Abstract
Forty-two patients with a clinical diagnosis of Bardet-Biedl syndrome ages 2-61 years were given a neuropsychological test battery to evaluate cognitive, sensory and behavioral functioning. These tests included the Wechsler scales of intelligence, Rey Auditory Verbal Learning Test, Boston Naming Test, D-KEFS Verbal Fluency Test, D-KEFS Color-Word Interference Test, D-KEFS Sorting Test, Wide Range Achievement Test: Math and Reading Subtests, Purdue Pegboard, The University of Pennsylvania Smell Identification Test, Social Communication Questionnaire, Social Responsiveness Scale, and Behavior Assessment System for Children, Second Edition, Parent Rating Scale. On the age appropriate Wechsler scale, the mean Verbal Comprehension was 81 (n=36), Working Memory was 81 (n=36), Perceptual Reasoning was 78 (n=24) and Full Scale IQ was 75 (n=26). Memory for a word list (Rey Auditory Verbal Learning Test) was in the average range with a mean of 89 (n=19). Fine motor speed was slow on the Purdue with mean scores 3-4 standard deviations below norms. All subjects were microsmic on the University of Pennsylvania Smell Identification Test. Of these 42 patients, only six were able to complete all auditory and visual tests; 52% were unable to complete the visual tests due to impaired vision. A wide range of behavioral issues were endorsed on questionnaires given to parents. Most had social skill deficits but no pattern of either externalizing or internalizing problems. We identify a characteristic neuro-behavioral profile in our cohort comprised of reduced IQ, impaired fine-motor function, and decreased olfaction.
Keywords: Bardet-Biedl syndrome, Intellectual disability, psychometric testing, brain development, olfaction
Introduction
Bardet-Biedl syndrome (BBS) is a pleiotropic multiple congenital syndrome characterized by obesity, polydactyly, rod-cone dystrophy, renal and genitourinary anomalies, and developmental delay [Beales et al., 1999]. Mutations in over a dozen different genes have been identified in this genetically heterogeneous autosomal recessive disorder, and few genotype-phenotype associations have been described [Ansley et al., 2003; Badano et al., 2003; Chiang et al., 2006; Chiang et al., 2004; Fan et al., 2004; Katsanis et al., 2000; Li et al., 2004; Mykytyn et al., 2001; Mykytyn et al., 2002; Nishimura et al., 2001; Nishimura et al., 2005; Slavotinek et al., 2000; Stoetzel et al., 2006; Stoetzel et al., 2007].
While neurocognitive impairments such as learning disabilities, speech delay, ataxia, and developmental delay are part of the diagnostic criteria for BBS developed by Beales et al. [1999], many of these features remain poorly characterized. The reported spectrum of intellectual disability in this population is broad; reports of patients' Full Scale and Verbal IQ scores range from <70 to >110 [Green et al., 1989; Barnett et al., 2002; Baker et al., 2011]. Emerging data suggest that patients with BBS have characteristic patterns of central nervous system (CNS) abnormalities, including reduced white matter throughout all regions of the brain and specific regional brain anomalies [Kepper-Noreuil et al., 2011; Baker et al., 2011]. Delineation of the specific neurocognitive deficits associated with this disorder is a necessary step in understanding the functional outcomes of BBS-associated CNS anomalies.
Though not a diagnostic criterion, impaired olfaction is another feature of BBS. Anosmia has been found in mouse models of the syndrome [Nishimura et al., 2004; Fath et al., 2005; Innaccone et al., 2005]. Although several studies associated BBS4 mutations with decreased olfaction [Iannaccone et al., 2005; Kulaga et al., 2004], others [Iannaccone et al., 2005] reported that approximately half of BBS patients with mutations in other BBS genes also had impaired olfaction.
Despite reports and observations of behavioral problems in patients with BBS, limited use of objective measures exists in the current literature. The most prominent behaviors reported anecdotally include: anxiety, depression, somatization, obsessive compulsive traits, attention problems, autistic-like symptoms, emotional immaturity, inability to recognize social cues, inappropriate/disinhibited mannerisms, and shallow affect [Barnett et al., 2002; Beales et al., 1999; Green et al., 1989; Moore et al., 2005]. Using an objective measure, Barnett et al. [2002] found internalizing behaviors (e.g., anxiety, depression) to be more prominent than externalizing behaviors (e.g., aggression, hyperactivity). In their study of ten individuals with BBS, Baker et al. [2011] reported that 90% of the patients exhibited autistic traits and/or mood and anxiety symptoms that significantly interfered with activities of daily living.
