Abstract
Hematopoiesis, the process by which the hematopoietic stem cells and progenitors differentiate into blood cells of various lineages, involves complex interactions of transcription factors that modulate the expression of downstream genes and mediate proliferation and differentiation signals. Despite the many controls that regulate hematopoiesis, mutations in the regulatory genes capable of promoting leukemogenesis may occur. The FLT3 gene encodes a tyrosine kinase receptor that plays a key role in controlling survival, proliferation and differentiation of hematopoietic cells. Mutations in this gene are critical in causing a deregulation of the delicate balance between cell proliferation and differentiation. In this review, we provide an update on the structure, synthesis and activation of the FLT3 receptor and the subsequent activation of multiple downstream signaling pathways. We also review activating FLT3 mutations that are frequently identified in acute myeloid leukemia, cause activation of more complex downstream signaling pathways and promote leukemogenesis. Finally, FLT3 has emerged as an important target for molecular therapy. We, therefore, report on some recent therapies directed against it.
Key words: FLT3 receptor, internal tandem duplication, acute myeloid leukemia, signal transduction networks, inhibitors.
Introduction
Recent advances in cell and molecular biology have revolutionized our understanding of normal hematopoiesis. It is generally accepted that survival, proliferation and differentiation are the three fundamental cellular processes that define normal hematopoietic cells. In acute myeloid leukemia (AML), a heterogeneous disorder of the hematopoietic progenitor cells, abnormalities have been identified that affect the balance between cell proliferation, survival and differentiation. These abnormalities result in the expansion of an abnormal stem cell clone. Over the last few years, several studies have concluded that leukemogenesis is a process in which multiple events involving independent genetic alterations in proto-oncogene or suppressor genes, together with epigenetic or environmental factors, contribute to the development of the full malignant phenotype.1 The genes involved in leukemogenesis are related to various cellular functions, including ligand-receptor interaction, signal transduction, intracellular localization, cell cycle control and apoptosis. In detail, the oncogenic events that underlie the onset of leukemia are often divided into two classes of mutations, following the two-hit model of leukemogenesis. Class I mutations confer a proliferation and survival advantage to blast cells, typically as a result of aberrant activation of signaling pathways. Otherwise, the class II mutations lead to an impaired differentiation via interference with transcription factors or co-activators. The cooperation between these two main classes of mutations leads to the emergence of leukemic cells capable of proliferation but not differentiation.2 In particular, epidemiological and genetic data have shown that the majority of AML present more than one recurrent alteration, including point mutations, gene rearrangements and chromosomal translocations. Furthermore, it has been suggested that point mutations in transcription factors are sufficient to confer a proliferative and survival advantage to the leukemic clone.
Mutations within the FMS-like tyrosine kinase 3 (FLT3) gene represent one of the most frequently identified genetic alterations that disturb intracellular signaling networks with a role in leukemia pathogenesis. FLT3 is a member of the class III receptor tyrosine kinase family that also includes platelet-derived growth factor receptor (PDGFR), macrophage colony-stimulating factor receptor (FMS) and stem cell factor receptor (c-KIT), with which it shares the same structure. Activating mutations in the FLT3 gene, including internal tandem duplications (ITDs) and missense point mutations in the tyrosine kinase domain (TKD), are the molecular abnormalities most frequently observed in the blood cells of AML patients. These mutations lead to the overexpression or constitutive activation of the tyrosine kinase receptor. Many studies indicate that patients with FLT3 mutations have a worse prognosis than patients without FLT3 alterations. In particular, the presence of an FLT3-ITD correlates with an increased risk of relapse and impaired overall survival. The effect on AML prognosis of the FLT3-TKD mutation has not yet been clearly defined; in several studies, the FLT3-TKD mutation did not seem to affect outcome while other investigations showed opposite results.3 In addition to cytogenetic abnormalities detected at diagnosis, which are the most important prognostic factor, FLT3 mutations are a significant independent prognostic factor that can influence outcome in terms of survival and duration of complete remission, even in patients with a normal karyotype.4 Because of its prognostic relevance, the current World Health Organization (WHO) classification recommends the assessment of FLT3 mutation status for all patients with AML, particularly in cytogenetically normal AML. However, because FLT3 frequently accompanies other genetic lesions, it is not included as a distinct entity in the 2008 revised WHO classification of myeloid neoplasms.5
Aberrantly activated FLT3 kinase is considered an attractive therapeutic target in AML. Therefore, specific small molecule FLT3 tyrosine kinase inhibitors (TKI) have been developed for AML therapy and are currently under investigation in the hope that they may revolutionize AML treatment.
Structure of the FLT3 receptor
The FLT3 receptor (Fms-like tyrosine kinase 3), also known as FLK2 (fetal liver tyrosine kinase 2), STK-1 (stem cell tyrosine kinase 1) or CD135, is encoded by the FLT3 gene located on chromosome 13q12.6–7 This gene consists of 24 exons and covers approximately 96 kb; the exact size is unknown because of the presence of a large intron (>50 kb) located between exons 2 and 3.8–10 The length of the transcript is 3.7 kb and it contains a pseudogene in the open reading frame of 2979 bp.8 The protein encoded is a transmembrane receptor of 933 amino acids with a molecular weight of 155–160 kDa that belongs to the class III family of receptor tyrosine kinase (RTK).8,11 The structure consists of four regions: i) a N-terminal extracellular region (541aa) consisting of five immunoglobulin-like domains, of which the three most distal from the plasma membrane are involved in ligand binding, while the proximal domains are involved in dimerization of the receptor; ii) a transmembrane portion (21aa); iii) a juxtamembrane (JM) domain; and iv) an intracellular C-terminal region (431aa) with a split kinase domain. The two substructures of this domain are called N-lobe and C-lobe and are connected by an interkinase domain. These lobes consist of a TKD and are also indicated as first tyrosine kinase (TK1) and second tyrosine kinase (TK2) domain, respectively9,12 (Figure 1). The extracellular region is highly glycosylated and contains a binding domain with high affinity for its ligand (FLT3 ligand or FL).13 The nonglycosylated isoform has a molecular weight of 130–143 kDa and is not associated with the plasma membrane.14
Figure 1.
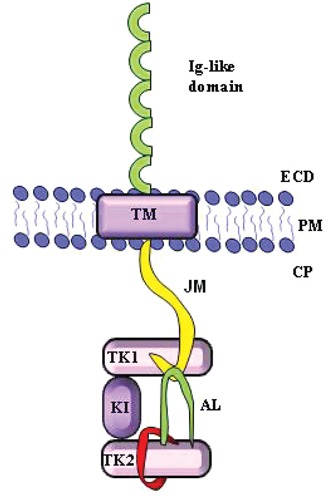
Schematic presentation of FLT3 receptor monomer. ECD, extracellular domain; PM, plasma membrane; CP, cytoplasm; TM, transmembrane domain; JM, juxtamembrane domain; TK1, first tyrosine kinase domain, N-lobe; KI, kinase insert; TK2, second tyrosine kinase domain, C-lobe; AL, activation loop.
Synthesis and activation of FLT3
In normal bone marrow, FLT3 is selectively expressed on CD34+ hematopoietic stem cells and immature hematopoietic progenitors, including the B-lymphoid progenitors, the myeloid precursors and monocytes, but it is virtually absent from the erythroid progenitors, playing an important role in the regulating processes of early hematopoiesis.11,15–18 Furthermore, it has been experimentally shown that CD34+ bone marrow cells can give rise to two different populations according to the level of FLT3 expression: cells expressing high levels of the receptor mature in the colony forming unit granulocyte-macrophage, while cells that express low levels preferentially mature in the burst forming unit erythroid colonies.19 FLT3 is also expressed in other hematopoietic organs such as spleen, liver, thymus, lymph nodes and placenta, and blood-forming organs such as gonads and brain.20–22
The processes of activation, internalization and degradation of FLT3 occur in a similar manner to other members of the same class of RTKs. The FLT3 gene transcription produces an mRNA that is translated into the FLT3 protein. This passes through the compartments of endoplasmic reticulum and Golgi undergoing glycosylation, with the addition of residues of N-acetylglucosamine, galactose, fucose and mannose to N-terminal code, which promotes the localization of the receptor on the plasma membrane.
