecologically important group following the end-Permian mass-extinction event (Kustatscher et al., 2014; Labandeira & Currano, 2013). Shcherbakov (2008a) even concluded that the entire class of insects was strongly reduced in diversity at the P-Tr boundary but following the end-Permian biotic crisis insect faunas already contained many elements common to modern insects (e.g., Aristov et al., 2013; Béthoux, Papier & Nel, 2005; Shcherbakov, 2008b; Lukashevich et al., 2010; Żyła et al., 2013; Haig et al., 2015) building the nucleus for the onset of the third pulse of herbivore expansion, coupled with an impressive adaptive radiation of herbivorous insects. Their associations with plants became significantly diverse being major elements for keystone communities in terrestrial ecosystems worldwide (e.g., Ash, 2014; Grauvogel-Stamm & Kelber, 1996; Kustatscher et al., 2014; Labandeira, 2006a; Labandeira, 2006b; Labandeira & Currano, 2013; McLoughlin, 2011; Moisan et al., 2012; Pott et al., 2008; Scott, Anderson & Anderson, 2004). Simultaneously, a major three-phased floral change has been proposed for Europe and probably worldwide (e.g., Grauvogel-Stamm & Ash, 2005). The first stage lasted from the Induan to early Anisian, which in Europe is characterized by a “survival” interval dominated by the lycopsid Pleuromeia Corda ex Giebel (1853) and conifers coupled with relatively low levels of plant–insect interactional diversity (Kustatscher et al., 2014); this is followed by a “recovery” interval characterized by the resurgence of lycopsids, sphenophytes, ferns, cycadophytes, conifers, ginkgophytes and seed ferns. The second stage occurred from the late Anisian to the Carnian. The third covers the Norian and Rhaetian stages, which is pivotal to understanding the evolution of trophically modern ecosystems (e.g., Benton, 2010; Labandeira, 2006b; Labandeira & Currano, 2013).
Table 1. Arthropod damage on Triassic plants.
List of published records of arthropod damage on Triassic plants.
| Study | Age | Formation and locality | Damage type |
|---|---|---|---|
| Nathorst (1876); Nathorst (1878) | Rhaetian (Late Triassic) | Pålsjö, Scania, Sweden | • Possible oviposition scars on Podozamites |
| Ghosh, Kar & Chatterjee (2015) | Norian/Rhaetian (Late Triassic) | Parsora Formation (Dhaurai Hill beds); South Rewa Gondwana Basin, central India | • Disc-like galls on Dicroidium hughesii |
| Walker (1938); Ash (1997); Ash (1999); Ash (2000); Ash (2001); Ash (2005); Ash & Savidge (2004); Ash (2014); Creber & Ash (2004) | Norian (Late Triassic) | Chinle Formation, Petrified Forest National Park, Arizona, USA | • Marginal and non-marginal feeding traces on Cynepteria, Marcouia, Zamites, Sphenopteris, Macrotaeniopteris, Dechellyia, Nilssoniopteris
• Possible oviposition scars and insect eggs on Dechellyia, ?Equisetites • Coprolite-bearing borings in Itopsidema, Araucarioxylon, Schilderia |
| Adami-Rodrigues, Gnaedinger & Gallego (2008) | Norian (Late Triassic) | El Tranquilo Group, Laguna Colorada Formation; Santa Cruz, Argentinia | • Specific and complex herbivory patterns of several FFG’s |
| Feng et al. (2014); Hsü et al. (1974) | Keuper (Late Triassic) | District Yungjen, Yunnan, China | • Crescent-shape bite marks on Mixopteris • Intense skeletonization Dictyophyllum nathorstii |
| Gallego et al. (2003); Gallego et al. (2004); Gnaedinger, Adami-Rodrigues & Gallego (2007); Gnaedinger, Adami-Rodrigues & Gallego (2008); Gnaedinger, Adami-Rodrigues & Gallego (2014) | Carnian-Norian (Late Triassic) | La Ternera Fm. (Quebrada La Cachivarita locality; La Ternera hill area, Copiapó Province), and the Las Breas Fm. (Punta del Viento locality, Vicuña, Elqui Province), Chile | • Oviposition scars on Heidiphyllum, Pseudoctenis, Taeniopteris |
| Strullu-Derrien et al. (2012) | Carnian (Late Triassic) | De Geerdalen Formation; Hopen Island, Svalbard Archipelago | • Aggregations of pellets or coprolites within bennettitalean roots • Gall-like structures within the cortical or pith tissues of the larger (probable bennettitalean) axes |
| Rozefelds & Sobbe (1987); Tillyard (1922); Webb (1982) | Carnian (Late Triassic) | Blackstone Formation, Ipswich Coal Measures Group; Sydney Basin, New South Wales, Australia | • Possible oviposition scars and insect eggs on Nilssoniopteris
• Possible galls or eggs on Dictyophyllum • Mining structures on Heidiphyllum, Ginkgoites |
| Meller et al. (2011); Pott et al. (2008); B Aschauer & T Wappler, 2012, unpublished data | Carnian (Late Triassic) | Lunz Formation; Lunz am See, eastern Northern Calcerous Alps, Austria | • Possible oviposition scars and insect eggs on Nilssoniopteris
• Possible mining structures on Nilssonia • Marginal and non-marginal feeding traces on Nilssoniopteris, and other bennettitalean leaves |
| Moisan et al. (2012) | Carnian (Late Triassic) | Madygen Formation; Turkestan Mountains, southwestern Kyrgyzstan, Central Asia | • Oviposition scars on Isoetites |
| Anderson & Anderson (1983); Anderson & Anderson (1985); Anderson & Anderson (2003); Labandeira & Anderson (2005); Scott, Anderson & Anderson (2004) | Carnian (Late Triassic) | Molteno Formation; Karoo Basin, KwaZulu- Natal, Eastern Cape and Northern Cape, South Africa | • Specific and complex herbivory patterns involving the frequency and diversity of 79 distinctive damage types (DTs) on about 220 whole-plant species (liverworts, lycopods, horsetails, ferns, cycads, peltasperms, corystosperms, hamshawvialeans, ginkgoaleans, voltzialean conifers, bennettitaleans, gnetophytes) |
| Linck (1949); Roselt (1954) | Carnian/Ladinian (Upper/Middle Triassic) | Bedheim, Germany | • Borings in Dadoxylon
• Possible oviposition scars on Equisetites |
| Geyer & Kelber (1987); Kelber & Geyer (1989) | Upper Ladinian (Middle Triassic) | Lettenkohle of Alsace, France; Lower Keuper of Franconia, Germany | • Crescent-shape bite marks on Schizoneura, Taeniopteris
• Possible oviposition scars and insect eggs on Equisetites |
| Heer (1877) | Ladinian (late Middle Triassic) | Neuewelt, Lettenkohle, Switzerland | • Possible oviposition scars on Equisetites |
| Minello (1994) | Ladinian (Middle Triassic) | Xinigua, Rio Grande do Sul, Santa Maria Formation (Rosario do Sul Group), Brazil | • Coprolite-bearing borings in Araucarioxylon |
| Grauvogel-Stamm & Kelber (1996) | Early Anisian (Early Middle Triassic) | Grès à Voltzia Formation; Grès-à-Voltzia, northern Vosges Mountains, France | • Crescent-shape bite marks on Neuropteridium
• possible eggs entangled in plant debris |
| McLoughlin (2011) | Anisian—Ladinian (Middle Triassic) | Wivenhoe Hill, Esk Trough, Esk Formation; Queensland, Australia | • Oviposition scars on Taeniopteris |
| McLoughlin (2011) | Olenekian—Anisian (late Early to early Middle Triassic) | Turrimetta Head, Sydney Basin; New South Wales, Australia | • Gall on Dicroidium |
| Kustatscher et al. (2014) | Olenekian (Lower Triassic) | Solling Formation; Bremke and Fürstenberg, Germany | • Specific herbivory patterns involving the frequency and diversity of 8 distinctive damage types (DTs) • External feeding damage on Tongchuanophyllum. Neuropteridium, Pelourdea • Mid-vein gall on Tongchuanophyllum • Linear series of lenticular or ovoidal oviposition scars on Tongchuanophyllum |
Thus, the late Middle Triassic (Ladinian) floras of the Dolomite Region in the Southern Alps of northeastern Italy provide an intriguing window into the early evidence for Herbivore Expansion 3. Ladinian floras from the Dolomites have been extensively studied in recent years (e.g., Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014; Kustatscher & Van Konijnenburg-van Cittert, 2005 and references therein), evidencing a dominance of conifers (Voltzia, Pelourdea), while cycadophytes, seed ferns, ferns, horsetails, and lycopsids are much rarer. Nevertheless, the flora from Monte Agnello is markedly distinct from other Ladinian floras of the Dolomites by its higher diversity and abundance in cycadophytes, seed ferns and ferns. It is currently the best documented and most diverse late Middle Triassic biota in the Alps documenting a rich vascular plant record, including moderate levels of external foliage feeding, piercing-and-sucking, galling, and ovipositional damage.