With these issues disrupting an affected individual's daily functioning and quality of life, it is important to better elucidate the cognitive, sensory, and psychosocial characteristics of Bardet-Biedl Syndrome. The purpose of the present study was to employ a prospective and comprehensive approach to quantify the cognitive, sensory, and behavioral features of the syndrome.
Materials and Methods
Participants
A total of 42 patients (19 male, 23 female) with Bardet-Biedl syndrome were assessed at the National Institutes of Health in Bethesda, Maryland between May 2004 and September 2010 as part of an ongoing natural history study approved by the NHGRI IRB (study # 04-HG-0123). Affected individuals were between 2 and 61 years old (mean 17 years) and the cohort included two sib-pairs. All met clinical diagnostic criteria for BBS [Beales et al., 1999] and 36 of the 42 patients had two mutations in a gene known to cause BBS (Supplemental eTable I in supporting information online). Most of the patients were described in Feuillan et al [2011] and the sequencing of the patients newly reported here was performed as described in that publication.
Procedures
A battery of neuropsychological tests was administered to each participant (vide infra); not all participants underwent the same testing battery and in some cases, measures were adapted as needed to accommodate participants' visual impairment or other special circumstances (i.e., one participant was not a native English speaker but could complete the non-verbal portions of the assessment). Behavioral questionnaires were completed by the participants' parent(s) for those participants under age 22 years.
Measures
A complete description of the battery of tests administered to participants is described in the Supplementary Methods section. Intellectual ability was assessed using age-appropriate Wechsler scales [Wechsler 2002; Wechsler 2004; Wechsler 2008], and six other memory, speech, and language assessments; the Rey Auditory Verbal Learning Test (RAVLT) [Spreen and Strauss, 1998], Boston Naming Test (BNT) [Heaton et al., 2004; Kaplan et al., 1983], D-KEFS Verbal Fluency Test (VFT), which is a subtest of the Delis-Kaplan Executive Functioning System (D-KEFS); [Delis et al., 2001], D-KEFS Color-Word Interference Test (CWIT), which is another subtest of the D-KEFS [Delis et al., 2001], D-KEFS Sorting Test, a third subset of the D-KEFS [Delis et al., 2001], and the math and reading subtests of the Wide Range Achievement Test (WRAT-4) [Wilkinson and Robertson, 2006]. Motor skills were evaluated using the Purdue Pegboard [Waber et al., 2007]. Olfaction was assessed with the University of Pennsylvania Smell Identification Test (UPSIT) [Doty et al., 1984], and three scales were used to achieve emotional behavioral assessments. The behavioral scales included: the Social Communication Questionnaire (SCQ) [Rutter et al., 2003]; the Social Responsiveness Scale (SRS) [Constantino and Gruber, 2005]; and Behavior Assessment System for Children, Second Edition (BASC-2) Parent Rating Scale (PRS) [Reynolds and Kamphaus, 2004], used for participants 22 years and younger. Statistical significance was assessed using unpaired t-tests and Pearson correlation analysis.
Results
Intellectual Ability
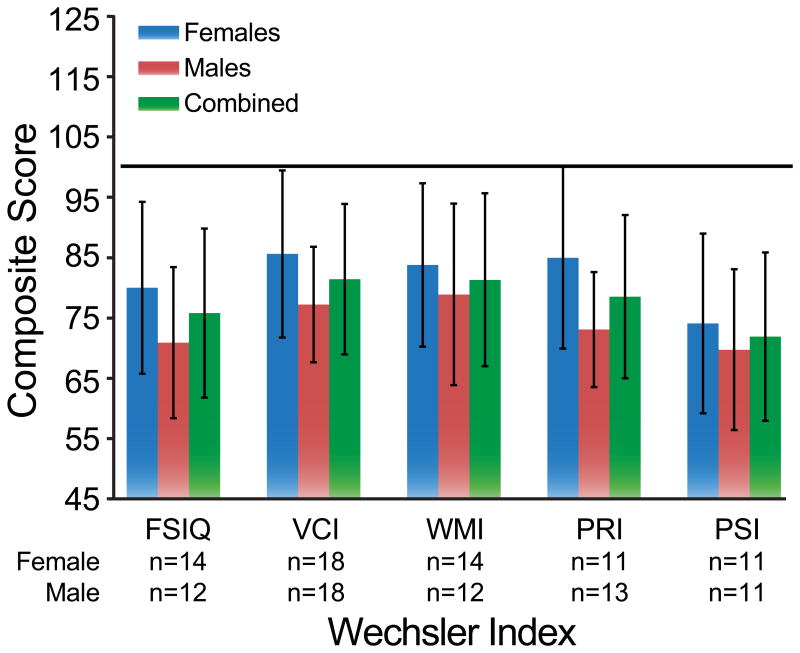