Wild-type FLT3 (FLT3-WT) remains in the inactive monomeric form; the binding of its ligand (FL or FLT3 ligand), probably in dimeric form, induces receptor dimerization that promotes the phosphorylation of the tyrosine kinase domain, activating the receptor and consequently the downstream effectors. Once activated, the dimerized receptor bound to the FL is rapidly internalized and degraded. The receptor remains in the inactive conformation thanks to the steric inhibition mediated by the JM domain. Upon FL binding, the receptor dimerization leads to the exposure of phosphorylated acceptor site of the tyrosine kinase domain. As previously indicated, the TKD of the FLT3 kinase is characterized by two catalytic lobes: N lobe (TK1) and C lobe (TK2), connected by a flexible peptide that can insert significant rotational movement of the kinase domain. When the N lobe is rotated away from the carboxy-terminal kinase domain, the receptor is in the catalytically inactive form. If the N lobe is rotated towards the C lobe, the key catalytic residues of both lobes are aligned and the kinase adopts an active conformation.12 The activating loop (A-loop), near to the C lobe, is a long flexible peptide segment which folds between the two catalytic domains and contains 1–3 tyrosine residues that can serve as a phosphorylation site (Figure 2). When these tyrosines are not phosphorylated, the A-loop adopts a closed conformation, folding back into the space between the two N and C lobes and blocking access for peptide substrates and the binding of the adenosine triphosphate (ATP). In the active state, when the tyrosines are phosphorylated, the A-loop adopts an open conformation and does not compromise the entry of the ATP and substrates.
Figure 2.
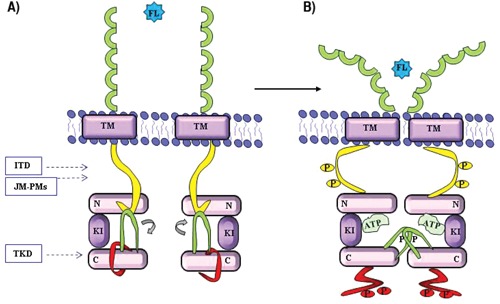
Activation of FLT3. A) Inactive conformation; B) Active conformation. Juxtamembrane domain (yellow), activation-loop (green), catalitic loop (red). P, phosphorylation site; N, N-lobe, TK1 domain; C, C-lobe, TK2 domain; ITD, internal tandem duplications; JM-PMs, point mutation in the juxtamembrane domain; TKD, point mutation in the tyrosine kinase domain; FL, FLT3 ligand.
Also, the state of phosphorylation of two key tyrosine residues of the JM domain is implicated in the activation and regulation of enzymatic activity of the receptor. The JM domain adopts a wedge shape that stabilizes the inactive state by establishing strong interactions with the rest of the molecule and preventing the rotation of the N lobe to C lobe. In the inactive form, the JM domain is close to the A loop which, therefore, cannot adopt an open conformation (Figure 2).
The binding of FL promotes the dimerization and the concomitant juxtaposition of the cytoplasmic domain of the FLT3 receptor. Once the dimer is formed, the trans-phosphorylation of specific tyrosine residues of the JM domain takes place, allowing activation of the kinase. When at least one of the JM tyrosines is phosphorylated, this domain cannot fold properly to maintain the inactive state, and the activating loop adopts an open conformation. This exposes the phosphate acceptor site residues of the catalytic domain. This conformation favors the entry of the ATP and the binding of the substrates, followed by the phosphorylation and the activation of downstream proteins. The protein dimerization stabilizes these conformational changes and promotes further activation of the receptor.
On the other hand, the kinase activity is negatively modulated by the tyrosine phosphatase that dephosphorylated the JM domain: when the phosphatase removes the phosphates, the JM domain is in the proper position around the kinase domain and correctly fits on its autoinhibitory site.12,23 The phosphorylation of FLT3 occurs after 5–15 min by the binding of FL, and the receptor-ligand complex is internalized and degraded after 20 min of stimulation. The frequency of FLT3 production, its rapid degradation and the downstream effects participates in a complex feedback loop that regulates normal receptor activity.20
FLT3 ligand
The exposure of FLT3 at its ligand is a crucial step in regulating the activity of the receptor. FL is a type 1 transmembrane protein, a member of a small family of growth factors that stimulate proliferation and differentiation of hematopoietic cells.24–25 There are three known isoforms: i) a 30-kDa glycoprotein with four transmembrane alpha-helix, an aminoterminal domain and a small cytoplasmic region, which is the most commonly expressed form, that binds to and activates the receptor; ii) a soluble, biologically active form, generated by the cut of the transmembrane isoform; and 3) a soluble form generated by alternative splicing that creates a premature stop codon.12,21–23,26 FL is expressed by most tissues, including blood-forming organs (spleen, thymus, peripheral blood and bone marrow), prostate, ovary, kidney, lung, colon, small intestine, testes, heart and placenta, with a higher level of expression in peripheral blood mononuclear cells. The wide expression of FL is in contrast to the limited pattern of expression of FLT3, suggesting that FLT3 expression is the limiting step in determining the tissue-specificity of receptor activation.27–29
FL activity is minimal when it acts alone, but it strongly synergizes with a number of other cytokines. In acute myeloid leukemias, FLT3 stimulation by its ligand promotes the proliferation and survival of leukemic blasts that express the receptor.21,30–31 The hematopoietic pro-genitors can be stimulated by the local secretion of FL or from direct contact with FL expressed on the surface of mononuclear cells, indicating the possibility of controlling the FLT3 activation through a paracrine loop or an autocrine feedback control.13,20
Signal transduction networks activated by FLT3-WT
FLT3 has a crucial role in many regulatory processes of hematopoietic cells, including phospholipid metabolism, transcription, proliferation, apoptosis, and establishing a connection with the RAS pathway; it is also involved in leukemogenesis14,20,30–33 (Figure 3). FLT3-WT causes the activation of signal transduction networks mainly through phospatidylinositol-3-kinase (PI3K) and the cascade of RAS, supporting the activation of AKT (protein kinase B, PKB), signal transducer and activator transcription factor (STAT) and extracellular-signal regulated kinase 1 and 2 (ERK1/2).34 Activated FLT3 is associated with growth factor receptor bound protein-2 (GRB2), a linker protein that binds to a diverse repertoire of signaling proteins, through SHC (Src homology 2 containing protein) via the SH2 domain. The adaptor protein GRB2 also contains an SH3 domain capable of binding proline-rich residues of other proteins, such as SOS (guanine nucleotide exchange factor), stimulating the dissociation of GDP and the subsequent binding of GTP to RAS. This leads to activation of RAS, which stimulates downstream effectors RAF, MAPK/ERK kinase and RSK (90-kDa ribosomal protein S6 kinase). These effectors activate CREB (cyclic adenosine monophosphate response element-binding protein), ELK and STAT leading to the transcription of genes involved in proliferation. Both the PI3K/AKT and RAS/ERK pathways are often activated in parallel and probably interact with many other anti-apoptotic and cell cycle proteins, such as WAF1, KIP1 and BRCA1. Activated FLT3 transduces the signal through the association and phosphorylation of various other cytoplasmic proteins, including PLCγ1 (phospholipase Cγ1), regulatory protein of the metabolism of phosphatidylinositol, VAV and FYN.
Figure 3.