Of particular importance, from a taphonomic viewpoint, the Dolomites were subject to significant volcanic activity, beginning in the late Ladinian. Consequently, conditions for exceptional preservation were high. Although most of the volcanic complexes were submarine, locally, such as in the area of Predazzo, subaerial eruptive centers existed (Hoernes, 1912; Leonardi, 1967), which alter the natural environment to variable extents and initiate very different effects on community composition, structure, function, and successional turnover on local and regional scales (e.g., Walker & Wardle, 2014). This makes the Monte Agnello ideal for examining the response that such environmental perturbation had on community structures and offers the possibility to study the ecological expansion of interactional diversity recorded from the varied habitats.
Geological and paleontological setting
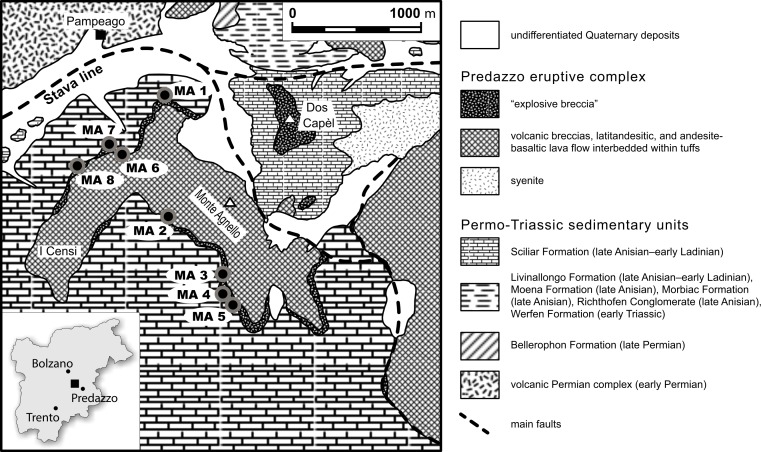
Monte Agnello (Fig. 1) represents an area that was marginally influenced by the Ladinian volcanic activity of the Predazzo volcano and is characterized by a well-preserved stratigraphic succession (e.g., Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014). The 250 m thick volcanic succession is composed of “explosion breccia” at the base, followed by lava breccia, and alternations of lava flows and tuffs (Calanchi, Lucchini & Rossi, 1977; Calanchi, Lucchini & Rossi, 1978; Lucchini, Rossi & Simboli, 1982). The “explosion breccia” comprises lithic fragments (calcareous, volcanic and metamorphic fragments, clastic rocks, isolated crystals), related to the Permo-Triassic volcano-sedimentary succession and the metamorphic basement (Vardabasso, 1930). The lithic fragments of the breccia are bound by carbonate and/or chlorite-serpentine cement (Calanchi, Lucchini & Rossi, 1977). The thickness of this “explosion breccia” varies between 25 m at Monte Agnello and 10 m at Censi. The volcanic succession accumulated mostly in a subaerial environment, and is related to explosive phreatic activity (e.g., bomb sags, antidunes, accretionary lapilli; Calanchi, Lucchini & Rossi, 1977; Lucchini, Rossi & Simboli, 1982).
Figure 1.
Simplified geological map of the Monte Agnello area (Dolomites, N-Italy), modified from Vardabasso (1930). MA1–MA8, fossil sites.
The flora is preserved in the tuff lenses at the base of the “explosion breccia” of the volcanic succession at Predazzo (Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014), which hinders an appropriate stratigraphic correlation between the diffrerent sites. Considering that they are related to one or perhaps a few phreatomagmatic events within the restricted time frame of late Ladinian volcanism the possible time difference between the single localities is however very reduced. The flora is composed of a large number of fronds, stems and reproductive organs of sphenophytes, ferns, seed ferns, cycadophytes and conifers. Due to the preservation in tuff layers, the organic material is missing and sometimes the remains are preserved only as impressions. Several stem fragments belong to the sphenophytes. The ferns are represented by Osmundaceae (Neuropteridium elegans (Brongniart) Schimper in Schimper & Schenk, 1879), Matoniaceae (Phlebopteris fiemmensis Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014) and Dipteridaceae (Thaumatopteris sp.). For the latter two families, it is the oldest fossil occurrence to date for the Northern Hemisphere. Additional ferns of unknown botanical affinity are Cladophlebis ladinica Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014, Cladophlebis sp. (Osmundaceae and/or the Dicksoniaceae) and Chiropteris monteagnellii Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014 (Dipteridaceae?). The seed ferns are represented by leaf fragments of Scytophyllum bergeri Bornemann, 1856. The cycadophyte leaf fragments probably belong to the genera Bjuvia Florin, 1933, Taeniopteris Brongniart, 1828 and/or Macrotaeniopteris Schimper, 1869 as well as Nilssonia Brongniart, 1828 and Apoldia Wesley, 1958. The conifers are represented by shoots of Voltzia Brongniart, 1828 and Pelourdea Seward, 1917 leaves. These plants grew probably during a humid spell, recently proposed for the late Ladinian of the Dolomites (Preto, Kustatscher & Wignall, 2010 and references therein).
Material and Methods
Data collection
Fossil plant assemblages were quantitatively censused from multiple sites at the base of the “explosive breccia,” that crops out on the northwestern slope of Monte Agnello—Censi, overlying a carbonate platform of late Anisian to Ladinian age (Sciliar Dolomite). About 684 specimens have been collected from eight distinctive sites denoted by the prefixes MA 1–MA 8 (Fig. 1 and Table 2). Sample size ranges from 2 to 244 plant remains, depending primarily on the quality and accessibility of the fossils. For the quantitative study, each identifiable plant fossil was counted. Of the plant fossil specimens collected at Monte Agnello, all that were adequately preserved and exceeded a minimum size of 0.5 cm2 were examined for insect damage. Parts and counterparts were matched whenever possible to avoid duplication. When possible, all specimens were assigned to a known species or plant morphotype. All analyzed specimens are housed at the Museo Geologico delle Dolomiti, Predazzo. Specimens occurring on the same rock slab are identified by different letters following the catalogue number whereas capital letters indicate parts and counterparts of the same specimen.
Table 2. Floral and insect damage composition late Ladinian flora from Monte Agnello, Dolomites, Italy.
| Species | # Leaves | % DMG | % Spec | % Gall | % Mine | % External | % PS | % Ovi | DTs | # FFGs | DTO all | DTO spec | DTO external | DT numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bjuvia cf. dolomitica | 113 | 15.93 | 0.89 | 0.89 | 14.16 | 0.89 | 9 | 5 | 21 | 1 | 18 | 1;2;3;12;14; 17;29;80;100 | ||
| Chiropteris montagnellii | 12 | |||||||||||||
| Cladophlebis ladinica | 24 | 4.17 | 4.17 | 1 | 1 | 101 | ||||||||
| Cladophlebis sp. | 4 | |||||||||||||
| Cone indet. | 3 | |||||||||||||
| Elatocladus sp. | 1 | |||||||||||||
| Equisetoid stem fragment | 1 | |||||||||||||
| Indet. | 5 | |||||||||||||
| Neuropteridium elegans | 3 | |||||||||||||
| Nilssonia cf. neuberi | 40 | 10.00 | 10.00 | 1 | 1 | 4 | 4 | 12 | ||||||
| Nilssonia sp. | 34 | 23.53 | 2.91 | 20.59 | 2.94 | 6 | 3 | 10 | 1 | 9 | 1;2;7;12; 13;128 | |||
| Pelourdea sp. | 4 | 25.00 | 25.00 | 1 | 1 | 1 | 1 | 12 | ||||||
| Phlebopteris fiemmensis | 6 | 33.33 | 16.67 | 16.67 | 16.67 | 2 | 2 | 2 | 1 | 1 | 2;80 | |||
| ?Podozamites sp. | 17 | 17.65 | 17.65 | 2 | 3 | 72;100 | ||||||||
| Pterophyllum sp. | 1 | |||||||||||||
| Radicites sp. | 1 | |||||||||||||
| Schizoneura paradoxa | 6 | |||||||||||||
| Scytophyllum bergeri | 55 | 54.55 | 7.27 | 3.64 | 1.82 | 49.09 | 8 | 4 | 37 | 4 | 34 | 3;5;12;13; 14;40;63;80 | ||
| Seed | 2 | |||||||||||||
| Sphenozamites sp. | 37 | 13.51 | 2.70 | 13.51 | 3 | 2 | 5 | 1 | 5 | 2;8;12 | ||||
| Stem indet. | 6 | |||||||||||||
| Taeniopteris sp. | 8 | 25.00 | 25.00 | 2 | 1 | 2 | 2 | 12;14 | ||||||
| Thaumatopteris sp. | 3 | |||||||||||||
| Voltzia sp. 1 | 84 | 3.57 | 1.19 | 2.38 | 1.19 | 2 | 2 | 3 | 1 | 48;121 | ||||
| Voltzia sp. 2 | 41 | 2.44 | 2.44 | 1 | 1 | 1 | 121 | |||||||
| Voltzia sp. indet. | 170 | 2.94 | 2.35 | 0.59 | 2 | 2 | 5 | 1 | 12;121 | |||||
| Wood | 3 | |||||||||||||
| Total | 684 | 12.14 | 1.32 | 1.61 | 0.15 | 9.36 | 0.29 | 0.73 | 20 | 7 | 95 | 9 | 75 | 1;2;3;5;7; 8;12;13;14; 17;29;40;48; 63;72;80;100; 101;121;128 |
Notes.