The Wechsler Scales provide measures of verbal ability and nonverbal ability as well as focused attention and speed of mental processing. Raw scores were converted into age corrected scaled scores and then combined to derive Index scores. The mean Full Scale IQ was 75.81 ± 14.01 (n=26), mean Verbal Comprehension Index (VCI) was 81.42 ± 12.50 (n=36), mean Working Memory Index (WMI) was 81.33 ± 14.33 (n=36), mean Perceptual Reasoning Index (PRI) was 78.54 ± 13.54 (n=24), and mean Processing Speed Index (PSI) was 71.91 ± 13.98 (n=22) (Table I). All score means fell more than 1 SD below the mean (Figure 1). Visual impairment precluded administration of the PRI (which includes block design reproduction and matrix reasoning) and/or PSI subtests (that require writing) to15 participants, VCI and WMI measures were obtained for a larger portion of patients. Of the 36 patients in whom VCI and WMI were measured, 22 and 24 (61% and 67%) scored more than 1 SD below the mean, respectively. There was a significant difference in mean scores by gender on both VCI (females 85.61 ± 13.86, n=18; males 77.22 ± 9.60, n=18, p=0.042) and PRI (females 85.00 ± 15.08, n=11; males 73.08 ± 9.55, n=13, p=0.028) scores, shown in figure 1. No correlation of the mutated BBS locus with IQ was found.
Table I. Descriptive statistics for the entire testing battery.
| n | Mean | (SD) | Range | |
|---|---|---|---|---|
| Wechsler IQ | ||||
| FSIQ | 26 | 75.81 | (14.01) | 48-100 |
| VCI | 36 | 81.42 | (12.50) | 55-108 |
| WMI | 36 | 81.33 | (14.33) | 56-108 |
| PRI | 24 | 78.54 | (13.54) | 55-106 |
| PSI | 22 | 71.91 | (13.98) | 50-92 |
| RAVLT | ||||
| Total Recall | 19 | 89.47 | (27.25) | 23-126 |
| Short Delay Recall | 19 | 100.32 | (19.97) | 53-131 |
| Long Delay Recall | 18 | 97.94 | (21.81) | 45-125 |
| WRAT-4 | ||||
| Math Computation | 9 | 83.44 | (10.54) | 66-97 |
| Word Reading | 9 | 88.11 | (9.83) | 77-102 |
| D-KEFS | ||||
| Word Fluency (Letter) | 17 | 7.53 | (3.37) | 1-14 |
| Word Fluency (Category) | 17 | 7.24 | (3.87) | 2-19 |
| Color-Word (Color) | 6 | 5.17 | (4.17) | 1-10 |
| Color-Word (Word) | 6 | 3.83 | (4.07) | 1-10 |
| Color-Word (Inhibition) | 6 | 7.33 | (5.68) | 1-15 |
| Color-Word (Inhibition/Switching) | 6 | 4.67 | (4.76) | 1-13 |
| Sorting (# Correct) | 6 | 5.83 | (3.66) | 1-10 |
| Sorting (Description) | 6 | 5.50 | (3.51) | 1-10 |
| Boston Naming Test | 12 | 60.50 | (30.74) | 0-104 |
| Purdue Pegboard | ||||
| Dominant Hand | 10 | 32.80 | (21.85) | 4-67 |
| Non-dominant Hand | 10 | 41.10 | (22.14) | 3-75 |
| Bimanual | 10 | 35.30 | (18.28) | 0-60 |
| UPSIT | ||||
| Raw Score | 17 | 22.12 | (8.99) | 9-34 |
| Percentage | 17 | 5.18 | (0.53) | 5-7 |
Note: standard scores are the measure reflected, except for D-KEFS (scaled) and UPSIT (raw).
Fig. 1.
Average composite scores of males, females, and combined group scores on Wechsler IQ tests. Black line represents the average score in the normal population (100). The data show that all sub scales for both genders are substantially lower than normal.
The likelihood of being able to complete enough subtests to obtain a FSIQ was inversely correlated with participants' ages (Table II). With the exception of one participant who was a non-native English speaker, we attributed this to progressive impaired vision.