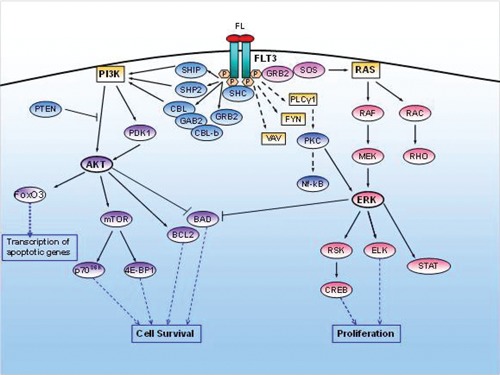
Signaling pathways activated by FLT3-WT.
PI3K activity is probably regulated through various interactions between FLT3, adaptor proteins SHC, one or more protein SHIP (SH2-containing inositol 5-phosphatase).35–36 Although PI3K activity is involved in the metabolism of phospholipids, it can also act as a regulator of proliferation in a competitive binding of phosphorylated protein SHC, SHP2 (SH2-containing phosphotyrosine phosphatase 2) and GRB2. The p85 subunit of PI3K kinase is associated with a protein of 100kDa and 120kDa represented by GAB2 (GRB2-associated binder 2) and CBL (casitas B-lineage lymphoma) proto-oncogene and, finally, with CBL-b, in a complex with SHP2 and SHIP. Receptor stimulation results in the phosphorylation of SHC proteins and CBL, with the formation of a complex with p85 subunit, and induces tyrosine phosphorylation of GAB1 and GAB2 and their association with SHP2 and GRB2.37 The PI3K stimulates downstream proteins such as PDK1 (protein kinase 1-dependent phosphoinositol-3) and AKT that phosphorylates mTOR (mammalian target of rapamycin), which activates the transcription of key genes through the activation of p70S6Kinase (p70S6K) and the inhibition of 4E-BP1 (eucaryotic transcription initiation factor 4E-binding protein).38–39 As p70S6K and 4E-BP1 regulate protein translation, activation of p70S6K results in an increase in overall protein synthesis and induction of cell survival. In addition, the activation of AKT blocks apoptosis through the phosphorylation of pro-apoptotic protein BAD (BCL2 antagonist of cell death, Bcl-XL/Bcl-2-association death promoter). Therefore, the pro-survival function of FLT3 is mediated by the phosphorylation of the pro-apoptotic BAD protein, the induction of anti-apoptotic BCL-2, and the prevention of the induction and activation of pro-apoptotic Bax.40 PI3K may still be deregulated by low-level expression of PTEN (phosphatase and tensin homolog deleted on chromosome 10).41 Among the important downstream targets of AKT, there are also members of the forkhead transcription factor family, including FoxO3. FoxO3 is involved in the transcription of apoptosis and cell cycle regulatory genes; the phosphorylation of these factors by AKT inactivates its function. The forkhead transcription factors control the survival of hematopoietic cells through their transcriptional activity on gene promoters of pro-apoptotic protein BIM and FAS-L, and it may influence the cell cycle progression through the transcription of cyclin dependent kinase inhibitors, such as p27kip1 and cycline D. Via the PI3K, FL activates anti-apoptotic signals in hematopoietic progenitors in a cytokine-dependent manner. The activation of PI3K and AKT is a major downstream pathway of intracellular signal transduction by which cytokines can support the survival of several cell-types.41–43
FLT3 and acute myeloid leukemia
FLT3-WT is expressed at high levels in a spectrum of several hematologic malignancies: 93% of AML cases, almost 100% of B-cell acute lymphoblastic leukemia (B-ALL), 87% of T-cell acute lymphoblastic leukemia (T-ALL) and in a small percentage of cases of chronic myeloid leukemia in blast crisis and chronic lymphoid leukemia. This suggests that the overexpression of this receptor may play a role in the survival and proliferation of leukemic cells.44–45 This abnormal expression is also found in human leukemia cell lines46–47 and, when combined with FLT3 ligand expression, leads to constitutive phosphorylation/activation of the receptor. These data indicate that the signaling of FLT3-WT may be important in certain subtypes of leukemia and may be involved in signaling pathways in an autocrine, paracrine or intracrine manner.37
In recent years, it has been shown that somatic activating mutations of the FLT3 gene are the most common genetic abnormalities in AML and have a significant impact on prognosis.44
About 30% of cases of AML at diagnosis have mutations of the FLT3 gene; in particular, 70% of patients with normal karyotype and 35% of patients with t(15;17), and the frequency is higher in de novo AML (26%) than in secondary AML (9%). Female patients are affected more frequently, and these mutations are associated with hypercellularity and a higher incidence of recurrence.48
Genetic alterations of FLT3 cause the expression of a constitutively activated tyrosine kinase receptor in AML blasts, and this activation is implicated in leukemogenesis, regulating the functional characteristics of leukemic blasts.49 In AML patients, two main types of activating mutations have been identified: ITD in the region coding for the JM domain, determined by the insertion of repeated amino acid sequences, and point mutations that cause amino acid substitution in the activation loop of the TKD.50–52
Internal tandem duplications
The ITDs are variable duplications of a number of bases that are multiples of three, preserving the reading frame, in exons 14 and 15, coding for the juxtamembrane domain.53 These duplications, whose length can vary from 3 to more than 400 base pairs, result in the insertion of repeated amino acid sequences in variable positions of the JM domain, and are not identifiable through traditional cytogenetic methods (Figure 2). Often there is also the insertion of three or six additional base pairs of unknown origin that leads to the addition of one or two new amino acids before the repeat region.22 The ITDs are formed when a fragment of the coding region for the JM domain is duplicated and inserted in direct head-to-tail orientation.20 Most ITDs occur at the 5’ of the JM domain (carboxy-terminal region of the domain) in exon 14; however, recent research has established that approximately 33% of ITDs occur within the tyrosine kinase domain.54 In addition, a new class of activating point mutations in the JM domain (JM-PMs) was reported in AML patients; although these mutations lead to constitutive autophosphorylation, their biological and clinical role still remains to be clarified55–56 (Figure 2).