- DMG
- percentage of damage
- Spec
- Specialized damage
- PS
- Piercing and sucking
- Ovi
- Oviposition
- FFG
- Functional Feeding groups
- DTO
- Damage type occurrence
The most recent approach toward understanding the patterns of herbivory in the fossil record involves quantification of both the richness and intensity of insect damage (Wilf & Labandeira, 1999; Labandeira, Johnson & Lang, 2002; Labandeira et al., 2007; Kustatscher et al., 2014). The richness of herbivory is determined first by establishing a classification system of distinctive, diagnosable damage types, or DTs, that can be used generally in studies of herbivore damage to plants. DTs then are grouped into functional feeding groups (FFG). Eight functional feeding groups are present in the Monte Agnello flora ((i) external foliage feeding, subdivided into hole, margin, surface feeding and skeleotization; (ii) piercing and sucking; (iii) oviposition, though not truly a feeding interaction but rather egg-laying that leaves a significant record of plant damage; (iv) mining and (v) galling). To date, over 290 fossil DTs have been identified (CC Labandeira, pers. comm., 2014). Finally the DTs are ranked by their host specificity (HS), ranging from 1 for generalists to 3 for high host-plant specialization, which then allows non-generalized DTs (e.g., those with HS of 2 and 3) to be analyzed separately.
Each foliar element was photographed using a Canon EOS 30D camera with a Canon EF-S 60 mm f/2.8 macro lens or a Nikon Coolpix E4500. All photographs were optimized using Abobe Photoshop CS6 and Adobe Lightroom 5.
Quantitative analysis
Quantitative analyses of insect damage were done using R version 3.1.0 (www.r-project.org). For damage diversity analyses, sample size was standardized by selecting random subsets of foliar elements without replacement and calculating the damage diversity for the subsample. Subsets of the data were subjected to rarefaction using an analytic method detailed below, which extends the solution found by Wappler et al. (2012) to cases where individuals may belong to multiple classes and allows the explicit reconstruction of probability distributions for the rarefied sample (Heck, van Belle & Simberloff, 1975). This process was repeated 5,000 times, and the results were averaged to obtain the standardized damage diversity for the bulk flora and four single sub-localities (MA1, MA5, MA7, MA8). The remaining sub-localities were removed from the census because the target sample size of at least 40 specimens was not reached. The standard deviations (SD) for the resamples were calculated to provide sample error bars.
Results
Damage on the bulk Monte Agnello flora
Of the 684 plant remains examined from the Monte Agnello flora, 83, or 12.13%, exhibit some sort of damage represented by 20 different damage types. The taxa or morphotypes examined were represented by foliage, axes, stem fragments, fructifications, and dispersed seeds (Table 2). A total of 95 damage type occurrences were observed throughout the bulk flora: 45 on cycadophytes (representing 36.5% of all specimens), 37 on seed ferns (8.0%), ten on conifers (44.3%), and three on ferns (7.6%) (Table 3), suggesting that selective feeding by insect herbivores preferentially targeted particular seed plants. This pattern of selectivity was also recognized within the early late Permian (Wuchiapingian) of the Gröden/Val Gardena Sandstone from the Bletterbach Gorge of the Dolomites (Northern Italy) (T Wappler, pers. obs., 2013). Herbivory recorded for the Monte Agnello sites represents nearly all of the fundamental modes of herbivory, excluding fungal infection, which was not observed (see Gunkel & Wappler, 2015). Multiple DTs or functional feeding groups were only recorded in 1.6% of the plant remains whereas the majority were only damaged in one way (∼11%). Seven distinctive functional feeding groups have been detected on the foliar elements from Monte Agnello, most of which occur on particular plant hosts. Types of the external foliage feeding constitute 78.9% of all DT occurrences and preferentially occurred on the seed fern S. bergeri and consists of the exophytic consumption of live plant tissues, subdivided into skeletonization and margin-, hole- and surface feeding; this is the most common ensemble of Triassic damage types (Labandeira & Prevec, 2014; T Wappler, pers. obs., 2013) (Fig. 2). Those of the galling FFG provided 11.5% of all DT occurrences and are more or less evenly distributed among conifers, ferns and seed ferns (Figs. 3C–3D and 3G). Galling represents the most biologically complex of all major interactions, and represents arthropod-induced abnormal cell proliferation that can occur on all major plant organs (e.g., Kustatscher et al., 2014; Scott, Anderson & Anderson, 2004); examples are widely known (e.g., Stone & Schönrogge, 2003). Oviposition, though not a feeding interaction, comprised 5.2% of all DT occurrences; examples are common (Ghosh, Kar & Chatterjee, 2015; McLoughlin, 2011) (Figs. 3E–3F and 1). Minor levels of insect damage were present for piercing-and-sucking (2.1% of all DT occurrences; Fig. 3J) and mining (1.1%; Fig. 3H) FFGs. Leafminers construct distinct leaf mines, most of which are quite conspicuous and represent a form of endophagous herbivory in which a herbivore targets and feeds on fluid tissues such as phloem, mesophyll or epidermal cell protoplasts (Sinclair & Hughes, 2010); examples are uncommon and the possible mining structure on the pteridosperm Scytophyllum bergeri (Fig. 3H) indicates that the origin and diversification of the leaf-mining habit occurred about 92 million years before the first appearance of fossil angiosperms (Ash, 1997; Gnaedinger, Adami-Rodrigues & Gallego, 2014; Kustatscher et al., 2014; McLoughlin, 2011; Moisan et al., 2012; Pott et al., 2008).
Table 3. Floral and insect damage composition of the late Ladinian flora from Monte Agnello, Dolomites, Italy on higher classification level.
| Plant groups | # Leaves | % DMG | % Spec | % Gall | % Mine | % External | % PS | % Ovi | DTs | # FFGs | DTO all | DTO spec | DTO external | DT numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conifer | 303 | 1.00 | 0.33 | 2.31 | 0.66 | 0.33 | 3 | 3 | 10 | 1 | 2 | 12;48;121 | ||
| Cycadophytes | 250 | 16.00 | 1.20 | 0.40 | 13.60 | 0.40 | 1.60 | 14 | 6 | 45 | 3 | 38 | 1;2;3;7;8; 12;13;14;17;29; 72;80;100;128 | |
| Indet. | 16 | |||||||||||||
| Ferns | 52 | 5.77 | 1.92 | 1.92 | 1.92 | 1.92 | 3 | 2 | 3 | 1 | 1 | 2;80;101 | ||
| Seed ferns | 55 | 54.55 | 7.27 | 3.64 | 1.82 | 49.09 | 8 | 4 | 37 | 4 | 34 | 3;5;12;13;14; 40;63;80 | ||
| Sphenophytes | 8 | |||||||||||||
| Total | 684 | 12.13 | 1.32 | 1.61 | 0.15 | 9.36 | 0.29 | 0.73 | 20 | 7 | 95 | 9 | 75 | 1;2;3;5; 7;8;12;13; 14;17;29;40; 48;63;72; 80;100;101; 121;128 |
Notes.