Table II. Summary of participants with FSIQs measured.
| Age Group | # FSIQs | # Assessed | % |
|---|---|---|---|
| < 10 | 14* | 15 | 93% |
| 10-19 | 8 | 13 | 62% |
| 20+ | 4 | 14 | 29% |
One participant could not have FSIQ assessed, due to being a non-native English speaker
Neuropsychological Tests
Memory
Nineteen participants completed the RAVLT (18 with the delayed recall trial). Memory standard scores (mean ± SD) were within the average range (total recall=89.47 ± 27.25, short delay recall=100.32 ± 19.97, long delay recall=97.94 ± 21.81).
Math/Reading (WRAT)
Nine participants completed the WRAT-4 and the mean math computation standard score was 83.44 ± 10.54, slightly more than 1 SD below the mean. The mean of the WRAT-4 word reading subtest was 88.11 ± 9.83, within normal limits.
Executive Functioning
Seventeen participants completed the letter and category conditions of the VFT subtest of the DKEFS and the group mean scaled scores were within normal limits at 7.53 ± 3.37 and 7.24 ± 3.87, respectively. Interestingly, VCI scores were not strongly correlated with verbal fluency (letter condition r=0.391, p=0.120; category condition r=0.368, p=0.146) but WMI scores were (letter condition r=0.724, p <0.001; category condition r=0.641, p=0.006).
Language
The performance of these participants on the BNT was characterized by a low mean standard score (60.67, n=12) and abroad range (0 - 104). Naming problems may have been affected by limited vision, although all participants indicated that they could discern the test pictures before commencing the task. No significant differences were identified by gender (p>0.05) and all scores were age corrected.
Motor Skills
Ten participants were able to complete the Purdue Pegboard test. Performance on this assessment was poor, with group averages falling three to four SD below the population mean. The group mean standard score for the dominant hand was 32.80 ± 21.85; non-dominant hand 41.10 ± 22.14; and bimanual 35.30 ± 18.28. There were no significant differences in male and female performance (p>0.05) nor any significant trending found with age.
Olfaction
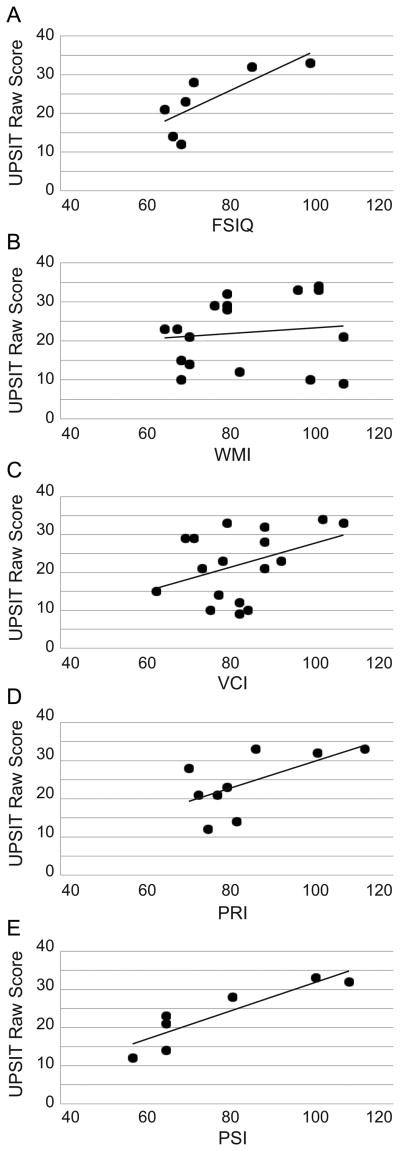
The mean UPSIT number correct score was 22.12 ± 8.99 (n=17) and when compared with published norms, none of the 17 patients were categorized as normosmic; all fell below the 7th centile (mean 5.18 ± 0.53). Six (35.3%) patients met criteria for mild microsmia, six (17.6%) with moderate microsmia, two (11.8%) with severe microsmia, and six (35.3%) with anosmia. There was no significant correlation of UPSIT scores to age (p>0.05), nor any significant differences in scores obtained by males versus females (p>0.05). As shown in Figure 2, positive correlations of UPSIT scores and both FSIQ (r=.774, p=0.041) and PSI (r=0.896, p=0.006) scores were identified.
Fig. 2.
University of Pennsylvania Smell Identification Test score correlations with IQ scores. (A) Full scale IQ; (B) Verbal Comprehension Index (VCI); (C) Working Memory Index (WMI); (D) Perceptual Reasoning Index (PRI); (E) Processing Speed Index (PSI).