The ITD mutation results in the ligand-independent dimerization and phosphorylation of the receptor causing phosphorylation of the WT receptor expressed by the same cell. While an unmutated receptor in the JM domain has an -helix conformation that blocks the activation of the kinase and can inhibit the self-dimerization, the presence of an ITD can prevent the association between the JM domain and the kinase domain, exposing it to constitutive activation.44 The presence of the duplication leads, therefore, to weak auto-inhibitory activity of the juxtamembrana domain, resulting in a conformational change from an inactive to a catalytically active state, even in the absence of the ligand.12
The conformational change in the JM domain promotes the ligand-independent dimerization and the constitutive autophosphorylation and activation of the receptor, resulting in cytokine-independent proliferation and in blocking myeloid differentiation of hematopoietic pro-genitors due to the inappropriate activation of signal pathways.57 It has been shown that the ITD and WT kinase receptors regulate downstream proteins in different ways: the mutated receptor activates the RAS and PI3K pathways in a manner similar to FLT3-WT. But, in this case, the activation of STAT5 (signal transducer and activator of transcription 5) plays a more critical role: STAT5 phosphorylated by FLT3-WT does not bind to the DNA, cells harboring FLT3-ITD have a high level of STAT5 phosphorylation and they show the binding of this transcription factor to DNA.20,23,58 Activation of STAT5 is critical for cell growth in association with the activation of MAPK, and its anti-apoptotic function is mediated by transcriptional regulation of cycline D1, BCL-XL,59 PIM serine-threonine kinases, p21WAF1/CIP1 (inhibitor of cyclin-dependent kinases) and c-MYC.60–64
Through a mechanism that is still not fully understood, FLT3-ITD is also able to inhibit the function of silencing mediator of retinoic acid tyroide hormone receptors (SMRT), a co-repressor that interacts with promyelocitic leukemia zinc finger (PLZF) and eight twenty one (ETO), and is involved in blocking proliferation through silencing of target genes involved in the regulation of cell growth.65
It has been reported that FLT3-ITD expression in Ba/f3 cells resulted in the activation of AKT and in the concomitant phosphorylation of the Forkhead family member FoxO3a. FoxO3a phosphorylation on threonine 32 through FLT3-ITD signaling promotes its translocation from the nucleus to the cytoplasm. Specifically, FLT3-ITD expression prevents FoxO3a-mediated apoptosis and the upregulation of p27Kip1 and Bim gene expression, suggesting that the oncogenic tyrosine kinase can negatively regulate FoxO transcription factors through FoxO3a phosphorylation, leading to the suppression of its function. This then promotes the survival and proliferation of AML cells.14,64–66
There are marked differences in expression and function of several myeloid transcription factors among the wild-type and mutated receptors. In FLT3-ITD expressing cells, the transcription factors PU.1, a member of the ETS family of transcription factors (EZB transformation-specific sequence), and C/EBP (CCAAT/enhancer-binding protein), both implicated in normal myeloid development, are repressed.67 Furthermore, the effects of FLT3-ITD on the activation of downstream signaling may be increased as a result of inhibition of cellular phosphatases, such as SHP-1, which can lead to amplification of the proliferative and anti-apoptotic effects.37,68
In addition, Tickenbrock et al. reported that FLT3-ITD induces expression of the receptor Frz-4 (Frizzled-4), making it active even in the absence of its natural ligand Wnt.69 The Wnt signaling pathway has important functions in cell fate decisions during embryonal development and in the adult organism, and it is implicated in hematopoietic stem cell self-renewal and proliferation. Deregulation of its activity is implicated in carcinogenic processes, and several molecules downstream of Wnt act as either tumor suppressors or proto-oncogenes.69 The activation of Frz-4 regulates the stability of the transcriptional coactivator β-catenin that, in the absence of Wnt ligand in the cytoplasm, is associated with protein APC (adenomatous polyposis coli), Axin and the serine-threonine kinase GSK 3b. After Wnt binding, the non-phosphorylated β-catenin accumulates in the cytoplasm, translocates into the nucleus and acts as a transcriptional coactivator of transcription factors TCF/LEF, target genes for transcription, including c-myc and cyclic D1. Activation of TCF transcriptional activity by FLT3-ITD is functionally relevant for the transforming activity of the mutant receptor. FLT3-ITD induces higher levels of β-catenin expression, associated with increased stability and, therefore, promotes the TCF/LEF-dependent transcriptional activity in the absence of Wnt ligand. The activation of this signal cascade is involved in leukemic progression since it causes a significant increase in cell proliferation.69 The FLT3-ITD receptor can still maintain its ability to respond to FL. Indeed, in the presence of the ligand, there is additional AKT phosphorylation and increased MAPK activation (Figure 4).
Figure 4.
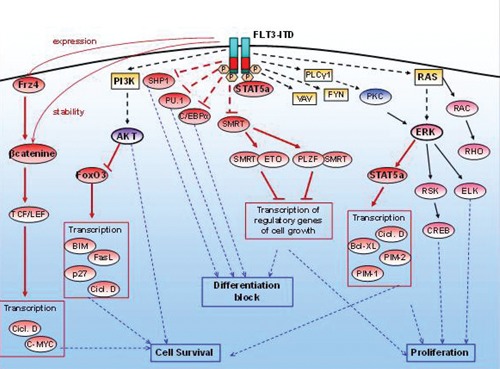
Signaling pathways activated by FLT3-ITD.
Tyrosine kinase domain mutations
The second type of mutations found in the FLT3 receptor is the replacement of an amino acid residue in the TKD (Figure 2). These substitutions are caused by missense mutations in the exon 20, involving the codons aspartic acid 835 (D835) and isoleucine 836 (I836). At least five different substitutions have been identified in the D835 codon: i) mainly tyrosine (D835Y), given by the mutation GAT → TAT; ii) less frequently, histidine (D835H), given by GAT → CAT mutation; iii) valine (D835V), given by the mutation GAT → GTT; vi) glutamate (D835E); and v) asparagine (D835N). The I836 codon is mutated to methionine (I836M) and asparagine (I836N).70–71
In addition, approximately 0–5% of de novo AML20,37,44,70 harbor a mutation in exon 20 that results in the insertion of a glycine and serine between amino acids 840 and 841. Recently, the replacement of tyrosine with a cysteine codon 842 (Y842C) and asparagine 841 with istidine (N841I) was discovered in patients with cytogenetic abnormalities who relapsed.72 Rarely, insertions of nucleotides and complex changes have been identified in the TKD domain deletions, but the sequence remains in frame. The amino acids replaced belong to the activation loop, which blocks the access of the ATP and the substrate to the kinase domain when the receptor is inactive. TKD mutations interfere with the inhibitory effect of the loop and lead to constitutive kinase activation, conferring growth factor independent proliferation through the activation of downstream target RAS/MAPK, PI3K/AKT and STAT5.41,73 In contrast to ITD, TKD mutations are not able to inhibit the transcription factors C/EBP and PU.1.74
Prognostic significance of FLT3 mutations in acute myeloid leukemia
Approximately 25–35% of adult AML patients and 10–17% of pediatric patients have ITD mutations in the FLT3 receptor, with a higher association with M3 subtype. In particular, these involve the variant BCR3, and M5 subtype with a lower frequency in M2, M6, and M7 sub-types.30,75–78 FLT3-ITD mutations are also closely correlated with specific cytogenetic abnormalities such as t(15;17) and MLL gene alterations. They are highly associated with increased white blood cell counts, high percentage of peripheral blood and bone marrow blasts, indicating that the mutation has a biological effect on proliferation.79 In addition, a significant proportion of patients (9–23%) have more than one ITD mutation, reflecting an underlying genetic instability.
FLT3-ITD has been found mainly in the heterozygous state, but there is also evidence of a partial or complete loss of wild-type allele; a high ratio of ITD/WT is a further adverse clinical prognostic factor. Indeed, the presence of a hemizygous genotype ITD/- that is found in 1% of pediatric patients and in 5% of adult patients is associated with a distinct phenotype with a significantly worse clinical outcome.80 The presence of an ITD in adults has no impact on achieving complete remission (CR) but it is significantly correlated with an increased risk of relapse (RR), and reduced disease free and overall survival (OS).20,44,81 Therefore, after the karyotype, FLT3-ITD was found to be the most significant independent prognostic factor in predicting survival in patients under the age of 60 years.