- DMG
- percentage of damage
- Spec
- Specialized damage
- PS
- Piercing and sucking
- Ovi
- Oviposition
- FFG
- Functional Feeding groups
- DTO
- Damage type occurrence
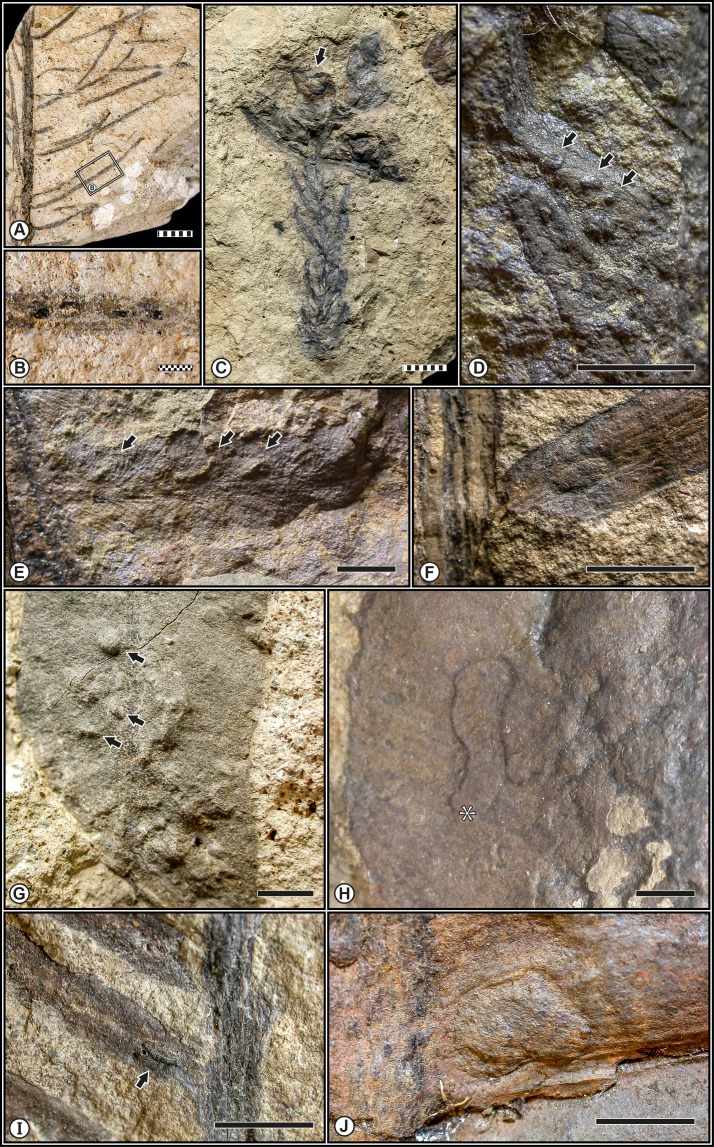
Figure 2. Examples of external foliage feeding at Monte Agnello (Dolomites, N-Italy).
(A), Scytophyllum bergeri Bornemann, 1856 with intensively consumed leaf margins (DT12, 14) (MGP63/97). (B) Hole feeding indicated by leaf removal on both sides of the primary veins (DT63) on S. bergeri Bornemann, 1856 (MGP196/39A-B). (C)–(D) Hole feeding on a Sphenophyte (DT8) (MGP194/106), enlarged in (D) (E) Marginal feeding on the cycadophyte Nilssonia cf. neuberi Stur ex Pott, Kerp & Krings, 2007 (DT12) (MGP191/6A). (F) Excision of leaf to primary vein (DT14) on Bjuvia cf. dolomitica Wachtler & Van Konijnenburg-van Cittert, 2000 (MGP181/11A). (G) Removal or abrasion of surface tissues with a weak reaction rim (DT29) indicated by the dotted lines on B. cf. dolomitica Wachtler & Van Konijnenburg-van Cittert, 2000 (MGP196/43). (H) Cuspate excision (DT81) on S. bergeri Bornemann, 1856 (MGP171/28), enlarged in (I). (J)–(L), External foliage feeding on B. cf. dolomitica Wachtler & Van Konijnenburg-van Cittert, 2000 (MGP195/69A), deep excision of leaf margin enlarged in K (DT12) and interveinal tissue removed in L (DT17). Scale bars: striped, 10 mm; solid, 5 mm; dotted, 1 mm.
Figure 3. Examples of internal foliage consumption at Monte Agnello (Dolomites, N-Italy).
(A)–(B) Elliptical piercing and sucking punctures on the conifer Voltzia sp. 1 (MGP196/35), enlarged in (B) (DT48). (C) Ellipsoidal, sessile bud gall from branchlet (DT121) on the unaffiliated Voltzia sp. 1 (MGP171/81). (D) Small, hemispherical, thoroughly carbonized structures (DT80) on Phlebopteris fiemmensis Kustatscher et al., 2014 (MGP181/57C), indicated by arrows. (E) Fern Speirocarpus sp. (MGP197/69B) showing lenticular-ovoidal foliar oviopsition scars (DT101), indicated by arrows. (F) and (I) Lenticular-ovoidal foliar oviopsition scars (DT100) on the unaffiliated cycadophytes (MGP196/6; MGP196/7A). (G) Undifferentiated galling structures (DT80) on a seed-fern (MGP63/94), indicated by arrows. (H) Semilinear, frass-laden, mining structure with a smooth and rimmed margin (DT40) on Scytophyllum bergeri Bornemann, 1856 (MGP63/98A), asterisk indicates initial place of oviposition. (J) Ellipsoidal scale impressions with roughened surface (DT128) on the cycadophyte Nilssonia cf. neuberi Stur ex Pott, Kerp & Krings, 2007 (DT128) (MGP194/72A). Scale bars: striped, 10 mm; solid, 5 mm; dotted, 1 mm.
Damage on individual species
Among the 28 taxa represented at Monte Agnello less than half indicate some kind of damage, whereas, three—Scytophyllum bergeri, Bjuvia cf. dolomitica and Nilssonia sp.—are the most herbivorized taxa (71,6% of all DT occurrences) but only representing one-third of the flora (Table 2). The most abundant plant species are the conifers Voltzia sp. (Fig. 3C) and Voltzia sp. 2, which have the lowest damage frequency (2.44–2.94%) of the common Monte Agnello taxa. Ferns are nearly equally as diverse as the seed ferns but damage frequency is at least ten times less abundant than among the seed ferns (Table 3 and Fig. 3D). Sphenophytes displayed no signs of insect-mediated herbivory but the small number of sampled leaves open the possibility that more collecting and study may yet reveal damage to this group also.
Damage at distinct sub-localities
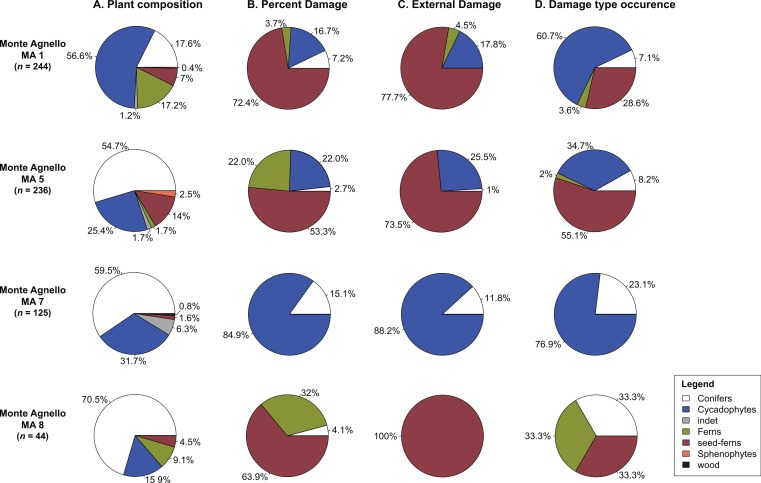
Plant material is generally preserved at the base of the “explosion breccia” at an angle to the bedding rather than compacted into a single horizon. Transport distance, therefore, must have been short, and burial was likely rapid. Thus, the fossil leaf assemblages must be considered as para-autochthonous (e.g., Hanley et al., 2007). Minimal transport allows us to document considerable changes in species composition and insect folivory over short distances and recognize possible heterogeneity in the structure and composition of the source plant communities and their associated herbivores. Large-scale disturbances may profoundly alter the composition and structure of plant communities and are rarely uniform in their influence on vegetation (Kustatscher, Dellantonio & Van Konijnenburg-van Cittert, 2014). Variations of floral composition and insect herbivore damage at the four sub-localities (MA1, MA5, MA7, MA8) censused are shown in Fig. 4 and Table 4. MA1 has the highest floral diversity (22 ssp.), followed by MA5 (16 ssp.) and MA7 (15 ssp.). MA8 is an extremely low-diversity flora (7 ssp.). Interestingly, all sites are strongly dominated by a single plant group representing in all cases over half of the characteristic plant material at that site. The most abundant plant lineage at MA5–MA8 is conifers, whereas at MA1 57% of the taphocoenosis is composed of cycadophytes. However, when analyzing herbivory on individual host groups at the four sub-localities, total damage frequency and external foliage feeding is overwhelmingly found on seed-fern hosts (Fig. 4), except MA7 where the preferred host-plants are cycadophytes.
Figure 4. Plant and damage composition within the single sub-localities.
Pie charts showing the frequency specimen data by (A). Host plant abundance (pooled in higher taxonomic ranks). (B)–(D) Damage composition. MA1, MA5, MA7, MA8, fossil sites.