Behavior
A summary of scores obtained for all behavioral measures can be found in Table III. The SCQ group mean score of 10.45 ± 5.99 (n=20) was below the ASD threshold (≥15). Although the mean score was higher in males (12.57 ± 3.99) as compared to females (9.31 ± 6.69) these differences were not statistically significant (p>0.05). There was no significant difference in SCQ score by gender (t=-1.174, p>0.05) and no correlation of SCQ score to age was found (r=0.124, p>0.05).
Table III. Summary scores for behavioral measures.
| n | Mean | (SD) | Range | |
|---|---|---|---|---|
| SCQ | 20 | 10.45 | (5.99) | 1-21 |
| SRS | ||||
| Total | 20 | 71.80 | (14.90) | 39-90 |
| Social Awareness | 20 | 65.95 | (16.65) | 34-90 |
| Social Cognition | 20 | 73.30 | (14.09) | 50-90 |
| Social Communication | 20 | 69.50 | (16.01) | 37-90 |
| Social Motivation | 20 | 63.75 | (15.49) | 38-90 |
| Autistic Mannerisms | 20 | 72.90 | (15.81) | 44-90 |
| BASC-2 | ||||
| Externalizing Problems | 13 | 46.92 | (9.72) | 36-67 |
| Internalizing Problems | 13 | 52.92 | (15.39) | 31-85 |
| Behavioral Symptoms Index | 13 | 53.31 | (12.32) | 35-79 |
| Adaptive Skills | 13 | 47.15 | (12.98) | 27-77 |
Note: T scores are the measure reflected, except for the SCQ (total score).
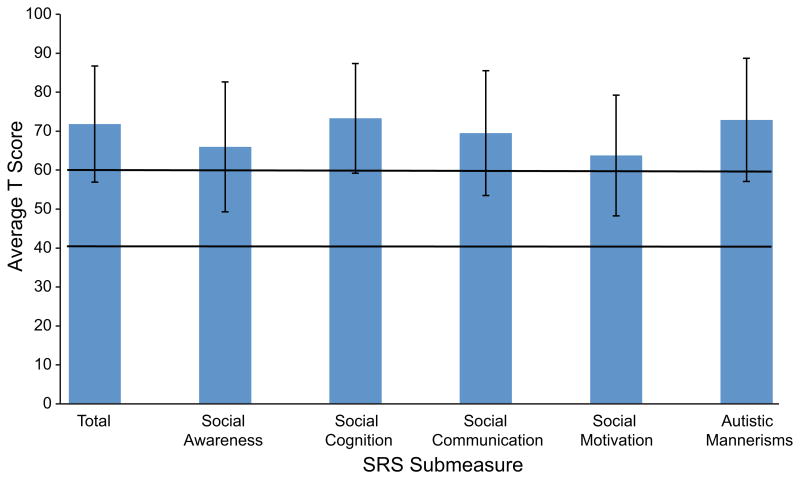
The total T-score (a standard score with a mean of 50 and a standard deviation of 10) as well as five subscale T-scores were obtained on the SRS and are shown in Figure 3. The group mean T-score (71.80 ± 14.90) fell in the mild/moderate range, as did the group means for all of the subscales (Social Awareness 65.95 ± 16.65, Social Cognition 73.30 ± 14.09, Social Communication 69.50 ± 16.01, Social Motivation 63.75 ± 15.49, Autistic Mannerisms 72.90 ± 15.81). Of the 20 participants who were assessed using this measure, 16 (80%) had scores that indicated a social skill deficit; eight (40%) had T-scores in the mild/moderate deficit range (60–75) and the remaining eight (40%) had T-scores in the severe range (≥76). No significant difference in SRS scores by gender was found (t=0.418, p>0.05). However, there was a slight positive trend in correlation of the SCQ to the SRS scores (r=0.621, p=0.003).
Fig. 3.
Average T scores on the Social Responsiveness Scale (SRS). The area between the black bars represent the average score range in the normal population (40-60).