In 2005, Falini et al. described a novel mutation within the NPM1 gene detected in 35% of AML patients. Like FLT3-ITDs, NPM1 mutations are significantly associated with cytogenetically normal AML, and a significant proportion of patients carry both FLT3-ITD and NPM1mut. NPM1 mutations are associated with a high rate of CR, an increase in event-free survival, and favorable OS. However, these positive effects are lost in the presence of a coexisting FLT3-ITD. Whether the genotype NPM1mut/FLT3-ITD is associated with intermediate or poor outcome is still under question.82
FLT3-TKD mutations occur more frequently in AML patients with MLL gene duplication, like the ITDs. The incidence of mutations in TKD is significantly lower than that of ITDs, with a frequency of 6–10% in AML, 2–5% in myelodysplastic syndromes (MDS) and 2–8% in ALL.70,83 Probably because of their low frequency, the prognostic impact of these mutations, both in terms of RR and OS, has not been definitively determined, but it was thought that it is still associated with reduced OS in intermediate risk patients under the age of 60 years; instead, there is no significant association with age, WBC count, percentage of blasts or cytogenetic profile.14,22,37,84–85
The prognostic significance of the differences between the ITDs and TKD mutations, which are supposed to have the same functional consequences, such as loss of auto-inhibitory control and constitutive kinase activity, might reflect different levels of constitutive activation. This might also be a consequence of the different signaling pathways triggered or might indicate that the ITD mutation is simply a marker of cellular genetic instability that causes other unknown mutations.44,70 The differences in the signaling pathway of FLT3 mutations could, therefore, have important implications for their transforming ability and for the design of mutation-specific therapeutic approaches.86
All changes in the kinase domain, however, induce same alterations in downstream signaling and have similar biological consequences other than tandem duplication. This suggests that ITDs and TKD mutations can be considered functionally different classes with different transforming potential and different sensitivity to the action of inhibitors.86 Both mutations (FLT3-ITD-TKD) on the same allele are detected in a rare percentage of AML patients at diagnosis (1–2%), with a worse clinical outcome than patients with single mutations. The dual expression of mutants in hematopoietic cells causes resistance to tyrosine kinase inhibitors and cytotoxic drugs. This is because the altered receptor shows increased kinase activity and causes cell cycle arrest at the G2/M checkpoint, and hyperactivity of STAT5 and its anti-apoptotic target genes BCL-XL and RAD51, already identified to form the basis of the drug resistance mechanism.87
A second alteration may be acquired in the course of the disease or during prolonged exposure to TK inhibitors, leading to the emergence of a resistant phenotype to the low affinity receptor inhibitor or increased catalytic activity.87–88
Targeted-therapies for FLT3 mutated cells
The main objective in the treatment of AML is inducing remission and preventing relapse. Conventional therapy is based on the administration of cytotoxic agents with the aim of reducing and eventually eradicating the leukemic population, and allowing the remaining stem cells to repopulate the bone marrow. Targeted therapy is a new type of cancer treatment using drugs directed against specific genetic or other abnormalities related to the leukemic cell clone. It is thought to diminish toxicity in healthy tissues and to increase the specificity of the target malignant cells.89 This therapy interferes with specific molecular pathways that are important for the genesis and/or maintenance of the malignant phenotype. This is in contrast to conventional chemotherapy agents which interfere with some aspects of the global cellular machinery, shared by non-malignant and malignant cells.
Some of the main inhibitors for targeted therapy of AML include: hypomethylating agents, multidrug resistance modulators, biological agents, and agents that modulate intracellular signaling pathways (Table 1).
Table 1. Summary of the main inhibitors for acute myeloid leukemia targeted therapy.
| Agents | Target and mechanisms of actions |
|---|---|
| Hypomethilating agents | Hypermethylation at CpG islands within promoter region can lead to silencing of tumor-suppressor genes and gene inactivation, acting as an alternative mechanism to deletions and mutations; the methylation of tumor-suppressor genes and silencing of DNA are chromatin remodeling factors that can contribute to carcinogenesis. |
| MDR modulators | Over-expression of multidrug resistance 1(MDR1) gene, encoding P-glycoprotein, reduces the intracellular drugs accumulation, causing resistance to chemotherapy treatment. |
| Biological agents | Anti-CD33 monoclonal antibodies (trigger downstream signaling cascade and lead to antiproliferative effect); histone deacetylases inhibitors (HDAC removing the acetyl group causes a tighter binding of histones to DNA, preventing gene transcription). |
| Agents that modulate intracellular signaling pathways | RTKs, docking and adapter proteins and transcription factors are the classes of proteins involved in the signaling, and an inappropriate function of the members of each group was associated with hematological malignancies. Several aberrations have been described in RTKs genes: c-abl, c-fms, flt3, c-kit, PDGFRα/β, and consequently inappropriate activation of downstream signaling cascades, such as Jak-Stat, Ras/MAPK e PI3K/AKT. Each of these proteins may be a therapeutic target, as well as factors directly involved in the regulation of apoptosis (BCL-2, NFkB, caspases, cyclins, inhibitors of cyclins, transcription factors). |
MDR, multidrug resistance; RTK, receptor tyrosine kinase; PI3K, phospatidylinositol-3-kinase; AKT, protein kinase B (PKB).
During the past decade, the identification of a constitutively active form of FLT3 as a possible mechanism to promote the progression of leukemogenesis has suggested exploiting this receptor as a therapeutic target, fueling the study and research for selective FLT3 tyrosine kinase inhibitors. Many of these, however, have inhibitory activity against other RTKs due to the structural homology of this receptor and causing possible side-effects because they bind to the active site of protein competing for the binding site for ATP. The mechanism of inhibition of tyrosine kinase inhibitors is similar, although they have a different structure, because it can bind the protein in a transition state, from the active to the inactive form.90
In this context, several promising compounds are undergoing clinical and experimental assessment. Lestaurtinib and midostaurin, known as CEP-701 and PKC412, respectively, are the most extensively studied FLT3 inhibitors and are of all clinical trials in AML the most advanced. Lestaurtinib is a multitargeted tyrosine kinase inhibitor that pre-clinical studies have shown to strongly inhibit FLT3 at nanomolar concentrations. In a phase I/II trial that examined patients with refractory or relapsed AML and FLT3 activating mutation, this agent showed a good tolerability profile and clinical activity. Fourteen patients received lestaurtinib 60 mg twice daily. Clinical activity was observed in 5 patients, including significant reductions in bone marrow and peripheral blood blasts from 25% to less than 5% at Day 28 of therapy. Lestaurtinib-related toxicities were minimal: grade ½ nausea and emesis (41% and 29%, respectively) and grade ¾ generalized weakness (18%).91 In a second separate phase II trial, lestaurtinib 60 mg twice daily was tested as first-line treatment in elderly patients with AML not considered fit for standard chemotherapy. Unlike the previous study, similar adverse events were observed. Clinical response has shown transient hematologic responses in 3 of 5 patients (60%) with FLT3 mutation and in 5 of 23 patients with FLT3 wild type.92–93 Other phase III studies are ongoing to determine the future of lestaurtinib as a treatment option for patients with FLT3-ITD AML, both in monotherapy and in combination with cytotoxic drugs. However, the results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutation in AML in first relapse are not encouraging. This trial reported that lestaurtinib treatment after chemotherapy did not increase response rate or prolong survival.94