Table 4. Floral diversity and evenness.
| Flora | N | S | Rarefied species diversity at 40 leaves | Rarefied external damage diversity at 40 leaves | Rarefied specialized damage diversity at 40 leaves | Pielou‘s J | Simpson D |
|---|---|---|---|---|---|---|---|
| MA1 | 244 | 22 | 12.89 ± 1.52 | 2.30 ± 1.13 | 0.63 ± 0.69 | 0.89 | 1.89 |
| MA5 | 236 | 16 | 9.03 ± 1.41 | 3.19 ± 1.08 | 0.68 ± 0.75 | 0.73 | 1.83 |
| MA7 | 125 | 15 | 9.38 ± 1.47 | 1.29 ± 0.49 | na | 0.63 | 1.70 |
| MA8 | 44 | 7 | 6.85 ± 0.36 | 0.93 ± 0.25 | na | 0.75 | 1.71 |
Discussion
Volcanogenic deposits can preserve spatio-temporal biotic patterns at levels of resolution not commonly represented in the fossil record. Consequently, the plant–insect assemblages recognized in this study appear compositionally and ecologically unique (Currano et al., 2011; Dale, Swanson & Crisafulli, 2005). The para-autochthonous early late Ladinian flora of the Monte Agnello (Dolomites, N-Italy) offers insights into the patterns of arthropod herbivory during the beginning of the third pulse of herbivore expansion (sensu Labandeira, 2006a; Labandeira, 2006b; Labandeira & Currano, 2013: Fig. 1). It also provides insights into the way herbivores responded to environmental perturbation and the reorganization of community structure. Even though our data are preliminary, the palaeoecological and temporal setting of the early late Ladinian flora of the Monte Agnello in the Dolomites supports three major conclusions that parallel those drawn from data known from intensively studied Gondwanan sites.
-
(1)
Dominance of seed plant herbivory. The dominance of seed plant herbivory by local arthropod herbivores, particularly that known since the Permian across western Euamerica (e.g., Schachat et al., 2014), Europe (Geyer & Kelber, 1987; T Wappler, pers. obs., 2013), Cathaysia (Glasspool et al., 2003), and in the extensive glossopterid-dominated floras across Gondwana (e.g., Cariglino & Gutiérrez, 2011; McLoughlin, 2011; Prevec et al., 2009) is also a conspicuous component of the late Anisian to Ladinian environments. This documents the persistence of the preferential targeting of selected groups of seed plants, like the cycadopytes in Monte Agnello, particularly by external foliage feeders. The pattern could be interpreted to support Feeny’s apparency hypothesis (Feeny, 1976), as seed plants were the most abundant and conspicuous, and therefore would have been the most apparent to herbivore consumption. However, for the Monte Agnello data, a more likely explanation favors increased herbivory on particular plants due to the anatomy of their leaves, suggesting that particular physical traits, like the scleromorphic structures of conifer taxa, reduce the palatability and digestibility of such plant material or act as a deterrent when more palatable plants are available (Labandeira & Anderson, 2005).
-
(2)
Increase of interactional diversity and rise of the leaf-mining habit. There is an increase in plant–insect interactional diversity during the Early to Late Triassic in eastern Euamerica and Gondwana regions (e.g., Kustatscher et al., 2014; Scott, Anderson & Anderson, 2004), coupled with an increase in the diversity of FFGs, DTs, and associated herbivore behaviors observed at Monte Agnello, compared to insect damage from earlier known floras (e.g., Kustatscher et al., 2014). Of particular importance is the presence of the leaf-mining habit in which holometabolous insect larvae consume the inner parenchymal, epidermal, vascular, or other tissues of a plant, leaving the outer wall of the epidermis undamaged (Hering, 1951). The earliest documented leaf-mining fossil records have been reported from Kyrgyzstan, Austria, Australia and South Africa in deposits of Middle to Late Triassic age (comp. Table 1).
-
(3)
Volcanic activity and site-specific habitat differences. The data presented here show that volcanogenic deposits are valuable for the creation and preservation of in situ sequential stages of biotic change not commonly represented in the fossil record. These episodic volcanic activities directly influenced the evolution of the environment, spatial structure and temporal dynamics of the plant community and the herbivores associated with the plants, resulting in vegetational heterogeneity had impact on both the likelihood and strength of interactions between plants and insect herbivores (e.g., Agrawal, Lau & Hambäck, 2006; Currano et al., 2011). Therefore, the heterogeneity among the sub-localities indicates that volcanic disturbance caused compositional and structural changes in the ecosystem during the time it occupied the site, which explain variations in plant physiognomy, plant and insect herbivore composition, and the overall paleoecology (Table 4). This conclusion is supported by (1) the spatial variability in the percentage of herbivorized plant host specimens, (2) the elevated number of DTs on each host plant, and (3) the differences in evenness and the relative abundance distributions of damage among the single sub-localities.
These conclusions warrant further verification from investigations of additional new sites to clarify patterns of arthropod herbivory during this crucial period of time where terrestrial ecosystems were beginning to become modern.
Acknowledgments
We thank Federica Angeli (Trento, Italy), Fulvio Boninsegna (Predazzo, Italy), Andrea Braito (Daiano, Italy), Daniele Ferrari (Museo Geologico delle Dolomiti, Predazzo, Italy), Christian Fontana (Vigo di Fassa, Italy) and Guido Roghi (CNR and University of Padova, Italy) for their help during fieldwork and the preparation of the fossils. Special thanks goes to Daniele Ferrari for the photographs of the plants. The authors acknowledge Ellen Currano, University of Wyoming, an anonymous reviewer, and the editor William DiMichele for their constructive and encouraging comments.
Institutional abbreviations
- MGP
Museo Geologico delle Dolomiti Predazzo Specimens occurring on the same rock slab are identified by different capital letters following the catalogue number.
Funding Statement
The work has been supported by the Comune di Predazzo (Provincia di Trento). This study is part of the project “The Permian-Triassic ecological crisis in the Dolomites: extinction and recovery dynamics in Terrestrial Ecosystems” financed by the Promotion of Educational Policies, University and Research Department of the Autonomous Province of Bolzano—South Tyrol. TW is supported by a Heisenberg grant from the German Science Foundation (1496/8-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Elio Dellantonio works for the Museo Ceologicao dell Dolomiti, and Evelyn Kustatscher is employed at the Naturmuseum Südtirol.
Author Contributions
Torsten Wappler conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Evelyn Kustatscher contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Elio Dellantonio contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
References
- Adami-Rodrigues et al. (2004).Adami-Rodrigues K, de Souza PA, Iannuzzi R, Pinto ID. Herbivoria em floras gonduânicas do neopaleózoico do Rio Grande do Sul: análise quantitativa. Revista Brasileira de Paleontologia. 2004;7:93–102. doi: 10.4072/rbp.2004.2.01. [DOI] [Google Scholar]
- Adami-Rodrigues, Gnaedinger & Gallego (2008).Adami-Rodrigues K, Gnaedinger S, Gallego OF. Registro de interações inseto-planta do grupo El Tranquilo (Triássico Superior) Provincia de Santa Cruz, Patagonia Argentina. In: Boardman DR, editor. Simpósio Brasileiro de Paleobotânica e Palinologia Boletim de Resumos. Florianópolis: Asociación Latinoamericana de Paleobotánica y Palinología; 2008. [Google Scholar]
- Adami-Rodrigues, Iannuzzi & Pinto (2004).Adami-Rodrigues K, Iannuzzi R, Pinto ID. Permian plant–insect interactions from a Gondwana flora of southern Brazil. Fossils and Strata. 2004;51:106–125. [Google Scholar]