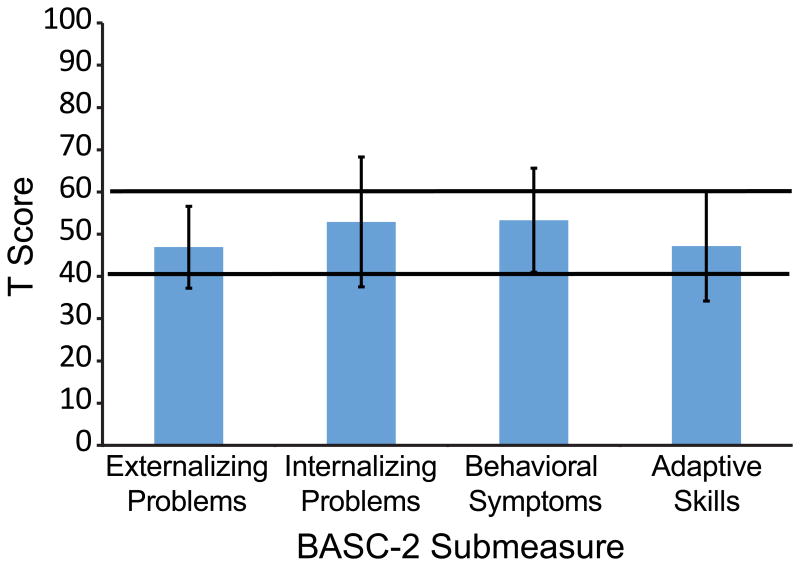
The BASC-2 Parent Rating Scale was analyzed for the 13 participants from ages 2 through 21 years, 11 months. All BASC-2 group mean T-scores fell within normal limits (40-60, Figure 4). The group mean T-score for externalizing problems was 46.92 ± 9.72, with two participants falling above the clinically significant cutoff (>60). The mean score for internalizing problems was 52.92 ± 5.39; five participants fell above the clinically significant cutoff for this subscale. There was no significant difference found when comparing externalizing to internalizing problems (p>0.05). In terms of the behavioral symptoms, the group mean was 53.31 ± 15.39, with three of the 13 participants scoring above the cutoff. Additionally, the mean group score for adaptive skills was 47.15 ± 12.98; three participants fell below the clinical cutoff (<40 {higher scores better}). There was no significant difference in BASC-2 scores by gender (Externalizing t=1.741, p>0.05, Internalizing t=-0.091, p>0.05, Behavioral Symptoms t=0.356, p>0.05, Adaptive Skills t=1.332, p>0.05) and no correlation of BASC-2 score to age was found (Externalizing r=-0.031, p>0.05, Internalizing r=0.066, p>0.05, Behavioral Symptoms r=0.073, p>0.05, Adaptive Skills r=0.259, p>0.05).
Fig. 4.
Average T scores on the Behavior Assessment System for Children, Second Edition (BASC-2) Parent Rating Scale. The area between the black bars represent the average score range in the normal population (40-60).
It should be noted that, across all intellectual and neuropsychological measures, there were no significant differences in the results when patients with BBS1 mutations were compared to those with BBS10 mutations.
Discussion
We used a battery of neurocognitive tests to quantify the cognitive, sensory and psychosocial characteristics of a group of individuals with BBS. The findings of the present study suggest that individuals with BBS exhibit a neurocognitive profile that is significantly different from that of the general population.
We found a characteristic cognitive and behavioral profile in our cohort comprised of reduced IQ, impaired fine-motor function, and decreased olfaction. The IQ scores in this group were in the borderline range (Standard Score between 70 and 79). Although participant age was not correlated with VCI score, our ability to measure a FSIQ was compromised in older participants. Impaired vision or blindness, a hallmark feature of the disorder and more prevalent in adult participants, precluded the administration of visual tests and, therefore, the generation of a FSIQ score. One important conclusion that we can draw from this finding is that testing done at a younger age when vision is better is more likely to provide a complete assessment of cognitive function in individuals with BBS. These data show similar, but slightly higher values when compared to the findings of Barnett et al [2002]. Memory for a list of words (RAVLT) was in the average range and may be explained by the fact that it is an adaptive capacity for those affected with BBS. Language and reading skills may not be fully evaluated by these data, a consequence of the low number of participants who could complete tasks in this area. Fine motor speed seemed to be impaired in patients with BBS; scores were poor on the Purdue with mean scores 3-4 standard deviations below the norm. A sensory deficit was also prominent, as all subjects were microsmic or worse on the UPSIT, with scores below the 7th centile.
Behaviorally, a wide range of issues were endorsed on questionnaires completed by parents. Most participants (80%) were reported to have social skill deficits but no pattern of either externalizing or internalizing problems emerged. Additionally, there seemed to be no indication of severe Autism Spectrum Disorder, as the majority of participants were within normal limits and below the threshold for this measure. Social skill deficits are likely better explained by cognitive and sensory differences between those with BBS and their peers.