Midostaurin, initially developed as a protein kinase C inhibitor, showed inhibitory activity against class III RTKs, such as c-KIT, FMS, PDGFR, VEGFR 2, as well as FLT3. As a derivate of staurosporine, midostaurin is biologically active by binding to the ATP-binding pocket of FLT3 so that it inhibits activation and tyrosine autophosphorylation. Midostaurin safety and tolerability were examined in a phase I study.95 A recent phase IIb trial reported on administration of midostaurin at two different dosages (50 or 100 mg twice daily) in 95 patients with AML or MDS with either FLT3-WT or FLT3 mutant. The investigators reported that the response rate of peripheral blood or bone marrow was 71% in FLT3 mutant patients and 42% in FLT3-WT patients. One partial response (PR) occurred in a patient with FLT3-mutant receiving the 100 mL dose regime.96 After these encouraging results, a multi-center phase III study has been started to test midostaurin in combination with daunorubicin and cytarabin in newly diagnosed FLT3 mutant AML patients.97
The MEK/MAPK pathway is a signaling cascade involved in the control of hematopoietic cell proliferation and differentiation. Downregulation of MEK phosphorylation inhibits proliferation and induces apoptosis of primary AML blasts.65 Sorafenib (BAY 43-9006) is a small oral molecule originally designed as an inhibitor of Raf-1 kinase targeting the RAF/MEK/ERK pathway. It has inhibitory properties against a number of other kinases, including FLT3 and vascular endothelial growth factor receptor.98–99 In pre-clinical studies, sorafenib induced dephosphorylation of MEK1/2 and ERK and induced apoptosis in AML cells.100 In a mouse leukemia model with mutant FLT3, sorafenib reduced leukemic burden and prolonged survival.101 A phase I study showed that sorafenib was well tolerated when investigated as single agent against FLT3-mutant AML. Although patients treated with one cycle of sorafenib achieved a marked decrease in peripheral blood and bone marrow blasts, this response was transient.102 In newly diagnosed cases of AML patients under the age of 65 years, the combination of sorafenib 400 mg twice daily with cytarabine and idarubicin has produced a high rate of CR in FLT3-ITD patients (93%) and inhibited FLT3 signaling.103
AC220 is the most recent kinase inhibitor of FLT3 under clinical investigation.104 AC220 inhibits FLT3 at low nanomolar concentrations in cellular assays and is highly selective when screened against the majority of the human kinome. Zarrinkar et al. showed that AC220 is much more selective than CEP-701, PKC-412, MLN-518, sunitinib or sorafenib.105 AC220 inhibits FLT3 activity in vivo, significantly extended survival in a mouse model of FLT3-ITD AML at doses as low as 1 mg/kg when given orally once a day, eradicated tumors in an FLT3-dependent mouse xenograft model at 10 mg/kg, and strongly inhibited FLT3 activity in primary human cells.105 A phase I study of single agent AC220 in 76 patients with FLT3-WT and FLT3-ITD in relapsed/refractory AML confirmed its significant clinical activity; 13% of patients achieved CR and 17% achieved PR. AC220 induced a rapid and durable response of up to 67 weeks, and higher overall response (56%) and CR (28%) rates were observed in FLT3-ITD mutations compared to FLT3-WT.106 Interim data from a phase II study of AC220 as single agent in relapsed/refractory patients with FLT3 mutations were presented at the 2011 Congress of the European Hematology Association. The clinical response data reported that a group of 53 patients achieved a CR rate of 45% and an additional 25% achieved PR.107
Another approach in target therapy is the development of anti-FLT3 monoclonal antibody, such as IMC-EB10 that blocks signaling by binding to the receptor and induces antibody-dependent cell-mediated cytotoxicity. Pre-clinical studies have shown the antiproliferative effects of IMC-EB10 against both wild-type and mutant FLT3 AML models.102
So far, several clinical trials have shown that the use of FLT3 inhibitors as monotherapy, although well tolerated, is limited by transient or partial clinical response. Furthermore, there is growing evidence to suggest that this partial clinical response is associated with multiple factors of resistance to FLT3 inhibitors.108 Consequently, in attempt to overcome resistance, several agents in combination with standard chemotherapy are under clinical investigation.
Discussion
The identification of FLT3 expression levels and its molecular mutations represent new opportunities in the treatment of AML. Over the last decade, the biology and the function of the wild-type and mutated FLT3 receptor have been well characterized. Equally, the relationship between FLT3 and new molecular alterations, such as NPM1, is well known. Various mechanisms of FLT3 activations may be present in different AML patients: FLT3 mutations are associated with a constitutive tyrosine kinase activity, consequently the inhibition of phosphorylated targets is growing in importance.109 Up to now, different compounds, including lestaurtinib, sunitinib, midostaurin and AC220 have been investigated in vitro and in vivo as FLT3 inhibitors.59 However, clinical trials have produced only partial and transitory results. Therefore, additional data are required to optimize treatment and, in particular, to investigate the underlying causes of resistance to FLT3 inhibitors, such as the role of the deregulation signaling pathways, the aberrant expression of antiapoptotic proteins or the acquisition of genetic mutations. Certainly, the role of the FLT3 ligand should be taken into consideration since recent evidence suggests that the ITD-mutated receptor is heavily influenced by FL.110 As reported by Sato et al., the FL ligand interferes with the ability of FLT3 inhibitors to block FLT3 signaling, at least in vitro.111 Current clinical trials are combining FLT3 inhibitors with conventional chemotherapy in an attempt to increase the cytotoxic effect against leukemia cells and reverse the poor prognosis for AML patients with FLT3 mutations. But treatment of patients with chemotherapy leads to high levels of FL that may be responsible for the generally poor level of in vivo FLT3 inhibition. Despite the considerable progress made in understanding the molecular mechanisms underlying the onset of AML, there is still no definitive cure. Therefore, based on our acquired knowledge, the challenge for the future will be to define appropriate therapeutic strategies that take into account the complex biological system of AML with an FLT3-ITD mutation.
Acknowledgements:
the authors would like to thank the Italian Association against Leukemia, Lymphomas and Myeloma (AIL) Campobasso for its continued support.
References
- 1.Irons RD, Stillman WS. The process of leukemogenesis. Environ Health Perspect. 1996;104:1239–46. doi: 10.1289/ehp.961041239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilliland GD. Molecular genetics of human leukemias: new insights into therapy. Semin Hematol. 2002;39:6–11. doi: 10.1053/shem.2002.36921. [DOI] [PubMed] [Google Scholar]
- 3.Motyckova G, Stone RM. The role of molecular tests in acute myelogenous leukemia treatment decisions. Curr Hematol Malig Rep. 2010;5:109–117. doi: 10.1007/s11899-010-0049-7. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Vardiman JW, Thiele J, Arber D, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukaemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 6.Rosnet O, Mattei MG, Marchetto S, Birnbaum D. Isolation and chromosomal localization of a novel FMS-like tyrosine kinase gene. Genomics. 1991;9:380–5. doi: 10.1016/0888-7543(91)90270-o. [DOI] [PubMed] [Google Scholar]
- 7.Carow CE, Kim E, Hawkins AL, et al. Localization of the human stem cell tyrosine kinase -1 gene (FLT3) to 13q12-->q13. Cytogenet Cell Genet. 1995;70:255–7. doi: 10.1159/000134046. [DOI] [PubMed] [Google Scholar]
- 8.Rosnet O. FLT3 (Fms-like tyrosine kinase3) Atlas Genet Cytogenet Oncol Haematol. 1999;3:73–4. [Google Scholar]
- 9.Agnes F, Shamoon B, Dina C, et al. Genomic structure of the downstream part of the human FLT3 gene: exon/intron structure conservation among gene encoding receptor tyrosine kinases (RTK) of subclass III. Gene. 1994;145:283–8. doi: 10.1016/0378-1119(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Genomic structure of human FLT3: implications for mutational analysis. Br J Haematol. 2001;113:1076–7. doi: 10.1046/j.1365-2141.2001.02821.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosnet O, Schiff C, Pebusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–9. [PubMed] [Google Scholar]