- Agrawal, Lau & Hambäck (2006).Agrawal AA, Lau JA, Hambäck PA. Community heterogeneity and the evolution of interactions between plants and insect herbivores. The Quarterly Review of Biology. 2006;81:349–376. doi: 10.1086/511529. [DOI] [PubMed] [Google Scholar]
- Anderson & Anderson (1983).Anderson JM, Anderson HM. Palaeoflora of southern Africa: Molteno Formation (Triassic). Volume 1: Part 1. Introduction: part 2. Dicroidium. Rotterdam: Balkema; 1983. [Google Scholar]
- Anderson & Anderson (1985).Anderson JM, Anderson HM. Palaeoflora of Southern Africa: prodromus of South African Megafloras: devonian to Lower Cretaceous: Rotterdam. Rotterdam: Balkema; 1985. [Google Scholar]
- Anderson & Anderson (2003).Anderson JM, Anderson HM. Heyday of the gymnosperms: systematics and biodiversity of the Late Triassic Molteno fructifications. Strelitzia. 2003;15:1–398. [Google Scholar]
- Aristov et al. (2013).Aristov DS, Bashkuev AS, Golubev VK, Gorochov AV, Karasev EV, Kopylov DS, Ponomarenko AG, Rasnitsyn AP, Rasnitsyn DA, Sinitshenkova ND, Sukatsheva ID, Vassilenko DV. Fossil insects of the middle and upper Permian of European Russia. Paleontological Journal. 2013;47:641–832. doi: 10.1134/S0031030113070010. [DOI] [Google Scholar]
- Ash (1997).Ash S. Evidence of arthropod-plant interactions in the Upper Triassic of the southeastern United States. Lethaia. 1997;29:237–248. doi: 10.1111/j.1502-3931.1996.tb01657.x. [DOI] [Google Scholar]
- Ash (1999).Ash S. An Upper Triassic Sphenopteris showing evidnce of insect predation from Petrified Forest National Park, Arizona. International Journal of Plant Sciences. 1999;160:208–215. doi: 10.1086/314115. [DOI] [Google Scholar]
- Ash (2000).Ash S. Evidence of oribatid mite herbivory in the stem of a Late Triassic tree fern from Arizona. Journal of Paleontology. 2000;74:1065–1071. doi: 10.1666/0022-3360(2000)074<1065:EOOMHI>2.0.CO;2. [DOI] [Google Scholar]
- Ash (2001).Ash S. New cycadophytes from the Upper Triassic Chinle Formation of the southwestern United States. Paleobios. 2001;21:15–28. [Google Scholar]
- Ash (2005).Ash S. A new Triassic flora and associated invertebrate fossils from the basal beds of the Chinle Formation, near Cameron, Arizona. Paleobios. 2005;25:17–34. [Google Scholar]
- Ash (2014).Ash SR. Contributions to the Upper Triassic Chinle flora in the American southwest. Palaeobiodiversity and Palaeoenvironments. 2014;94:279–294. doi: 10.1007/s12549-014-0150-3. [DOI] [Google Scholar]
- Ash & Savidge (2004).Ash S, Savidge RA. The bark of the Late Triassic Araucarioxylon arizonicum tree from the Petrified Forest National Park, Arizona. IAWA Journal. 2004;25:349–368. doi: 10.1163/22941932-90000371. [DOI] [Google Scholar]
- Benton (2010).Benton MJ. The origins of modern biodiversity on land. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2010;365:3667–3679. doi: 10.1098/rstb.2010.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton & Emerson (2007).Benton MJ, Emerson BC. How did life become so diverse? The dynamics of diversification according to the fossil record and melecular phylogenetics. Palaeontology. 2007;50:23–40. doi: 10.1111/j.1475-4983.2006.00612.x. [DOI] [Google Scholar]
- Béthoux, Papier & Nel (2005).Béthoux O, Papier F, Nel A. The Triassic radiation of the entomofauna. Comptes Rendus Palevol. 2005;4:609–621. doi: 10.1016/j.crpv.2005.06.005. [DOI] [Google Scholar]
- Bornemann (1856).Bornemann JG. Über organische Reste der Lettenkohlengruppe Thüringens—Ein Beitrag zur Fauna und Flora dieser Formation. Leipzig: W. Engelmann Verlag; 1856. [Google Scholar]
- Brongniart (1828).Brongniart AT. Histoire des végétaux fossiles, ou recherches botaniques et géologiques sur les végétaux renfermés dans les divers couches du globe. Paris, Amsterdam: Dufour/d’Ocagne; 1828–1837. [Google Scholar]
- Calanchi, Lucchini & Rossi (1977).Calanchi N, Lucchini F, Rossi PL. M. Agnello: un apparato vulcanico a condotto centrale nelle Dolomiti. Minerologica Petrografica Acta. 1977;21:221–229. [Google Scholar]
- Calanchi, Lucchini & Rossi (1978).Calanchi N, Lucchini F, Rossi PL. The Volcanic Rocks from the Mount Agnello Area (Fiemme Valley, Italy): a contribution to the knowledge of the Mid-Triassic magmatism of the Southern Alps. Tschermaks mineralogische und petrographische Mitteilungen. 1978;25:131–143. doi: 10.1007/BF01082858. [DOI] [Google Scholar]
- Cariglino & Gutiérrez (2011).Cariglino B, Gutiérrez PR. Plant-insect interactions in a Glossopteris flora from the La Golondrina Formation (Guadalupian–Lopingian), Santa Cruz Province, Patagonia, Argentina. Ameghiniana. 2011;48:103–112. doi: 10.5710/AMGH.v48i1(321). [DOI] [Google Scholar]
- Creber & Ash (2004).Creber GT, Ash SR. The Late Triassic Schilderia adamanica and Woodworthia arizonica trees of the Petrified Forest National Park, Arizona, USA. Palaeontology. 2004;47:21–38. doi: 10.1111/j.0031-0239.2004.00345.x. [DOI] [Google Scholar]
- Currano et al. (2011).Currano ED, Jacobs BF, Pan AD, Tabor NJ. Inferring ecological disturbance in the fossil record: a case study from the late Oligocene of Ethiopia. Palaeogeography, Palaeoclimatology, Palaeoecology. 2011;309:242–252. doi: 10.1016/j.palaeo.2011.06.007. [DOI] [Google Scholar]
- Dale, Swanson & Crisafulli (2005).Dale VH, Swanson FJ, Crisafulli CM. Disturbance, survival, and succession: understanding ecological responses to the 1980 eruption of Mount St. Helens. New York: Springer; 2005. [Google Scholar]
- Feeny (1976).Feeny P. Plant apparency and chemical defense. In: Wallace JW, Mansell RL, editors. Biochemical interaction between plants and insects. New York: Plenum Press; 1976. pp. 1–40. [Google Scholar]
- Feng et al. (2014).Feng Z, Su T, Yang JY, Chen YX, Wei HB, Dai J, Guo Y, Liu JR, Ding JH. Evidence for insect-mediated skeletonization on an extant fern family from the Upper Triassic of China. Geology. 2014;42:407–410. doi: 10.1130/G35369.1. [DOI] [Google Scholar]
- Florin (1933).Florin R. Studien über die Cycadales des Mesozoicums nebst Erörterungen über die Spaltöffnungsapparate der Bennettitales. Kungliga Svenska Vetenskapsakademiens Handlingar, Ser. 3. 1933;12:1–119. [Google Scholar]
- Gallego, Cúneo & Escapa (2014).Gallego J, Cúneo R, Escapa I. Plant–arthropod interactions in gymnosperm leaves from the Early Permian of Patagonia, Argentina. Geobios. 2014;47:101–110. doi: 10.1016/j.geobios.2014.01.002. [DOI] [Google Scholar]
- Gallego et al. (2003).Gallego OF, Gnaedinger S, Kirsten O, Giovanelli S. Primera cita de trazas fósiles de insectos en hojas del Pérmico de Uruguay y Triásico de Chile. Universidad Nacional Del Nordeste, Comunicaciones Científicas y Tecnológicas Biológicas. 2003;B032:1–4. [Google Scholar]
- Gallego et al. (2004).Gallego OF, Gnaedinger S, Labandeira CC, Martins-Neto RG, Kirsten O. Permian and Triassic insect traces on fossil leaves from Uruguay and Chile. Abstract 35International Congress on Ichnology ICHNIA Abstract Book. 2004;1 [Google Scholar]
- Geyer & Kelber (1987).Geyer G, Kelber K-P. Fluügel und Lebensspuren von Insekten aus dem Unteren Keuper Mainfrankens. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 1987;174:331–355. [Google Scholar]
- Ghosh, Kar & Chatterjee (2015).Ghosh AK, Kar R, Chatterjee R. Leaf galls on Dicroidium hughesii (Feistmantel) Lele from the Triassic of India—a new record. Alcheringa. 2015;39:92–98. doi: 10.1080/03115518.2015.958285. [DOI] [Google Scholar]
- Giebel (1853).Giebel CG. Referat über Spieker. Zeitschrift für die gesamten Naturwissenschaften. 1853;2(7):34. [Google Scholar]
- Glasspool et al. (2003).Glasspool I, Hilton J, Collinson ME, Wang S-J. Foliar herbivory in Late Palaeozoic Cathaysian gigantopterids. Review of Palaeobotany and Palynology. 2003;127:125–132. doi: 10.1016/S0034-6667(03)00107-6. [DOI] [Google Scholar]