Our data suggest that there may be better ways to both classify and identify the neurocognitive, sensory, and behavioral characteristics associated with BBS and ways to use this knowledge to better manage the syndrome in affected individuals. For example, BBS has traditionally been described as coincident with severe intellectual disability but this was not supported by the findings of this study, nor by those of Barnett et al, [2002]; there is emerging evidence that the intellectual spectrum is broader than once thought.
Our study also informs our understanding of the severity of the loss of olfaction related to BBS. This is important to keep in mind for clinical purposes, as there are many risk factors involved with a diminished capacity to detect odor and ultimately this deficit can be a safety issue (e.g., the ability to smell scented natural gas). Further, the olfactory deficits found in this sample are believed to be a result of the disorder itself, as previous research has indicated that olfactory dysfunction is not a result of decreased cognitive ability [McKeown et al., 1996].
Although anecdotal and parental descriptions of behaviors on the autism spectrum appear in the BBS literature [Barnett et al., 2002], the data presented here, derived from validated and normed questionnaires given to parents and patients, suggest that while individuals with BBS may have mild to moderate impairments in social functioning, behavioral and emotional functional deficits were not clinically significant for a substantial number of patients. In terms of management, while the results of carefully designed studies are lacking in the Pervasive Developmental literature, social skills training, similar to that used for PDD, beginning early may be beneficial to help these children negotiate adolescence [Koenig et al., 2009]. Thus, behavioral deficits should be addressed early on in patients with BBS and social skills training may be effective at enhancing their quality of life as young adults if started early.
There are several limitations to this study. Comprehensive neuropsychological testing can be difficult when assessing individuals with BBS. Poor and progressive loss of vision precluded use of several tests and may also have affected the participant's ability to complete some processing speed tests. Thus, the data set for these tests was small and this may have affected our statistical power. Deciphering the origin of behavioral and social issues that BBS patients struggle with can be equally difficult. It was difficult to fully determine if these issues are true manifestations of BBS or secondary to the social implications of other manifestations of the disorder including obesity and impaired vision. A literature review of childhood obesity studies reported that while behavioral problems were observed within subgroups of obese children, there was no clear indication of higher rates of psychiatric comorbidity in the population of obese children [Zametkin et al., 2004]. In contrast, visual impairment may be a confounding factor. In a study of 269 patients (107 visually impaired or blind), Evenhuis et al. [2008] found that although intellectual disability itself impaired daily functioning, visual impairment diminished an individual's daily functioning—including social and communication skills–even more [Evenhuis et al., 2008]. Finally, there may be potential selection biases for the study because of self-referral and convenience sampling recruitment to this study (recruitment of participants was typically via their unaffected family members).
Developing a better understanding of the cognitive, sensory, and psychosocial characteristics of patients with BBS is important for management and provision of the appropriate services to improve quality of life. Future investigations to refine the neurocognitive profile of individuals with BBS would benefit from larger sample sizes to increase statistical power. Utilizing more non-visual means of testing could also help to assess these individuals and increase study power. Finally, correlating assessments of cognitive and behavioral functioning with brain morphology studies may refine and improve our understanding of the complete neurocognitive profile of this and other rare disorders.
Supplementary Material
Acknowledgments
The authors are grateful to the participants in this study, to the Laurence-Moon Bardet-Biedl Family Network for their enthusiastic support of the NIH Clinical Study of BBS. This study was supported by the Intramural Research Program of the National Human Genome Research Institute. This project would not have been possible without the contributions of Dr. Penelope Feuillan, who passed away during the course of this study. We also thank Julia Fekecs for assistance with graphic illustrations.