- 12.Griffith J, Black J, Faerman L, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13:169–78. doi: 10.1016/s1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 13.Lyman SD, Jacobsen SE. c-kit ligand and flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91:1101–34. [PubMed] [Google Scholar]
- 14.Markovic A, Mackenzie KL, Lock RB. FLT3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol. 2005;37:1168–72. doi: 10.1016/j.biocel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Rosnet O, Buhring HJ, Marchetto S, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia. 1996;10:238–48. [PubMed] [Google Scholar]
- 16.Maroc N, Rottapel R, Rosnet O, et al. Biochemical characterization and analysis of the transforming potential of the FLT3/FLK2 receptor tyrosine kinase. Oncogene. 1993;8:909–18. [PubMed] [Google Scholar]
- 17.Kikushige Y, Yoshimoto G, Miyamoto T, et al. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/ macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180:7358–67. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 18.Kindler T, Lipka DB, Fisher T. FLT3 as a therapeutic target in AML: still changes after all these years. Blood. 2010;116:5089–102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 19.Gotze K, Ramirez M, Tabor K, et al. Flt3 high and Flt3 low CD34+ progenitor cell isolated from human bone marrow are functionally distinct. Blood. 1998;91:1947–58. [PubMed] [Google Scholar]
- 20.Stirewalt DL, Radich JP. The role of FLT3 in hematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 21.Del Zotto G, Luchetti F, Zamai L. CD135. J Biol Regul Homeost Agents. 2001;15:103–16. [PubMed] [Google Scholar]
- 22.Brown P, Small D. FLT3 inhibitors: a paradigm of the development of targeted therapeutics for pediatric cancer. Eur J Cancer. 2004;40:707–21. doi: 10.1016/j.ejca.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Cheetham GM. Novel protein kinases and molecular mechanisms of autoinhibition. Curr Opin Struct Biol. 2004;14:700–5. doi: 10.1016/j.sbi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Lyman SD. Biology of flt3 ligand and receptor. Int J Hematol. 1995;62:63–73. doi: 10.1016/0925-5710(95)00389-a. [DOI] [PubMed] [Google Scholar]
- 25.Wodnar-Filipowicz A. FLT3 ligand: role in control of hematopoietic and immune functions of the bone marrow. News Physiol Sci. 2003;18:247–51. doi: 10.1152/nips.01452.2003. [DOI] [PubMed] [Google Scholar]
- 26.Lisovsky M, Braun SE, Ge Y, et al. Flt3-ligand production by human bone marrow stromal cells. Leukemia. 1996;10:1012–8. [PubMed] [Google Scholar]
- 27.Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kKinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157–67. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 28.Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–801. [PubMed] [Google Scholar]
- 29.Yonemura Y, Ku H, Lyman SD, Ogawa M. In vitro expansion of hematopoietic progenitors and maintenance of stem cells: comparison between FLT3/FLK-2 ligand and KIT ligand. Blood. 1997;89:1915–21. [PubMed] [Google Scholar]
- 30.Gilliland GD, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 31.Jonssön M, Engström M, Jönsson JI. FLT3 Ligand regulates apoptosis through AKT-dependent inactivation of transcription factor FoxO3. Biochem Biophys Res Commun. 2004;318:899–903. doi: 10.1016/j.bbrc.2004.04.110. [DOI] [PubMed] [Google Scholar]
- 32.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–74. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 33.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–74. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 34.Scholl C, Gilliland DG, Frohling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol. 2008;35:336–45. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Mantel C, Broxmeyer HE. Flt3 signaling involves tyrosylphosphorylation of SHP-2 and SHIP and their association with Grb2 and Shc in Baf3/Flt3 cells. J Leukoc Biol. 1999;65:372–80. doi: 10.1002/jlb.65.3.372. [DOI] [PubMed] [Google Scholar]
- 36.Marchetto S, Fournier E, Beslu N, et al. SHC and SHIP phosphorylation and interaction in response to activation of the FLT3 receptor. Leukemia. 1999;13:1374–82. doi: 10.1038/sj.leu.2401527. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Broxmeyer HE. Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp2, grb2 and PI3 kinase. Biochem Biophys Res Commun. 2000;277:195–9. doi: 10.1006/bbrc.2000.3662. [DOI] [PubMed] [Google Scholar]
- 38.Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1:89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altmann JK, Sassano A, Platanias LC. Targeting mTor for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–7. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through pim1. Br J Haematol. 2006;134:500–9. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 41.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 42.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–7. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Gan B, Liu D, Paik J. FOXO family member in cancer. Cancer Biol Ther. 2011;12:253–9. doi: 10.4161/cbt.12.4.15954. [DOI] [PubMed] [Google Scholar]
- 44.Kottaridis PD, Gale RE, Linch DC. FLT3 mutations and leukaemia. Br J Hematol. 2003;122:523–38. doi: 10.1046/j.1365-2141.2003.04500.x. [DOI] [PubMed] [Google Scholar]
- 45.Martinelli G, Piccaluga PP, Lo Coco F. FLT3 Inhibition as tailored therapy for acute myeloid leukemia. Haematologica. 2003;88:4–8. [PubMed] [Google Scholar]
- 46.Dasilva N, Hu ZB, Ma W, et al. Expression of the FLT3 gene in human-lymphoma cell lines. Leukemia. 1994;8:885–8. [PubMed] [Google Scholar]
- 47.Meierhoff G, Dehmel U, Gruss HJ, et al. Expression of the FLT3 receptor and FLT3 ligand in human leukemia-lymphoma cell lines. Leukemia. 1995;9:1368–72. [PubMed] [Google Scholar]
- 48.Fenski R, Flesch K, Serve S, et al. Costitutive activation of FLT3 in acute myeloid leukemia and its consequences for growth of 32D cells. Br J Haematol. 2000;108:322–30. doi: 10.1046/j.1365-2141.2000.01831.x. [DOI] [PubMed] [Google Scholar]
- 49.Bruserud O, Hovland R, Wergeland L, et al. Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: a functional characterization of Flt3-ligand the effects in AML cell populations with and without genetic Flt3 abnormalities. Haematologica. 2003;88:416–28. [PubMed] [Google Scholar]
- 50.Bianchini M, Ottaviani E, Grafone T, et al. Rapid detection of Flt3 mutations in acute myeloid leukemia patients by denaturing HPLC. Clin Chem. 2003;49:1642–50. doi: 10.1373/49.10.1642. [DOI] [PubMed] [Google Scholar]
- 51.Piccaluga PP, Bianchini M, Martinelli G. Novel FLT3 point mutation in acute myeloid leukaemia. Lancet Oncol. 2003;4:604–604. doi: 10.1016/s1470-2045(03)01219-1. [DOI] [PubMed] [Google Scholar]
- 52.Sritana N, Auewarakul C. KIT and FLT3 receptor tyrosine kinase mutations in acute myeloid leukaemia with favorable cytogenetics: two novel mutations and selective occurrence in leukaemia subtypes and age groups. Exp Mol Pathol. 2008;85:227–31. doi: 10.1016/j.yexmp.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the gene flt3 found in acute myeloid leukemia. Leukemia. 1996;10:1911–8. [PubMed] [Google Scholar]
- 54.Breitenbuecher F, Schnittger S, Grundler R, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113:4074–7. doi: 10.1182/blood-2007-11-125476. [DOI] [PubMed] [Google Scholar]
- 55.Reindl C, Bagrintseva K, Vempati S, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. 2006;107:3700–7. doi: 10.1182/blood-2005-06-2596. [DOI] [PubMed] [Google Scholar]
- 56.Gianfelici V, Diverio D, Breccia M, et al. A novel point mutation within the juxtamembrane domain of the FLT3 gene in acute myeloid leukemia. Ann Hematol. 2010;90:845–6. doi: 10.1007/s00277-010-1092-0. [DOI] [PubMed] [Google Scholar]
- 57.Chung KY, Morrone G, Schuringa JJ, et al. Enforced expression of a Flt3 internal tandem duplication in human CD34+ cells confers properties of self-renewal and enhanced erythropoiesis. Blood. 2005;105:77–84. doi: 10.1182/blood-2003-12-4445. [DOI] [PubMed] [Google Scholar]
- 58.Hayakawa F, Towatari M, Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP Kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–31. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 59.Yonemura Y, Ku H, Lyman SD, Ogawa M. In vitro expansion of hematopoietic progenitors and maintenance of stem cells: comparison between FLT3/FLK-2 ligand and KIT ligand. Blood. 1997;89:1915–21. [PubMed] [Google Scholar]