- Gnaedinger, Adami-Rodrigues & Gallego (2007).Gnaedinger SC, Adami-Rodrigues K, Gallego OF. Evidencias de trazas de oviposición de insectos (Odonata) en hojas del Triásico de Chile. Ameghiniana. 2007;44:94R. [Google Scholar]
- Gnaedinger, Adami-Rodrigues & Gallego (2008).Gnaedinger SC, Adami-Rodrigues K, Gallego OF. Insect egg ovipositions on leaves from the Upper Triassic from northern Chile. In: Boardman DR, editor. Simpósio Brasileiro de Paleobotânica e Palinologia Boletim de Resumos. Florianópolis: Asociación Latinoamericana de Paleobotánica y Palinología; 2008. [Google Scholar]
- Gnaedinger, Adami-Rodrigues & Gallego (2014).Gnaedinger SC, Adami-Rodrigues K, Gallego OF. Endophytic oviposition on leaves from the Late Triassic of northern Chile: ichnotaxonomic, palaeobiogeographic and palaeoenvironment considerations. Geobios. 2014;47:221–236. doi: 10.1016/j.geobios.2014.06.003. [DOI] [Google Scholar]
- Grauvogel-Stamm & Ash (2005).Grauvogel-Stamm L, Ash SR. Recovery of the Triassic land flora from the end-Permian life crisis. Comptes Rendus Palevol. 2005;4:593–608. doi: 10.1016/j.crpv.2005.07.002. [DOI] [Google Scholar]
- Grauvogel-Stamm & Kelber (1996).Grauvogel-Stamm L, Kelber K-P. Plant-insect interactions and coevolution during the Triassic in western Europe. Paleontologica Lombarda, NS. 1996;5:5–23. [Google Scholar]
- Gunkel & Wappler (2015).Gunkel S, Wappler T. Plant-insect interactions in the upper Oligocene of Enspel (Westerwald, Germany), including an extended mathematical framework for rarefaction. Palaeobiodiversity and Palaeoenvironments. 2015;95:55–75. doi: 10.1007/s12549-014-0176-6. [DOI] [Google Scholar]
- Haig et al. (2015).Haig DW, Martin SK, Mory AJ, McLoughlin S, Backhouse J, Berrell RW, Kear BP, Hall R, Foster CB, Shi GR, Bevan JC. Early Triassic (early Olenekian) life in the interior of East Gondwana: mixed marine–terrestrial biota from the Kockatea Shale, Western Australia. Palaeogeography, Palaeoclimatology, Palaeoecology. 2015;417:511–533. doi: 10.1016/j.palaeo.2014.10.015. [DOI] [Google Scholar]
- Hanley et al. (2007).Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:157–178. doi: 10.1016/j.ppees.2007.01.001. [DOI] [Google Scholar]
- Heck, van Belle & Simberloff (1975).Heck KL, Van Belle G, Simberloff D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56:1459–1461. doi: 10.2307/1934716. [DOI] [Google Scholar]
- Heer (1877).Heer O. Die vorweltliche Flora der Schweiz. Zürich: J. Wurster; 1877. [Google Scholar]
- Hering (1951).Hering EM. Biology of the leaf miners. Dordrecht: Springer; 1951. [Google Scholar]
- Hoernes (1912).Hoernes R. Zur Geologie von Predazzo. Sitzungsberichte der königlichen Akademie der Wissenschaft. 1912;121:3–31. [Google Scholar]
- Hsü et al. (1974).Hsü J, Chu C-N, Chen Y, Tuan S-Y, Hu Y-F, Chu W-C. New genera and species of the late triassic plants from Yungjen, Yunnan I. Acta Botanica Sinica. 1974;16:266–278. [Google Scholar]
- Iannuzzi & Labandeira (2008).Iannuzzi R, Labandeira CC. The oldest record and early history of insect folivory. Annals of the Entomological Society of America. 2008;101:79–94. doi: 10.1603/0013-8746(2008)101[79:TOROEF]2.0.CO;2. [DOI] [Google Scholar]
- Kelber & Geyer (1989).Kelber K-P, Geyer G. Lebensspuren von Insekten an Pflanzen des Unteren Keupers. Courier Forschungsinstitut Senckenberg. 1989;109:165–174. [Google Scholar]
- Kustatscher, Dellantonio & Van Konijnenburg-van Cittert (2014).Kustatscher E, Dellantonio E, Van Konijnenburg-van Cittert JHA. The ferns of the late Ladinian, Middle Triassic flora from Monte Agnello, Dolomites, N-Italy. Acta Palaeontologica Polonica. 2014;59:741–755. doi: 10.4202/app.2012.0076. [DOI] [Google Scholar]
- Kustatscher et al. (2014).Kustatscher E, Franz M, Heunisch C, Reich M, Wappler T. Floodplain habitats of braided river systems: depositional environment, flora and fauna of the Solling Formation (Buntsandstein, Lower Triassic) from Bremke and Fürstenberg (Germany) Palaeobiodiversity and Palaeoenvironments. 2014;94:237–270. doi: 10.1007/s12549-014-0161-0. [DOI] [Google Scholar]
- Kustatscher & Van Konijnenburg-van Cittert (2005).Kustatscher E, Van Konijnenburg-van Cittert JHA. The Ladinian flora (Middle Triassic) of the Dolomites: palaeoenvironmental reconstructions and palaeoclimatic considerations. GeoAlp. 2005;2:31–51. [Google Scholar]
- Labandeira (2006a).Labandeira CC. The four phases of plant-arthropod associations in deep time. Geologica Acta. 2006a;4:409–438. [Google Scholar]
- Labandeira (2006b).Labandeira CC. Silurian to Triassic plant and hexapod clades and their associations: new data, a review, and interpretations. Arthropod Systematics & Phylogeny. 2006b;64:53–94. [Google Scholar]
- Labandeira & Anderson (2005).Labandeira CC, Anderson JM. Insect leaf-mining in Late Triassic gymnospermous floras from the Molteno Formation of South Africa. Abstract 15Geological Society of America Abstracts with Programs. 2005;37 [Google Scholar]
- Labandeira & Currano (2013).Labandeira CC, Currano ED. The fossil record of plant–insect dynamics. Annual Review of Earth and Planetary Sciences. 2013;41:287–311. doi: 10.1146/annurev-earth-050212-124139. [DOI] [Google Scholar]
- Labandeira, Johnson & Lang (2002).Labandeira CC, Johnson KR, Lang PJ. Preliminary assessment of insect herbivory across the Cretaceous-Tertiary boundary: major extinction and minimum rebound. In: Hartman JH, Johnson KR, Nichols DJ, editors. The Hell Creek formation of the northern Great Plains. Boulder: Geological Society of America Special Paper; 2002. pp. 297–327. [Google Scholar]
- Labandeira & Prevec (2014).Labandeira CC, Prevec R. Plant paleopathology and the roles of pathogens and insects. International Journal of Paleopathology. 2014;4:1–16. doi: 10.1016/j.ijpp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Labandeira et al. (2007).Labandeira CC, Wilf P, Johnson KR, Marsh F. Guide to insects (and other) damage types on compressed plant fossils. Version 3.0. Washington, D.C.: Smithsonian Institution; 2007. [Google Scholar]
- Leonardi (1967).Leonardi P. Le Dolomiti: geologia dei monti tra Isarco e Piave. Rovereto: Consiglio Nazionale delle Ricerche e della Giunta Proviniciale di Trento; 1967. [Google Scholar]
- Linck (1949).Linck O. Fossile Bohrgänge (Anobichnium simile ngn sp.) an einem Keuperholz. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie B. 1949;90:180–185. [Google Scholar]
- Looy et al. (1999).Looy CV, Brugman WA, Dilcher DL, Visscher H. The delayed resurgence of equatorial forests after the Permian–Triassic ecologic crisis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13857–13862. doi: 10.1073/pnas.96.24.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini, Rossi & Simboli (1982).Lucchini F, Rossi PL, Simboli G. Il magmatismo triassico dell’area di Predazzo. In: Castellarin A, Vai GB, editors. Guida alla Geologia del Sudalpino centro-orientale. Roma: Societá Geologica Italiana, Guide Geologiche Regionali; 1982. pp. 221–229. [Google Scholar]
- Lukashevich et al. (2010).Lukashevich ED, Przhiboro AA, Marchal-Papier F, Grauvogel-Stamm L. The oldest occurrence of immature Diptera (Insecta), Middle Triassic, France. Annales de la Société Entomologique de France (Nouvelle série) 2010;46:4–22. doi: 10.1080/00379271.2010.10697636. [DOI] [Google Scholar]
- McLoughlin (2011).McLoughlin S. New records of leaf galls and arthropod oviposition scars in Permian–Triassic Gondwanan gymnosperms. Australian Journal of Botany. 2011;59:156–169. doi: 10.1071/BT10297. [DOI] [Google Scholar]
- Meller et al. (2011).Meller B, Ponomarenko AG, Vasilenko DV, Fischer TC, Aschauer B. First beetle elytra, abdomen (Coleoptera) and a mine trace from Lunz (Carnian, Late Triassic, Lunz-am-See, Austria) and their taphonomical and evolutionary aspects. Palaeontology. 2011;54:97–110. doi: 10.1111/j.1475-4983.2010.01009.x. [DOI] [Google Scholar]
- Minello (1994).Minello LF. As “florestas petrificadas” da região de São Pedro do Sul e Mata, RS. III—análise morfológica megascópica, afinidades e considerações paleoambientais. Acta Geologica Leopoldensia. 1994;39:75–91. [Google Scholar]