References
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet. 2003;72:650–658. doi: 10.1086/368204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Northam GB, Chong WK, Banks T, Beales P, Baldeweg T. Neocortical and hippocampal volume loss in a human ciliopathy: A quantitative MRI study in Bardet–Biedl syndrome. Am J Med Genet A. 2011;155:1–8. doi: 10.1002/ajmg.a.33773. [DOI] [PubMed] [Google Scholar]
- Barnett S, Reilly S, Carr L, Ojo I, Beales PL, Charman T. Behavioural phenotype of Bardet-Biedl syndrome. J Med Genet. 2002;39:e76. doi: 10.1136/jmg.39.12.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36:437–446. [PMC free article] [PubMed] [Google Scholar]
- Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, Sheffield VC. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proc Natl Acad Sci U S A. 2006;103:6287–6292. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;75:475–484. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles: Western Psychological Corporation; 2005. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function [Monograph] Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Evenhuis HM, Sjoukes L, Koot HM, Kooijman AC. Does visual impairment lead to additional disability in adults with intellectual disabilities? J Intellect Disabil Res. 2008;53:19–28. doi: 10.1111/j.1365-2788.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, Green JS, Lewis RA, van Haelst MM, Parfrey PS, Baillie DL, Beales PL, Katsanis N, Davidson WS, Leroux MR. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet. 2004;36:989–993. doi: 10.1038/ng1414. [DOI] [PubMed] [Google Scholar]
- Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, Zheng YC, Caruso RC, Brooks BP, Johnston JJ, Yanovski JA, Biesecker LG. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab. 2011;96:528–535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, McManamon PJ, O'Leary E, Pryse-Phillips W. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. New Engl J Med. 1989;321:1002–1009. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults, Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Iannaccone A, Mykytyn K, Persico AM, Searby CC, Baldi A, Jablonski MM, Sheffield VC. Clinical evidence of decreased olfaction in Bardet-Biedl syndrome caused by a deletion in the BBS4 gene. Am J Med Genet A. 2005;132:343–346. doi: 10.1002/ajmg.a.30512. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, Segal O. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat Genet. 2000;26:67–70. doi: 10.1038/79201. [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil KM, Blumhorst C, Sapp JC, Brinckman D, Johnston J, Nopoulos PC, Biesecker LG. Brain tissue- and region-specific abnormalities on volumetric MRI scans in 21 patients with Bardet-Biedl syndrome (BBS) BMC Med Genet. 2011;12:101. doi: 10.1186/1471-2350-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K, De Los Reyes A, Cicchetti D, Scahill L, Klin A. Group Intervention to Promote Social Skills in School-age Children with Pervasive Developmental Disorders: Reconsidering Efficacy. J Autism Dev Disord. 2009;39:1163–1172. doi: 10.1007/s10803-009-0728-1. [DOI] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- McKeown DA, Doty RL, Perl DP, Frye RE, Simms I, Mester A. Olfactory function in young adolescents with Down's syndrome. J Neurol Neurosurg Psychiatry. 1996;61:412–414. doi: 10.1136/jnnp.61.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132:352–360. doi: 10.1002/ajmg.a.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, Riise R, Baldi A, Raas-Rothschild A, Gorman SW, Duhl DM, Jacobson SG, Casavant T, Stone EM, Sheffield VC. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–191. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–438. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, Haider NB, Kwitek-Black AE, Ying L, Duhl DM, Gorman SW, Heon E, Iannaccone A, Bonneau D, Biesecker LG, Jacobson SG, Stone EM, Sheffield VC. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2) Hum Mol Genet. 2001;10:865–874. doi: 10.1093/hmg/10.8.865. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, Sheffield VC. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77:1021–1033. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 2nd. Minneapolis, MN: NCS Pearson Inc.; 2004. [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ The Social Communication Questionnaire Manual. Los Angeles: Western Psychological Corporation; 2003. [Google Scholar]
- Sapp JC, Nishimura D, Johnston JJ, Stone EM, Héon E, Sheffield VC, Biesecker LG. Recurrence risks for Bardet-Biedl syndrome: Implications of locus heterogeneity. Genet Med. 2010;12:623–627. doi: 10.1097/GIM.0b013e3181f07572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG. Mutations in MKKS cause Bardet-Biedl syndrome. Nat Genet. 2000;26:15–16. doi: 10.1038/79116. [DOI] [PubMed] [Google Scholar]
- Smith RS, Doty RL, Burlingame GK, McKeown DA. Smell and taste function in the visually impaired. Percept Psychophys. 1993;54:649–655. doi: 10.3758/bf03211788. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Second. New York: Oxford University Press; 1998. [Google Scholar]
- Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, Jalk N, Vicaire S, Sarda P, Hamel C, Lacombe D, Holder M, Odent S, Holder S, Brooks AS, Elcioglu NH, Silva ED, Rossillion B, Sigaudy S, de Ravel TJ, Lewis RA, Leheup B, Verloes A, Amati-Bonneau P, Megarbane A, Poch O, Bonneau D, Beales PL, Mandel JL, Katsanis N, Dollfus H. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, de Ravel TJ, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue Pegboard: norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Waber DP, DeMoor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J, the Brain Development Cooperative Group J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence—Third Edition (WPPSI-III) San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) San Antonio: The Psychological Corporation; 2004. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV) San Antonio: Pearson; 2008. [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT4 Wide Range Achievement Test Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- Zametkin AJ, Zoon CK, Klein HW, Munson S. Psychiatric aspects of child and adolescent obesity: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43:134–150. doi: 10.1097/00004583-200402000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.