- 60.Kim KT, Baird K, Ahn JY, et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105:1759–67. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi S, Harigae H, Kaku M, et al. FLT3 mutation activates p21WAF1/CIP1 gene expression through the action of STAT5. Biochem Biophys Res Commun. 2004;316:85–92. doi: 10.1016/j.bbrc.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Nosaka T, Kawashima T, Misawa K, et al. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–65. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizuki M, Fenski R, Halfter H, et al. FLT3 Mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–14. [PubMed] [Google Scholar]
- 64.Spiekermann K, Bagrintseva K, Schwab R, et al. Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res. 2003;9:2140–50. [PubMed] [Google Scholar]
- 65.Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol. 2011;4:13–13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheijen B, Ngo HT, Kang H, Griffin JD. FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene. 2004;23:3338–49. doi: 10.1038/sj.onc.1207456. [DOI] [PubMed] [Google Scholar]
- 67.Zheng R, Friedman AD, Levis M, et al. Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBP expression. Blood. 2004;103:1883–90. doi: 10.1182/blood-2003-06-1978. [DOI] [PubMed] [Google Scholar]
- 68.Chen P, Levis M, Brown P, et al. FLT3/ITD Mutation signaling includes suppression of SHP-1. J Biol Chem. 2005;280:5361–9. doi: 10.1074/jbc.M411974200. [DOI] [PubMed] [Google Scholar]
- 69.Tickenbrock L, Schwable J, Wiedehage M, et al. Flt3 tandem duplication mutations cooperate with Wnt signaling in leukemic signal transduction. Blood. 2005;105:3699–706. doi: 10.1182/blood-2004-07-2924. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 71.Sholl S, Krause C, Loncarevic IF, et al. Specific detection of FLT3 point mutations by highly sensitive real time polymerase chain reaction in acute myeloid leukemia. J Lab Clin Med. 2005;145:295–304. doi: 10.1016/j.lab.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Jiang J, Paez JG, Lee JC, et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood. 2004;104:1855–8. doi: 10.1182/blood-2004-02-0712. [DOI] [PubMed] [Google Scholar]
- 73.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–8. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 74.Choudhary C, Schwable J, Brandts C, et al. AML-associated Flt3 kinase domain mutations show signal transduction differences compared with ITD mutations. Blood. 2005;106:265–73. doi: 10.1182/blood-2004-07-2942. [DOI] [PubMed] [Google Scholar]
- 75.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 76.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 77.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 78.Levis M, Small D. ITDoes matter leukemia. 2003;17:1738–52. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 79.Au WY, Fung A, Chim CS, et al. FLT3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004;125:463–9. doi: 10.1111/j.1365-2141.2004.04935.x. [DOI] [PubMed] [Google Scholar]
- 80.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–9. [PubMed] [Google Scholar]
- 81.Shih LY, Lin TL, Wang PN, et al. Internal tandem duplication of Fms-like tyrosine kinase 3 is associated with poor outcome in patients with myelodysplastic syndrome. Cancer. 2004;101:989–98. doi: 10.1002/cncr.20440. [DOI] [PubMed] [Google Scholar]
- 82.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 83.Griffin DJ. Point mutations in the FLT3 gene in AML. Blood. 2001;97:2193–2193. doi: 10.1182/blood.v97.8.2193a. [DOI] [PubMed] [Google Scholar]
- 84.Carnicer MJ, Nomdedeu JF, Lasa A, et al. FLT3 mutations are associated with other molecular lesions in AML. Leuk Res. 2004;28:19–23. doi: 10.1016/s0145-2126(03)00125-5. [DOI] [PubMed] [Google Scholar]
- 85.Bagrintseva K, Schwab R, Kohl TM, et al. Mutations in the tyrosine kinase domain of FLT3 define a new molecular mechanism of acquired drug resistance to PTK inhibitors in FLT3-ITD-transformed hematopoietic cells. Blood. 2004;103:2266–75. doi: 10.1182/blood-2003-05-1653. [DOI] [PubMed] [Google Scholar]
- 86.Choudhary C, Schwable J, Brandts C, et al. AML-associated FLT3 kinase domain mutations show signal transduction differences in comparison to FLT3 ITD mutations. Blood. 2005;106:265–73. doi: 10.1182/blood-2004-07-2942. [DOI] [PubMed] [Google Scholar]
- 87.Bagrintseva K, Geisenhof S, Kern R, et al. FLT3-ITD-TKD dual mutants associated AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L) Blood. 2005;105:3679–85. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 88.Tiesmeier J, Muller-Tidow C, Westermann A, et al. Evolution of FLT3-ITD and D835 activating point mutations in relapsing acute myeloid leukemia and response to salvage therapy. Leuk Res. 2004;28:1069–74. doi: 10.1016/j.leukres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 89.Zwaan CM, Kaspers GJ. Possibilities for tailored and targeted therapy in pediatric acute myeloid leukaemia. Br J Hematol. 2004;127:264–79. doi: 10.1111/j.1365-2141.2004.05167.x. [DOI] [PubMed] [Google Scholar]
- 90.Sawyers CL. Finding the next Gleevec: FLT3 targeted kinase inhibitor therapy for acute myeloid leukemia. Cancer Cell. 2002;1:413–5. doi: 10.1016/s1535-6108(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 91.Shabbir M, Stuart R. Lestaurtinib, a multitargeted tyrosine kinase inhibitor: from bench to bedside. Expert Opin Invest Drugs. 2010;19:427–36. doi: 10.1517/13543781003598862. [DOI] [PubMed] [Google Scholar]
- 92.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chempotherapy. Blood. 2006;108:3262–70. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 93.Fathi A, Chen YB. Treatment of FLT3-ITD acute myeloid leukemia. Am J Blood Res. 2011;1:175–89. [PMC free article] [PubMed] [Google Scholar]
- 94.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvatage chemotherapy followed by lestaurtinib for patients with FLT3 mutant in AML in first relapse. Blood. 2011;117:3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PK412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–92. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 96.Fisher T, Stone MR, DeAngelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–45. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stölzel F, Steudel C, Oelschlägel U, et al. Mechanisms of resistance against PKC412 in resistant FLT3-ITD positive human acute myeloid leukemia cells. Ann Hematol. 2010;89:653–62. doi: 10.1007/s00277-009-0889-1. [DOI] [PubMed] [Google Scholar]
- 98.Wilhelm S, Carter C, Tang L, et al. Bay 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 99.Wilhelm S, Adnane L, Newell P, et al. Preclinical overwiev of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signalling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of intrinsecapoptotic pathway. Leukemia. 2008;22:808–18. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Konopleva M, Shi Y, et al. Mutant FLT3: a direct target of sorafenib in AML. J Natl Cancer Inst. 2008;100:184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 102.Pemmaraju N, Kantarjian H, Ravandi F, Cortes J. FLT3 inhibitors in the treatment of acute myeloid leukemia. Cancer. 2011;117:3293–304. doi: 10.1002/cncr.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–60. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fathi A, Lewis M. Flt3 inhibitors: a story of the old and the new. Curr Opin Hematol. 2011;18:71–6. doi: 10.1097/MOH.0b013e3283439a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zarrinkar PP, Gunawardane RN, Cramer Gardner MF, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–92. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cortes J, Foran J, Ghirdaladze D, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a firstin-human (FIH) phase 1 AML study. Blood (ASH Annual Meeting Abstracts) 2009;114:636–636. Abstract. [Google Scholar]
- 107.Levis MA. Phase II open-label, AC220 monotherapy efficacy (ACE) study in patients with acute myeloid leukemia (AML) with FLT3-ITD activating mutations: interim results. Congress of the European Hematology Association. 2011:1019–1019. Abstract. [Google Scholar]
- 108.Weisberg E, Sattler M, Ray A, Griffin JD. Drug resistance in mutant FLT3 positive AML. Oncogene. 2010;29:5120–34. doi: 10.1038/onc.2010.273. [DOI] [PubMed] [Google Scholar]
- 109.Grafone T, Palmisano M, Nicci C, et al. Monitoring of FLT3 phosphorylation status and its response to drugs by flow cytometry in AML blast cells. Hematol Oncol. 2008;26:159–66. doi: 10.1002/hon.854. [DOI] [PubMed] [Google Scholar]
- 110.Levis M. FLT3/ITD AML and the law of unintended consequences. Blood. 2011;117:6987–90. doi: 10.1182/blood-2011-03-340273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117:3286–93. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]