- Moisan et al. (2012).Moisan P, Labandeira CC, Matushkina NA, Wappler T, Voigt S, Kerp H. Lycopsid-arthropod associations and odonatopteran oviposition on Triassic herbaceous Isoetites. Palaeogeography Palaeoclimatology Palaeoecology. 2012;344–345:6–15. doi: 10.1016/j.palaeo.2012.05.016. [DOI] [Google Scholar]
- Nathorst (1876).Nathorst AG. Bidrag till Sveriges fossila Flora. Kongliga Svenska Vetenskaps-Akademiens Handlingar. 1876;14:1–82. [Google Scholar]
- Nathorst (1878).Nathorst AG. Beiträge zur fossilen Flora Schwedens. Über einige rhätische Pflanzen von Pålsjö in Schonen. Stuttgart: E. Schweizerbart’sche Verlagshandlung (E. Koch); 1878. [Google Scholar]
- Pott, Kerp & Krings (2007).Pott C, Kerp H, Krings M. Morphology and epidermal anatomy of Nilssonia (cycadalean foliage) from the Upper Triassic of Lunz (Lower Austria) Review of Palaeobotany and Palynology. 2007;143:197–217. doi: 10.1016/j.revpalbo.2006.07.007. [DOI] [Google Scholar]
- Pott et al. (2008).Pott C, Labandeira CC, Krings M, Kerp H. Fossil insect eggs and ovipositional damage on bennettitalean leaf cuticles from the Carnian (Upper Triassic) of Austria. Journal of Paleontology. 2008;82:778–789. doi: 10.1666/06-094.1. [DOI] [Google Scholar]
- Preto, Kustatscher & Wignall (2010).Preto N, Kustatscher E, Wignall PB. Triassic climates—state of the art and perspectives. Palaeogeography, Palaeoclimatology, Palaeoecology. 2010;290:1–10. doi: 10.1016/j.palaeo.2010.03.015. [DOI] [Google Scholar]
- Prevec et al. (2009).Prevec R, Labandeira CC, Neveling J, Gastaldo RA, Looy CV, Bamford M. Portrait of a Gondwanan ecosystem: a new late Permian fossil locality from KwaZulu-Natal, South Africa. Review of Palaeobotany and Palynology. 2009;156:454–493. doi: 10.1016/j.revpalbo.2009.04.012. [DOI] [Google Scholar]
- Roopnarine et al. (2007).Roopnarine PD, Angielczyk KD, Wang SC, Hertog R. Trophic network models explain instability of Early Triassic terrestrial communities. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2077–2086. doi: 10.1098/rspb.2007.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselt (1954).Roselt G. Ein neuer Schachtelhalm aus dem Keuper und Beiträge zur Kenntnis von Neocalamites meriani Brongn. Geologie. 1954;3:617–643. [Google Scholar]
- Rozefelds & Sobbe (1987).Rozefelds AC, Sobbe I. Problematic insect leaf mines from the Upper Triassic Ipswich Coal Measures of southeastern Queensland, Australia. Alcheringa. 1987;11:51–57. doi: 10.1080/03115518708618979. [DOI] [Google Scholar]
- Schachat et al. (2014).Schachat S, Labandeira CC, Gordon J, Chaney D, Levi S, Halthore MS, Alvarez J. Plant–insect interactions from the Early Permian (Kungurian) Colwell Creek Pond, North-Central Texas: the early spread of herbivory in riparian environments. International Journal of Plant Sciences. 2014;175:855–890. doi: 10.1086/677679. [DOI] [Google Scholar]
- Schimper (1869).Schimper WP. Traité de Paléontologie végétale ou la flore du monde primitif dans ses rapports avec les formations géologiques et la flore du monde actuel. Tome premier. Paris: Bailliére J.B. et Fils; 1869. [Google Scholar]
- Schimper & Schenk (1879).Schimper WP, Schenk A. In: Handbuch der Palaeontologie, Teil II. Palaeophytologie. Lief. 1. Zittel KA, editor. Leipzig: R. Oldenbourg; 1879. pp. 1–152. [Google Scholar]
- Scott, Anderson & Anderson (2004).Scott AC, Anderson JM, Anderson HM. Evidence of plant–insect interactions in the Upper Triassic Molteno Formation of South Africa. Journal of the Geological Society, London. 2004;161:401–410. doi: 10.1144/0016-764903-118. [DOI] [Google Scholar]
- Seward (1917).Seward AC. Fossil plants, III pteridospermae, cycadofilices, cordaitales, cycadophyta. Cambridge: Cambridge University Press; 1917. [Google Scholar]
- Shcherbakov (2008a).Shcherbakov DE. On Permian and Triassic insect faunas in relation to biogeography and the Permian–Triassic crisis. Paleontological Journal. 2008a;42:15–31. doi: 10.1007/s11492-008-1003-1. [DOI] [Google Scholar]
- Shcherbakov (2008b).Shcherbakov DE. Insect recovery after the Permian/Triassic crisis. Alavesia. 2008b;2:125–131. [Google Scholar]
- Sinclair & Hughes (2010).Sinclair RJ, Hughes L. Leaf miners: the hidden herbivores. Austral Ecology. 2010;35:300–313. doi: 10.1111/j.1442-9993.2009.02039.x. [DOI] [Google Scholar]
- Slater, McLoughlin & Hilton (2012).Slater BJ, McLoughlin S, Hilton J. Animal–plant interactions in a Middle Permian permineralised peat of the Bainmedart Coal Measures, Prince Charles Mountains, Antarctica. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;363–364:109–126. doi: 10.1016/j.palaeo.2012.08.018. [DOI] [Google Scholar]
- Stone & Schönrogge (2003).Stone GN, Schönrogge K. The adaptive significance of insect gall morphology. Trends in Ecology & Evolution. 2003;18:512–522. doi: 10.1016/S0169-5347(03)00247-7. [DOI] [Google Scholar]
- Strullu-Derrien et al. (2012).Strullu-Derrien C, McLoughlin S, Philippe M, Mørk A, Strullu DG. Arthropod interactions with bennettitalean roots in a Triassic permineralized peat from Hopen, Svalbard Archipelago (Arctic) Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;348–349:45–58. doi: 10.1016/j.palaeo.2012.06.006. [DOI] [Google Scholar]
- Tillyard (1922).Tillyard RJ. Mesozoic insects of Queensland. No. 9. Orthoptera, and additions to the Protorthoptera, Odonata, Hemiptera and Plannipennia. Proceedings of the Linnean Society of New South Wales. 1922;47:447–470. [Google Scholar]
- Tong et al. (2007).Tong J, Zhang S, Zuo J, Xiong X. Events during Early Triassic recovery from the end-Permian extinction. Global and Planetary Change. 2007;55:66–80. doi: 10.1016/j.gloplacha.2006.06.015. [DOI] [Google Scholar]
- Vardabasso (1930).Vardabasso S. Carta geologica del territorio eruttivo di Predazzo e Monzoni nelle Dolomiti di Fiemme e Fassa. Padova: R. Scuola d’Ingegneria; 1930. [Google Scholar]
- Wachtler & Van Konijnenburg-van Cittert (2000).Wachtler M, Van Konijnenburg-van Cittert J. The fossil flora of the Wengen Formation (Ladinian) in the Dolomites (Italy) Beiträge zur Paläontologie. 2000;25:105–141. [Google Scholar]
- Walker (1938).Walker MV. Evidence of Triassic insects in the Petrified Forest National Monument, Arizona. Proceedings of the United States National Museum. 1938;85:137–140. doi: 10.5479/si.00963801.85-3033.137. [DOI] [Google Scholar]
- Walker & Wardle (2014).Walker LR, Wardle DA. Plant succession as an integrator of contrasting ecological time scales. Trends in Ecology & Evolution. 2014;29:504–510. doi: 10.1016/j.tree.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Wappler et al. (2012).Wappler T, Labandeira CC, Rust J, Frankenhäuser H, Wilde V. Testing for the effects and consequences of mid Paleogene climate change on insect herbivory. PLoS ONE. 2012;7:e921. doi: 10.1371/journal.pone.0040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb (1982).Webb JA. Triassic species of Dictyophyllum from eastern Australia. Alcheringa. 1982;6:79–91. doi: 10.1080/03115518208566988. [DOI] [Google Scholar]
- Wesley (1958).Wesley A. Contributions to the Knowledge of the flora of the Grey Limestones of Veneto, Part II. A revision of the Flora fossilis formationis oolithicae of De Zigno. Memorie degli Istituti di Geologia e Mineralogia dell’Università di Padova. 1958;21:1–57. [Google Scholar]
- Wilf & Labandeira (1999).Wilf P, Labandeira CC. Response of plant–insect associations to Paleocene-Eocene warming. Science. 1999;284:2153–2156. doi: 10.1126/science.284.5423.2153. [DOI] [PubMed] [Google Scholar]
- Żyła et al. (2013).Żyła D, Wegierek P, Owocki K, Niedźwiedzki G. Insects and crustaceans from the latest Early–early Middle Triassic of Poland. Palaeogeography, Palaeoclimatology, Palaeoecology. 2013;371:136–144. doi: 10.1016/j.palaeo.2013.01.002. [DOI] [Google Scholar]